Abstract
Objective:
A small but growing proportion of lung transplant recipients survive longer than a decade post-transplant. The aim of this study was to identify factors associated with survival beyond a decade following lung transplant.
Methods:
We queried the United Network for Organ Sharing registry for adult (age ≥18) recipients undergoing first-time isolated lung transplantation between the introduction of the lung allocation score (LAS) in 2005 and 2009. Recipients were stratified into three cohorts: those that survived less than 1, 1–10, and greater than 10 years. Multivariable logistic regression was used to identify factors independently associated with early mortality (<1 year) and long-term (>10 years) survival.
Results:
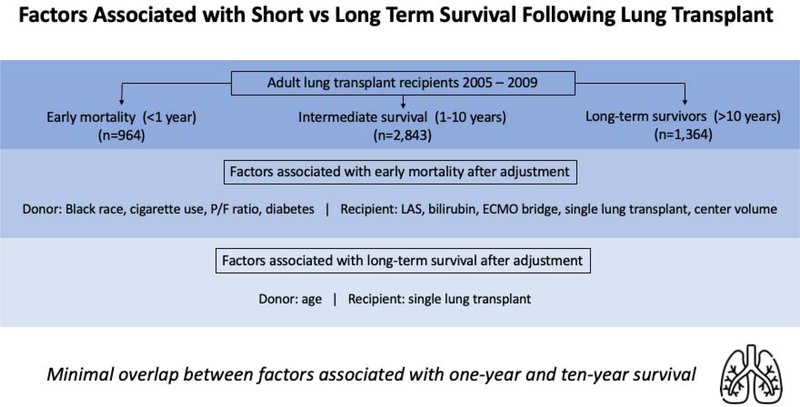
5,171 lung transplant recipients and their associated donors met inclusion criteria, including 964 (18.6%) with early mortality, 2,843 (55.0%) with intermediate survival and 1,364 (26.3%) long-term survivors. Factors independently associated with early mortality included donor Black race, cigarette use, PaO2/FiO2 ratio, diabetes, recipient LAS, total bilirubin, ECMO bridge requirement, single lung transplantation, and annual lung transplant center volume. The only factors independently associated with long-term survival among those who survived at least one year was donor age and single lung transplantation.
Conclusions:
Of patients undergoing lung transplantation after the implementation of the LAS, approximately one quarter survived ten years post-transplant. There was minimal overlap between the factors associated with one-year and ten-year survival. Importantly, the LAS was not associated with long-term survival. Further research is needed to better refine patient selection and optimize management strategies to increase the number of long-term survivors.
Keywords: Lung transplantation, long term survival, LAS, lung allocation score
Background
Despite a growing global experience with lung transplantation, recipient median survival in the modem era remains a modest 6.5 years, which is the lowest survival rate amongst recipients of the five most-commonly transplanted organs in the US [1]. While early mortality is common, refinement of patient selection, surgical technique, management of perioperative complications, and immunosuppression regimens have led to a small but growing population of lung allograft recipients with prolonged survival beyond a decade post-transplant [2–4]. Due to regulatory requirements and current publicly reported outcomes, much of the lung transplant literature and the widely used lung allocation score (LAS), focuses primarily on 1-year survival [5]. Studying the unique cohort of patients with prolonged survival enhances the understanding of factors that influence outcomes following lung transplantation and ultimately improves the quality of patient care.
Although several validated risk indices for recipient survival following lung transplantation have been developed, factors associated with long-term survival are less well elucidated [6]. We aimed to examine donor and recipient factors associated with long-term survival in a modern cohort using a large national transplant registry and compare these factors with those associated with the commonly utilized endpoint of one-year survival.
Methods
Data source & study population
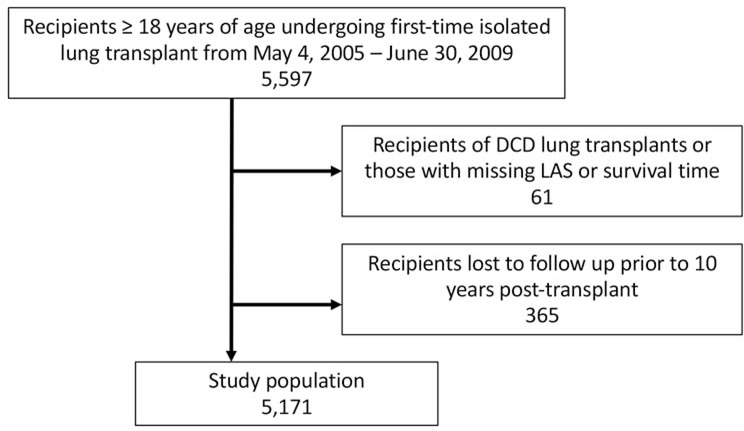
The United Network for Organ Sharing (UNOS) provided deidentified recipient and donor transplant data for all organ transplants performed in the US. The database was queried for all adult (age ≥18) recipients undergoing first-time single or bilateral lung transplantation between May 4, 2005 and June 30, 2009 along with their associated donors (Figure 1). This period was selected to capture a modern cohort of patients since the implementation of the LAS with at least 10 years of follow up potentially recorded. Recipients with prior transplants, those undergoing multiorgan transplantation, and those with missing survival data were excluded. Donation after circulatory death (DCD) donors were also excluded. Lastly, to minimize bias, patients who were lost to follow up in the 10 years following transplant were also excluded.
