Highlights
-
•
Literature review constraints included FMD, parasites, brucellosis, and PPR.
-
•
Expert workshops added CCPP, CBPP, mastitis, and reproductive disorders.
-
•
Most constraints manageable with existing technologies / best husbandry practices.
-
•
Systemic challenges limit livestock keepers’ access to vet services and inputs.
Keywords: Veterinary, Livestock disease, Global animal health, Poor, Poverty, Prioritisation
Abstract
Managing the health needs of livestock contributes to reducing poverty and improving the livelihoods of smallholder and pastoralist livestock keepers globally. Animal health practitioners, producers, policymakers, and researchers all must prioritize how to mobilize limited resources. This study employed three approaches to prioritize animal health needs in East and West Africa and South Asia to identify diseases and syndromes that impact livestock keepers. The approaches were a) systematic literature review, b) a series of expert workshops, and c) a practitioner survey of veterinarians and para-veterinary professionals. The top constraints that emerged from all three approaches include endo/ ectoparasites, foot and mouth disease, brucellosis, peste des petits ruminants, Newcastle disease, and avian influenza. Expert workshops additionally identified contagious caprine pleuropneumonia, contagious bovine pleuropneumonia, mastitis, and reproductive disorders as constraints not emphasized in the literature review. Practitioner survey results additionally identified nutrition as a constraint for smallholder dairy and pastoralist small ruminant production. Experts attending the workshops agreed most constraints can be managed using existing veterinary technologies and best husbandry practices, which supports a shift away from focusing on individual diseases and new technologies towards addressing systemic challenges that limit access to veterinary services and inputs. Few research studies focused on incidence/ prevalence of disease and impact, suggesting better incorporation of socio-economic impact measures in future research would better represent the interests of livestock keepers.
1. Introduction
Healthy livestock support a growing human population, contribute to economic growth, and for the world’s poor, can offer a pathway out of poverty. Disease is a threat to these benefits, and humans have been concerned with the control of animal disease for thousands of years. A collection of papyri from the ancient Egyptian town of Kahun dating back to 2nd millennium BC containing detailed descriptions of cattle diseases and their treatment showcases our long history managing animal disease (Steverding, 2008). In modern times, motivations to control animal disease range from public health concerns in the case of zoonoses (Sherikar and Waskar, 2005; Kahn, 2006; Katz et al., 2009), economic gain (Singh et al., 2015; Webb et al., 2018; Barratt et al., 2019), and improvement of livelihoods (Peacock, 2005; Randolph et al., 2007; Kryger et al., 2010), especially for people around the world who depend on livestock. Controlling disease is a worthy but costly investment. Motivations for control often dictate how research and control efforts are prioritized.
1.1. Prioritization frameworks
This research contributes to an ongoing conversation about prioritization of animal health needs to maximize the impact of livestock on alleviating poverty. A seminal study described by Perry et al. (2002) was the first large-scale attempt to assess disease impacts with this goal in mind (Perry et al., 2002). The study considered all livestock species, all types of diseases (endemic, epidemic, zoonotic, and food-borne), and three major geographies where major concentrations of poverty exist: South-east Asia, South Asia, and Sub-Saharan Africa. The result was a ranking of priority diseases based on literature review and semi-quantitative input from groups of experts working in veterinary services, NGOs, and international organizations. Disease categorizations were overlaid on databases of livestock production systems and poverty.
Heffernan (2009) contributed a data-driven livestock disease prioritization framework that broadened the data inputs beyond a group of experts. The framework incorporates standard poverty measures, rankings of the importance of respective livestock species, income data, and mortality and morbidity rates of disease to yield a ‘disease gap’ or change in the poverty status of a household due to the impact of influence of a livestock disease. The study found large variations in the impact of the same disease across the wealth groups and within the same production system. East Coast fever had the greatest impact on poverty amongst pastoralists and smallholder farmers, while foot and mouth disease was more problematic for the better-off pastoralists (Heffernan, 2009). The framework was informed by data from livestock keeping households in Kenya. Data availability is a constraint to expanding this framework to new geographies. The Global Burden of Animal Diseases (GBAD) program, launched in 2018, is addressing this gap through the creation of a dataset used to estimate direct and indirect losses caused by animal diseases worldwide (Rushton et al., 2018). While the GBAD program will involve some data collection, it relies on exploiting existing public and private datasets.
This study contributes to the animal health needs prioritization conversation by identifying the most important animal health constraints affecting the livelihoods of commercially oriented smallholder farmers and pastoralists using three methods. By assessing the incidence/ prevalence and impact of smallholder animal health constraints using multiple methods across three geographic regions, we identify priorities and compare methods of prioritization.
2. Methods
The study contains three activities: a) a systematic literature review, b) a series of expert workshops, and c) a practitioner survey of veterinarians and para-veterinary professionals (Table 1). The geographic focus of the study was South Asia, West Africa, and East Africa. This study was designed as an initiative within the Global Alliance for Livestock Veterinary Medicines (GALVmed) 2030 strategy development process. GALVmed is a not-for-profit company operating as a livestock health product development and access partnership and a UK registered charity (“GALVmed”, 2020).
Table 1.
