Abstract
Rural, ethnically diverse residents face at least twice the risk of Alzheimer’s disease than urban residents. Chronic diseases such as diabetes and hypertension which increase dementia risk are more prevalent in rural areas with less access to specialty providers. A home-based approach for increasing dementia detection and treatment rates was tested among rural residents of government-assisted independent living facilities (N = 139; 78% non-White, and 70% with health literacy below 5th grade). Of 28 residents identified at risk during cognitive screening, 25 agreed to further in-depth assessment by adult gerontological nurse practitioners (AGNP). Fifteen of 25 (60%) completing consequent primary provider referrals were diagnosed with dementia and receiving new care (statistically significant; [χ2(1) = 76.67, p < .001, Phi = 0.743]). Home-based dementia management through a community engagement approach can help to meet the Healthy People 2030 goals of earlier detection and treatment and reduce the length of costly institutionalizations.
Keywords: Rural, Ethnically diverse older adults, Dementia detection, AGNP, Subsidized Housing
Of the 5.7 million Americans with Alzheimer’s disease and related dementias,1 60% are not detected until the moderate stage of the illness. 1–3 This diagnostic, social, and financial burden of ADRD may be magnified in rural, ethnically diverse older residents.4–6 Rural residents face twice the risk of developing ADRD than urban residents for multiple reasons including increased risk factors for ADRD such as diabetes, smoking, and hypertension.7–12 Hispanic and African Americans experience 1.8 to 2.5 times greater risk of ADRD than Whites.1,4–6 Root causes contributing to these increased risks include less education, low health literacy, low income, poor nutrition, insufficient access to primary and specialty providers, lack of insurance, and inadequate knowledge navigating a limited healthcare system.9–12
Alzheimer’s disease incidence increases with advancing age, and a higher percentage of older adults are found in rural rather than urban communities.9 Rural areas are also becoming increasingly more diverse. Racial and ethnic minorities contributed up to 75% of rural and small-town growth between 2000 and 2010.10 ADRD diagnoses among minorities has been recognized as occurring less and/or later in the illness than among Whites.13 Furthermore, researchers found that missed opportunities for dementia detection among ethnic minorities combined with rural residence led to 11% lower Medicare-associated diagnostic rate in rural counties (95% CI: 9%–13%).9 This disparity is compounded by low rates of education and low health literacy in rural areas.14 Lack of education has recently been linked to less cognitive reserve and triggering of neuropathological developments by leading experts in the field of dementia prevention.15,16 Low levels of health literacy have been linked to increased risk of dementia.17 In a recent systematic review of health literacy and incident dementia, researchers discovered that adjusted models revealed a bidirectional relationship between health literacy and dementia.18 In a study of 853 persons aged 55–74, low health literacy was strongly associated with participants who tested as having cognitive impairment not dementia (CIND).19 In another recent study, researchers discovered that higher levels of health literacy was associated with reduced conversion of mild cognitive impairment to dementia.20 Other investigators recently surmised that education about dementia is a public health priority, as level of knowledge about illness is often linked to risk perception, which influences health behaviors such as screening.21
Early and accurate diagnosis of ADRD at the mild cognitive impairment (MCI) stage, especially in higher risk rural underserved settings, could save up to 7.9 trillion dollars in health and long term care costs by 2050.1 Early detection of ADRD could offer benefits, such as discovering potentially reversible causes that can be treated, providing patients and families adequate time for advanced care planning, offering opportunities to participate in clinical trials, and initiating earlier treatment with currently available medications which may slow symptom progression.22,23 Although the debate regarding the usefulness of routine dementia screening continues,24 numerous protocols, algorithms, and policies to facilitate ADRD detection and treatment following brief cognitive assessment have been released by established national medical organizations.25 Despite these resources, few physicians appear to be accessing these tools or conducting brief cognitive assessments in persons over 65.1,26 Although 80% of surveyed primary care practitioners (PCP) believed there was strong rationale for dementia screening, including financial, medical, and social reasons, only a few PCPs regularly conducted dementia screening.26 Brief, more accessible screening can address these problems.
Recent studies have focused largely on disclosing dementia diagnoses in populations outside the U.S. We located two occurring within the past five years in the U.S. which have important implications for nurses and adult gerontological nurse practitioners (AGNP) providing home-based care. Investigators conducting a recent systematic review of 54 qualitative, quantitative, and mixed method studies revealed that only 34% of primary care providers and 48% of specialists usually/routinely tell the person with dementia their diagnosis.27 Most providers (89% of providers and 97% of specialists) tell the family the diagnosis instead, to avoid causing the patient distress.27,28 However, researchers found that even a small amount of time building rapport with patients prior to disclosing a dementia diagnosis led to reduced distress.29 They stated that dementia disclosure should emphasize patient-centered communication to minimize psychological distress following diagnosis.29 Community based-nurses are well positioned for providing compassionate patient-centered care.
Support for home-based visits by nurse practitioners
Further support for the need for the current study was identified in a systematic review of research focusing on the impact of nurse-led home health promotion activities. They reported that several health outcomes were positively impacted by at-home nursing care for older adults.30 Although cardiorespiratory, neurological, and orthopedic conditions were investigated, ADRD was not included. In the present study, cognitive function was the primary focus of the AGNP assessment.
A sentinel study featuring home visits by gerontological nurse practitioners (AGNP) provided context for the current of AGNP-led home-based care. To improve early detection of health problems and risk factors in older adults living at home, AGNPs performed a comprehensive assessment for cognitive impairment and depression followed by referrals to community providers as needed for further evaluation for dementia. Post-intervention, participants stayed in their homes significantly longer (p = .02) with fewer unmet care needs at 18 months as compared to control participants. Cost savings in 1995 were estimated at $48,000 per 100 persons, with savings of approximately $6000 for each year of life without disability/nursing home admission.31
Aims and hypothesis
The primary aim guiding this inquiry was to evaluate the feasibility of home-based memory assessment (HBA) by AGNPs to confirm cognitive risk for rural, older, and ethnically diverse adults when identified to be at risk via a brief dementia screening protocol conducted by research assistants. The objectives in meeting this aim were as follows: 1) Determine the proportion of potential participants who complete cognitive screening and screened positive for risk of cognitive impairment, 2) Determine the proportion of participants at risk for cognitive impairment who accepted a HBA conducted by the AGNP, 3) Determine the proportion of those referred by an AGNP who completed a follow up diagnostic evaluation with community provider, 4) Determine the degree of agreement between RA screening results, AGNP confirmation of cognitive risk, and community provider treatment for cognitive impairment, and 5) Assess the satisfaction, acceptance, and follow-though with the HBA in research participants and healthcare providers. We hypothesized that older age, greater years living in rural settings, less years of education, and lower health literacy will predict higher cognitive risk.
