Abstract
Objective
Coronavirus disease 2019 [COVID-19] infection in patients with chronic liver disease [CLD] may precipitate acute-on-chronic liver failure [ACLF]. In a large multi-center cohort of COVID-19-infected patients, we aim to analyze (1) the outcomes of patients with underlying CLD [with and without cirrhosis] and (2) the development and impact of ACLF on in-hospital mortality.
Design
We identified 192 adults with CLD from among 10,859 patients with confirmed COVID-19 infection (admitted to any of 12 hospitals in a New York health care system between March 1, 2020 and April 27, 2020). ACLF was defined using the EASL-CLIF Consortium definition. Patient follow-up was through April 30, 2020, or until the date of discharge, transfer, or death.
Results
Of the 84 patients with cirrhosis, 32 [38%] developed ACLF, with respiratory failure [39%] and renal failure [26%] being the most common. Hispanic/Latino ethnicity was particularly at higher risk of in-hospital mortality [adjusted HR 4.92, 95% 1.27–19.09, p < 0.02] in cirrhosis despite having lower risk of development of ACLF [HR 0.26, 95% CI 0.08–0.89, p = 0.03]. Hypertension on admission predicted development of ACLF [HR 3.46, 95% CI 1.12–10.75, p = 0.03]. In-hospital mortality was not different between CLD patients with or without cirrhosis [p = 0.24] but was higher in those with cirrhosis who developed ACLF [adjusted HR 9.06, 95% CI 2.63–31.12, p < 0.001] with a trend for increased mortality by grade of ACLF [p = 0.002]. There was no difference in in-hospital mortality between the CLD cohort compared to matched control without CLD (log rank, p = 0.98) and between the cirrhosis cohort compared to matched control without cirrhosis (log rank, p = 0.51).
Conclusion
Development of ACLF is the main driver of increased in-hospital mortality in hospitalized patients with COVID-19 infection and cirrhosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-021-10181-y.
Keywords: Acute-on-chronic liver failure, COVID-19, Chronic liver disease, Cirrhosis, Liver chemistries, Mortality, Organ failure
Introduction
Since the start of the outbreak of coronavirus disease 2019 [COVID-19] in Wuhan, China in December 2019, there have been more than 90,673,898 cases diagnosed globally with more than 1,940,321 deaths as of January 11, 2021 [1–3].
The prevalence of chronic liver disease [CLD] among patients with COVID-19 has been reported to range from 2 to 11% [4]. A recent meta-analysis of COVID-19 infection in patients with CLD concluded that CLD does not increase the risks of severe COVID-19 infection complications or mortality [5]. However, a another international registry of patients with CLD and COVID-19 infection reported that 36% of patients experienced hepatic decompensation, and the highest risk was among patients with Child–Turcotte–Pugh class B and C cirrhosis [6]. Notably, 24% of patients with new hepatic decompensation did not have respiratory symptoms at the time of diagnosis [6].
COVID-19 infection in patients with CLD may precipitate acute-on-chronic liver failure [ACLF]. In this study, we report on [1] the outcomes of patients [both with and without cirrhosis] with COVID-19 infection and underlying CLD in a multi-hospital healthcare system within the United States epicenter of the pandemic and [2] the development and impact of ACLF in those with Cirrhosis.
Methods
Study population
We identified patients hospitalized with confirmed COVID-19 infection using compiled data from the inpatient electronic health record [EHR; Sunrise Clinical Manager, Allscripts, Chicago, IL] for patients admitted to 12 hospitals in New York City and Long Island within the Northwell Health system in New York from the period of March 1, 2020, to April 27, 2020. A confirmed case of COVID-19 was defined as a positive reverse transcriptase polymerase chain reaction on a specimen obtained through nasopharyngeal swabbing. We excluded children under the age of 18 years from this study.
We screened all patients hospitalized with confirmed COVID-19 infection for known CLD by searching our database for ICD-10 diagnostic codes (Fig. 1 and Supplementary Fig. 1) or any radiologic report [regardless of whether prior to or during the index COVID-19 hospitalization] including the term “cirrhosis.” Due to the poor specificity of ICD-10 codes in identifying patients with cirrhosis [7], a single transplant hepatologist [with greater than 10 years of experience, SKS] manually reviewed outpatient, emergency department, and inpatient electronic records for each patient who was identified as having CLD on the initial screen. We followed a systematic approach to confirm whether patients had CLD with or without cirrhosis (described in Supplementary Fig. 1). Any discrepancies were resolved with consensus after re-review by a second transplant hepatologist [NR]. Patients with a positive hepatitis C antibody and negative hepatitis C ribonucleic acid [RNA]—regardless of whether due to false-positive antibody test, prior spontaneous viral clearance, or virologic response to previously received antiviral therapy—were excluded from our study population unless they had cirrhosis or an etiology of CLD aside from hepatitis C. We also excluded solid-organ transplant recipients [heart, liver, or kidney] and patients with pre-hospitalization dependence on renal replacement therapy.
Fig. 1.
Approach used to identify patients with chronic liver disease and cirrhosis. *ICD-10 diagnostic codes used to screen for chronic liver disease were K70 [alcoholic liver disease], K71 [toxic liver disease], K73 [chronic hepatitis, not elsewhere classified], K74 [fibrosis and cirrhosis of liver], K75 [other inflammatory liver diseases], K76 [other diseases of liver], B18 [chronic viral hepatitis], and B19 [unspecified viral hepatitis]. **Patients with alternate explanations for radiologic findings [such as liver surface nodularity due to hepatic metastases, splenomegaly due to known hematologic disorder, or ascites due to peritoneal carcinomatosis] were not included as having cirrhosis unless they also fulfilled other criteria for cirrhosis
The Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health approved this study as minimal-risk research using data collected for routine clinical practice and waived the requirement for informed consent [Approval #20–0200].
Data collection
We collected the following demographic information: age, sex, race, ethnicity, presence of co-morbid conditions, and body mass index [BMI]. Race and ethnicity data were collected by self-report in pre-specified fixed categories. For laboratory parameters, we collected baseline values [defined as the first measurement for each parameter within 48 h of hospital presentation] of creatinine, international normalized ratio [INR], bilirubin, albumin, aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase, leukocyte count, hemoglobin, platelet count, lactate, ferritin, lactate dehydrogenase [LDH], D-dimer, C-reactive protein [CRP], and procalcitonin. For the three key laboratory parameters used to calculate Model for End-Stage Liver Disease [MELD] scores and to define ACLF [namely, INR, creatinine, and bilirubin], baseline measurements were verified and peak measurements [defined as the highest measurement for each parameter within the hospitalization] were collected through manual chart review. Manual chart review by an experienced transplant hepatologist was also used to identify the etiology of CLD for patients, collect data on arterial blood gas respiratory parameters, and assess for in-hospital development of severe [West-Haven grade III or IV] hepatic encephalopathy. Follow-up was through date of discharge, transfer, death or May 7, 2020, for patients that remained hospitalized.
