Summary
Vasopressor use during esophagectomy has been reported to increase the risk of postoperative anastomotic leak and associated morbidity. We sought to assess the association between vasopressor use and fluid (crystalloid and colloid) administration and anastomotic leak following open esophagectomy. Patients who underwent open Ivor Lewis esophagectomy were identified from a prospective institutional database. The primary outcome was postoperative anastomotic leak (any grade) and analyzed using logistic regression models. Postoperative anastomotic leak developed in 52 of 327 consecutive patients (16%) and was not significantly associated with vasopressor use or fluid administered in either univariable or multivariable analyses. Increasing body mass index was the only significant characteristic of both univariable (P = 0.004) and multivariable analyses associated with anastomotic leak (odds ratio, 1.05; 95% confidence interval, 1.01–1.09; P = 0.007). Of the 52 patients that developed an anastomotic leak, 12 (23%) were grade 1, 21 (40%) were grade 2 and 19 (37%) were grade 3. In our cohort, only body mass index, and not intraoperative vasopressor use and fluid administration, was significantly associated with increased odds of postoperative anastomotic leak following open Ivor Lewis esophagectomy.
Keywords: complications, thoracic surgery, esophagogastrectomy, norepinephrine, phenylephrine
INTRODUCTION
Despite the increased use of neoadjuvant chemotherapy and radiation, surgical resection remains the standard of treatment for resectable esophageal malignancies. The 5-year survival for all patients with esophageal cancer is 10 to 15%, increasing to 40% for patients who receive curative surgery.1 However, surgical resection of esophageal malignancies was associated with perioperative mortality of approximately 3% and major morbidity of up to 30% in a recent Society of Thoracic Surgeons analysis.2 Leakage at the esophageal anastomotic site remains a major cause of postoperative morbidity and occurs in 5–20% of cases.3,4 Ninety-day mortality among patients who develop anastomotic leak following Ivor Lewis esophagectomy is reported to be as high as 18%, whereas overall mortality for patients without leak is considerably lower, at 6%.5
Multiple risk factors for the development of anastomotic leak have been identified, including age, male sex, emergent surgery, smoking, alcohol use, higher American Society of Anesthesiologists (ASA) physical status classification, obesity, prolonged operative time, decreased serum albumin level, intraoperative blood loss, acidotic pH, elevated pCO2, diabetes, renal failure, and cardiovascular disease.3,6–13 A meta-analysis including more than 3000 patients observed that surgical technique may also influence leak rate, with hand-sewn anastomoses associated with a higher leak rate than stapled anastomoses.14 Many of these known risk factors are difficult to mitigate in the perioperative period, and the identification of interventions that decrease rates of anastomotic leak and postoperative complications could play an important role in reducing overall patient morbidity.
Several small and conflicting studies have shown that surgical, hemodynamic, and postoperative factors interact in a complex manner to affect rates of anastomotic leak and other postoperative complications.7–9,13 In particular, the use of vasopressors has been proposed to adversely influence the rate of anastomotic leak.15 The primary goal of this study was to assess the effect of patient and perioperative factors, including intraoperative vasopressor use and fluid administration, on the rate of anastomotic leak following open esophagectomy.
