Abstract
Background:
Patients with adhesive capsulitis are evaluated for pain and progressive contracture of the glenohumeral joint. Whether endocrine, immune, or inflammatory processes are involved in its definite pathogenesis is still under debate. Some cross-sectional studies with a small sample size have noted that hyperlipidemia is a possible risk factor for frozen shoulders.
Purpose/Hypothesis:
The purpose was to conduct a longitudinal population-based study to investigate the risk of adhesive capsulitis among patients with hyperlipidemia. It was hypothesized that patients with hyperlipidemia would have a higher risk of adhesive capsulitis and that the use of statin drugs could reduce the rate.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Using data from the National Health Insurance Research Database (NHIRD) of Taiwan, the authors obtained the records of patients with hyperlipidemia who received a diagnosis between 2004 and 2005 and were followed up until the end of 2010. The control cohort comprised age- and sex-matched patients without hyperlipidemia. Propensity score matching was performed for the other comorbidities. A Cox multivariate proportional hazards model was applied to analyze the risk factors of adhesive capsulitis. The hazard ratio (HR) and adjusted HR were estimated between the study and control cohorts after adjustment for confounders. The effects of statin use on adhesive capsulitis risk were also analyzed.
Results:
The NHIRD records of 28,748 patients and 114,992 propensity score–matched controls were evaluated. A higher incidence rate of adhesive capsulitis was revealed in the hyperlipidemia cohort, with a crude HR of 1.70 (95% CI, 1.61-1.79; P < .001) and adjusted HR of 1.50 (95% CI, 1.41-1.59; P < .001). Patients with hyperlipidemia who used a statin still had higher crude and adjusted HRs compared with controls. Statin use did not exert protective effects on patients with hyperlipidemia.
Conclusion:
Patients with hyperlipidemia had a 1.5-fold higher risk of adhesive capsulitis than did healthy controls. Statin use did not provide protection against adhesive capsulitis in patients with hyperlipidemia.
Keywords: hyperlipidemia, adhesive capsulitis, frozen shoulder, population-based study
Patients with adhesive capsulitis are often evaluated for symptoms such as severe shoulder pain, progressive fibrosis, and contracture of the glenohumeral joint (resulting in limited range of motion).27 The prevalence of adhesive capsulitis is between 2% and 5%, and it is more common in women.30 The symptoms associated with adhesive capsulitis result in disability in patients and increased public health care expenditure. To date, the involvement of endocrine, immune, and inflammatory processes in its definite pathogenesis is still under debate.30 Studies have reported many risk factors for adhesive capsulitis, such as cervical disc discectomy, upper extremity trauma, thyroid disease, diabetes, Dupuytren contracture, breast cancer, and autoimmune disease.†† Moreover, patients diagnosed as having myocardial infarction and cerebrovascular disease have been reported to be at risk of adhesive capsulitis.24,25 Identifying possible risk factors is crucial for elucidating the pathogenesis of adhesive capsulitis.
Hyperlipidemia is a systemic metabolic disease characterized by abnormal blood lipid levels and has a well-known effect on the vascular system and internal organs.7 A recent study reported that patients with hyperlipidemia had a higher risk of rotator cuff diseases than did control patients without hyperlipidemia.20 In addition, a systematic review demonstrated an association between hyperlipidemia and rotator cuff disease.37 These 2 studies have mentioned that statin use can reduce the risk of rotator cuff disease and incidence of rotator cuff revision surgery.20,37
Bunker and Anthony5 noted that hyperlipidemia is a risk factor for adhesive capsulitis because of the similarity between the pathological findings of adhesive capsulitis and those of Dupuytren contracture (known to be associated with hyperlipidemia). In a subsequent study, Bunker and Esler6 reported higher serum cholesterol and triglyceride levels in patients with frozen shoulder than in healthy controls. However, most studies have used a cross-sectional design, data from a single medical service delivery system, and a relatively small sample size and have not investigated the time sequence of the causal relationship. Therefore, the implications of the findings of these studies may be limited.
