Abstract
Background:
Our understanding of how multimorbidity progresses and changes is nascent.
Objectives:
Assess multimorbidity changes among racially/ethnically diverse middle-aged and older adults.
Design, Setting, and Participants:
Prospective cohort study using latent class analysis to identify multimorbidity combinations over 16-years, and multinomial logistic models to assess change relative to baseline class membership. Health and Retirement Study respondents (age ≥51 years) in 1998 and followed through 2014 (N = 17,297).
Measures:
Multimorbidity latent classes of: hypertension, heart disease, lung disease, diabetes, cancer, arthritis, stroke, high depressive symptoms.
Results:
Three latent classes were identified in 1998: minimal disease (45.8% of participants), cardiovascular-musculoskeletal (34.6%), cardiovascular-musculoskeletal-mental (19.6%); and three in 2014: cardiovascular-musculoskeletal (13%), cardiovascular-musculoskeletal-metabolic (12%), multisystem multimorbidity (15%). Remaining participants were deceased (48%) or lost to follow-up (12%) by 2014. Compared to minimal disease, individuals in cardiovascular-musculoskeletal in 1998 were more likely to be in multisystem multimorbidity in 2014 (OR=1.78, p < .001), and individuals in cardiovascular-musculoskeletal-mental in 1998 were more likely to be deceased (OR=2.45, p<.001) or lost to follow-up (OR=3.08, p<.001). Hispanic and Black Americans were more likely than White Americans to be in multisystem multimorbidity in 2014 (respectively, OR=1.67, p=.042; OR=2.60, p<.001). Black compared to White Americans were more likely to be deceased (OR=1.62, p=.01) or lost to follow-up (OR=2.11, p<.001) by 2014.
Conclusions and Relevance:
Racial/ethnic older adults are more likely to accumulate morbidity and die compared with White peers, and should be the focus of targeted and enhanced efforts to prevent and/or delay progression to more complex multimorbidity patterns.
Keywords: Multimorbidity, Multiple Chronic Conditions, Latent Class Analysis
INTRODUCTION
Multimorbidity (two or more co-occurring chronic diseases in any given patient) is associated with adverse health outcomes and important to understanding racial and ethnic disparities in health (1–3). A growing body of work corroborates the compounding effects of combinations of chronic diseases on health outcomes beyond the risk attributable to individual diseases (4), including high rates of disability and institutionalization, high healthcare expenditures, and earlier mortality (5–8). In addition, disparities in multimorbidity burden have been documented, including a higher burden and faster accumulation of multimorbidity among minority racial and ethnic groups, even after controlling for differences in socioeconomic status (9). Thus, it is widely understood that reducing the disproportionate multimorbidity observed for minority racial and ethnic adults earlier in the life course will be necessary to support successful aging initiatives in the US (10).
Yet, multimorbidity is not a stagnant phenomenon. As older adults age, they may develop new chronic diseases that are added to existing ones to form new multimorbidity combinations. Individuals who increase in their morbidity from one period to the next may be at increased risk of multimorbidity-related consequences, such as poorer functional health, greater health care utilization, and earlier mortality. Much about how specific multimorbidity combinations emerge and change over time is poorly understood, particularly whether these changes between combinations vary for minority ethnic middle-aged and older adults. Tracing these disease groupings as they occur in the population and, in particular, mapping racial/ethnic variations in the development and progression of multimorbidity over time holds important clinical and policy significance (11,12). For instance, understanding how specific disease combinations change within and between racial/ethnic groups may help identify specific groupings of diseases that accelerate the development of disability, incur substantial health care costs, and lead to earlier mortality for segments of the population who are most at risk (13).
This study aims to understand how multimorbidity combinations change over time within and between racial/ethnic groups of middle-age and older adults. To accomplish this aim, we first identify latent classes of chronic diseases—distinct multimorbidity combination groups—for community-dwelling participants from a nationally representative study, who were interviewed in 1998 and then followed through 2014. Second, we evaluate changes in membership between the identified latent classes, as well as to two known states—death and loss to follow-up—over the 16-year interval and determine whether these changes differ across racial/ethnic groups. Understanding how changes in specific multimorbidity combinations differ among individuals from various racial/ethnic backgrounds provides important insight into inequities in health, health care, and mortality.
METHODS
Data
The Health and Retirement Study (HRS) is a nationally representative survey of non-institutionalized middle- and older-aged adults (51 years and older), which explores the transitions in health that occur toward the end of an individual’s work life and into retirement (15). Publicly available and anonymized HRS survey data in the 1998 and 2014 waves were used in this study. Assessing multimorbidity latent classes over this 16-year period permitted sufficient elapsed time to observe substantive changes to chronic disease burden and multimorbidity profiles.
The Health Sciences Institutional Review Board (IRB) at the University of Michigan has approved the HRS and the IRB at Oregon Health & Science University has approved this study. This is a secondary data analysis of de-identified publicly available data, and as such it was not appropriate or possible to involve patients or the public in the design, conduct, reporting, or dissemination plans of our research.
Study Population
We included study participants from the 1998 HRS interview who were followed until 2014. Of the 19,785 respondents in the 1998 wave who were alive, age-eligible, and not in nursing homes, 17,621 individuals met study criteria of providing consistent disease response patterns (see Chronic Diseases below for detail) and non-missing race/ethnicity. We excluded 324 respondents due to small group sizes for American Indian, Asian or “other” race/ethnicities. The resulting total sample in 1998 was 17,297 individuals. We estimated latent disease classes in 1998 for all of these individuals. By 2014, 8,301 and 2,052 participants died or were lost to follow-up, respectively. Latent disease classes in 2014 were then estimated with the surviving 6,944 respondents.