MATERIALS AND METHODS
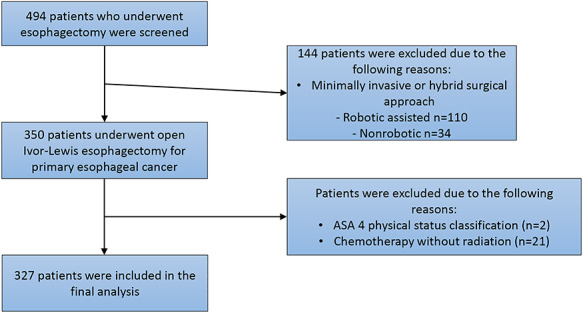
Following approval and a waiver of informed consent from the institutional review board at Memorial Sloan Kettering Cancer Center, we retrospectively reviewed the charts of 494 consecutive patients who underwent Ivor Lewis esophagectomy between February 2010 and December 2015. Although minimally invasive esophagectomy (MIE) is currently our favored approach, we excluded patients undergoing MIE during this period (robotic assisted n = 110; nonrobotic assisted n = 34) due to the small numbers, variability in operator experience, and evolution of surgical techniques. Anastomotic reconstruction was performed using a circular stapler. We included patients with open oncologic surgical resection and ASA physical status classification 2 or 3. Patients with ASA physical status classification 4 (considered to be outliers; n = 2) were excluded, as were patients who received only preoperative chemotherapy without radiation treatment (n = 21), leaving 327 patients for analysis (Fig. 1, study flow diagram). All patients received general anesthesia with endotracheal intubation. Anesthesia was maintained with sevoflurane volatile anesthetic and neuromuscular blockade, and an epidural infusion with hydromorphone 8 mcg/mL and bupivacaine 0.05% was used to supplement intraoperative analgesia in all patients. Additional boluses of intraoperative intravenous fentanyl were given as needed and at the discretion of the anesthesia care team. Fluid was administered at the discretion of the anesthesiology attending, but in accordance with our group’s routine practice, liberal fluid administration was avoided. Total fluid was defined as the sum of crystalloid and colloid administered in the operating room as well as that given in the postanesthesia care unit. Our practice was to transfuse for a hemoglobin level < 8 g/dL and < 9 g/dL for patients with known coronary artery disease. Vasopressors were used with the goal of maintaining systolic blood pressure > 90 mmHg, which usually correlated with a mean arterial pressure > 60 mmHg. A similar practice of blood pressure management was followed in the postanesthesia care unit. Vasopressors used were ephedrine boluses or phenylephrine either as bolus or by continuous infusion. We calculated the total estimated “equipotent” doses of these vasopressors as norepinephrine equivalents using the formula of ([phenylephrine [μg/kg]/10] + [ephedrine [mg] × 2]).16 Similarly, management of the epidural infusion during surgery was at the discretion of the anesthesiology attending. Intermittent disruptions in the continuous use of the epidural regimen were not recorded. Patients were extubated in the operating room at the conclusion of the procedure. Following surgery, all patients received continuous epidural analgesia for pain relief and were transferred from the postanesthesia care unit to the thoracic surgical floor on postoperative day 1.
The primary outcome was postoperative anastomotic leak within 30 days of surgery (any grade). Patients were further identified by the severity of the anastomotic leak based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0: grade 1: asymptomatic diagnostic finding; intervention not indicated; grade 2: symptomatic; medical intervention indicated; grade 3: severe symptoms; invasive intervention indicated (see below).17,18 The secondary outcome was any major complications within 30 days (grade ≥ 3, as assessed by the CTCAE version 5.0).17,18 Grade 3 complications include any postoperative complications that require surgical, radiologic, or endoscopic intervention or multitherapy; grade 4 complications require intensive care unit management and life support; and grade 5 complications result in death.
Statistical analysis
The study sample size was determined by the set of surgeries that met the inclusion criteria predefined for this study. However, we conducted a poststudy power calculation justification to assess the power to detect clinically important odds ratio (OR) given the expected incidence of leak of 20% at the mean value of norepinephrine equivalents. Our hypothesis was that higher levels of norepinephrine equivalents would lead to higher odds of leak. We had 327 patients in this study; this sample size would, therefore, provide 80% power to detect an OR of approximately 1.48 or stronger at the two-sided 0.05 significance level and 48% power to detect an OR of 1.3 or stronger.
Associations between patient characteristics and each outcome were assessed using logistic regression, with robust standard error, clustered by the provider. Multivariable models were built using a backward selection process, starting with factors with P < 0.1 in the univariable analyses. All multivariable models included a set of factors that were determined to be clinically relevant regardless of statistical significance: in the multivariable model for the primary endpoint, we included surgical duration, vasopressor use, and amount of fluid administered; in the multivariable model for any major complications, we included surgical duration and amount of fluid administered. All statistical tests were two-sided, and P < 0.05 was considered to indicate statistical significance. Statistical tests were conducted using Stata 13.1 (StataCorp, College Station, TX).