Study outcomes
The primary outcome of interest was the development and grade of ACLF, defined using the EASL-CLIF Consortium definitions (Supplementary Table 1) [8, 9]. Organ failures were likewise defined using the EASL-CLIF Consortium definitions [8, 9]. Secondary outcomes included in-hospital mortality, development of individual organ failures, need for invasive mechanical ventilation, need for intensive-care-unit [ICU] level of care, need for renal replacement therapy, and hospital length of stay. Due to the COVID-19 pandemic, many additional ICUs were created in non-traditional hospital areas and units. Hence, need for ICU level of care was defined by any one of the following criteria: need for invasive mechanical ventilation, need for vasopressor or inotrope support, or being under the care of an ICU service or in a known ICU location. None of our patient received ECMO (Extra Corporeal Membrane Oxygenation).
Statistical analysis
Descriptive statistics was calculated for all the key variables. Continuous variables were expressed as medians with interquartile ranges [IQRs] and categorical variables as counts with percentages. We used non-parametric Wilcoxon rank sum tests, Yates corrected Chi-squared tests, and Fisher’s exact tests, as indicated, to compare parameters between the patients with and without cirrhosis or, among patients with cirrhosis, between those with and without ACLF. Predictors of ACLF were determined using univariate and multivariate logistic regressions. Multivariate logistic regression analysis was performed using Stepwise approach, entry criteria for inclusion of the variables in the final model was p < 0.1, and the stay criteria was p < 0.35. Co-variates were considered significant if p < 0.1 on univariate analyses and stepwise elimination was used to select the variables included in the final multivariate model.
To assess the relative effect of underlying CLD and cirrhosis on in-hospital mortality, we performed a sensitivity analysis by building two propensity score-matched (PSM) control cohorts (CLD and cirrhosis) (1:1 match). Variables used in the PSM model included age, gender, ethnicity, and pre-existing comorbidities including diabetes, hypertension, chronic liver disease, coronary artery disease (CAD), heart failure (HF), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), and malignancy. Matching was carried out using a local SAS macro “gmatch” [10]. Controls were selected randomly without replacement.
For the survival analyses, we censored patients as alive without the event of interest on their date of hospital discharge or transfer or at 21 days of follow-up, whichever was earlier. We analyzed survival by testing Kaplan–Meier survival curves using log-rank tests as well as estimated hazard ratios [HRs] and 95% confidence intervals [CIs] using univariate and multivariate Cox proportional hazards models. Multivariate Cox regression models were adjusted for potential confounding variables identified a priori: age, sex, ethnicity, and race. We checked the proportional hazards assumption for each variable included in our Cox regression models using graphical assessment of the Kaplan–Meier survival curves and log[– log(survival)] versus log[time] graphs to look for parallel curves while ensuring that Schoenfeld residuals were independent of time. For both logistic regression and Cox regression analyses, we used simplified categorizations for ethnicity [Hispanic/Latino ethnicity versus Non-Hispanic/Latino or other/unknown] and race [white versus black versus other/unknown] due to limited sample sizes. We used two-sided tests with alpha equaling 0.05. Statistical analyses were performed using SAS version 9.4 [SAS Institute, Cary, NC].
Results
From a total of 11,265 patients hospitalized with COVID-19, we excluded 106 children under 18 years of age and 300 patients without baseline liver chemistries; this left 10,859 eligible patients (Fig. 1). From this adult cohort, 295 patients were screened as having underlying CLD using ICD-10 diagnostic codes or a radiologic report of “cirrhosis.” After further manual chart review, 51 patients were deemed not to have underlying CLD and were excluded. We further excluded patients with end-stage renal disease [n = 17], prior heart transplant [n equal to 2], prior kidney transplant [n = 2], or prior liver transplant [n = 1], and we excluded one patient with non-cirrhotic portal hypertension due to hepatic amyloidosis. We also excluded 29 patients who had a positive hepatitis C antibody but negative hepatitis C RNA, and no evidence of cirrhosis or secondary etiology of CLD. Liver biopsy data were available in 13 patients, only 3 of them had cirrhosis by histology. The final cohort consisted of 192 CLD patients, representing 1.8% of the original cohort of hospitalized adults with COVID-19 infection: 84 patients with cirrhosis and 108 patients without cirrhosis.
The median age of patients with CLD was 63 years [range 27–97 years, IQR 55–72 years]. The majority were male [67%], non-Hispanic/Latino ethnicity [69%] and had co-morbid hypertension [63%]. Compared to CLD patients without cirrhosis, patients with cirrhosis were older, less likely to be obese, and more likely to have co-morbid chronic obstructive pulmonary disease (Table 1). There were differences in the etiology of CLD between patients with or without cirrhosis, but nonalcoholic fatty liver disease [NAFLD] was the most common etiology in both groups. CLD patients with cirrhosis more likely to have HCV and Alcohol-Related Liver Diseases whereas non-cirrhotic patients were more likely to have NAFLD and Hepatitis B. Baseline laboratory parameters of both groups are presented in Table 1. Patients with cirrhosis had similar creatinine levels but higher INRs and bilirubin levels than patients without cirrhosis; this led to higher MELD scores. The median baseline MELD score for patients with cirrhosis was 13 [IQR 10–20]. Patients with cirrhosis also had lower baseline levels of albumin, hemoglobin, platelets, ferritin, and LDH, but higher levels of AST, alkaline phosphatase, lactate, and D-dimer.
