Abstract
The oral poly(adenosine diphosphate-ribose) polymerase inhibitor olaparib is approved for the treatment of patients with human epidermal growth factor 2-negative (HER2−) metastatic breast cancer (mBC) and a germline breast cancer susceptibility gene (BRCA) mutation who have been treated with chemotherapy. This case report describes a 63-year-old postmenopausal woman with somatic BRCA2-mutated mBC who responded to olaparib treatment following multiple prior lines of therapy. The patient presented in January 2012 with locally advanced, hormone receptor-positive (HR+), HER2− BC which, despite initial response to neoadjuvant chemotherapy, recurred as bone disease in February 2014, and subsequently skin (June 2016) and liver (October 2016) metastases. A comprehensive 592-gene next-generation sequencing panel (Caris Life Sciences), performed on a skin biopsy, detected a pathogenic frameshift mutation in BRCA2 (H3154fs, c.9460delC), which was not identified in a 28-gene hereditary cancer germline analysis (Myriad Genetics, Inc.), and was therefore considered to be a somatic mutation. In January 2017, cell-free DNA (cfDNA) analysis (Guardant Health, Inc.) confirmed the BRCA2 H3154fs mutation in plasma. After several lines of chemotherapy and endocrine therapy, deriving clinical benefit from eribulin and capecitabine, the disease progressed by October 2017, and olaparib (300 mg orally twice daily) was initiated in January 2018. By April 2018, the liver lesions had shrunk by 80% and a >90% response in multiple skin lesions was noted. Clinical response was maintained for 8 months, followed by progression in the skin in September 2018. Biopsy of recurrent lesions revealed a novel BRCA2 mutation, E3152del (c.9455_9457delAGG), predicted to restore the open reading frame and presumably the mechanism of resistance to olaparib. Further likely resistance mutations were noted in subsequent cfDNA analyses. This case demonstrated a clinical response with olaparib as a later-line therapy for HR+, HER2− mBC with a somatic BRCA2 mutation.
Keywords: breast cancer, metastasis, olaparib, PARP inhibitor, somatic BRCA2 mutations
Introduction
Breast cancer (BC) susceptibility genes (BRCA1 and BRCA2) encode proteins essential to high-fidelity repair of DNA double-strand breaks (DSBs).1 Deletions or mutations in these genes, particularly in breast and ovarian cancer,2 result in compromised homologous recombination repair (HRR), posing significant risks to genome integrity.3,4
Poly(adenosine diphosphate-ribose) polymerase (PARP) is a major factor in the repair of DNA single-strand breaks (SSBs).5 Olaparib, an oral PARP inhibitor (PARPi),6 traps PARP at DNA SSBs, thereby stalling replication forks, leading to their collapse and creating irreparable DNA DSBs implicated in tumour-specific cell death in BRCA-mutated cancers.4,7
In January 2018, olaparib was approved by the United States (US) Food and Drug Administration (FDA) for the treatment of patients with deleterious or suspected deleterious germline BRCA-mutated human epidermal growth factor receptor 2-negative (HER2−) metastatic BC (mBC) treated with chemotherapy in the neoadjuvant, adjuvant or metastatic setting.8 Approval was based on the results of the phase III OlympiAD trial, in which olaparib monotherapy improved median progression-free survival (PFS; 7.0 months) compared with standard chemotherapy (4.2 months; hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.43–0.80; p < 0.001).6
In a cohort study of 273 unselected patients with BC,9 germline BRCA mutations were twice as likely as somatic BRCA mutations; 3% of patients harboured only somatic mutations in BRCA1 or BRCA2, for which there is no approved therapy in the USA. HRR deficiency has also been reported in a significant proportion of patients with BRCA-wildtype BC,7 as hereditary and somatic mutations can occur in other genes involved in the HRR pathway, such as PALB2 and PTEN.10–13 This suggests the clinical benefit of PARPi in mBC could extend to somatic BRCA mutations, and conceivably to other non-BRCA mutation HRR-deficient cells, regardless of germline versus somatic origin.4,7
We present the case of a postmenopausal woman with oestrogen receptor-positive (ER+), HER2− mBC and a somatic BRCA2 mutation in tumour tissues and plasma, who after several lines of chemotherapy, with responses and ultimately progression, had a clinically meaningful response to olaparib.
