Abstract
Background:
Cytotoxic T-lymphocyte (CTL) infiltration into tumor is a positive prognostic factor in breast cancer. High tumor mutational burden (TMB) is also considered as a predictor of tumor immunogenicity and response to immunotherapy. However, it is unclear whether the infiltration of functional CTL simply reflects the TMB or represents an independent prognostic value.
Methods:
Utilizing The Cancer Genome Atlas (TCGA) breast cancer cohort, we established the Functional Hotness Score (FHS). The associations of FHS and breast cancer patient prognosis as well as distinct immunity markers were analyzed in a total of 3011 breast cancer patients using TCGA, METABRIC and metastatic breast cancer (MBC) cohort GSE110590.
Results:
We established FHS, based on CD8A, GZMB and CXCL10 gene expression levels of bulk tumors, which delivered the best prognostic value among some gene combinations. Breast cancer patients with the high-FHS tumors showed significantly better survival. FHS was lower in the MBCs. Triple-negative breast cancer (TNBC) showed the highest FHS among subtypes. FHS predicted patient survival in hormone receptor (HR)-negative, especially in TNBC, but not in HR-positive breast cancer. FHS predicted patient prognosis independently in TNBC. The high-FHS TNBCs showed not only higher CD8+ T cell infiltration, but also enhanced broader type-1 anti-cancer immunity. The patients with the high-FHS tumors showed better prognosis not only in high-TMB tumors but also in low-TMB TNBCs. The combination of high-TMB with high-FHS identified a unique subset of patients who do not recur over time in TNBC.
Conclusion:
TNBCs with high FHS based on the expression levels of CD8A, GZMB and CXCL10 showed improved prognosis with enhanced anti-cancer immunity regardless of TMB. FHS constitutes an independent prognostic marker of survival, particularly robustly when combined with TMB in TNBC.
Keywords: breast cancer, cytokine profile, T-cell infiltration, triple-negative breast cancer, tumor microenvironment, tumor mutational burden
Background
Accumulating data demonstrate the association of the elevated numbers of tumor infiltrating lymphocytes (TILs) with improved clinical outcomes in breast cancer.1–3 Among TILs, cytotoxic T-lymphocytes (CTLs) play a crucial role in anti-cancer immunity. Cancer cell recognition by the CTLs is driven by neoantigens which mainly reflect tumor mutational burden (TMB).4,5 Triple-negative breast cancer (TNBC) has a higher TMB and accumulates more CTLs as compared with other breast cancer subtypes.5 As a result, TNBC is more sensitive to immune checkpoint inhibitors (ICIs), such as PD1/PD-L1 blockade, which enhance CTL survival and cytolytic activity,6,7 resulting in their Food and Drug Administration approval in TNBC.8 However, PD-L1 blockade is effective in only a small portion of TNBC patients, and the predictive value of PD-L1 immunohistochemistry (IHC) is very limited, with some of the PD-L1 negative patients still responding to PD-L1 blockade.9 Similarly, the predictive value of individual CTL markers of IHC is not reliable and limited by their variation and spatial heterogeneity within individual tumors.10 These considerations highlight the importance of identification of the improved markers predicting the ability of the immune system to control breast cancer progression and responsiveness to treatments.
CTLs are identified by CD8 surface marker, which is encoded by CD8A gene.11 Granzyme B (GZMB) is a serine protease that is secreted by activated CTLs and natural killer (NK) cells to induce apoptosis of the target cells.12,13 Chemokines, such as CXCL10 and CCL5, are key to the selective attraction of activated (effector, effector–memory and memory) CTLs into tumors, as shown in multiple cancers.12,13 In addition to mutation-dependent neoantigens, CTLs can also recognize elevated levels of self-antigens,14–16 raising the possibility that their influx may be also important in the control of weakly immunogenic cancers with limited TMB. However, it remains unknown whether tumor infiltrating functional CTL levels correlate with improved patient survival and are independent of TMB. In order to investigate it, we developed the Functional Hotness Score (FHS), combining gene expressions of markers and attractants of activated CTL.
Methods
Study design and patient cohorts
A total of 3011 breast cancer patients were analyzed. We used the breast cancer cohort from The Cancer Genome Atlas (TCGA)17 as a testing cohort to establish FHS and to characterize the high-FHS cohort. As a validation cohort, we used the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohort.18,19 There are 1091 and 1904 primary breast cancer tissues with gene expression, in TCGA and METABRIC, respectively, and patient demographics are shown in Table 1. TCGA provisional and METABRIC datasets were downloaded through cBioportal.20,21 Mutation count data was from comprehensive DNA sequence in TCGA and from 40 targeted DNA sequences in METABRIC. The patients with a mutation count of more than or equal to 50 were classified as high-TMB group in TCGA, and more than or equal to 20 in METABRIC. Metastatic breast cancer (MBC) cohort GSE11059022 (n = 16) was downloaded through the Gene Expression Omnibus repository. Since all the patients analyzed in this study were from de-identified publicly available cohorts, institutional review board approval was waived, and informed consents were obtained by the researchers of the original publication (TCGA,17 METABRIC,18,19 GSE11059022), which was the case in our previous publications.23,24
Table 1.
