Abstract
Acute kidney injury is a serious health hazard disease due to its complex etiology and lack of effective treatments, resulting in high medical costs and high mortality. At present, a large number of basic research studies on acute kidney injury have been carried out. However, acute kidney injury models established in rodents sometimes do not simulate the course of human disease well. Research in large animal models of acute kidney injury is relatively rare, and methods to build a mature model of acute kidney injury have failed. Because its kidney anatomy and morphology are very similar to those in humans, the mini pig is an ideal animal in which to model kidney disease. Nephrotoxic drug-induced acute kidney injury has a high incidence. In this study, we established models of acute kidney injury induced by two drugs (gentamicin and cisplatin). Finally, the model of cisplatin-induced acute kidney injury was developed successfully, but we found the model of gentamycin-induced acute kidney injury was not reproducible. Compared to other models, these models better represent acute kidney injury caused by antibiotics and chemotherapeutic drugs and provide a basis for the study of new treatments for acute kidney injury in a large animal model.
Keywords: Acute kidney injury, drug-induced model, mini pig
Impact statement
At present, effective treatment methods for AKI are lacking, so it is particularly important to find effective treatment measures and targets. However, AKI models established in rodents do not always simulate the course of human disease well. To the best of our knowledge, only few previous studies have explored the establishment of drug-induced AKI in a large animal model. In this study, we established models of AKI induced by gentamicin and cisplatin with the hope of finding a large animal model that can better represent the occurrence and development of AKI caused by antibiotics and chemotherapeutic drugs and to provide a basis for the study of new treatments for AKI in a large animal model. Our findings indicate a mature and repeatable method for the establishment of a large animal model of cisplatin-induced AKI. And it serves as a reference to clinical doctors in the field of nephrology. Due to the poor repeatability, the model of gentamycin-induced AKI still needs further exploration.
Introduction
Acute kidney injury (AKI) is an important disease that seriously endangers health because of its complex etiology and lack of effective treatment, resulting in high medical costs and high mortality.1,2 The use of nephrotoxic drugs is an important cause of AKI. Aminoglycosides and chemotherapeutic drugs are commonly used in clinical practice. However, due to their serious side effects, such as nephrotoxicity, the clinical application of these drugs is limited.3,4 Current nephroprotective measures are also unsatisfactory in these patients. Therefore, it is essential to find possible protective measures through the cisplatin-AKI model to mitigate cisplatin-induced AKI.
At present, drug-induced AKI models are mostly constructed in rodents (rats, mice).5 However, rodents and humans obviously differ in their genetics, body size, behavior, life span and so on. Models of kidney disease in rodents do not simulate human kidney disease well. Thus, rodent models do not reflect the effectiveness of nephroprotective measures in humans well. In addition, drug-induced AKI models in rodents are usually constructed by intraperitoneal (i.p.) injection, which obviously differs from the method used in clinical treatment.
Therefore, there is an urgent need for a drug-induced AKI model that is closer to human beings in terms of genetics, renal anatomy and morphology, physiology, mode of administration and pathogenesis. In this study, we established a model of AKI induced by two drugs (gentamicin and cisplatin). Compared to other models, this model better represents the occurrence of AKI caused by antibiotics and chemotherapeutic drugs, providing a basis for the study of new treatments for AKI in a large animal model.
Materials and methods
Animals
Male Chinese experimental mini pigs aged 15 weeks were obtained from the Institute of Zoology of the Chinese Academy of Sciences. The animals were housed two per cage in a room maintained at 18–22°C and a relative humidity of 30–70% with artificial lighting from 8:00 to 20:00. The experimental protocol carried out was in accordance with the approved guidelines of the Institutional Animal Care and Use Committee at the Chinese PLA General Hospital. And the experiments conform to the ARRIVE guidelines.6
Gentamicin was purchased from Guangzhou Baiyunshan Tianxin Phamaceutical Co., Ltd. In the gentamicin dose–response study, mini pigs received a daily intramuscular (i.m.) injection of gentamicin sulfate (60 or 80 mg/kg) in distilled water. The mini pigs were randomly assigned to sham and experimental groups (n = 3 per group). For the gentamicin (60 mg/kg) repeatability experiment, the mini pigs received an i.m. injection of 60 mg/kg gentamicin daily.
Cisplatin was purchased from Jiangsu Haosen Pharmaceutical Group Co., Ltd. In the cisplatin dose–response study, mini pigs received an i.p. injection of cisplatin (2.5, 3.5, 4, and 6 mg/kg) in distilled water. After the injection mode was changed, mini pigs received an intravenous (i.v.) injection of cisplatin (3.7, 3.8, 3.9 and 4 mg/kg) (n = 3 per group). Before intravenous injection, the pig ear was sterilized under respiratory anesthesia. Next, the pig's ear was held and flattened, and the tip was raised slightly. The needle was held in the right hand to make the needle tip penetrate into the skin and blood vessels. The syringe was gently drawn back to allow the return of blood. After the injection, the needle was pulled out, and the needle hole was compressed with a sterilized cotton ball so that no bleeding occurred.
Serum biochemistry analysis
Serum samples were collected by centrifugation at 3000 r/min for 10 min and stored at −80°C before analyses. Serum biochemical parameters were measured using an autoanalyzer (Cobas 8000, Roche, Germany).