Measures
Chronic Diseases
Eight self-reported chronic diseases, each prompted by “Has a doctor ever told you that you have…,” were available in this study: three cardiovascular conditions - heart disease (composite including myocardial infarction, coronary heart disease, angina, congestive heart failure, or other heart problems), hypertension (i.e., high blood pressure), and stroke (but not TIA), and 5 other diseases: diabetes, arthritis, lung disease (including chronic bronchitis or emphysema, and excluding asthma), cancer (including any malignant tumors with the exception of skin cancer), and high depressive symptoms (CES-D8 ≥ 4) (14). Cognitive impairment was assessed but excluded from consideration because of model convergence difficulties most likely due to the low prevalence of this condition in this sample.
Clinically-inconsistent chronic disease reports exist across HRS waves. To settle inconsistencies, we applied a previously developed multistep adjudication method (15); briefly, discrepancies were resolved using disease-specific follow-up questions (i.e., “evidence” of disease such as reporting disease-specific medications or treatments). Respondents with unresolved inconsistencies were excluded.
Race/Ethnicity
Race/ethnicity comprised mutually-exclusive categories: non-Hispanic White, non-Hispanic Black, and Hispanic.
Covariates
Sociodemographic covariates included age, sex, education (years of schooling), and net worth (total wealth minus total debt).
Statistical Analysis
Latent class analyses were used to classify the eight chronic diseases using Mplus Version 8.4 (16). Full information maximum likelihood estimation was used allowing for missing data. The bootstrap test of Vuong-Lo-Mendell-Rubin (VLMR) likelihood ratio test was used to select the number of latent classes (17). Bayesian information criterion (BIC) was also examined for selecting the optimal number of classes. After selection of the number of classes, probable class membership was assigned based on the highest probability of membership, which was used in subsequent repeated measures and multinomial logistic regression models to examine likely change in class membership or to known states of mortality or lost to follow-up over the 16-year interval using SAS Version 9.4. To interpret each identified latent class, we assessed response probabilities for each of the chronic diseases. For the latent classes, higher response probabilities indicate that the disease is more central to the interpretation of the class. Multinomial models added two additional known states, death or lost to follow-up, to the probable latent classes as outcomes categories in 2014. The multinomial logistic models controlled baseline class membership as well as covariates to provide information about the association between race/ethnicity and each of the outcome categories in 2014, taking into account initial class membership differences among groups at baseline. We estimated a series of models (Models 1–3) to examine the relationship between race/ethnicity and outcome category in 2014. These sequential models first examine overall unadjusted changes (Model 1), then examine race/ethnic group differences unadjusted for covariates (Model 2), and finally examine these differences after full covariate adjustment for age, sex, education, and net worth (Model 3). In additional analyses (Supplemental Digital Content), we present stratified multinomial logistic models reporting within racial/ethnic group changes to later-period final disposition (i.e., 2014 latent classes, death, or lost to follow-up). Weighting and sampling design adjustments appropriate for the HRS (18) were used for latent class models and for the logistic regression models (PROC SURVEYLOGISTIC).
RESULTS
Fifty-seven percent of the study sample was female and the mean age in 1998 was 67 years. Seventy-eight percent of the sample was non-Hispanic White, 14% were non-Hispanic Black, and 7% were Hispanic. Additional sample descriptive characteristics are summarized in Table 1 and Table A1 (Supplemental Digital Content).
Table 1.
Baseline Characteristics of the Total Study Sample and by Initial Latent Classes, Health and Retirement Study 1998.
| Total (n = 17,297) |
1998 Class 1: MINIMAL DISEASE (n =7,928) |
1998 Class 2: CV-MSK MM (n =5,986) |
1998 Class 3: CV-MSK-MENTAL MM (n =3,383) |
Deceased by 2014 (n = 8,301) |
Lost to Follow Up by 2014 (n = 2,052) |
|
|---|---|---|---|---|---|---|
| Age, mean (SD) | 66.8 (10.1) | 64.7 (9.6) | 67.1 (9.8) | 71.0 (10.1) | 72.3 (9.9) | 63.2 (8.1) |
| Female sex, n (%) | 9800 (56.7) | 4489 (56.6) | 3396 (56.7) | 1915 (56.6) | 4383 (52.8) | 1233 (60.1) |
| Hispanic, n (%) | 1283 (7.4) | 572 (7.2) | 465 (7.8) | 246 (7.3) | 560 (6.7) | 164 (8.0) |
| Non-Hispanic White, n (%) | 13566 (78.4) | 6600 (83.2) | 4391 (73.4) | 2575 (76.1) | 6478 (78.0) | 1660 (80.9) |
| Non-Hispanic Black, n (%) | 2448 (14.2) | 756 (9.5) | 1130 (18.9) | 562 (16.6) | 1263 (15.2) | 228 (11.1) |
| Years education, median (IQR) | 12.0 (11.0, 14.0) | 12.0 (12.0,14.0) | 12.0 (10.0, 14.0) | 12.0 (9.0, 12.0) | 12.0 (9.0, 13.0) | 12.0 (12.0, 4.0) |
| Net worth (USD), median (IQR) | 1.3 (0.4, 3.3) | 1.7 (0.6, 4.0) | 1.2 (0.4, 3.1) | 0.7 (0.1, 2.0) | 1.0 (0.3, 2.5) | 1.6 (0.6, 4.1) |
| Diseases, n (%) | ||||||
| Hypertension | 8270 (47.8) | 58 (0.7) | 5592 (93.4) | 2620 (77.4) | 4676 (56.3) | 845 (41.2) |
| Diabetes | 2465 (14.3) | 197 (2.5) | 1171 (19.6) | 1097 (32.4) | 1694 (20.4) | 190 (9.3) |
| Cancer | 1897 (11.0) | 685 (8.6) | 591 (9.9) | 621 (18.4) | 1272 (15.3) | 138 (6.7) |
| Lung disease | 1481 (8.6) | 427 (5.4) | 0 (0.0) | 1054 (31.2) | 1101 (13.3) | 94 (4.6) |
| Cardiovascular disease | 3976 (23.0) | 775 (9.8) | 598 (10.0) | 2603 (76.9) | 2833 (34.1) | 274 (13.4) |
| Stroke | 1100 (6.4) | 0 (0.0) | 379 (6.3) | 721 (21.3) | 883 (10.6) | 74 (3.6) |
| Arthritis | 9175 (53.0) | 3259 (41.1) | 2939 (49.1) | 2977 (88.0) | 4910 (59.1) | 986 (48.1) |
| High depressive symptoms (CES-D8 ≥4) | 2539 (14.7) | 690 (8.7) | 622 (10.4) | 1227 (36.3) | 1487 (17.9) | 238 (11.6) |
| No. diseases, median (IQR) | 2.0 (1.0, 3.0) | 1.0 (0.0, 1.0) | 2.0 (1.0, 3.0) | 4.0 (3.0, 4.0) | 2.0 (1.0, 3.0) | 1.0 (0.0, 2.0) |
Abbreviations: CV-MSK MM = Cardiovascular-Musculoskeletal Multimorbidity, CV-MSK-MENTAL MM = Cardiovascular-Musculoskeletal-Mental Multimorbidity, SD = standard deviation; IQR = interquartile range; USD = United States dollars, in hundreds of thousands of dollars; CES-D8 = Eight item version of the Center for Epidemiological Studies Depression Scale. Notes: Sample weights, strata, and probability sampling unit not applied.