Patient demographics.
| TCGA (n = 1091) | METABRIC (n = 1904) | |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 58.5 ± 13.2 | 61.1 ± 13.0 |
| Menopause | ||
| Post | 703 | 1493 |
| Pre | 226 | 411 |
| Indeterminate | 73 | 0 |
| Unknown | 89 | 0 |
| ER | ||
| ER+ | 805 | 1459 |
| ER− | 237 | 445 |
| Indeterminate | 2 | 0 |
| Unknown | 47 | 0 |
| PR | ||
| PR+ | 697 | 1009 |
| PR− | 342 | 895 |
| Indeterminate | 4 | 0 |
| Unknown | 48 | 0 |
| HER2 | ||
| HER2+ | 185 | 236 |
| HER2− | 767 | 1668 |
| Indeterminate | 25 | 0 |
| Unknown | 114 | 0 |
| Histology | ||
| IDC | 781 | 1500 |
| ILC | 203 | 142 |
| Others | 107 | 262 |
| Stage | ||
| Stage 0 | 0 | 4 |
| Stage I | 180 | 475 |
| Stage II | 618 | 800 |
| Stage III | 249 | 115 |
| Stage IV | 20 | 9 |
| Stage X | 24 | 501 |
IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma; METABRIC, Molecular Taxonomy of Breast Cancer International Consortium; TCGA, The Cancer Genome Atlas.
CIBERSORT
The infiltrating immune cell fractions into tumors were estimated by the CIBERSORT algorithm.25 The calculated data was downloaded through the TCIA website (https://tcia.at/home).26 The TNBC patients were divided into high and low CD8+ T cell groups using same percentage of high and low FHS groups.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) was conducted comparing high and low FHS TNBCs among 50 hallmark gene sets27 using software provided by the Broad Institute (http://software.broadinstitute.org/gsea/index.jsp) as previously described.23,28,29 False discovery rate <0.01 was considered as significant.
Statistical analysis
Score and TMB differences between two groups were analyzed using Student’s t-test, and one-way analysis of variance was used for the comparison of more than two groups. Pearson correlations were calculated based on the expression levels of the genes and plotted. The survival analyses were conducted by Kaplan–Meier curve with log-rank test, and univariate and multivariate analyses were conducted by Cox regression model. The data of infiltrating immune cell fractions was compared by Wilcoxon test. All statistical analyses were performed using R software (http:///www.r-project.org/) and Bioconductor (http://bioconductor.org/).
Results
Development of the FHS
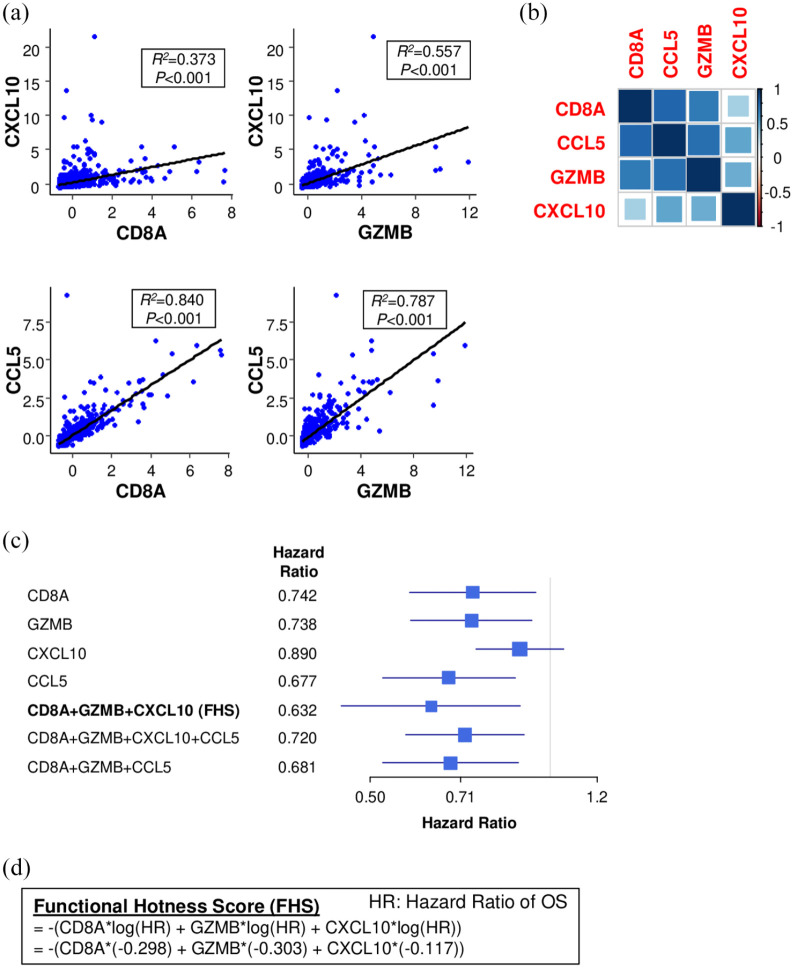
The FHS was developed using the combination of CD8A, GZMB and chemokine gene expression that deliver the best hazard ratio of overall survival (OS) in TCGA. Hazard ratio of CCL5 is 0.677, which was much lower than that of CXCL10 (0.890). However, CCL5 expression was highly correlated with CD8A and GZMB (R2 = 0.840 and R2 = 0.787, respectively) [Figure 1(a) and (b)] and combination of CCL5 together with CD8A and GZMB did not improve the prediction of prognosis (hazard ratio 0.681) compared with CXCL10 (hazard ratio 0.632) [Figure 1(c)]. Therefore, we established FHS combining CD8A, GZMB and CXCL10 expression (hazard ratio 0.632) [Figure 1(c)]. FHS predicted breast cancer patient prognosis better than each single gene expression [Figure 1(c)]. Further, adding CCL5 did not enhance the prognostic power (hazard ratio 0.720) [Figure 1(c)]. FHS was calculated using the log hazard ratio of OS in each gene, using the following formula [Figure 1(d)]:
Figure 1.