Figure 1.
Derivation of the final study cohort, which included 5,171 adults undergoing first-time isolated lung transplant from May 4, 2005 to June 30, 2009. DCD, donation after circulatory death; LAS, lung allocation score.
Data analysis
Recipients were stratified into three primary cohorts for descriptive analysis: those that survived <1 year (early mortality), 1–10 years (intermediate survival), and >10 years (long-term survival) following transplantation. This definition of long-term survival is in concordance with an earlier analysis of the UNOS registry and is also a commonly used clinical benchmark for other solid organ transplants [7]. Baseline recipient and donor characteristics were compared between groups using the Wilcoxon rank sum test for continuous variables and the Pearson χ2 test or Fisher’s exact test for categorical variables. Donor-recipient predicted total lung capacity (pTLC) ratios were calculated using validated regression equations that include height, age, and sex [8, 9]. Documented recipient cause of death was also compared between groups. The primary outcome was survival at least ten years following index lung transplantation. Adjusted logistic regression models were created with model covariates selected a priori. Since we aimed to examine the independent association between LAS and survival, LAS was entered as a covariate instead of its individual components (lung disease diagnosis group, recipient age, body mass index (BMI), history of diabetes, functional status, percent predicted forced vital capacity, pulmonary artery systolic pressure, pulmonary capillary wedge pressure, oxygen requirement at rest, 6-min walking distance, need for mechanical ventilation, and serum creatinine). A sensitivity analysis was performed examining factors associated with long-term survival using multivariable logistic regression as above, with the covariate LAS replaced with many of its components: lung disease diagnosis group, recipient age, BMI, diabetes, forced vital capacity, and serum creatinine. Linearity of continuous variables with the logit of the outcome was assessed using restricted cubic splines. For ease of interpretation, non-linear continuous variables were modeled using piecewise linear splines. Center-level clustering was accounted for with centers entered as a random intercept. Missing covariate data were handled using multiple imputation. Two-sided p-values ≤0.05 were considered statistically significant. Statistical analyses were performed using R version 3.5.1 (Vienna, Austria). This study was deemed exempt by the Duke University Institutional Review Board.
Results
Baseline characteristics
5,171 lung transplant recipients and their associated donors met inclusion criteria including 964 (18.6%) with early mortality, 2,843 (55.0%) with intermediate survival and 1,364 (26.3%) long-term survivors (Figure 1). Baseline recipient characteristics stratified by cohort are presented in Table 1. Recipients in the three cohorts were of similar sex, race/ethnicity, had similar rates of diabetes, and of size mismatch based upon predicted total lung capacity (pTLC) ratios. Long-term survivors were younger at the time of transplant, had a lower body mass index (BMI), were less likely to undergo single lung transplantation, and were transplanted at higher volume centers.
Table 1.
Recipient baseline characteristics stratified by survival
| Variable | <1 year survival | 1–10 year survival | >10 year survival | p-value |
|---|---|---|---|---|
| (n=964) | (n=2,843) | (n=1,364) | ||
| Male sex | 590 (61.2%) | 1,660 (58.4%) | 777 (57.0%) | 0.120 |
| Age (years, median, IQR) | 59 (51–64) | 58 (49–63) | 55 (45–61) | <0.001 |
| BMI (kg/m2, median, IQR) | 25.6 (21.6–28.7) | 25.3 (21.5–28.5) | 24.7 (20.7–28.1) | 0.001 |
| Donor-recipient pTLC ratio (median, IQR) | 1.02 (0.89–1.16) | 1.03 (0.91–1.17) | 1.03 (0.91–1.16) | 0.133 |
| Race/Ethnicity | 0.719 | |||
| White | 820 (85.1%) | 2,416 (85.0%) | 1,162 (85.2%) | |
| Black | 74 (7.7%) | 244 (8.6%) | 120 (8.8%) | |
| Hispanic | 47 (4.9%) | 131 (4.6%) | 62 (4.5%) | |
| Other | 23 (2.4%) | 52 (1.8%) | 20 (1.5%) | |
| Diabetes | 170 (17.7%) | 475 (16.7%) | 221 (16.2%) | 0.636 |
| Malignancy | 68 (7.3%) | 168 (6.0%) | 59 (4.5%) | 0.015 |
| Diagnosis group | <0.001 | |||
| A | 325 (33.7%) | 1,124 (39.5%) | 500 (36.7%) | |
| B | 34 (3.5%) | 75 (2.6%) | 39 (2.9%) | |