Table 1.
Admission characteristics of 192 patients with COVID-19 infection and chronic liver disease
| Characteristics | Chronic liver disease [n = 192] | ||
|---|---|---|---|
| No cirrhosis [n = 108] | Cirrhosis [n = 84] | p | |
| Vital signs | |||
| Systolic blood pressure < 90 mmHg | 5 [4.6] | 5 [5.6] | 0.68 |
| Temperature > 38 °C | 32 [29.6] | 11 [13.1] | 0.006 |
| Heart rate > 90 bpm | 79 [73.2] | 45 [53.6] | 0.005 |
| Respiratory rate > 20 bpm | 38 [35.2] | 28 [33.3] | 0.79 |
| SpO2 < 90% | 16 [14.8] | 12 [14.3] | 0.92 |
| SpO2 < 80% | 4 [3.7] | 3 [3.6] | 0.96 |
| Leukocytes < 4 or > 12 [× 109/L] | 28 [26] | 28 [33.3] | 0.26 |
| Sex | 0.63 | ||
| Male | 71 [65.7] | 58 [69.0] | |
| Female | 37 [34.3] | 26 [31.0] | |
| Age [years] | 61 [49–70] | 69 [60–78] | < 0.001 |
| Race | 0.18 | ||
| White | 42 [38.9] | 29 [34.5] | |
| Black | 16 [14.8] | 16 [19.0] | |
| Asian | 18 [16.0] | 5 [6.0] | |
| Other/multiracial | 31 [28.7] | 33 [39.3] | |
| Unknown | 3 [2.8] | 1 [1.2] | |
| Ethnicity | 0.39 | ||
| Non-Hispanic or Latino | 78 [72.2] | 54 [64.3] | |
| Hispanic or Latino | 26 [24.1] | 24 [28.6] | |
| Other or unknown | 4 [3.7] | 6 [7.1] | |
| Esophageal varices | 0 [0] | 25 [29.8] | < 0.001 |
| Ascites | 0 [0] | 28 [33.3] | < 0.001 |
| Co-morbid conditions | |||
| Hypertension | 64 [59.3] | 57 [67.9] | 0.22 |
| Diabetes mellitus | 45 [41.7] | 44 [52.4] | 0.14 |
| Coronary artery disease | 21 [19.5] | 8 [9.5] | 0.07 |
| Chronic kidney diseasea | 11 [10.2] | 8 [9.5] | 1.00 |
| Heart failure | 8 [7.4] | 10 [11.9] | 0.33 |
| Malignancy | 8 [7.4] | 14 [16.7] | 0.07 |
| COPD | 6 [5.6] | 12 [14.3] | 0.047 |
|
Body mass index [kg/m2]b < 30 ≥ 30 |
30 [26–34] 38 [46.3] 44 [53.7] |
27 [25–31] 45 [69.2] 20 [30.8] |
0.005 |
| Etiology | |||
| NAFLD | 60 [55.6] | 29 [34.5] | 0.005 |
| Hepatitis C | 14 [12.9] | 23 [27.4] | 0.012 |
| Alcohol-related liver disease | 10 [9.3] | 18 [21.4] | 0.018 |
| Hepatitis B | 21 [19.5] | 3 [3.6] | 0.008 |
| Otherc | 3 [2.8] | 11 [13.1] | 0.009 |
| Laboratory measurements [first within 48 h]b | |||
| MELD score | 10 [8–13] | 13 [10–20] | < 0.001 |
| Creatinine [mg/dL] | 1.0 [0.8–1.5] | 1.1 [0.8–1.6] | 0.63 |
| INR | 1.2 [1.1–1.3] | 1.3 [1.2–1.6] | < 0.001 |
| Bilirubin [mg/dL] | 0.5 [0.4–0.7] | 1.0 [0.5–1.9] | < 0.001 |
| Albumin [g/dL] | 3.6 [3.0–4.0] | 3.0 [2.4–3.5] | < 0.001 |
| AST [IU/L] | 48 [36–81] | 60 [43–112] | 0.01 |
| ALT [IU/L] | 40 [22–64] | 33 [23–54] | 0.41 |
| Alkaline phosphatase [IU/L] | 70 [52–94] | 119 [80–183] | < 0.001 |
| Leukocytes [× 109/L] | 7.1 [4.8–9.2] | 6.3 [4.1–8.2] | 0.10 |
| Hemoglobin [g/dL] | 13.6 [12.2–14.8] | 12.1 [10.1–14.3] | 0.002 |
| Platelets [× 109/L] | 206 [155–265] | 115 [70–164] | < 0.001 |
| Lymphocyte count [× 109/L] | 0.95 [0.69–1.33] | 0.72 [0.42–1.24] | 0.009 |
| Lactate [mmol/L] | 1.6 [1.3–2.2] | 2.3 [1.6–3.6] | 0.009 |
| Ferritin [μg/L] | 696 [340–1232] | 395 [122–1151] | 0.007 |
| LDH [U/L] | 418 [314–518] | 334 [282–455] | 0.048 |
| D-Dimer [ng/mL] | 381 [199–802] | 652 [384–2170] | 0.003 |
| CRP [mg/L] | 6.7 [3.7–14.3] | 6.4 [2.5–9.7] | 0.11 |
| Procalcitonin [ng/mL] | 0.2 [0.1–0.4] | 0.3 [0.1–0.8] | 0.07 |
Data are presented as n [%] for categorical variables and median [IQR] for continuous variables
ALT alanine aminotransferase, AST aspartate aminotransferase, COPD chronic obstructive pulmonary disease, CRP C-reactive protein, INR international normalized ratio, LDH lactate dehydrogenase, MELD model for end-stage liver disease, NAFLD nonalcoholic fatty liver disease
aPatients with chronic kidney disease stage V [end-stage renal disease dependent on hemodialysis] were excluded from the study population
bMissing data for some patients: body mass index [n = 45], MELD score [n = 40], INR [n = 40], lactate [n = 106], ferritin [n = 53], lactate dehydrogenase [n = 72], D-dimer [n = 77], CRP [n = 46], and procalcitonin [n = 56]
cConsisted of: autoimmune hepatitis [n = 5: 2 without cirrhosis, 2 with cirrhosis without ACLF, and 1 with cirrhosis and ACLF], primary biliary cholangitis [n = 2: 1 without cirrhosis and 1 with cirrhosis without ACLF], cardiac cirrhosis [n = 3 with cirrhosis: 1 without ACLF and 2 with ACLF], cryptogenic cirrhosis [n = 3 with cirrhosis: 1 without ACLF and 2 with ACLF], and secondary biliary cirrhosis [n = 1 with cirrhosis without ACLF]
Of the 84 patients with cirrhosis, 32 [38%] developed ACLF. Differences between patients with cirrhosis who did or did not develop ACLF are shown in Table 2. The majority of patients with ACLF had non-Hispanic/Latino ethnicity [81%]. Patients who developed ACLF were older [72 versus 68 years, p = 0.04] and more likely to have co-morbid hypertension [84% versus 58%, p = 0.01] and chronic kidney disease [25% versus 0%, p < 00.001; median baseline creatinine 1.8 versus 0.8 mg/dL, p < 0.001]. Etiologies of cirrhosis were similar between patients who did or did not develop ACLF. On multivariate logistic regression analysis (Table 3), Hispanic/Latino ethnicity [HR 0.26, 95% CI 0.08–0.89, p = 0.03] was negatively associated with development of ACLF whereas hypertension on admission predicted development of ACLF [HR 3.46, 95% CI 1.12–10.75, p = 0.03]. Compared to patients with cirrhosis who did not develop ACLF, those with ACLF had higher baseline MELD scores [18 versus 11, p < 0.001], leukocyte and platelet counts, and levels of CRP and procalcitonin but lower hemoglobin levels. Baseline levels of INR, bilirubin, albumin, liver enzymes, lactate, ferritin, LDH, and D-dimer were not significantly different between the two groups of patients with cirrhosis.
Table 2.