RESULTS
Demographic and clinical characteristics by leak status are presented in Table 1. Postoperative anastomotic leak occurred in 52 of 327 patients (16%). Of the 52 patients that developed an anastomotic leak, 12 (23%) were grade 1, 21 (40%) were grade 2, and 19 (37%) were grade 3. Total vasopressor use as estimated by norepinephrine equivalents (median [25th–75th IQR]) among the following subsets of patients were as follows—no leak: 9.8 μg/kg (0.1–20.8); grade 1: 0.3 μg/kg (0.0–10.1); grade 2: 0.2 μg/kg (0.0–10.5); grade 3: 20.1 μg/kg (0.0–30.4)—and did not differ significantly between the groups, respectively. Results of the univariable logistic regression analysis showed that body mass index (BMI) (OR, 1.04; 95% confidence interval [CI], 1.01–1.07; P = 0.004) was significantly associated with increased odds of leak (Table 1). After adjustment for surgical duration, red blood cell transfusion, cumulative vasopressor use, and total fluid administration, increasing BMI (OR, 1.05; 95% CI, 1.01–1.09; P = 0.007) remained significantly associated with increased odds of leak (Table 2). The model had good fit to the data, with an area under the curve of 0.631. Neither cumulative vasopressor uses nor total fluid (crystalloid and colloid) administration nor any of the other variables tested were found to be significantly associated with increased odds of leak in the multivariable regression analysis.
Table 1.
Demographic and clinical characteristics by leak status and results of univariable logistic regression
| Characteristic | No Leak (n = 276 [84%]) | Leak (n = 52 [16%]) | Univariable logistic regression | |
|---|---|---|---|---|
| OR (95% CI) | P | |||
| Age | 63.0 (56.0–70.0) | 59.0 (54.0–65.0) | 0.98 (0.96–1.00) | 0.1 |
| Male | 229 (83) | 45 (87) | 1.29 (0.832) | 0.3 |
| ASA 2 | 50 (18) | 13 (26) | Reference | |
| ASA 3 | 225 (82) | 39 (75) | 0.67 (0.34–1.3) | 0.2 |
| BMI | 27.7 (24.9–31.1) | 28.7 (26.0–31.8) | 1.04 (1.01–1.07) | 0.004 |
| Smoking, ever | 173 (63) | 32 (62) | 0.94 (0.53–1.69) | 0.8 |
| Asthma | 14 (5.1) | 4 (7.7) | 1.55 (0.78–3.09) | 0.2 |
| COPD | 15 (5.5) | 1 (1.9) | 0.34 (0.07–1.55) | 0.2 |
| HTN | 77 (28) | 17 (33) | 1.25 (0.68–2.13) | 0.5 |
| CAD | 24 (8.7) | 4 (7.7) | 0.87 (0.38–2.00) | 0.7 |
| DM | 45 (16) | 10 (19) | 1.12 (0.70–2.13) | 0.5 |
| CKD | 1 (0.4) | 0 (0) | ||
| Surgical duration, h | 5.8 (4.8–7.6) | 5.3 (4.6–6.6) | 0.80 (0.55–1.17)† | 0.2 |
| Estimated blood loss, L | 0.3 (0.2–0.5) | 0.3 (0.2–0.6) | 1.10 (0.66–1.83) | 0.7 |
| RBCs per 100 mL | 0.0 (0.0–10.5)* | 0.0 (0.0–10.5) | 1.04 (0.96–1.13) | 0.3 |
| FFP | NA | NA | ||
| Yes | 2 (0.7) | 0 (0) | ||
| Platelets | NA | NA | ||
| Yes | 2 (0.7) | 0 (0) | ||
| Ephedrine, yes | 134 (49) | 23 (44) | 0.83 (0.47–1.49) | 0.5 |
| Ephedrine, mg (if >0 mg) | 10.0 (10.0–20.0) | 10.0 (5.0–20.0) | ||
| Phenylephrine, yes | 200 (73) | 35 (67) | 0.77 (0.50–1.18) | 0.2 |
| Phenylephrine, mg (if >0 mg) | 0.3 (0.2–0.7) | 0.3 (0.1–1.4) | ||
| Norepinephrine equivalent dose, μg/kg | 9.8 (0.1–20.8) | 0.8 (0.0–20.0) | 1.0 (0.98–1.02) | 0.8 |
| Total fluid, L | 6.5 (5.1–7.7) | 5.9 (5.0–7.2) | 0.81 (0.81–1.20)‡ | 0.9 |
| Preoperative chemoradiation | 225 (82) | 46 (85) | 1.23 (0.57–2.62) | 0.6 |
Values are presented as no. (%) or median (25th–75th percentile). ASA, American Society of Anesthesiologists physical status classification; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; FFP, fresh frozen plasma; HTN, hypertension; NA, not applicable; OR, odds ratio; RBCs, red blood cells transfused.
