Abstract
Background: Chronic suppurative otitis media (CSOM) is a common otological problem in daily clinical practice. It is crucial to know the bacterial pathogens and their antimicrobial susceptibilities in patients with CSOM to achieve a good clinical outcome.
Objectives: To identify the aerobic bacterial pathogens and their antibiotic sensitivities in subjects with CSOM in Al-Ramadi Teaching Hospital.
Materials and Methods: A cross-sectional, descriptive study included 102 subjects with a clinical diagnosis of CSOM (aural discharge >2 weeks, eardrum perforation, and conductive deafness). Purulent discharge was obtained from the middle ear with sterile swabs and cultured for bacterial microorganisms. The sensitivity of the isolated agents to antimicrobials was evaluated by a culture and sensitivity method. SPSS version 22 was used for statistical analysis of the data. Differences were considered statistically significant at p < 0.05.
Results: Out of 3634 outpatient subjects, 102 (2.8%) presented with active CSOM. The age range of the patients was 1–70 years (mean 28.90 ± 19.8). There were 58 females (56.9%). Out of 117 ear swab specimens, 107 (91.5%) yielded positive cultures. The majority (101, 94.4%) of the specimens yielded a single organism. There was a high statistically significant difference (p < 0.001) between gram-positive (n = 77, 68.1%) and gram-negative pathogens (n = 36, 31.9%). Pseudomonas aeruginosa in 65 (57.5%) cases and Staphylococcus aureus in 19 (16.8%) cases were the two most commonly isolated organisms. The drugs imipenem (93.8%), amikacin (86.1%), azteronam (83.1%), and ciprofloxacin (81.5%) were effective against P. aeruginosa (p < 0.001). Amikacin (100%), imipenem (94.7%), ciprofloxacin (68.4%), and gentamicin (63.1%) were the most effective antibiotics against Staph. aureus (p < 0.001).
Conclusion: The prevalence rate of active CSOM was 2.8%. Ciprofloxacin showed high effectiveness against the two most common isolated pathogens (P. aeruginosa and Staph. aureus); therefore, it could be used as empirical therapy for active CSOM cases.
Keywords: Otitis media, Chronic suppurative otitis media, Antibiotic sensitivity, Aerobic bacteria, Ciprofloxacin
Introduction
Otitis media (OM) is defined as a group of complex inflammatory conditions involving the middle ear and caused by infection.1 OM is a common disease in Ramadi city with a prevalence of 13.65%.2 One-third of OM is of the chronic suppurative OM (CSOM) type.2
CSOM is characterized by continuous or intermittent ear discharge and conductive deafness. The disease might injure the internal ear, leading to permanent sensorineural deafness, as well as other extra or intracranial complications.3
CSOM occurs due to different bacteria affecting the middle ear cleft. Different investigations from various nations have shown that common microorganisms cause CSOM. Pseudomonas aeruginosa is the common agent in some studies, while other researchers have reported that Staphylococcus aureus is the predominant microorganism.4 The differences among these types of studies might be due to differences in the studied patients’ samples and geographic locations. CSOM is usually treated by aural cleansing and topical antibiotic drops. Systemic antimicrobial drugs are reserved for those cases with complications or failure of the initial treatment. According to the clinical background of the disease, empirical treatment with antibiotics is started while awaiting the results of culture and sensitivities. However, this regime might result in the emergence of resistant microorganisms. Early studies have evaluated the antibiotic susceptibility and resistance patterns of bacterial microorganisms.5–7
The pathogenesis and causes of CSOM are still poorly understood.4 Therefore, it is of the utmost importance to assess the frequencies of involved microorganisms in various geographical locations. Moreover, knowing the different bacterial pathogens and their antibiotic sensitivity is necessary before choosing the appropriate empirical antibiotic. Therefore, we aimed to determine the causative aerobic bacterial agents and their antibiotic sensitivities in CSOM cases in Al-Ramadi Teaching Hospital.