| C | 100 (10.4%) | 347 (12.2%) | 222 (16.3%) | |
| D | 505 (52.4%) | 1,297 (45.6%) | 603 (44.2%) | |
| Creatinine (mg/dL, median, IQR) | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | <0.001 |
| Total bilirubin (mg/dL, median, IQR) | 0.5 (0.3–0.8) | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | <0.001 |
| Pre-transplant status | <0.001 | |||
| Intensive care unit | 157 (16.3%) | 157 (5.5%) | 63 (4.6%) | |
| Hospitalized (non-ICU) | 89 (9.2%) | 198 (7.0%) | 86 (6.3%) | |
| Not hospitalized | 718 (74.5%) | 2,488 (87.5%) | 1,215 (89.1%) | |
| IV antibiotics in two weeks before transplant | 115 (12.5%) | 268 (9.8%) | 153 (11.6%) | 0.039 |
| Ventilator support at transplant | 89 (9.2%) | 112 (3.9%) | 51 (3.7%) | <0.001 |
| ECMO support at transplant | 23 (2.4%) | 11 (0.4%) | 6 (0.4%) | <0.001 |
| Lung allocation score (LAS, median, IQR) | 39.3 (34.5–49.0) | 37.7 (33.7–44.4) | 37.7 (33.8–45.0) | <0.001 |
| Days on waitlist (median, IQR) | 81(26–263) | 80 (24–253) | 87 (25–293) | 0.349 |
| Single lung transplant | 375 (38.9%) | 1,210 (42.6%) | 352 (25.8%) | <0.001 |
| HLA match | 0.624 | |||
| 0 antigen match | 177 (22.0%) | 541 (22.7%) | 233 (20.2%) | |
| 1 antigen match | 306 (38.0%) | 876 (36.7%) | 441 (38.2%) | |
| 2 antigen match | 202 (25.1%) | 631 (26.5%) | 301 (26.1%) | |
| 3+ antigen match | 120 (14.9%) | 337 (14.1%) | 180 (15.6%) | |
| Center annual lung transplant volume (median, IQR) | 28 (17–45) | 30 (22–45) | 30 (22–48) | <0.001 |
IQR, interquartile range; BMI, body mass index; pTLC, predicted total lung capacity; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; HLA, human leukocyte antigen
Donor characteristics are presented in Table 2. Compared with early mortality recipients and those with intermediate survival, donors associated with long-term survivors were younger, more likely white, and were less likely to have a history of cigarette use or hypertension. Donors in both cohorts were of similar sex, BMI, and had similar causes of death. Transplants of long-term survivors had a longer graft ischemic time than those of intermediate and short-term survivors.
Table 2.
Donor and graft characteristics stratified by recipient survival
| Variable | <1 year survival | 1–10 year survival | >10 year survival | p-value |
|---|---|---|---|---|
| (n=964) | (n=2,843) | (n=1,364) | ||
| Male sex | 559 (58.0%) | 1,713 (60.3%) | 848 (62.2%) | 0.126 |
| Age (years, median, IQR) | 31 (21–46) | 31 (21–47) | 29 (20–45) | 0.046 |
| BMI (kg/m2, median, IQR) | 24.5 (21.9–27.6) | 24.7 (22.0–27.8) | 24.6 (21.9–27.5) | 0.453 |
| Race/ethnicity | <0.001 | |||
| White | 556 (57.7%) | 1,825 (64.2%) | 881 (64.6%) | |
| Black | 222 (23.0%) | 496 (17.4%) | 225 (16.5%) | |
| Hispanic | 157 (16.3%) | 416 (14.6%) | 199 (14.6%) | |
| Other | 29 (3.0%) | 106 (3.7%) | 59 (4.3%) | |
| Cigarette use | 167 (17.5%) | 429 (15.2%) | 176 (13.0%) | 0.010 |
| Cocaine use | 112 (11.7%) | 323 (11.5%) | 137 (10.2%) | 0.396 |
| Diabetes | 72 (7.5%) | 158 (5.6%) | 59 (4.3%) | 0.005 |
| Hypertension | 229 (23.9%) | 571 (20.2%) | 253 (18.6%) | 0.007 |
| Cancer | 13 (1.4%) | 60 (2.1%) | 25 (1.8%) | 0.323 |
| Pulmonary infection | 314 (32.6%) | 861 (30.3%) | 395 (29.0%) | 0.173 |
| Creatinine (mg/dL, median, IQR) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 0.518 |
| Total bilirubin (mg/dL, median, IQR) | 0.8 (0.5–1.3) | 0.8 (0.5–1.2) | 0.7 (0.5–1.2) | 0.022 |
| P/F ratio | 456 (387–511) | 445 (376–506) | 447 (383–502) | 0.048 |
| Cause of death | 0.615 | |||
| Anoxia | 90 (9.3%) | 281 (9.9%) | 145 (10.6%) | |
| Cerebrovascular/stroke | 383 (39.7%) | 1,048 (36.9%) | 475 (34.8%) | |
| Head trauma | 462 (47.9%) | 1,430 (50.3%) | 700 (51.3%) | |
| CNS tumor | 8 (0.8%) | 22 (0.8%) | 12 (0.9%) | |
| Other | 21 (2.2%) | 62 (2.2%) | 32 (2.3%) | |
| Graft ischemic time (hours, median, IQR) | 4.9 (4.0–6.1) | 4.8 (3.8–6.0) | 5.1 (4.2–6.2) | <0.001 |
IQR, interquartile range; BMI, body mass index; CNS, central nervous system
Recipient cause of death
Table 3 includes recipient cause of death stratified by short-, intermediate-, and long-term survival. Of recipients with a documented cause of death, infection and multiple organ failure were more common among those with early mortality while malignancy and renal failure were more commonly the cause of death for recipients who survived at least 10-years post-transplant.