*Data are median (minimum–maximum).
†Per hour increase in surgical duration.
‡Per liter increase in total fluid.
Table 2.
Multivariable logistic regression for odds of an esophageal anastomotic leak (any grade)
| Variable | OR (95% CI) | P |
|---|---|---|
| BMI | 1.05 (1.01–1.09) | 0.007 |
| RBCs, yes | 0.74 (0.45–1.21) | 0.2 |
| Norepinephrine equivalent | 1.00 (0.98–1.01) | 0.9 |
| Total fluid, per L increase | 1.09 (0.88–1.34) | 0.5 |
| Surgery time, per h | 0.75 (0.50–1.10) | 0.14 |
BMI, body mass index; CI, confidence interval; OR, odds ratio; RBCs, red blood cells.
Fig. 1.
Study flow diagram.
Secondary outcomes
Major postoperative complications (CTCAE classification system grade ≥ 3) occurred in 61 of 327 patients (19%). The number of events within major complications could be grouped in five categories: cardiovascular (n = 5), pulmonary (n = 21), gastrointestinal (n = 34), vascular (n = 5), and infectious (n = 3). Demographic and clinical characteristics by major complication status and results of the univariable logistic regression analyses are presented in Table 3. On multivariable logistic regression analysis (with adjustment for ASA physical status classification 3 versus 2, BMI, receipt of red blood cell transfusion, and surgical duration), history of asthma, greater estimated blood loss, and increasing red blood cell transfusion were significantly associated with increased odds of experiencing a major complication (Table 4). Preoperative chemoradiation remained associated with reduced odds of experiencing a major complication in the multivariable setting (OR, 0.36; 95% CI, 0.24–0.55; P < 0.0001) (Table 4). The model had good fit for the data, with an area under the curve of 0.688.
Table 3.
Demographic and clinical characteristics by major postoperative complication status and results of univariable logistic regression
| Characteristic | No major complication (n = 266 [82%]) | Major complication (n = 61 [18%]) | Univariable logistic regression | |
|---|---|---|---|---|
| OR (95% CI) | P | |||
| Age | 62.0 (56.0–69.0) | 63.0 (56.0–73.0) | 1.01 (0.99–1.04) | 0.3 |
| Male | 229 (86) | 45 (74) | 0.45 (0.15–1.37) | 0.2 |
| ASA 2 | 58 (22) | 5 (8.2) | Reference | |
| ASA 3 | 208 (78) | 56 (92) | 3.128 (0.89–11.02 | 0.077 |
| BMI | 27.6 (24.9–31.1) | 28.6 (26.0–32.5) | 1.04 (1.01–1.07) | 0.003 |
| Smoking, ever | 169 (64) | 36 (59) | 0.83 (0.54–1.28) | 0.4 |
| Asthma | 10 (3.8) | 8 (13) | 3.86 (2.01–7.42) | <0.0001 |
| COPD | 14 (5.3) | 2 (3.3) | 0.61 (0.29–2.16) | 0.4 |
| HTN | 81 (30) | 13 (21) | 0.62 (0.29–1.32) | 0.2 |
| CAD | 22 (8.3) | 6 (10) | 1.21 (0.71–2.05) | 0.5 |
| DM | 43 (16) | 12 (20) | 1.27 (0.93–1.74) | 0.14 |
| CKD | 1 (0.4) | 0 (0) | ||
| Preoperative chemoradiation | 230 (86) | 41 (69) | 0.38 (0.24–0.58) | <0.0001 |
| Surgical duration, h | 5.7 (4.7–7.3) | 6.0 (5.0–7.4) | 1.09 (0.96–1.24)† | 0.2 |
| Estimated blood loss, L | 0.3 (0.2–0.5) | 0.3 (0.2–0.6) | 2.25 (1.46–3.46) | 0.0002 |
| RBCs per 100 mL | 0.0 (0.0–10.5)* | 0.0 (0.0–10.5) | 1.21 (1.11–1.31) | <0.0001 |
| FFP | NA | NA | ||
| Yes | 1 (0.4) | 1 (1.6) | ||
| Platelets | NA | NA | ||
| Yes | 2 (0.8) | 0 (0) | ||
| RBCs, yes | 10 (3.8) | 7 (11) | 3.32 (1.34–8.23) | 0.01 |
| Total fluid, L | 6.4 (5.1–7.6) | 6.7 (5.4–7.9) | 1.09 (0.96–1.25)‡ | 0.2 |
| Norepinephrine equivalent dose, μg/kg | 3.6 (0.1–20.5) | 10.0 (0.1–22.3) | 1.00 (0.99–1.02) | 0.5 |