Latent class models
Latent class models of the eight chronic diseases were estimated for 1–4 classes for both the 1998 (N = 17,297) and 2014 (N = 6,944) waves. Models with 4 classes did not converge, suggesting a four class solution was not feasible. Table 2 presents the BIC fit results and bootstrap VLMR tests that compare the fit of each model to the model with one fewer classes. The BIC values improved for each model with an additional class and the VLMR tests indicated significant improvement in fit for the two-class model relative to the single-class model (p < .001) and the three-class model relative to the two-class model (p < .05).
Table 2.
Multimorbidity Latent Classes in 1998 and 2014.
| 1998 (N = 17,297) | 2014 (N = 6,944) | |||
|---|---|---|---|---|
| Number of Classes | BIC | VLMR LR χ2, df (p) | BIC | VLMR LR χ2, df (p) |
| 1 | 121548.00 | – | 58440.431 | – |
| 2 | 117987.536 | 3409.668, 9 (< 0.001) | 57133.955 | 1216.451, 9 (<0.001) |
| 3 | 117818.965 | 257.036, 9 (< 0.001) | 57030.059 | 150.501, 9 (< 0.001) |
| Disease | Class in 1998 | Conditional probability (p) | Class in 2014 | Conditional probability (p) |
| Hypertension | MINIMAL | 0.12 (0.309) | CV-MSK | 0.43 (<0.001) |
| Diabetes | DISEASE | 0.03 (0.084) | 0.08 (0.005) | |
| Cancer | 0.08 (<0.001) | 0.22 (<0.001) | ||
| Lung disease | 0.05 (<0.001) | 0.09 (<0.001) | ||
| Heart disease | 0.07 (<0.001) | 0.17 (<0.001) | ||
| Stroke | 0.01 (0.274) | 0.03 (0.001) | ||
| Arthritis | 0.36 (<0.001) | 0.66 (<0.001) | ||
| Depressive symptoms | 0.09 (<0.001) | 0.06 (<0.001) | ||
| Hypertension | CV-MSK | 0.75 (<0.001) | CV-MSK-METAB | 0.92 (<0.001) |
| Diabetes | 0.18 (0.005) | 0.42 (<0.001) | ||
| Cancer | 0.10 (<0.001) | 0.20 (<0.001) | ||
| Lung disease | 0.02 (0.324) | 0.04 (0.267) | ||
| Heart disease | 0.19 (0.001) | 0.32 (<0.001) | ||
| Stroke | 0.06 (0.05) | 0.10 (<0.001) | ||
| Arthritis | 0.52 (<0.001) | 0.73 (<0.001) | ||
| Depressive symptoms | 0.12 (<0.001) | 0.05 (<0.001) | ||
| Hypertension | CV-MSK-MENTAL | 0.71 (<0.001) | MULTISYSTEM | 0.92 (<0.001) |
| Diabetes | 0.29 (<0.001) | 0.40 (<0.001) | ||
| Cancer | 0.17 (<0.001) | 0.27 (<0.001) | ||
| Lung disease | 0.26 (<0.001) | 0.32 (<0.001) | ||
| Heart disease | 0.60 (<0.001) | 0.70 (<0.001) | ||
| Stroke | 0.19 (<0.001) | 0.24 (<0.001) | ||
| Arthritis | 0.80 (<0.001) | 0.95 (<0.001) | ||
| Depressive symptoms | 0.36 (<0.001) | 0.28 (<0.001) | ||
Abbreviations: BIC = Bayesian Information Criteria, VLMR LR = Vuong-Lo-Mendell-Rubin likelihood ratio test, df = degrees of freedom; CV-MSK = Cardiovascular-Musculoskeletal; CV-MSK-MENTAL = Cardiovascular-Musculoskeletal-Mental; CV-MSK-METAB = Cardiovascular-Musculoskeletal-Metabolic. Notes: p-values based on bootstrapped VLMR test, which is supported by simulation work showing better performance at class comparison test and was used without complex sampling design adjustments because these are not available for these tests.