Table 3.
Recipient cause of death among those with cause of death documented stratified by length of survival post-transplant
| Cause of death | <1 year survival | 1–10 year survival | >10 year survival |
|---|---|---|---|
| (n=911) | (n=2,639) | (n=337) | |
| Graft failure | 139 (15.3%) | 539 (20.4%) | 54 (16.0%) |
| Pulmonary | 122 (13.4%) | 491 (18.6%) | 40 (14.8%) |
| Cardiovascular | 72 (7.9%) | 148 (5.6%) | 22 (6.5%) |
| Infection | 288 (31.6%) | 412 (15.6%) | 39 (11.6%) |
| Malignancy | 30 (3.3%) | 389 (14.7%) | 65 (19.3%) |
| Renal failure | 3 (0.3%) | 58 (2.2%) | 9 (2.7%) |
| Liver failure | 5 (0.5%) | 18 (0.7%) | 3 (0.9%) |
| Multiple organ failure | 82 (9.0%) | 87 (3.3%) | 9 (2.7%) |
| Other | 170 (18.7%) | 497 (18.8%) | 86 (25.5%) |
Adjusted analysis
Recipient, donor, and transplant factors independently associated with early mortality were identified using adjusted logistic regression with the outcome of 1-year survival (Table 4). Donor factors independently associated with recipient <1 year mortality included Black race (aOR 0.67, 95% CI 0.55–0.82), cigarette use (aOR 0.75, 95% CI 0.61–0.93), history of diabetes (aOR 0.65, 95% CI 0.48–0.90), increasing graft ischemic time over 5 hours (aOR 0.88 per hour, 95% CI 0.81–0.95), and increasing P/F ratio (aOR 0.99 per 10 units, 95% CI 0.99–1.00). Significant recipient factors associated with early mortality included increasing LAS (aOR 0.94 per 5 units, 95% CI 0.91–0.96), increasing total bilirubin less than 0.75mg/dL (aOR 0.59, 95% CI 0.39–0.90), ECMO requirement at transplant (aOR 0.26, 95% CI 0.13–0.53), and single lung transplantation (aOR 0.82, 95% CI 0.68–1.00). Increasing center annual adult lung transplant volume below 40 (aOR 1.09 per 5 transplants, 95% CI 1.03–1.15) was associated with an increased odds of 1-year survival, while increasing center volume above 40 averaged over the study period was not associated with an increased or decreased odds of 1-year survival (aOR 0.97, 95% CI 0.90–1.04).
Table 4.