Methods
Design
A quantitative descriptive, correlational research design was used to evaluate the effectiveness of and satisfaction with a novel program to increase detection of previously undiagnosed ADRD in a sample of ethnically diverse rural older Floridians and to identify factoors that predicted cohniotive risk. All sociodemographic data, cognitive screening results, dementia-specific evaluations, follow-up appointments, and medical management were tracked and recorded in SPSS v24 (IBM, Armonk, NY)32 on a password protected computer with an encrypted server. The study was approved by the PI’s university Institutional Review Board.
Sample/Setting
This study took place in a culturally diverse, medically underserved area of southcentral rural Florida locally referred to as “The Glades”. Most of the community (78%) is comprised of African American, Afro-Caribbean, and Hispanic residents with 26.5% over age 65 compared to the national average of 18%.33,34 Persons aged 50 or older were recruited from three government-supported senior independent living communities. A total of 293 residents occupied the facilities at the time of the study.
Inclusion and exclusion criteria.
Community-dwelling residents age 50 or older, who spoke English, Spanish, or Creole, and were not previously diagnosed with dementia or depression, were eligible to enroll in the study. Participants who scored > 15 on the Center for Epidemiologic Studies (CES-D)35 were included and additionally referred for mental health assessment for depression. Visiting family members, friends, persons reporting a diagnosis of a dementia-related illness, or persons younger than 50 did not qualify for the study.
The sample size for this study was calculated using the same parameters as a prior study31 to increase health care access using nurse practitioners, with an effect size of 0.5, power of 0.8, alpha level of 0.05 (two-tailed), and GPower336 (Mann–Whitney–Wilcoxon test for Means between groups).37 With six independent variables (age, gender, race, ethnicity, health literacy, and education) and three dependent variables (screening, diagnosis, and treatment) the resulting recommendation for a′ priori sample size was 134. Based on our prior studies in similar ethnically diverse older adult populations,38,39 we expected an attrition rate of approximately 10%, and we recruited 140 participants.
Recruitment procedures included posting information about the study in the residents’ weekly bulletins written in English, Spanish, and Creole, which asked “Do you want to have your memory checked”? Facilities managers encouraged residents to attend if they were interested. After sociodemographic surveys were completed to determine eligibility, informed consent was obtained from all willing participants prior to study enrollment. Five-dollar gift cards to the dollar store within walking distance to the communities were offered as a thank-you for participating in the study.
Intervention
Research assistants (RAs) were sought from the surrounding community. Requirements included a high school degree, some type of engagement or experience in the health care field, and familiarity with the community. In addition to an English-speaking health services coordinator, a social worker and an LPN who spoke Haitian Creole or Spanish completed the research team. The RAs completed human protections training and an education program provided by the PI that addressed ADRD, study protocols, and correct administration of study measures. Return demonstrations of study procedures were conducted between research team members, and corrections made accordingly.
The trained RA’s pre-screened participants using the Mini-CogTM40 in common areas such as dining rooms and lounges reserved during study activity. Four separate stations were established at each facility to offer privacy during the interviews. The RAs also administered instruments measuring health literacy, depression risk, and basic knowledge of Alzheimer’s disease. This initial interview process lasted 20–30 min for each participant. The RAs immediately notified the PI of any Mini-Cog result below three, and the PI entered the station and asked the participant if they would like a visit by the nurse practitioner to receive a more in-depth health assessment. The PI scheduled the follow-up visits by telephone.
The AGNP conducted HBA with dementia-specific evaluations in the homes of the at-risk participants. The AGNP’s holistic approach included assessments of home safety, eye and ear, musculoskeletal, sleep and hygiene, social, spiritual, and mental health, including dementia-specific screening. Upon completion of the AGNP assessments, reports were mailed to providers. Participants were encouraged to follow-up with their provider, which was not an unrealistic expectation as almost all were being seen at least quarterly for management of diabetes and/or hypertension. Treatment results were evaluated by phone calls and visits to the participants within six months of the intervention. “Treatment” was operationalized as participants following up with the community provider and changes to medications or recommendations by the provider.
Measures
All measures were previously available and tested in Spanish, and all were translated into Haitian Creole for this study, using a three-stage process of translation, back translation, and piloted among older adults in a Haitian Creole church. The measures were administered in the lay participant’s preferred language by a trained tri-lingual lay health educator, was available during all study activities. Instruments administered during risk screening included the sociodemographic survey, the Center for Epidemiological Studies-Depression scale (CES-D)35, Mini-Cog,TM40 measure of health literacy (REALM-SV)41 and the basic knowledge of Alzheimer’s disease (BKAD).42,43
The sociodemographic survey
This instrument consisted of 13 questions about the six independent variables (age, gender, race, ethnicity, language spoken, education, and health literacy level) as well as questions regarding years lived in a rural area, marital and caregiver status, and if a healthcare provider had asked them about their memory or tested their memory during an office or emergency room visit.
The Mini-Cog™
The Mini-Cog™ is a brief (3–5 min) screening tool40 included in the Alzheimer’s Association workgroup suggestions for detecting cognitive impairment in older adults.44 The Mini-Cog™ demonstrated an original sensitivity39 of 0.85/95%; CI: 0.71, 0.98, with a specificity of 0.58/95%; CI: 0.46, 0.71, and has been used in multiple studies, including ethnically diverse populations.45–47 Potential participants are asked to listen to three spoken words and then draw a clock and place the numbers and hands on the clock. Following this, they are asked to repeat the three words. If they remember all three, they are determined to have normal cognitive function. If they missed any of the words, the Clock Drawing Test (CDT) is scored. A determination of normal cognitive function requires correct positioning of all the numbers. Two points are awarded if all numbers are drawn in the correct sequence and position, and the hands display the requested time. For scoring of the recall portion, one point is given for each correct word, with a possible score between 0 and 3. Combined with the CDT, an overall maximum Mini-Cog score is five.40 A score of 0–2 indicates a positive screen for dementia.40 In this study, if potential participants scored below 3, they were referred to the AGNP for further geriatric assessment and dementia-specific evaluation.