Materials And Methods
A cross-sectional, descriptive study was conducted at Al-Ramadi Teaching Hospital, and the Microbiology Laboratory in the College of Medicine, University of Anbar, Anbar province, Iraq. The study covered the period from November 2018 to April 2019. One hundred two patients with a clinical diagnosis of CSOM (unilateral or bilateral) were enrolled. The study was approved by the Ethical Committee of the University of Anbar (reference number 68 on 29-5-2019), and written informed consent was obtained from every patient or their parents. Patient selection was performed according to the flow chart (Figure. 1).
Figure 1.
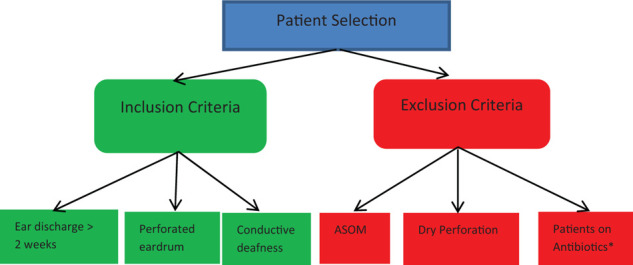
Flow chart of patient selection (ASOM = acute suppurative otitis media, * = patients on antibiotics 7 days before presentation).
The samples were taken by an otolaryngologist after cleaning the ear canal of the excess purulent exudate with a spirit swab. The swabs were collected from the discharging ears by inserting a sterile specimen stick deep in the canal. The collected samples were placed in transport media (Amies media) and then transported to the microbiology laboratory. The samples from bilaterally discharging ears were collected separately. The swabs were inoculated on nutrient agar plates, blood agar plates, MacConkey agar plates, and Sabouraud dextrose agar plates then incubated for 48 hours at 37°C. The agar plates were examined after 24 and 48 hours. Plates showing no growth with 48 hours were recorded as negative cultures. Bacteria showing growth was identified by standard techniques based on morphological, cultural, and biochemical characteristics. Antimicrobial sensitivities were carried out using the standard disc diffusion technique8 as shown in Figure 2.
Figure 2.

Sensitivity of P. aeruginosa isolated from a patient with CSOM to 12 antibiotics.
Data were analyzed using IBM SPSS software version 22. The results were presented in tables as frequencies and percentages. A chi-square test was used for comparisons between variables, and differences were considered statistically significant at p < 0.05.
Results
Out of 3634 patients seen during the studied period, 102 (2.8%) presented with active unilateral or bilateral CSOM and were enrolled in the study. The age of our patients was between 1 and 70 years (mean 28.90, SD ± 19.8). The youngest age group affected was < 10 (26, 25.5%), and the oldest was ≥ 70 years 1 (1%). There were 44 males (43.1%) and 58 females (56.9%), and the male-to-female ratio was 1/1.31. There were no statistically significant differences between the age and gender of the CSOM patients (p>0.05) (Table 1).
Table 1.
Distribution of age and gender in 102 patients with CSOM.
| Age group (years) | Total | Males | Females | p value | |||
|
| |||||||
| N | % | N | % | N | % | ||
|
| |||||||
| < 10 | 26 | 25.5% | 14 | 31.8% | 12 | 20.7% | 0.912 |
|
| |||||||
| 10–19 | 14 | 13.7% | 7 | 15.9% | 7 | 12.1% | 0.619 |
|
| |||||||
| 20–29 | 13 | 12.7% | 5 | 11.4% | 8 | 13.8% | 0.797 |
|
| |||||||
| 30–39 | 12 | 11.8% | 2 | 4.5% | 10 | 17.2% | 0.395 |
|
| |||||||
| 40–49 | 18 | 17.6% | 8 | 18.2% | 10 | 17.2% | 0.809 |
|
| |||||||
| 50–59 | 11 | 10.8% | 4 | 9.1% | 7 | 12.1% | 0.572 |
|
| |||||||
| 60–69 | 7 | 6.9% | 3 | 6.8% | 4 | 6.9% | 0.421 |
|
| |||||||
| ≥ 70 | 1 | 1% | 1 | 2.3% | 0 | 0 | . |
|
| |||||||
| Total | 102 | 100% | 44 | 100% | 58 | 100% | 0.946 |
|
| |||||||
N=Number
Out of 102 patients, 78 (76.5%) had unilateral active disease (39 for each side) and 24 (23.5%) had bilateral (15 of them had bilateral, and 9 had unilateral active disease). Therefore, 117 ear discharge specimens were collected. One hundred seven (91.5%) were positive cultures and 10 (8.5%) were negative. The majority (101, 94.4%) of the specimens yielded a single organism, and 2 pathogens were isolated from 6 (5.6%); therefore, the total number of isolates was 113. The three most commonly isolated microorganisms were P. aeruginosa 65 (57.5%), Staph. aureus 19 (16.8%), and Staph. epidermidis 17 (15.1%). The least commonly isolated organism was E. coli, in 3 (2.7%). There was a high statistically significant difference between gram-negative and gram-positive microorganisms (p < 0.001) (Table 2).
