Abstract
Background.
T-cell–mediated rejection (TCMR) is the most frequent type of acute rejection and is associated with kidney allograft failure. Almost 40% of TCMR episodes are nonresponsive to therapy and molecular mechanisms for the nonresponsiveness are unknown. Our single-center study identified that urinary cell FOXP3 mRNA abundance predicts TCMR reversibility and allograft survival.
Methods.
We developed PCR assays and measured absolute copy numbers of transcripts for FOXP3, CD25, CD3E, perforin, and 18S rRNA in 3559 urines from 480 kidney allograft recipients prospectively enrolled in the multicenter Clinical Trials in Organ Transplantation-04. In this replication study, we investigated the association between mRNA profile and TCMR diagnosis, TCMR reversibility and allograft survival.
Results.
18S rRNA normalized levels of mRNA for FOXP3 (P=0.01, Kruskal-Wallis test), CD25 (P=0.01), CD3E (P<0.0001), and perforin (P<0.0001) were diagnostic of TCMR, but only FOXP3 mRNA level predicted TCMR reversibility (ROC AUC=0.764; 95% confidence interval, 0.611 to 0.917; P=0.008). Multivariable logistic regression analyses showed that urinary cell FOXP3 mRNA level predicted reversal, independent of clinical variables. A composite model of clinical variables and FOXP3 mRNA (AUC = 0.889; 95% CI, 0.781 to 0.997; P<0.001) outperformed FOXP3 mRNA or clinical variables in predicting TCMR reversibility (P=0.01, likelihood ratio test). Multivariable Cox proportional hazards regression analyses showed that FOXP3 mRNA level predicts kidney allograft survival (P=0.047), but not after controlling for TCMR reversal (P=0.477).
Conclusions.
Urinary cell level of FOXP3 mRNA is diagnostic of TCMR, predicts TCMR reversibility, and is prognostic of kidney allograft survival via a mechanism involving TCMR reversal.
INTRODUCTION
T-cell–mediated rejection (TCMR) is the most frequent type of acute rejection.1–4 Antirejection therapy has evolved over the years, but in a comprehensive study of 256 kidney allograft recipients with kidney allograft biopsies showing TCMR, Bouatou et al. found that 40% of TCMR episodes fail to respond to therapy.5 Moreover, Banff tubulitis and interstitial inflammation scores at the time of TCMR biopsy diagnosis were not determinants of kidney allograft loss, whereas Banff inflammation and peritubular capillaritis scores observed in the biopsy performed 3 months after antirejection treatment were independent predictors of kidney allograft failure.5 The need for posttreatment parameters to improve prognostic accuracy is problematic from the perspective of clinical decision making at the time of TCMR diagnosis. Also, the need for multiple biopsies – one to diagnose TCMR and a second one to better prognosticate TCMR outcome – is challenging in view of complications related to the invasive biopsy procedure. The well-documented interobserver variability in the grading of biopsies is yet another challenge.6,7
A deficiency in FOXP+ regulatory T cells (Tregs) is a potential mechanism for recalcitrant TCMR. To test this hypothesis, we examined whether urinary cell FOXP3 mRNA profiles predict TCMR responsiveness to antirejection treatment. The focus on FOXP3 was informed by: (i) our earlier single-center study of 83 kidney allograft recipients demonstrating that urinary cell level of FOXP3 mRNA predicts TCMR reversal and identifies patients at risk for allograft failure8; (ii) naturally occurring CD4+FOXP3+ regulatory T cells (Tregs) playing a nonredundant role in immune homeostasis and self-tolerance9,10; (iii) preclinical data showing that Tregs prevent rejection and promote transplant tolerance11–13; and (iv) ongoing clinical evaluation of adoptive Treg therapy to control autoimmunity or promote allograft tolerance.14,15 We also considered it important to replicate our earlier findings8 in view of the existing crisis in reproducing scientific observations.16,17
In the current investigation, we measured levels of mRNAs in 3505 urine specimens collected from an independent cohort of 480 kidney transplant recipients enrolled in the multicenter Clinical Trials in Organ Transplantation-04 (CTOT-04) and determined whether urinary cell level of FOXP3 mRNA predicts functional reversal of TCMR and predicts kidney allograft survival following an episode of TCMR.
MATERIALS AND METHODS
Kidney Allograft Recipients
In the parent CTOT-04 study, 485 kidney allograft recipients were prospectively enrolled at 5 transplant sites. Urine was prospectively collected on days 3, 7, 15, and 30 and in months 2, 3, 4, 5, 6, 9, and 12 posttransplantation and at the time of each kidney allograft biopsy and 2 weeks thereafter. Urine cell pellets, prepared at each clinical site, were shipped to the Gene Expression Monitoring core at Weill Cornell Medicine. RNA was isolated from the urinary cell pellets, reverse transcribed to cDNA, and checked for transcript quality thresholds – at least 100 copies of TGFB1 mRNA and 5x107 copies of 18S rRNA per 1 microgram of total RNA – prior to downstream data analysis. Absolute levels of mRNA for CD3E, perforin, granzyme B, proteinase inhibitor-9, CD103, interferon inducible protein-10 (IP-10), CXCR3, TGFB1, and 18S rRNA (CTOT-04 Prespecified mRNA Panel) were measured. A total of 3559 urine specimens from 485 kidney allograft recipients passed the RNA quality thresholds. The primary objectives of the parent CTOT-04 study were to determine whether the urinary cell mRNA levels, measured at the time of biopsy, is diagnostic of TCMR and whether the levels in sequential samples predict future development of TCMR.4 The parent CTOT-04 study did not investigate whether mRNA levels predict TCMR reversal or are associated with kidney allograft survival. Urinary cell levels of FOXP3 mRNA and CD25 mRNA were not measured in the parent CTOT-04 study.
We obtained independent funding (RO1 AI072790, PI, M. Suthanthiran) to perform this ancillary study. In the current investigation, cDNA prepared from the 3559 urine cell pellets from the parent CTOT-04 were retrieved for the measurement of FOXP3 mRNA and CD25 mRNA. Prior to measurement of mRNAs, the cDNAs were assessed for RNA quality thresholds and 3505 of the 3559 cDNAs (98.5%) prepared from the urine specimens from 480 of 485 kidney allograft recipients from the CTOT-04 study met the quality thresholds. The validated cDNAs were used to measure urinary cell level of mRNAs using customized preamplification-enhanced real-time quantitative (customized) PCR assays.4 Supplementary Table S1, provided as Supplemental Digital Content, lists the sequences of the oligonucleotide primers and TaqMan probes we designed for the absolute quantification of mRNAs. Additional details of the customized PCR assays have been published.4
In this replication study, we applied the same functional criterion we used in our discovery study8 to classify an episode of TCMR as reversible or nonreversible.
Statistical Methods
Copy number for each mRNA was analyzed before and after normalization with 18S rRNA copies (x10−6) and both with and without log10-transformation. Kruskal-Wallis and Mann-Whitney statistical tests were used to compare mRNA levels across diagnoses. Receiver-operating-characteristic (ROC) curves were used to determine the predictive accuracy of each log10-transformed 18S rRNA normalized mRNA and the sensitivity and specificity were determined for the threshold that maximized Youden’s index.18 Multivariable logistic regression was used to evaluate whether urinary cell FOXP3 mRNA level predicts TCMR reversal after controlling for potential confounders.
Kaplan-Meier survival curves were used to analyze graft survival rates stratified by biopsy status, TCMR reversibility, mRNA levels and serum creatinine measured at the time of TCMR biopsy, and antirejection therapy with antithymocyte globulin (ATG). Time to event was calculated from time of TCMR biopsy until graft failure or censoring. Patients were censored if they died prior to experiencing graft failure or were lost to follow-up. Log-rank tests were used to compare survival curves across strata. Long-term follow-up information on graft outcomes, beyond the 3-year duration of the CTOT-04 study, was obtained from the Organ Procurement and Transplantation Network (OPTN). Multivariable Cox proportional hazards regression was used to evaluate urinary cell mRNA as a predictor of graft failure after controlling for potential confounders.
All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC), except for the creation of ROC curves using the pROC R package.19 The datasets and programming code generated for the current analyses are available from the corresponding author on request.
Study Approval
The institutional review board at Weill Cornell Medicine approved the study, “Multicenter Study: Use of PCR to Evaluate Immune Regulatory Molecules”, Protocol Number 9608002317.
RESULTS
Patients and Biopsies
Figure 1 shows the distribution of the 3505 urine samples with urinary cell mRNA data from the 480 kidney allograft recipients enrolled in CTOT-04. Among the 480 patients, 218 underwent kidney allograft biopsies, which were classified by the on-site pathologist using the updated Banff 97 classification schema.20 Supplementary Table S2 is the Biopsy Form developed by the NIAID Statistical and Clinical Coordinating Center (SACCC) for the CTOT studies and used by the pathologists to record the biopsy findings.
Figure 1.