Values are presented as no. (%) or median (25th–75th percentile). ASA, American Society of Anesthesiologists physical status classification; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; FEV1, forced expiratory volume in 1 second; FFP, fresh frozen plasma; HTN, hypertension; NA, not applicable; OR, odds ratio; RBCs, red blood cells; total fluid, crystalloid + colloid.
*Data are median (minimum–maximum).
†Per hour increase in surgical duration.
‡Per liter increase in total fluid.
Table 4.
Multivariable logistic regression for odds of a major (grade ≥ 3) postoperative complication
| Variable | OR (95% CI) | P |
|---|---|---|
| ASA (3 vs. 2) | 2.82 (0.84–9.541) | 0.095 |
| BMI | 1.00 (0.97–1.03) | 0.9 |
| History of asthma | 4.12 (1.99–8.53) | 0.0001 |
| Estimated blood loss, L | 1.75 (1.11–2.76) | 0.015 |
| RBCs per 100 mL | 1.19 (1.09–1.30) | 0.0001 |
| Total fluid, per L increase | 1.19 (0.92–1.23) | 0.4 |
| Preoperative chemoradiation | 0.36 (0.24–0.55) | <0.0001 |
| Surgical duration, per h | 1.02 (0.88–1.19) | 0.8 |
ASA, American Society of Anesthesiologists physical status classification; BMI, body mass index; CI, confidence interval; OR, odds ratio; RBCs, red blood cells transfused.
DISCUSSION
The main findings of this study are that intraoperative vasopressor use and total crystalloid fluid administration were not associated with increased odds of postoperative anastomotic leak following open Ivor Lewis esophagectomy. However, increasing BMI was associated with an increased incidence of anastomotic leak, and this observation is supported by previous findings.3,6–13 We examined vasopressor either as an estimate of norepinephrine equivalents using the totals of ephedrine and phenylephrine or by either of these drugs individually. Furthermore, we also could not find a significant association between vasopressor use and grade severity with the primary outcome. None of the other variables examined was associated with an increased incidence of leak in our cohort. The overall leak rate in our study population was 17%, which is comparable with rates reported recently.3,5,19,20 For our secondary outcome, a history of asthma, greater estimated blood loss, and increasing red blood cell transfusion were independently associated with increased odds of major postoperative complications. Several of these factors have previously been demonstrated to affect outcomes following esophagectomy.3,7,12 We observed a decrease in major postoperative complications in patients who had received preoperative chemoradiation. We speculate that this may be related to a better oncologic response to this neoadjuvant therapy or be specific to this cohort of patients; therefore, this finding requires confirmation in a larger population.
The use of vasopressors during esophagectomy has been a source of controversy among surgeons and anesthesiologists. The tip of the stomach, which is used for the anastomosis of the conduit, is thought to be most vulnerable to ischemia, since that portion of the stomach is perfused only by the gastroepiploic artery, which often terminates much lower than the tip itself. Hence, some surgeons believe that splanchnic vasoconstriction caused by vasopressors may make the conduit—with its already limited vascular supply, as a result of multiple blood vessels being divided for transposition—at risk of ischemia.