Establishment of Functional Hotness Score (FHS). (a) Correlation plots of CXCL10 and CCL5 to CD8A as well as granzyme B (GZMB) in The Cancer Genome Atlas (TCGA). (b) Correlation matrix of CD8A, GZMB, CXCL10 and CCL5 in TCGA. (c) Forest plot of multiple gene combination to estimate patient overall survival (OS) in TCGA. (d) Formula of FHS calculation.
Following the previous reports,30,31 we defined the top 15% of FHS tumors in the whole cohort as the “high-FHS” tumors. As we expected, the patients with the high-FHS tumors showed significantly better OS in the testing cohort, TCGA (p = 0.006) [Figure 2(a)]. This finding was also confirmed in the validation cohort, METABRIC, in which the breast cancer patients with the high-FHS tumors showed significantly better prognosis (p = 0.034) [Figure 2(b)].
Figure 2.
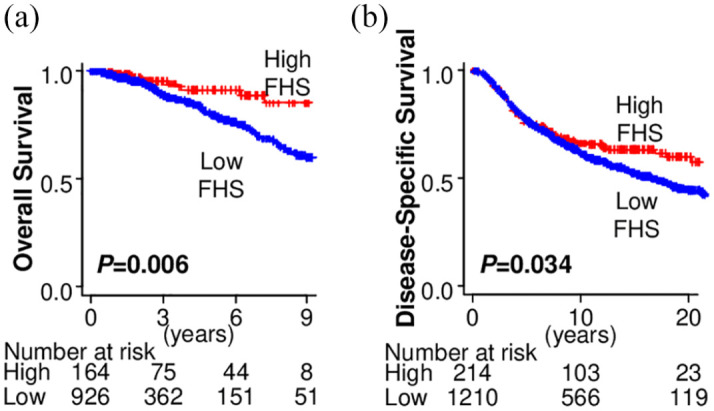
The association of Functional Hotness Score (FHS) and breast cancer patient prognosis. (a) Overall survival by FHS in The Cancer Genome Atlas (TCGA). (b) Disease-specific survival by FHS in Molecular Taxonomy of Breast Cancer International Consortium (METABRIC).
Decreased FHS in MBC
Since MBC is known to be particularly immunosuppressed, we investigated the association of FHS with MBC. Among the primary tumors, the stage IV tumors that have metastasis showed trend towards lower FHS than stage I/II/III tumors in TCGA, although it did not reach statistical significance (p = 0.082) [Figure 3(a)]. In the MBC cohort (GSE110590), which contains 16 primary breast cancers and 67 metastatic tissues from the same patients, the metastatic tumors showed significantly lower FHS than the primary tumors (p = 0.018) [Figure 3(b)]. Most of the metastatic tumors showed lower FHS as compared with its matched primary tumors, particularly in the liver (p = 0.003) and the lung (p = 0.031) [Figure 3(b) and (c)]. On the other hand, many of the brain metastases showed similar levels of FHS compared with their matched primary tumors (p = 0.068) [Figure 3(b) and (c)].
Figure 3.
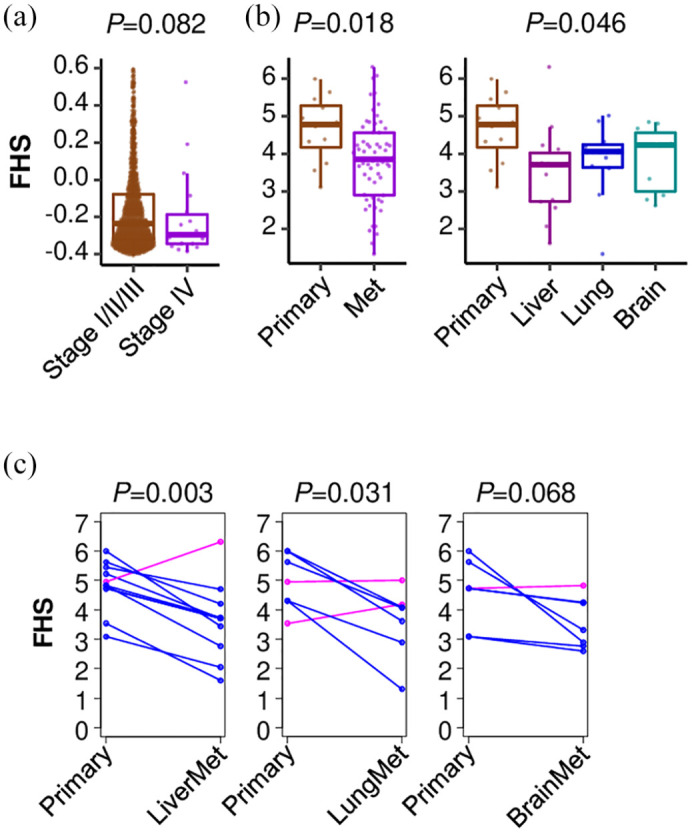
The association of Functional Hotness Score (FHS) and metastatic (Met) breast cancer (MBC). (a) FHS comparison between stage I/II/III and stage IV primary breast tumors in The Cancer Genome Atlas. (b) FHS comparison between primary and metastatic tumors in MBC cohort, GSE110590. (c) Matched pair comparison between primary and metastatic tumors in GSE110590.