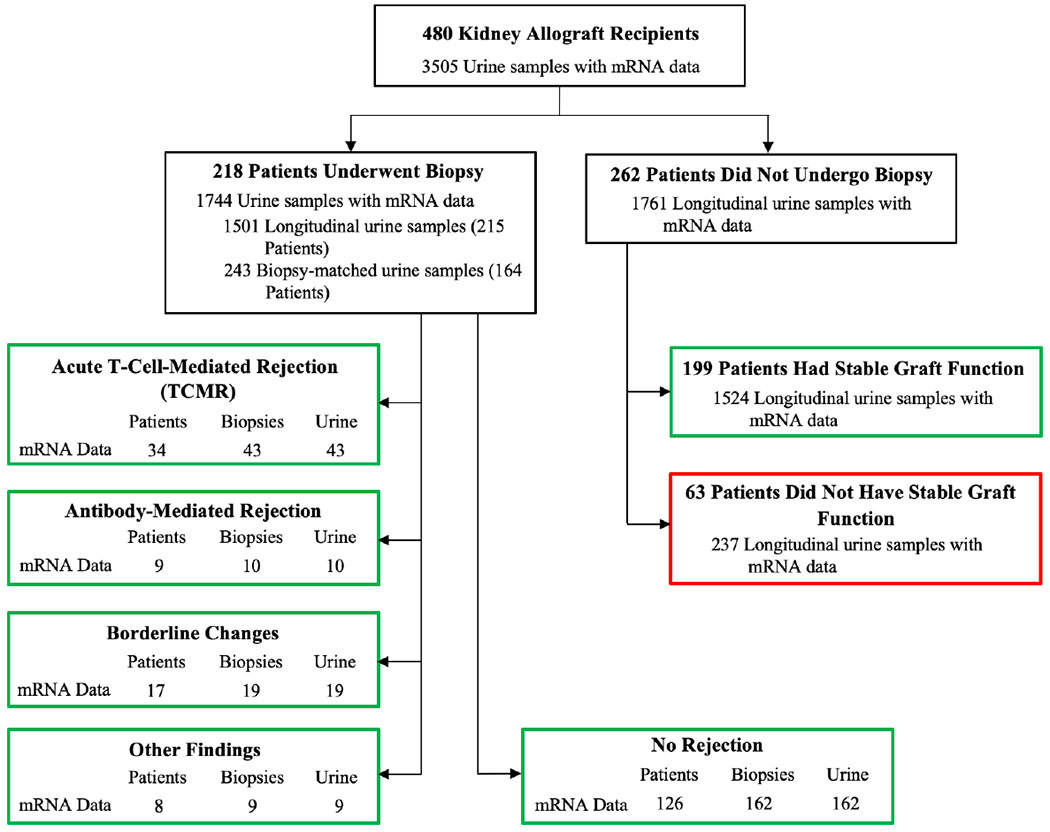
Patients, biopsy results, and urine samples. The distribution of 3505 urine samples from 480 kidney allograft recipients enrolled in the CTOT-04 study is shown. The number of patients with biopsy-matched urine samples (urine collected from 3 days before to 1 day after biopsy) are shown for patients with Banff TCMR grade 1A or higher, AMR, Borderline Changes, Other Findings, and those without any rejection features in their biopsies (No Rejection). Among patients who did not undergo a biopsy, 199 patients met criteria for stable graft function based on (i) average of recorded serum creatinine values at 6, 9, and 12 months ≤ 2.0 mg/dl, (ii) no graft loss or death, (iii) no treatment for acute rejection, and (iv) no evidence of cytomegalovirus or polyomavirus type BK infection during the first 12 months posttransplantation and contributed 1524 urine samples. Sixty-three patients failed to meet criteria for stable graft function and contributed 237 urine specimens. Green boxes represent samples included in this study, whereas the red box represents samples not included in the data analysis.
The TCMR group consisted of 43 biopsies from 34 patients. Among the 43 TCMR biopsies, 38 were for-cause biopsies and 5 were surveillance biopsies. Nineteen of the 43 TCMR biopsies were graded as Banff IA, 10 as grade IB, 11 as IIA, 2 as IIB, and 1 as grade III.
The Supplemental Digital Content (SDC) provides information regarding the 10 AMR biopsies, 19 Borderline Changes biopsies, and 9 biopsies classified as Other Findings; 7 of 9 Other Findings were diagnosed as BK virus nephropathy.
The No Rejection group consisted of 162 biopsies from 126 patients. Among the 162 biopsies, 107 were for-cause biopsies and 55 were surveillance biopsies. This group was designated as “No Rejection” group because none of the biopsies displayed features characteristic of TCMR, AMR, or Borderline Changes. However, several of the No Rejection biopsies displayed features that were recorded in the NIAID SACCC Biopsy Form using Banff 97 Other nonrejection diagnoses.21 The Banff Other changes included acute tubular necrosis (n=66), tubular atrophy (n=61), interstitial fibrosis (n=51), glomerulosclerosis (n=23), vascular narrowing (n=17), calcineurin toxicity (n=11), and/or recurrent disease (n=2). Several of the No Rejection biopsies displayed more than 1 abnormality such as the presence of both interstitial fibrosis and tubular atrophy (n=47).
Among the 480 kidney allograft recipients, 262 did not have a recorded biopsy. Among these 262 patients, 199 patients were classified as having Stable Graft Function by virtue of meeting the following criteria: (i) average of serum creatinine assessed at 6, 9, and 12 months posttransplantation less than or equal to 2.0 mg/dl; (ii) no graft loss or death during the first 12 months following transplantation; (iii) no treatment for acute rejection; (iv) no cytomegalovirus or BK virus infection; and (v) no clinical indication for a biopsy. An additional 63 patients also did not have a biopsy; information regarding these patients who did not meet the criteria for a Stable Graft Function is provided as SDC.
Urinary Cell mRNAs Diagnostic of TCMR
We compared urinary cell levels of mRNAs in (i) 43 urines matched to TCMR biopsies from 34 patients, (ii) 162 urines matched to No Rejection biopsies from 126 patients, and (iii) 1524 urines from 199 patients with stable graft function (Stable). The biopsy matched urine samples were collected within minus 3 days to plus 1 day of biopsy. The 1524 urines from the Stable group were prospectively collected on days 3, 7, 15, and 30 and in months 2, 3, 4, 5, 6, 9, and 12 posttransplantation. Table 1 summarizes the baseline characteristics of the kidney allograft recipients included in this analysis.
Table 1.
Characteristics of the kidney allograft recipients and their organ donorsa
| Recipient Characteristics | Acute T-Cell–Mediated Rejection (N of Patients = 34) | No Rejection (N of Patients = 126) | Stable (N of Patients = 199) | P-valueb |
|---|---|---|---|---|
| Biopsy samples | 43 | 162 | ||
| Urine samples | 43 | 162 | 1524 | |
| Age, years | 0.2652 | |||
| Mean (SD) | 45.0 (11.8) | 48.0 (13.0) | 49.0 (13.9) | |
| Median | 43 | 48 | 50 | |
| Min, Max | 24, 73 | <1, 76 | <1, 78 | |
| Sex, N (%) | 0.0146 | |||
| Female | 8 (23.5) | 43 (34.1) | 92 (45.8) | |
| Male | 26 (76.5) | 83 (65.9) | 109 (54.2) | |
| Ethnicity, N (%) | 0.5532 | |||
| Hispanic or Latino | 3 (8.8) | 20 (15.9) | 31 (15.4) | |
| Not Hispanic or Latino | 30 (88.2) | 101 (80.2) | 161 (80.1) | |
| Unknown or Not Reported | 1 (2.9) | 5 (4.0) | 9 (4.5) | |
| Race, N (%) | 0.0497 | |||
| Black or African American | 13 (38.2) | 49 (38.9) | 45 (22.4) | |
| White | 20 (58.8) | 62 (49.2) | 119 (59.2) | |
| Asian | 1 (2.9) | 9 (7.1) | 10 (5.0) | |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 2 (1.0) | |
| Other | 0 (0) | 4 (3.2) | 20 (10.0) | |
| Unknown or Not Reported | 0 (0) | 2 (1.6) | 5 (2.5) | |
| Induction Therapy, N (%) | <0.0001 | |||
| IL-2 Receptor Antibody | 6 (17.6) | 12 (9.5) | 20 (10.1) | |
| CAMPATH-1H | 10 (29.4) | 58 (46.0) | 29 (14.6) | |
| Thymoglobulin | 15 (44.1) | 38 (30.2) | 135 (67.8) | |
| More than 1 induction therapy | 2 (5.9) | 7 (5.6) | 6 (3.0) | |
| No Induction Therapy | 1 (2.9) | 2 (1.6) | 1 (0.5) | |
| Missing Information | 0 (0) | 9 (7.1) | 10 (5.0) | |
| BMI | 0.0022 | |||
| Mean (SD) | 30.5 (6.2) | 28.5 (6.3) | 27.0 (5.6) | |
| Median | 29 | 28 | 26 | |
| Min, Max | 22, 43 | 17, 45 | 16, 43 | |
| < 18.5 | 0 (0) | 2 (1.6) | 4 (2.0) | |
| 18.5 - 24.9 | 9 (26.5) | 35 (27.8) | 58 (28.9) | |
| 25.0 - 29.9 | 7 (20.6) | 41 (32.5) | 56 (27.9) | |
| ≥ 30.0 | 14 (41.2) | 40 (31.7) | 44 (21.9) | |
| Missing | 4 (11.8) | 8 (6.3) | 39 (19.6) | |
| Donor Characteristics | ||||
| Age, years | 0.5344 | |||
| Mean (SD) | 41.8 (11.5) | 39.8 (14.8) | 38.9 (14.7) | |
| Median | 40 | 41 | 39 | |
| Min, Maxb | 20, 65 | 5, 66 | 1, 73 | |
| Missing | 0 | 0 | 3 | |
| Sex, N (%) | 0.3621 | |||
| Female | 17 (50.0) | 61 (48.4) | 83 (41.3) | |
| Male | 17 (50.0) | 65 (51.6) | 118 (58.7) | |
| Ethnicity, N (%) | 0.3895 | |||
| Hispanic or Latino | 4 (11.8) | 21 (16.7) | 36 (17.9) | |
| Not Hispanic or Latino | 29 (85.3) | 92 (73.0) | 140 (69.7) | |
| Unknown or Not Reported | 1 (2.9) | 13 (10.3) | 25 (12.4) | |
| Race, N (%) | 0.0013 | |||
| Black or African American | 10 (29.4) | 34 (27.0) | 19 (9.5) | |
| White | 22 (64.7) | 82 (65.1) | 154 (76.6) | |
| Asian | 1 (2.9) | 3 (2.4) | 5 (2.5) | |
| American Indian or Alaska Native | 0 | 1 (0.8) | 1 (0.5) | |
| Other | 0 | 0 (0) | 3 (1.5) | |
| Unknown or Not Reported | 1 (2.9) | 6 (4.8) | 19 (9.5) | |
| Source of Donor, N (%) | 0.6900 | |||
| Deceased | 15 (44.1) | 57 (45.2) | 85 (42.3) | |
| Living/related | 9 (26.5) | 39 (31.0) | 73 (36.3) | |
| Living/unrelated | 10 (29.4) | 30 (23.8) | 43 (21.4) | |
| Cause of Death, N (%) | 0.0455 | |||
| Anoxia | 4 (26.7)) | 9 (15.8) | 15 (17.6) | |
| Cerebrovascular Accident/Injury/Stroke | 6 (40.0) | 27 (47.4) | 16 (18.8) | |
| Head Injury/Trauma | 2 (13.3) | 8 (14.0) | 24 (28.2) | |
| Intracranial Bleed | 1 (6.7)) | 2 (3.5) | 6 (7.1) | |
| Motor Vehicle Accident | 0 (0) | 1 (1.8) | 2 (2.4) | |
| Other | 1 (6.7) | 4 (7.0) | 11 (12.9) | |
| Unknown | 0 (0) | 3 (5.3) | 10 (11.8) | |
| Missing | 1 (6.7) | 3 (5.3) | 1 (1.2) | |
Demographics of recipients and their organ donors are shown. 34 patients underwent 43 kidney allograft biopsies and contributed 43 urine samples matched to acute T-cell–mediated rejection biopsies. 126 patients underwent 162 biopsies and contributed 162 urine samples matched to No Rejection biopsies. 199 patients did not undergo a biopsy and contributed 1524 urine samples. These 199 patients were classified as Stable based on meeting the following criteria: average serum creatinine values at 6, 9 and 12 months ≤ 2.0 mg/dl, no treatment for acute rejection, and no evidence of cytomegalovirus or polyomavirus type BK infection during the first 12 months posttransplantation. The biopsy matched urine samples were collected within minus 3 days to plus 1 day of biopsy. The 1524 urines from the Stable group were prospectively collected on days 3, 7, 15, and 30 and in months 2, 3, 4, 5, 6, 9, and 12 posttransplantation.