Summary of study methods with associated geographies, livestock categories, and production systems.
| Method | Geography | Livestock categories | Production systems |
|---|---|---|---|
| 1. Systematic literature review |
South Asia: India, Nepal, Bangladesh (only if paired with India/ Nepal) West Africa: Senegal, Mali, Ghana, Burkina Faso, Ivory Coast, Togo, Benin, Nigeria East Africa: Tanzania, Kenya, Uganda, Ethiopia, South Sudan, Malawi, Mozambique, Zambia |
Bovids: Cattle, dairy cows, buffalo, yaks Small ruminants: Sheep, goats Poultry: Chickens, ducks, guinea fowl |
Smallholders, agro-pastoralists, pastoralists, small-scale commercial |
| 2. Expert workshops |
South Asia: Held in India, participants from India and Nepal West Africa: Held in Ghana, participants from Chad, Mali, Côte d’Ivoire, Nigeria, Ghana, Sierra Leone East Africa: Held in Ethiopia, participants from Kenya, Tanzania, Ethiopia, Uganda |
Poultry, small ruminants, cattle, dairy (including buffalo) | Smallholder and pastoralists |
| 3. Veterinary practitioner survey | East Africa: Practitioners from East Africa, Uganda best represented | Emergent commercial poultry farming Low input small ruminant farming Smallholder large ruminant (non-dairy) Small scale dairy farming Pastoralist large ruminant farming Pastoralist small ruminant farming |
|
2.1. Systematic literature review
A systematic literature review was conducted to understand the current state of published knowledge regarding smallholder animal health needs and the impact of diseases and syndromes (Fig. 1). We conducted searches in PubMed, CAB Abstracts, and Web of Science databases (Supplementary material 1). Document eligibility criteria included relevant location and livestock species, primary or secondary data, written in English, and published between 2002 and 2019. Each article was tagged with animal health constraint, location, and livestock category tags (Supplementary material 2 and 3). We were unable to consistently determine whether studies represented smallholder farmers, pastoralists, and/ or both so we did not disaggregate. The animal health tags were standardized based on health constraints that came up in the literature. All articles were given preliminary tags using keyword searches of the title and abstract. After the eligibility screening, a subset of articles with a focus on impact were selected and systematically tagged after reading the full text. These articles were purposively chosen for their consideration of incidence/ prevalence, impact, and smallholder perspective.
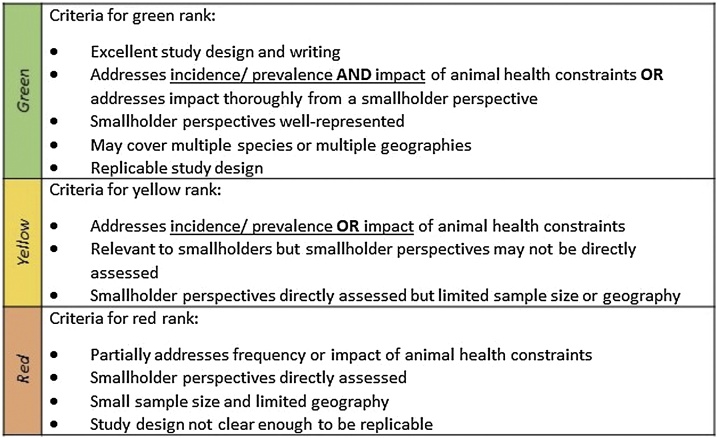
Fig. 1.
Ranking criteria used for the subset of articles focusing on impact of animal health constraints on smallholders and pastoralists.
Summarized articles were given a ranking (green, yellow, red) to indicate the extent of the article’s focus on incidence/ prevalence of animal health constraints and their impact (Fig. 1). Green-ranked articles are best, focusing on incidence/ prevalence and impact. Four researchers trained on a shared set of about 20 articles, discussed conflicts, then subsequent articles were ranked by a single researcher. A team of two researchers worked together on each geographic area with one member of each team professionally based in or responsible for the relevant geographic area. Incidence/ prevalence included disease seroprevalence, outbreak incidence, number of cases, or percentage of self- reported disease. Impact included mortality, morbidity, production losses, economic losses, monetary quantification of disease burden, or qualitative descriptions of impact by smallholder livestock keepers. The principal summary measure used in the results was whether a given animal health constraint was mentioned in the article regardless of the type of study conducted. We did not attempt to assess bias of individual studies that may have affected their study outcome although the quality of the impact articles is reflected in the ranking criteria.
2.2. Expert workshops
Three workshops were held with sector experts in Ethiopia, Ghana, and India in 2019.
Experts were selected based on having extensive, first-hand knowledge of smallholder livestock production systems in relevant settings within the specific regions of interest (East Africa, West Africa, India & Nepal) and operational knowledge of veterinary provision to smallholder livestock keepers. The objective was to identify big picture priorities from the perspective of a commercially oriented smallholder livestock keeper across a large geographic area. By default, such an approach fails to capture finer scale variation and doesn’t include first-hand testimonials from farmers, farmer groups, or value chain actors who interact with farmers. The workshop relied on experts to synthesize their experiences working with livestock keepers into succinct identification of constraints that are important on a large scale.
Workshop sessions were structured to achieve a consensus of animal health constraint ranking while attempting to address inherent biases brought by the experts (e.g. resulting from their research focus or those diseases prioritized by government veterinary services) and in the literature (e.g. focus on specific diseases without examining the broader impact to the smallholder). To assist the experts in developing independent opinions without being biased by the group during the workshop, each participant was asked to rank the top three animal health priorities before the meeting, using a standard form. Attention was drawn to the “animal health constraint” so the experts could prioritize syndromes and not be restricted to individual diseases. The literature review was shared with the attendees, after they had completed their survey, but before travelling to the workshop. The workshop itinerary is presented in Supplementary material 4.
