Abstract
Holmes tremor (HT) is a rare symptomatic movement disorder characterized by a combination of resting, postural, and action tremors. HT is usually caused by lesions in the brain stem, thalamus, and cerebellum, and the pathogenesis is believed to be related to the nigrostriatal pathway and/or the cerebello–thalamo–cortical pathway. Many medications have been used to treat HT with various degrees of effectiveness. We herein present a case involving an elderly woman who developed atypical HT 23 months after cerebral hemorrhage. The atypical HT manifested as a tremor of the right limb with involuntary flexion of the distal five fingers of the right upper limb. Imaging findings suggested the existence of an old hemorrhage in the left thalamus. Specifically, diffusion tensor imaging data of the whole brain and multimodal three-dimensional medical imaging revealed significant white matter microstructural changes in the centromedian nucleus of the left thalamus. Treatment with high-dose oral levodopa was not efficient, but the symptoms gradually decreased in severity and disappeared 1 month after switching to oral clonazepam treatment.
Keywords: Holmes tremor, central nucleus, clonazepam, atypical, cerebral hemorrhage, case report
Introduction
Holmes tremor (HT) is an irregular, slow-frequency (<4.5 Hz) tremor that is predominantly unilateral and often occurs in the upper limbs with occasional involvement of the lower limbs. Lesions in the brain stem, thalamus, and cerebellum are associated with different etiologies of HT, but all ultimately lead to development of the disease. The differential effects according to lesion location include ischemic or hemorrhagic cerebrovascular disorders, bleeding secondary to vascular malformations, demyelination, and infection. The pathogenesis of HT is generally believed to be related to the nigrostriatal pathway and/or the cerebello–thalamo–cortical pathway. We herein report a case involving an elderly woman with right limb tremor caused by white matter microstructural damage after hemorrhage in the centromedian nucleus of the left thalamus. In addition, atypical HT manifested as a tremor of the right limb with involuntary flexion of the distal five fingers of the right upper limb. These symptoms gradually decreased in severity and disappeared after the patient began treatment with oral clonazepam.
Case report
At 29 months prior to our consultation, a 73-year-old woman presented with left thalamic hemorrhage (bleeding volume of about 10 mL) and numbness of the right limb with no signs of limb movement disorder. The limb numbness disappeared after treatment for cerebrovascular disease. About 6 months prior to our consultation, she suddenly developed a tremor of the proximal and distal ends of the right upper limbs as well as the proximal lower limbs. The tremor was irregular, high in amplitude, and slow in frequency. We analyzed the patient’s tremors by electromyography and found that the peak frequency of the tremor was 4.2 Hz in the resting state, 4.0 Hz in the posture state, 4.2 Hz in the intention state, and 4.5 Hz in the holding 1000 g state. The half-width power of the tremor was 60.84 mg2/μV2 in the resting state, 135.79 mg2/μV2 in the posture state, 112.87 mg2/μV2 in the intention state, and 280.46 mg2/μV2 in the 1000 g state. The patient showed synchronous contraction of the flexors and extensors in the resting state and alternate contractions in the remaining states. In addition, the involuntary flexion of the five fingers at the distal end of the right upper limb was consistent with the rhythm of the proximal tremor of the right upper limb. This movement did not show oscillation or rhythm, and it persisted during posture, rest, and movement. There was no obvious aggravation when trying to maintain a normal posture, nor was there aggravation due to emotional tension, mental stimulation, or voluntary exercise. Moreover, no further change in muscle tension from high to low occurred during involuntary movement. We also performed an electromyographic examination and found no symptoms consistent with myoclonus (Video 1). Overall, the tremor presented most of the time during rest, posture, and action; worsened when attempts were made to inhibit it; and disappeared during sleep. No associated rigidity or bradykinesia was observed, and laboratory parameters showed no abnormalities. The patient had a >2-year history of hypertension, with blood pressure controlled in the normal range by regular treatment with antihypertensive drugs. Magnetic resonance imaging (Figure 1, Video 2) revealed an old hemorrhage in the left thalamus. Diffusion tensor imaging of the whole brain and multimodal three-dimensional medical imaging showed significant white matter microstructural changes in the centromedian nucleus of the left thalamus. After the patient failed to respond to a high dose of oral levodopa (250 mg four times daily) for 3 months, the treatment regimen was changed to oral clonazepam (2 mg once daily). After this treatment change, her symptoms gradually decreased in severity and finally disappeared after continuous oral administration for 1 month. At the 3-month follow-up, the patient’s symptoms disappeared without recurrence.