Case report
In January 2012, a 57-year-old, postmenopausal, white woman presented with a left breast mass and family history of later-onset breast and prostate cancer. Examination revealed a 13 × 13 cm mass, an ulcerating satellite mass in the left inframammary fold and a matted 4 cm mass in the left axillary lymph nodes.
A biopsy of the left breast demonstrated invasive lobular carcinoma, which was ER+ (96%), progesterone receptor-positive (PR+; 95%), HER2− by immunohistochemistry (IHC) and Ki-67 40%.
A computed tomography (CT) scan of the chest and abdomen and a bone scan were negative for distant metastases. The patient was staged as having locally advanced, initially inoperable, stage 3 (cT4, cN3, cM0) cancer. Neoadjuvant chemotherapy with docetaxel and bevacizumab was initiated (Table 1). Complete clinical response was achieved after six cycles of therapy.
Table 1.
Time course of anticancer therapies before initiating treatment with olaparib.
| Treatment | Start treatment | End treatment | Treatment duration, months |
|---|---|---|---|
| Neoadjuvant docetaxel + bevacizumab | 13 February 2012 | 7 June 2012 | 3.8 |
| Letrozole | 12 September 2012 | 17 March 2014 | 18.3 |
| Cyclophosphamide + methotrexate + fluorouracil | 17 March 2014 | 28 April 2014 | 1.4 |
| Dose-dense adriamycin + cyclophosphamide + paclitaxel | 30 April 2014 | 11 June 2014 | 1.4 |
| Everolimus + exemestane | 22 July 2014 | 21 August 2014 | 1 |
| Eribulin | 17 September 2014 | 20 July 2016 | 22 |
| Fulvestrant + palbociclib | 27 July 2016 | 14 December 2016 | 4.6 |
| Capecitabine | 10 January 2017 | 21 December 2017 | 11.5 |
| Olaparib, 300 mg twice daily | 26 January 2018 | 25 November 2018 | 10 |
In July 2012, the patient underwent a left mastectomy with axillary lymph node dissection and a contralateral right prophylactic mastectomy. Pathologic stage was ypT1a(m), pN2a. Postoperatively, she received left chest wall and regional nodal irradiation; adjuvant letrozole was initiated in September 2012. A bone scan in 2013 was negative for metastatic disease.
Following the development of right hip pain in February 2014, a bone scan revealed multiple new sites of osseous metastatic disease in the right iliac wing, multiple thoracic vertebrae, both scapulae, right ribs and sternum. A CT-guided biopsy of the right iliac crest showed metastatic, poorly differentiated adenocarcinoma consistent with breast origin: ER+ (100%), PR+ (100%) and HER2− (0/3) by IHC. The patient underwent chemotherapy with one cycle of cyclophosphamide, methotrexate and fluorouracil, followed by three cycles of adriamycin, cyclophosphamide and paclitaxel.
Re-evaluation in July 2014 showed new metastatic foci in the mid-thoracic spine and right femur. A subsequent switch to everolimus plus exemestane was poorly tolerated, and was discontinued after 1 month.
Eribulin, initiated in September 2014, was generally well tolerated. Bone scans revealed mild improvement at some sites in March 2015 and September 2015, and stable disease in December 2015 and April 2016. CT scans in April 2016 were negative for visceral disease.
Physical examination in June 2016 revealed two new skin lesions on the left chest and back; skin punch biopsies were positive for breast carcinoma. A comprehensive 592-gene next-generation sequencing (NGS) panel (Caris Life Sciences, Phoenix, AZ, USA), performed on the skin biopsy, detected a pathogenic frameshift mutation in BRCA2 (H3154fs, c.9460delC, variant allele frequency [VAF] 74%). IHC testing confirmed continued ER- and PR-positivity, with HER2− by IHC and chromogenic in situ hybridization. Key NGS panel findings are summarized in Table 2. One month later, a 28-gene hereditary cancer germline analysis (Myriad Genetics, Inc., Salt Lake City, UT, USA) of a blood specimen was negative, confirming that the BRCA2 mutation identified in the tumour tissue was somatic, not hereditary.