To the best of our knowledge, large-scale epidemiological studies identifying hyperlipidemia as a risk factor for adhesive capsulitis are lacking. In addition, the effect of statin use on the risk of adhesive capsulitis among patients with hyperlipidemia remains unclear. The purpose of this research was therefore to conduct a longitudinal population-based study to investigate the risk of adhesive capsulitis among patients with hyperlipidemia with or without statin use. Our hypothesis was that patients with hyperlipidemia have a higher risk of adhesive capsulitis and the use of statin drugs can reduce the rate in these patients.
Methods
Study Database
This retrospective population-based cohort study used data from the National Health Insurance (NHI) Research Database (NHIRD), managed by the National Health Research Institute of Taiwan. Since the NHI program was introduced in Taiwan in 1995, the National Health Research Institute has managed the medical benefit claims of all 22.9 million of its residents, covering >99% of the population. The completeness and accuracy of the NHIRD is guaranteed by the Department of Health and the NHI Bureau, and the insurance authority releases insured medical records as deidentified secondary data to the public for research purposes.
The data for this study were obtained from the Longitudinal Health Insurance Database 2005 (LHID2005), a subset of the NHIRD. The LHID2005 contains the complete original claims data of 1 million insured individuals who were randomly sampled from the NHIRD registry in 2005. Until the end of 2010, all sampled individuals were followed up for outcome identification by using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). However, detailed outcomes and examination reports cannot be obtained from this database. The data of all insured individuals are deidentified in the LHID2005. Institutional review board approval was received for this study.
Study Group Selection
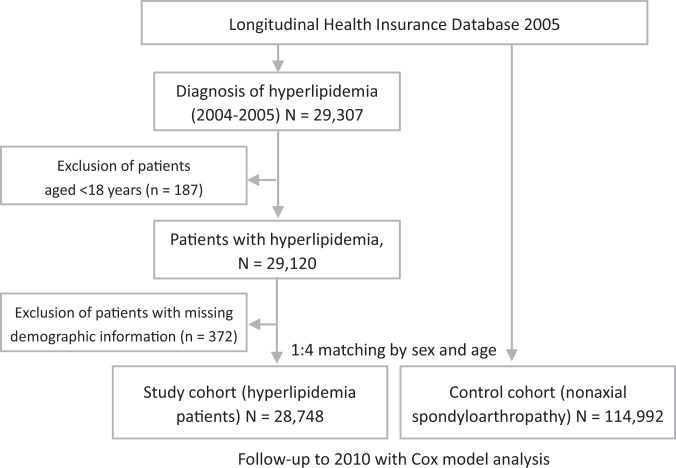
The study cohort included all patients who received a major diagnosis of hyperlipidemia (ICD-9-CM codes 272.0-272.4) between January 1, 2004, and December 31, 2005, according to ambulatory medical visit data. To improve the accuracy of diagnosis, only those who received a diagnosis of hyperlipidemia in at least 2 consecutive outpatient visits were included in the analysis. Excluded were patients with a diagnosis of adhesive capsulitis (ICD-9-CM code 726.0) before a diagnosis of hyperlipidemia, whose records were missing data such as date of birth and sex, and who were younger than 18 years. A total of 28,748 patients with hyperlipidemia met the inclusion criteria and were included in the study (Figure 1). By age group (≤30, 31-40, 41-50, 51-60, 61-70, and >70 years) and sex, patients in the control group (n = 114,992; all without hyperlipidemia) were matched with patients in the study cohort (4 control patients per 1 study patient); this matching was performed by using the remaining patient records in the LHID.
Figure 1.
Study flowchart.
Patient Variables and Comorbidities
In LHID2005, the patient variables of age, sex, urbanization level, and prescription of statin (simvastatin, lovastatin, atorvastatin, fluvastatin, pravastatin, and rosuvastatin; used for >28 days) were obtained for analysis. The comorbidities were diabetes mellitus (ICD-9-CM codes 250 and 251), hypertension (codes 401-405), coronary heart disease (codes 410 and 412), thyroid disorder (codes 240-246), gout (code 274), stroke (codes 430-438), and autoimmune disease (rheumatoid arthritis and systemic lupus erythematosus; codes 714.0 and 710.0). The endpoint of follow-up was determined to be the occurrence of adhesive capsulitis (adhesive capsulitis of the shoulder; code 726.0) from the index date to the endpoint or until December 31, 2010, whichever came first, and the final date observations were censored observations.