2.3. Veterinary practitioner survey
An animal health priority questionnaire (Supplementary material 5) was administered to veterinarians and para-veterinary professionals working in East Africa. They were asked to rank animal health constraints in a series of categories of species/ production systems (Table 1). The survey administration was managed by two GALVmed regional veterinary consultants on contract for a larger survey project. The consultants reached out to the veterinary practitioners and requested they complete the 30- minute survey using an online link or by printing and scanning a paper form. A payment of $10 USD was set as an incentive to return the survey within one week. The target was to request a completed survey from 20 practitioners from each of the 3 countries (Kenya, Tanzania, and Uganda) for a total of 60 people contacted.
3. Results
3.1. Systematic literature review
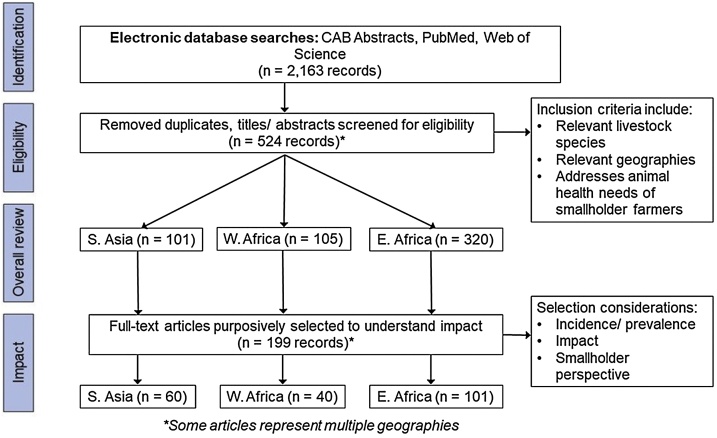
The literature search resulted in 524 eligible documents for the overall literature review and a subset of 199 documents that focused on impact (see Fig. 2 and Supplementary materials 3, 6–8). Of the 199 impact articles, only 17 articles across all geographies met the criteria for the “best” green rank, “addressing incidence/ prevalence AND impact of animal health constraints OR addressed impact thoroughly from a smallholder perspective”.
Fig. 2.
PRISMA flow diagram showing the selection of articles for the overall literature review and more targeted impact study.
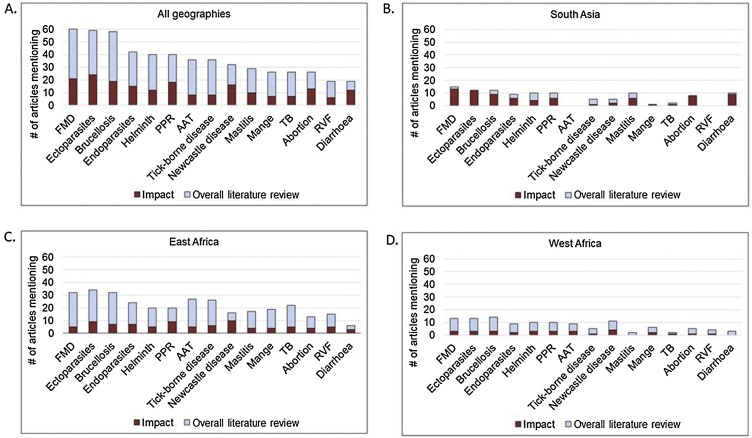
The top 15 animal health constraints for all geographies in order of number of overall literature review articles that mentioned them were foot and mouth disease (FMD), ectoparasites, brucellosis, endoparasites, helminths, peste des petits ruminants (PPR), animal African trypanosomiasis (AAT), tick-borne diseases, Newcastle disease (ND), mastitis, mange, tuberculosis, abortion, Rift Valley Fever (RVF), and diarrhea. The number of documents mentioning each health concern ranged from 60 for FMD to 19 for diarrhea (Fig. 3). A full list of all health constraints and annotated bibliographies by region and livestock type are presented in Supplementary material 6. Five green-ranked articles mentioned FMD and trypanosomiasis respectively (three articles discussing animal African trypanosomiasis plus two discussing trypanosomiasis in South Asia) and two mentioned lumpy skin disease (LSD) (Supplementary material 8). Health constraints mentioned by a single, green-ranked article included ectoparasites, abortion, East Coast fever, Newcastle disease, PPR, and black quarter.
Fig. 3.
Top 15 animal health constraints by geographic area ordered by number of articles mentioning the concern. (n = 524 articles) The height of the bar represents the total number of articles, the upper, light blue bar represents the number of articles in the overall literature review, and the lower, dark red bar represents the subset of articles focusing on impact. Abbreviations: Foot and mouth disease (FMD), peste des petits ruminants (PPR), animal African trypanosomiasis (AAT), tuberculosis (TB), Rift Valley Fever (RVF), lumpy skin disease (LSD). Note: Some constraints do not apply to all geographic areas, such as Rift Valley Fever in South Asia. Some articles mention multiple health constraints.
When all impact articles are broken down by geography and production system, as shown in Table 2, important health concerns include FMD and brucellosis for bovids (which includes cattle and dairy), PPR for small ruminants, and Newcastle disease for poultry with the addition of avian influenza in West Africa and South Asia. Broad categories including endoparasites, exoparasites, and helminths are top concerns in all geographies, especially for bovids and small ruminants.
Table 2.