Admission characteristics of 84 patients with COVID-19 infection and cirrhosis, stratified by whether or not they developed ACLF
| Characteristics | Cirrhosis [N = 84] | ||
|---|---|---|---|
| No ACLF [n = 52] | ACLF [n = 32] | p | |
| Vital signs | |||
| Systolic blood pressure < 90 mHg | 3 [5.8] | 2 [6.3] | 0.93 |
| Temperature > 38 °C | 5 [9.6] | 6 [18.8] | 0.32 |
| Heart rate > 90 bpm | 29 [55.8] | 16 [50] | 0.61 |
| Respiratory rate > 20 bpm | 14 [26.9] | 14 [43.8] | 0.11 |
| SpO2 < 90% | 5 [9.6] | 7 [21.9] | 0.19 |
| SpO2 < 80% | 1 [1.9] | 2 [6.3] | 0.55 |
| Leukocytes < 4 or > 12 [× 109/L] | 22 [42.3] | 6 [18.8] | 0.03 |
| Sex | 0.47 | ||
| Male | 34 [65.4] | 24 [75.0] | |
| Female | 18 [34.6] | 8 [25.0] | |
| Age [years] | 67 [57–73] | 72 [67–78] | 0.04 |
| Race | 0.02 | ||
| White | 15 [28.9] | 14 [43.8] | |
| Black | 7 [13.5] | 9 [28.1] | |
| Asian | 2 [3.8] | 3 [9.4] | |
| Other/multiracial | 27 [51.9] | 6 [18.8] | |
| Unknown | 1 [1.9] | 0 [0.0] | |
| Ethnicity | 0.02 | ||
| Non-Hispanic or Latino | 28 [53.8] | 26 [81.3] | |
| Hispanic or Latino | 20 [38.5] | 4 [12.5] | |
| Other or unknown | 4 [7.7] | 2 [6.3] | |
| Esophageal varices | 16[30.8] | 9[28.1] | 0.80 |
| Ascites | 16[30.8] | 12[37.5] | 0.52 |
| Co-morbid conditions | |||
| Hypertension | 30 [57.7] | 27 [84.4] | 0.01 |
| Diabetes mellitus | 28 [53.8] | 16 [50.0] | 0.73 |
| Coronary artery disease | 3 [5.8] | 5 [15.6] | 0.14 |
| Chronic kidney diseasea | 0 [0.0] | 8 [25.0] | < 0.001 |
| Heart failure | 4 [7.7] | 6 [18.8] | 0.13 |
| Malignancy | 10 [19.2] | 4 [12.5] | 0.42 |
| COPD | 6 [11.5] | 6 [18.8] | 0.36 |
|
Body mass index [kg/m2]b < 30 ≥ 30 |
27 [25–31] 29 [76.3] 9 [23.7] |
27 [24–31] 16 [59.3] 11 [40.7] |
0.94 0.178 |
| Etiology | |||
| NAFLD | 17 [32.7] | 12 [37.5] | 0.65 |
| Hepatitis C | 15 [28.8] | 8 [25.0] | 0.80 |
| Alcohol-related liver disease | 14 [26.9] | 4 [12.5] | 0.17 |
| Hepatitis B | 0 [0.0] | 3 [9.4] | 0.052 |
| Otherc | 6 [11.5] | 5 [15.6] | 0.74 |
| Laboratory measurements [first within 48 h]b | |||
| MELD score | 11 [9–14] | 18 [14–23] | < 0.001 |
| Child–Turcotte–Pugh score | 0.21 | ||
| CTP A | 26 (50) | 10 (31.3) | |
| CTP B | 22 (56.4) | 17 (53.1) | |
| CTP C | 4 (7.7) | 5 (15.6) | |
| Creatinine [mg/dL] | 0.8 [0.7–1.1] | 1.8 [1.3–2.7] | < 0.001 |
| INR | 1.3 [1.2–1.5] | 1.4 [1.2–2.0] | 0.57 |
| Bilirubin [mg/dL] | 1.0 [0.7–1.8] | 1.1 [0.4–2.5] | 0.77 |
| Albumin [g/dL] | 3.2 [2.5–3.5] | 2.7 [2.3–3.5] | 0.19 |
| AST [IU/L] | 56 [43–101] | 75 [42–116] | 0.39 |
| ALT [IU/L] | 32 [24–52] | 35 [22–65] | 0.70 |
| Alkaline phosphatase [IU/L] | 119 [82–177] | 124 [80–200] | 0.62 |
| Leukocytes [× 109/L] | 5.1 [3.6–7.8] | 7.1 [6.0–9.3] | 0.005 |
| Hemoglobin [g/dL] | 12.7 [10.7–14.8] | 10.8 [9.4–13.2] | 0.01 |
| Platelets [× 109/L] | 112 [60–149] | 129 [97–232] | 0.02 |
| Lymphocyte count [× 109/L] | 0.72 [0.41–1.18] | 0.74 [0.49–1.25] | 0.16 |
| Lactate [mmol/L] | 2.1 [1.5–2.7] | 2.6 [1.6–4.1] | 0.25 |
| Ferritin [μg/L] | 272 [102–1113] | 459 [308–1297] | 0.18 |
| LDH [U/L] | 323 [271–423] | 378 [313–473] | 0.15 |
| D-Dimer [ng/mL] | 606 [340–1744] | 800 [429–2499] | 0.54 |
| CRP [mg/L] | 4.5 [1.8–7.5] | 9.0 [5.7–14.6] | 0.001 |
| Procalcitonin [ng/mL] | 0.2 [0.1–0.5] | 0.7 [0.3–1.5] | 0.006 |
Data are presented as n [%] for categorical variables and median [IQR] for continuous variables
ALT alanine aminotransferase, AST aspartate aminotransferase, COPD chronic obstructive pulmonary disease, CRP C-reactive protein, INR international normalized ratio, LDH lactate dehydrogenase, MELD model for end-stage liver disease, NAFLD nonalcoholic fatty liver disease
aPatients with chronic kidney disease stage V [end-stage renal disease dependent on hemodialysis] were excluded from the study population
bMissing data for some patients: body mass index [n = 45], MELD score [n = 40], INR [n = 40], lactate [n = 106], ferritin [n = 53], lactate dehydrogenase [n = 72], D-dimer [n = 77], CRP [n = 46], and procalcitonin [n = 56]
cConsisted of: autoimmune hepatitis [n = 5: 2 without cirrhosis, 2 with cirrhosis without ACLF, and 1 with cirrhosis and ACLF], primary biliary cholangitis [n = 2: 1 without cirrhosis and 1 with cirrhosis without ACLF], cardiac cirrhosis [n = 3 with cirrhosis: 1 without ACLF and 2 with ACLF], cryptogenic cirrhosis [n = 3 with cirrhosis: 1 without ACLF and 2 with ACLF], and secondary biliary cirrhosis [n = 1 with cirrhosis without ACLF]
Table 3.
Predictors of ACLF on univariate and multivariate logistic regression analyses
| ACLF | ||||
|---|---|---|---|---|
| Unadjusted analysis | Adjusted analysisa | |||
| HR [95% CI] | p | HR [95% CI] | p | |
| Sex | ||||
| Male | 1 | |||
| Female | 0.63 [0.24–1.68] | 0.36 | ||
| Age [years] | 1.03 [0.99–1.07] | 0.09 | ||
| Race | ||||
| White [REF] | 1 | |||
| Black | 1.38 [0.40–4.70] | 0.12 | ||
| Other | 0.32 [0.11–0.91] | 0.009 | ||
| Ethnicity | ||||
| Non-Hispanic or non-Latino [REF] | 1 | 1 | ||
| Hispanic or Latino | 0.23 [0.07–0.75] | 0.01 | 0.26 [0.08–0.89] | 0.03 |
| Esophageal varices | 0.88 [0.33–2.32] | 0.80 | ||
| Ascites | 1.35 [0.53–3.41] | 0.53 | ||
| Co-morbid conditions | ||||
| Hypertension [REF = No] | 3.96 [1.32–11.91] | 0.01 | 3.46 [1.12–10.75] | 0.03 |
| Diabetes mellitus [REF = No] | 0.86 [0.36–2.07] | 0.73 | ||
| Coronary artery disease [REF = No] | 3.02 [0.67–13.64] | 0.15 | ||
| Chronic kidney diseaseb [REF = No] | > 99.99 [< 0.01– > 99.99] | 0.96 | ||
| Heart failure [REF = No] | 2.77 [0.72–10.70] | 0.14 | ||
| Malignancy [REF = No] | 0.60 [0.17–2.10] | 0.43 | ||
| COPD [REF = No] | 1.77 [0.52–6.05] | 0.36 | ||
| Obesity | ||||
| Body mass index [kg/m2] < 30 [REF] | 1 | |||
| Body mass index [kg/m2] ≥ 30 | 2.22 [0.76–6.47] | 0.15 | ||
| Etiology | ||||
| NAFLD | ||||
| No | 1 | |||
| Yes | 1.24 [0.49–3.10] | 0.65 | ||
aMultivariate logistic regression analysis was performed using Stepwise approach, entry criteria for inclusion of the variables in the final model was P < 0.2, and the stay criteria was P < 0.35. Age, race, ethnicity and hypertension were included in the initial model
bPatients with chronic kidney disease stage V [end-stage renal disease dependent on hemodialysis] were excluded from the study population
The incidence and type of organ failures among patients with and without cirrhosis are presented in Table 4. On hospital admission, there was no difference in the prevalence of tachypnea (RR > 20) in the CLD patients with and without cirrhosis although more patients in the non-cirrhotic group had fever [Temp > 38 C], and tachycardia [HR > 90]. The majority of patients with CLD experienced at least one organ failure [54%], with respiratory failure [39%], renal failure [26%], and circulatory failure [18%] being the most common. Cerebral failure and liver failure were rare and occurred exclusively among patients with cirrhosis [7% and 4% of patients with cirrhosis, respectively]. There were no significant differences in the number of organ failures, need for mechanical ventilation, need for ICU care, need for renal replacement therapy, or length of stay between patients with and without cirrhosis. The evolution of ACLF on admission and during the hospital course among the 84 patients with cirrhosis is summarized in Table 5. On admission, 14 [16.7%] patients presented with ACLF and an additional 18 [21.4%] developed ACLF during their hospitalization. Not surprisingly, respiratory failure was the predominant organ failure considering the mode of infection of the virus, followed by renal failure, circulatory failure, coagulation failure, and liver failure, respectively. On admission, only 4.8% required mechanical ventilation, but 44.1% will ultimately require mechanical ventilation during the hospitalization with almost 33.3% developing multi-organ failure.