Renal histopathological studies
Kidneys were excised, fixed in 4% paraformaldehyde and embedded in paraffin. For light microscopy, paraffin-embedded renal sections (4 mm thick) were stained with periodic acid-Schiff (PAS) solution and examined under a microscope. Histological examinations were performed independently in a blinded fashion by two observers. All group samples were evaluated, and 10 randomly selected fields from each sample were scored at a total magnification of 400×, the average scores were calculated. The main indicators were (1) loss of the brush border, (2) a protein/cell cast, (3) The degeneration of renal tubular epithelial cells (granular, vacuolation), (4) cytolysis, (5) cell necrosis or apoptosis, and (6) the infiltration of interstitial inflammatory cells. Scoring was based on the percentage of the renal interstitial area showing the lesions mentioned above as follows: 0%, 0 points; 0–25%, 1 point; 25–50%, 2 points; 50–75%, 3 points; and >75%, 4 points.
TUNEL assay
An in situ cell death detection kit was used according to the manufacturer’s instructions for TUNEL assays (Roche). TUNEL-positive tubular cells were counted in 10 nonoverlapping fields of each sample.
Western blot analysis and antibodies
The frozen kidney tissues were lysed in RIPA lysis buffer and centrifuged at 12,000 ×g for 30 min at 4°C to obtain the cellular proteins, which is contained in the supernatant. Equal amounts of protein from each sample were resolved by SDS-PAGE, transferred to nitrocellulose (NC) membranes and blocked with 5% skim milk for 1 h at room temperature. The membranes were then probed with the following primary antibodies at 4°C overnight: anti-Ataxia telangiectasia mutated (ATM), anti-puma (Santa Cruz Biotechnology), anti-E-Cadherin, anti-p53, anti-ATM and Rad-3-related (ATR), anti-CHK1, anti-CHK2, anti-caspase-3 (Cell Signaling Technology), anti-alpha smooth muscle (abcam) and anti-actin (Proteintech).
The blots were subsequently probed with horseradish peroxidase-conjugated anti-rabbit IgG (Beyotime Biotechnology) at 1:1000–1:5000. Immunoreactive bands were visualized by enhanced chemiluminescence, and densitometry was performed using Quantity One software (Bio-Rad Laboratories).
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS, Chicago, IL). Comparisons among groups were analyzed by analysis of variance (ANOVA). Values of P < 0.05 indicated statistical significance.
Results
Gentamicin-induced AKI
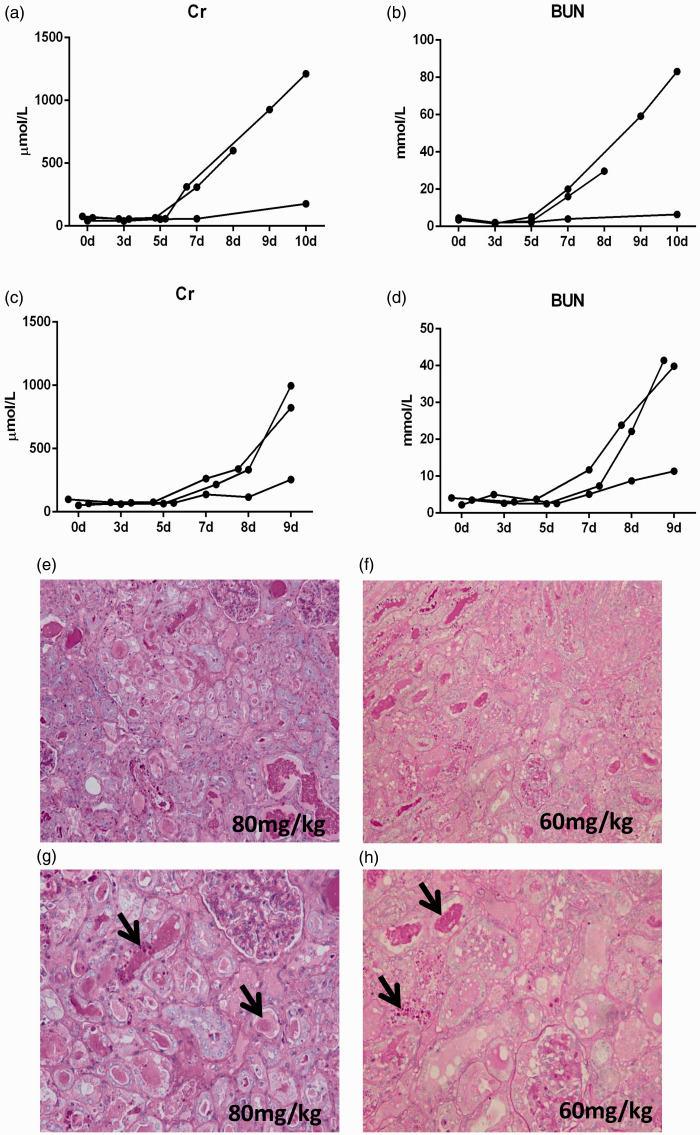
For the gentamicin-induced AKI model, based on previous studies, we first administered 80 mg/kg/day gentamicin to the mini pigs by i.m. injection. The observation time was 10 days. However, the results showed that one pig died on the eighth day of modeling. Although biochemical indexes were obviously increased on the tenth day (Figure 1(a) and (b)), these increases were greater than those seen in a previous studys.7 Based on the symptoms of overdose in the literature, we changed the dosage to 60 mg/kg/day and shortened the observation time to nine days. After these two experiments, the group administered 60 mg/kg/day gentamicin began to show abnormal blood biochemistry on the sixth to seventh days of modeling. On the 9th day, serum creatinine (Cr) and blood urea nitrogen (BUN) levels were obviously increased (Figure 1(c) and (d)), and the renal pathology also revealed obvious renal tubular necrosis (Figure 1(e) and (h)).