Table 2.
Summary of common cancer-associated somatic genetic variants detected in skin specimens using NGS.
| Gene | 592-gene NGS panel #1, 22 June 2016 | 592-gene NGS panel #2, 16 October 2018 | ||||||
|---|---|---|---|---|---|---|---|---|
| Detected variants |
Functional classification | VAF, % | Detected variants |
Functional classification | VAF, % | |||
| Protein alteration | Nucleotide change | Protein alteration | Nucleotide change | |||||
| ALK | P40H | NA | VUS | 25 | — | — | — | — |
| BRCA2 | H3154fs | c.9460delC | Pathogenic | 74 | — | — | — | — |
| BRCA2 | — | — | — | — | E3152del | c.9455_9457delAGG | Probable reversion | 35 |
| ESR1 | Y537S | NA | Pathogenic | 36 | Y537S | c.1610 _1611delinsCC | Pathogenic | 22 |
| NOTCH1 | R176Q | NA | VUS | 16 | — | — | — | |
| RB1 | G840R | NA | VUS | 14 | — | — | — | |
| WT1 | E154* | NA | Pathogenic | 36 | — | — | — | — |
| WT1 | E153D | NA | VUS | 37 | — | — | — | — |
| WT1 | — | — | — | — | E153 _E154 delinsD* | c.459_460delinsCT | Pathogenic | 27 |
A total of 592 genes was tested using NGS. Alterations prefaced by: p., protein; c., complementary DNA.
ALK, anaplastic lymphoma receptor kinase gene; BRCA2, breast cancer type 2 susceptibility gene; ESR1, oestrogen receptor alpha gene; NA, not available; NGS, next-generation sequencing; NOTCH1, notch homologue 1, translocation-associated gene; RB1, retinoblastoma-1 gene; VAF, variant allele frequency; VUS, variant of uncertain significance; WT1, Wilms tumour 1.
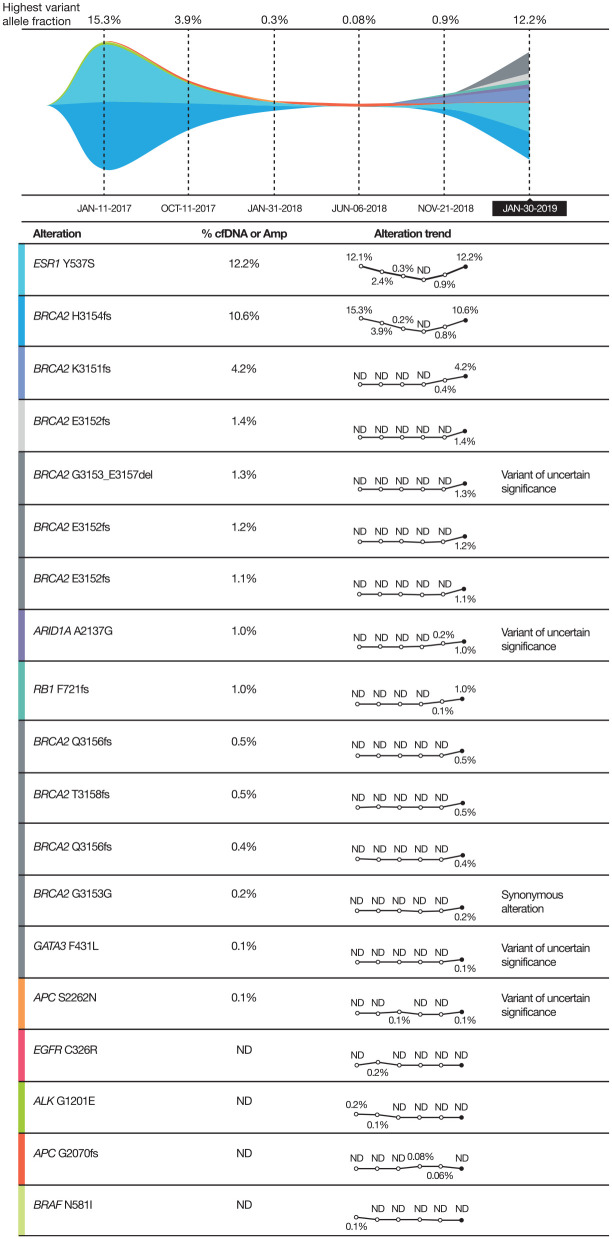
Fulvestrant and palbociclib were initiated in July 2016. By October 2016, bone lesions (mixed lytic and blastic disease) were stable, but subtle liver metastases were suspected in a CT scan. A further CT scan in January 2017 showed enlarging liver metastases, with the largest measuring 2.2 × 1.6 cm in segment 6. Plasma cell-free DNA (cfDNA) analysis (Guardant Health, Inc., Redwood City, CA, USA) confirmed the somatic BRCA2 H3154fs mutation (VAF, 15.3%; Figure 1). The patient was switched to capecitabine chemotherapy, with prompt dose reduction due to hand–foot syndrome.