The rapid estimate of literacy in medicine, short form (REALM-SV)
The short form of the REALM-SV,41 a seven-word recognition test that has been used in multiple settings with a reported Cronbach’s alpha of 0.91 was used to test health literacy.48 Participants were asked to read the words aloud, and to say “blank” after five seconds for items that are problematic. The seven words were Menopause, Antibiotics, Exercise, Jaundice, Rectal, Anemia, and Behavior. The results were analyzed by grade levels from below third grade to high school and functional ability.
The center for epidemiological studies-depression scale (CES-D)34
As depression can be confused with, be comorbid with, or mask symptoms of ADRD the CES-D35 was used to screen participants for potential depression symptomatology, addressing limitations noted in other studies of dementia screening.35 The CES-D is a 20-item Likert measure of symptoms associated with depression. Overall scores range from 0 to 60. Higher scores indicate more depressive symptomatology; a cutoff score of 16 or greater indicates risk for clinical depression and need for referral for further evaluation.35 Participants in this study with scores of 16 or higher were referred by the nurse practitioner to a psychiatric mental healthcare provider. The CES-D has consistently demonstrated good specificity and high internal consistency with a Cronbach’s alpha of 0.90 across wide age ranges and is sensitive to differences between caregivers and non-caregivers.49 Prior tests suggest that the CES-D can be used appropriately with diverse populations,49 rendering it a good fit for this study.
The basic knowledge of Alzheimer’s disease (BKAD)
The BKAD is an AD knowledge measure designed by the principal investigator and colleagues. Results from the BKAD Phase 1 tests for stability, reliability including Rasch modeling, discrimination and point biserial indices, and concurrent, divergent, and construct were favorable: The test discriminated well between persons with higher and lower levels of education [F(2, 226) = 170.51, p = .001].42 Phase 2 testing in ethnically diverse Florida and Appalachian populations of Hispanic, Haitian, and African American residents resulted in a Cronbach’s alpha of 0.84.43 Significant relationships between education, health literacy and BKAD scores supported validity
Dementia-specific evaluation measures
The two measures that were administered to persons who were identified as needing, and agreed to, follow-up by the AGNPs for more-in depth gerontological and cognitive assessment were the Montreal Cognitive Assessment – Basic (MoCA-B)50 and Quick Dementia Rating System (QDRS).51 Both were available previously in Spanish. The QDRS was translated into Haitian Creole in the same manner as the screening measures.
The montreal cognitive assessment-basic (MoCA-B)
The Montreal Cognitive Assessment-Basic50 is a 15 to 20-minute test adapted from the MoCA52 to allow adjustments for those with low levels of education and health literacy. It also contains culturally relevant terms and images, and is available in 35 languages. The MoCA-B includes assessment for orientation, short-term memory through delayed recall, naming and language abilities, abstraction, executive function through visuospatial ability, and attention.50 A total score of 30 can be earned by completing all tests accurately. One point each is added to the test-taker’s score if he or she has four years or less of formal education, or is illiterate. Thus, a potential two points could be added to the total raw score. Internal consistency was averaged across studies with strong results of Cronbach’s alpha of 0.82 and test-retest of 0.91.50 It was used in this study to conduct further cognitive assessment in those scoring below 3 on the Mini-Cog.TM46
The quick dementia rating system (QDRS)
The QDRS,51 which gauges study eligibility and cognitive impairment without requiring extensive training, assisted with identifying persons with probable dementia. The 10 item QDRS is sensitive to detecting early cognitive changes regardless of etiology, and can be administered as either a self-report for informant interview.53 In psychometric testing, QDRS scores increased with higher clinical dementia ratings (CDR) staging and poorer neuropsychological performance (Ps < 0.001). The QDRS demonstrated construct validity against cognitive, behavioral, and functional measures (Ps 0.004 to < 0.001); and reliability (Cronbach α: 0.86–0.93). The QDRS demonstrated differential scores across different dementia etiologies. The AUC for the QDRS was 0.911 (95% confidence interval or CI 0.86–0.96) and for the CDR-SB was 0.996 (95% CI 0.99–1.0) demonstrating comparable ability to discriminate normal controls from dementia.51, p.1
There are ten QDRS domains 1) memory and recall; 2) orientation; 3) decision-making and problem-solving; 4) activities outside the home; 5) function at home, including hobbies 6) toileting and personal hygiene; 7) behavior and personality changes; 8) language and communication; 9) mood; and 10) attention and concentration. Scores range from 0 to 30; a higher score signals a greater risk of cognitive impairment.
Scores > 1.5 indicate a need for further assessment to establish a formal diagnosis. Scores 0 – 1.5 suggest that a dementing disorder is unlikely, but a very early disease process cannot be ruled out. More advanced assessment may be warranted in cases where other objective evidence of impairment exists.51,53 Although it has been tested among diverse ethnic participants for community surveys and preventive health, the QDRS had not been used widely in rural settings. The QDRS was completed for the participants scoring lower than 3 on the Mini-Cog™ with the assistance of the nurse and/or day manager who knew the residents.