Figure 1.
Renal function and kidney histology after gentamicin injection. (a–b) Changes in creatinine and urea nitrogen levels in the group treated with 80 mg/kg/day gentamicin. (c–d) Changes in creatinine and urea nitrogen levels in the group treated with 60 mg/kg/day gentamicin. (e–f) Representative photographs of kidney sections from animals given various dosages of gentamicin (periodic acid-Schiff staining; magnification, 200×). (g–h) The black arrows indicate casts, tubular cell degeneration, and necrosis (periodic acid-Schiff staining; magnification, 400×). (A color version of this figure is available in the online journal.)
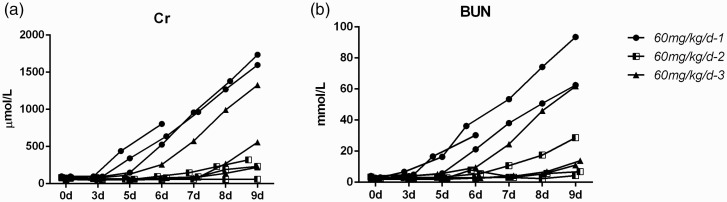
However, a series of problems in the later stage of the modeling process were revealed when the modeling was repeated: instability within the model group, unsuccessful modeling, and the poor overall state of the mini pigs due to long-term i.m. injection. In the next experiment, we repeated the method three times with three mini pigs each. However, the results were very different (Figure 2). The timing of the biochemical changes was very inconsistent among the three experiments, as shown by the observation of marked abnormality or even death in one group and no significant changes in the other group. In the first group, the biochemical indexes increased significantly from the fourth to the fifth day of modeling. One pig died on the sixth day. The excessive increase of Cr and BUN may reflect the rapid damage of kidney, which leading to death. Moreover, the muscle at the injection site gradually became stiff as the result of continuous injection into the neck muscle. This made the animals more difficult to handle, and the mini pigs became more difficult to control during injections in the later stage of modeling. Through three repeated experiments, we saw that the repeatability of the gentamicin-induced AKI model was poor. The times at which biochemical indexes changed were inconsistent, and these indexes were both too high and too low in different animals at the same time. Therefore, the gentamicin-induced mini pig AKI model requires a long modeling period and shows poor reproducibility, and the time point at which each biochemical index changes is not consistent. Determination of the optimal treatment time would be difficult, and this model is not suitable for the observation of drug efficacy.