P-values are based on 1-way ANOVA for continuous variables and Chi-square tests for categorical variables.
Violin plots with in-laid box-and-whisker plots in Figure 2 portray the distribution of log10-transformed ratios of mRNA to 18S rRNA copies (x10−6). Urinary cell levels of all 4 mRNAs were significantly higher in urines matched to TCMR biopsies than in urines matched to No Rejection biopsies or in urines collected prospectively from the Stable group. Table S3 shows the median and lower and upper quartiles of the log10-transformed 18S rRNA normalized ratios of mRNAs in urines matched to TCMR biopsies, No Rejection biopsies, and urines from the Stable group. Table S3 also shows the log10-transformed 18S rRNA normalized ratios of mRNAs in urines matched to Borderline Changes biopsies, AMR biopsies, and BKVN biopsies. Table S4 shows the median and lower and upper quartiles of the absolute copy number of mRNAs in urines for the same diagnostic categories.
Figure 2.
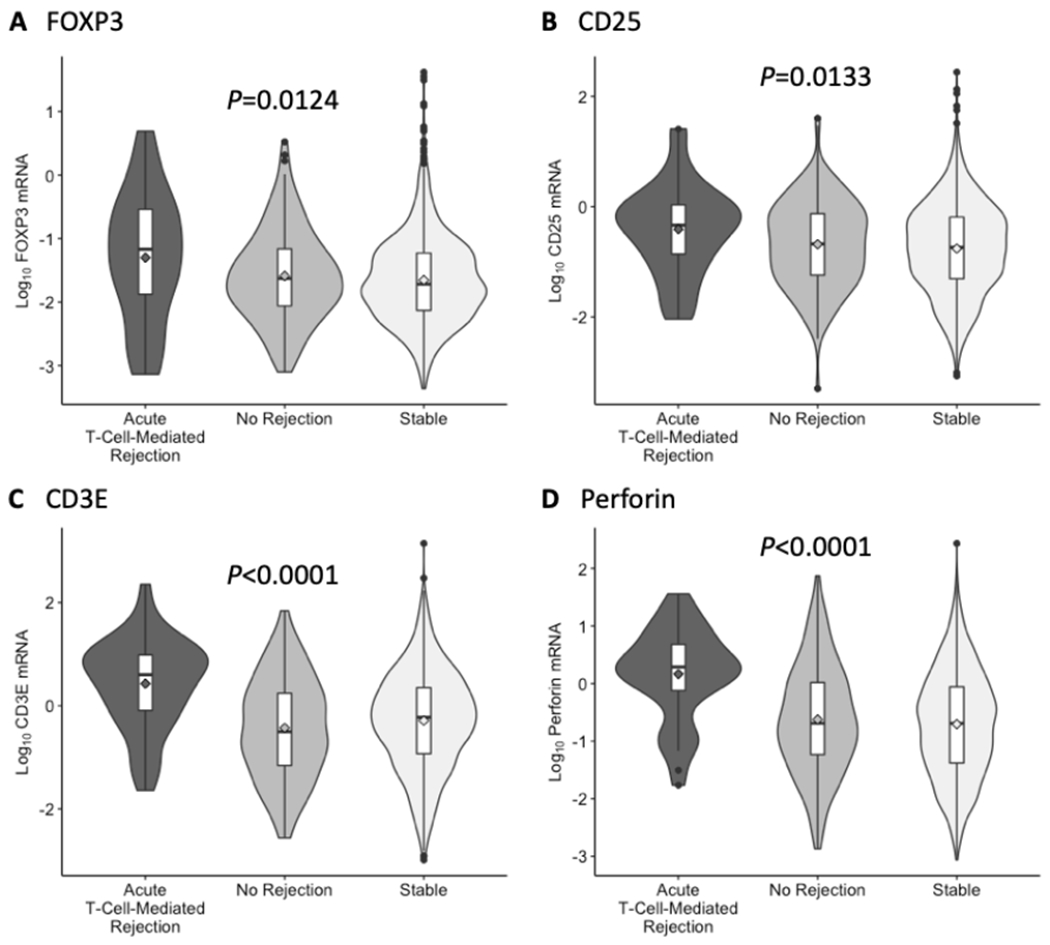
Levels of mRNA in urinary cells. Violin plots with in-laid box-and-whisker plots show the distribution of log10-transformed ratios of mRNA copies to 18S ribosomal RNA (rRNA) copies (x10−6) for FOXP3, CD25, CD3E, and perforin in 43 urine samples matched to 43 biopsy specimens (from 34 subjects) diagnosed as acute T-cell–mediated rejection, 162 urine samples matched to 162 biopsy specimens (from 126 subjects) without any rejection features in the biopsy (No Rejection), and 1524 urine samples collected longitudinally from 199 subjects with stable graft function who did not undergo biopsy (Stable). The in-laid box-and-whisker plots display the 25th, 50th, and 75th percentile values via the bottom, middle, and top lines in the box, respectively, and the 10th and 90th percentile values via the ends of the bottom and top whiskers, respectively; the diamonds represent the mean and circles indicate outliers. The violin plots display the distribution and spread of observations in each diagnostic group. The P-value from the Kruskal-Wallis test of the null hypothesis of no group differences in the distributions is presented above each set of violin plots.
Supplementary Figure S1 shows ROC curves comparing levels of mRNA for FOXP3, CD3E, CD25, and perforin in urines matched to TCMR biopsies vs. urine matched to No Rejection biopsies; urines matched to TCMR vs. urines from the Stable group; and urines matched to No Rejection biopsies vs. urines from the Stable group. All 4 mRNAs distinguished patients with TCMR biopsies from those with No Rejection biopsies and patients with TCMR biopsies from the Stable patients. Levels of all 4 mRNAs in urines were not different between the No Rejection biopsy group and the Stable group.
Urinary Cell mRNA Levels Stratified by Reversibility of TCMR
In our earlier discovery study,8 an episode of TCMR was classified as reversible if the serum creatinine level returned to within 15% of the prerejection level within 4 weeks after initiation of antirejection therapy. In this replication study, we used the same functional criterion to classify an episode of TCMR as reversible. With the use of prespecified criterion, we classified 39 of 43 TCMR episodes as reversible (n=24) or nonreversible (n=15). Four of 43 TCMR episodes were not classified due to missing creatinine values (n=2), proximity to an earlier TCMR biopsy (n=1), or proximity to BKV nephropathy diagnosis (n=1).
Table 2 summarizes recipient and donor characteristics by TCMR reversibility status. Recipient age was higher in those with reversible TCMR compared to those with nonreversible TCMR (P=0.033, Mann-Whitney) and deceased donor organ was less common among those with reversible TCMR compared to those with nonreversible TCMR (P=0.055). Additional characteristics analyzed are provided as SDC. Table 3 summarizes biopsy-associated characteristics by TCMR reversibility status. The median time from transplantation to biopsy was 117 days in those with reversible TCMR versus 269 days in those with nonreversible TCMR (P=0.015). Additional characteristics analyzed, including Banff biopsy grade and serum creatinine at the time of TCMR biopsy, were not significantly different between the 2 groups and are provided as SDC.
Table 2.
