Abstract
Patient: Male, 58-year-old
Final Diagnosis: Cardiomyopathy
Symptoms: Arthritis • dynpnea • rash
Medication: —
Clinical Procedure: —
Specialty: Cardiology • Dermatology • Rheumatology
Objective:
Rare co-existance of disease or pathology
Background:
Psoriasis is a chronic inflammatory skin disease associated with multiple comorbidities including psoriatic arthritis (PsA), atherosclerotic disease, metabolic syndrome, diabetes, hypertension, obesity, and depression. Interestingly, nonischemic cardiomyopathy, especially dilated cardiomyopathy (DCM), has been associated with psoriasis and reported in only in a few cases in the literature.
Case Report:
We report the rare case of a 58-year-old man with a medical history of untreated severe psoriasis and PsA who presented with a sudden onset of shortness of breath. Laboratory and radiographic studies showed an elevated level of B-type natriuretic peptide and acute bilateral pulmonary edema. The patient had normal coronary arteries on cardiac catheterization and echocardiography showed newly diagnosed DCM with systolic and diastolic dysfunction. Cardiac magnetic resonance imaging was consistent with nonischemic DCM (NIDCM) with no evidence of hypertrophy, infiltrative process, or edema. The patient was diagnosed with acute congestive heart failure secondary to NIDCM in the setting of long-standing untreated psoriasis. He responded well to diuretics, was placed on guideline-directed medical therapy, and was discharged with a LifeVest personal cardiac defibrillator. As an outpatient, the patient was started on secukinumab, a monoclonal antibody against inter-leukin-17A. At his last follow-up appointment, the patient reported improvement in his cardiac symptoms and resolution of his psoriatic skin lesions; repeat echocardiography showed improvement in his ejection fraction.
Conclusions:
Although studies have shown a higher prevalence of cardiovascular disease in patients with psoriasis, an association with NIDCM has not been studied sufficiently. We recommend further studies of the prevalence, pathogenesis, screening, and management of NIDCM in patients with psoriasis.
Keywords: Arthritis, Psoriatic; Autoimmune Diseases; Cardiomyopathies; Psoriasis
Background
Psoriasis is a chronic immune-mediated disease of unclear etiology [1]. However, genetic background has been implicated along with other factors, including environmental and immune system components [1]. Psoriasis is one of the most common autoimmune diseases, affecting 3.2% of the population in the United States and 1% to 3% of the population worldwide [2]. The disease is seen in people of any age or sex and can be considered multisystemic, although it mainly affects the skin, the joints in 11% to 30% of cases, and less commonly, the cardiovascular system [1–3]. Previous studies have strongly suggested that prevalence of cardiovascular risk factors and diseases is higher in patients with psoriasis – especially those with associated psoriatic arthritis (PsA) – than in the general population. Interestingly, rare reports exist in the literature of nonischemic cardiomyopathy (NICM), particularly dilated cardiomyopathy (DCM), in patients with psoriasis [4]. Nevertheless, there is still limited understanding about the underlying shared pathophysiological process between psoriasis and cardiovascular disease (CVD) in this population [2].