Figure 2.
Effect of 60 mg/kg/day gentamicin on renal function in mini pigs in three repetitive experiments. (a–b) Changes in the creatinine and urea nitrogen levels of each pig.
Cisplatin-induced AKI
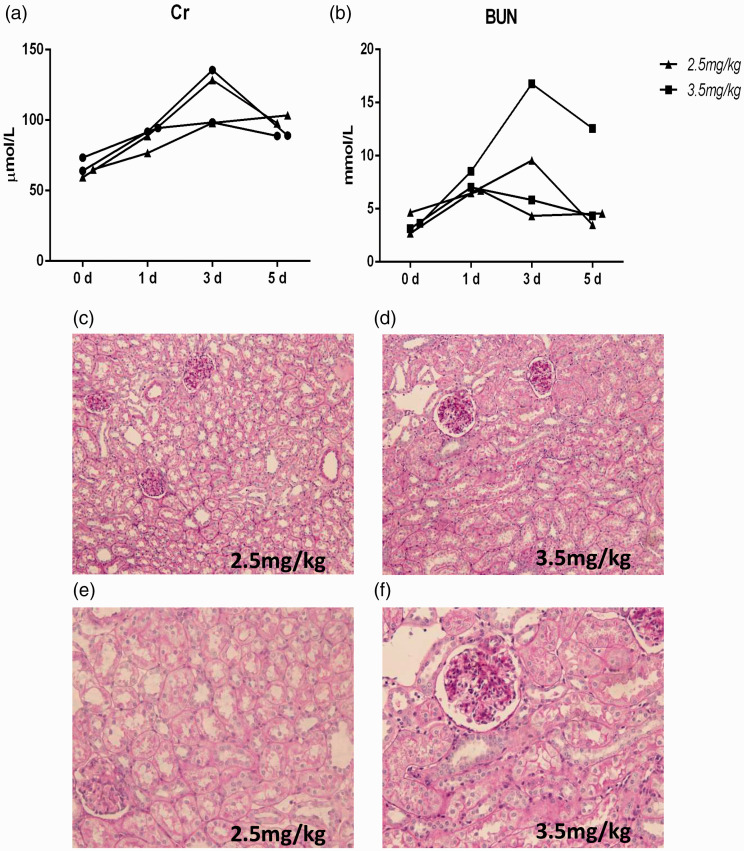
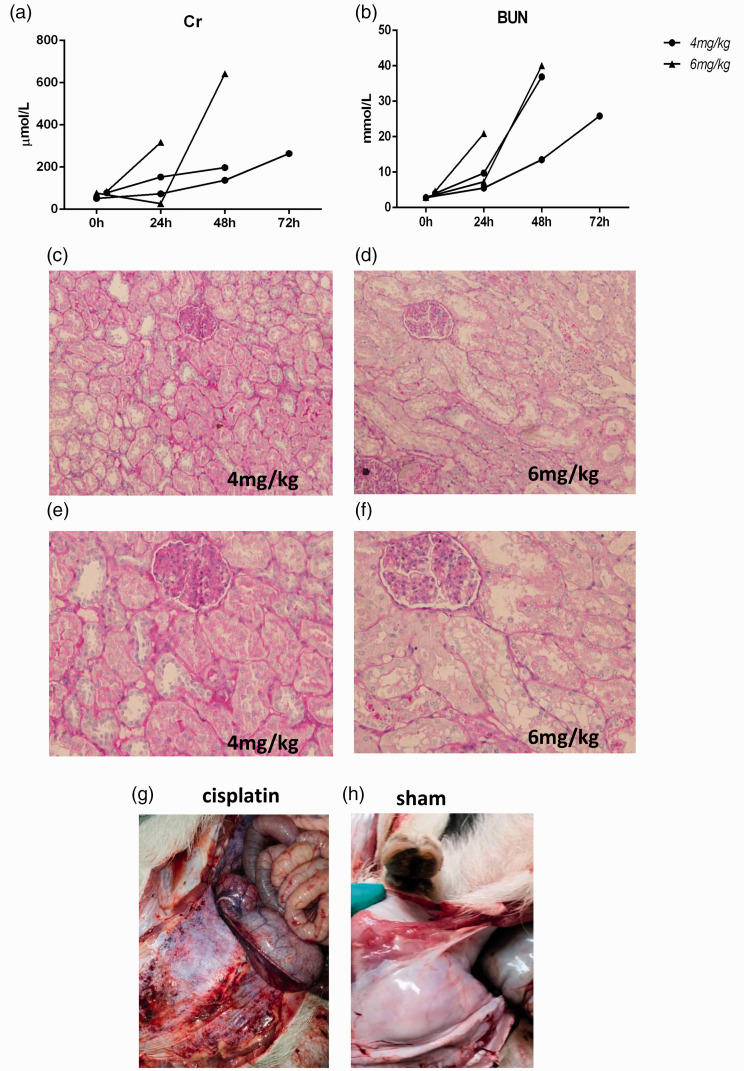
For the cisplatin-induced AKI model, due to the lack of references, we started with a small dose of 2.5 mg/kg or 3.5 mg/kg and referred to a mouse model of cisplatin-induced AKI for the use of i.p. injection. We found that doses of 2.5 mg/kg and 3.5 mg/kg cisplatin were not successful. Although the creatinine urea nitrogen levels was obviously abnormal on the third day of modeling (Figure 3(a) and (b)), in accordance with the general trend observed in the cisplatin-induced AKI model, the results of pathological detection of the kidney tissue did not change (Figure 3(c) to (f)). Therefore, we increased the dose to 4 mg/kg and 6 mg/kg. However, the groups treated with 4 mg/kg and 6 mg/kg cisplatin exhibited a high mortality rate. Although one pig died, the renal pathology still did not change significantly (Figure 4(a) to (f)). When we sacrificed the pigs, we found that the abdominal wall was covered with bleeding points and exhibited ascites (Figure 4(g) and (h)). These results suggest that systemic lesions were more severe than the renal pathological changes.
Figure 3.
The effects of 2.5 mg/kg and 3.5 mg/kg i.p. cisplatin on renal function and histology in mini pigs. (a–b) Changes in creatinine and urea nitrogen levels after cisplatin injection. (c–f) Representative photographs of kidney sections from animals given 2.5 mg/kg and 3.5 mg/kg cisplatin (periodic acid-Schiff staining; magnification, 200× and 400×). (A color version of this figure is available in the online journal.)
Figure 4.
The effects of 4 mg/kg and 6 mg/kg i.p. cisplatin on renal function and histology in mini pigs. (a–b) Changes in creatinine and urea nitrogen levels after cisplatin injection. (c–f) Representative photographs of kidney sections from animals given 4 mg/kg and 6 mg/kg cisplatin (periodic acid-Schiff staining; magnification, 200× and 400×). (g–h) The abdominal wall of mini pigs after cisplatin or saline injection. (A color version of this figure is available in the online journal.)