Table 2 also presents the conditional probabilities of each disease for each class of the three-class model for 1998 and 2014. The pattern of response probabilities suggests the three classes in 1998 are characterized as minimal disease (relatively disease-free), cardiovascular-musculoskeletal multimorbidity (elevated probability of hypertension and arthritis), and cardiovascular-musculoskeletal-mental multimorbidity (elevated probability of hypertension, heart disease, arthritis, and depressive symptoms). The three classes in 2014 are characterized as cardiovascular-musculoskeletal multimorbidity (elevated probability of hypertension and arthritis), cardiometabolic-musculoskeletal multimorbidity (elevated probability of hypertension, diabetes, heart disease, and arthritis), and multisystem multimorbidity (elevated probability of hypertension, diabetes, lung disease, heart disease, and arthritis). Figure 1 illustrates the number of respondents that change from each of the three identified latent classes in 1998 to the identified classes in 2014, or to death or lost to follow-up.
Figure 1.
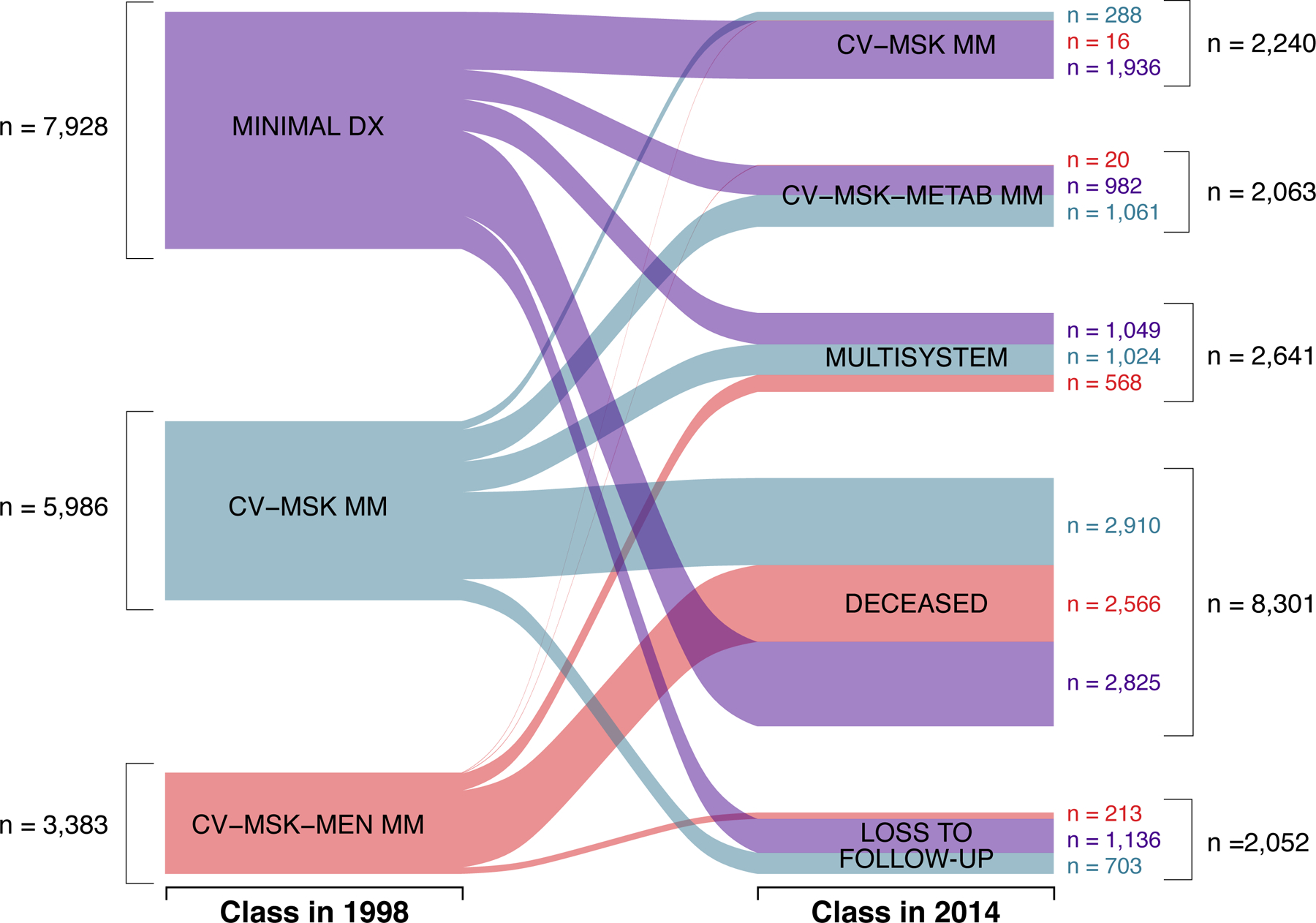
Changes for Study Participants from 1998 to 2014.
Abbreviations:
MINIMAL DX = Minimal Disease
CV-MSK MM = Cardiovascular-Musculoskeletal Multimorbidity
CV-MSK-MEN MM = Cardiovascular-Musculoskeletal-Mental
CV-MSK-METAB MM = Cardiometabolic-Musculoskeletal
Multimorbidity Class Changes
Table 3 summarizes the percentage of respondents, by racial/ethnic group, who change from their initial 1998 class to the multisystem multimorbidity class, death, or lost to follow-up by 2014. Percentages between racial/ethnic groups are relatively equivalent with a few notable exceptions: a slightly higher proportion of Black respondents shifted from cardiovascular-musculoskeletal-mental multimorbidity to the multisystem multimorbidity class (24% vs. 18% White, 20% Hispanic), and a slightly higher proportion of Hispanic respondents changed from cardiovascular-musculoskeletal multimorbidity to the multisystem multimorbidity class (24% vs. 20% White, 18% Black). With regard to within-racial/ethnic group changes, participants in the cardiovascular-musculoskeletal and cardiovascular-musculoskeletal-mental initial 1998 classes were significantly more likely to move to mortality or lost to follow-up relative to the minimal disease class across all three racial/ethnic groups.