Characteristics of kidney transplant recipients with acute T-cell–mediated rejection by reversible statusa
| Recipient Characteristics | Total | Reversible TCMR | Nonreversible TCMR | P-valueb |
|---|---|---|---|---|
| Kidney allograft recipientsc | 33 | 21 | 12 | |
| Number of biopsiesd | 39 | 24 | 15 | |
| Age, years | 0.033 | |||
| Mean (SD) | 45.0 (12.0) | 48.0 (10.9) | 39.8 (12.5) | |
| Median | 43 | 46 | 36 | |
| Min, Max | 24, 73 | 35, 69 | 24, 73 | |
| Sex, N (%) | 0.730 | |||
| Female | 8 (24.2) | 6 (28.6) | 2 (16.7) | |
| Male | 25 (75.8) | 15 (71.4) | 10 (83.3) | |
| Ethnicity, N (%) | 0.898 | |||
| Hispanic or Latino | 2 (6.1) | 1 (4.8) | 1 (8.3) | |
| Not Hispanic or Latino | 30 (90.9) | 19 (90.5) | 11 (91.7) | |
| Unknown or Not Reported | 1 (3.0) | 1 (4.8) | 0 (0) | |
| Race, N (%) | 0.455 | |||
| Black or African American | 13 (39.4) | 6 (28.6) | 7 (58.3) | |
| White | 19 (57.6) | 14 (66.7) | 5 (41.7) | |
| Asian | 1 (3.0) | 1 (4.8) | 0 (0) | |
| American Indian or Alaska Native | 0 (0) | 0 (0) | 0 (0) | |
| Other | 0 (0) | 0 (0) | 0 (0) | |
| Unknown or Not Reported | 0 (0) | 0 (0) | 0 (0) | |
| Induction Therapy, N (%) | 0.679 | |||
| IL-2 Receptor Antibody | 6 (18.2) | 5 (23.8) | 1 (8.3) | |
| CAMPATH-1H | 9 (27.3) | 7 (33.3) | 2 (16.7) | |
| Thymoglobulin | 15 (45.5) | 8 (38.1) | 7 (58.3) | |
| More than 1 induction therapy | 2 (6.1) | 0 (0) | 2 (16.7) | |
| No Induction Therapy | 1 (3.0) | 1 (4.8) | 0 (0) | |
| Missing Information | 0 (0) | 0 (0) | 0 (0) | |
| BMI | 0.772 | |||
| Mean (SD) | 30.6 (6.3) | 30.8 (6.7) | 30.2 (5.6) | |
| Median | 30 | 29 | 30 | |
| Min, Max | 22, 43 | 22, 43 | 24, 38 | |
| < 18.5 | 0 (0) | 0 (0) | 0 (0) | |
| 18.5 - 24.9 | 9 (27.3) | 6 (28.6) | 3 (25.0) | |
| 25.0 - 29.9 | 6 (18.2) | 4 (19.1) | 2 (16.7) | |
| ≥ 30.0 | 14 (42.4) | 10 (47.6) | 4 (33.3) | |
| Missing | 4 (12.1) | 1 (4.8) | 3 (25.0) | |
| Donor Characteristics | ||||
| Age, years | 0.409 | |||
| Mean (SD) | 41.8 (11.7) | 40.6 (12.4) | 44.0 (10.5) | |
| Median | 40 | 40 | 43 | |
| Min, Max | 20, 65 | 20, 65 | 26, 61 | |
| Missing | 0 | 0 | 0 | |
| Sex, N (%) | 0.554 | |||
| Female | 17 (51.5) | 10 (47.6) | 7 (58.3) | |
| Male | 16 (48.5) | 11 (52.4) | 5 (41.7) | |
| Ethnicity, N (%) | 0.852 | |||
| Hispanic or Latino | 3 (9.1) | 2 (9.5) | 1 (8.3) | |
| Not Hispanic or Latino | 29 (87.9) | 19 (90.5) | 10 (83.3) | |
| Unknown or Not Reported | 1 (3.0) | 0 (0) | 1 (8.3) | |
| Race, N (%) | 0.940 | |||
| Black or African American | 10 (30.3) | 7 (33.3) | 3 (25.0) | |
| White | 21 (63.6) | 13 (61.9) | 8 (66.7) | |
| Asian | 1 (3.0) | 0 (0) | 1 (8.3) | |
| American Indian or Alaska Native | 0 | 0 (0) | 0 (0) | |
| Other | 0 | 0 (0) | 0 (0) | |
| Unknown or Not Reported | 1 (3.0) | 1 (4.8) | 0 (0) | |
| Source of Donor, N (%) | 0.055 | |||
| Deceased | 15 (45.5) | 6 (28.6) | 9 (75.0) | |
| Living/related | 8 (24.2) | 8 (38.1) | 0 (0) | |
| Living/unrelated | 10 (30.3) | 7 (33.3) | 3 (25.0) | |
| Cause of Death, N (%) | 0.932 | |||
| Anoxia | 4 (26.7) | 2 (9.5) | 2 (15.4) | |
| Cerebrovascular Accident/Injury/Stroke | 7 (46.7) | 2 (9.5) | 5 (38.5) | |
| Head Injury/Trauma | 2 (13.3) | 1 (4.8) | 1 (7.7) | |
| Intracranial Bleed | 1 (6.7) | 0 (0) | 1 (7.7) | |
| Motor Vehicle Accident | 0 (0) | 0 (0) | 0 (0) | |
| Other | 0 (0) | 0 (0) | 0 (0) | |
| Unknown | 0 (0) | 0 (0) | 0 (0) | |
| Missing | 1 (6.7) | 1 (4.8) | 0 (0) | |
An episode of TCMR was classified as reversible if the serum creatinine level returned to within 15% of the prerejection level within 4 weeks after initiation of antirejection therapy. This was the functional criterion used to classify an episode of TCMR as reversible or nonreversible in our earlier discovery study8. Using this criterion, 39 of 43 TCMR episodes were classified as reversible (n=24) or nonreversible (n=15). Four of 43 TCMR episodes were not classified due to missing creatinine values (n=2), proximity to an earlier TCMR biopsy (n=1), or proximity to BKV nephropathy diagnosis (n=1).
P-value based on Mann-Whitney test for continuous variables and chi-square tests for categorical variables.
Thirty-three of 34 patients with TCMR were analyzed for TCMR reversible versus nonreversible; a single patient with TCMR biopsy was excluded from analysis because of BKVN diagnosis in proximity to TCMR diagnosis.
Thirty nine of 43 TCMR biopsy matched urine samples were analyzed for TCMR reversible vs. nonreversible; 4 urine samples matched to TCMR biopsies were excluded from data analysis because serum creatinine level was not available after antirejection treatment in 2, 2 episodes of TCMR occurred in proximity in 1 patient and 1 TCMR occurred in close proximity to BKVN.
Table 3.
Biopsy associated characteristics of kidney transplant recipients with acute T-cell–mediated rejection by reversible statusa
| Total | Reversible TCMR | Nonreversible TCMR | P-valueb | |
|---|---|---|---|---|
| Study subjectsc | 33 | 21 | 12 | |
| Number of biopsiesd | 39 | 24 | 15 | |
| Time from Transplant to Biopsy, days | 0.015 | |||
| Mean (SD) | 217 (188) | 159 (160) | 310 (196) | |
| Median | 180 | 117 | 269 | |
| Min, Max | 3, 701 | 3, 491 | 18, 701 | |
| Banff Grade | 0.911 | |||
| Grade IA | 16 | 10 | 6 | |
| Grade IB | 9 | 5 | 4 | |
| Grade IIA | 11 | 8 | 3 | |
| Grade IIB | 2 | 0 | 2 | |
| Grade III | 1 | 1 | 0 | |
| Serum Creatinine at Baseline, mg/dL | 0.436 | |||
| Mean (SD) | 1.9 (1.2) | 2.0 (1.4) | 1.7 (0.5) | |
| Median | 1.6 | 1.6 | 1.6 | |
| Min, Max | 0.9, 8.2 | 0.9, 8.2 | 1.1 (3.3) | |
| Serum Creatinine at Time of Biopsy, mg/dL | 0.315 | |||
| Mean (SD) | 3.1 (2.8) | 2.8 (2.3) | 3.7 (3.4) | |
| Median | 2.3 | 2.3 | 2.5 | |
| Min, Max | 1.1, 13.3 | 1.1, 12.2 | 1.7, 13.3 | |
| Serum Creatinine 4 weeks postbiopsy, mg/dL | 0.011 | |||
| Mean (SD) | 2.2 (1.2) | 1.8 (1.2) | 2.8 (0.9) | |
| Median | 1.9 | 1.6 | 2.7 | |
| Min, Max | 0.9, 6.6 | 0.9, 6.6 | 1.6, 4.5 | |
| Antirejection Regimene | N/A | |||
| Glucocorticoids | 37 | 23 | 14 | |
| Antilymphocyte antibodies | 14 | 9 | 5 | |
| Other | 6 | 2 | 4 |
An episode of TCMR was classified as reversible if the serum creatinine level returned to within 15% of the prerejection level within 4 weeks after initiation of antirejection therapy. This was the functional criterion used to classify an episode of TCMR as reversible or nonreversible in our earlier discovery study8. Using this criterion, 39 of 43 TCMR episodes were classified as reversible (n=24) or nonreversible (n=15). Four of 43 TCMR episodes were not classified due to missing creatinine values (n=2), proximity to an earlier TCMR biopsy (n=1) or proximity to BKV nephropathy diagnosis (n=1).
P-value based on Mann-Whitney test for continuous variables and chi-square tests for categorical variables.
Thirty-three of 34 patients with TCMR were analyzed for TCMR reversible versus nonreversible; a single patient with TCMR biopsy was excluded from analysis because of BKVN diagnosis in proximity to TCMR diagnosis.
Thirty nine of 43 TCMR biopsy matched urine samples were analyzed for TCMR reversible vs. nonreversible; 4 urine samples matched to TCMR biopsies were excluded from data analysis because serum creatinine level was not available after antirejection treatment in 2, 2 episodes of TCMR occurred in proximity in 1 patient and 1 TCMR occurred in close proximity to BKVN. Three patients had 3 TCMR biopsies and contributed 3 biopsy-matched urine samples. One of the 3 patients contributed 1 urine sample matched to a nonreversible episode based on absence of improvement in serum creatinine followed by 2 urine samples matched to 2 episodes of reversible TCMR. The 2 remaining patients contributed 1 urine sample matched to a reversible episode of TCMR followed by 2 urine samples matched to 2 episodes of nonreversible TCMR each (5 months apart in 1 patient and 12 months apart in the second patient).
The sum of antirejection treatments for biopsies within a particular column exceeds the total number of biopsies (TCMR diagnoses) because several TCMR episodes were treated with multiple antirejection regimens.