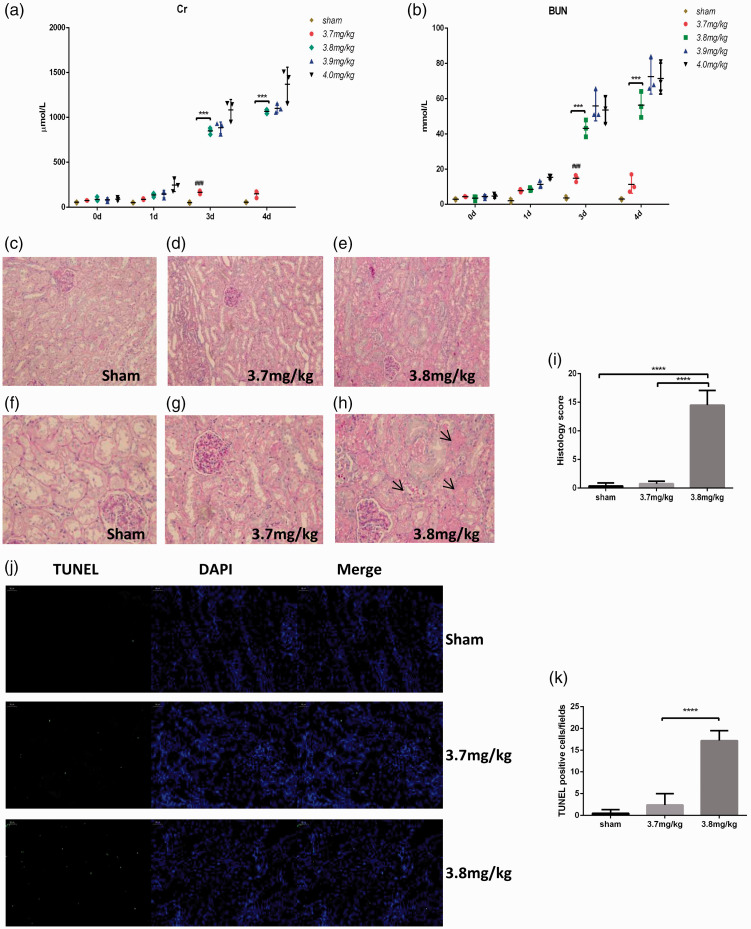
We did not think that i.p. injection is a suitable method. Therefore, we changed the method of injection. We used a single i.v. injection of 3.7 mg/kg, 3.8 mg/kg, 3.9 mg/kg, and 4 mg/kg cisplatin. Compared to those of previous experiments, the results were improved, and the survival rate of the animals on day 6 was 100%. The biochemical indexes of the groups treated with 3.8 mg/kg, 3.9 mg/kg, and 4 mg/kg cisplatin were significantly changed on day 4 (Figure 5(a) and (b)). Unlike that of the sham group, the renal pathology of the group treated with 3.8 mg/kg cisplatin showed obvious tubular necrosis on day 4 (Figure 5(c) to (i)). We evaluated apoptosis by TUNEL staining. The results showed that when the dose of cisplatin was 3.8 mg/kg, cell apoptosis was significantly increased (Figure 5(j) and (k)).
Figure 5.
Effect of various doses of i.v. cisplatin on renal function and histology in mini pigs. (a–b) Changes in creatinine and urea nitrogen levels after saline or 3.7–4 mg/kg cisplatin injection. (c–h) Representative photographs of kidney sections from animals given 3.7 mg/kg and 3.8 mg/kg cisplatin (periodic acid-Schiff staining; magnification, 200× and 400×). The black arrows indicate casts, tubular cell degeneration, and necrosis. (i) Histology scores based on standard procedures were significantly different in the groups administered 3.8 mg/kg cisplatin. The quantitative analysis was carried out in 10 random fields per pig. (j) Representative images of TUNEL staining. Scale bar, 50 μm. (k) Quantification of TUNEL-positive cells in the kidney. (A color version of this figure is available in the online journal.)
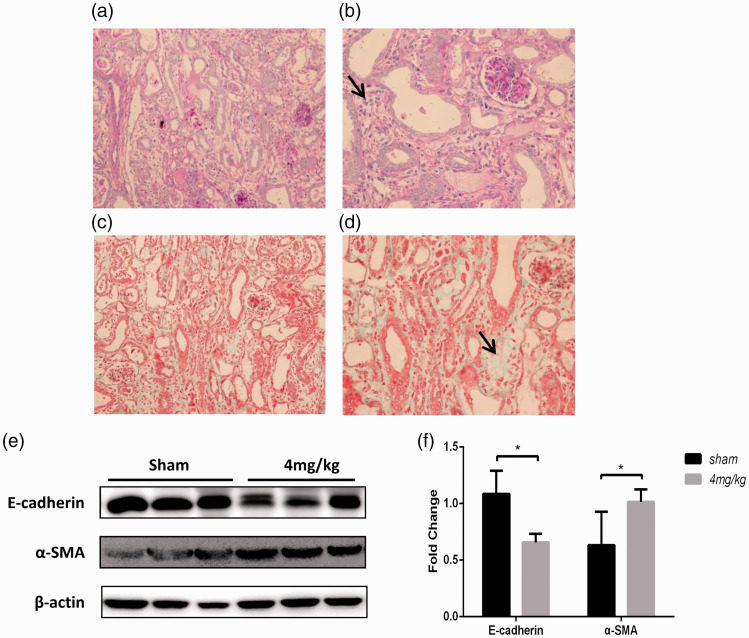
To clarify the pathological changes after cisplatin-induced injury, we extended the observation time of the group treated with 4 mg/kg cisplatin to the sixth day of modeling. Pathological analysis of the group treated with 4 mg/kg cisplatin showed obvious interstitial lesions and that the necrotic renal tubules began to be replaced by an intercellular substance (Figure 6(a) to (d)). We also detected markers of renal fibrosis, such as E-cadherin and alpha-smooth muscle actin (α-SMA) in tissue (Figure 6(e) and (f)). The results showed that at 6 days after cisplatin injection, the level of fibrosis increased significantly. Therefore, to observe acute injury, the modeling time should be maintained at three to four days.
Figure 6.
Change in histology in mini pigs administered 4 mg/kg i.v. cisplatin. (a–b) Periodic acid-Schiff staining of kidney sections (magnification, 400×). (c–d) Masson staining of kidney sections (magnification, 400×). The black arrows indicate interstitial changes. (e–f) Levels of fibrosis were analyzed by Western blotting. (A color version of this figure is available in the online journal.)
Figure 7.