Several studies have examined the effect of various interventions on esophageal conduit blood flow, which may play a role in the development of anastomotic leak. In 2008, Theodorou et al. 15 observed a reduction in gastric graft perfusion in pigs given norepinephrine infusion. In contrast, a small prospective study that examined 10 patients undergoing esophagectomy showed a significant improvement in blood flow at both ends of the gastric tube with the administration of intravenous epinephrine,21 and a similar study demonstrated reversal of the epidural-bolus–induced reduction of blood flow with the administration of an intravenous phenylephrine infusion,22 indicating that inotropes or vasopressors may have a role to play in maintaining gastric graft perfusion. A more recent prospective study that examined the blood pressure of 84 patients undergoing esophagectomy found an increased incidence of anastomotic leak in patients who had intraoperative hypotensive episodes with a decrease in systolic blood pressure of at least 30% for >5 minutes, suggesting that hypotension may play a role in influencing anastomotic integrity.23 Our practice is to maintain systolic blood pressure > 90 mmHg in conjunction with conservative but not restrictive fluid management. Although the current study cohort was treated before the adoption of a formal enhanced recovery program at our institution, our group’s practice was to administer fluids judiciously and blood products only when clearly indicated. Under these study conditions, we did not find a significant association between vasopressor use and development of anastomotic leak or other significant postoperative cardiopulmonary complications.
It has been proposed that the use of vasodilators could improve conduit blood flow, but several small studies have failed to demonstrate a benefit.24 Intraoperative evaluation of blood flow using indocyanine green fluorescence in the gastric conduit seems to hold promise for predicting the risk of anastomotic leak following esophagectomy, but its routine application clinically remains under investigation.25–27 The complete avoidance of vasopressors in patients undergoing esophagectomy is unfounded and may cause harm by leading to excess fluid administration, a known precipitant of morbidity,28 whereas excessive administration of vasopressors may contribute to conduit ischemia through vasoconstriction.
When used, thoracic epidural analgesia is often started during surgery as an adjunct to general anesthesia and maintained after surgery for postoperative pain management. Epidural infusions as used by our group typically contain a combination of opioid and low concentration of local anesthetic and may affect the leak rate through changes in blood pressure and thus graft perfusion pressure, inflammatory mediators, postoperative mobility, and respiratory status. However, few studies have examined the role epidural analgesia may play in influencing anastomotic leak. A retrospective review of 207 patients found that the use of thoracic epidural analgesia was independently associated with a decreased incidence of anastomotic leak,29 and in a recent retrospective review of a prospective database of 587 patients who underwent open esophagectomy, Li et al. 30 found that epidural analgesia may attenuate the inflammatory response and reduce the incidence of anastomotic leak following esophagectomy, despite lower blood pressure in the epidural analgesia group.
Our study has several limitations. The clinical data were gathered in a database in real-time, but the data for analysis were examined retrospectively. All the patients in this study underwent open esophagectomy; our results may therefore not be generalizable to patients undergoing MIE. Vasopressor and fluid management were done at the discretion of the anesthesia care team, without a standard protocol such as an enhanced recovery program. However, the patients were managed by an experienced group of anesthesiologists who followed common departmental practices. Our findings could benefit from confirmation in a larger cohort of patients.
In conclusion, in this retrospective study, we did not observe evidence that intraoperative use of perioperative vasopressors or total fluid administration was associated with increased odds of perioperative anastomotic leak following open Ivor Lewis esophagectomy. Increasing BMI was associated with higher odds of developing postoperative anastomotic leak. The complete avoidance of vasopressors in patients undergoing esophagectomy is unfounded and may cause harm by leading to excess fluid administration, a known precipitant of morbidity.
ACKNOWLEDGMENTS
We thank David B. Sewell of the Department of Surgery, Memorial Sloan Kettering Cancer Center, for editorial assistance.