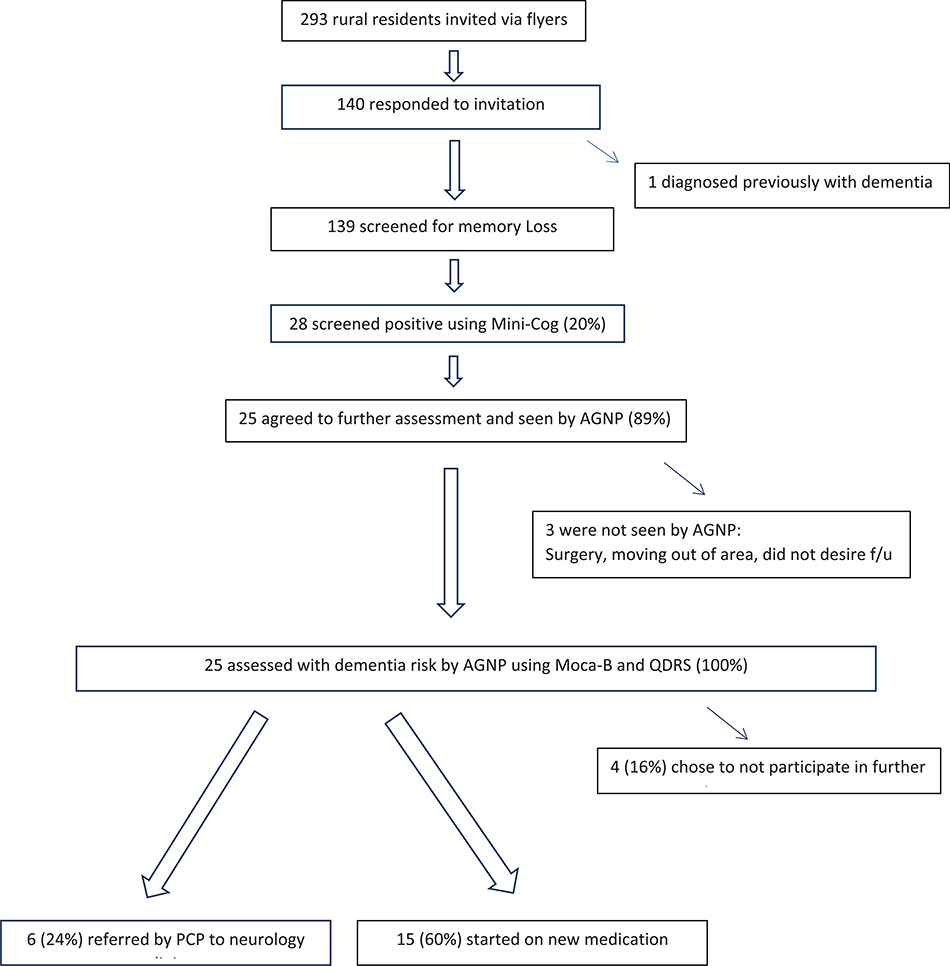
To solicit provider feedback regarding the AGNP home-based dementia-specific assessment approach, a brief survey of five structured questions with self-addressed and stamped envelopes and gift cards were sent to each of the study participants’ PCPs (n = 10). The questions, created with a local social worker who was well-known to the providers and residents, asked three yes/no questions asking the provider if 1) it was helpful to receive AGNP focused cognitive and physical assessment for their patients completed in the home setting, 2) if they did annual cognitive screening, and 3) if they were aware of the numerous tools and algorithms regarding detecting and treating Alzheimer’s disease and related dementias available on the National Institute of Aging’s (NIA) website.54 Two open-ended questions were what advice or feedback they had regarding the approach, and if there were programs that they would like to see offered in the community to support their care of older adults at risk for dementia. Fig. 1
Fig. 1.
Flowchart showing screening, dementia detection, referral, and diagnoses.
Results
Table 1 describes the sample characteristics by cognitive status. Participants were mostly women (60%) with a mean age of 69 (SD = 8.9). Years of education (M = 8.2; SD = 4.9) and health literacy were low (M = 3.3; SD = 2.7). Women comprised 60% of the sample and only 32% were married. All those enrolled in the study agreed to participate in pre-screening.
Table 1.
Sample characteristics related to cognitive status.
| Variable | Normal Cognition (n = 111) | Impaired Cognition (n = 28) | p value |
|---|---|---|---|
| Age, years | 69.2 (8.6) | 67.4 (8.7) | .336 |
| Education, years | 8.2 (5.0) | 8.2 (4.7) | .964 |
| Gender,% Female | 64.9 | 42.9 | .033 |
| Race/Ethnicity,% | .045 | ||
| White, Non-Hispanic | 4.5 | 3.6 | |
| African American | 85.6 | 78.6 | |
| Afro-Caribbean | 8.1 | 3.6 | |
| Hispanic | 1.8 | 10.7 | |
| Marital Status,% never married | 25.2 | 39.3 | .241 |
| Years living rural | 37.3(22.7) | 31.9 (18.9) | .246 |
| ADRD knowledge1 | 23.1 (5.1) | 19.2 (6.2) | .001 |
| Depression | 7.5 (6.9) | 10.7 (12.3) | .076 |
| Health Literacy | 3.3 (2.7) | 3.2 (2.8) | .885 |
Note: AD + Alzheimer’s disease.
Sample characteristics related to cognitive status
AD knowledge and health literacy
Mean BKAD scores indicating AD knowledge was 22 of 32 possible items suggesting moderate knowledge level. Health literacy was similar between groups. Fourteen (9.5%) of 133 participants completing the CES-D scored above the cutoff for depression,49 (M = 8.19; SD = 8.2) and were referred to their primary care provider for followup.
Objective 1
One hundred and forty of the 293 residents voluntarily appeared at either of the two screening days, and were invited to participate in determining if they met the inclusion criteria. One hundred thirty-nine participants meet the criteria and participated in the study. One participant disclosed that they had been previously diagnosed with Alzheimer’s disease. Twenty-eight participants out of the 139 pre-screened were identified as being at risk for cognitive impairment based on Mini-Cog™ scores. These residents were offered HBA follow-up visits by the AGNP (20%).
Objective 2
Twenty-five of 28 participants recommended for HBA agreed to participate in further evaluation (89.3%). One of the participants declined the HBA because of surgery the following week, another was moving to a different facility in northern Florida, and one did not want to have any further assessment, stating “I am doing fine”.
Objective 3
The results of HBA revealed that 25 participants were at risk for cognitive decline as determined by scores below the optimal cut-off of 26 on the 30-point MoCA-B, and QDRS scores above the cut-off of 1.5. MoCA-B scores were 17.4 ± 5.2 (range 9–25) suggesting individuals had very mild to moderately-severe cognitive performance. The QDRS scores were 5.8 ± 2.9 (range 1.0–11.0) suggested individuals had no impairment to moderate cognitive impairment. The QDRS and MoCA-B scores were correlated (r = − 0.88, p < .01). All 25 of the participants found to be at risk for ADRD by the AGNPs based on results of the MoCA-B and QDRS were referred to their primary care provider (Table 2).
Table 2.
Chi square analysis of dementia risk, provider contact, and dementia diagnosis (Dx).
| Not Seen |
Seen |
||||||
| f | % | f | % | χ2(1) | p | Phi | |
| No Dementia Risk | 110 | 99.1% | 1 | 0.9% | 109.03 | <0.001 | .886 |
| Dementia Risk | 4 | 14.3% | 24 | 85.7% | |||
| No Dx of Dementia |
Dx of Dementia |
||||||
| f | % | f | % | χ2(1) | p | Phi | |
| Not Seen | 114 | 0.0% | 0 | 0.0% | 76.67 | <0.001 | .743 |
| Seen by Provider | 10 | 40.0% | 15 | 60.0% | |||
Objective 4
There was 100% agreement between the RA results of the prescreening using the Mini-Cog, and AGNP determination of cognitive risk using the MoCA-B and QDRS. Of the 28 referred to PCP, 25 completed appointments (89%). Fifteen of those seen by their PCP were started on new medication (60%) (Table 2).