We compared mRNA levels in urines matched to reversible TCMR to levels in urines matched to nonreversible TCMR (Table 4). FOXP3 mRNA level was higher in urines matched to reversible TCMR than in urines matched to nonreversible TCMR (P=0.0096, Mann-Whitney Test). In contrast, levels of mRNA for CD25 (P=0.1531), CD3E (P=0.1887), and perforin (P=0.4322) were not different between the 2 groups.
Table 4.
18S rRNA normalized, log10-transformed levels of mRNA in urinary cells from reversible TCMR group, nonreversible TCMR group and No Rejection groupa
| Type of mRNA | Reversible TCMR Group | Nonreversible TCMR Group | No Rejection Group | P-Valueb Reversible TCMR Vs. | P-Valueb Nonreversible TCMR Vs. | |
|---|---|---|---|---|---|---|
| (N = 24 samples, 21 patients) | (N = 15 samples, 12 patients) | (N = 162 samples, 126 patients) | ||||
| FOXP3 | −0.82 | −1.637 | −1.628 | Nonreversible TCMR | 0.0096 | |
| (−1.445, −0.322) | (−2.579, −0.982) | (−2.061, −1.161) | No Rejection | <0.0001 | 0.7212 | |
| CD25 | −0.156 | −0.385 | −0.675 | Nonreversible TCMR | 0.1531 | |
| (−0.739, 0.255) | (−1.286, −0.180) | (−1.247, −0.122) | No Rejection | 0.0066 | 0.7623 | |
| CD3E | 0.858 | 0.312 | −0.504 | Nonreversible TCMR | 0.1887 | |
| (0.199, 1.061) | (−0.285, 0.995) | (−1.159, 0.242) | No Rejection | <0.0001 | 0.0026 | |
| Perforin | 0.35 | 0.197 | −0.69 | Nonreversible TCMR | 0.4322 | |
| (0.116, 0.817) | (−0.219, 0.822) | (−1.232, 0.019) | No Rejection | <0.0001 | 0.0017 |
Median (lower, upper quartiles) log-transformed ratio of mRNA copies to 18S rRNA copies (x 10−6) is shown for each mRNA measure. The number of patients with biopsy-matched urine samples (urine collected from 3 days before to 1 day after biopsy) are shown for patients with functional reversal of acute T-cell–mediated rejection (reversible TCMR), nonreversible TCMR, and those without any rejection features in the biopsy (No Rejection). An episode of TCMR was classified as reversible if the serum creatinine level returned to within 15% of prerejection level within 4 weeks after initiation of antirejection therapy.
P-values for pairwise differences are based on Mann-Whitney test.
We compared levels of mRNAs in urines matched to reversible or nonreversible TCMR to levels in urines matched to No Rejection biopsies (Table 4). Levels of mRNA for FOXP3 (P<0.0001), CD25 (P=0.0066), CD3E (P<0.0001), and perforin (P<0.0001) were higher in urines matched to reversible TCMR than in urines matched to No Rejection biopsies. Levels of FOXP3 mRNA (P=0.7212) and CD25 mRNA (P=0.7623) were not different between urines matched to nonreversible TCMR and urines matched to No Rejection biopsies, whereas levels of mRNA for CD3E (P=0.0026) and perforin (P<0.0017) were significantly higher (Table 4).
ROC Curve Analysis of TCMR Reversal
We performed ROC curve analysis to determine the predictive performance of urinary cell mRNA levels. ROC curve analysis yielded an AUC of 0.764 (95% CI, 0.611 to 0.917; P=0.008) for the 18S rRNA normalized values of FOXP3 mRNA (Figure 3A). The cut-point that maximized Youden’s index18 was −1.33; at this threshold, FOXP3 mRNA predicted TCMR reversal with 75% (95% CI, 53% to 90%) sensitivity and 67% (95% CI, 45% to 77%) specificity; the positive and negative predictive values were 78% (95% CI, 63% to 88%) and 62% (43% to 78%), respectively. Levels of mRNA for CD25 (P=0.100) (Figure 3B), CD3E (P=0.173) (Figure 3C), and perforin (P=0.399) (Figure 3D) were not predictive. The CTOT-04 urinary cell 3-gene signature of CD3E mRNA, IP-10 mRNA, and 18S rRNA, previously shown to be diagnostic and anticipatory of TCMR,4 did not predict TCMR reversal (P=0.253) (Figure 3E). Serum creatinine, measured at the time biopsy, did not predict TCMR reversal (P=0.308) (Figure 3F).
Figure 3.
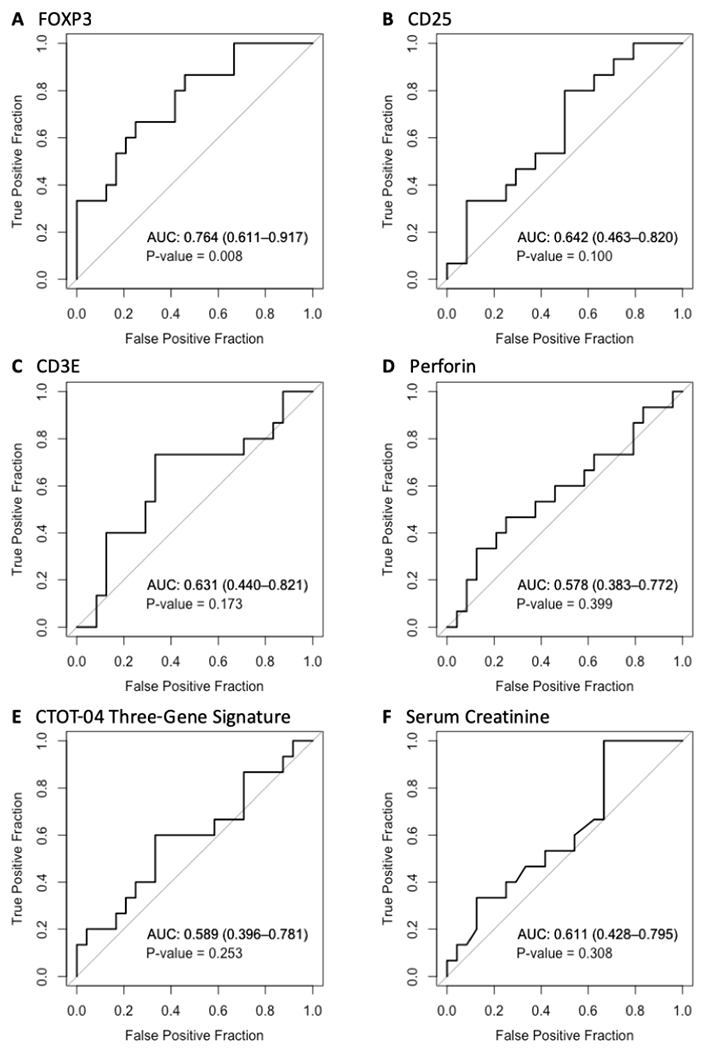
Receiver-operating-characteristic (ROC) curve analyses for TCMR reversal. ROC curves for (A) 18S ribosomal RNA (rRNA) normalized FOXP3 mRNA; (B) 18S rRNA normalized CD25 mRNA; (C) 18S rRNA normalized CD3E mRNA; (D) 18S rRNA normalized perforin mRNA; (E) the CTOT-04 3-gene TCMR diagnostic signature (calculated from 18S rRNA normalized CD3E and IP-10 mRNAs and 18S rRNA); and (F) serum creatinine level measured at time of TCMR biopsy. In addition to the ROC curve (a plot of the fraction of true positive results [sensitivity] and the fraction of false positive results [1- specificity] for discriminating reversal versus nonreversal of an episode of TCMR using different thresholds of a predictor), each panel gives the area under the receiver operating characteristic curve (AUC) with its 95% confidence interval and the P-value for the test of the null hypothesis that the AUC=0.5. An AUC value of 0.5 is no better than that expected by chance (the null hypothesis) whereas a value of 1.0 reflects a perfect discriminator. P-values are obtained from Wald tests from logistic regression analyses predicting reversible status from the measure of interest. Among the variables tested, only urinary cell FOXP3 mRNA level, measured at the time of biopsy, predicted TCMR reversal (ROC AUC: 0.764, 95%CI, 0.611 to 0.917, P=0.008).
A Composite Model for Predicting TCMR Reversibility
We examined whether a combination of clinical variables and urinary cell mRNAs predict TCMR reversal better than clinical variables or mRNAs alone. A stepwise logistic regression analysis using a combination of urinary cell levels of mRNA for FOXP3, CD3, CD25, and perforin and kidney allograft function reflected by serum creatinine level measured at time of TCMR biopsy showed that the most parsimonious and best fitting model is the model containing only FOXP3 mRNA. Recipient age (P=0.033, Mann-Whitney test), time from transplantation to biopsy (P=0.015), and type of donor graft (P=0.055) differed significantly between reversible and nonreversible TCMR groups by univariable analysis (Tables 2 and 3). Multivariable logistic regression analyses showed that FOXP3 mRNA level continued to significantly predict TCMR reversal after controlling for recipient age (P=0.0090), time from transplantation to biopsy (P=0.0130), and type of donor graft (P=0.0170) (Table 5). We examined whether a composite model that included the clinical variables and urinary cell FOXP3 mRNA level yields a higher AUC than the AUC of FOXP3 mRNA alone or the AUC of all 3 clinical variables without FOXP3 mRNA. The prediction model that included clinical variables and FOXP3 mRNA yielded an AUC of 0.889 (95% CI, 0.781–0.997, P<0.001). By likelihood ratio test, the composite model was significantly better than (i) the model with FOXP3 mRNA alone (P=0.012) and (ii) the model that included the 3 clinical variables (P=0.006). The regression equations for the models are provided as SDC.
Table 5.