Statistical Analysis
We used the Pearson chi-square test to analyze patient variables and comorbidities in the study and control groups. To analyze the risk and incidence of adhesive capsulitis between these 2 groups, we used the Cox model after adjusting for possible confounding factors. In addition, the effect of medication for hyperlipidemia, such as statins, and adjusted hazard ratios (HRs) were analyzed in this model. Moreover, the effect of statin use on the risk of adhesive capsulitis was analyzed in the study cohort during the follow-up period, and the results are presented using Kaplan-Meier hazard curves. All data analyses were performed using the Stata package (Version 11; StataCorp) and SAS statistical package (Version 9.1.3; SAS Institute). A P value <.05 was considered statistically significant.
Results
In both cohorts, 45.1% of the patients were men, and the prevalence of comorbidities such as diabetes mellitus, coronary heart disease, hypertension, stroke, chronic obstructive pulmonary disease, autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus), thyroid disease, and gout was higher in the hyperlipidemia cohort than in the control cohort (Tables 1 and 2).
Table 1.
Characteristics of Age- and Sex-Matched Patients in the Study Cohortsa
| Characteristic | Nonhyperlipidemia Cohort (n = 114,992) | Hyperlipidemia Cohort (n = 28,748) | P Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, y | >.999 | ||||
| 18-30 | 4904 | 4.3 | 1226 | 4.3 | |
| 31-40 | 11,920 | 10.4 | 2980 | 10.4 | |
| 41-50 | 25,868 | 22.5 | 6467 | 22.5 | |
| 51-60 | 31,680 | 27.5 | 7920 | 27.5 | |
| 61-70 | 24,280 | 21.1 | 6070 | 21.1 | |
| >70 | 16,340 | 14.2 | 4085 | 14.2 | |
| Sex | >.999 | ||||
| Male | 51,808 | 45.1 | 12,952 | 45.1 | |
| Female | 63,184 | 54.9 | 15,796 | 54.9 | |
| Urbanization level | <.001 | ||||
| Urban | 39,838 | 34.6 | 17,440 | 60.7 | |
| Suburban | 20,839 | 18.1 | 7771 | 27.0 | |
| Rural | 54,315 | 47.2 | 3537 | 12.3 | |
aBoldface P value indicates statistically significant between-group differences (P < .05).
Table 2.
Comorbidities for Age- and Sex-Matched Patients in the Study Cohortsa
| Comorbid Medical Disorder | Nonhyperlipidemia Cohort (n = 114,992) | Hyperlipidemia Cohort (n = 28,748) | P Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Diabetes mellitus | .007 | ||||
| Yes | 19,535 | 17.0 | 9761 | 34.0 | |
| No | 95,457 | 83.0 | 18,987 | 66.0 | |
| Coronary heart disease | <.001 | ||||