Multivariable logistic regression model for donor and recipient factors independently associated with recipient one-year survival
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Odds Ratio | Lower | Upper | p-value |
| Donor/graft characteristics | ||||
| Age (per 5 years) | 1.00 | 0.96 | 1.03 | 0.817 |
| Male sex (vs female) | 1.14 | 0.93 | 1.38 | 0.207 |
| Race/ethnicity (reference: white) | ||||
| Black | 0.67 | 0.55 | 0.82 | <0.001 |
| Hispanic | 0.80 | 0.64 | 1.00 | 0.054 |
| Other | 1.16 | 0.73 | 1.83 | 0.541 |
| BMI (per unit) | 1.01 | 0.99 | 1.03 | 0.267 |
| Cigarette use | 0.75 | 0.61 | 0.93 | 0.009 |
| Diabetes | 0.65 | 0.48 | 0.90 | 0.008 |
| Donor cause of death (reference: head trauma) | ||||
| Anoxia | 1.01 | 0.77 | 1.34 | 0.922 |
| Cerebrovascular/stroke | 0.94 | 0.76 | 1.17 | 0.592 |
| CNS tumor | 0.87 | 0.37 | 2.05 | 0.750 |
| Other | 0.92 | 0.55 | 1.54 | 0.743 |
| Ischemic time | ||||
| <5 hours (per hour) | 1.03 | 0.92 | 1.15 | 0.592 |
| ≥5 hours (per hour) | 0.88 | 0.81 | 0.95 | 0.001 |
| P/F ratio (per 10 units) | 0.99 | 0.99 | 1.00 | 0.019 |
| Recipient characteristics | ||||
| Male sex (vs female) | 0.87 | 0.72 | 1.05 | 0.139 |
| Lung allocation score (LAS, per 5 units) | 0.94 | 0.91 | 0.96 | <0.001 |
| Donor-Recipient pTLC ratio | ||||
| <1 (per 0.1 unit increase) | 1.04 | 0.94 | 1.14 | 0.450 |
| ≥1 | 1.00 | 0.93 | 1.06 | 0.903 |
| Race/ethnicity (reference: white) | ||||
| Black | 1.05 | 0.78 | 1.39 | 0.765 |
| Hispanic | 1.09 | 0.76 | 1.57 | 0.641 |
| Other | 0.85 | 0.50 | 1.46 | 0.559 |
| Total bilirubin | ||||
| <0.75 mg/dL (per mg/dL) | 0.59 | 0.39 | 0.90 | 0.015 |
| ≥0.75 mg/dL (per mg/dL) | 0.96 | 0.87 | 1.06 | 0.400 |
| ECMO at transplant | 0.26 | 0.13 | 0.53 | <0.001 |
| Center annual lung transplant volume | ||||
| <40 (per 5 transplants) | 1.09 | 1.03 | 1.15 | 0.007 |
| ≥40 (per 5 transplants) | 0.97 | 0.90 | 1.04 | 0.367 |
| Single lung transplant | 0.82 | 0.68 | 1.00 | 0.045 |
CNS, central nervous system; P/F, PaO2/FiO2; BMI, body mass index; ECMO, extracorporeal membrane oxygenation
Factors associated with long-term survival among recipients with conditional survival to at least 1-year were identified using adjusted logistic regression with the outcome of 10-year survival (Table 5, Figure 2). Increasing donor age (aOR 0.96 per 5 years, 95% CI 0.93–1.00) and single lung transplantation (aOR 0.50, 95% CI 0.42–0.60) were both independently associated with mortality prior to 10 years post-transplant. LAS was not associated with 10-year survival (aOR 0.99 per 5 units, 95% CI 0.96–1.02). Supplemental Table 1 includes a similar logistic regression model, with the LAS covariate replaced with several of its components. In this model, recipient age and FVC were significantly associated with 10-year survival but recipient BMI, diagnosis, diabetes status, and creatinine were not. Kaplan-Meier survival analyses of the entire cohort, as well as the cohort of recipients who survived at least one year stratified by single vs. bilateral lung transplantation are presented in Supplemental Figure 1. An adjusted logistic regression model for 10-year survival among all recipients (regardless of whether 1-year survival was achieved) is presented in Supplemental Table 2. Factors associated with <10 year mortality included donor age, Black race, LAS, and single lung transplantation while increasing center volume less than 40 was associated with increased odds of 10-year survival.
Table 5.
Multivariable logistic regression model for donor and recipient factors independently associated with recipient ten-year survival among recipients who survived at least one year
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Odds Ratio | Lower | Upper | p-value |
| Donor/graft characteristics | ||||
| Age (per 5 years) | 0.96 | 0.93 | 1.00 | 0.019 |
| Male sex (vs female) | 1.13 | 0.94 | 1.35 | 0.189 |
| Race/ethnicity (reference: white) | ||||
| Black | 0.90 | 0.74 | 1.09 | 0.285 |
| Hispanic | 0.98 | 0.79 | 1.21 | 0.836 |
| Other | 0.95 | 0.65 | 1.39 | 0.796 |
| BMI (per unit) | 1.00 | 0.99 | 1.02 | 0.622 |
| Cigarette use | 0.88 | 0.72 | 1.09 | 0.251 |
| Diabetes | 0.85 | 0.61 | 1.19 | 0.346 |
| Donor cause of death (reference: head trauma) | ||||
| Anoxia | 1.08 | 0.84 | 1.38 | 0.557 |
| Cerebrovascular/stroke | 1.13 | 0.93 | 1.37 | 0.237 |
| CNS tumor | 1.00 | 0.46 | 2.18 | 0.998 |
| Other | 1.29 | 0.80 | 2.08 | 0.298 |
| Ischemic time | ||||
| <5 hours (per hour) | 1.11 | 1.00 | 1.24 | 0.053 |
| ≥5 hours (per hour) | 0.98 | 0.91 | 1.06 | 0.563 |
| P/F ratio | ||||
| <450 (per 10 units) | 1.01 | 1.00 | 1.02 | 0.105 |
| ≥450 (per 10 units) | 0.99 | 0.98 | 1.00 | 0.207 |
| Recipient characteristics | ||||
| Male sex (vs female) | 0.87 | 0.74 | 1.03 | 0.108 |
| Lung allocation score (LAS, per 5 units) | 0.99 | 0.96 | 1.02 | 0.351 |
| Donor-Recipient pTLC ratio | ||||
| <1 (per 0.1 unit increase) | 1.02 | 0.93 | 1.12 | 0.617 |
| ≥1 | 0.99 | 0.93 | 1.05 | 0.669 |
| Race/ethnicity (reference: white) | ||||
| Black | 1.00 | 0.78 | 1.30 | 0.980 |
| Hispanic | 1.14 | 0.82 | 1.59 | 0.446 |
| Other | 0.90 | 0.52 | 1.56 | 0.713 |
| Total bilirubin (per mg/dL) | 1.02 | 0.94 | 1.12 | 0.610 |
| ECMO at transplant | 1.23 | 0.43 | 3.54 | 0.704 |
| Center annual lung transplant volume | ||||
| <40 (per 5 transplants) | 1.04 | 1.00 | 1.09 | 0.087 |
| ≥40 (per 5 transplants) | 1.00 | 0.95 | 1.04 | 0.855 |
| Single lung transplant | 0.50 | 0.42 | 0.60 | <0.001 |
CNS, central nervous system; P/F, PaO2/FiO2; BMI, body mass index; ECMO, extracorporeal membrane oxygenation
Figure 2.