Highest FHS in TNBC among breast cancer subtype
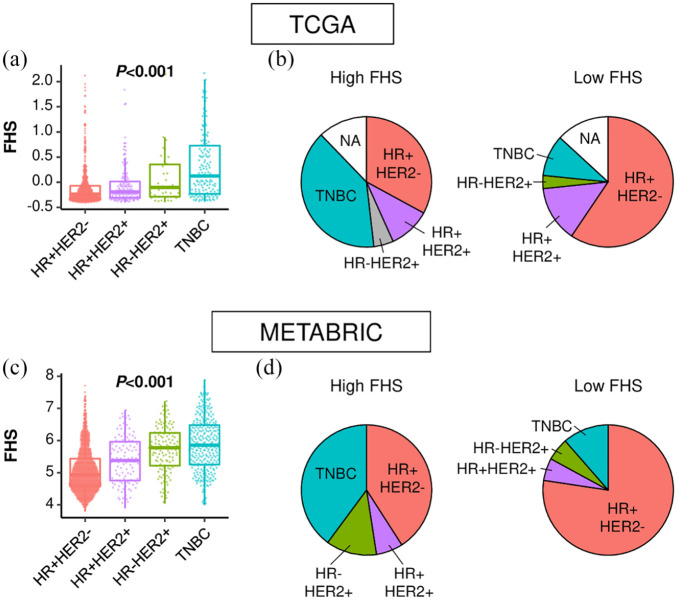
Since TNBC is the most immunogenic subtype, we hypothesized that FHS is higher in TNBC than in other subtypes. As expected, TNBC showed the highest FHS among all subtypes in TCGA (p < 0.001) [Figure 4(a)]. Consistently, TNBC accounted for the largest portion of the high-FHS tumors in TCGA (39.6%) [Figure 4(b)]. These findings were validated in the METABRIC cohort, where the highest FHS was seen in TNBC among subtypes; TNBC was the most dominant in the high-FHS tumors [Figure 4(c) and (d)].
Figure 4.
Functional Hotness Score (FHS) by subtype. (a) FHS comparison among breast cancer subtypes in The Cancer Genome Atlas (TCGA). (b) Distribution of each subtype in the high- and the low-FHS breast cancers in TCGA. (c) FHS comparison among breast cancer subtypes in Molecular Taxonomy of Breast Cancer International Consortium (METABRIC). (d) Distribution of each subtype in the high- and the low-FHS breast cancers in METABRIC.
HR, hormone receptor; NA, not available; TNBC, triple-negative breast cancer.
TNBC patients with high-FHS tumors demonstrate prolonged survival
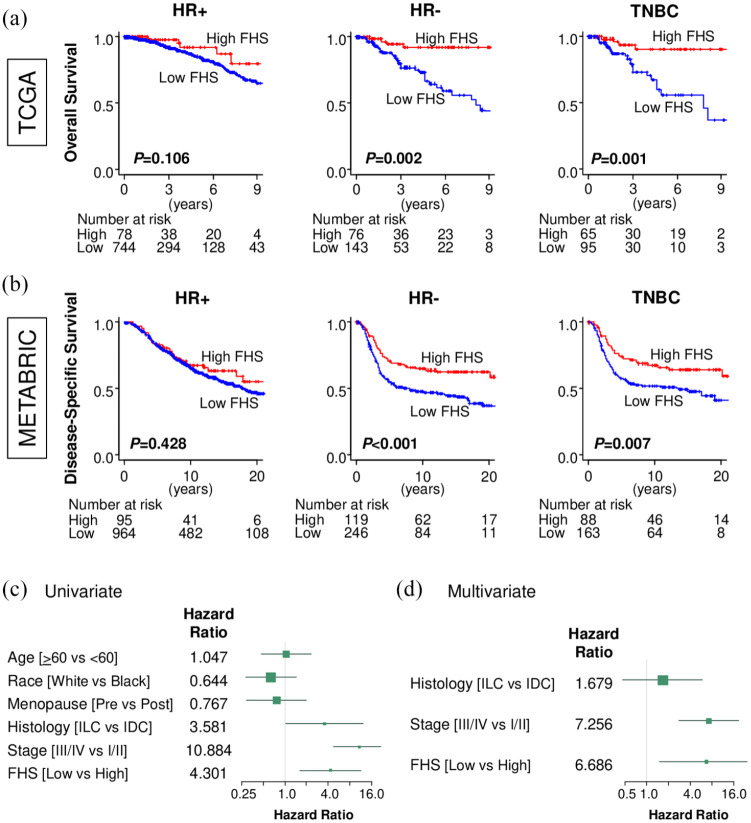
We further investigated the impact of FHS on patient survival in each subtype. In the hormone receptor (HR)-positive patients, there was no statistical difference in OS between the high- and the low-FHS tumors in TCGA (p = 0.106) [Figure 5(a)]. Further, HR-positive/HER2-negative (p = 0.250) and HR-positive/HER2-positive (p = 0.117) cohort did not show significant difference in OS by FHS in TCGA (Supplemental material Figure S1A online). On the other hand, the high-FHS tumor showed significantly better OS in the HR-negative patients in TCGA (p = 0.002) [Figure 5(a)]. When we focused on only TNBC, the patients with high-FHS tumor also showed significantly better prognosis (p = 0.001) [Figure 5(a)], but not in the HR-negative/HER2-positive cohort (p = 0.279) (Supplemental Figure S1a). These findings were validated in the METABRIC cohort, in which FHS did not predict patient survival in the HR-positive patients (p = 0.428) [Figure 5(b)], as well as HR-positive/HER2-negative (p = 0.309) or HR-positive/HER2-positive patients (p = 0.654) (Supplemental Figure S1b), but did in the HR-negative (p < 0.001) as well as TNBC patients (p = 0.007) [Figure 5(b)]. In the METABRIC cohort, the HR-negative/HER2-positive cohort also showed significant difference in survival by FHS (p = 0.049) (Supplemental Figure S1b), thus, the impact of FHS on patient survival in HR-negative/HER2-positive cohort is inconclusive. In the univariate analysis, invasive lobular carcinoma in histological subtype, advanced stage (stage III/IV) and low-FHS were significant risk factors for OS in TNBCs of TCGA cohort [Figure 5(c)]. Further, multivariate analysis demonstrated that only advanced stage (stage III/IV) and lower FHS were independent prognostic factors [Figure 5(d)]. FHS showed clinical significance in TNBC, thus, we further focused on TNBCs.