Contributor Information
Kevin J Walsh, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Hao Zhang, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Kay See Tan, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Alessia Pedoto, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Dawn P Desiderio, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Gregory W Fischer, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Manjit S Bains, Thoracic Surgery Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
David R Jones, Thoracic Surgery Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Daniela Molena, Thoracic Surgery Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
David Amar, Department of Anesthesiology and Critical Care Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Financial support
This work was supported, in part, by NIH Cancer Center Support Grant P30 CA008748.
Disclosures
David R. Jones serves as a senior medical advisor for Diffusion Pharmaceuticals and a consultant for Merck and AstraZeneca. All other authors have no potential conflicts of interest with the contents of this study.
Author contributions
Kevin Walsh: conceptualization, data curation, investigation, writing original draft writing review & editing; Hao Zhang: conceptualization, data curation, investigation, validation; Kay See Tan: conceptualization, investigation, formal analysis, writing original draft, review & editing; Alessia Pedoto: conceptualization, investigation, writing review & editing; Dawn Desiderio: conceptualization, investigation, writing review & editing; Gregory Fischer: conceptualization, investigation, writing review & editing; Manjit Bains: conceptualization, investigation, writing review & editing; David Jones: methodology, data curation, investigation, writing review & editing; Daniela Molena: methodology, investigation, writing original draft, review & editing; David Amar: conceptualization, supervision, data curation, methodology, writing original draft, review & editing.
References
- 1. Mariette C, Markar S R, Dabakuyo-Yonli T S et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med 2019; 380(2): 152–62. [DOI] [PubMed] [Google Scholar]
- 2. Raymond D P, Seder C W, Wright C D et al. Predictors of major morbidity or mortality after resection for esophageal cancer: a society of thoracic surgeons general thoracic surgery database risk adjustment model. Ann Thorac Surg 2016; 102(1): 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kassis E S, Kosinski A S, Ross P Jr, Koppes K E, Donahue J M, Daniel V C. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013; 96(6): 1919–26. [DOI] [PubMed] [Google Scholar]
- 4. Van Daele E, Van de Putte D, Ceelen W, Van Nieuwenhove Y, Pattyn P. Risk factors and consequences of anastomotic leakage after ivor Lewis oesophagectomydagger. Interact Cardiovasc Thorac Surg 2016; 22(1): 32–7. [DOI] [PubMed] [Google Scholar]
- 5. Rutegard M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012; 19(1): 99–103. [DOI] [PubMed] [Google Scholar]
- 6. Sauvanet A, Mariette C, Thomas P et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005; 201(2): 253–62. [DOI] [PubMed] [Google Scholar]
- 7. Sunpaweravong S, Ruangsin S, Laohawiriyakamol S, Mahattanobon S, Geater A. Prediction of major postoperative complications and survival for locally advanced esophageal carcinoma patients. Asian J Surg 2012; 35(3): 104–9. [DOI] [PubMed] [Google Scholar]
- 8. Haga Y, Wada Y, Takeuchi H, Ikejiri K, Ikenaga M. Prediction of anastomotic leak and its prognosis in digestive surgery. World J Surg 2011; 35(4): 716–22. [DOI] [PubMed] [Google Scholar]
- 9. Junemann-Ramirez M, Awan M Y, Khan Z M, Rahamim J S. Anastomotic leakage post-esophagogastrectomy for esophageal carcinoma: retrospective analysis of predictive factors, management and influence on longterm survival in a high volume Centre. Eur J Cardiothorac Surg 2005; 27(1): 3–7. [DOI] [PubMed] [Google Scholar]
- 10. Wright C D, Kucharczuk J C, O'Brien S M, Grab J D, Allen M S, Society of Thoracic Surgeons general thoracic surgery Database . Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a society of thoracic surgeons general thoracic surgery database risk adjustment model. J Thorac Cardiovasc Surg 2009; 137(3): 587–95 discussion 596. [DOI] [PubMed] [Google Scholar]