Case Report
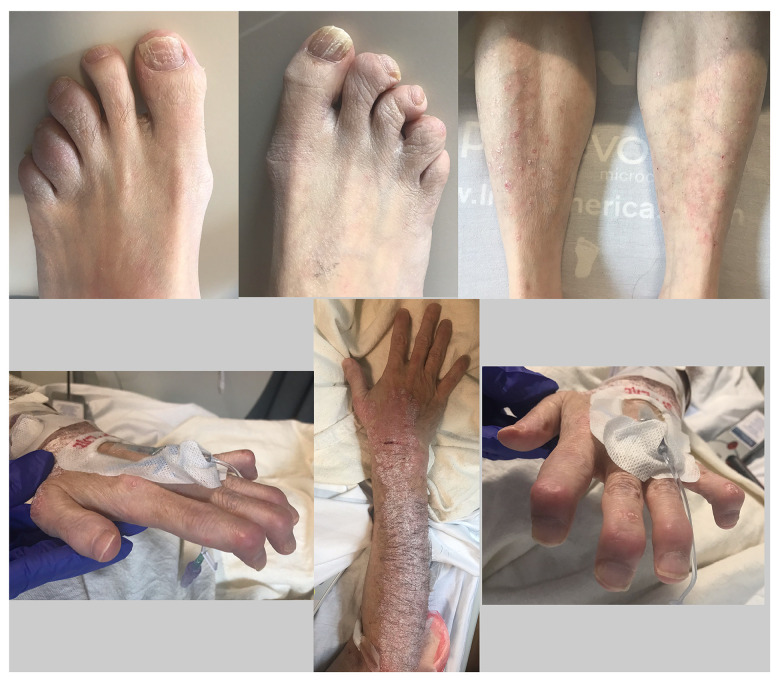
A 58-year-old fairly active man with poor medical follow-up and a long history of untreated severe psoriasis and PsA presented to the Emergency Department (ED) with sudden onset of shortness of breath associated with mid-back pain and a productive cough that had started about 5 h prior to admission. He was found to have extensive skin psoriasis (>50% of the body) with deformities in the joints of his of hands and feet secondary to PsA (Figure 1).
Figure 1.
These images of our patient show swelling and enlargement of most of the distal interphalangeal joints in his hands and feet and significant flexed deformities in those joints. They also reveal onycholysis and a scaly, silvery, erythematous rash with sharply defined margins on his forearms, wrists, fingers, toes, and legs.
On admission, the patient’s vital signs were a temperature of 37.1°C, blood pressure of 211/128 mmHg, heart rate of 136 beats per minute, respiratory rate of 35 breaths per minute, and oxygen saturation of 89% on room air. Physical examination was significant for increased work of breathing and bilateral rales on auscultation of the lung fields. The patient was noted to have deformities of the distal interphalangeal (DIP) joints in his hands and feet, with restricted range of motion. Onycholysis also was present. On the patient’s arms, legs, flanks, and abdomen, there was a scaly, silvery, erythematous rash with sharpy defined margins.
Laboratory results on admission were only remarkable for B-type natriuretic peptide (BNP) at 865 pg/mL (normal value, 0–100 pg/mL), with negative troponins. An electrocardiogram revealed sinus tachycardia with left bundle branch block. Chest X-ray showed acute bilateral pulmonary edema. An emergency computed tomography angiogram was obtained, which ruled out aortic dissection and pulmonary embolism but showed ground-glass opacities bilaterally and dependent atelectatic changes at the lung bases, greater on the right than on the left side. The patient was placed on bilevel positive airway pressure. He received furosemide and labetalol in the ED and Cardiology and Rheumatology were consulted. An echocardio-gram performed the following day showed severely reduced left ventricular systolic function with an ejection fraction (EF) of 21% to 25%, grade III (severe) diastolic dysfunction, and severe global hypokinesis (Figure 2).
Figure 2.
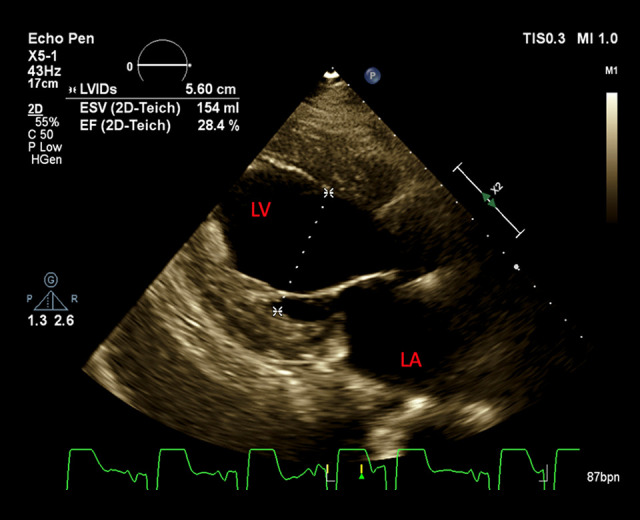
A transthoracic echocardiogram reveals severely reduced left ventricular systolic function, severe diastolic dysfunction, and severe global hypokinesis.