Table 2.
The microorganisms isolated from 107 specimens.
| Pathogens | Frequency | Percentage | p value |
|
| |||
| Gram-negative | |||
|
| |||
| P. aeruginosa | 65 | 57.5 | |
|
| |||
| K. pneumonia | 5 | 4.4 | |
|
| |||
| Proteus spp. | 4 | 3.5 | |
|
| |||
| E. coli | 3 | 2.7 | < 0.001 |
|
| |||
| Total | 77 | 68.1 | |
|
| |||
| Gram-positive | |||
|
| |||
| Staph. aureus | 19 | 16.8 | |
|
| |||
| Staph. epidermidis | 17 | 15.1 | |
|
| |||
| Total | 36 | 31.9 | |
|
| |||
P. aeruginosa isolates were mostly sensitive to the drugs imipenem (93.8%), amikacin (86.1%), aztreonam (83.1%), and ciprofloxacin (81.5%). The least effective antibiotic was metronidazole (1.5%). There was a high statistically significant difference in the antibiotic sensitivities of P. aeruginosa (p < 0.001) (Table 3).
Table 3.
The 12 antibiotics susceptibilities against the 65 isolated P. aeruginosa.
| Antibiotic | Sensitive | Resistant | Percentage of sensitive pathogens |
|
| |||
| Imipenem | 61 | 4 | 93.8 |
|
| |||
| Amikacin | 56 | 9 | 86.1 |
|
| |||
| Aztreonam | 54 | 11 | 83.1 |
|
| |||
| Ciprofloxacin | 53 | 12 | 81.5 |
|
| |||
| Gentamicin | 38 | 27 | 58.4 |
|
| |||
| Amoxicillin /Clavulanic acid | 8 | 57 | 12.3 |
|
| |||
| Ceftriaxone | 6 | 59 | 9.2 |
|
| |||
| Cefixime | 6 | 59 | 9.2 |
|
| |||
| Amoxicillin | 4 | 61 | 7.6 |
|
| |||
| Ceftazidime | 4 | 61 | 7.6 |
|
| |||
| Metronidazole | 1 | 64 | 1.5 |
|
| |||
| Penicillin G | 1 | 64 | 1.5 |
|
| |||
p < 0.001
The majority of Staph. aureus isolates were more sensitive to amikacin (100%), imipenem (94.7%), ciprofloxacin (68.4%), and gentamicin (63.1%). On the other hand, the least effective antimicrobial was metronidazole (5.2%). There was a high statistically significant difference in the antibiotic sensitivities of Staph. aureus (p < 0.001) (Table 4).
Table 4.
Susceptibilities of 19 Staph. aureus isolates to 12 antibiotics.