Standardized odds ratios for TCMR reversal: multivariable logistic regression analysesa
| Covariatesb | FOXP3 OR | P-Value |
|---|---|---|
| Unadjusted | 3.33 (1.36, 8.11) | 0.0083 |
| Serum Creatininec | 3.32 (1.38, 7.99) | 0.0075 |
| Age at Transplant | 3.88 (1.40, 10.7) | 0.0090 |
| Time from Transplant to Biopsy | 3.73 (1.32, 10.5) | 0.0130 |
| Type of Transplant | 2.97 (1.21, 7.24) | 0.0170 |
| Fully Adjusted Modeld | 3.88 (1.28, 11.8) | 0.0168 |
Standardized odds ratios (per 1-SD difference in log10-transformed, 18S rRNA normalized FOXP3 mRNA) are presented along with estimated 95% confidence intervals in parentheses. Urinary cell level of FOXP3 mRNA remained predictive of TCMR reversal after individual adjustment for serum creatinine measured at time of biopsy, age at transplant, time from transplant to biopsy, and type of transplant (deceased donor, unrelated live donor, related live donor. Data are derived from 39 urine samples matched to 39 TCMR biopsies categorized as reversible (n=24) or nonreversible (n=15) from 33 patients.
For each row, TCMR is regressed on FOXP3 mRNA level plus the covariate listed.
Serum creatinine level (mg/dL) measured at time of biopsy.
Adjusted for serum creatinine, age at transplant, time from transplant to biopsy, and type of transplant.
Prospective Trajectory
We examined the impact of the antirejection therapy on the postbiopsy prospective trajectories of urinary cell FOXP3 mRNA level and on the CTOT-04 3-gene diagnostic signature score. The prospective trajectory of FOXP3 mRNA for the reversible TCMR group started above the log10-transformed 18S normalized FOXP3 mRNA threshold of −1.33 for TCMR reversal (represented by the red dashed line) at the time of biopsy and remained close to the threshold throughout the subsequent 30 days (Figure 4A). In contrast, the prospective trajectory of FOXP3 mRNA for nonreversible TCMR group started below the threshold at the time of biopsy and remained below the threshold throughout the next 30 days (Figure 4B). The prospective trajectory of CTOT-04 urinary cell 3-gene signature decreased from the time of biopsy and crossed the diagnostic threshold of −1.213 (represented by the red dashed line) within 15 days of initiation of antirejection therapy in those with reversible TCMR (Figure 4C), but remained consistently above the threshold in those with nonreversible TCMR (Figure 4D).
Figure 4.
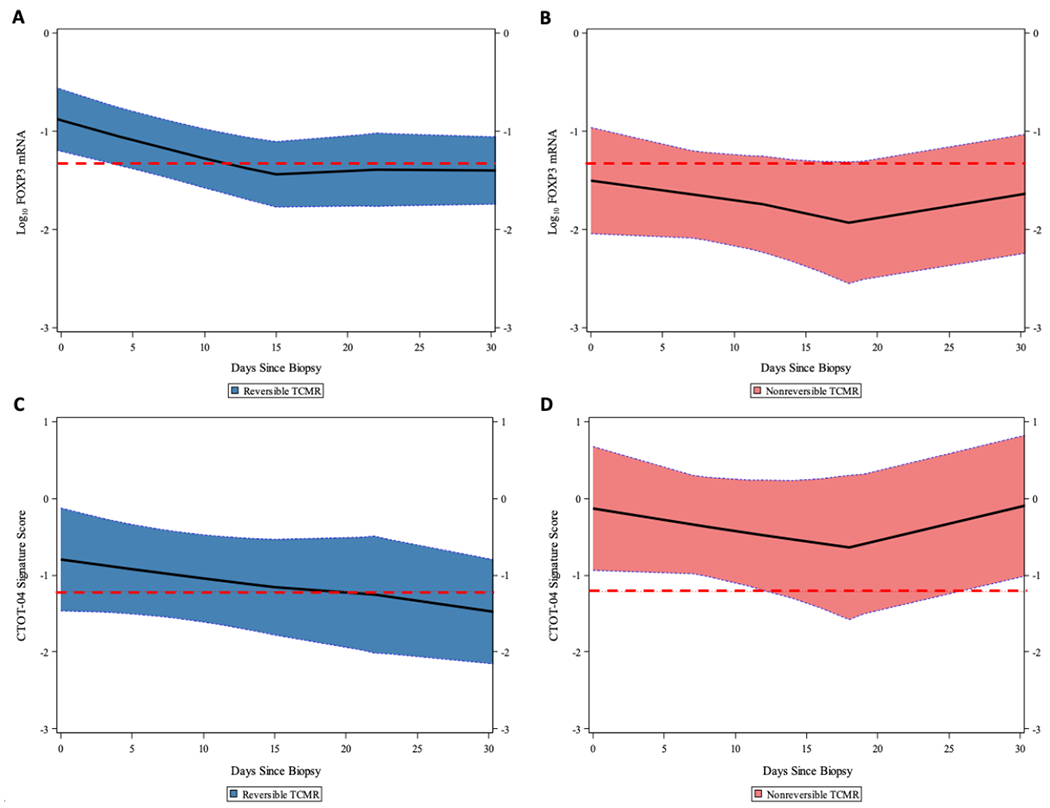
Prospective trajectories of 18S rRNA normalized FOXP3 mRNA level and CTOT-04 3-gene TCMR diagnostic signature score as a function of time since TCMR biopsy. The loess-smoothed average within-person trajectories and 95% confidence bands of the urinary cell log10-transformed, 18S rRNA normalized FOXP3 mRNA level and the median score of CTOT-04 3-gene TCMR diagnostic signature are shown for the reversible TCMR group (A, C) and the nonreversible TCMR group (B, D). Levels of mRNA in 81 urines from 21 patients with reversible TCMR and 43 urines from 12 patients with nonreversible TCMR were used to generate the prospective trajectories. (A) The median level of urinary cell FOXP3 mRNA at the time of TCMR biopsy was significantly higher in patients with reversible TCMR than in patients with nonreversible TCMR. The prospective trajectory in the reversible TCMR group started above the −1.33 threshold (for discriminating reversible from nonreversible TCMR) at time of TCMR biopsy and remained close to the threshold throughout the 30 days after the biopsy. (B) The prospective trajectory in patients with nonreversible TCMR started below the threshold at time of TCMR biopsy and remained well below the threshold through 30 days after the biopsy. (C) The prospective trajectory of the CTOT-04 3-gene TCMR diagnostic signature at time of TCMR biopsy did not differ significantly between those with reversible TCMR or nonreversible TCMR. Among the patients with reversible TCMR, the average score decreased from time of TCMR biopsy and crossed the diagnostic threshold of −1.213 within 15 days of initiation of antirejection therapy. (D) The CTOT-04 3-gene TCMR diagnostic signature prospective trajectory remained consistently above the threshold among the patients with nonreversible TCMR.
Survival Analyses
Survival probabilities for the entire cohort of 480 kidney allograft recipients at 1, 3, 5, and 10 years were 99%, 95.6%, 91.2%, and 81.5%, respectively (Figure 5A). Survival probabilities, at the same time points, were 100%, 100%, 99%, and 93.1%, respectively, for the Stable group; 97.7%, 93%, 86.7%, and 75.2%, respectively, for the No Rejection biopsy group; and 100%, 83.1%, 77.3%, and 58.8%, respectively, for the TCMR group (P<0.0001) (Figure 5B). The Kaplan-Meier survival curve for the TCMR group was significantly different than the curves for Stable group (P<0.0001, by log-rank test) and No Rejection group (P=0.0510).
Figure 5.
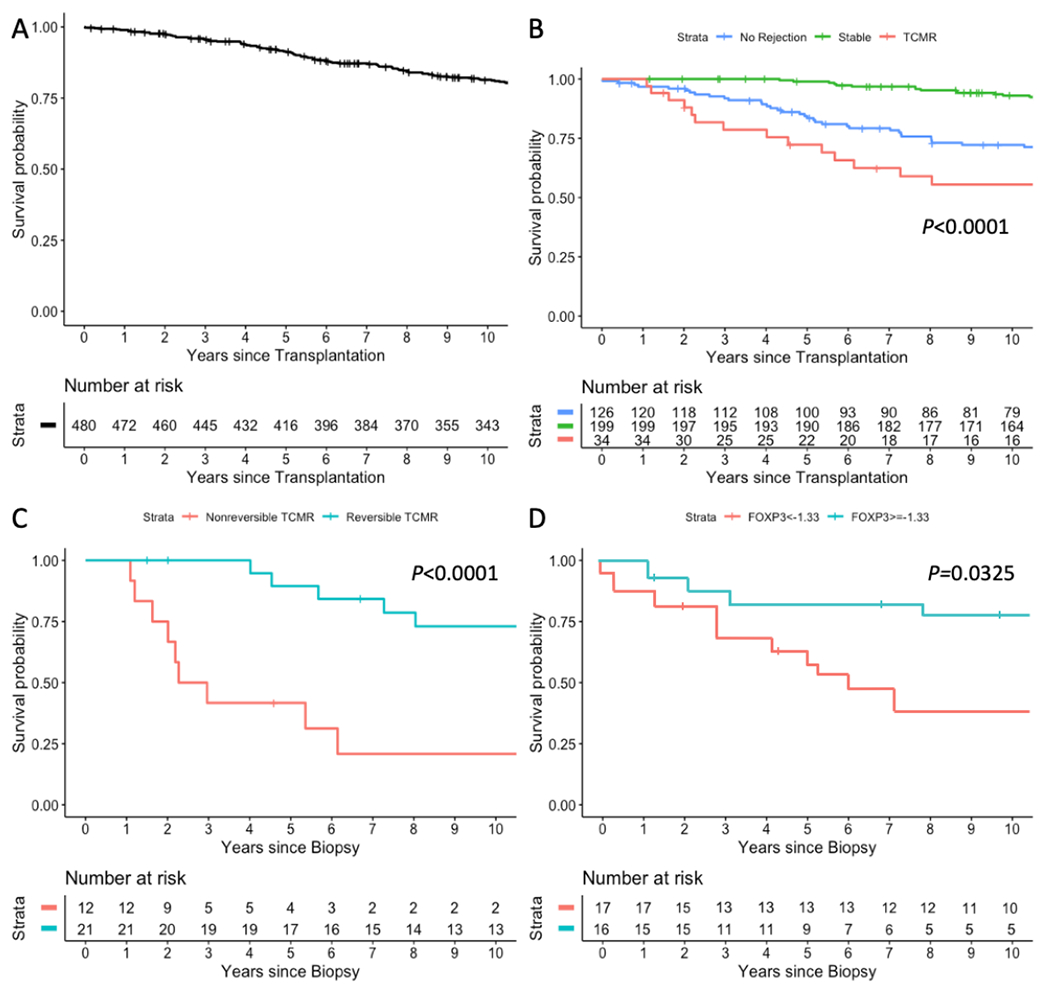
Kaplan-Meier kidney allograft survival curves. (A) Survival curve, from time of transplantation, for the entire cohort of 480 kidney allograft recipients (patients); (B) Survival curves, from time of transplantation, for the 199 patients with stable graft function (Stable), for the 126 patients with 162 biopsies showing no rejection features in their biopsies (No Rejection) and for the 34 patients with 43 biopsies classified as TCMR Banff grade IA or higher (TCMR); (C) Survival curves of 33 patients, from time of TCMR biopsy, stratified by TCMR reversibility status. One patient with BKVN diagnosis in close proximity to TCMR could not be classified as reversible or nonreversible TCMR and is excluded in this analysis; (D) Survival curves for the 33 patients, from the time of biopsy, stratified by log10-transformed 18S rRNA normalized FOXP3 mRNA threshold of −1.33 for TCMR reversal. The patient with BKVN diagnosis in close proximity to TCMR is excluded in this analysis. Time to event was calculated from date of TCMR biopsy (or the date of last TCMR biopsy for the 3 patients with multiple episodes) until graft failure or last follow-up date. Subjects were censored if they experienced death prior to graft failure or were lost to follow-up. P-values are based on log-rank tests. At-risk tables are shown in each plot, just above the X-axis.