- 11. Tabatabai A, Hashemi M, Mohajeri G, Ahmadinejad M, Khan I A, Haghdani S. Incidence and risk factors predisposing anastomotic leak after transhiatal esophagectomy. Ann Thorac Med 2009; 4(4): 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasegawa T, Kubo N, Ohira M et al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg 2015; 19(2): 226–33. [DOI] [PubMed] [Google Scholar]
- 13. Bootsma B T, Huisman D E, Plat V D et al. Towards optimal intraoperative conditions in esophageal surgery: a review of literature for the prevention of esophageal anastomotic leakage. Int J Surg 2018; 54(Pt A): 113–23. [DOI] [PubMed] [Google Scholar]
- 14. Deng X F, Liu Q X, Zhou D, Min J X, Dai J G. Hand-sewn vs linearly stapled esophagogastric anastomosis for esophageal cancer: a meta-analysis. World J Gastroenterol 2015; 21(15): 4757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theodorou D, Drimousis P G, Larentzakis A, Papalois A, Toutouzas K G, Katsaragakis S. The effects of vasopressors on perfusion of gastric graft after esophagectomy. An experimental study. J Gastrointest Surg 2008; 12(9): 1497–501. [DOI] [PubMed] [Google Scholar]
- 16. Khanna A, English S W, Wang X S et al. Angiotensin ii for the treatment of vasodilatory shock. N Engl J Med 2017; 377(5): 419–30. [DOI] [PubMed] [Google Scholar]
- 17. Seely A J, Ivanovic J, Threader J et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010; 90(3): 936–42 discussion 942. [DOI] [PubMed] [Google Scholar]
- 18. Dantoc M, Cox M R, Eslick G D. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg 2012; 147(8): 768–76. [DOI] [PubMed] [Google Scholar]
- 19. Biere S S, Maas K W, Cuesta M A, van der Peet D L. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg 2011; 28(1): 29–35. [DOI] [PubMed] [Google Scholar]
- 20. Markar S R, Arya S, Karthikesalingam A, Hanna G B. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013; 20(13): 4274–81. [DOI] [PubMed] [Google Scholar]
- 21. Al-Rawi O Y, Pennefather S H, Page R D, Dave I, Russell G N. The effect of thoracic epidural bupivacaine and an intravenous adrenaline infusion on gastric tube blood flow during esophagectomy. Anesth Analg 2008; 106(3): 884–7. [DOI] [PubMed] [Google Scholar]
- 22. Pathak D, Pennefather S H, Russell G N et al. Phenylephrine infusion improves blood flow to the stomach during oesophagectomy in the presence of a thoracic epidural analgesia. Eur J Cardiothorac Surg 2013; 44(1): 130–3. [DOI] [PubMed] [Google Scholar]
- 23. Fumagalli U, Melis A, Balazova J, Lascari V, Morenghi E, Rosati R. Intra-operative hypotensive episodes may be associated with post-operative esophageal anastomotic leak. Updates Surg 2016; 68(2): 185–90. [DOI] [PubMed] [Google Scholar]
- 24. Buise M, van Bommel J, Jahn A, Tran K, Tilanus H, Gommers D. Intravenous nitroglycerin does not preserve gastric microcirculation during gastric tube reconstruction: a randomized controlled trial. Crit Care 2006; 10(5): R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koyanagi K, Ozawa S, Oguma J et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: new predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore) 2016; 95(30): e4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell C, Reames M K, Robinson M, Symanowski J, Salo J C. Conduit vascular evaluation is associated with reduction in anastomotic leak after esophagectomy. J Gastrointest Surg 2015; 19(5): 806–12. [DOI] [PubMed] [Google Scholar]
- 27. Ladak F, Dang J T, Switzer N et al. Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 2019; 33(2): 384–94. [DOI] [PubMed] [Google Scholar]
- 28. Chau E H, Slinger P. Perioperative fluid management for pulmonary resection surgery and esophagectomy. Semin Cardiothorac Vasc Anesth 2014; 18(1): 36–44. [DOI] [PubMed] [Google Scholar]
- 29. Michelet P, D'Journo X B, Roch A et al. Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest 2005; 128(5): 3461–6. [DOI] [PubMed] [Google Scholar]
- 30. Li W, Li Y, Huang Q, Ye S, Rong T. Short and long-term outcomes of epidural or intravenous analgesia after esophagectomy: a propensity-matched cohort study. PLoS One 2016; 11(4): e0154380. [DOI] [PMC free article] [PubMed] [Google Scholar]