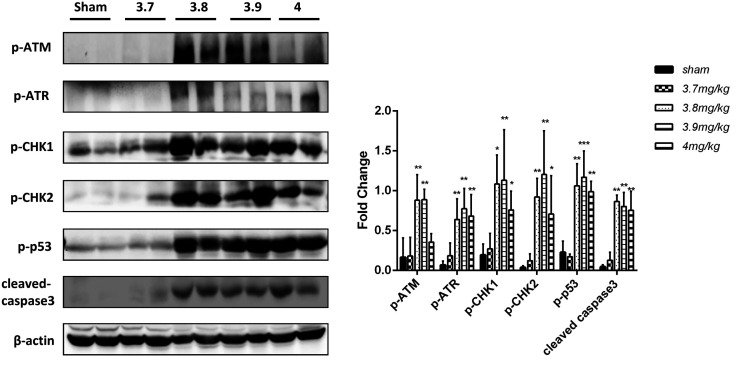
DNA damage/p53 expression and apoptosis in cisplatin-induced AKI mini pigs. (a) Levels of DNA damage/p53-related proteins were analyzed by Western blotting. (b) Three replicates were used for Western blot quantification. Values are presented as the means ± SDs. *P < 0.05, **P < 0.01, ***P < 0.001 versus the sham group.
The DNA damage/p53 pathway in cisplatin-induced AKI mini pigs
The DNA damage/p53 pathway is the main mechanism of renal tubular cell apoptosis induced by cisplatin-AKI, but previous studies were conducted in rodents.8,9 In this study, for the first time, we tested this pathway in the kidney of mini pigs with AKI, and the results were consistent with those of previous studies. The main markers of the DNA damage/p53 pathway and apoptosis were upregulated on the fourth day after 3.8 mg/kg and 3.9 mg/kg cisplatin injection. The levels of these markers in the group treated with 4 mg/kg cisplatin on the sixth day were still significantly higher than those of the control group (Figure 7).
Discussion
Aminoglycosides and chemotherapeutic drugs are common causes of drug-induced kidney injury. In basic research studies, gentamicin and cisplatin are mainly selected to establish animal models of drug-induced AKI. At present, gentamicin and cisplatin nephrotoxicity models have been established in mice and rats,5 but the results in rodents do not simulate the process of the human disease well because of the large differences between rodents and humans. A few studies have been conducted in non-rodent model animals, such as dogs and monkeys.10 However, although rodents, which are easy to obtain and maintain, are the most commonly used animals for small animal disease models, rodents and humans obviously differ in their genetics, body shape, behavior, longevity, and so on. Disease models established in rodents sometimes do not mimic the course of human disease well. For example, streptomycin can cause vestibular and auditory cell damage in patients. In mouse models, streptomycin did not cause these side effects. However, streptomycin had side effects on pigs similar to those on humans.11 Among other animals, primates are genetically closest to humans, but they are expensive and difficult to maintain. Because of their strong genetic, anatomical and physiological similarities with humans, mini pigs have advantages over rodent models as pharmacokinetic models.12 Studies have shown that in many cases, mini pigs are more suitable for toxicology tests than dogs or monkeys.13
The various organ systems of mini pigs and humans not only are morphologically similar but also have essentially the same physiological function, especially the skin and cardiovascular, gastrointestinal and urinary systems. In terms of anatomy, kidney size in the mini pig is similar to that in humans, and the kidney accounts for 0.5% of the body weight. Mini pig and human kidneys are multi-papillary (the mouse kidney has a single lobe), and the number of kidney lobes in mini pigs is similar to that in humans. Furthermore, the diameters of the proximal tubules, distal tubules and collecting tubules in mini pigs are similar to those in humans.14
The incidence of aminoglycoside-induced AKI varies from 5% to 25%.15,16 Gentamicin and other aminoglycoside antibiotics are the most commonly used antibiotics to treat Gram-negative bacterial infections.17 Among all aminoglycoside antibiotics, gentamicin may be the most commonly used and studied.18 Serious gentamicin-induced complications, such as nephrotoxicity and ototoxicity, greatly limit its clinical application.4 The pathogenesis of these effects may include immune complex damage and direct cytotoxic effects. In previous studies, gentamicin models have primarily been established in rats and mice. A small number of studies have used dogs as a model. However, there are no standard methods of model building in rodents or non-rodents. For example, some doses are determined based on body surface area, while some are determined based on body weight. Some methods use i.p. injection, while some use i.m. injection. In addition, drugs are administered through both single and multiple injections.5 This may lead to the occurrence of different pathological processes in different models or an inability to determine the optimal timing for treatment in drug screening. Our study used mini pig as a model developed with gentamicin at a dose of 80 mg/kg, which is used in most studies. However, the results showed a high mortality rate. Although reducing the dose ensured survival and model mini pigs developed with a reduced dose showed obvious biochemical and pathological manifestations of AKI, the damage in each group of model animals was inconsistent. Regardless of the use of i.p. or i.m. injection, continuous injection will make large animals, such as mini pigs, impossible to fully manage and difficult to maintain. Furthermore, if the injection time is reduced, there is a risk of model failure. Several clinical studies have shown that short-term or single-dose injection of gentamicin had no significant relationship with decreased renal function.4,19 Therefore, building animal models may still require long-term injection. However, the tolerance and stability of long-term injection still need to be ensured. This is an issue that needs to be addressed in the establishment of a stable gentamicin-induced AKI mini pig model. In addition, the dose of gentamicin was much higher than that used in human patients (60 mg/kg in pigs vs. 1.0∼1.7 mg/k three times a day in patients). This may not accurately reflect the condition in humans. It raises the question of whether the pathological effects of gentamycin in the pig are the same as those in humans. Therefore, whether the mini pigs are suitable for gentamycin-induced AKI model remains to be studied.