Objective 5
When calling participants to determine results of the follow-up visit with their community provider, the nurses reported the following: Of the fifteen who were started on new medication, four were taking new prescriptions of donepezil hydrochloride, and one both donepezil hydrochloride and memantine hydrochloride. One each were started on an antihypertensive, bronchodilator, glaucoma treatment, and antidepressant. The remainder (n = 6) were referred to a neurology clinic at a hospital about 30 min away (with county transportation). The four participants who received a dementia diagnosis but did not report a change in management were either 1) angry about the diagnosis (n = 2): “the doctor told me I have developed a problem with my memory” and because of you “el doctor me dijo que estaba loco” (doctor told me I was crazy), or 2) frustrated because they were unable to comply with the recommentations (n = 2): ‘the doctor told me I needed some new medication to help with my memory, but I already can’t pay for my other medication; trying to get it sorted,” or told that “I need to start getting out more; quit hiding in my apartment all day, and be more careful about my sugar.”
Of the ten PCP to whom the AGNPs referred patients, six responded to the open-ended surveys by return-mail with the self-addressed and stamped envelope. Not all providers answered all of the yes-no questions, and not all provided written feedback. However, those who responded (n = 6) indicated that it was helpful to have the results of the dementia-specific evaluations conducted by the AGNP when attempting to discuss the evidence of memory loss with their patients. This agreed with previous work investigating the usefulness of nurse practitioners in conducting cognitive assessments in primary care.55 Three of the PCP made notations on the surveys that receiving a copy of the actual scored MoCA-B and QDRS documents rather than just the summary was particularly helpful. However, two PCP reported that “most” of their patients were resistant to new therapies, believing that it was normal age-related loss. This corresponds with many residents (68%) incorrectly answering the BKAD item asking if “memory loss is a normal part of aging.”
Only one physician provider surveyed reporting knowing about the NIA’s website that offers a range of information from ADRD diagnosis, treatment, and management algorithms to a 9-module CE-Credit training for providers in medically underserved areas.54 Two of the six surveys included requests by the providers to offer more screening and services for the older adult population in the rural area in general, not just dementia evaluations.
Predictors of risk of cognitive impairment
We had hypothesized that greater age or years lived rural, and lower levels of education and health literacy would predict higher cognitive risk, however, regression analyses revealed that none of these factors correlated with cognitive risk as indicated by the Mini-Cog™ in the first stage screening. We did find that female sex was associated with higher cognitive risk. Results of the QDRS conducted during the HBA were independent of age, education, health literacy, or years rural, which was not unexpected as it is an intra-individual measure.51,53 When examining a composite score of the QDRS and MoCA-B, we found that dementia knowledge was weakly negatively correlated with cognitive impairment (r = −0.34; p = .01). After determining there was no multicollinearity, stepwise regression demonstrated that female sex and low dementia knowledge predicted risk of cognitive impairment: R2 = 0.488, F(8,125) = 3.9, p < .001 (Table 3).
Table 3.
Stepwise regression results: model summary, ANOVA*, and coefficients.
| Model | R | R Square | Adjusted R Square | Std. Error of the Estimate | ||
| 1 | .459a | .211 | .205 | 1.38137 | ||
| 2 | .500b | .250 | .239 | 1.35169 | ||
| Model | Sum of Squares | df | Mean Square | F | Sig. | |
| 1 | Regression | 68.236 | 1 | 68.236 | 35.759 | .000b |
| Residual | 255.698 | 134 | 1.908 | |||
| Total | 323.934 | 135 | ||||
| 2 | Regression | 80.934 | 2 | 40.467 | 22.149 | .000c |
| Residual | 243.000 | 133 | 1.827 | |||
| Total | 323.934 | 135 | ||||
| Model | Unstandardized Coefficients |
Standardized Coefficients | t | Sig. | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | 2.885 | .190 | 15.199 | .000 | |
| Literacy (Health) Test | .265 | .044 | .459 | 5.980 | .000 | |
| 2 | (Constant) | 3.446 | .282 | 12.203 | .000 | |
| Literacy (Health) Test | .250 | .044 | .434 | 5.729 | .000 | |
| Years lived in Glades (or rural area) | −0.014 | .005 | −0.200 | −2.636 | .009 | |
Predictors: (Constant), Literacy (Health) Test.
Predictors: (Constant), Literacy (Health) Test, Years lived in Glades (or rural area).
ANOVA = Analysis of Variance.
Discussion
Key findings of this study included that 1) brief cognitive screening conducted by a trained research assistant was a cost-efficient way to provide information and referrals to healthcare providers; 2) follow-up HBA with dementia-specific evaluations by AGNP were effective in detecting cognitive risk and improving the likelihood of treatment by providers in rural underserved communities, and notably, 3) 60% of participants had changes in medication regimen based on the HBA supporting that dementia screening provided benefits to participants. These findings agree with recent study results of over 4000 primary care largely urban patients screened for dementia using a DemTect score < 9.50 Seventeen percent screened positive for dementia, and 59% signed an informed consent for further evaluation, of which 49% were newly diagnosed with ADRD. We found that more females than males presented with cognitive decline. This is similar to findings reported by other studies such as Kalbe56 and colleagues who found that female sex was significantly associated with dementia detection. These researchers concluded that screening was a valuable means of detecting dementia, and also as Eichner and colleagues57 found, additional diagnostic assessment was imperative. In prior work with 288 multicultural older adults, we found that screening for MCI and ADRD was feasible, participants were satisfied with the program and discussion of their results, showed interest in sharing results with their family and providers, 50% attempted behavioral change based on their results, and 25% had their providers order additional tests and medications.58
The overall willingness to screen in this cohort of older persons with fewer years of education was similar to new findings by Harrawood and colleagues59 who surveyed 954 urban and suburban primary adults aged 65 and older. Their findings that persons with more education and greater healthcare access were less interested in screening could be explained as those patients who are well known to their provider would trust the provider to inform them if they needed screening.59 Results of Harrawood’s study and this project add weight to the argument that persons at risk will be in favor of routine cognitive screening. Of interest is that in all participants seen by the AGNP in this study, only one self-reported as having been previously diagnosed with any type of dementia. This individual had ceased taking an ADRD medication after being advised by an insurance representative seeking to enroll for additional coverage that the dementia diagnosis was incorrect.