There was minimal overlap between the identified factors associated with short-term (<1-year) and long-term (>10-year) survival. Accordingly, current prognostic factors are not applicable to assess and guide improvement in survival beyond the first-year post-transplant. P/F, PaO2/FiO2; LAS, lung allocation score; ECMO, extracorporeal membrane oxygenation.
Discussion
In this retrospective analysis of the UNOS registry, we examined the cohort of patients who have experienced long-term survival following lung transplantation since the introduction of the LAS in 2005. In addition to a descriptive comparison with short and intermediate-term survivors, we identified factors independently associated with long-term survival using adjusted logistic regression. We demonstrated that there was minimal overlap between the factors associated with 1-year survival and those associated with 10-year survival among the cohort of recipients who survived at least one year. Further, documented causes of death different significantly among recipients with short-, intermediate-, and long-term survival following lung transplant. These findings have important implications as we move towards more patient-centric quality metrics, such as long-term survival.
We identified 1,364 lung transplant recipients transplanted between May 2005 and June 2009 that survived at least 10 years post-transplant, representing approximately 26% of the total number of transplants performed during this period. By comparison, in the 2010 study by Weiss and colleagues examining long-term survivors between 1987–1997, only approximately 17% of recipients survived past 10 years, indicating a gradual improvement of post-lung transplant survival during this period [7]. This improvement in survival, which occurred despite a trend towards an older pool of donors and recipients during this time, may be the result of better rescue following complications and better immunosuppression management [10].
Many recipient characteristics have been previously linked to short-term recipient outcomes in prior UNOS registry analyses as well as with data from the International Society for Heart and Lung Transplantation (ISHLT) registry [11, 12]. The LAS, which is a weighted score from 0–100 derived from multiple recipient diagnosis, oxygenation, and functional status measures, was introduced in 2005 as a means of stratifying lung transplant candidates based on the risk of waitlist and 1-year mortality for organ allocation purposes [13]. In this study, as expected, we demonstrated a significant association between LAS and short-term posttransplant survival. However, the LAS was not associated with long-term recipient survival among those who survived at least one-year post-transplant. While not originally developed to stratify candidates based upon their potential for longer-term post-transplant survival, the significant emphasis placed on LAS in the organ allocation process may not be optimal for maximizing the number of long-term survivors. As the lung transplant community places a greater emphasis on prolonged survival, we will need to consider factors beyond the frequently emphasized LAS.
Similar to many previously published studies, we demonstrated a strong association between donor Black race as well as donor smoking history with recipient short-term survival [11, 12, 14–16]. The mechanism of the relationship between donor race and recipient survival cannot be fully elucidated in a retrospective registry analysis. Prior research has, however, suggested that donor/recipient race matching significantly influences recipient short-term outcomes, with limited impact on longer-term survival [14]. Our finding of no association between cause of donor brain death and recipient survival is also in congruence with prior studies [17, 18]. Likewise, our results demonstrating a relationship between donor diabetes status and short-term lung transplant recipient survival has been previously reported among US and international lung transplant recipient cohorts [12, 16, 19, 20]. The finding that none of these factors were associated with long-term survival among recipients who survived at least one year is a novel finding, however. In an examination of 1,800 lung transplants from the UNOS registry, Meyer and colleagues previously demonstrated that donor age was not associated with short-term 2 year survival [21]. Our data again demonstrates that donor age is less important for short term survival, although our findings suggest that donor age is indeed a significant factor associated with long-term 10-year survival. The association between donor oxygenation and recipient outcomes has been controversial [22, 23]. In this study, we demonstrated that donor P/F ratios are significantly associated with the risk of short-term mortality, however this association was no longer apparent when examining factors associated with survival a decade post-transplant.