Patients with reversible TCMR had significantly better survival than those with nonreversible TCMR (Figure 5C, P<0.0001). Patients with FOXP3 mRNA levels above −1.33 (the Youden index threshold for TCMR reversal) at the time of a TCMR biopsy had significantly better graft survival compared to those with levels below the threshold (Figure 5D, P=0.0325). Multivariable Cox proportional hazards regression analysis showed that FOXP3 mRNA level remains significantly predictive of kidney allograft outcomes after adjustment for age and serum creatinine measured at time of biopsy, but not after adjustment for time from transplant to biopsy, type of transplant, or TCMR reversibility (Table 6).
Table 6.
Kidney allograft survival: multivariable Cox proportional hazards regression analysesa
| Covariatesb | FOXP3 HR | P-Value |
|---|---|---|
| Unadjusted | 0.55 (0.30, 0.99) | 0.0474 |
| Serum Creatininec | 0.55 (0.30, 0.99) | 0.0475 |
| Age at Transplant | 0.53 (0.28, 0.98) | 0.0445 |
| Time from Transplant to Biopsy | 0.64 (0.30, 1.33) | 0.2309 |
| Type of Transplant | 0.65 (0.36, 1.18) | 0.1584 |
| Reversible Status | 0.78 (0.39, 1.56) | 0.4771 |
| Fully Adjusted Modeld | 0.69 (0.30, 1.61) | 0.3986 |
Standardized hazard ratios (per 1-SD change difference in log10-transformed, 18S rRNA normalized FOXP3 mRNA) for kidney allograft survival are presented along with estimated 95% confidence intervals in parentheses. Urinary cell FOXP3 mRNA level remained predictive of graft survival after individual adjustment for serum creatinine measured at time of biopsy and age at transplant, but not after adjustment for time from transplant to biopsy, and type of transplant. Data are derived from 39 urine samples matched to 39 TCMR biopsies categorized as reversible or nonreversible from 33 patients.
For each row, graft survival is regressed on FOXP3 mRNA level plus the covariate listed.
Serum creatinine level (mg/dL) measured at time of biopsy.
Adjusted for serum creatinine, age at transplant, time from transplant to biopsy, and type of transplant.
The relationship between FOXP3 mRNA level and graft outcome may be a direct effect of FOXP3+ Tregs (reflected by FOXP3 mRNA abundance) on graft survival and/or by an indirect effect through the association between FOXP3+ Tregs and TCMR reversibility (Figure 6). Our analysis showed that: (i) FOXP3 mRNA level is significantly associated with TCMR reversibility after adjustment for covariates (a-path; OR=3.88; 95% CI, 1.28 to 11.8, P=0.0168, Table 5); (ii) TCMR reversible status is significantly associated with graft survival (P<0.0001, Figure 5C) and the association of reversible status with graft failure (HR=0.16; 95% CI, 0.05-0.51; P=0.0017) remains statistically significant after adjustment for FOXP3 mRNA level (b-path; HR=0.21; 95% CI, 0.06-0.73; P=0.0139); and (iii) the significant association between FOXP3 mRNA level and graft survival (HR=0.55, 95% CI, 0.30-0.99, P=0.0474, Table 6) is no longer statistically significantly associated with graft survival after adjustment for TCMR reversibility (c′-path; HR=0.78; 95% CI, 0.39-1.56; P=0.4771, Table 6). Altogether, our data support the hypothesis that the association of FOXP3 mRNA level with graft outcome is mediated through TCMR reversal.
Figure 6.
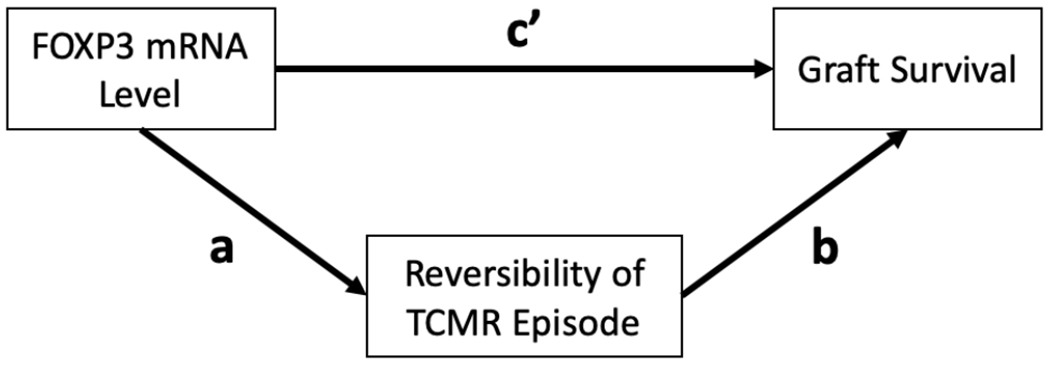
Proposed mechanism for the association between urinary cell FOXP3 mRNA abundance and kidney allograft outcome. FOXP3 mRNA level may impact graft outcome via TCMR reversal (path a), through a direct effect that is independent of its effect on TCMR reversal (path c’) or both. Data analysis showing that: i) after adjustment for covariates, FOXP3 mRNA level is significantly associated with TCMR reversibility (a-path; OR=3.88; 95% CI, 1.28 −11.8, P=0.0168); (ii) TCMR reversible status is significantly associated with graft survival after adjustment for FOXP3 mRNA level and covariates (path b; HR=0.21; 95% CI, 0.06-0.73; P=0.0139) and (iii) the association between FOXP3 mRNA level and graft survival is negligible after adjustment for TCMR reversibility (path cȲ; HR=0.78; 95% CI, 0.39-1.56; P=0.4771) support the hypothesis that the association of FOXP3 mRNA level with graft outcome is primarily mediated through TCMR reversal.
Figure S2 shows that antithymocyte globulin as antirejection therapy (P=0.6903, Figure S2) or serum creatinine level, measured at time of biopsy, is not associated with kidney allograft survival (P=0.7084). Figure S2 also shows that urinary cell levels of mRNA for CD25 (P=0.2915), CD3E (P=0.3826), and perforin (P=0.8542), or the CTOT-04 3-gene TCMR diagnostic signature score (P=0.2138), all measured at the time of TCMR biopsy, are not associated with kidney allograft survival.
DISCUSSION
FOXP3+ Tregs play a pivotal role in preventing autoimmunity and maintaining immune homeostasis.9,10 Preclinical studies suggest that Tregs prevent or delay the onset of allograft rejection and may induce tolerance.11–13 In our earlier single-center clinical study,8 we found that urinary cell FOXP3 mRNA level is diagnostic of TCMR, predicts TCMR reversal, and is associated with kidney allograft survival. In the current investigation, we have replicated these findings using urine samples collected from an external cohort of 480 kidney allograft recipients enrolled in the multicenter CTOT-04 study. Our replication of earlier observations is significant from a biological perspective regarding the potential role of FOXP3+ Tregs in regulating kidney allograft rejection, and is reassuring in the context of the existing crisis in replicating published data.16,17
We found that a composite signature of clinical variables and urinary cell FOXP3 mRNA level is a better predictor of TCMR reversal than either the clinical variables alone or FOXP3 mRNA level alone. In this regard, whereas the finding that urinary cell FOXP3 level alone predicts TCMR reversal represents replication of our earlier finding,8 the new composite signature requires validation in a future study.
The prospective trajectory of FOXP3 mRNA started above the threshold for TCMR reversal at the time of biopsy in the reversible TCMR group and remained close to this threshold throughout the subsequent 30 days whereas the prospective trajectory started below the threshold at the time of biopsy in the nonreversible TCMR group and remained below the threshold throughout the next 30 days. Strikingly, the prospective trajectory of CTOT-04 urinary cell 3-gene signature decreased from the time of biopsy and crossed the TCMR diagnostic threshold of −1.213 within 15 days of initiation of antirejection therapy in those with reversible TCMR, but remained consistently above the TCMR rejection threshold among those with nonreversible TCMR. These differential trajectories suggest that the balance between Tregs (reflected in this study by FOXP3 mRNA abundance) and T effectors (reflected in this study by CTOT-04 urinary cell 3-gene signature score) may impact TCMR responsiveness to therapy.