Figure 5.
The association of Functional Hotness Score (FHS) and breast cancer patient prognosis by subtype. (a) Overall survival (OS) by FHS in hormone receptor (HR)-positive, HR-negative, and triple-negative breast cancer (TNBC) in The Cancer Genome Atlas (TCGA). (b) Disease-specific survival by FHS in HR-positive, HR-negative, and TNBC in Molecular Taxonomy of Breast Cancer International Consortium (METABRIC). (c) Forest plot of hazard ratio for OS in the univariate analysis of TCGA TNBC patients. (d) Forest plot of hazard ratio for OS in the multivariate analysis of TCGA TNBC patients.
IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma.
Anti-cancer immune signature in high-FHS TNBC
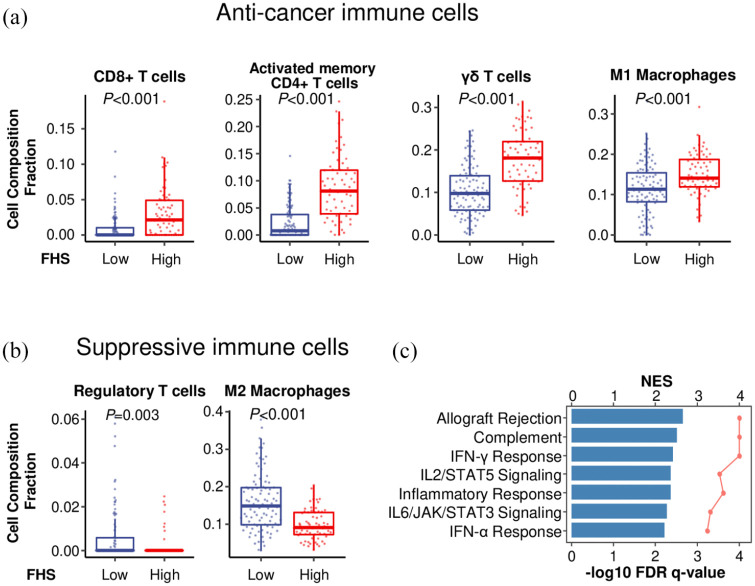
Cell composition fraction estimation analysis revealed that the high-FHS TNBCs were significantly associated with higher infiltration of anti-cancer immune cells, such as CD8+ T cells (p < 0.001), activated memory CD4+ T cells (p < 0.001), γδ T cells (p < 0.001) and M1 macrophages (p < 0.001) [Figure 6(a)], and with lower infiltration of suppressive immune cells, such as regulatory T cells (p = 0.003), and M2 macrophages (p < 0.001) in TCGA [Figure 6(b)]. Further, GSEA demonstrated that the high-FHS TNBCs were significantly associated with immune response-related gene sets, including allograft rejection, complement, interferon (IFN)-γ response, IL2/STAT5 signaling, inflammatory response, IL6/JAK/STAT3 signaling, and IFN-α response in TCGA [Figure 6(c)]. These findings indicate that FHS reflects not only CD8+ T cell infiltration, but also a broader type-1 anti-cancer immunity.
Figure 6.
The association of Functional Hotness Score (FHS) and cancer immunity in triple-negative breast cancers (TNBCs). (a) Comparison of anti-cancer immune cell infiltration between the low- and the high-FHS TNBCs in The Cancer Genome Atlas (TCGA). (b) Comparison of suppressive immune cell infiltration between the low- and the high-FHS TNBCs in TCGA. (c) Gene sets enriched in the high-FHS TNBCs in TCGA [false discovery rate (FDR) q <0.01].
NES, normalized enrichment score
High FHS predicts improved survival of TNBC patients regardless of TMB
Last, we investigated FHS in the association with TMB. Unexpectedly, there was no significant difference in TMB level between the low- and the high-FHS TNBCs (p = 0.276) [Figure 7(a)]. When we stratified patients into high- (⩾50) and low-TMB (<50), the proportions of the high- and the low-TMB tumors were also not significantly different between the high- and the low-FHS TNBCs [Figure 7(b)]. To investigate whether the prognostic value of FHS depends on TMB, we analyzed the significance of FHS in the high- and the low-TMB TNBCs. Interestingly, the patients with high-FHS TNBC showed better OS not only in the high-TMB group (p = 0.019), but also in the low-TMB group (p = 0.049) in TCGA [Figure 7(c) and (d). Strikingly, when both FHS and TMB were considered, TNBCs with the high FHS and the high TMB demonstrated a particularly long OS compared with the others (p = 0.008), highly unusual for the aggressive characteristics of TNBC [Figure 7(e)]. Contrary, when we used CD8+ T cell count instead FHS, there was no difference in patient survival between the high CD8+ T cell with high TMB tumor and the other tumors (p = 0.452) [Figure 7(f)]. Similar findings were shown in the METABRIC cohort; however, this is limited by mutation data with only representative genes rather than the whole genome (Figure S2). The high-FHS group showed better survival only in the low-TMB group (p = 0.003) and not in the high-TMB group, most likely because it has only 40 targeted DNA mutation data, thus, there were only 30 patients in total in the high-TMB group (Figure S2). These findings suggest that high FHS associates with better prognosis in TNBC regardless of TMB.