Table 4.
Evolution of ACLF on admission and in-hospital in the 84 patients with cirrhosis based on EASL-CLIF definition
| On admission | In-hospital | |
|---|---|---|
| All patients | N = 84 | N = 84 |
| ACLF | 14 (16.7) | 32 (38.1) |
| ACLF grades | ||
| ACLF grade 0 | 70 (83.3) | 52 (61.9) |
| ACLF grade 1 | 7 (8.3) | 10 (11.9) |
| ACLF grade 2 | 5 (6.0) | 8 (9.5) |
| ACLF grade 3 | 2 (2.4) | 14 (16.7) |
| Organ failures | ||
| Liver failure | 2 (2.4) | 3 (3.6) |
| Renal failure | 12 (14.3) | 25 (29.8) |
| Coagulation failure | 6 (7.1) | 7 (8.3) |
| Circulatory failure | 5 (6) | 16 (19.1) |
| Respiratory failure | 13 (15.5) | 37 (44.1) |
| Number of organ failures | ||
| 0 | 53 (63.1) | 33 (39.3) |
| 1 | 20 (23.8) | 23 (27.2) |
| 2 | 9 (10.7) | 14 (16.7) |
| ≥ 3 | 2 (2.4) | 14 (16.7) |
| Mechanical ventilation | 4 (4.8) | 37 (44.1) |
Table 5.
Evolution of ACLF on admission and in-hospital in the 84 patients with cirrhosis based on EASL-CLIF definition
| On admission | In-hospital | |
|---|---|---|
| All patients | N = 84 | N = 84 |
| ACLF | 14 (16.7) | 32 (38.1) |
| ACLF grades | ||
| ACLF grade 0 | 70 (83.3) | 52 (61.9) |
| ACLF grade 1 | 7 (8.3) | 10 (11.9) |
| ACLF grade 2 | 5 (6.0) | 8 (9.5) |
| ACLF grade 3 | 2 (2.4) | 14 (16.7) |
| Organ failures | ||
| Liver failure | 2 (2.4) | 3 (3.6) |
| Renal failure | 12 (14.3) | 25 (29.8) |
| Coagulation failure | 6 (7.1) | 7 (8.3) |
| Circulatory failure | 5 (6) | 16 (19.1) |
| Respiratory failure | 13 (15.5) | 37 (44.1) |
| Number of organ failures | ||
| 0 | 53 (63.1) | 33 (39.3) |
| 1 | 20 (23.8) | 23 (27.2) |
| 2 | 9 (10.7) | 14 (16.7) |
| ≥ 3 | 2 (2.4) | 14 (16.7) |
| Mechanical ventilation | 4 (4.8) | 37 (44.1) |
Figure 2 shows Kaplan–Meier curves for in-hospital mortality according to the presence of cirrhosis and/or ACLF (Fig. 2a) or, among patients with cirrhosis, by grade of ACLF (Fig. 2b). There was no significant difference in mortality between patients with and without cirrhosis [unadjusted HR 1.84, 95% CI 0.92–3.68, p equal to 0.08; adjusted HR 1.54, 95% CI 0.75–3.18, p = 0.24]. Among patients with cirrhosis, those who developed ACLF were five times as likely to die while hospitalized (Table 6, HR 5.05, 95% CI 1.83–13.86, p = 0.002). After adjustment for age, sex, race, and ethnicity, risk of in-hospital mortality was nine times higher among patients who developed ACLF (Table 7, adjusted HR 9.06, 95% CI 2.63–31.12, p < 0.001). Hispanic/Latino ethnicity was particularly at higher risk of in-hospital mortality (Table 7, adjusted HR 4.92, 95% CI 1.27–19.09, p < 0.02) in cirrhosis despite having lower risk of development of ACLF as noted earlier. There was a trend for incrementally worse survival by increased grade of ACLF (Table 7, p for trend equal to 0.002), with adjusted HRs of 3.09 [95% CI 0.54–17.34, p = 0.20] for ACLF grade I, 9.99 [95% CI 1.49–66.95, p equal to 0.02] for ACLF grade II, and 18.31 [95% CI 4.50–74.46, p < 0.001] for ACLF grade III compared to patients with cirrhosis who did not develop ACLF (Table 7).
Fig. 2.
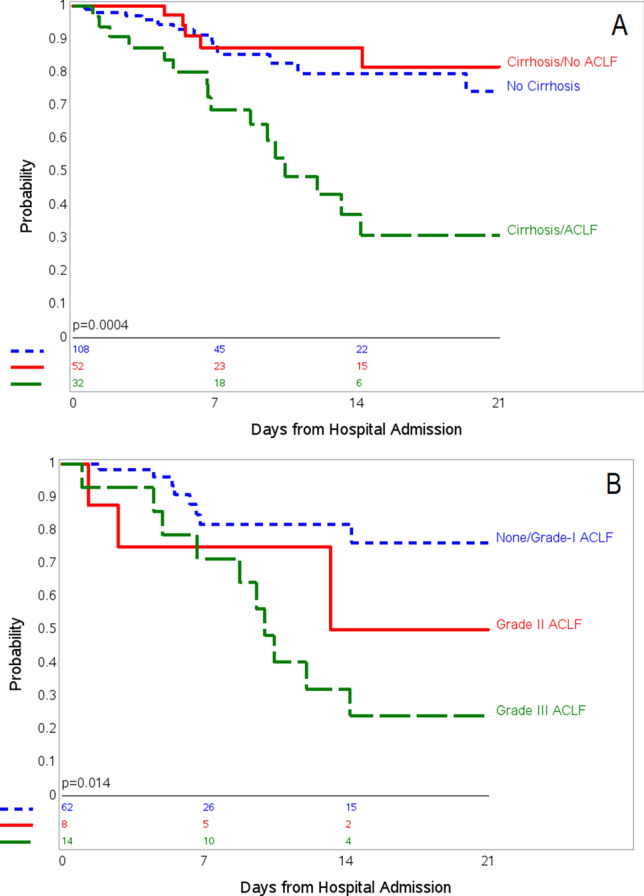
Kaplan–Meier curves for in-hospital mortality among a all patients with chronic liver disease, based on no cirrhosis, cirrhosis without ACLF, and cirrhosis with ACLF; and b patients with cirrhosis, based on grade of ACLF
Table 6.