Table 3.
Racial/Ethnic Differences in Changing to Multisystem Multimorbidity, Deceased, or Lost-to-Follow up status by 2014, by Initial Multimorbidity Class in 1998.
| % Changing to MULTISYSTEM Class in 2014 | |||||||
|---|---|---|---|---|---|---|---|
| Class in 1998 | Total | Black | Hispanic | White | |||
| MINIMAL | 15.00 | 13.36 | 13.43 | 15.22 | |||
| CV-MSK | 19.91 | 18.43* | 24.86 | 19.76*** | |||
| CV-MSK-MENTAL | 18.76 | 24.12*** | 19.79*** | 17.96*** | |||
| % Changing to DECEASED by 2014 | |||||||
| Total | Black | Hispanic | White | ||||
| MINIMAL | 33.73 | 37.51 | 29.83 | 33.73 | |||
| CV-MSK | 48.32 | 49.08* | 40.69** | 48.77*** | |||
| CV-MSK-MENTAL | 76.41 | 72.75** | 74.84* | 77.01*** | |||
| % Changing to LOST TO FOLLOW-UP by 2014 | |||||||
| Total | Black | Hispanic | White | ||||
| MINIMAL | 7.37 | 4.48 | 7.96 | 7.54 | |||
| CV-MSK | 5.96 | 3.30** | 4.54* | 6.47*** | |||
| CV-MSK-MENTAL | 3.55 | 2.01*** | 3.82*** | 3.74*** | |||
Abbreviations: MINIMAL= Minimal Disease; CV-MSK= Cardiovascular-Musculoskeletal; CV-MSK-MENTAL= Cardiovascular-Musculoskeletal-Mental; MULTISYSTEM= Multisystem. Notes: MINIMAL class is referent category for significance tests conducted within racial/ethnic group to each status in 2014,
p < .05,
p <.01,
p<.001.
For example, 18.43% of Black participants move from CV-MSK in 1998 to MULTISYSTEM in 2014, and are significantly more likely to move to MULTISYSTEM 2014 relative to Black participants in MINIMAL 1998.
A multinomial logistic regression model was used to examine racial/ethnic differences in membership in the three probable latent classes, lost-to-follow-up, and mortality categories in 2014, controlling for baseline class membership; the comparison category was the least morbid class (minimal disease in 1998 and cardiovascular-musculoskeletal in 2014). Two dummy variables representing comparisons in 1998 for the two other identified classes and the least morbid class were entered as predictors (cardiovascular-musculoskeletal vs. minimal and cardiovascular-musculoskeletal-mental vs. minimal) with a multiple categorical outcome of each of the three predicted classes in 2014 using a multinomial logistic model. Results are presented in Table 4, in which separate columns provide odds ratios for the outcome comparisons of change from the initial 1998 class to the cardiovascular-musculoskeletal-metabolic and the multisystem multimorbidity class (in 2014) compared with the predicted cardiovascular-musculoskeletal class (least morbid class in 2014), respectively. Model 3 demonstrates that participants predicted to be in the cardiovascular-musculoskeletal class in 1998 were significantly more likely to be assigned to the multisystem class (OR = 1.78, p < .001) in 2014 than those initially assigned in 1998 to the minimal disease class.
Table 4.
Odds of Changing to Later-Period Multimorbidity Classes, or to Deceased and Lost to Follow-Up Between 1998 and 2014, by Initial Multimorbidity Class and Race/Ethnicity.
| Status in 2014** OR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Status in 1998* | CV-MSK-METAB | MULTISYSTEM | DECEASED | LOST TO FOLLOW-UP | |||
| Model 1 | |||||||
| CV-MSK | 0.24 (0.19, 0.30) | 1.78 (1.40, 2.25) | 1.64 (1.36, 2.00) | 1.77 (1.47, 2.14) | |||
| CV-MSK-MENTAL | 0.04 (0.02,0.07) | 0.11 (0.07 0.17) | 2.60 (2.03, 3.33) | 4.71 (3.75, 5.91) | |||
| Model 2 | |||||||
| CV-MSK | 0.24 (0.19, 0.30) | 1.70 (1.34, 2.14) | 1.60 (1.32, 1.94) | 1.73 (1.44, 2.08) | |||
| CV-MSK-MENTAL | 0.04 (0.02, 0.07) | 0.11 (0.07, 0.16) | 2.54 (1.98, 3.26) | 4.60 (3.67, 5.77) | |||
| Black vs. White | 1.33 (0.94, 1.88) | 2.62 (1.78, 3.86) | 1.84 (1.27, 2.68) | 1.85 (1.28, 2.68) | |||
| Hispanic vs. White | 0.99 (0.64, 1.54) | 1.56 (0.96, 2.54) | 1.14 (0.81, 1.59) | 0.94 (0.64, 1.39) | |||
| Model 3 | |||||||
| CV-MSK | 0.25 (0.20, 0.32) | 1.78 (1.41, 2.26) | 1.62 (1.33, 1.97) | 1.44 (1.21, 1.71) | |||
| CV-MSK-MENTAL | 0.05 (0.03, 0.08) | 0.11 (0.07, 0.18) | 2.45 (1.87, 3.21) | 3.08 (2.44, 3.90) | |||
| Black vs. White | 1.40 (0.98, 2.00) | 2.60 (1.77, 3.82) | 1.62 (1.12, 2.36) | 2.11 (1.44, 3.08) | |||
| Hispanic vs. White | 1.22 (0.76, 1.96) | 1.67 (1.02, 2.74) | 0.97 (0.70, 1.35) | 0.91 (0.59, 1.39) | |||
| Age in 1998 | 0.97 (0.96, 0.98) | 0.98 (0.96, 0.99) | 0.99 (0.98, 1.01) | 1.12 (1.11, 1.13) | |||
| Female sex | 1.23 (1.04, 1.45) | 1.23 (1.05, 1.44) | 1.26 (1.09, 1.47) | 0.68 (0.60, 0.78) | |||
| Years of education | 1.09 (1.04, 1.13) | 1.04 (1.00, 1.08) | 0.98 (0.94 1.01) | 0.96 (0.93, 0.99) | |||
| Net worth in 1998 | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | 1.00 (1.00, 1.00) | |||
Abbreviations: CV-MSK= Cardiovascular-Musculoskeletal; CV-MSK-MENTAL= Cardiovascular-Musculoskeletal-Mental; CARDIOMET-MSK= Cardiometabolic-Musculoskeletal; MULTISYSTEM= Multisystem Multimorbidity.