Figure 7.
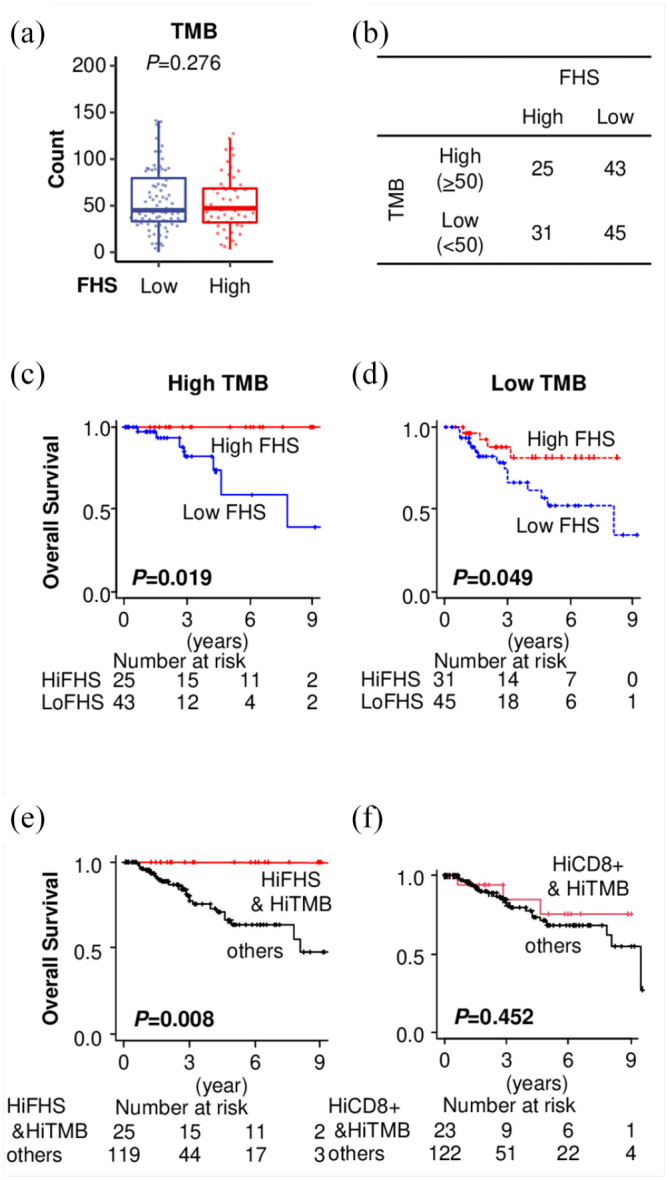
Functional Hotness Score (FHS) and tumor mutational burden (TMB) in triple-negative breast cancers (TNBCs). (a) Comparison of TMB between the low- and the high-FHS TNBCs in The Cancer Genome Atlas (TCGA). (b) Patients distribution in association with FHS and TMB in TCGA TNBCs. (c) and (d) Overall survival (OS) estimation by FHS in the high- and the low-TMB TNBC patients in TCGA with full DNA sequence data. (e) OS estimation comparing the high-FHS–high-TMB and the others and (f) high-CD8+ T cell–high-TMB and the others in TCGA TNBCs.
HiFHS, high Functional Hotness Score; LoFHS, low Functional Hotness Score.
Discussion
In this study, we established FHS combining CD8A, GZMB and CXCL10 gene expression levels of a bulk tumor of TNBC to identify “hot tumors” with improved prognosis despite high risk subtype. The FHS has a stronger prognostic value than each individual gene. The FHS is lower in stage IV than earlier stage tumors, and lower in metastatic tumors than primary tumors from the same patients. TNBC has the highest FHS among breast cancer subtypes, and high FHS predicted prolonged survival in TNBCs. The high FHS is associated with not only CD8+ T cell infiltration, but also a broader type-1 anti-cancer immunity in TNBCs. Importantly, the prognostic value of the FHS is independent of the TMB. In fact, FHS used jointly with the TMB index allowed us to identify the unique subset of TNBC patients with particularly good prognosis.
FHS combines the expression levels of CTL lineage marker CD8A,12 and GZMB, an enzyme secreted by CTLs and NK cells to induce apoptosis target cells.12,13 In addition, it includes CXCL10. CXCL10 is a chemokine which attracts not only activated CTLs (effector, effector-memory and central memory, but not naïve or suppressed cells), but also multiple immune cells, including NK cells, dendritic cells, and macrophages towards cancer lesions.32 Consequently, CXCL10 is involved in modulating both innate and adaptive immunity, but selectively their desirable effector, rather than suppressive, components.33 Indeed, our results demonstrated that high-FHS tumors showed not only enhanced markers of cellular immunity, such as higher CD8+ T cell infiltration, but also indications of enhanced humoral immunity, judging by activated memory CD4+ T cells. Further, high-FHS tumors are associated with M1 macrophages, which can produce CXCL10 and attract Th1 CD4+ helper T cells. This is in agreement with the commonly accepted notion that tumor infiltrating immune cells are highly correlated with each other.11 CCL5 is also a chemokine which attracts effector T cells,34 but it is also produced by CTLs themselves in tumor tissues.12 Accordingly, CCL5 alone predicts patient prognosis better than CXCL10 alone, but, since its expression is tightly correlated with CD8A and GZMB, it does not provide additional prognostic value as a part of composite FHS.