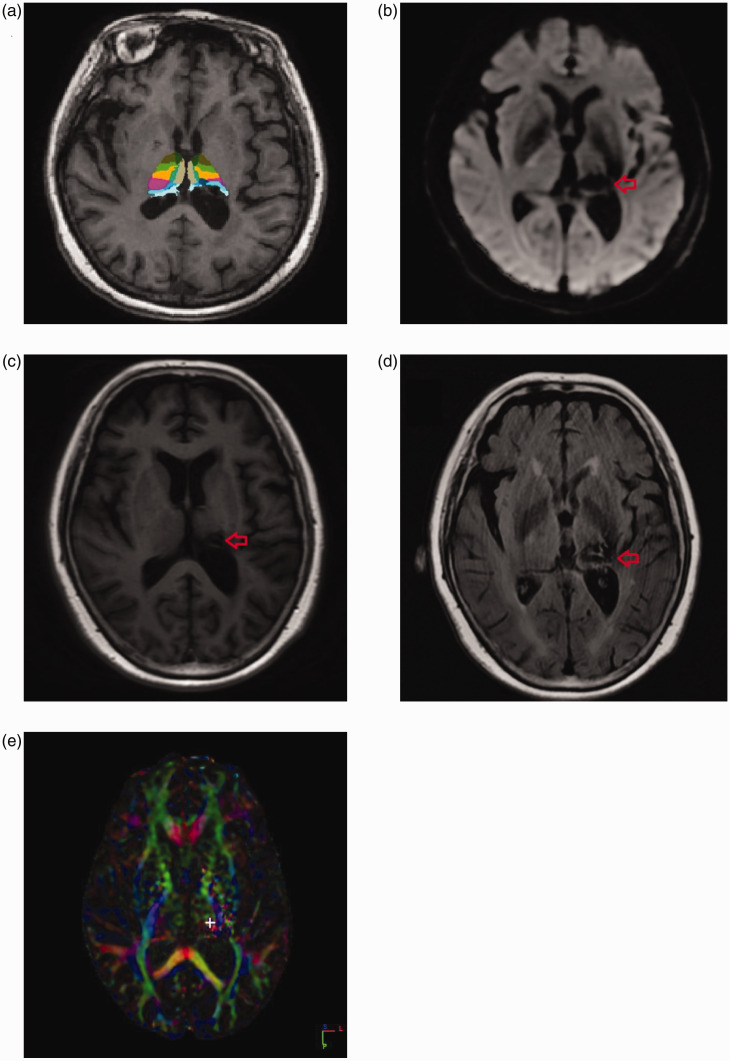
Figure 1.
(a) FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) was used to analyze the brain imaging data. The software showed that the central nucleus of the thalamus was damaged (red arrow). (b) A diffusion-weighted imaging sequence revealed an old hemorrhage in the left thalamus (red arrow). (c) A magnetic resonance imaging T1-weighted pulse sequence also showed the old hemorrhage in the left thalamus (red arrow). (d) A magnetic resonance imaging T2-weighted pulse sequence also showed the old hemorrhage in the left thalamus (red arrow). (E) Whole-brain diffusion tensor imaging showed that the FA value of the left central thalamic nucleus was 0.0378 (white cross symbol), whereas the FA value of the right central thalamic nucleus was 0.2345.