Animal health constraints: Findings from overall literature review documents including subset of impact articles. Health constraints mentioned by only one article not listed.
| Overall review | Region | n | 1. | 2. | 3. | 4. | 5. |
|---|---|---|---|---|---|---|---|
| Poultry | East Africa | 29 | ND (16) | IBD (10) | *Fowl pox (4) | *Coccidiosis (4) | *Endo/exoparasites (4) |
| West Africa | 24 | Avian influenza (9) | ND (8) | IBD (4) | |||
| South Asia | 16 | ND (5) | Avian influenza (3) | Coccidiosis (2) | Salmonellosis (2) | ||
| Small ruminants | East Africa | 100 | PPR (20) | Ectoparasites (19) | Mange (16) | Helminths/ endoparasites (12) | RVF (12) |
| West Africa | 34 | Ectoparasites (8) | Helminths (7) | Endoparasites (6) | Brucellosis (6) | ||
| South Asia | 35 | PPR (9) | Ectoparasites (8) | Helminths (7) | Endoparasites (6*) | Brucellosis (6*) | |
| Cattle/dairy | East Africa | 240 | FMD (31) | Brucellosis (29) | AAT (27) | Ectoparasites (25) | TBDs (22) |
| West Africa | 67 | FMD (13) | Brucellosis (12) | AAT (9*) | Ectoparasites (9*) | Helminths (9*) | |
| (w. buffalo) | South Asia | 61 | Ectoparasites (12*) | FMD (12*) | Brucellosis (11) | Mastitis (10) | Theileriosis (8) |
| Impact | Region | 1. | 2. | 3. | 4. | 5. | |
| Poultry | East Africa | 12 | ND (10) | IBD (3) | Fowl pox (4) | Endo/exoparasites (3*) | Coccidiosis (3*) |
| West Africa | 10 | Avian influenza (5) | ND (4) | CRD (2) | |||
| South Asia | 9 | Avian influenza (3) | ND (2) | Coccidiosis (2) | |||
| Small ruminants | East Africa | 35 | PPR (10) | Ectoparasites (9) | Mange (9) | Endoparasites (6) | |
| West Africa | 24 | Helminths (4) | Ectoparasites (3) | Endoparasites (3) | Haemonchosis (3) | ||
| South Asia | 24 | Helminths (4) | PPR (3*) | Endoparasites (3*) | Ectoparasites (3*) | Haemonchosis (3*) | |
| Cattle/dairy | East Africa | 67 | Brucellosis (9) | FMD (8*) | AAT (8*) | Ectoparasites (8*) | TBDs/ LSD (8*) |
| West Africa | 25 | Brucellosis (5*) | Helminths (5*) | FMD (4*) | Endoparasites (4*) | ||
| (w. buffalo) | South Asia | 37 | FMD (10) | Ectoparasites (8*) | Brucellosis (8*) | Diarrhea (7) |
Abbreviations: Newcastle disease (ND), Infectious bursal disease (IBD), peste des petits ruminants (PPR), Rift Valley Fever (RVF), African trypanosomiasis (AAT), tick-borne diseases (TBDs), lumpy skin disease (LSD). Notes: TBDs include heartwater, ECF, anaplasmosis, babesiosis. Helminths is a subset of the endoparasite category.
Indicates a tie.
3.2. Expert workshops
A total of 23 participants attended the three expert workshops (12 in South Asia, 13 in West Africa, and 8 in East Africa). The experts included academic researchers, government veterinarians, and representatives from private sector companies and NGOs delivering veterinary products and services to smallholder livestock keepers. Only three participants were women and there were no women participants in the South Asia workshop. The workshops, unlike the systematic literature review, differentiated between animal health constraints for smallholder farmers and pastoralists and divided dairy and cattle into two separate categories.
Top concerns included FMD, PPR, Newcastle disease, avian influenza, consistent with the top results of the systematic literature review, with the addition of Contagious Caprine Pleuropneumonia (CCPP), Contagious Bovine Pleuropneumonia (CBPP), mastitis, and reproductive disorders (Table 3). The workshop results contained additional health constraints not mentioned in the top 15 animal health constraints of the systematic literature review, including dermatophilosis, Gumboro disease (infectious bursal disease), sheep and goat pox, respiratory problems, abortion, coccidiosis, lameness, orf, and foot rot.
Table 3.
Animal health constraints: Consensus from East Africa, West Africa, and South Asia expert workshops.
| Smallholders | Region | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|---|
| Poultry | East Africa | ND | Nutrition | Diarrhea | Gumboro | Fowl typhoid | Fowl pox |
| West Africa | ND | Gumboro | Diarrhea | Avian influenza | Fowl pox | ||
| South Asia | Avian Influenza | ND | Fowl pox | Coccidiosis | Ecto/Endoparasites | Gumboro | |
| Small ruminants | East Africa | CCPP | PPR | SGP | Helminths | Abortion | Foot rot |
| West Africa | PPR | Helminths | Mange | Abortion | Heartwater | Lameness | |
| South Asia | PPR | Endoparasites/ diarrhea | Respiratory problems | Vesicular disease | Zoonoses | Abortion | |
| Cattle | East Africa | FMD | TBDs | LSD | Helminths | AAT | |
| West Africa | CBPP | Dermatophilosis | Abortion | Ecto/Endo parasites | Lameness | ||
| Dairy | East Africa | Mastitis | Abortion | Brucellosis | FMD | LSD | TBDs |
| West Africa | Masititis | Abortion | FMD | Helminths | Dermatophilosis | ||
| (w/ buffalo) | South Asia | Reproductive disorders | FMD | Mastitis | Ecto/Endo/ Haemo parasites | Metabolic diseases | Zoonoses |
| Pastoralists | |||||||
| Small ruminants | East Africa | CCPP | Helminths | PPR | SGP | Orf | Abortion |
| West Africa | PPR | Helminths | Abortion | Mange | Heartwater | ||
| Cattle | East Africa | FMD | TBDs | AAT | LSD | Helminths | Anthrax |
| West Africa | CBPP | Dermatophilosis | FMD | Bovine TB | Abortion | Ecto/ Endoparasites |
Abbreviations: Newcastle disease (ND), contagious caprine pleuropneumonia (CCPP), peste des petits ruminants (PPR), sheep and goat pox (SGP), foot and mouth disease (FMD), tick-borne diseases (TBDs), lumpy skin disease (LSD), animal African trypanosomiasis (AAT), contagious bovine pleuropneumonia (CBPP), bovine tuberculosis (bovine TB). Note:Haemo parasites include theileria, trypanosomes, and babesia; metabolic diseases include milk fever and ketosis; vesicular diseases include Orf, contagious ecthyma, FMD, SGP; zoonoses include Johne’s disease and brucellosis; reproductive disorders include symptoms resulting from brucellosis, Infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD), poor diet and infertility from unknown causes.