Platinum drugs, which are commonly used in chemotherapy, have long been proposed to cause nephrotoxicity.10 Cisplatin, a platinum drug, is also an important cause of drug-induced AKI. In various studies, the incidence of cisplatin-induced AKI ranged from 10% to 30% due to the use of inconsistent criteria for AKI.20–23 Basic research on cisplatin nephrotoxicity has already begun, and this research is conducted mostly in mouse models but occasionally in dog and monkey models.10 At present, a small number of studies have used long-term, low-dose, multiple injection of cisplatin to build chronic kidney disease (CKD) models,24 and most of these studies used cisplatin to study AKI. The basic method is the application of single dose of 20–25 mg/kg cisplatin via i.p. injection in mice and serves as the conventional method of cisplatin-induced AKI modeling.
Among previous studies in AKI models, only Santiago et al.25 used mini pigs to build an AKI model in 2016. However, this study conducted pediatric experiments, so the mini pigs were younger than those used in this study, and the mini pigs were administered a lower dose of cisplatin than that used in our study by injection. Our study showed that increasing the injected dose to 3.7 mg/kg still failed to cause renal injury. In addition, the observation time was at least three days. Pathological analysis showed obvious tubular casting and necrosis. The pathological results of Santiago showed that at 2 days after 3 mg/kg cisplatin injection, only a small number of tubular casts appeared in the pathological analysis, and the renal tubular cells did not change. Because of the mild degree of injury caused by this modeling method, this model is not conducive to studying the mechanism and treatment of AKI. Furthermore, a large amount of tubular necrosis occurred after 5 mg/kg cisplatin injection, indicating that the dose was obviously too high. The number of pigs used was small, and the sex was not standardized, so their study did not provide sufficient reference for the establishment of a cisplatin-induced AKI model in normal mini pigs. According to our experimental results, it is recommended to use five to six-month-old male adult pigs with 3.8 mg/kg cisplatin injection. After at least three to four days of observation, biochemical indexes and the renal pathology significantly change. Although interstitial lesions were observed on the sixth day of modeling in this study, a single high-dose injection is still not suitable for modeling chronic kidney injury. A recent study developed chronic renal injury models in pigs using consecutive low-dose injections. However, the authors did not test common indicators of chronic renal injury.26 Therefore, it was impossible to determine whether their model is appropriate for the study of chronic kidney injury. The best method to establish a CKD model in mini pigs remains to be elucidated.
The study on the risk factors for cisplatin nephrotoxicity is complex, and the application method of cisplatin, dosage, antihypertensive drugs, and age have been studied deeply.27–29 In addition, the risk of AKI in cancer patients with chronic kidney disease (CKD) is also increased.21 Angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEI/ARB) drugs may inhibit the activity of the renin-angiotensin (RAS) system, lead to the aggravation of renal ischemia, and delay the excretion of cisplatin.30 Age and CKD complications may increase the possibility of AKI through reduced renal function. However, the animal model established in our study cannot simulate the occurrence of AKI with these risk factors. It can only be used to study the basic pathogenesis and treatment of AKI, which may be one of the limitations of this study. In the future, we hope to establish corresponding animal models with renal lesions for the study of the risk factors for cisplatin nephrotoxicity.
The mechanism of AKI has not been fully elucidated. p53 is a well-known tumor suppressor protein whose induction and activation contribute critically to the pathogenesis of cisplatin-induced nephrotoxic AKI. P53 activation may lead to autophagy, cell cycle arrest, and apoptosis.31 Recently, Zhu also emphasized the important role of DNA damage and p53 in the regulation of cisplatin-induced AKI.8 Cisplatin can cause DNA cross-linking when taken in by renal tubular cells, generating a DNA damage response. The key DNA damage response sensors are the kinases ATM and ATR. They further recruit “transducer” protein kinases, such as checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2), which phosphorylate downstream factors, called “effectors”, such as p53, which further induces renal cell apoptosis. Therefore, the DNA damage response and p53 activation are important pathogenic mechanisms of AKI following cisplatin treatment. In our study, the importance of these factors was verified in large animals for the first time. We detected the expression of DNA damage/p53 pathway-related proteins, the results of which showed that when the dose of cisplatin was 3.8–4 mg/kg, this pathway was significantly upregulated, consistent with the previous study.
In conclusion, this study explored the establishment of two drug-induced AKI models. Gentamicin was not suitable for development of a large animal AKI model due to the long modeling period required and poor reproducibility. The cisplatin-induced AKI modeling method consisted of a single i.v. injection of 3.8 mg/kg cisplatin with an observation time of day 4. Single i.v. injection of cisplatin is easy to perform, and the model was stable. This model can thus be used as a large animal model to study drug-induced AKI.
Footnotes
Authors’ contributions: W SY, ZH CY, C GY and C XM contributed to the conception of the study. W SY and ZH CY performed the analysis and experiments. W SY contributed significantly to analysis and manuscript preparation. All authors approve the final version
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: This work is supported by the National Key R&D Program of China (2018YFA0108803), the Science and Technology Project of Beijing (D181100000118004).