While residents were overwhelmingly willing to participate in follow-up visits, we acknowledge that there is a distinct practical difference between informing residents that the screening tests revealed the need for follow-up and providers informing referred patients that they were actually experiencing cognitive decline. Researchers found that while 132 patients were willing to undergo screening, their support lessened after learning of consequences to screening.60 A recent policy brief by the Alzheimer’s Impact Movement (AIM) highlights additional provider and consumer resources.61 Using available practice guidance for sharing a dementia diagnosis with patients such as the The KAER protocol44 may help providers to avoid negative responses to cognitive screening and treatment.
Strengths
This study was representative of rural residents with a lower socioeconomic status who received government subsidies for housing and food, and many for whom English was their second language. The study population had adequate social support as a result of living in established senior residences where the residents often sat outside and talked with one another, and often checked on neighbors. The manager had a personal relationship with the residents, was aware of their needs, and had regular contact with community resources. These factors, in addition to the upgraded quality of home settings not previously experienced by migrant workers prior to retirement, may have contributed to less self-report of depression and increased participation. We believe that multiunit subsidized housing can be an ideal setting for early screening and detection and for increasing participation in research, particularly in rural areas where access to primary care and specialty care may be limited.
Limitations
This sample was not representative of all underserved groups. This study included African Americans, Afro Caribbeans, and Hispanics living in a rural area. Other racial, ethnic, socioeconomic, and geographic groups may differ. Another limitation was that although provider offices were nearby, most of the participants were accustomed to waiting many hours to be seen. Areas with less access to health care providers may have different outcomes. In addition, most (n = 91) of the original participants lived alone, which may have increased participants’ willingness to welcome AGNP visits to their homes more readily than the traditional lengthy clinic visit common to the area. It may be possible that the results of this study would have been different if participants were recruited from housing units that were not subsidized, where issues associated with navigating healthcare access might be of less concern, or where persons may be more hesitant to allow an unknown provider into their home. Some of the positive reception to nurse visits in the home may also have been related to the desire for socialization.62
Although rural residents may be at risk for cognitive impairment, community providers may not be willing or able to conduct additional in-depth cognitive evaluations or initiate treatment for various reasons. These reasons may stem from the belief that receiving a dementia diagnosis could be more harmful than beneficial, as disease modifying agents to treat this progressive disease are not always effective.63,64 In addition, current available treatments have modest treatment effects, may be too expensive, and cause side effects. Receiving an ADRD diagnosis may have social stigma attached to it and may have different representations, belief systems, and cultural norms across different racial, ethnic, and geographic groups.
Implications
In a recent multi-country study of over 1300 primary care and specialty physicians, providers reported that patienst believed that dementia was a normal part of aging and not reporting symptoms were the most common patient-related barriers.64 The most common barrier (32%) for primary care providers in diagnosing dementia was the challenge of identifying whether cognitive decline was related to normal aging.64 Policy changes are needed to support adult gerontological nurse practitioners to bill for dementia detection and management during home visits and conduct sustained follow-up, while coordinating care with other healthcare providers.
Future directions also should include meeting with rural, ethnically diverse providers to raise awareness of NIA resources for dementia detection and treatment beginning with the AIM Policy Brief.61 Nurses specializing in geriatric nursing are well-positioned to make a real difference in underserved populations by leading interprofessional collaborative efforts in the management of dementia detection and diagnosis. Community engagement will help to meet the Healthy People (2020/2030) goals of earlier detection and treatment, thereby reducing length of costly institutionalizations65,66 through an emphasis on home-based dementia management. The Hartford Institute of Geriatric Education67 offers a complete resource for Nurse Practitioners and other PCP to facilitate these Healthy People 2030 goals through the following objectives to identify:
processes for diagnosis of dementia by PCPs in the primary care setting
goals of management of dementia in primary care
benefits of a collaborative approach to care of patients with dementia
online resources for PCPs, Patients and Families
considerations for hospice67
Conclusion
This home-based approach to dementia detection by AGNPs with primary care provider follow-through was effective for rural, culturally diverse, retired farmworkers who face an increased risk of cognitive decline due to socioeconomics, comorbidities and reduced access to care. Our project and others58 support that dementia screening has more benefits than harms. Research implications include expanding the study to both rural and urban subsidized housing residents, and testing the feasibility and effectiveness of culturally relevant education regarding dementia detection and benefits. Subsidized housing for older adults may be an effective recruitment site for research. Community-based participatory research would be one method of developing acceptable methods of recruitment and new pathways for building culturally appropriate care partnerships.
Acknowledgments
This project was funded the Florida Department of Health, Ed and Ethel Moore Alzheimer’s Disease Research Program #7AZ28 (Wiese PI) and The National Institutes of Health R01 AG040211-A1 (Galvin PI). The National Institutes of Health R01 NS101483-01A1 (Galvin PI). There are no HIPAA concerns (data was blinded for this report). The Institutional Review Board of Florida Atlantic University, granted approval for this project: (#719852-6). We are grateful for the assistance by Ms. Fredrica Christie, our Research Assistant.
Footnotes
Declaration of Competing Interest
We have no Conflicts of Interest.
References
- 1.Gaugler J, James B, Johnson T, Marin A, Weuve J. 2019. Alzheimer’s disease facts and figures. Alzheimers Dement 2019 Mar 1;15(3):321–387. [Google Scholar]
- 2.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018. March 5:1–8. 10.1007/s1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ open. 2017. February 1;7:(2) e011146. 10.1136/bmjopen-2016-011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216–224. 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lines LM, Sherif NA, Wiener JM. Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: a literature review. RTI Press Publ. 2014. 10.3768/rtipress.2014.RR.0024.1412. Vol RR-0024–1412. [DOI] [Google Scholar]
- 6.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged≥ 65 years. Alzheimers Dement 2018. September 19. 10.1016/j.jalz.2018.06.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards Iii GA, Gamez N, Escobedo G Jr, Calderon O, Moreno-Gonzalez I. Modifiable risk factors Alzheimer’s disease. Front Aging Neurosci. 2019;11:146. 10.3389/fnagi.2019.00146. Published 2019 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol 2017. October 1;74(10):1246–1254. 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abner EL, Jicha GA, Christian WJ, Schreurs BG. Rural–urban differences in Alzheimer’s disease and related disorders diagnostic prevalence in kentucky and West Virginia. J Rural Health. 2016;32(3):314–320. 10.1111/jrh.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker K, Horowitz JM, Brown A, Fry R, Cohn D, Igielnik R. Demographic and economic trends in urban, suburban and rural communities. What unites and divides urban, suburban, and rural communities. Pew Res Cent: Soc Demogr Trends. May 2018. Retrieved from; https://www.pewsocialtrends.org/2018/05/22/demographic-and-economic-trends-in-urban-suburban-and-rural-communities/.