| Antibiotic | Sensitive | Resistant | Percentage of sensitive pathogens |
|
| |||
| Amikacin | 19 | 0 | 100 |
|
| |||
| Imipenem | 18 | 1 | 94.7 |
|
| |||
| Ciprofloxacin | 13 | 6 | 68.4 |
|
| |||
| Gentamicin | 12 | 7 | 63.1 |
|
| |||
| Aztreonam | 7 | 12 | 36.8 |
|
| |||
| Ceftriaxone | 5 | 14 | 26.3 |
|
| |||
| Amoxicillin /Clavulanic acid | 3 | 16 | 15.7 |
|
| |||
| Amoxicillin | 3 | 16 | 15.7 |
|
| |||
| Cefixime | 2 | 17 | 10.5 |
|
| |||
| Penicillin G | 2 | 17 | 10.5 |
|
| |||
| Ceftazidime | 1 | 18 | 5.3 |
|
| |||
| Metronidazole | 1 | 18 | 5.3 |
|
| |||
p < 0.001
Discussion
The prevalence ratio of active CSOM in this study was 2.8%. This ratio was lower than that reported by Al-Ani (one-third of the total prevalence ratio of OM 13.65%)2 who had reported the prevalence rate of OM in the same city (Ramadi city). This difference was due to the difference in the inclusion criteria between the two studies (active diseases in our study vs active and inactive diseases in Al- Anis' study). CSOM is usually developed following the first attack of ASOM and is characterized by an intermittent or continuous aural discharge through a perforated eardrum. It is an important cause of conductive deafness, especially in developing countries.9 Failure to eradicate the infection might result in extracranial or intracranial complications. Therefore, one of the essential steps in the management of this disease is the identification of the causative pathogen and the appropriate antimicrobial therapy through culture and sensitivity tests.
Approximately 20% of our patients were the age group < 10 years. Mofatteh et al., have reported that the most common age group affected was 21–30.6 A recent study from China showed that the age group 51–60 was the most affected.10 In contrast, many investigations have reported that the majority of CSOM cases were < 20 years of age.11–14 Despite the various studies have related to the age of patients, this disease is more common in children.4 These studies have not taken into account the duration of the disease; therefore, we unable to determine the beginning of the disease and the actual age of infection. From these results, we could conclude that in studies with a high prevalence of CSOM in children, the disease is still common in their geographical locations. The opposite is true for studies that recorded a high percentage of adults in their regions; the disease is less common in their countries, and its control has improved.
In the current study, there was a predominance (but statistically insignificant difference) of female compared with male CSOM subjects, which is consistent with previous investigations.6,11,15 The reason for this is unclear, but it might be related to the difference in propensity to seek medical advice between the two sexes, as CSOM has been reported to affect the two sexes equally.4 However, other studies have shown a predominance of males in CSOM subjects.7,16 The difference among various studies may be attributed to the samples studied in different geographical areas.
The pathogenesis and different causative microorganisms of CSOM are not well understood. Therefore, it is necessary to study the prevalence of different microorganisms in different geographical locations.4 Understanding the local prevalence of the disease with their causative pathogens and antibiotics susceptibilities is crucial for choosing the most effective empirical antibiotic as well as understanding the general course of the infection.17 Moreover, the dominant microorganisms and their antimicrobial susceptibility have changed from time to time. Therefore, it is essential to perform continuous and periodic surveillance to guide the selection of appropriate antibiotics.5
Our results showed that 91.5% culture positivity, which is consistent with other investigations6,12,13,18,19 and in contrast to other studies.10,20 These differences may be due to differences in technique, sample size, infection by slowly replicating microbes, and/or local hygienic conditions. Previous studies16 reported that polymicrobial cultures are most commonly isolated from the middle ear discharge of CSOM cases. Other studies have reported nearly equal percentages of single and multiple organisms isolated from aural pus specimens.12,13 Our results and the prior investigation11 concluded that the majority of ear swabs yielded single pathogens.
The majority of our isolated microorganisms were gram-negative bacteria, similar to results from Nigeria,7 Tanzania,13 Malawi,12 and Angola16 but in contrast to results from Korea,5 China,10 Bangladesh,21 and Iran.6,22 This could be explained by the fact that different pathogens cause CSOM in various geographic areas.
The study by Neeff et al.,23 was the first investigation to report a comprehensive microbiological profile of the middle ear cleft in healthy controls and subjects with CSOM groups. The study used conventional culture and polymerase chain reaction (PCR) techniques for the identification of microorganisms from both the middle ear and mastoid cavity. The major outcome of the study was that many healthy middle ears contain bacteria; this finding opposed the findings of the prior investigation.24 The study by Neeff et al., also showed that culture-based methods underestimated the presence of different pathogens within the middle ear cleft in comparison with real-time PCR. The PCR technique reported that Staph. aureus and other gram-positive and gram-negative pathogens were found in both healthy controls and patients with CSOM.