Association between ACLF and in-hospital mortality among cirrhosis patients hospitalized with confirmed COVID-19 infection on unadjusted univariate analysis
| In-hospital mortality on unadjusted analysis | ||
|---|---|---|
| HR [95% CI] | p | |
| ACLF | ||
| No | 1 | |
| Yes | 5.05 [1.83–13.86] | 0.002 |
| ACLF grades | ||
| ACLF grades | 1 | |
| ACLF grade I | 5.64 [1.27–24.83] | 0.02 |
| ACLF grade II | 3.29 [0.78–13.81] | 0.10 |
| ACLF grade III | 5.84 [1.98–17.16] | < 0.001 |
| Sex | ||
| Male | 1 | |
| Female | 2.18 [0.91–5.25] | 0.08 |
| Age [years] | 1.03 [1.00–1.07] | 0.049 |
| Race | ||
| White [REF] | 1 | |
| Black | 0.36 [0.08–1.67] | 0.19 |
| Other | 0.62 [0.25–1.52] | 0.33 |
| Ethnicity | ||
| Non-Hispanic or non-Latino | 1 | |
| Hispanic or Latino | 1.72 [0.69–4.23] | 0.25 |
| Esophageal varices | 0.48 [0.18–1.31] | 0.15 |
| Ascites | 0.76 [0.31–1.88] | 0.55 |
| Co-morbid conditions | ||
| Hypertension [REF = No] | 1.52 [0.56–4.15] | 0.42 |
| Diabetes mellitus [REF = No] | 1.70 [0.68–4.23] | 0.26 |
| Coronary artery disease [REF = No] | 1.42 [0.48–4.23] | 0.53 |
| Chronic kidney disease* [REF = No] | 2.51 [0.84–7.50] | 0.10 |
| Heart failure [REF = No] | 2.35 [0.86–6.44] | 0.09 |
| Malignancy [REF = No] | 0.36 [0.08–1.56] | 0.17 |
| COPD [REF = No] | 1.97 [0.66–5.87] | 0.23 |
| Obesity | ||
| Body mass index [kg/m2] < 30 [REF] | 1 | |
| Body mass index [kg/m2 ≥ 30] | 1.29 [0.48–3.49] | 0.62 |
| Etiology | ||
| NAFLD | ||
| No | 1 | |
| Yes | 1.53 [0.65–3.62] | 0.33 |
* Patients with chronic kidney disease stage V [end-stage renal disease dependent on hemodialysis] were excluded from the study population
Table 7.
Association between ACLF and in-hospital mortality among cirrhosis patients hospitalized with confirmed COVID-19 infection on adjusted multivariate analysis
| Variables | In-hospital mortality on adjusted analysisa | |||
|---|---|---|---|---|
| Multivariate model 1 | Multivariate model 1 | |||
| HR [95% CI] | p | HR [95% CI] | p | |
| ACLF | ||||
| No | 1 | |||
| Yes | 9.06 [2.63–31.12] | < 0.001 | ||
| ACLF Grades | ||||
| No ACLF [REF] | 1 | |||
| ACLF grade I | 3.09 [0.54–17.34] | 0.20 | ||
| ACLF grade II | 9.99 [1.49–66.95] | 0.02 | ||
| ACLF grade III | 18.31 [4.50–74.46] | < 0.001 | ||
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.40 [0.50–3.92] | 0.53 | 1.93 [0.63–5.95] | 0.25 |
| Age [years] | 1.01 [0.97–1.05] | 0.58 | 1.09 [0.99–1.07] | 0.21 |
| Race | ||||
| White [REF] | 1 | 1 | ||
| Black | 0.34 [0.07–1.69] | 0.19 | 0.45 [0.09–2.33] | 0.34 |
| Other | 0.53 [0.15–1.82] | 0.31 | ||
| Ethnicity | ||||
| Non-Hispanic or non-Latino | 1 | 1 | ||
| Hispanic or Latino | 4.92 [1.27–19.09] | 0.02 | 8.10 [1.81–36.25] | 0.006 |
aMultivariate Cox regression models were adjusted for potential confounding variables identified a priori: age, sex, ethnicity, and race
Baseline characteristics of the propensity score-matched controls without underlying CLD or cirrhosis compared to patients with CLD or cirrhosis (1:1 match) used in the sensitivity analysis are shown on Supplemental Tables 2 and 3. There were no significant differences in baseline demographics or comorbidities. Kaplan–Meier survival curves showed no difference in-hospital mortality according to the presence of CLD compared to matched control without CLD (log rank, p = 0.98) (Fig. 3a) and presence of cirrhosis compared to matched control without cirrhosis (log rank, p = 0.51) (Fig. 3b).
Fig. 3.
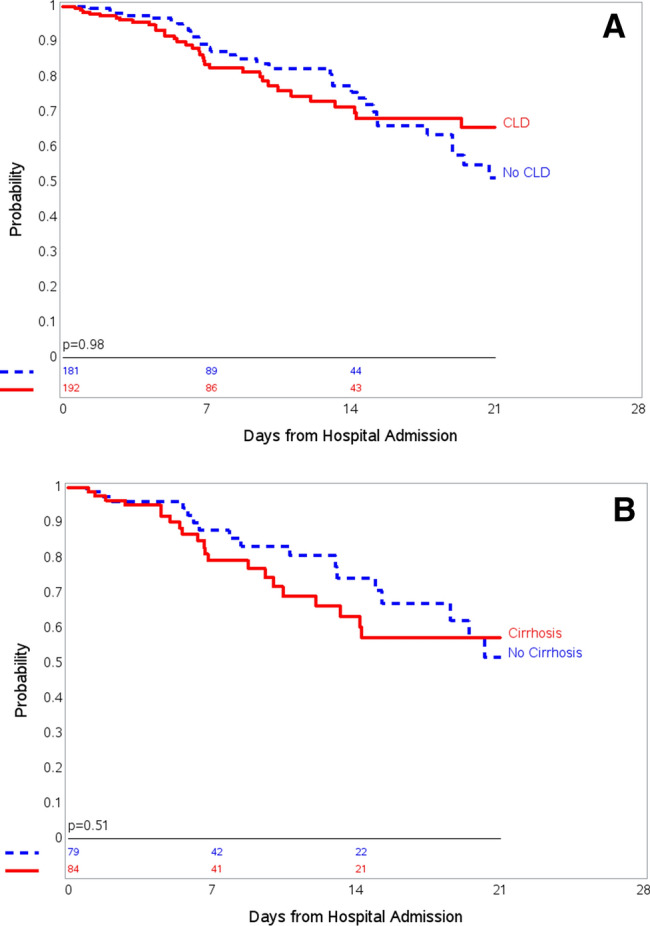
Kaplan–Meier curves for in-hospital mortality among a all patients with chronic liver disease (CLD] and propensity-matched control without CLD; and b patients with cirrhosis and propensity-matched control without cirrhosis
Discussion
We have highlighted several important observations from the current analysis amongst a large cohort of CLD patients hospitalized with COVID-19 infection. First, the development of ACLF among those with cirrhosis and COVID-19 led to worse in-hospital mortality and it was incremental with higher grades of ACLF. Second, patients with cirrhosis who developed ACLF had a higher median MELD score at hospital admission. Third, cirrhotic patients of Hispanic/Latino ethnicity were particularly at higher risk of in-hospital mortality despite having lower risk of development of ACLF. Hypertension on admission predicted development of ACLF. Finally, the presence of CLD or cirrhosis by itself is not associated with a difference in in-hospital mortality after comparison with an age, gender and comorbidity-matched control group using propensity control methods.