- 11.Mattos MK, Snitz BE, Lingler JH, Burke LE, Novosel LM, Sereika SM. Older rural – and urban–dwelling Appalachian adults with mild cognitive impairment. J Rural Health. 2017;33(2):208–216. 10.1111/jrh.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudson A, Meit M, Tanenbaum E, et al. Exploring rural and urban mortality differences. Bethesda, MD: Rural Health Reform Policy Res Cent. 2015. [Google Scholar]
- 13.Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292–312. 10.1016/j.jalz.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aljassim N, Ostini R. Health literacy in rural and urban populations: a systematic review. Patient Educ Couns 2020. June 20. 10.1016/j.pec.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017. December 16;390(10113):2673–2734. 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 16.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet. 2020. August 8;396 (10248):413–446. 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YB, Chen YL, Xue HP, Hou P. Health literacy risk in older adults with and without mild cognitive impairment. Nurs Res. 2019. Nov;68(6):433. 10.1097/NNR.0000000000000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira D, Bosco A, di Lorito C. Is poor health literacy a risk factor for dementia in older adults? Systematic literature review of prospective cohort studies. Maturitas 2019. June 1;124:8–14. 10.1016/j.maturitas.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Lovett RM, Curtis LM, Persell SD, et al. Cognitive impairment no dementia and associations with health literacy, self-management skills, and functional health status. Patient Educ Couns. 2020. March 12. 10.1016/j.pec.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson RS, Yu L, James BD, Bennett DA, Boyle PA. Association of financial and health literacy with cognitive health in old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2017;24(2):186–197. 10.1080/13825585.2016.1178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo RY. Lo RY. Uncertainty and health literacy in dementia care. Tzu-Chi Med J 2020;32(1):14. 10.4103/tcmj.tcmj_116_19.2020 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois B, Padovani A, Scheltens P, Rossi A, Dell’Agnello G. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis. 2016;49(3):617–631. 10.3233/JAD-150692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piton M, Hirtz C, Desmetz C, et al. Alzheimer’s disease: advances in drug development. J Alzheimers Dis. 2018;1–11:69. 10.3233/JAD-180145. [DOI] [PubMed] [Google Scholar]
- 24.Lin JS, O’Connor E, Rossom RC, et al. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. Nov. (Evidence Syntheses, No. 107.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK174643/. [PubMed] [Google Scholar]
- 25.Cordell CB, Borson S, Boustani M, et al. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement 2013;9 (2):141–150. 10.1016/j.jalz.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Fowler NR, Perkins AJ, Turchan HA, et al. Older primary care patients’ attitudes and willingness to screen for dementia. J Aging Res. 2015;2015:1–7. 10.1155/2015/42326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low LF, McGrath M, Swaffer K, Brodaty H. Communicating a diagnosis of dementia: a systematic mixed studies review of attitudes and practices of health practitioners. Dementia. 2019. November;18(7–8):2856–2905. [DOI] [PubMed] [Google Scholar]
- 28.Harada K, Lee Ss, Shimada H, et al. Psychological predictors of participation in screening for cognitive impairment among community–dwelling older adults. Geriatr Gerontol Int. 2017. August;17(8):1197–1204. 10.1111/ggi.12841. [DOI] [PubMed] [Google Scholar]
- 29.Wynn MJ, Carpenter BD. Discourse features among providers, patients, and companions and their effect on outcomes of dementia diagnosis disclosure. J Gerontol: Ser B. 2019. June 14;74(5):756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tappenden P, Campbell F, Rawdin A, Wong R, Kalita N. The clinical effectiveness and cost-effectiveness of home-based, nurse-led health promotion for older people: a systematic review. Health Technol Assess (Winchester, Engl). 2012;16(20):1. 10.3310/hta16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuck AE, Aronow HU, Steiner A, et al. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med. 1995. November 2;333(18):1184–1189. 10.1056/NEJM199511023331805. [DOI] [PubMed] [Google Scholar]
- 32.IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016. [Google Scholar]
- 33.Florida department of elder affairs . Alzheimer’s disease initiative 2020. Available at:http://elderaffairs.state.fl.us/doea/alz.php. [Google Scholar]
- 34.U.S. Census Bureau (2018). State and county quick facts. Retrieved from:https://www.census.gov/quickfacts/fact/table/gladescountyflorida,US/PST045218.
- 35.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977. June;1(3):385–401. 10.1177/014662167700100306. [DOI] [Google Scholar]
- 36.Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007. May 1;39(2):175–191. 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 37.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947. Mar> 1:50–60. https://www.jstor.org/stable/2236101. [Google Scholar]
- 38.Tappen RM, Gibson SE, Williams CL. Explanations of AD in ethnic minority participants undergoing cognitive screening. Am J Alzheimers Dis Other Demen. 2011. June;26(4):334–339. 10.1177/1533317511412047. Epub 2011 Jun 21. PMID: 21697141.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiese L, Williams CL, Galvin J. Ethnically diverse rural Florida stakeholder perceptions about cognitive screening. Journal of Aging and Mental Health. 2018. 10.1080/13607863.2018.1525607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borson S, Scanlan JM, Chen PJ, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 2003;51:1451–1454. PMID: 14511167. [DOI] [PubMed] [Google Scholar]
- 41.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007. November 1;45 (11):1026–1033. 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 42.Wiese LK, Williams CL, Tappen R, Newman D, Rosselli M. Assessment of basic knowledge about Alzheimer’s disease among older rural residents: A pilot test of a new measure. Journal of Nursing Measurement. 2017;25(3):519–548. 10.1891/1061-3749.25.3.519. [DOI] [PubMed] [Google Scholar]
- 43.Wiese LK, Williams CL, Newman D, Tappen RM. An Updated Measure of Alzheimer’s Disease Knowledge for use in Underserved Rural Settings. Journal of Aging and Mental Health. 2019. 10.1080/13607863.2019.1584880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortinsky R Chair of GSA workgroup on cognitive impairment detection and earlier diagnosis. Toolkit: 4-step process to detecting cognitive impairment and earlier diagnosis of dementia. 2020. Retrieved from: https://www.geron.org/programsservices/alliances-and-multi-stakeholder-collaborations/cognitive-impairmentdetection-and-earlier-diagnosis.