The current study reported that P. aeruginosa was the most common isolated microorganism in individuals with CSOM. This finding is similar to other investigations.11,14,25 A study from Iran6 found that Staphylococci spp. were the most isolated pathogens, with a percentage of 64.9%. Staph. aureus was the most isolated microorganism from middle ear discharge of CSOM subjects in other studies.7,21,22,26 Uddén et al.,16 have found that Proteus spp. were the most prevalent isolated organisms. The study by Chirwa et al.,12 has reported that Proteus mirabilis was the most commonly isolated organism. While Abraham et al.,13 have shown that K. pneumonia was the commonly cultured pathogen. In a systematic review by Coleman et al.,27 it was reported that P. aeruginosa and Staph. aureus were commonly identified in aural discharge of children with CSOM. These differences among various studies might be attributed to differences among geographical locations. As a result of these differences among various locations, otolaryngologists cannot depend solely on empirical antibiotics to eradicate infection in the affected CSOM subjects. Therefore, local continuous and periodic surveillance are essential steps in the management of this disease.
Bacterial biofilm in the middle ear cavity may act as a reservoir for certain bacteria in the causation of recurrent or continuous ear discharge in subjects with CSOM.28 Successful eradication of such bacterial behavior may have the benefit of controlling this type of infection, its consequences and complications, and avoiding the development of antibiotics resistance. A prior study28 from Greenland showed that biofilm was detected in 81% (17/21) of the initial aural discharge episodes. Non-typeable Haemophilus influenzae (NTHI), Staph. aureus, and anaerobes were the most frequent isolates. Of the 13 recurrent cases, 3 subjects had the same genotype of pathogen in a subsequent episode, 2 had pathogens of the same phenotype (NTHI), and the remaining 8 had novel pathogens. The study concluded that there was a high rate of biofilm in CSOM, but with limited clinical benefit.
In our study, P. aeruginosa showed excellent sensitivity to imipenem and very good sensitivity to amikacin, aztreonam, and ciprofloxacin. However, it had a high resistance to the following antibiotics: amoxicillin/clavulanic acid, ceftriaxone, cefixime, amoxicillin, ceftazidime, metronidazole, and penicillin G. The most sensitive antibiotics to P. aeruginosa were imipenem, ciprofloxacin, and amikacin in a study from Iran,6 which was consistent with our findings. A study from India11 reported that the most effective antimicrobial against P. aeruginosa was amikacin, followed by imipenem and piperacillin plus tazobactam. The most effective antibiotics against P. aeruginosa as reported by Garba et al., from Nigeria7 were ciprofloxacin and nitrofurantoin. P. aeruginosa showed 100% susceptibility to piperacillin, piperacillin/tazobactam, and meropenem in a recent study from China.10 Another study from Iran showed high sensitivity of P. aeruginosa to ciprofloxacin and cephalexin.22 The most effective antibiotic against P. aeruginosa was gentamicin, as reported by a study from Southeastern Nigeria.25 Various studies have reported different susceptibilities of Staph. aureus to antimicrobials. Mofatteh et al.,6 have shown that clindamycin and ciprofloxacin were the most effective drugs. A prior study from India has also reported that vancomycin was effective against all Staph. aureus isolates.11 A study from China found that Staph. aureus showed the highest sensitivity to vancomycin (100%), gentamicin (98.1%), and rifampicin (97.2%),10 while our results showed that amikacin was effective against all Staph. aureus isolates, imipenem was effective against 94.7%, and ciprofloxacin against 68.4%. The differences in the effectiveness of antibiotics against the pathogens causing CSOM in different investigations may be related to differences in the geographical areas, local prescription of antibiotics, and the prevalence of resistant pathogen species. Ciprofloxacin can be used as an empirical antibiotic for the treatment for CSOM cases in our locality because it is effective in considerable numbers of the two most common pathogens.