Notes:
Multinomial logistic regression models to assess change from the CV-MSK or CV-MSK-MENTAL 1998 classes as compared with change from MINIMAL 1998 class (reference).
Change to the CV-MSK-METAB or MULTISYSTEM 2014 classes, or deceased or lost to follow-up by 2014, as compared with change to CV-MSK 2014 class (reference).
Racial/ethnic Differences in Morbidity Classes
A multinomial logistic regression model, using the cardiovascular-musculoskeletal class in 2014 as the referent category, examined differences among racial/ethnic groups in the likelihood of being assigned to the cardiometabolic-musculoskeletal or multisystem multimorbidity class in 2014 after controlling for predicted class membership in 1998, represented by two dummy variables, with White respondents as the referent group (Table 4). This analysis tests for significant differences in shifting to the cardiometabolic-musculoskeletal or multisystem multimorbidity class between racial/ethnic groups, accounting for initial class membership (19). After adjustment for covariates, Hispanic respondents had 1.7 greater odds (OR=1.67, p=0.042) and Black respondents had 2.6 greater odds (OR=2.60, p<.001) than White respondents to be assigned to the multisystem multimorbidity class in 2014 after controlling for class membership in 1998.
Differences in Death or Lost to Follow-up
Participants predicted to be in the cardiovascular-musculoskeletal-mental class in 1998 were 2.5 times more likely to have died (OR=2.45, p<.001) and over 3 times more likely to have been lost to follow-up (OR=3.08, p<.001) by 2014 than those initially assigned in 1998 to the minimal disease class. With regard to racial/ethnic differences among decedents and non-respondents in the follow-up period, Black respondents were 1.6 times more likely to have died (OR=1.62, p=.01) and over 2 times more likely to have been lost to follow-up (OR=2.11, p<.001) than White respondents after controlling for class membership in 1998. However, Hispanic respondents were similar to White respondents in their likelihood of death or being lost to follow-up by 2014.
DISCUSSION
This study identified latent disease classes in a nationally representative sample of middle-age and older adults across a 16-year period and estimated the likelihood of changing from initial to later disease classes or to the known disposition of death or loss to follow-up by the end of the observation window. Our findings demonstrated that individuals in the cardiovascular-musculoskeletal multimorbidity class in the beginning of the observation period were more likely to change to higher morbidity classes characterized either by additional clinically-concordant diseases (e.g., heart disease and diabetes) or to the multisystem multimorbidity class, characterized by multiple diseases, some of which may be non-concordant (e.g., lung disease). Unsurprisingly, those in the highest morbidity class in 1998 (cardiovascular-musculoskeletal-mental multimorbidity) were more likely to move to the highest morbidity class in 2014 (multisystem multimorbidity), and were also more likely to have died or been lost to follow-up by 2014. Changing from cardiovascular-musculoskeletal-mental multimorbidity in 1998 to the identified classes in 2014 is also noteworthy because of the absence of high depressive symptoms characterizing any of the 2014 classes, a clinically-plausible change given fluctuations and potential reversibility of depressive symptomatology.
There are additional important considerations when contextualizing these changes in multimorbidity profiles over this 16-year period. Approximately three-fourths of those in the most burdensome or “complex” class in 1998, cardiovascular-musculoskeletal-mental, die by the end of the study period. Similarly, approximately half of those in the “intermediate” initial-period cardiovascular-musculoskeletal class die in the intervening follow-up period, and approximately one-sixth progress to each of the two more burdensome classes in 2014, characterized by advanced cardiovascular (heart disease), metabolic (diabetes), or respiratory (lung disease) conditions. Interestingly, approximately one-quarter of those in the minimal class of the initial period experience “healthy aging”: after 16 years of follow-up, they are likely to be in a relatively healthy class characterized only by elevated probabilities of hypertension and arthritis. Approximately one-third of the minimal class transition to death, indicating that the intervening period saw the development of a deadly event that may be related or unrelated to their chronic disease burden, such as a stroke, heart attack, or a fatal injury.
There were also important racial/ethnic differences in changes to multimorbidity classes characterized by higher morbidity. Hispanic and Black Americans were more likely than White Americans to change to the multisystem multimorbidity class, and Black Americans were more likely to have died or to have been lost to follow-up over the intervening period than White Americans. This finding is in agreement with prior studies that showed an earlier and more rapid progression of chronic disease accumulation for Black middle-aged and older adults (2) and suggests that these demographic groups should be at the center of preventive clinical and public health efforts aimed at slowing the accumulation of chronic diseases and reducing inequities in multimorbidity and its consequences in later life.
This represents an important contribution with substantial clinical implications, particularly for higher risk populations experiencing substantial changes in their health status over time. Several recent studies have explored empirical methods to identify multimorbidity combinations, including latent class methodologies (11,17,20–25). However, few studies have focused on understanding changes in multimorbidity combinations over time in a given population (20,26,27). Further, only a small contingent attempt to account for social factors associated with class membership in the more burdensome and potentially deleterious multimorbidity combinations.