Calculation of FHS requires only three genes in a bulk unseparated tumor tissue, which can be measured by quantitative polymerase chain reaction (qPCR). It is more time, cost and labor efficient than IHC and minimizes the evaluation bias. It addresses complementary aspects of CTL (numbers/expansion, effector function and migratory function) and differentiates between patients with good and poor prognosis within the same histological tumor cohorts. The prognostic value of FHS will be confirmed by qPCR in our upcoming prospective study.
The number of TILs is a known prognostic biomarker in some cancers, including breast cancer and melanoma.1–3,35 The relationship between TILs and PD-1/PD-L1 expression has been reported in multiple types of cancer. PD-1 expression in lymphocytes correlates with PD-L1 expression in cancer cells in breast cancer and melanoma.36,37 The number of TILs correlates with PD-1 expression in lymphocyte and PD-L1 expression in cancer cells in breast cancer and melanoma,36,37 whereas it also inversely correlates with plasma PD-1 levels in melanoma.35 Combination of PD-L1 expression in cancer cells and the number of infiltrating CTLs predicts patient prognosis in gastric cancer.38 PD-1 positive T cell characteristics, including cytokine and chemokine productions, are different in the tumor (TIL) and in the peripheral blood in lung cancer.39 Positive stainings of PD-L1 in cancer cells and PD-1 in lymphocytes are associated with aggressive cancer biology, on the other hand, they are also associated with increased pathological complete response to the neoadjuvant chemotherapy in breast cancer.36
ICI is only effective in a small portion of TNBC patients. Although PD-1/PD-L1 expressions can be utilized as a prognostic biomarker and predict cancer aggressiveness in association with TIL levels as mentioned above, the predictive value of PD-L1 expression by IHC is limited because some PD-L1 negative patients still respond to PD-1 blockade.9 Similarly, the predictive value of individual CTL markers is not reliable, partially due to a huge variation and spatial heterogeneity within individual tumors.10 We believe that FHS may provide a way to overcome these challenges, to identify patients who respond to ICI treatment. Therefore, our follow-up study will evaluate the association of FHS with the response to immunotherapy.
TMB has been proposed as a key factor in the generation of immunogenic tumor-associated antigenic epitopes, acting as primary targets for CTLs in many types of tumors.40 Indeed, it has been shown that TMB and CD8+ T cell infiltration are correlated with each other in several types of cancers, including renal cell carcinoma, pancreatic, thyroid, skin and uterine cancers.41 TMB is also known to be associated with higher sensitivity of ICIs in breast cancer, which is thought to be due to enhanced anti-cancer immune response.42 Thus, it was of interest to investigate the relationship of our FHS with TMB. Unexpectedly, we found that the TMB, although it is a prognostic biomarker by itself, does not determine the FHS, which can be used independently in a complementary fashion to predict prognosis in both the high- and low-TMB patient cohorts. What was particularly striking was that even the low-TMB tumors showed improved prognosis, and with the combination of high FHS with high TMB showed an optimal prognostic value to identify a unique subset of TNBC patients with uniformly long survival.
This study has limitations. Since publicly available cohorts were analyzed using a bioinformatical approach alone, this is a retrospective study with its known biases. Future prospective studies to investigate the utility of qPCR to easily measure FHS and to investigate the association between FHS and outcome of immunotherapy are necessary to confirm the findings.
In summary, we demonstrated that high FHS based on the gene expression levels of CD8A, GZMB and CXCL10 predicts excellent long-term TNBC patient survival with enhanced anti-cancer immunity regardless of TMB. FHS constitutes an independent prognostic marker of survival that is particularly robust when combined with TMB in TNBCs.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211006680 for Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer by Eriko Katsuta, Li Yan, Mateusz Opyrchal, Pawel Kalinski and Kazuaki Takabe in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-2-tam-10.1177_17588359211006680 for Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer by Eriko Katsuta, Li Yan, Mateusz Opyrchal, Pawel Kalinski and Kazuaki Takabe in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-3-tam-10.1177_17588359211006680 for Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer by Eriko Katsuta, Li Yan, Mateusz Opyrchal, Pawel Kalinski and Kazuaki Takabe in Therapeutic Advances in Medical Oncology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant R01CA160688, R01CA250412, R37CA248018, as well as the Edward K. Duch Foundation and Paul & Helen Ellis Charitable Trust to KT, DOD grant W81XWH-19-1-0674 (BC180510) to PK and KT, and National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Comprehensive Cancer Center Bioinformatics and Biostatistics Shared Resources. KT is the Alfiero Foundation Chair of Breast Oncology at Roswell Park Comprehensive Cancer Center.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Eriko Katsuta, Breast Surgery, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Li Yan, Department of Biostatistics and Bioinformatics, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Mateusz Opyrchal, Department of Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA; Division of Oncology, Department of Internal Medicine, Washington University in Saint Louis, Saint Louis, MO, USA.
Pawel Kalinski, Department of Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Kazuaki Takabe, Breast Surgery, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Elm & Carlton Streets, Buffalo, NY 14263, USA; Department of Surgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo the State University of New York, Buffalo, NY, USA; Department of Breast Surgery and Oncology, Tokyo Medical University, Tokyo, Japan; Department of Surgery, Yokohama City University, Yokohama, Japan; Department of Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan; Department of Breast Surgery, Fukushima Medical University, Fukushima, Japan.