The patient was continued on 40 mg of furosemide during his admission and was weaned off supplemental oxygen and remained asymptomatic thereafter. Further work-up by a rheumatologist revealed a C-reactive protein level of 1.83 mg/dL (normal value, 0–0.74 mg/dL), an erythrocyte sedimentation rate of 29 mm/h (normal value, 0–20 mm/h), and an antinu-clear antibody count of 0.11 AU (normal value, <0.90 AU). Due to extensive skin psoriasis, it was determined that he would need treatment with interleukin (IL)-17, IL-12/23 inhibitors, or a phosphodiesterase-4 inhibitor with avoidance of tumor necrosis factor inhibitors because of cardiomyopathy with low EF. A cardiac catheterization performed on day 4 of hospitalization showed normal coronary vasculature but severe left ventricular systolic dysfunction. The patient responded well to diuretics and was placed on guideline-directed medical therapy (GDMT), including losartan, carvedilol, and spironolactone, and was discharged with a LifeVest defibrillator with outpatient follow-up by a cardiologist and a rheumatologist.
At his cardiology follow-up, cardiac magnetic resonance imaging was performed with results consistent with mild biventricular nonischemic cardiomyopathy and no evidence of hypertrophy, an infiltrative process, ischemia, or edema. The left ventricle was mildly dilated with a left ventricular end-diastolic volume index of 102 mL/m2. During the patient’s 2-week follow-up appointment with the rheumatologist, he was started on an anti-IL-17A monoclonal antibody (mAb) (secukinumab) via subcutaneous injection at a dosage of 300 mg weekly for 5 weeks and monthly thereafter. At his 3-month follow-up visit, the patient reported significant improvement in his cardiac symptoms with a BNP of 8.1 pg/mL (normal value, 0–100 pg/ mL), resolution of his psoriatic skin lesions, and an EF of 41% to 45% on a repeat echocardiogram.
Discussion
Psoriasis is the most common chronic autoimmune disease in the United States with an idiopathic etiology [2]. Several studies have reported a predominance of traditional cardiovascular risk factors, including diabetes, hyperlipidemia, hypertension, and premature atherosclerosis, and a higher prevalence of cardiovascular sequalae such as myocardial infarction, stroke, and cardiovascular death in patients with psoriasis than in the general population [1–3]. Furthermore, investigators have noticed a frequent association between psoriasis (particularly PsA) and several forms of CVD, such as aortitis, cardiac conduction dys-function, pericarditis, myocarditis, and valvular insufficiencies [1]. The risk of cardiovascular involvement increases with the severity and duration of the disease, co-existence of PsA, and use of disease-modifying antirheumatic drugs (DMARDs) [2,3,5].
McDonald and Calbresi found that 11.5% of hospitalized patients with psoriasis had ≥1 cardiovascular or cerebrovascular events in comparison with 5% of patients who did not have psoriasis [6]. A study conducted by El-Mongy et al also found an increased risk of CVD in patients with psoriasis in the absence of cardiovascular risk factors [7].
However, an interesting and prominent association between psoriasis and nonischemic cardiomyopathy, commonly NIDCM, has been increasingly reported despite the scarceness of data available about this topic in the literature [2]. Generally, the incidence of DCM has been reported as 10 times higher in patients with psoriasis [2,3].
It has been suggested that there is a common genetic background between psoriasis/PsA and DCM; therefore, an autoimmune process may have a role in the pathogenesis of DCM [2]. To the best of our knowledge, few proposed mechanisms have been reported in the literature as a potential cause of NIDCM in patients with psoriasis [2]. One is development of an inflammatory cardiomyopathy secondary to underlying cytokine-induced myocardial inflammation [2]. Certain growth factors and cytokines produced by inflammatory cells are prominently involved in this mechanism. such as transforming growth factor-β, IL-1, and IL-17A [2]. Furthermore, an article by Ryabkova et al entertained an interesting autoantibody-mediated process involving several antibodies, such as cytotoxic or cell-modulating antibodies with different possible effects according to their targets, leading to contractility and/or conduction disorders [8]. Recently, autoimmunity based on autoantibodies directed against G-protein-coupled receptors (functional autoantibodies), particularly those directed against the alpha1 receptor, has been increasingly accepted as one of the pathogenic drivers of psoriasis-associated CVD [9].