Figure 1.
Summary of genetic mutations showing the allele frequencies of cfDNA alterations in plasma across multiple time points.
ALK, anaplastic lymphoma receptor kinase gene; Amp, amplified by polymerase chain reaction; APC, adenomatous polyposis coli gene; ARID, AT-rich interactive domain gene; BRAF, B-Raf gene; BRCA2, breast cancer type 2 susceptibility gene; cfDNA, cell-free DNA; EGFR, epidermal growth factor receptor gene; ESR1, oestrogen receptor alpha gene; GATA3, GATA binding protein 3 gene; ND, not detected; RB1, retinoblastoma-1 gene.
By March 2017, near-complete resolution of skin lesions was noted. CT scans demonstrated 80% reduction in liver lesion diameters and stable bone disease. CT scans in July 2017 were stable.
In October 2017, skin lesions recurred and progressed, with a CT scan revealing substantial progression of multiple metastases in the liver. The capecitabine dosage was increased, and the cutaneous lesions responded briefly, but a CT scan in January 2018 showed multiple new liver lesions and doubling in size of the largest lesion (Figure 2). The skin lesions had progressed, and new satellite nodules were detected. Bone scan results remained stable. Capecitabine was discontinued after almost 12 months of therapy.
Figure 2.
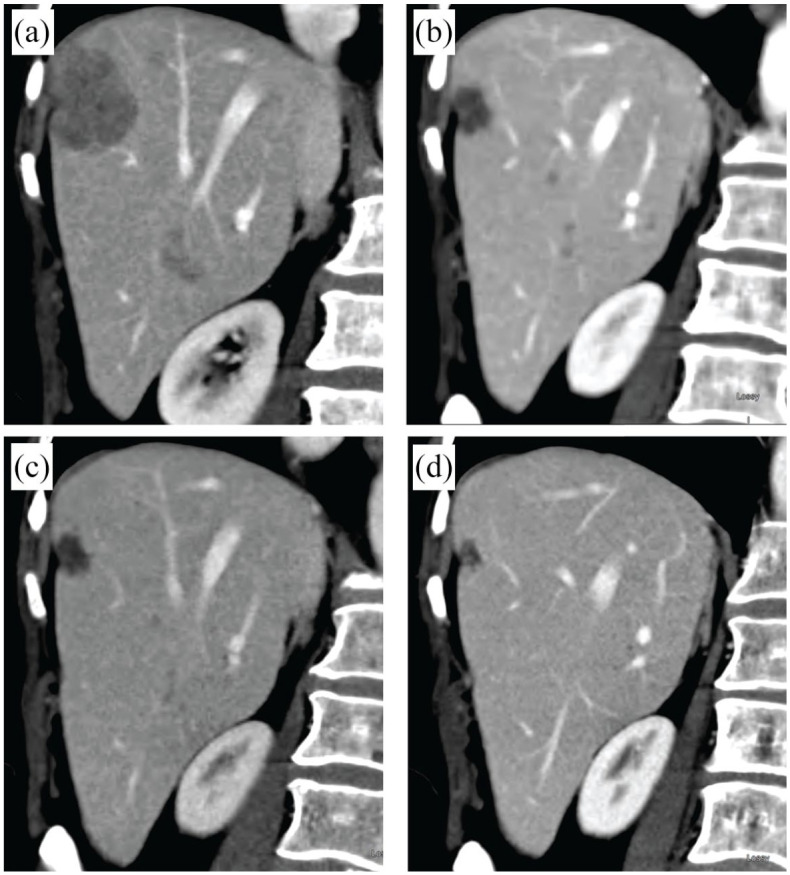
CT scans of liver metastases. (a) January 2018, before olaparib therapy. (b) April 2018, following interruption to olaparib therapy. (c) June 2018, during olaparib therapy. (d) January 2019, after stopping olaparib therapy.