The association between graft ischemic time and recipient outcomes has also been controversial, with some studies finding improved recipient survival associated with longer ischemic times. We report a decreased likelihood of one-year survival associated with longer ischemic times but no association with long-term 10-year survival, which may be partially explained by the increasingly strong correlation between bilateral lung transplantation and improved survival in later years following transplant.
In the present study, the only factor that was significantly associated with both one-year survival as well as ten-year survival among those who survived at least one year was bilateral lung transplantation. Several prior studies have also demonstrated strong associations between bilateral lung transplantation and recipient outcomes [24–26]. Given significant allograft shortages, however, the benefit associated with bilateral lung transplantation must be weighed against the benefit of transplanting two recipients with a single lung each [27].
Lastly, in this study we demonstrated a correlation between increasing hospital lung transplant volume up to 40 transplants per year and improved short-term survival. After 40 or more annual transplants performed, a “ceiling effect” was observed where additional transplants did not result in increasingly better one-year survival, although the number of transplant centers meeting this volume threshold was small. Prior studies have also found that hospital procedural volume is an important component of center-specific variation in lung transplant survival [28, 29]. We also demonstrated that increasing annual volume up to 40 transplants per year was associated with survival a decade post-transplant among all comers, however the association was no longer significant when limited to only recipients who survived at least one year. The data presented here suggests that the impact of higher volume centers is most salient in the weeks and months following transplant and may influence survival a decade later, although to a lesser degree. This likely reflects improved complication management by high volume centers, including titration of immunosuppression regimens following BOS and CLAD diagnoses, as well as the availability of expert transplant infectious disease and oncology specialists. These findings further support the notion of regionalization of care, especially given the high degree of complexity involved in managing this patient population.
As a retrospective analysis of national registry data, this study is limited by the quantity and accuracy of available variables within the dataset. In particular, we could not reliably examine recipient immunosuppression data as well as the impact of post-transplant complications on short- and long-term survival. In addition, other factors including medical non-compliance, allograft rejection, gastroesophageal reflux disease, environmental exposures, and socioeconomic status likely influence long-term outcomes as well. However, given the sheer size of the registry, containing records of all transplants performed in the US since 1987, the UNOS database is likely the best source for examining factors associated with long-term survival in lung transplantation. Lastly, this analysis of long-term survivors necessitated examining the cohort of patients that were transplanted at least 10 years ago. As such, we could not evaluate the impact of advancements in the management of lung transplant patients made over the last decade. Future studies should also be conducted examining factors associated with long-term survival among recipients of allografts from donation after circulatory death donors.
Conclusion
In this analysis of greater than 5,000 lung transplants performed in the US, we identified factors associated with survival greater than ten years following lung transplantation and compared these factors to those associated with short-term one-year survival. While several donor, recipient, and hospital factors were significantly independently associated with one-year survival, there was minimal overlap with factors associated with long-term survival. Importantly, the widely emphasized LAS was not associated with long-term recipient survival among those who survived at least one-year post-transplant. Accordingly, current prognostic factors used to stratify lung transplant candidates may not be applicable to assess and guide improvement in survival beyond the first year. Further study of this important cohort of patients is warranted to better understand drivers of long-term survival.
Supplementary Material
Central Picture.
There was minimal overlap between factors associated with short- and long-term survival.
Central Message
Current prognostic factors used to stratify lung transplant candidates, including the LAS, may not be applicable to assess and guide improvement in survival beyond the first year.
Perspective Statement.
Due to regulatory requirements and current publicly reported outcomes, much of the lung transplant literature focuses on 1-year survival. In this analysis, there was minimal overlap between factors associated with 1- and 10-year survival. As the lung transplant community places a greater emphasis on prolonged survival, we will need to consider factors beyond the frequently emphasized LAS.