The increasing endemicity of avian influenza in West Africa and India was highlighted as a threat to smallholder poultry production, with broader veterinary public health implications. FMD was recognized as a constraint to large ruminant production in all three workshops. The availability of FMD vaccines, either through public immunization campaigns or private sector delivery, was identified as a major factor in controlling disease for smallholder livestock keepers.
The constraints in Table 3 can be grouped into three classes: vaccine preventable (registered vaccines available in the region/country), treatable with pharmaceuticals (registered pharmaceuticals available within the region/country), and largely avoidable through best practice in husbandry (recognized methods in production which if followed will reduce the risk of certain conditions). The experts were challenged to reflect whether a smallholder livestock keeper with the knowledge and financial resources could address these constraints within their country, given the existing private and public sector set-ups as they currently stand. For most of the constraints, the answer was “yes”.
One exception to the constraints acknowledged to be avoidable through vaccination, treatment, or good husbandry, was reproductive disorders. In all workshops, it was recognized there was a poor understanding of the etiology underlying these disorders, particularly for abortions and neo-natal mortality. While Brucella species were commonly implicated, the evidence base was often poor, with those few studies conducting more rigorous diagnostic investigations suggesting a much more complicated and unclear picture as to the main reasons for abortions and neo-natal mortality.
3.3. Workshop discussion
In addition to the prioritization of animal health constraints, workshop participants brought up additional, cross-cutting concerns including improved vaccines, regulatory reform, product quality and correct application, and emerging trends.
3.3.1. Improved vaccines
A common theme from across all three workshops was the desire for combination vaccines. The idea was discussed particularly with reference to the global programme for the control and eradication of PPR, which will provide an important platform for controlling other diseases including CCPP and SGP. The profile of a combination eye-drop vaccine for poultry disease was also supported. Reduced vial size and simplification of administration (e.g. pre- diluted) were also common themes, widely recognized as helping to improve uptake by smallholder livestock keepers.
3.3.2. Regulatory reform
Reforms in specific legislation were identified as important in addressing several of the animal health constraints. In Kenya, the mismatch between the desire for smallholder livestock keepers to vaccinate against FMD privately, and the regulated role of the state in providing all FMD vaccinations (without enough resources to meet demand) was one such example. Across both Africa meetings, the role of community animal health workers was discussed. The need for improved standards for artificial insemination operatives, and the ability for them to increase income from the provision of other services, such as vaccination, was highlighted. Similar concerns were expressed for the standardization of para-veterinarians.
3.3.3. Product quality and correct application
Quality issues with vaccines, pharmaceuticals and supplements/feed were highlighted as a contributory factor for many of the constraints listed in Table 3. It was recognized that private animal health product and service providers should be able to address these issues through well managed supply chains. Developing a recognizable and enforceable mark for veterinary inputs, such as the Pan African Veterinary Centre of the African Union (PANVAC) stamp on vaccines, was proposed as a way for smallholder livestock keepers to assess quality. With intensification of smallholder livestock production, issues of feed quality and the need for supplementation of incomplete diets is increasingly necessary to capitalize on market opportunities.
Outside of the quality of product, correct application was identified as another important contributory factor to the persistence of avoidable constraints. Well-recognized examples included under-application of acaricides and underdosing with antimicrobials leading to increased problems of resistance. Linked to this, on the off-take side, poor practices drive antimicrobial and acaricide/insecticide contamination of eggs, milk, meat, and hides.
The growth of both antimicrobial drug resistance and acaricide resistance were addressed in all three workshops. It was recognized that lack of knowledge on correct administration, as well as poor quality or even counterfeit products, were major contributors to the trend.
3.3.4. Emerging trends
Participants in all three workshops brought up two additional emerging trends: the growing importance of goats and climate change.
Although the drivers were different (e.g., export trade to the Middle East in East Africa, land pressures and migration pattern change in West Africa, rise of popularity of goat milk in India), goats were seen as an increasingly important source of income for smallholder livestock keepers. Traditionally, goats have been perceived as a livestock option that requires minimal investment, with smallholders not investing much in animal health inputs. The PPR vaccination programs, coupled with increased market demand for goat meat and milk, provide an opportunity to alter these perceptions.
Increased climatic variability and increased frequency of extreme weather events were considered important trends in all three settings. This is contributing to changes in land use which greater pressure on water and grazing land; this in turns drives driving intensification and changes in production systems, such as the switch from cattle to goats.