ORCID iD: Si-Yang Wang https://orcid.org/0000-0002-4009-0800
References
- 1.Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013; 84:457–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8:1482–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, Kim RW, Parikh CR. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med 2011; 39:1493–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayward RS, Harding J, Molloy R, Land L, Longcroft-Neal K, Moore D, Ross JDC. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol 2018; 84:223–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vicente-Vicente L, Casanova AG, Hernández-Sánchez MT, Pescador M, López-Hernández FJ, Morales AI. A systematic Meta-analysis on the efficacy of pre-clinically tested nephroprotectants at preventing aminoglycoside nephrotoxicity. Toxicology 2017; 377:14–24 [DOI] [PubMed] [Google Scholar]
- 6.Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol 2020; 177:3617–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Bai XY, Sun X, Cai G, Hong Q, Ding R, Chen X. Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep 2015; 5:11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu S, Pabla N, Tang C, He L, Dong Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch Toxicol 2015; 89:2197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol 2014; 25:2278–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madias NE, Harrington JT. Platinum nephrotoxicity. Am J Med 1978; 65:307–14 [DOI] [PubMed] [Google Scholar]
- 11.Madsen LB, Thomsen B, Sølvsten CAE, Bendixen C, Fredholm M, Jørgensen AL, Nielsen AL. Identification of the porcine homologous of human disease causing trinucleotide repeat sequences. Neurogenetics 2007; 8:207–18 [DOI] [PubMed] [Google Scholar]
- 12.Cooper DKC, Hara H, Iwase H, Yamamoto T, Jagdale A, Kumar V, Mannon RB, Hanaway MJ, Anderson DJ, Eckhoff DE. Clinical pig kidney xenotransplantation: how close are we? J Am Soc Nephrol 2020; 31:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr., Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012; 49:344–56 [DOI] [PubMed] [Google Scholar]
- 14.Swindle MM, Smith AC, Hepburn BJ. Swine as models in experimental surgery. J Invest Surg 1988; 1:65–79 [DOI] [PubMed] [Google Scholar]
- 15.Picard W, Bazin F, Clouzeau B, Bui HN, Soulat M, Guilhon E, Vargas F, Hilbert G, Bouchet S, Gruson D, Moore N, Boyer A. Propensity-based study of aminoglycoside nephrotoxicity in patients with severe sepsis or septic shock. Antimicrob Agents Chemother 2014; 58:7468–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 2011; 79:33–45 [DOI] [PubMed] [Google Scholar]
- 17.Choi JJ, Moffett BS, McDade EJ, Palazzi DL. Altered gentamicin serum concentrations in obese pediatric patients. Pediatr Infect Dis J 2011; 30:347–9 [DOI] [PubMed] [Google Scholar]
- 18.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol Res 2010; 62:179–86 [DOI] [PubMed] [Google Scholar]
- 19.Carlsen S, Boel J, Jarløv JO, Gjørup I, Søborg C, Arpi M. The effect of short-course gentamicin therapy on kidney function in patients with bacteraemia – a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2018; 37:2307–12 [DOI] [PubMed] [Google Scholar]
- 20.Muraki K, Koyama R, Honma Y, Yagishita S, Shukuya T, Ohashi R, Takahashi F, Kido K, Iwakami S-I, Sasaki S, Iwase A, Takahashi K. Hydration with magnesium and mannitol without furosemide prevents the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non-small cell lung cancer. J Thorac Dis 2012; 4:562–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Watanabe S, Ohtsubo A, Shoji S, Ishikawa D, Tanaka T, Nozaki K, Kondo R, Okajima M, Miura S, Tanaka J, Sakagami T, Koya T, Kagamu H, Yoshizawa H, Narita I. Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer 2016; 16:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasaja Y, Sutandyo N, Andrajati R. Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pac J Cancer Prev 2015; 16:1117–22 [DOI] [PubMed] [Google Scholar]
- 23.Mousavi SSB, Zadeh MH, Shahbazian H, Khanzadeh A, Hayati F, Ghorbani A, Golzari K, Valavi E, Motemednia F, Mousavi MB. The protective effect of theophyline in cisplatin nephrotoxicity. Saudi J Kidney Dis Transpl 2014; 25:333–37 [DOI] [PubMed] [Google Scholar]
- 24.Shi M, McMillan KL, Wu J, Gillings N, Flores B, Moe OW, Hu MC. Cisplatin nephrotoxicity as a model of chronic kidney disease. Lab Invest 2018; 98:1105–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santiago MJ, Fernández SN, Lázaro A, González R, Urbano J, López J, Solana MJ, Toledo B, Del Castillo J, Tejedor A, López-Herce J. Cisplatin-induced non-oliguric acute kidney injury in a pediatric experimental animal model in piglets. PLoS One 2016; 11:e0149013–e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y-J, Li K-Y, Wang P-J, Huang H-W, Chen M-J. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced lanyu pig model. J Food Drug Anal 2020; 28:103–14 [DOI] [PubMed] [Google Scholar]
- 27.Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD. Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: relevance of age and dose as risk factors. Eur J Cancer 2009; 45:3213–9 [DOI] [PubMed] [Google Scholar]
- 28.Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol 2016; 11:1173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tixier F, Ranchon F, Iltis A, Vantard N, Schwiertz V, Bachy E, Bouafia-Sauvy F, Sarkozy C, Tournamille JF, Gyan E, Salles G, Rioufol C. Comparative toxicities of 3 platinum-containing chemotherapy regimens in relapsed/refractory lymphoma patients. Hematol Oncol 2017; 35:584–90 [DOI] [PubMed] [Google Scholar]
- 30.Kurt E, Manavoglu O, Dilek K, Orhan B, Evrensel T. Effect of cisplatin on plasma renin activity and serum aldosterone levels. Clin Nephrol 1999; 52:397–8 [PubMed] [Google Scholar]
- 31.Tang C, Ma Z, Zhu J, Liu Z, Liu Y, Liu Y, Cai J, Dong Z. P53 in kidney injury and repair: mechanism and therapeutic potentials. Pharmacol Ther 2019; 195:5–12 [DOI] [PubMed] [Google Scholar]