Given that no clear pathophysiological process has been confirmed, more studies are recommended to better understand the underlying pathogenesis of NIDCM in patients with psoriasis [2].
A review of the literature shows few case reports describing an association between psoriasis and nonischemic cardiomyopathy [2]. Most of the cases were associated with DCM; however, few reports described hypertrophic cardiomyopathy in patients with psoriasis [2]. In a 2008 retrospective study performed at a large hospital in Israel, Eliakim-Raz et al performed a computerized search for cardiomyopathy in the records of 2292 patients with psoriasis [1]. They found cardiomyopathy of different types in 20 of these patients (0.87% prevalence). Ten of the 20 patients were diagnosed with DCM (0.43% prevalence), most of whom had patent coronary arteries [1]. A case report by Fukuhara et al revealed an association between PsA, Takayasu disease, and NIDCM, which presumably suggests that the 3 conditions share a similar underlying immune-mediated and inflammatory process [10]. Pietrzak et al reported on a patient with psoriasis and severe DCM who had significantly more ventricular impairment than would have resulted from myocardial ischemia [11]. Abdelaoui et al also reported on DCM in a patient with a history of psoriasis and normal coronary arteries [4].
A large cohort study by Zhao et al demonstrated that patients with severe psoriasis had more subclinical left ventricular systolic dysfunction (LVSD), as detected by 2-dimensional speckle tracking-derived strain analysis, than the control group [12]. Milaniuk et al analyzed the echocardiography of patients with psoriasis and PsA and found valvular defects in 40.7%, LVSD in 27.8%, and left ventricular hypertrophy in 11.1%. LVSD, increased aorta stiffness index, and increased pulmonary artery blood pressure also were reported [13].
In the present report, the patient had a history of untreated severe psoriasis and long-term PsA and presented with acute congestive heart failure (CHF) due to NIDCM. Although he was newly diagnosed with hypertension on admission, he had no other classic risk factors for CVD, such as obesity, smoking, or diabetes. In the setting of normal coronary vasculature on cardiac catheterization and cardiac MRI indicating NICM, with no evidence of hypertrophy, infiltrative process, or edema, psoriasis-induced NIDCM has been suggested. Our patient had improvement in cardiac symptoms, psoriatic skin lesions, and EF following treatment with GDMT for CHF and a mAb against IL-17.
To sum up, recent studies and observations have increasingly suggested that psoriasis is an independent risk factor for cardiovascular events (ischemic heart disease, stroke, and sudden death) mediated by psoriasis-induced inflammation, endothelial injury, atherogenesis, and eventually premature atherosclerosis. Investigators also have observed that the more severe the psoriasis and the longer its duration, the worse the cardiovascular outcomes. However, the pathogenesis and association between psoriasis and NICM have not been well studied, despite the presumption that a flare-up of chronic inflammation and proinflammatory cytokines may initiate development of NIDCM in patients with psoriasis [5]. This theory was supported by Boehncke et al, who suggested that continuous anti-inflammatory therapy with a DMARD may have a potential cardioprotective effect in patients with psoriasis [14].
Therefore, we encourage physicians to: 1. increase their index of suspicion about a potential increased risk of CV events, both ischemic and nonischemic, associated with psoriasis; 2. counsel their patients with the disease about the importance of eliminating any traditional CVD risk factors, such as obesity, smoking, and sedentary lifestyle; and 3. institute early treatment with anti-psoriasis agents to reduce CVD-related morbidity and mortality.