3.4. Veterinary practitioner survey
The results of the veterinary practitioner survey are shown in Table 4. Constraints match those mentioned in the workshop, with the addition of chronic respiratory disease for poultry, anaplasmosis in smallholder dairy operations, and the inclusion of nutrition as a constraint, mentioned in relation to smallholder dairy and pastoralist small ruminants. Out of 60 practitioners contacted, only 20 returned surveys (response rate of 33 %). The survey was sent soon after another extensive survey done by GALVmed which likely led to contact and practitioner fatigue and contributed to the low response rate. Most practitioners were from Uganda (13), followed by Tanzania and Kenya (3 each), and one from Ethiopia. The practitioners had an average of 15 years of field experience. The gender of the practitioners was not recorded.
Table 4.
Animal health constraints: Consensus from veterinary practitioner survey conducted in East Africa.
| Smallholders | 1. | 2. | 3. | 4. | 5. |
|---|---|---|---|---|---|
| Poultry | ND | Coccidiosis | Ecto/Endo parasites | Fowl pox | Chronic respiratory disease |
| Small ruminants | Helminths | TBDs | CCPP | PPR | Orf |
| Cattle | FMD | TBDs | LSD | Helminths | Trypanosomiasis |
| Dairy | Mastitis | Infertility & abortion | Nutrition | ECF | Anaplasmosis |
| Pastoralists | |||||
| Small ruminants | CCPP | PPR | Endoparasites | Nutrition | Orf & pasteurellosis |
| Cattle | FMD | CBPP | ECF | TBDs | Endoparasites |
Abbreviations: Newcastle disease (ND), tick-borne diseases (TBDs), contagious caprine pleuropneumonia (CCPP), peste des petits ruminants (PPR), foot and mouth disease (FMD), lumpy skin disease (LSD), East Coast fever (ECF), contagious bovine pleuropneumonia (CBPP).
4. Discussion
The objective of this study was to assess the incidence/ prevalence and impact of smallholder animal health needs using multiple methods across three geographic regions, identify priorities, and compare methods of prioritization.
4.1. Incidence, prevalence, and impact
Less than 10 % (17/199) of the impact articles met the criteria for green ranking, evidence that few studies were able to address impact thoroughly from a smallholder perspective or incorporate measures of incidence/ prevalence and impact of animal health constraints. In this section, we briefly discuss the types of articles found in the literature review, highlight two excellent, green-ranked studies, and identify opportunities for better incorporating impact measures in future research.
Many articles were characterizations of production systems with limited sample sizes in a single geographic area (Kamal et al., 2012; Seyoum et al., 2013; Olobatoke et al., 2015; Satisha et al., 2018). Others addressed incidence/ prevalence with a larger sample size in a broader geographic area (for example serosurveys for a specific disease) but failed to connect incidence or prevalence estimates to the impact on smallholder livestock keepers (Jagun and Onoja, 2011; Kabi et al., 2015; Abdela, 2017; Chengat Prakashbabu et al., 2017; Okumu et al., 2019). A few articles estimated economic impact of specific diseases or outbreak events at household level (Sitawa et al., 2016), farm level (Govindaraj et al., 2018), or herd level (Alhaji and Isola, 2018).
Examples of green-ranking articles include Jemberu et al. (2014) with an outbreak investigation of FMD in Ethiopia which reported on morbidity/ mortality rates and economic losses due to milk loss, draft power loss, and mortality (Jemberu et al., 2014). The study also disaggregated data based on production system, differentiating between pastoralists and crop-livestock mixed system. A second example of a green ranking article is a study by Kumar et al. (2017), who conducted retrospective risk analyses to estimate economic losses in India due to trypanosomiasis. In addition to reporting an estimated total annual loss of US $ 671.1 million (US $ 344–US $ 1209 million at 95 % confidence interval), they included loss parameters relevant to smallholder livestock keepers including reduction in milk yield (36 % of total loss), reproductive losses (26 %), reduction in growth (10 %), reduction in draught power (8 %), and additional opportunity cost (3 %) (Kumar et al., 2017).
Opportunities for contributing to the current body of literature for smallholder animal health needs include studies from interdisciplinary teams that connect results from epidemiology studies with socio-economic impact assessments identify priorities and compare methods of prioritization and consideration and inclusion of impact indicators that are relevant for a smallholder context. All three prioritization methods identified constraints such as endo and ectoparasites, mange, mastitis, reproductive disorders, and even nutrition in the veterinary practitioner survey, that contribute to morbidity rather than mortality. Impact on smallholder livestock keepers may come in the form of production losses, less profitable sales, increased veterinary costs, or increased labor or time required to care for animals. Thoughtful identification of relevant indicator measurements of impact and measurement of these indicators will allow for more useful impact estimates. Interdisciplinary teams that include economists and social scientists are more likely to have a broader vocabulary and include a wider range of impact indicators that studies undertaken by veterinarians or epidemiologists only. The conspicuous absence of gender as a consideration in this and other studies is evidence of the limited capacity of many animal health research teams. The role of women in agriculture and livestock production is well-documented (FAO, 2010) and the different roles played by men and women in livestock keeping means they may be differentially impacted by disease (Kristjanson et al., 2010), prioritize livestock species differently (Wieland et al., 2016), and face different challenges in accessing information and veterinary services such as vaccines (Mutua et al., 2019). While this study does not provide a metric for the number of articles that incorporated a gender perspective, it is clearly an area for improvement in future research projects. Lastly, stratifying disease priorities by production system is useful in interpreting animal health priorities, however not all studies in the systematic literature review were specific enough to distinguish between pastoralists and smallholder farmers or even identify the level of intensification. Clearly indicating this type of information makes studies more useful for prioritizing smallholder animal health constraints.