| Yes | 17,995 | 15.6 | 8123 | 28.3 | |
| No | 96,997 | 84.4 | 20,625 | 71.7 | |
| Hypertension | <.001 | ||||
| Yes | 41,956 | 36.5 | 15,555 | 54.1 | |
| No | 73,036 | 63.5 | 13,193 | 45.9 | |
| Stroke | <.001 | ||||
| Yes | 12,208 | 10.6 | 4038 | 14.0 | |
| No | 102,784 | 89.4 | 24,712 | 86.0 | |
| COPD | <.001 | ||||
| Yes | 25,423 | 22.1 | 8499 | 29.6 | |
| No | 89,569 | 77.9 | 20,249 | 70.4 | |
| Autoimmune disease (RA, SLE) | <.001 | ||||
| Yes | 4168 | 3.6 | 1620 | 5.6 | |
| No | 110,824 | 96.4 | 27,128 | 94.4 | |
| Thyroid disorder | <.001 | ||||
| Yes | 3515 | 3.1 | 1539 | 5.4 | |
| No | 111,477 | 96.9 | 27,209 | 94.6 | |
| Gout | <.001 | ||||
| Yes | 8541 | 7.4 | 7576 | 26.4 | |
| No | 106,451 | 92.6 | 21,172 | 73.6 | |
aBoldface P values indicate statistically significant between-group differences (P < .05). COPD, chronic obstructive pulmonary disease; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
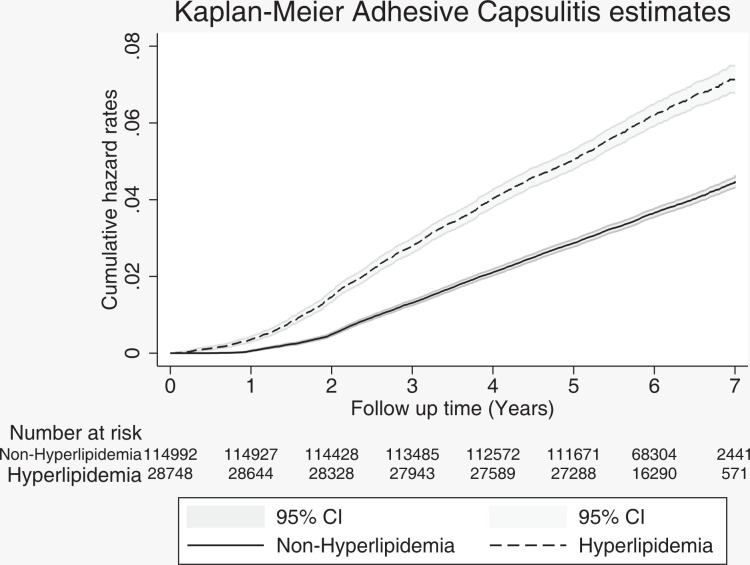
The incidence of adhesive capsulitis was 631 per 100,000 person-years in the control cohort and 1057 per 100,000 person-years in the hyperlipidemia cohort. The crude HR was 1.70 (95% CI, 1.61-1.79), and the adjusted HR was 1.50 (95% CI, 1.41-1.59) for the risk of adhesive capsulitis in the study cohort compared with the control cohort (P < .001 for both) (Table 3). Figure 2 presents the Kaplan-Meier hazard curves for the risk of adhesive capsulitis in the hyperlipidemia and control cohorts during the 7-year follow-up period.
Table 3.
Incidence and Hazard Ratios for Adhesive Capsulitis Between the Study Cohorts During the 7-Year Follow-upa
| Presence of Adhesive Capsulitis | Nonhyperlipidemia Cohort (n = 114,992) | Hyperlipidemia Cohort (n = 28,748) |
|---|---|---|
| Patients with adhesive capsulitis | 4579 | 1813 |
| Person-years | 725,763 | 171,480 |
| Incidence per 100,000 person-years | 631 | 1057 |
| Crude HR (95% CI) | 1.00 | 1.70 (1.61-1.79)b |
| Adjusted HR (95% CI) | 1.00 | 1.50 (1.41-1.59)b |
aAdjusted for patient age, sex, urbanization level, and diagnosis of autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus), diabetes mellitus, hypertension, coronary heart disease, stroke, thyroid, gout, and chronic obstructive pulmonary disease. HR, hazard ratio.
bP < .001 for risk of adhesive capsulitis compared with the nonhyperlipidemia cohort.
Figure 2.
Kaplan-Meier hazard curves for adhesive capsulitis between the hyperlipidemia and nonhyperlipidemia cohorts during the 7-year follow-up.