The overall survival of kidney allografts in our multicenter CTOT-04 study cohort was similar to the US kidney graft survival rates22 suggesting that our study participants are representative of the US kidney transplant population. Kidney graft survival was significantly inferior in patients with biopsy confirmed TCMR, with most graft failures occurring in those with nonreversible TCMR. In the current study, we validate prior findings that urinary cell FOXP3 mRNA level, measured at the time of TCMR, is associated with kidney allograft survival. The association between FOXP3 mRNA level and graft outcome is likely to be mediated by an indirect effect through the association between FOXP3 mRNA level and TCMR reversibility since the significant association between FOXP3 mRNA level and graft survival was no longer statistically significant after adjustment for TCMR reversibility.
Our study has limitations. We characterized TCMR reversal based on functional recovery rather than by histological confirmation with follow-up biopsy. This may not be a significant limitation since graft survival in our study was strongly associated with the functional criterion used to classify an episode of TCMR. We did not assess the functional activity of Tregs, and we inferred Treg deficiency based on FOXP3 mRNA abundance and this could be considered a limitation as well. We note that mRNA expression patterns in themselves have helped inform therapeutic decisions.23 Another limitation of our study is that the donor specific antibody status was unknown at the time of TCMR biopsy.
We measured FOXP3 mRNA level in urine as a surrogate for intragraft FOXP3 expression. Our whole genome RNA sequencing of urinary cells and kidney allograft biopsies demonstrating that kidney allograft gene signatures are enriched in urinary cells supports the idea that urine is excellent surrogated for the kidney allograft biopsy.24 In this study, we did not assess intragraft FOXP3 protein level. However, the existing literature suggests a positive correlation between intragraft FOXP3 mRNA level and protein expression.25–27 It would be important to investigate the relationship between intragraft FOXP3+ cells and urinary cell FOXP3 mRNA levels especially in the context of TCMR reversibility and graft survival.
The current study focused on TCMR reversal. In a comprehensive study of biopsies diagnosed as Borderline TCMR, Nankievell et al. identified differential outcomes ranging from minimal impact to deleterious consequences including progressive tubular injury and fibrosis, an increased risk for acute rejection, allograft failure and even death.28 It would be important to investigate the association between urinary cell mRNA profiles and the outcome of biopsies classified as Borderline TCMR.
The parent CTOT-04 study identified and validated a urinary cell 3-gene signature of CD3E mRNA, IP-10 mRNA, and 18S rRNA that is diagnostic of TCMR and anticipatory of a future episode of TCMR.4 The current investigation extends the utility of urinary cell mRNA profiling by demonstrating that urinary cell FOXP3 mRNA level predicts functional reversal of TCMR and graft survival following an episode of TCMR.
Supplementary Material
Acknowledgments:
The authors gratefully acknowledge Dr. Nancy D. Bridges, Transplantation Branch, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA for her careful review of the manuscript and key insights. The authors thank Ms. Christina Chang and Ms. Christine Hoang (Weill Cornell Medicine, New York, NY) for their superb technical assistance in performing the RT-QPCR assays. The authors thank the United Network for Organ Sharing for providing the clinical data for the calculation of survival curves of kidney allograft recipients. The data reported here have been supplied by UNOS as the contractor for the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by all members of OPTN. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as reflecting official policy of or interpretation by the OPTN or the U.S. Government.
Financial Disclosure: This investigation was supported by awards from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (RO1 AI072790 and R37 AI051652 to MS). The Clinical Trials in Organ Transplantation Study-04 (ClinicalTrials.gov NCT00337220) was supported by an award (UO1AI63589) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health to Abraham Shaked, University of Pennsylvania School of Medicine, Philadelphia, PA.
Abbreviations:
- AUC
area under the curve
- BMI
body mass index
- CI
confidence interval
- CTOT-04
Clinical Trials in Organ Transplantation 04
- cDNA
complementary DNA
- IP-10
interferon inducible protein-10
- PI-9
proteinase inhibitor-9
- ROC
receiver operating characteristic
- RT-QPCR
real-time quantitative polymerase chain reaction
- rRNA
ribosomal RNA
- TCMR
T-cell–mediated rejection
- Tregs
regulatory T cells
- TGFB1
transforming growth factor beta 1
Footnotes
Publisher's Disclaimer: Disclaimer: M. Suthanthiran has a Consultancy Agreement with CareDx, Inc. Brisbane, CA and with Sparks Therapeutics, Philadelphia, PA. The other authors of this manuscript declare no conflicts of interest.
References
- 1.Opelz G, Döhler B; Collaborative Transplant Study Report. Influence of time of rejection on long-term graft survival in renal transplantation. Transplantation. 2008;85(5):661–666. doi: 10.1097/TP.0b013e3181661695 [DOI] [PubMed] [Google Scholar]
- 2.El Ters M, Grande JP, Keddis MT, et al. Kidney allograft survival after acute rejection, the value of follow-up biopsies. Am J Transplant. 2013;13(9):2334–2341. doi: 10.1111/ajt.12370 [DOI] [PubMed] [Google Scholar]
- 3.Hricik DE, Nickerson P, Formica RN, et al. Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am J Transplant. 2013;13(10):2634–2644. doi: 10.1111/ajt.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suthanthiran M, Schwartz JE, Ding R, et al. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med. 2013;369(1):20–31. doi: 10.1056/NEJMoa1215555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouatou Y, Viglietti D, Pievani D, et al. Response to treatment and long-term outcomes in kidney transplant recipients with acute T cell-mediated rejection. Am J Transplant. 2019;19:1972–1988. doi: 10.1111/ajt.15299 [DOI] [PubMed] [Google Scholar]
- 6.Furness PN, Taub N, Convergence of European Renal Transplant Pathology Assessment Procedures (CERTRAP) Project. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60(5):1998–2012. doi: 10.1046/j.1523-1755.2001.00030.x [DOI] [PubMed] [Google Scholar]
- 7.Furness PN, Philpott CM, Chorbadjian MT, et al. Protocol biopsy of the stable renal transplant: a multicenter study of methods and complication rates. Transplantation. 2003;76(6):969–973. doi: 10.1097/01.TP.0000082542.99416.11 [DOI] [PubMed] [Google Scholar]
- 8.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342–2351. doi: 10.1056/NEJMoa051907 [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Mikami N, Wing JB, et al. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–566. doi: 10.1146/annurev-immunol-042718-041717 [DOI] [PubMed] [Google Scholar]
- 10.Georgiev P, Charbonnier LM, Chatila TA. Regulatory T cells: the many faces of Foxp3. J Clin Immunol. 2019;39(7):623–640. doi: 10.1007/s10875-019-00684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobbold SP, Castejon R, Adams E, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172(10):6003–6010. doi: 10.4049/jimmunol.172.10.6003 [DOI] [PubMed] [Google Scholar]
- 12.Albert MH, Liu Y, Anasetti C, et al. Antigen-dependent suppression of alloresponses by Foxp3-induced regulatory T cells in transplantation. Eur J Immunol. 2005;35(9):2598–2607. doi: 10.1002/eji.200526077 [DOI] [PubMed] [Google Scholar]
- 13.Li W, Gauthier JM, Higashikubo R, et al. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J Clin Invest. 2019;129(2):556–568. doi: 10.1172/JCI122083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano M, Fanelli G, Albany CJ, et al. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. doi: 10.3389/fimmu.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira LMR, Muller YD, Bluestone JA, et al. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18(10):749–769. doi: 10.1038/s41573-019-0041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker M Is there a reproducibility crisis? Nature. 2016;533(7604):452–454. [DOI] [PubMed] [Google Scholar]
- 17.Reproducibility McNutt M.. Science. 2014;343(6168):229. doi: 10.1126/science.1250475 [DOI] [PubMed] [Google Scholar]
- 18.Le CT. A solution for the most basic optimization problem associated with an ROC curve. Stat Methods Med Res. 2006;15(6):571–584. doi: 10.1177/0962280206070637 [DOI] [PubMed] [Google Scholar]
- 19.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708–714. doi: 10.1034/j.1600-6143.2003.00072.x [DOI] [PubMed] [Google Scholar]
- 21.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x [DOI] [PubMed] [Google Scholar]
- 22.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 annual data report: kidney. Am J Transplant. 2019;19 Suppl 2:19–123. doi: 10.1111/ajt.15274 [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Muthukumar T, Yang Y, et al. Urinary cell transcriptomics and acute rejection in human kidney allografts. JCI Insight. 2020;5:e131552. doi: 10.1172/jci.insight.131552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunami M, Rosales IA, Adam BA, et al. Long-term kinetics of intragraft gene signatures in renal allograft tolerance induced by transient mixed chimerism. Transplantation. 2019;103(11):e334. doi: 10.1097/TP.0000000000002911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yapici U, Bemelman FJ, Scheepstra CG, et al. Intragraft FOXP3 protein or mRNA during acute renal allograft rejection correlates with inflammation, fibrosis, and poor renal outcome. Transplantation. 2009;87(9):1377–1380. doi: 10.1097/TP.0b013e3181a24a4b [DOI] [PubMed] [Google Scholar]
- 27.Dummer CD, Carpio VN, da Silva Loreto M, et al. Analysis of FOXP3 gene and protein expressions in renal allograft biopsies and their association with graft outcomes. Ren Fail. 2013;35(4):521–530. doi: 10.3109/0886022X.2013.766568 [DOI] [PubMed] [Google Scholar]
- 28.Nankivell BJ, Agrawal N, Sharma A, et al. The clinical and pathological significance of borderline T cell-mediated rejection. Am J Transplant. 2019;19(5):1452–1463. doi: 10.1111/ajt.15197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