The present study is largely consistent with studies that identify multimorbidity classes characterized by relatively healthy, cardiovascular, musculoskeletal, and high multimorbidity disease combinations (20,23,25) and also supports recent work documenting earlier onset, greater burden of chronic disease, more rapid accumulation, and earlier mortality among racial/ethnic minority populations in the U.S. (2). That Black and Hispanic Americans are more likely to change to the multisystem multimorbidity class relative to White Americans speaks to the accumulation of cardiovascular and metabolic diseases for this demographic group. To this point, a recent investigation (2) of racial/ethnic differences in multimorbidity accumulation noted that middle-aged Black Americans reached the multimorbidity threshold earlier than White Americans—approximately 4 years sooner—and as a result are entering late middle-age with a greater likelihood of being in high burden multimorbidity classes. In contrast, White Americans spend a greater proportion of their midlife relatively disease-free, thus staying relatively healthy for extended periods of their lifespan.
This study benefits from numerous strengths. First, the HRS represents a longstanding and robust set of longitudinal data that largely generalize to middle-aged and older adult populations in the U.S. Second, this study leverages the longitudinal design of the HRS to identify underlying multimorbidity combinations and changes over a 16-year period, a substantial period that follows participants from middle to later life. Third, this study identifies distinct latent classes of chronic disease at the beginning and end of this 16-year period and specifies whether Black, White, or Hispanic adults are more likely to change to end-period latent classes, death or are lost to follow-up. Thus, this study provides insight into health disparities in middle-age to older-age that stem from disease combinations that reflect greater progression and sequelae.
Several limitations should also be noted. First, while cognitive impairment and dementia represent important disease domains in the conceptualization of multimorbidity, cognitive impairment was not included in our assessment of latent classes due to low prevalence in a community-dwelling population and subsequent model convergence issues. Given the vast challenges of managing cognitive impairment alongside additional chronic diseases, it will be important for future studies to assess changes in multimorbidity combinations that include cognitive impairment utilizing other methods. Second, chronic disease diagnoses were self-reported, which may be subject to underreporting. Still, several studies have shown adequate concordance between patient reports of various physician-diagnosed chronic diseases and administrative or clinical data sources (28,29), and highlight the importance of documenting patient-reported outcomes of disease status (30–32). Further, to address clinically-inconsistent longitudinal patterns of disease reporting, we applied a previously developed adjudication procedure to minimize the extent of these inconsistent patterns. Third, we were constrained by software limitations. While the parametric bootstrapped likelihood ratio test (33) is known to have greater statistical power for selecting the number of latent classes (13, 22, 23), this method was not available with sampling design adjustments. Finally, while we assess shifts from initial-period multimorbidity classes to known states of death or lost to follow-up, this study is not designed to examine the contribution of disease combinations that precede mortality events or time to mortality events. These remain important points for future study.
CONCLUSIONS
Our findings have important clinical and research implications. Understanding how multimorbidity classes and combinations change over time is an important advancement with implications for programs, policy, and care provided by clinicians and the health care systems charged with treating and managing increasingly complex chronic disease profiles for a growing and heterogeneous population of older adults. Future work should focus on specific health-related consequences of multimorbidity combinations and identifying temporal changes that are predictive of rapid health downturns and earlier mortality.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging at the National Institutes of Health (R01AG055681 to ARQ, R01AG047891 to HGA who contributed from the Yale Claude D. Pepper Older Americans Independence Center P30AG021342). Content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Footnotes
Competing interest
All authors declare that they have no competing interests.
Ethics Committee Approval: The Health Sciences Institutional Review Board (IRB) at the University of Michigan has approved the HRS and the IRB at Oregon Health & Science University has approved this study.
REFERENCES
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet. 2012;380(9836):37–43. [DOI] [PubMed] [Google Scholar]
- 2.Quiñones AR, Botoseneanu A, Markwardt S, Nagel CL, Newsom JT, Dorr DA, et al. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLOS ONE. 2019. June 17;14(6):e0218462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salive ME. Multimorbidity in older adults. Epidemiologic reviews. 2013;35:75–83. [DOI] [PubMed] [Google Scholar]
- 4.Tinetti ME, Fried T. The end of the disease era. The American Journal of Medicine. 2004;116(3):179–85. [DOI] [PubMed] [Google Scholar]