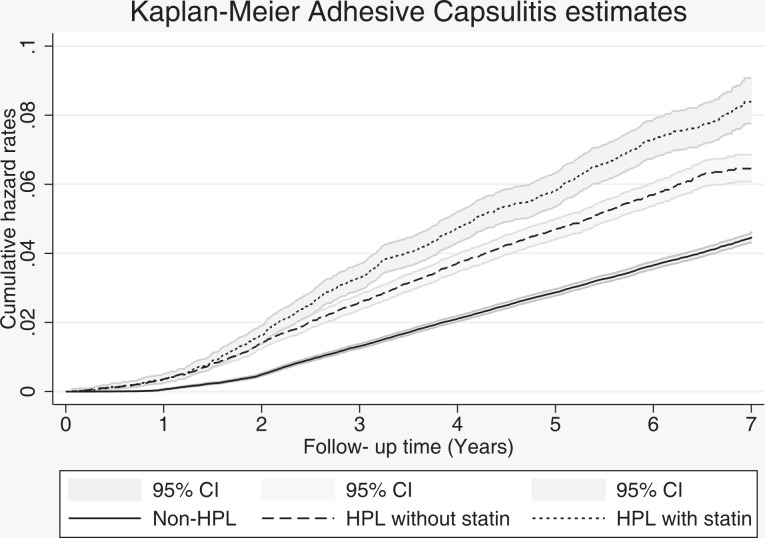
When comparing groups by statin use, the crude and adjusted HRs were 1.56 (95% CI, 1.47-1.67) and 1.46 (95% CI, 1.37-1.57), respectively, in the hyperlipidemia cohort without statin and the control cohort, and they were 1.98 (95% CI, 1.83-2.15) and 1.56 (95% CI, 1.43-1.70), respectively, in the hyperlipidemia cohort with statin and the control cohort (P < .001 for all) (Table 4). Figure 3 presents the Kaplan-Meier hazard curves of the risk of adhesive capsulitis among patients with hyperlipidemia without statin use, patients with hyperlipidemia with statin use, and patients without hyperlipidemia during the 7-year follow-up period.
Table 4.
Hazard Ratios for Adhesive Capsulitis by Statin Usea
| Presence of Adhesive Capsulitis | Nonhyperlipidemia Cohort | Hyperlipidemia Cohortb | |
|---|---|---|---|
| No Statin Use | Statin Use | ||
| Crude HR (95% CI) | 1.00 | 1.56 (1.47-1.67) | 1.98 (1.83-2.15) |
| Adjusted HR (95% CI) | 1.00 | 1.46 (1.37-1.57) | 1.56 (1.43-1.70) |
aAdjusted for patient age, sex, urbanization level, and diagnosis of autoimmune disease (rheumatoid arthritis, systemic lupus erythematosus), diabetes mellitus, hypertension, coronary heart disease, stroke, thyroid, gout, and chronic obstructive pulmonary disease. HR, hazard ratio.
bP < .001 for risk of adhesive capsulitis compared with the nonhyperlipidemia cohort.
Figure 3.
Kaplan-Meier hazard curves for adhesive capsulitis between the hyperlipidemia cohort with and the cohort without statin use and the nonhyperlipidemia cohort during the 7-year follow-up. HPL, hyperlipidemia.
Discussion
This large-scale, longitudinal, population-based, real-world study revealed that hyperlipidemia was a risk factor for adhesive capsulitis. To date, evidence regarding the association between hyperlipidemia and adhesive capsulitis has been limited. Our study provides evidence that patients with hyperlipidemia are at higher risk of adhesive capsulitis than are those without hyperlipidemia. Our results further show that patients with hyperlipidemia who use a statin to control their lipid profile are still at risk of adhesive capsulitis. Identifying the possible pathogenesis and association between hyperlipidemia and adhesive capsulitis could help improve the quality of care for patients with hyperlipidemia.
The main causes of adhesive capsulitis of the shoulder are inflammatory contracture of the shoulder capsule and accumulation of inflammatory cytokines.11 Kim et al16 noted that the level of intercellular adhesion molecule-1 (ICAM-1) was significantly greater in capsular tissue from the glenohumeral joint of patients with adhesive capsulitis. Synoviocytes treated using ICAM-1 will express more cytokines, such as transforming growth factor beta 1 (TGF-β1) and connective tissue growth factor.16 Increased expression of TGF-β1 and platelet-derived growth factor has been found in adhesive capsulitis capsule tissue. This fibrous inducing factor (TGF-β1 and platelet-derived growth factor) contributes to shoulder synovitis, leading to fibrous reactions.31
Several studies have reported an association between hyperlipidemia and adhesive capsulitis. Bunker and Esler6 observed significant increases in fasting serum triglyceride and cholesterol levels in patients with shoulder adhesive capsulitis. In a study conducted by Hand et al,12 17% of patients with primary shoulder inflammation had hypercholesterolemia, which was designated as a risk factor. A longitudinal study that used Taiwan’s NHI data without determining the lipid profile of each patient reported that diabetes with accompanying hyperlipidemia is an independent risk factor for adhesive capsulitis.21 Sung et al34 indicated that hypercholesterolemia and inflammatory lipoproteinemia, especially high levels of low-density lipoprotein (LDL) and non–high-density lipoprotein (non-HDL) cholesterol, were significantly associated with adhesive capsulitis. LDL is prone to oxidative modification by reactive oxygen species, resulting in oxidized LDL, which can induce endothelial cell activation in vessel walls.35 The endothelial cell activation leads to the expression of ICAM-1 and vascular cell adhesion molecule-1, which attract inflammatory cells and inflammation-related cytokines.14 On the other hand, ICAM-1 also plays an important role in the molecular mechanisms of developing adhesive capsulitis.