CT, computed tomography
Olaparib therapy (300 mg orally twice daily) was initiated on 26 January 2018. A cfDNA assay on 31 January 2018 again showed the original BRCA2 H3154fs (c.9460delC), this time at a much lower VAF of 0.2%. Skin lesions improved by 50% within 3 weeks. Initial symptomology (nausea, fatigue, irritated mouth) also improved. Olaparib treatment was paused briefly in February 2018, during hospitalization for Klebsiella bacteraemia complicating febrile pancytopenia, and was restarted at a full dose (300 mg twice daily) following recovery of the white blood cell count.
In April 2018, a CT scan showed liver lesion shrinkage of 80% (Figure 2). Multiple lytic lesions in the spine remained unchanged. Physical examination showed >90% response in multiple skin lesions. Olaparib treatment was continued, with mild fatigue and resolution of nausea. Periodic packed red blood cell transfusions were required.
By June 2018, a >95% improvement in the metastatic skin lesions and further reduction in liver lesions was noted; bone scan results remained stable. A cfDNA analysis at this time demonstrated undetectable levels of BRCA2 and of other mutations, including ESR1 and ALK (Figure 1). CT scans on 30 August 2018 showed further reduction in the hepatic metastases.
One month later, a lesion on the left back had grown from approximately 7 mm to 20 mm, although no new skins lesions were noted. A skin biopsy (16 October 2018) showed GATA3-positive, ER+ (90%), PR+ (90%), HER2− mBC. A repeat 592-gene NGS panel on the skin biopsy detected a novel BRCA2 mutation, E3152del (c.9455_9457delAGG), at a VAF of 35%, predicted to restore the open reading frame and potentially lead to resumption of functional activity (Table 2).
cfDNA analysis on 21 November 2018 showed co-occurring BRCA2 H3154fs (c.9460delC; VAF, 0.8%) and emerging K3151fs (c.9452_9453delAA; VAF, 0.4%) mutations (Figure 1). A CT scan showed minimal liver disease, stable bone disease, and small left pleural effusion. New satellite skin lesions on the back were detected on physical examination. Olaparib treatment was discontinued after 10 months of therapy, and vinorelbine was initiated.
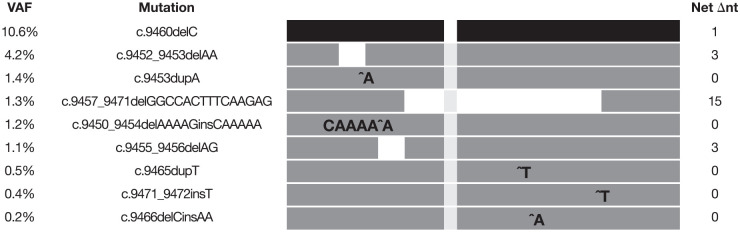
By January 2019, multiple skin nodules had progressed, with unchanged bone disease. Eastern Cooperative Oncology Group performance status remained at 1. Pain was controlled with long-acting narcotics, vinorelbine treatment was discontinued and liposomal doxorubicin treatment was initiated, with poor tolerance. A subsequent cfDNA analysis (30 January 2019) showed VAF increases in the original BRCA2 H3154fs to 10.6% and in the recently emerging BRCA2 K3151fs to 4.2%, as well as three new genomically distinct variants of BRCA2 E3152fs (c.9453dupA, c.9450_9454delAAAAGinsCAAAAA, and c.9455_9456delAG) at 1.4%, 1.2% and 1.1% VAF, respectively, and three genomically distinct BRCA2 Q3156fs variants (c.9465dupT, c.9471_9472insT, and c.9466delCinsAA), at 0.5%, 0.4%, and 0.2% VAF, respectively (Figure 3). A CT scan demonstrated a slight increase in pleural effusion, with stable liver and bone disease. Carboplatin and radiation therapy for ulcerated skin lesions began on 30 January 2019, and continued until April 2019, when hepatic progression was observed. The patient received palliative care and died in August 2019.