Nowadays, advanced molecular-based techniques are in current use, and can depend on PCR, microfluidics, and nanotechnology for rapid and perfect testing.29 Some of them are able to detect the causative pathogens and their antibiotic susceptibilities in less than an hour.30 Unfortunately, due to the limited use of real-time PCR and the unavailability of other modern techniques in our city, we did not use them in the identification of the pathogens from ear discharge. Therefore, we could not cover all microorganisms in our study. The other shortcoming of the current study was the small sample size.
In conclusion, the prevalence of active CSOM was 2.8%. P. aeruginosa was the most common isolated pathogen followed by Staph. aureus. Ciprofloxacin could be used as an empirical treatment for CSOM because our results have shown that it could be effective in eradicating the two most common pathogens in a considerable number of isolates. However, ear discharge should be sent for culture and sensitivity testing to determine antimicrobial sensitivity patterns.
References
- 1. Dickson G. Acute otitis media. Prim Care Clin Off Pract. 2014; 41(1):11-8. [DOI] [PubMed]
- 2. Al-Ani R. Prevalence of otitis media among Patients attending otorhinolaryngology clinic in Ramadi City/Iraq. Egypt J Ear Nose Throat Allied Sci. 2020; 21(1):17-21.
- 3. Verhoeff M, van der Veen EL, Rovers MM, Sanders EAM, Schilder AGM. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol. 2006; 70(1):1-12. [DOI] [PubMed]
- 4. Mittal R, Lisi CV, Gerring R, Mittal J, Mathee K, Narasimhan G, et al. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J Med Microbiol. 2015; 64(Pt 10):1103-16. [DOI] [PMC free article] [PubMed]
- 5. Yeo SG, Park DC, Hong SM, Cha CIl, Kim MG. Bacteriology of chronic suppurative otitis media–a multicenter study. Acta Otolaryngol. 2007; 127(10):1062-7. [DOI] [PubMed]
- 6. Mofatteh MR, Shahabian Moghaddam FS, Yousefi M, Namaei MH. A study of bacterial pathogens and antibiotic susceptibility patterns in chronic suppurative otitis media. J Laryngol Otol. 2018; 132(1):41-5. [DOI] [PubMed]
- 7. Bilkisu IG, Bello AM, Fatima M, Murtala R, Usman MS, Khadija OI, et al. Antibiotic susceptibility pattern of bacterial isolates in children with otitis media in Zamfara, North-Western Nigeria. Afr J Microbiol Res. 2017; 11(43):1558-63.
- 8. Maugeri G, Lychko I, Sobral R, Roque ACA. Identification and antibiotic-susceptibility profiling of infectious bacterial agents: a review of current and future trends. Biotechnol J. 2019; 14(1):e1700750. [DOI] [PMC free article] [PubMed]
- 9. Acuin J. Chronic suppurative otitis media: burden of illness and management options. In: Chronic suppurative otitis media: burden of illness and management options. 2004:83.
- 10. Xu J, Du Q, Shu Y, Ji J, Dai C. Bacteriological profile of chronic suppurative Otitis media and antibiotic susceptibility in a tertiary care hospital in Shanghai, China. Ear, Nose Throat J. 2020; 0145561320923823. [DOI] [PubMed]
- 11. Nazir A, Kadri SM. Aerobic bacteriology of chronic suppurative otitis media: a hospital based study. Int J Res Med Sci. 2014; 2(4):1521-5.
- 12. Chirwa M, Mulwafu W, Aswani JM, Masinde PW, Mkakosya R, Soko D. Microbiology of chronic suppurative otitis media at Queen Elizabeth central hospital, Blantyre, Malawi: a cross-sectional descriptive study. Malawi Med J. 2015; 27(4):120-4. [PMC free article] [PubMed]
- 13. Abraham ZS, Ntunaguzi D, Kahinga AA, Mapondella KB, Massawe ER, Nkuwi EJ, et al. Prevalence and etiological agents for chronic suppurative otitis media in a tertiary hospital in Tanzania. BMC Res Notes. 2019; 12(1):429. [DOI] [PMC free article] [PubMed]
- 14. Shyamala R, Reddy PS. The study of bacteriological agents of chronic suppurative otitis media–aerobic culture and evaluation. J Microbiol Biotechnol Res. 2012; 2:152-62.