In the current analysis including 10,859 adults with confirmed COVID-19 infection who had been admitted to any one of our 12 hospitals in the New York metropolitan area, we noted a prevalence of CLD of 1.8%. Few studies have evaluated whether patients with CLD are at increased risk for poor outcomes due to COVID-19 infection [11–14]. The presence of underlying chronic liver disease was significantly associated with more severe COVID-19 infections and mortality in a recent meta-analysis [14]. A recent study from Asia, which included 228 patients (185 CLD without cirrhosis and 43 with cirrhosis) reported that 43% of those with CLD without cirrhosis presented with acute liver injury and 20% of those with cirrhosis presented with either ACLF (11.6%) or acute hepatic decompensation (9%) [11]. A recent meta-analysis noted that the presence of CLD was associated with more severe COVID-19 infection (pooled OR 1.48) and overall mortality (pooled OR 1.78) [14]. In contrast, two other meta-analyses failed to find an association between CLD and increased COVID-19 severity or mortality [15, 16]. In the present cohort of patients with CLD, we have noted that the presence of CLD or cirrhosis by itself is not associated with a difference in in-hospital mortality after comparison with an age, gender and comorbidity-matched control using propensity control methods, but the presence of ACLF significantly increased the in-hospital mortality in those with cirrhosis and COVID-19 infection.
ACLF is a syndrome associated with a high risk of short-term death [i.e., death less than 28 days after hospital admission] in patients with acutely decompensated cirrhosis [17]. Three major features characterize this syndrome: it occurs in the context of intense systemic inflammation, it frequently develops in close temporal relationship with proinflammatory precipitating events [e.g., infections or alcoholic hepatitis], and it is associated with single- or multiple-organ failure [18]. Elevated serum levels of several cytokines, including soluble Fas (sFas) antigen [19], TNF-α, sTNF-αR1, sTNF-αR2, IL-2, IL-2R, IL-6, IL-8, IL-10, and interferon-γ, have been described in patients with ACLF [20–23]. Elevated levels of circulating cytokines in ACLF may be the result of increased production due to endotoxemia [24, 25].
We previously reported the first case of ACLF related to COVID-19 infection in a patient with cirrhosis without significant respiratory symptoms [26], which led to our interest in further exploring the impact of ACLF in this population. Consistent with the typical course of critical COVID-19 infection, in the current study, the high prevalence of ACLF [38%] among patients with cirrhosis was predominantly driven by the development of respiratory failure. As in other studies of patients with critical COVID-19 infection, renal failure and the need for vasopressors were not uncommon [27–29]. Acute kidney injury occurs early and in temporal association with respiratory failure and is associated with a poor prognosis [29]. Differences in the MELD scores between patients with cirrhosis who did or did not develop ACLF in our study were largely due to a higher prevalence of chronic kidney disease and higher serum creatinine levels [possibly secondary to a combination of chronic kidney disease and superimposed acute kidney injury] in the ACLF group. In our study, although liver failure and cerebral failure were exclusively seen among patients with underlying cirrhosis, patients with and without cirrhosis had similar number of organ failures. These groups also had a similar in-hospital mortality as well as a similar need for invasive mechanical ventilation and an ICU level of care.
The mechanism of liver injury in COVID-19 infection is postulated to be a result of direct cytopathic effects of the virus; immune imbalance and cytokine storm-related multi-organ damage; hypoxia-reperfusion dysfunction; and idiosyncratic, drug-induced liver injury due to medications used for the management of COVID-19 infection [30, 31]. Early retrospective data from China and Italy showed that elevated inflammatory markers—particularly ferritin, CRP, LDH, interleukin 6 [IL-6], and D-dimer—were associated with severe illness and increased mortality [32]. A severely dysregulated immune response to local inflammation (COVID-19-induced pneumonia) with heightened production of cytokines and chemokines that in turn act as chemoattractants for other inflammatory cells result in an exaggerated immune response with consequent systemic manifestations and multi-organ involvement [30]. This sequence of events, termed cytokine storm, has been shown to be a major determinant of poor survival in patients with COVID-19 infection [30]. In a retrospective, single-center study of 148 COVID-19-infected patients, there were higher levels of procalcitonin and CRP in patients presenting with abnormal liver chemistries [33]. In the current study, patients who developed ACLF had significantly higher baseline levels of procalcitonin and CRP. Although levels of ferritin, LDH, and D-Dimer were also numerically higher in the ACLF group than in cirrhosis patients who did not develop ACLF, these differences did not reach statistical significance. Nonetheless, the finding of increased inflammatory markers in patients with ACLF suggests that cytokine storm may be a precipitant of ACLF in cirrhosis patients infected with COVID-19. A recent study from our center has evaluated the role of combination of corticosteroids with tocilizumab showing superior survival outcome when compared with SoC (Standard of Care) treatment as well as treatment with corticosteroids alone or in combination with anakinra [34]. These encouraging results should be evaluated in the context of preventing ACLF in cirrhosis.
In a multivariate analysis, hypertension and non-Hispanic/Latino ethnicity were independent predictors of ACLF. Our finding of hypertension as a risk factor for ACLF and poor outcomes is consistent with results from a pooled analysis of 6,560 patients with COVID-19 that showed an association between hypertension and risk of a composite outcome including mortality, severe COVID-19, acute respiratory distress syndrome, the need for ICU care, and disease progression [35]. Due to genetic polymorphism or the use of angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker therapy [36], hypertensive patients may have increased susceptibility to the development of severe COVID-19 infection as a result of increased angiotensin-converting enzyme 2 expression. NAFLD was the most common etiology of underlying CLD in both patients with and without cirrhosis. However, etiology of underlying liver disease did neither impact the development of ACLF in cirrhosis patients in our cohort nor did it impact the in-hospital mortality. Hispanic/Latino ethnicity, although associated with a lower risk of developing ACLF, was associated with a higher risk for in-hospital mortality due to COVID-19 on adjusted analysis—consistent with the reported literature [37].
An important limitation of our study is its retrospective nature and lack of pre-hospitalization data for many patients. In addition, data on viral kinetics and viral persistence were not available, though these might have differed among patients with CLD and cirrhosis and might have impacted their outcomes. Finally, due to limited sample sizes, we were unable to develop robust multivariate models adjusting for multiple baseline covariates such as MELD score, and our comparisons of survival between groups might have residual confounding.