- 45.Borson S, Scanlan JM, Watanabe J, et al. Improving identification of cognitive impairment in primary care. Int J Geriatr Psychiatry. 2006;21:349–355. 10.1002/gps.1470. [DOI] [PubMed] [Google Scholar]
- 46.Borson S The Mini-Cog: a cognitive “vitals signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021. . [DOI] [PubMed] [Google Scholar]
- 47.McCarten J, Anderson P, et al. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc. 2011;59:309. 10.1111/j.1532-5415.2010.03249.x. −213. [DOI] [PubMed] [Google Scholar]
- 48.Bass PF,Wilson JF, Griffith CH. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18(12):1036–1038. 10.1111/j.1525-1497.2003.10651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cosco TD, Prina M, Stubbs B, Wu YT. Reliability and validity of the center for epidemiologic studies depression scale in a population-based cohort of middle-aged US adults. J Nurs Meas 2017. January 1;25(3):476–485. 10.1891/1061-3749.25.3.476. [DOI] [PubMed] [Google Scholar]
- 50.Julayanont P, Tangwongchai S, Hemrungrojn S, et al. The montreal cognitive assessment-basic (MoCA-B): a new mild cognitive impairment screening test for illiterate and low educated elderly. Alzheimers Dement. 2018;11(7):P442–P443. 10.1016/j.jalz.2015.06.435. [DOI] [PubMed] [Google Scholar]
- 51.Galvin JE. The Quick Dementia Rating System (QDRS): a rapid dementia staging tool. Alzheimer’s & Dem: Diagnosis, Assessment & Disease Monitoring. 2015;1 (2):249–259. 10.1016/j.dadm.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Roeck EE, De Deyn PP, Dierckx E, Engelborghs S. Brief cognitive screening instruments for early detection of Alzheimer’s disease: a systematic review. Alzheimers Res Ther. 2019;11(1):21. 10.1186/s13195-019-0474-3. Published 2019 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galvin JE, Tolea MI, Chrisphonte S. Using a Patient-Reported Outcome to Improve Detection of Cognitive Impairment and Dementia: The Patient Version of the Quick Dementia Rating System (QDRS). PloS One 2020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Institutes of Health: National Institute of Aging’s Alzheimer’s and Dementia Resources for Professionals. 2018. Retrieved from: https://www.nia.nih.gov/health/alzheimers-dementia-resources-for-professionals.
- 55.Yang Y, Xiao LD, Deng L, Wang Y, Li M, Ullah S. Nurse–led cognitive screening model for older adults in primary care. Geriatr Gerontol Int 2015. June;15(6):721–728. 10.1111/ggi.12339. [DOI] [PubMed] [Google Scholar]
- 56.Kalbe E, Kessler J, Calabrese P, Smith R, Passmore AP, Brand M. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19:136–143. 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- 57.Eichler T, Thyrian JR, Hertel J, et al. Rates of formal diagnosis of dementia in primary care: the effect of screening. Alzheimers Dement (Amst). 2015;1(1):87–93. 10.1016/j.dadm.2014.11.007. Published 2015 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galvin JE, Tolea MI, Chrisphonte S. What older adults do with the results of dementia screening programs. PLoS One. 2020;15: e0235534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrawood A, Fowler NR, Perkins AJ, LaMantia MA, Boustani MA. Acceptability and results of screening among older adults in the United States. Geriatr Gerontol Int. 2018. Jan 1;15(1):51–55. 10.2174/156720501466617090810090553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly S, Martin S, Kuhn I, Cowan A, Brayne C, Lafortune L. Barriers and facilitators to the uptake and maintenance of healthy behaviours by people at mid-life: a rapid systematic review. PLoS ONE. 2016;11:(1) e0145074. 10.1371/journal.pone.0145074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Most F Early detection and diagnosis of Alzheimer’s dementia. AIM Policy Brief 2017. Alzheimer’s.org. Retrieved from; https://alzimpact.org/img/Policy_Brief_Early_Detection_and_Diagnosis_Brief_AIM.pdf. [Google Scholar]
- 62.Robinson SM, Canavan M, O’Keeffe ST. Preferences of older people for early diagnosis and disclosure of Alzheimer’s disease (AD) before and after considering potential risks and benefits. Arch Gerontol Geriatr. 2014;59(3):607–612. 10.1016/j.archger.2014.07.01. [DOI] [PubMed] [Google Scholar]
- 63.Judge D, Roberts J, Khandker R, Ambegaonkar B, Black CM. Physician perceptions about the barriers to prompt diagnosis of mild cognitive impairment and Alzheimer’s disease. Int J Alzheimers Dis. 2019. 10.1155/2019/3637954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Portacolone E, Johnson JK, Covinsky KE, Halpern J, Rubinstein RL. The effects and meanings of receiving a diagnosis of mild cognitive impairment or Alzheimer’s disease when one lives alone. J Alzheimers Dis. 2018. January 1;61(4):1517–1529. 10.3233/JAD-170723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Black CM, Fillit H, Xie L, et al. Economic burden, mortality, and institutionalization in patients newly diagnosed with Alzheimer’s disease. J Alzheimers Dis. 2018:1–9. 10.3233/JAD-170518. (Preprint). [DOI] [PubMed] [Google Scholar]
- 66.Geldmacher DS, Kirson NY, Birnbaum HG, et al. Implications of early treatment among Medicaid patients with Alzheimer’s disease Alzheimers dement. 2014. March 1;10(2):214–24. 10.1016/j.jalz.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Hartford Institute of geriatric nursing and consortium of New York geriatric education centers (CNYGEC) dementia and collaborative care. E-learning module. 2014. Retrieved from: https://hign.org/consultgeri-resources/elearning.