A relationship between abnormal lipid metabolism and rotator cuff tear has been identified in the literature. Patients with rotator cuff tear have high blood cholesterol levels.1,2 Animal studies have indicated that a high-cholesterol diet has adverse effects on rotator cuff healing3,9 and results in more severe lipid infiltration; treating hypercholesterolemia using statins can alleviate this condition.9
According to a population-based analysis of the Taiwan NHI database, diabetes and hyperlipidemia are risk factors for rotator cuff disease.20 Statin use might provide protection against rotator cuff disease in patients with hyperlipidemia.20 However, according to the present analysis, although patients with hyperlipidemia had a higher risk of adhesive capsulitis, those who used statins to treat hyperlipidemia did not have a lower risk of adhesive capsulitis than did those who did not use statins. By contrast, patients using statins were more likely to develop adhesive capsulitis than were patients not using statins. We believe that this finding may be related to the current treatment guidelines for hyperlipidemia.18,19 Generally, hyperlipidemia is defined as an LDL level >100. People with hyperlipidemia but without risk factors (hypertension, men ≥45 years old, women ≥55 years old, a family history of early-onset coronary heart disease, HDL level <40 mg/dL, and smoking habit) require treatment if they exhibit an LDL level >190. Patients with multiple risk factors require treatment when they exhibit an LDL level >130. In addition, patients with coronary atherosclerosis, such as cardiovascular disease, cardiac catheterization, or coronary artery bypass surgery, require treatment when they exhibit an LDL level >70. These results indicate that people using statins have more severe hyperlipidemia, greater risk factors, and obvious comorbidities. This may explain why patients with hyperlipidemia receiving statin treatment were at higher risk of adhesive capsulitis, or there is a possibility that statin drugs had a direct adverse result toward developing adhesive capsulitis. However, there is no current literature discussing the relationship between statin use and adhesive capsulitis. Statin drugs have a side effect of statin-related myopathy. The incidence is 7% to 29%.33 There are several clinicopathological phenotypes including any degree of benign myalgia or self-limited toxic myopathy that improves with drug withdrawal up to the more severe and even life-threatening forms such as rhabdomyolysis or immune-mediated necrotizing myopathy.4 The exact pathophysiology of statin myopathy is not fully known. One theory is that statins inhibit the synthesis of mevalonate, a precursor of both cholesterol and coenzyme Q10 (CoQ10), and that the statin-induced CoQ10 deficiency is involved in the pathogenesis of statin myopathy.22 CoQ10 is a vitamin-like organic compound widely expressed in humans; it plays a key role in electron transport in oxidative phosphorylation of mitochondria.10 The depletion of CoQ10 in myocyte mitochondria may disturb cellular respiration and consequently cause muscle-related toxicities, including rhabdomyolysis.29 Several CoQ10 supplementation clinical trials investigating the effects of CoQ10 supplementation in heart failure have been conducted. A significant enhancement in serum HDL and decreased ICAM-1 and interleukin 6 in serum were noted in the CoQ10 supplementation group.26 As mentioned, ICAM-1 also has an important role in the develop of adhesive capsulitis. This can possibly explain why patients with hyperlipidemia taking statins will have a lower CoQ10 level, which results in increased ICAM-1 and the development of adhesive capsulitis.