In this large cohort of hospitalized COVID-19 patients with CLD, development of ACLF among those with cirrhosis led to worse in-hospital mortality. COVID-19-related ACLF remains the main driver of mortality and critical illness for COVID-19-infected patients with cirrhosis. The presence of hypertension on admission may increase the risk of ACLF in cirrhotic patients. Although Hispanic/Latino ethnicity may be at lower risk for the development of ACLF due to COVID-19, they remain at higher risk of in-hospital mortality. The presence of CLD or cirrhosis by itself is not associated with a difference in in-hospital mortality after comparison with an age, gender and comorbidity-matched control group. Preventative strategies for the COVID-19 infection with early vaccination strategies should be prioritized in patients with cirrhosis and an early institution of treatment should be strongly considered.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Algorithm for identification of the study population. *ICD-10 diagnostic codes used to screen for chronic liver disease were: K70 [alcoholic liver disease], K71 [toxic liver disease], K73 [chronic hepatitis, not elsewhere classified], K74 [fibrosis and cirrhosis of liver], K75 [other inflammatory liver diseases], K76 [other diseases of liver], B18 [chronic viral hepatitis], and B19 [unspecified viral hepatitis]
Acknowledgements
We would like to acknowledge the contributions of the Northwell Health COVID-19 Research Consortium. We also acknowledge and honor all of our Northwell team members who consistently put themselves in harm’s way during the COVID-19 pandemic; this article is dedicated to them, as their vital contribution to knowledge about COVID-19 and sacrifices on behalf of the patients made it possible.
Author contributions
SKS conceptualized the study; SKS, CK, NR and JH collected the data; SKS, NR did formal analysis of the data; SKS, DB, and NR prepared the first draft. All the authors participated in intellectual input, critical revision, and approval of the manuscript under supervision of SKS.
Funding
This work was supported by grants R24AG064191 from the National Institute on Aging of the National Institutes of Health and R01LM012836 from the National Library of Medicine of the National Institutes of Health. Neither source of funding had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The views expressed in this paper are those of the authors and do not represent the views of the National Institutes of Health, the United States Department of Health and Human Services, or any other government entity.
Availability of data
The data that support the findings of this study are available on request from COVID19@northwell.edu. The data are not publicly available due to restrictions as it could compromise the privacy of research participants.
Code availability
The code that support the findings of this study are available on request from COVID19@northwell.edu.
Declarations
Conflict of interest
Sanjaya K. Satapathy, Nitzan C. Roth, Charlotte Kvasnovsky, Jamie S. Hirsch, Arvind J. Trindade, Ernesto Molmenti, Matthew Barish, David Hirschwerk, Ben L. Da, David Bernstein, Northwell Health COVID-19 Research Consortium declare no conflicts of interest related to the content of the manuscript.
Ethics approval
The Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health approved this study as minimal-risk research using data collected for routine clinical practice [Approval #20–0200].
Consent to participate
The Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health waived the requirement for informed consent to participate in the study.
Consent for publication
The Institutional Review Board for the Feinstein Institutes of Medical Research at Northwell Health waived the requirement for informed consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Retrieved June 3, 2020, from https://coronavirus.jhu.edu/map.html. Accessed on 3 June 2020.
- 4.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol. 2020;33:114–115. doi: 10.1097/MEG.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive predictive value of international classification of diseases, 10th revision, codes for cirrhosis and its related complications. CliniGastroenterol Hepatol. 2018;16:1677–1678. doi: 10.1016/j.cgh.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, et al. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516–1523. doi: 10.1111/liv.12597. [DOI] [PubMed] [Google Scholar]
- 9.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(1426–1437):1437.e1421–1429. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Matching using the GREEDY algorithm. (mayoclinic.com:https://www.mayo.edu/research/documents/gmatchsas/DOC-10027248,10/2003). Accessed on 5 Jan 2021.
- 11.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int 2020; p. 1–11. [DOI] [PMC free article] [PubMed]
- 12.Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(768–771):e763. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2020;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hep Intl. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, et al. comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash DC). 2020; p. 2402961. [DOI] [PMC free article] [PubMed]
- 16.Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol 9000 (Publish Ahead of Print) [DOI] [PMC free article] [PubMed]
- 17.Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–261. doi: 10.1159/000047017. [DOI] [PubMed] [Google Scholar]
- 18.Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 19.Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276:49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 20.Maher JJ, Scott MK, Saito JM, Burton MC. Adenovirus-mediated expression of cytokine-induced neutrophil chemoattractant in rat liver induces a neutrophilic hepatitis. Hepatology (Baltimore, MD) 1997;25:624–630. doi: 10.1002/hep.510250322. [DOI] [PubMed] [Google Scholar]
- 21.Dorman RB, Gujral JS, Bajt ML, Farhood A, Jaeschke H. Generation and functional significance of CXC chemokines for neutrophil-induced liver injury during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2005;288:G880–886. doi: 10.1152/ajpgi.00317.2004. [DOI] [PubMed] [Google Scholar]
- 22.Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg. 2003;16:141–147. doi: 10.1080/08941930390205782. [DOI] [PubMed] [Google Scholar]
- 23.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology (Baltimore, MD) 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 24.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology (Baltimore, MD) 1998;27:507–512. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 25.Jaeschke H, Bajt ML. Critical role of CXC chemokines in endotoxemic liver injury in mice. J Leukoc Biol. 2004;76:1089–1090. doi: 10.1189/jlb.0504309. [DOI] [PubMed] [Google Scholar]
- 26.Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Liver Int. 2020;40:1590–1593. doi: 10.1111/liv.14506. [DOI] [PubMed] [Google Scholar]
- 27.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anirvan P, Narain S, Hajizadeh N, Aloor FZ, Singh SP, Satapathy SK. Cytokine-induced liver injury in coronavirus disease-2019 (COVID-19): untangling the knots. Eur J Gastroenterol Hepatol. 2021 doi: 10.1097/MEG.0000000000002034. [DOI] [PubMed] [Google Scholar]
- 31.Thuluvath PJ, Alukal JJ, Ravindran N, Satapathy SK. What GI physicians need to know during COVID-19 pandemic. Dig Dis Sci 2020; p. 1–11. [DOI] [PMC free article] [PubMed]
- 32.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest. 2020;159:933–948. doi: 10.1016/j.chest.2020.09.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320926899. doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Chaar M, King K, Galvez A. Are black and Hispanic persons disproportionately affected by COVID-19 because of higher obesity rates? Surg Obesity Relat Dis. 2020;16:1096–1099. doi: 10.1016/j.soard.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Algorithm for identification of the study population. *ICD-10 diagnostic codes used to screen for chronic liver disease were: K70 [alcoholic liver disease], K71 [toxic liver disease], K73 [chronic hepatitis, not elsewhere classified], K74 [fibrosis and cirrhosis of liver], K75 [other inflammatory liver diseases], K76 [other diseases of liver], B18 [chronic viral hepatitis], and B19 [unspecified viral hepatitis]
Data Availability Statement
The data that support the findings of this study are available on request from COVID19@northwell.edu. The data are not publicly available due to restrictions as it could compromise the privacy of research participants.
The code that support the findings of this study are available on request from COVID19@northwell.edu.