Figure 3.
Summary of BRCA2 genetic mutations showing the allele frequencies of cfDNA alterations in plasma from the analysis on 30 January 2019 and predicted net change in nucleotides highlighting predicted restoration of the open reading frame.
BRCA2, breast cancer type 2 susceptibility gene; cfDNA, cell-free DNA; Net ∆nt, net change in nucleotides; VAF, variant allele frequency.
Discussion
These findings indicate that olaparib was effective as later-line therapy in a 63-year-old woman with a somatic BRCA2 mutation, who was diagnosed 7 years earlier with ER+, PR+ and HER2− invasive lobular carcinoma. Metastatic recurrence in bone developed <2.5 years after neoadjuvant chemotherapy, while she was receiving adjuvant endocrine therapy. She subsequently received multiple therapeutic lines, deriving clinical benefit from eribulin and capecitabine, followed by cutaneous and hepatic progression prior to olaparib treatment.
Genomic alterations were detected in skin biopsies performed more than 2 years apart and in sequential plasma cfDNA analyses. The most pertinent and potentially actionable mutation found was BRCA2 H3154fs, a pathogenic mutation leading to a non-functional BRCA2 protein. A subsequent multigene panel for germline mutations in blood was negative for BRCA2, confirming the somatic origin of the BRCA2 pathogenic mutation, concordant with genomic testing across breast, skin and plasma specimens (Table 2 and Figure 1). The mutational analyses provided a strong rationale for treatment with olaparib, considering the known sensitivity of germline BRCA mutations to the effects of PARPi therapy.14 The patient had a clinical response to olaparib that lasted 8 months, with tolerable toxicity.
Non-detection of the original somatic BRCA2 H3154fs mutation in skin in the second NGS panel and in sequential plasma cfDNA assays suggests a positive effect of olaparib in suppressing the pathogenic clone, leading to prolongation of the clinical response.
Somatic BRCA2 reversion mutations and treatment resistance have been reported in metastatic prostate cancer exposed to PARPi.15,16 cfDNA analysis has the advantage of detecting heterogeneous alterations from multiple tumour sites with a minimally invasive approach, and quantitative VAFs can serve as a measure of the tumour burden and therapeutic response, especially when used sequentially.15,17
In this patient, sequential cfDNA analyses revealed multiple mutations over time, each predicted to restore the open reading frame. Concurrent identification of the original H3154fs mutation with reversion mutations likely represents differing subclonal tumours’ responses to olaparib. These polyclonal alterations were probable mechanisms of resistance, emerging after months of olaparib therapy, as previously reported in patients with pathogenic germline and somatic BRCA mutations in breast and ovarian cancers.18–21 To our knowledge, this is the first case of clinical response to olaparib documented for somatic BRCA2-mutated mBC, with presumed resistance emerging from reversion mutations. Further research into PARPi therapy in patients with somatic BRCA-mutated, germline BRCA-wildtype mBC is underway.
This case report demonstrates clinical response to olaparib as later-line therapy for ER+, HER2− mBC, with germline wildtype BRCA genes and an actionable somatic BRCA mutation, for which PARPi could provide substantial clinical benefit.
Acknowledgments
Written informed consent for publication of this case report was provided by the patient’s next of kin (husband). Medical writing support was provided by Laura Fullerton-Batten, CMC Connect, a division of McCann Health Medical Communications Ltd, Macclesfield, UK, and by Michael Riley, of Oxford PharmaGenesis, Cardiff, UK. Medical writing support, under the direction of the authors, was in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461–464).