This research has several possible limitations. First, this analysis used ICD-9-CM codes in the LHID2005 to determine diagnosis of adhesive capsulitis and hyperlipidemia. However, the accuracy of diagnoses in the database could not be confirmed. To enhance diagnostic accuracy, the NHI has established several verification committees that regularly review medical records and verify the accuracy of the diagnostic codes. In addition, we used only consecutively coded cases to avoid using inaccurate codes in the database records. These methods might have improved the accuracy of the registered diagnoses of adhesive capsulitis and hyperlipidemia. Second, our results were based on a retrospective cohort study. Information regarding possible confounding factors such as lifestyle, obesity, cigarette smoking, and alcohol consumption could not be obtained from the administrative database. Also, patients in our hyperlipidemia cohort had significantly more underlying disease processes compared with the control group. This may have added to the variance in results between cohorts. Third, the severity of hyperlipidemia and laboratory lipid profile data could not be obtained from the LHID2005 database. Therefore, future prospective studies stratified by the severity of hyperlipidemia and risk of adhesive capsulitis are recommended.
Conclusion
The results of our 7-year, longitudinal, population-based, case-control cohort study revealed that patients with hyperlipidemia had a 1.5-fold higher risk of adhesive capsulitis than did those without hyperlipidemia. Statin use did not provide protection against adhesive capsulitis in patients with hyperlipidemia.
Footnotes
Final revision submitted August 21, 2020; accepted September 30, 2020.
The authors declared that they have are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Taipei (ref No. IRB-2018-007).
References
- 1. Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ. Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res. 2011;29(3):380–383. [DOI] [PubMed] [Google Scholar]
- 3. Beason DP, Tucker JJ, Lee CS, Edelstein L, Abboud JA, Soslowsky LJ. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elbow Surg. 2014;23(6):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–414. [DOI] [PubMed] [Google Scholar]
- 5. Bunker TD, Anthony PP. The pathology of frozen shoulder: a Dupuytren-like disease. J Bone Joint Surg Br. 1995;77(5):677–683. [PubMed] [Google Scholar]
- 6. Bunker TD, Esler CN. Frozen shoulder and lipids. J Bone Joint Surg Br. 1995;77(5):684–686. [PubMed] [Google Scholar]
- 7. Chen YQ, Zhao SP, Zhao YH. Efficacy and tolerability of coenzyme A vs pantethine for the treatment of patients with hyperlipidemia: a randomized, double-blind, multicenter study. J Clin Lipidol. 2015;9(5):692–697. [DOI] [PubMed] [Google Scholar]
- 8. Cheville AL, Tchou J. Barriers to rehabilitation following surgery for primary breast cancer. J Surg Oncol. 2007;95(5):409–418. [DOI] [PubMed] [Google Scholar]
- 9. Chung SW, Park H, Kwon J, Choe GY, Kim SH, Oh JH. Effect of hypercholesterolemia on fatty infiltration and quality of tendon-to-bone healing in a rabbit model of a chronic rotator cuff tear: electrophysiological, biomechanical, and histological analyses. Am J Sports Med. 2016;44(5):1153–1164. [DOI] [PubMed] [Google Scholar]
- 10. Di Lorenzo A, Iannuzzo G, Parlato A, et al. Clinical evidence for Q10 coenzyme supplementation in heart failure: from energetics to functional improvement. J Clin Med. 2020;9(5):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Georgiannos D, Markopoulos G, Devetzi E, Bisbinas I. Adhesive capsulitis of the shoulder: is there consensus regarding the treatment? A comprehensive review. Open Orthop J. 2017;11:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hand C, Clipsham K, Rees JL, Carr AJ. Long-term outcome of frozen shoulder. J Shoulder Elbow Surg. 2008;17(2):231–236. [DOI] [PubMed] [Google Scholar]
- 13. Huang SW, Lin JW, Wang WT, Wu CW, Liou TH, Lin HW. Hyperthyroidism is a risk factor for developing adhesive capsulitis of the shoulder: a nationwide longitudinal population-based study. Sci Rep. 2014;4:4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96(12):4219–4225. [DOI] [PubMed] [Google Scholar]