Conclusions
We have presented the case of a patient with an unusual association between long-term psoriasis and NIDCM who had a favorable outcome in response to GDMT for CHF and biologic DMARDs, with resolution of cardiac symptoms, psoriatic skin lesions, and improved EF on echocardiography. Despite increased reporting in the literature about NIDCM in patients with psoriasis, there is limited understanding and insufficient data about the exact incidence, risk factors, pathogenesis, reversibility, screening, and management of the condition in these individuals. Certainly, further studies and research are recommended about this topic to better prevent and treat CVD in patients with psoriasis.
Footnotes
Conflict of Interest
None.
References:
- 1.Eliakim-Raz N, Shuvy M, Lotan C, Planer D. Psoriasis and dilated cardiomyopathy: coincidence or associated diseases? Cardiology. 2008;111(3):202–6. doi: 10.1159/000121605. [DOI] [PubMed] [Google Scholar]
- 2.Hashim T, Ahmad A, Chaudry A, et al. Psoriasis and cardiomyopathy: A review of the literature. South Med J. 2017;110(2):97–100. doi: 10.14423/SMJ.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 3.Pinto M, Shenoy MM, Hegde SP, et al. Psoriasis: Not just skin deep. Arch Med Health Sci. 2015;3(2):266–71. [Google Scholar]
- 4.Abdelaoui B, Benlafkih O, Ztati M, et al. The diagnosis of dilated cardiomyopathy during extended psoriasis: case report. Int J Adv Res. 2020;8(01):596–600. [Google Scholar]
- 5.Pietrzak A, Bartonsinska J, Chodorowska G, et al. Cardiovascular aspects of psoriasis: an updated review. Int J Dermatol. 2013;52(2):153–62. doi: 10.1111/j.1365-4632.2012.05584.x. [DOI] [PubMed] [Google Scholar]
- 6.McDonald CJ, Calabresi P. Thromboembolic disorders associated with psoriasis. Arch Dermatol. 1973;107(6):918. [PubMed] [Google Scholar]
- 7.El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: A potential association. J Eur Acad Dermatol Venereol. 2010;24(6):661–66. doi: 10.1111/j.1468-3083.2009.03481.x. [DOI] [PubMed] [Google Scholar]
- 8.Ryabkova VA, Shubik YV, Erman MV, et al. Lethal immunoglobulins: Autoantibodies and sudden cardiac death. Autoimmun Rev. 2019;18(4):415–25. doi: 10.1016/j.autrev.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Wallukat G, Schimke I. Lethal immunoglobulins: Autoantibodies and sudden cardiac death. Autoimmun Rev. 2019 Feb 14;pii:S1568-9972(19)30037-0. doi: 10.1016/j.autrev.2018.12.005. [Epub ahead of print] of Ryabkova VA et al. Autoimmun Rev. 2019; 18(7):749–50. [DOI] [PubMed] [Google Scholar]
- 10.Fukuhara K, Urano Y, Akaike M, et al. Psoriatic arthritis associated with dilated cardiomyopathy and Takayasu’s arteritis. Br J Dermatol. 1998;138(2):329–33. doi: 10.1046/j.1365-2133.1998.02085.x. [DOI] [PubMed] [Google Scholar]
- 11.Pietrzak A, Brzozowska A, Lotti T, et al. Psoriasis and cardiovascular. Dermatol Ther. 2013;26:489–92. doi: 10.1111/dth.12021. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C-T, Yeung C-K, Siu C-W, et al. Relationship between parathyroid hormone and subclinical myocardial dysfunction in patients with severe psoriasis. J Eur Acad Dermatol Venereol. 2014;28:461–68. doi: 10.1111/jdv.12123. [DOI] [PubMed] [Google Scholar]
- 13.Milaniuk S, Pietrzak A, Mosiewicz B, et al. Influence of psoriasis on circulatory system function assessed in echocardiography. Arch Dermatol Res. 2015;307(10):855–61. doi: 10.1007/s00403-015-1586-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehncke S, Salgo R, Garbaraviciene J, et al. Effective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: Results of a prospective longitudinal observational study. J Eur Acad Dermatol Venereol. 2011;25(10):1187–93. doi: 10.1111/j.1468-3083.2010.03947.x. [DOI] [PubMed] [Google Scholar]