- 5.Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity Combinations and Disability in Older Adults. Journals of Gerontology: Medical Sciences. 2016;71(6):823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quiñones A, Markwardt S, Thielke S, Rostant O, Vásquez E, Botoseneanu A. Prospective Disability in Different Combinations of Somatic and Mental Multimorbidity. J Gerontol A Biol Sci Med Sci. 2018. January 16;73(2):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei MY, Mukamal KJ. Multimorbidity, Mortality, and Long-Term Physical Functioning in 3 Prospective Cohorts of Community-Dwelling Adults. Am J Epidemiol. 2018. January 1;187(1):103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei MY, Levine DA, Zahodne LB, Kabeto MU, Langa KM. Multimorbidity and Cognitive Decline Over 14 Years in Older Americans. J Gerontol A Biol Sci Med Sci [Internet]. [cited 2019 Aug 12]; Available from: https://academic.oup.com/biomedgerontology/advance-article/doi/10.1093/gerona/glz147/5512423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quiñones AR, Liang J, Bennett JM, Xu X, Ye W. How does the trajectory of multimorbidity vary across Black, White, and Mexican Americans in middle and old age? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(6):739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson-Lawrence V, Zajacova A, Sneed R. Education, race/ethnicity, and multimorbidity among adults aged 30–64 in the National Health Interview Survey. SSM - Population Health. 2017. December;3:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitson HE, Johnson KS, Sloane R, Cigolle CT, Pieper CF, Landerman L, et al. Identifying Patterns of Multimorbidity in Older Americans: Application of Latent Class Analysis. J Am Geriatr Soc. 2016. August 1;64(8):1668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiñones AR, Allore HG, Botoseneanu A, Newsom JT, Nagel CL, Dorr DA. Tracking Multimorbidity Changes in Diverse Racial/Ethnic Populations Over Time: Issues and Considerations. J Gerontol A Biol Sci Med Sci. 2020. January 20;75(2):297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koroukian SM, Schiltz NK, Warner DF, Sun J, Stange KC, Given CW, et al. Multimorbidity: constellations of conditions across subgroups of midlife and older individuals, and related Medicare expenditures. Journal of Comorbidity. 2017. April 10;7(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. Ann Arbor, Michigan: Institute for Social Research, University of Michigan; 2000. [Google Scholar]
- 15.Cigolle CT, Nagel CL, Blaum CS, Liang J, Quiñones AR. Inconsistency in the Self-report of Chronic Diseases in Panel Surveys: Developing an Adjudication Method for the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2018. June 14;73(5):901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muthén LK, Muthen B. Mplus User’s Guide, Eigth Ediition. Los Angeles, CA: Muthén & Muthén; 2017. [Google Scholar]
- 17.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural equation modeling. 2007;14(4):535–69. [Google Scholar]
- 18.Heeringa SG, West BT, Berglund PA, West BT, Berglund PA. Applied Survey Data Analysis [Internet]. Chapman and Hall/CRC; 2017. [cited 2019 Mar 13]. Available from: https://www.taylorfrancis.com/books/9781498761611 [Google Scholar]
- 19.Newsom JT. Basic longitudinal analysis approaches for continuous and categorical variables. In: Newsom JT, Jones RN, Hofer SM, editors. Longitudinal Data Analysis: A Practical Guide for Researchers in Aging, Health, and Social Science. New York: Routledge; 2012. p. 143–79. [Google Scholar]
- 20.Strauss VY, Jones PW, Kadam UT, Jordan KP. Distinct trajectories of multimorbidity in primary care were identified using latent class growth analysis. Journal of clinical epidemiology. 2014;67(10):1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen FB, Pedersen MH, Friis K, Glümer C, Lasgaard M. A Latent Class Analysis of Multimorbidity and the Relationship to Socio-Demographic Factors and Health-Related Quality of Life. A National Population-Based Study of 162,283 Danish Adults. PLOS ONE. 2017. January 5;12(1):e0169426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olaya B, Moneta MV, Caballero FF, Tyrovolas S, Bayes I, Ayuso-Mateos JL, et al. Latent class analysis of multimorbidity patterns and associated outcomes in Spanish older adults: a prospective cohort study. BMC Geriatrics. 2017. August 18;17(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonsoulin ME, Durazo-Arvizu RA, Goldstein KM, Cao G, Zhang Q, Ramanathan D, et al. A Health Profile of Senior-Aged Women Veterans: A Latent Class Analysis of Condition Clusters. Innov Aging [Internet]. 2017. September 1 [cited 2019 Aug 13];1(2). Available from: https://academic.oup.com/innovateage/article/1/2/igx024/4643008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and Comorbidity of Chronic Diseases among the Senior Australians: Prevalence and Patterns. PLOS ONE. 2014. January 8;9(1):e83783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen OK, Makam AN, Halm EA. National Use of Safety-Net Clinics for Primary Care among Adults with Non-Medicaid Insurance in the United States. PLOS ONE. 2016. March 30;11(3):e0151610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng C, Ellis JL, Steiner JF, Shoup JA, McQuillan DB, Bayliss EA. Assessment of morbidity over time in predicting health outcomes. Medical care. 2014. March;52 Suppl 3:S52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vos R, van den Akker M, Boesten J, Robertson C, Metsemakers J. Trajectories of multimorbidity: exploring patterns of multimorbidity in patients with more than ten chronic health problems in life course. BMC Family Practice. 2015. January 22;16(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of clinical epidemiology. 2004;57(10):1096–103. [DOI] [PubMed] [Google Scholar]
- 29.Skinner KM, Miller DRS, Lincoln EM, Lee A, Kazis LES. Concordance Between Respondent Self-reports and Medical Records for Chronic Conditions: Experience From the Veterans Health Study. Journal of Ambulatory Care Management Health Status Measurement and Ambulatory Space Management. 2005. June;28(2):102–10. [DOI] [PubMed] [Google Scholar]
- 30.Petrie KJ, Jago LA, Devcich DA. The role of illness perceptions in patients with medical conditions. Current opinion in psychiatry. 2007. March;20(2):163–7. [DOI] [PubMed] [Google Scholar]
- 31.Leventhal EA, Crouch M. Are there differences in perceptions of illness across the lifespan? In: Petrie KJ, Weinman JA, editors. Perceptions of health and illness. New York, NY: Routledge; 1997. p. 77–102. [Google Scholar]
- 32.Giles WH, Croft JB, Keenan NL, Lane MJ, Wheeler FC. The validity of self-reported hypertension and correlates of hypertension awareness among blacks and whites within the stroke belt. American Journal of Preventive Medicine. 1995. June;11(3):163–9. [PubMed] [Google Scholar]
- 33.McLachlan G, Peel D. Finite Mixture Models. John Wiley & Sons; 2004. 450 p. [Google Scholar]
- 34.Asparouhov T, Muthen B. Using Mplus TECH11 and TECH14 to test the number of latent classes. MPlus Web Notes. 2012;14. [Google Scholar]
- 35.Bray BC, Lanza ST, Tan X. Eliminating Bias in Classify-Analyze Approaches for Latent Class Analysis. Structural Equation Modeling: A Multidisciplinary Journal. 2015. January 2;22(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