- 15. Kang JH, Lin HC, Tsai MC, Chung SD. Increased risk for adhesive capsulitis of the shoulder following cervical disc surgery. Sci Rep. 2016;6:26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim YS, Kim JM, Lee YG, Hong OK, Kwon HS, Ji JH. Intercellular adhesion molecule-1 (ICAM-1, CD54) is increased in adhesive capsulitis. J Bone Joint Surg Am. 2013;95(4):e181–e188. [DOI] [PubMed] [Google Scholar]
- 17. Lancaster ST, Grove TN, Woods DA. Management of post-traumatic stiffness of the shoulder following upper limb trauma with manipulation under anaesthetic. Shoulder Elbow. 2017;9(4):258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li YH, Chao TH, Liu PY, Ueng KC, Yeh HI. Lipid lowering therapy for acute coronary syndrome and coronary artery disease: highlights of the 2017 Taiwan lipid guidelines for high risk patients. Acta Cardiol Sin. 2018;34(5):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li YH, Ueng KC, Jeng JS, et al. 2017 Taiwan lipid guidelines for high risk patients. J Formos Med Assoc. 2017;116(4):217–248. [DOI] [PubMed] [Google Scholar]
- 20. Lin TT, Lin CH, Chang CL, Chi CH, Chang ST, Sheu WH. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med. 2015;43(9):2126–2132. [DOI] [PubMed] [Google Scholar]
- 21. Lo SF, Chu SW, Muo CH, et al. Diabetes mellitus and accompanying hyperlipidemia are independent risk factors for adhesive capsulitis: a nationwide population-based cohort study (version 2). Rheumatol Int. 2014;34(1):67–74. [DOI] [PubMed] [Google Scholar]
- 22. Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49(23):2231–2237. [DOI] [PubMed] [Google Scholar]
- 23. Massoud SN, Pearse EO, Levy O, Copeland SA. Operative management of the frozen shoulder in patients with diabetes. J Shoulder Elbow Surg. 2002;11(6):609–613. [DOI] [PubMed] [Google Scholar]
- 24. Miller MD, Wirth MA, Rockwood CA, Jr. Thawing the frozen shoulder: the “patient” patient. Orthopedics. 1996;19(10):849–853. [DOI] [PubMed] [Google Scholar]
- 25. Minter WT III. The shoulder-hand syndrome in coronary disease. J Med Assoc Ga. 1967;56(2):45–49. [PubMed] [Google Scholar]
- 26. Mohseni M, Vafa M, Zarrati M, Shidfar F, Hajimiresmail SJ, Rahimi Forushani A. Beneficial effects of coenzyme Q10 supplementation on lipid profile and intereukin-6 and intercellular adhesion molecule-1 reduction, preliminary results of a double-blind trial in acute myocardial infarction. Int J Prev Med. 2015;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neviaser AS, Hannafin JA. Adhesive capsulitis: a review of current treatment. Am J Sports Med. 2010;38(11):2346–2356. [DOI] [PubMed] [Google Scholar]
- 28. Ogilvie-Harris DJ, Myerthall S. The diabetic frozen shoulder: arthroscopic release. Arthroscopy. 1997;13(1):1–8. [DOI] [PubMed] [Google Scholar]
- 29. Owczarek J, Jasińska M, Orszulak-Michalak D. Drug-induced myopathies: an overview of the possible mechanisms. Pharmacol Rep. 2005;57(1):23–34. [PubMed] [Google Scholar]
- 30. Redler LH, Dennis ER. Treatment of adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 2019;27(12):e544–e554. [DOI] [PubMed] [Google Scholar]
- 31. Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997;15(3):427–436. [DOI] [PubMed] [Google Scholar]
- 32. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10(2):149–151. [DOI] [PubMed] [Google Scholar]
- 33. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sung CM, Jung TS, Park HB. Are serum lipids involved in primary frozen shoulder? A case-control study. J Bone Joint Surg Am. 2014;96(21):1828–1833. [DOI] [PubMed] [Google Scholar]
- 35. van Diepen JA, Berbée JF, Havekes LM, Rensen PC. Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis. 2013;228(2):306–315. [DOI] [PubMed] [Google Scholar]
- 36. Wohlgethan JR. Frozen shoulder in hyperthyroidism. Arthritis Rheum. 1987;30(8):936–939. [DOI] [PubMed] [Google Scholar]
- 37. Yang Y, Qu J. The effects of hyperlipidemia on rotator cuff diseases: a systematic review. J Orthop Surg Res. 2018;13(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zreik NH, Malik RA, Charalambous CP. Adhesive capsulitis of the shoulder and diabetes: a meta-analysis of prevalence. Muscles Ligaments Tendons J. 2016;6(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]