Acknowledgements
Dr. Jawitz was supported by NIH grant 5T32HL069749 and Dr. Raman was supported by NIH grant 5T32CA093245. The authors have no disclosures relevant to the presented work. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Funding for this study was provided by NIH grants 5T32HL069749 (OKJ) and 5T32CA093245 (VR)
Glossary of abbreviations
- LAS
lung allocation score
- UNOS
United Network for Organ Sharing
- DCD
donation after circulatory death
- pTLC
predicted total lung capacity
- BMI
Body mass index
- P/F ratio
PaO2/FiO2 ratio
- BOS
bronchiolitis obliterans syndrome
- CLAD
chronic lung allograft dysfunction
Footnotes
The authors have no relevant disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe RA, Roys EC, and Merion RM, Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant, 2010. 10(4 Pt 2): p. 961–72. [DOI] [PubMed] [Google Scholar]
- 2.Thabut G, et al. , Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med, 2013. 187(12): p. 1335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santacruz JF and Mehta AC, Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc, 2009. 6(1): p. 79–93. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan AK, et al. , The Diagnosis and Management of Airway Complications Following Lung Transplantation. Chest, 2017. 152(3): p. 627–638. [DOI] [PubMed] [Google Scholar]
- 5.Egan TM, et al. , Development of the new lung allocation system in the United States. Am J Transplant, 2006. 6(5 Pt 2): p. 1212–27. [DOI] [PubMed] [Google Scholar]
- 6.Gries CJ, et al. , Development of a predictive model for long-term survival after lung transplantation and implications for the lung allocation score. J Heart Lung Transplant, 2010. 29(7): p. 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss ES, et al. , Factors indicative of long-term survival after lung transplantation: a review of 836 10-year survivors. J Heart Lung Transplant, 2010. 29(3): p. 240–6. [DOI] [PubMed] [Google Scholar]
- 8.Eberlein M, et al. , Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc, 2013. 10(5): p. 418–25. [DOI] [PubMed] [Google Scholar]
- 9.Mason DP, et al. , Matching donor to recipient in lung transplantation: How much does size matter? J Thorac Cardiovasc Surg, 2009. 137(5): p. 1234–40 e1. [DOI] [PubMed] [Google Scholar]
- 10.Studer SM, et al. , Lung transplant outcomes: a review of survival, graft function, physiology, health-related quality of life and cost-effectiveness. Eur Respir J, 2004. 24(4): p. 674–85. [DOI] [PubMed] [Google Scholar]
- 11.Scientific Registry of Transplant Recipients. SRTR Risk Adjustment Model Documentation: Posttransplant Outcomes. 2019. [cited 2019 February 19]. [Google Scholar]
- 12.Chambers DC, et al. , The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant, 2018. 37(10): p. 1169–1183. [DOI] [PubMed] [Google Scholar]
- 13.Russo MJ, et al. , High lung allocation score is associated with increased morbidity and mortality following transplantation. Chest, 2010. 137(3): p. 651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen JG, et al. , Impact of donor-recipient race matching on survival after lung transplantation: analysis of over 11,000 patients. J Heart Lung Transplant, 2009. 28(10): p. 1063–71. [DOI] [PubMed] [Google Scholar]
- 15.Bonser RS, et al. , Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet, 2012. 380(9843): p. 747–55. [DOI] [PubMed] [Google Scholar]
- 16.Chambers DC, et al. , The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant, 2017. 36(10): p. 1047–1059. [DOI] [PubMed] [Google Scholar]
- 17.Ciccone AM, et al. , Does donor cause of death affect the outcome of lung transplantation? J Thorac Cardiovasc Surg, 2002. 123(3): p. 429–34; discussion 434–6. [DOI] [PubMed] [Google Scholar]
- 18.Crawford TC, et al. , Traumatically Brain-Injured Donors and the Impact on Lung Transplantation Survival. Ann Thorac Surg, 2018. 106(3): p. 842–847. [DOI] [PubMed] [Google Scholar]
- 19.Ambur V, et al. , The impact of lungs from diabetic donors on lung transplant recipientsdagger. Eur J Cardiothorac Surg, 2017. 51(2): p. 285–290. [DOI] [PubMed] [Google Scholar]
- 20.Courtwright A and Cantu E, Evaluation and Management of the Potential Lung Donor. Clin Chest Med, 2017. 38(4): p. 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer DM, et al. , Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest, 2000. 118(5): p. 1255–62. [DOI] [PubMed] [Google Scholar]
- 22.Zafar F, et al. , Does donor arterial partial pressure of oxygen affect outcomes after lung transplantation? A review of more than 12,000 lung transplants. J Thorac Cardiovasc Surg, 2012. 143(4): p. 919–25. [DOI] [PubMed] [Google Scholar]
- 23.Chaney J, et al. , Lung donor selection criteria. J Thorac Dis, 2014. 6(8): p. 1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits JM, et al. , Predictors of lung transplant survival in eurotransplant. Am J Transplant, 2003. 3(11): p. 1400–6. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer JM, et al. , Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA, 2015. 313(9): p. 936–48. [DOI] [PubMed] [Google Scholar]
- 26.Black MC, et al. , Double lung transplants have significantly improved survival compared with single lung transplants in high lung allocation score patients. Ann Thorac Surg, 2014. 98(5): p. 1737–41. [DOI] [PubMed] [Google Scholar]
- 27.Aryal S and Nathan SD, Single vs. bilateral lung transplantation: when and why. Curr Opin Organ Transplant, 2018. 23(3): p. 316–323. [DOI] [PubMed] [Google Scholar]
- 28.Thabut G, et al. , Survival differences following lung transplantation among US transplant centers. JAMA, 2010. 304(1): p. 53–60. [DOI] [PubMed] [Google Scholar]
- 29.Weiss ES, et al. , The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg, 2009. 88(4): p. 1062–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.