4.2. Identifying priorities
Globally, ten constraints emerge from all three methods as having high impact on smallholder livestock keepers: endo/ ectoparasites, FMD, brucellosis, PPR, Newcastle disease, avian influenza, CCPP, CBPP, mastitis, and reproductive disorders. This list is in no way exhaustive and it may be more useful to look at the full list of disease constraints identified by region or production system.
As noted by the workshop participants, many of these constraints, except for reproductive disorders, can be prevented or treated using best practices with existing technologies available in the respective geographies. This supports the prioritization of addressing systemic limitations that make it challenging for smallholder livestock keepers to access veterinary services and inputs. The conclusions about regulatory reform and product quality/ correct application raised by workshop participants are examples of such challenges. Improved vaccines, especially combination vaccines that prevent multiple diseases, may be a valuable contribution for populations with limited access to veterinary services. Supporting infrastructural improvements to veterinary systems through support of paraprofessionals or strengthening public private partnerships may also be a valuable entry-point for addressing animal health constraints. Prioritizing the systems and infrastructure that delivery existing veterinary technologies is another direct invitation to incorporate gender analyses into future studies. While women livestock keepers may face more barriers to access, they can be powerful allies in disease control when given access to the appropriate tools, as evidenced by a study in Kenya linking the formation of women’s groups with improved control of transboundary livestock diseases (Kristjanson et al., 2010).
4.3. Comparing methods
Lastly, we compare methods of prioritization within the study and across similar studies. While there is some consensus across the three methods of prioritizing animal health constraints within this study, the following constraints were not in the top 15 identified in the literature review overall or for any geographic region but were identified as important in the workshops and/ or practitioner survey: CBPP, CCPP, heartwater, and lumpy skin disease.
Published literature focused more on individual diseases rather than syndromes, such as reproductive disorders (although abortion was a top constraint), or production constraints, such as nutrition. Funding and research priorities are not necessarily driven by improving the welfare of smallholder livestock keepers and using published studies to inform priorities may lead to bias towards zoonoses such as zoonotic tuberculosis, biologically interesting diseases such as trypanosomiasis, or well-recognized diseases such as FMD at the expensive of chronic and low-mortality constraints such as mange or syndromes such as reproductive disorders. Workshop participants may also introduce biases stemming from their personal research interests. Veterinary practitioners may underestimate the danger of outbreaks of rare diseases or constraints that have economic and public health consequences beyond the impact on the clients they work with.
Conclusions of this study shared by Perry et al. (2002) and Heffernan (2009) , two other studies prioritizing animal health concerns as a function of their impact on the poor, are the need for improved impact measures and the promise of using existing technologies and best practices in conjunction with improving the delivery of animal health services. A key finding from Heffernan’s study was that for diseases with a high morbidity but comparatively low mortality, poor households spent much less on treatment and prevention than the better off, and are therefore disproportionately affected (Heffernan, 2009). The delivery of animal health services and the need to make existing technologies “more effective and appropriate for the poor” was addressed by Perry et al. (2002). Debates about how best to serve farmers in smaller scale production systems continue, as evidenced by the theme of the 2018 Tanzania Veterinary Association conference “Veterinary Profession as a Catalyst for Transformative Change of the Animal Industry”, where veterinary practitioners discussed the changing landscape of veterinary governance and extension service delivery system (Tanzania Veterinary Association, 2018).
While some overarching challenges remain constant, the systematic literature review revealed some notable progress managing animal health constraints since publication of the first systematic prioritization study by Perry et al in 2002. Examples include the global eradication of rinderpest (Roeder and Rich, 2009), a disease that ranked within the top 20 conditions with impact on the poor, and commitment from the international community to begin working towards the eradication of peste des petits ruminants (OIE/FAO, 2015). There have also been beneficial technological advances, such as the development of a thermotolerant Newcastle disease vaccine (I-2), which is now produced and available in many developing countries (Fisher, 2014).
4.4. Limitations
Limitations to this study include lack of representation of grey literature published outside of peer-reviewed journals, potential exclusion of relevant articles from Francophone countries in West Africa by limiting the search to articles published in English, having only one reviewer assigning impact score for each article in the systematic literature review, the selection of a small number of experts for the workshops, lack of representation of livestock keepers themselves in the workshop, and the limited geography of the veterinary practitioner survey, which ideally would have also been administered in West Africa and South Asia. Despite literature documenting the gender gap in agriculture and the role of women in livestock production, the under-representation of women in the stakeholder workshops (only 3 of 23 experts were women) perpetuates the status quo where the priorities and interests of men dominate discussions about how resources should be prioritized. Pigs were not included in this study due to the organizational prioritizations of GALVmed and its funders, however they play an important role in smallholder livelihoods globally. Nutrition and feeding strategies and antimicrobial resistance were excluded in the systematic literature review, but nutrition was identified as an important constraint in the veterinary practitioner survey and antimicrobial resistance was brought up in the expert workshops. There were few representatives of broader animal management constraints such as nutritionists or welfare specialists in the expert workshops, which likely contributed to their underrepresentation in the findings. Lastly, there were some small differences in the disease constraint definitions across the three methods because a categorization system was independently created for each method. For example, the systematic literature review included ectoparasites and endoparasites separately while the stakeholder workshop combined them. This allowed the disease constraints to more closely match the priorities that emerged from each method, however, introduces minor challenges when comparing results across methods.