References
- 1. Pruneri G, Vingiani A, Denkert C. Tumor infiltrating lymphocytes in early breast cancer. Breast 2018; 37: 207–214. [DOI] [PubMed] [Google Scholar]
- 2. Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2016; 13: 228–241. [DOI] [PubMed] [Google Scholar]
- 3. Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 2019; 37: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med 2019; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas A, Routh ED, Pullikuth A, et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. Oncoimmunology 2018; 7: e1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 7. Caruso C. Early pembrolizumab ups TNBC responses. Cancer Discov 2019; 9: 1638. [Google Scholar]
- 8. Tokumaru Y, Joyce D, Takabe K. Current status and limitations of immunotherapy for breast cancer. Surgery 2020; 167: 628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res 2018; 24: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salgado R, Denkert C, Demaria S, et al. The evaluation of Tumor-Infiltrating Lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26: 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katsuta E, Rashid OM, Takabe K. Clinical relevance of tumor microenvironment: immune cells, vessels, and mouse models. Human Cell. Epub ahead of print 7 June 2020. DOI: 10.1007/s13577-020-00380-4. [DOI] [PubMed] [Google Scholar]
- 12. Muthuswamy R, Berk E, Junecko BF, et al. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res 2012; 72: 3735–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, Li F, Ping Y, et al. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget 2015; 6: 24978–24989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finn OJ. A believer’s overview of cancer immunosurveillance and immunotherapy. J Immunol 2018; 200: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finn OJ. Human tumor antigens yesterday, today, and tomorrow. Cancer Immunol Res 2017; 5: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinez-Usatorre A, Donda A, Zehn D, et al. PD-1 blockade unleashes effector potential of both high- and low-affinity tumor-infiltrating T cells. J Immunol 2018; 201: 792–803. [DOI] [PubMed] [Google Scholar]
- 17. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pereira B, Chin SF, Rueda OM, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016; 7: 11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siegel MB, He X, Hoadley KA, et al. Integrated RNA and DNA sequencing reveals early drivers of metastatic breast cancer. J Clin Invest 2018; 128: 1371–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsuta E, Yan L, Takeshita T, et al. High MYC mRNA expression is more clinically relevant than MYC DNA amplification in triple-negative breast cancer. Int J Mol Sci 2019; 21: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katsuta E, Rashid OM, Takabe K. Fibroblasts as a biological marker for curative resection in pancreatic ductal adenocarcinoma. Int J Mol Sci 2020; 21: 3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015; 12: 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charoentong P, Finotello F, Angelova M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep 2017; 18: 248–262. [DOI] [PubMed] [Google Scholar]
- 27. Liberzon A, Birger C, Thorvaldsdottir H, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015; 1: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katsuta E, Yan L, Nagahashi M, et al. Doxorubicin effect is enhanced by sphingosine-1-phosphate signaling antagonist in breast cancer. J Surg Res 2017; 219: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katsuta E, Maawy AA, Yan L, et al. High expression of bone morphogenetic protein (BMP) 6 and BMP7 are associated with higher immune cell infiltration and better survival in estrogen receptorpositive breast cancer. Oncol Rep 2019; 42: 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018; 19: 40–50. [DOI] [PubMed] [Google Scholar]
- 31. Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 2015; 1: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Enderlin M, Kleinmann EV, Struyf S, et al. TNF-α and the IFN-γ-inducible protein 10 (IP-10/CXCL-10) delivered by parvoviral vectors act in synergy to induce antitumor effects in mouse glioblastoma. Cancer Gene Ther 2009; 16: 149–160. [DOI] [PubMed] [Google Scholar]
- 33. Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer (review). Oncol Lett 2011; 2: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mlecnik B, Tosolini M, Charoentong P, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology 2010; 138: 1429–1440. [DOI] [PubMed] [Google Scholar]
- 35. Incorvaia L, Badalamenti G, Rinaldi G, et al. Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? Ther Adv Med Oncol 2019; 11: 1758835919848872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitano A, Ono M, Yoshida M, et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017; 2: e000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bai Y, Niu D, Huang X, et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn Pathol 2017; 12: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi E, Chang MS, Byeon SJ, et al. Prognostic perspectives of PD-L1 combined with tumor-infiltrating lymphocytes, Epstein-Barr virus, and microsatellite instability in gastric carcinomas. Diagn Pathol 2020; 15: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gu Y, Sheng SY, Tang YY, et al. PD-1 expression and function of T-cell subsets in TILs from human lung cancer. J Immunother 2019; 42: 297–308. [DOI] [PubMed] [Google Scholar]
- 40. Capietto AH, Jhunjhunwala S, Delamarre L. Characterizing neoantigens for personalized cancer immunotherapy. Curr Opin Immunol 2017; 46: 58–65. [DOI] [PubMed] [Google Scholar]
- 41. Varn FS, Wang Y, Mullins DW, et al. Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment. Cancer Res 2017; 77: 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valero C, Lee M, Hoen D, et al. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat Genet 2021; 53: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211006680 for Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer by Eriko Katsuta, Li Yan, Mateusz Opyrchal, Pawel Kalinski and Kazuaki Takabe in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-2-tam-10.1177_17588359211006680 for Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer by Eriko Katsuta, Li Yan, Mateusz Opyrchal, Pawel Kalinski and Kazuaki Takabe in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-3-tam-10.1177_17588359211006680 for Cytotoxic T-lymphocyte infiltration and chemokine predict long-term patient survival independently of tumor mutational burden in triple-negative breast cancer by Eriko Katsuta, Li Yan, Mateusz Opyrchal, Pawel Kalinski and Kazuaki Takabe in Therapeutic Advances in Medical Oncology