Footnotes
Conflict of interest statement: Lee S. Schwartzberg is a consultant for AstraZeneca, Genentech, Helsinn, Lilly, Novartis, Pfizer and Spectrum. He has received research funding from Amgen and Pfizer and is on the speakers’ bureau for Amgen and Puma. Lesli A. Kiedrowski is an employee and shareholder of Guardant Health, Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing support was funded by AstraZeneca, Cambridge, UK.
ORCID iD: Lee S. Schwartzberg  https://orcid.org/0000-0002-7433-3428
https://orcid.org/0000-0002-7433-3428
Contributor Information
Lee S. Schwartzberg, West Cancer Center, 7945 Wolf River Blvd., Germantown, TN 38138, USA
Lesli A. Kiedrowski, Department of Medical Affairs, Guardant Health, Inc., Redwood City, CA, USA.
References
- 1. Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer 2016; 60: 49–58. [DOI] [PubMed] [Google Scholar]
- 2. Chen CC, Feng W, Lim PX, et al. Homology-directed repair and the role of BRCA1, BRCA2, and related proteins in genome integrity and cancer. Annu Rev Cancer Biol 2018; 2: 313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gunderson CC, Moore KN. BRACAnalysis CDx as a companion diagnostic tool for Lynparza. Expert Rev Mol Diagn 2015; 15: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 4. Murata S, Zhang C, Finch N, et al. Predictors and modulators of synthetic lethality: an update on PARP inhibitors and personalized medicine. Biomed Res Int 2016; 2016: 2346585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell 2015; 60: 547–560. [DOI] [PubMed] [Google Scholar]
- 6. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017; 377: 523–533. [DOI] [PubMed] [Google Scholar]
- 7. den Brok WD, Schrader KA, Sun S, et al. Homologous recombination deficiency in breast cancer: a clinical review. JCO Precis Oncol 2017; 1: 1–13. [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. Lynparza prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf. (accessed 7 September 2020).
- 9. Winter C, Nilsson MP, Olsson E, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol 2016; 27: 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendes-Pereira AM, Martin SA, Brough R, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med 2009; 1: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ming M, He Y-Y. PTEN in DNA damage repair. Cancer Lett 2012; 319: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nepomuceno TC, De Gregoriis G, de Oliveira FMB, et al. The role of PALB2 in the DNA damage response and cancer predisposition. Int J Mol Sci 2017; 18: 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ngeow J, Sesock K, Eng C. Breast cancer risk and clinical implications for germline PTEN mutation carriers. Breast Cancer Res Treat 2017; 165: 1–8. [DOI] [PubMed] [Google Scholar]
- 14. Yi T, Feng Y, Sundaram R, et al. Antitumor efficacy of PARP inhibitors in homologous recombination deficient carcinomas. Int J Cancer 2019; 145: 1209–1220. [DOI] [PubMed] [Google Scholar]
- 15. Quigley D, Alumkal JJ, Wyatt AW, et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov 2017; 7: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carneiro BA, Collier KA, Nagy RJ, et al. Acquired resistance to poly (ADP-ribose) polymerase inhibitor olaparib in BRCA2-associated prostate cancer resulting from biallelic BRCA2 reversion mutations restores both germline and somatic loss-of-function mutations. JCO Precis Oncol 2018; 2: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lupini L, Moretti A, Bassi C, et al. High-sensitivity assay for monitoring ESR1 mutations in circulating cell-free DNA of breast cancer patients receiving endocrine therapy. Sci Rep 2018; 8: 4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noordermeer SM, van Attikum H. PARP inhibitor resistance: a tug-of-war in BRCA-mutated cells. Trends Cell Biol 2019; 29: 820–834. [DOI] [PubMed] [Google Scholar]
- 19. Weigelt B, Comino-Mendez I, de Bruijn I, et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res 2017; 23: 6708–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gornstein EL, Sandefur S, Chung JH, et al. BRCA2 reversion mutation associated with acquired resistance to olaparib in estrogen receptor-positive breast cancer detected by genomic profiling of tissue and liquid biopsy. Clin Breast Cancer 2018; 18: 184–188. [DOI] [PubMed] [Google Scholar]
- 21. Christie EL, Fereday S, Doig K, et al. Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol 2017; 35: 1274–1280. [DOI] [PubMed] [Google Scholar]