Discussion
HT is a symptomatic, low-frequency (<4.5 Hz) tremor that predominantly affects the proximal limbs, upper limbs, and occasionally the lower limbs.1–3 However, a tremor of the right limb with involuntary flexion of the distal five fingers of the right upper limb is not mentioned in the current literature. A delay of 4 weeks to 2 years is commonly observed between lesion onset and the occurrence of a tremor and may be related to nerve remodeling.1,4 The pathogenesis of HT is believed to be related to the nigrostriatal pathway and/or the cerebello–thalamo–cortical pathway.5 According to the mechanism of action of the nigrostriatal pathway, the patient in the current case was treated with a high dose of oral levodopa for 3 months, which was ineffective. Dopaminergic medication is a first-line treatment for HT, but the nigrostriatal tract was not a key part of the HT circuit in our patient.6 Current research shows that involvement of the substantia nigra and striatum is not necessary for the occurrence of HT.7 Specifically, many patients with HT have normal dopamine levels as measured via neuroimaging, and only half of such patients respond to dopaminergic medication.4,8
The treatment regimen in the present case was changed to oral clonazepam, upon which the patient’s symptoms disappeared, and they had not recurred at the 3-month follow-up. Whole-brain diffusion tensor imaging and multimodal three-dimensional medical imaging suggested the presence of significant white matter microstructural damage in the centromedian nucleus of the left thalamus. Based on the relevant literature, the centromedian nucleus is the largest nucleus among the intralaminar nuclei of the thalamus and is the primary nonspecific projection of the thalamus. The afferent impulses of the intralaminar nuclei originate from the ascending fibers of the brain stem reticular formation, the emboliform nuclei of the cerebellum, and the medial globus pallidus and other thalamic nuclei. Instead of projecting to the cerebral cortex, the centromedian nucleus projects to the caudate nucleus, putamen, and globus pallidus and may also diffuse to many thalamic nuclei, which in turn transmit impulses to secondary centers in the cerebral cortex.6 In summary, the centromedian nucleus of the left thalamus connects not only to the cerebellum but also to the cerebral cortex. Joutsa et al.9 found that the damage that causes HT is not limited to any single brain area. Rather, such damage is located in a functionally connected brain circuit that includes eight specific brain areas: the red nucleus, globus pallidus interna, ventral oralis posterior and pulvinar nuclei of the thalamus, pontomedullary junction, and three regions in the cerebellum. This circuit may therefore have therapeutic significance for HT9 and coincides with the imaging evidence observed in this case. Thus, we speculate that the centromedian nucleus is the main functional thalamic nucleus in the cerebello–thalamo–cortical pathway. The destruction of white matter fibers in the centromedian nucleus will cause dysfunction of this cerebello–thalamo–cortical pathway, which may in turn cause HT. In our patient, remission was likely due to treatment with clonazepam. We speculate that the mechanism of action of clonazepam may be related to the cerebello–thalamo–cortical pathway.
Supplementary Material
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Huan Zhao https://orcid.org/0000-0002-7459-6922
Xuhong Yang https://orcid.org/0000-0002-0702-3075
Ethics
This study was approved by the Ethical Review Committee of the Hospital of Chengdu University of Traditional Chinese Medicine (Chengdu, China) (approval no. 2019KL-061). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent to participate in this study was provided by the patient. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Samie MR, Selhorst JB, Koller WC. Post-traumatic midbrain tremors. Neurology 1990; 40: 62–66. [DOI] [PubMed] [Google Scholar]
- 2.Liu ZQ, Wan ZR, Jia XT, et al. [Clinical features and short-term prognosis of Holmes' tremor]. Zhonghua Yi Xue Za Zhi 2019; 99: 801–805. [DOI] [PubMed] [Google Scholar]
- 3.Holmes G. On certain tremors in organic cerebral lesions. Brain 1904; 27: 327–375. [Google Scholar]
- 4.Gajos A, Bogucki A, Schinwelski M, et al. The clinical and neuroimaging studies in Holmes tremor. Acta Neurol Scand 2010; 122: 360–366. [DOI] [PubMed] [Google Scholar]
- 5.Kamble N, Pal PK. Tremor syndromes: a review. Neurol India 2018; 66: S36–S47. [DOI] [PubMed] [Google Scholar]
- 6.Raina GB, Cersosimo MG, Folgar SS, et al. Holmes tremor: clinical description, lesion localization, and treatment in a series of 29 cases. Neurology 2016; 86: 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajos A, Budrewicz S, Koszewicz M, et al. Is nigrostriatal dopaminergic deficit necessary for Holmes tremor to develop? The DaTSCAN and IBZM SPECT study. J Neural Transm (Vienna) 2017; 124: 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertel F, Züchner M, Decker C, et al. Unilateral Holmes tremor, clearly responsive to cerebrospinal fluid release, in a patient with an ischemic midbrain lesion and associated chronic hydrocephalic ventricle enlargement. Case report. J Neurosurg 2006; 104: 448–451. [DOI] [PubMed] [Google Scholar]
- 9.Joutsa J, Shih LC, Fox MD. Mapping Holmes tremor circuit using the human brain connectome. Ann Neurol 2019; 86: 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.