- 15. Al-Marzoqi AH, Al-Janabi HSO, Hussein HJ, Taee ZMAl, Yheea SK. Otitis media; etiology and antibiotics susceptibility among children under ten years old in Hillah city, Iraq. J Nat Sci Res. 2013; 3(3):2224-3186.
- 16. Uddén F, Filipe M, Reimer Å, Paul M, Matuschek E, Thegerström J, et al. Aerobic bacteria associated with chronic suppurative otitis media in Angola. Infect Dis Pover. 2018; 7(1):42. [DOI] [PMC free article] [PubMed]
- 17. Brook I. The role of anaerobic bacteria in chronic suppurative otitis media in children: implications for medical therapy. Anaerobe. 2008; 14(6):297-300. [DOI] [PubMed]
- 18. Prakash M, Lakshmi K, Anuradha S, Swathi GN. Bacteriological profile and their antibiotic susceptibility pattern of cases of chronic suppurative otitis media. Asian J Pharm Clin Res. 2013; 6(3):210-2.
- 19. Agrawal A, Kumar D, Goyal A, Goyal S, Singh N, Khandelwal G. Microbiological profile and their antimicrobial sensitivity pattern in patients of otitis media with ear discharge. Indian J Otol. 2013; 19(1):5.
- 20. Kim SH, Kim MG, Kim SS, Cha SH, Yeo SG. Change in detection rate of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa and their antibiotic sensitivities in patients with chronic suppurative otitis media. J Int Adv Otol. 2015; 11(2):151-6. [DOI] [PubMed]
- 21. Akhtar N, Datta PG, Yasmin M, Naha A, Jewel AM, Hossen F, et al. Microbiology of chronic suppurative otitis media in a tertiary care hospital in Bangladesh. Mymensingh Med J MMJ. 2017; 26(3):592-9. [PubMed]
- 22. Mozafari Nia KM, Sepehri G, Khatmi H, Shakibaie MR. Isolation and antimicrobial susceptibility of bacteria from chronic suppurative otitis media patients in Kerman, Iran. Iran Red Crescent Med J. 2011; 13(12):891-4. [PMC free article] [PubMed]
- 23. Neeff M, Biswas K, Hoggard M, Taylor MW, Douglas R. Molecular microbiological profile of chronic suppurative otitis media. J Clin Microbiol. 2016; 54(10):2538-46. [DOI] [PMC free article] [PubMed]
- 24. Westerberg BD, Kozak FK, Thomas EE, Blondel-Hill E, Brunstein JD, Patrick DM. Is the healthy middle ear a normally sterile site? Otol Neurotol. 2009; 30(2):174-7. [DOI] [PubMed]
- 25. Orji FT, Dike BO. Observations on the current bacteriological profile of chronic suppurative otitis media in South eastern Nigeria. Ann Med Health Sci Res. 2015; 5(2):124-8. [DOI] [PMC free article] [PubMed]
- 26. Sahu MC, Swain SK. Surveillance of antibiotic sensitivity pattern in chronic suppurative otitis media of an Indian teaching hospital. World J Otorhinolaryngol Head Neck Surg. 2019; 5(2):88-94. [DOI] [PMC free article] [PubMed]
- 27. Coleman A, Wood A, Bialasiewicz S, Ware RS, Marsh RL, Cervin A. The unsolved problem of otitis media in indigenous populations: a systematic review of upper respiratory and middle ear microbiology in indigenous children with otitis media. Microbiome. 2018; 6(1):199. [DOI] [PMC free article] [PubMed]
- 28. Jensen RG, Johansen HK, Bjarnsholt T, Eickhardt-Sørensen SR, Homøe P. Recurrent otorrhea in chronic suppurative otitis media: is biofilm the missing link? Eur Arch Otorhinolaryngol. 2017; 274(7):2741-7. [DOI] [PubMed]
- 29. Labib S. Sepsis care pathway. No. 2-Qatar Critical Care Conference Proceedings. Qatar Med J. 2020; 2019:4. [DOI] [PMC free article] [PubMed]
- 30. Singer M. Beta-blockers in sepsis. No. 2-Qatar Critical Care Conference Proceedings. Qatar Med J. 2020; 2019:42.