4.5. Conclusion
Managing animal health constraints is a pathway to improving the livelihoods of smallholder and pastoralist livestock keepers globally. By employing three methods of prioritization, we identify diseases and syndromes that have the greatest impact on livestock keepers. Most of the top priorities can be managed using existing technologies and best practices which supports a shift away from focusing on individual diseases and new technologies and towards addressing systemic challenges that limit access to veterinary services and inputs. Few research studies focused on incidence/ prevalence of disease and impact on smallholders suggesting opportunities for interdisciplinary studies that include gender analyses and define impact using socioeconomic indicators in addition to epidemiology indicators.
Declaration of Competing Interest
Paul Coleman, Andrea Guest, Brian Perry, and Zoë Campbell contributed as consultants for GALVmed to complete these research activities.
Acknowledgements
We appreciate the contributions of the participants in the workshops and the veterinary practitioners who shared their opinions and expertise. Thank you to the International Livestock Research Institute in Nairobi, Kenya and the Paul G. Allen School for Global Animal Health in Pullman, Washington for supporting the corresponding author in drafting this manuscript. Thanks to Suzanne Fricke in the Washington State University Animal Health Library for her useful advice on search strategies, Sarah Cleaveland for supporting an early career scientist, Mark Caudell for friendly competition, and Scott Hotaling for the reminder that if you think someone should write it, probably you should write it.
Acknowledgments
Funding
This is based on research funded in part by the Bill & Melinda Gates Foundation (Investment ID OPP1176784) and with UK aid from the UK Government (Project300504) through GALVmed. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation or the UK Government.
Footnotes
Indicates article is referenced in the paper but not part of the systematic literature review
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.prevetmed.2021.105279.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References1
- Abdela N. Sero-prevalence, risk factors and distribution of foot and mouth disease in Ethiopia. Acta Trop. 2017;169:125–132. doi: 10.1016/j.actatropica.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Alhaji N.B., Isola T.O. Pastoralists’ knowledge and practices towards clinical bovine dermatophilosis in cattle herds of North-Central Nigeria: the associated factors, burden and economic impact. Trop. Anim. Health Prod. 2018;50:381–391. doi: 10.1007/s11250-017-1445-y. [DOI] [PubMed] [Google Scholar]
- *Barratt A.S., Rich K.M., Eze J.I., Porphyre T., Gunn G.J., Stott A.W. Framework for estimating indirect costs in animal health using time series analysis. Front. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengat Prakashbabu B., Thenmozhi V., Limon G., Kundu K., Kumar S., Garg R., Clark E.L., Srinivasa Rao A.S.R., Raj D.G., Raman M., Banerjee P.S., Tomley F.M., Guitian J., Blake D.P. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet. Parasitol. 2017;233:62–72. doi: 10.1016/j.vetpar.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *FAO . Women in Agriculture: Closing the Gender Gap for Development. FAO; 2010. Documenting the gender gap in agriculture; pp. 23–38. [Google Scholar]
- *Fisher H. 2014. Newcastle Disease Control in Africa, ACIAR Impact Assessment Series. [Google Scholar]
- *GALVmed [WWW Document] 2020. GALVmed [WWW Document] URL https://www.galvmed.org/ (Accessed 6.25.20) [Google Scholar]
- Govindaraj G., Sridevi R., Nandakumar S.N., Vineet R., Rajeev P., Binu M.K., Balamurugan V., Rahman H. Economic impacts of avian influenza outbreaks in Kerala, India. Transbound. Emerg. Dis. 2018;65:e361–e372. doi: 10.1111/tbed.12766. [DOI] [PubMed] [Google Scholar]
- *Heffernan C. Panzootics and the poor: devising a global livestock disease prioritisation framework for poverty alleviation. OIE Rev. Sci. Tech. 2009;28:897–907. doi: 10.20506/rst.28.3.1934. [DOI] [PubMed] [Google Scholar]
- Jagun A., Onoja A.B. The current status of Peste des Petits Ruminant (PPR) in sheep in Ibadan southwestern Nigeria. J. Commonw. Vet. Assoc. 2011;27:133–138. [Google Scholar]
- Kabi F., Muwanika V., Masembe C. Spatial distribution of Brucella antibodies with reference to indigenous cattle populations among contrasting agro-ecological zones of Uganda. Prev. Vet. Med. 2015;121:56–63. doi: 10.1016/j.prevetmed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- *Kahn L.H. Confronting zoonoses, linking human and veterinary medicine. Emerg. Infect. Dis. 2006;12:556–561. doi: 10.3201/eid1204.050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M., Mondal S., Islam S., Islam M. Present status of goat rearing under rural conditions in south-west regions of Bangladesh. In: Rahmann G., Godinho D., editors. Organic Animal Husbandry Challenges. 2012. Hamburg/ Trenthorst, Germany. [Google Scholar]
- *Katz J.M., Veguilla V., Belser J.A., Maines T.R., van Hoeven N., Pappas C., Hancock K., Tumpey T.M. The public health impact of avian influenza viruses. Poult. Sci. 2009;88:872–879. doi: 10.3382/ps.2008-00465. [DOI] [PubMed] [Google Scholar]
- *Kristjanson P., Waters-Bayer A., Johnson N., Tipilda A., Njuki J., Baltenweck I., Grace D., Macmillan S. 2010. Livestock and Women’s Livelihoods: A Review of the Recent Evidence. Nairobi, Kenya. [Google Scholar]
- *Kryger K., Thomsen K., Whyte M., Dissing M. Smallholder Poultry Production; Rome: 2010. Smallholder Poultry Production – Livelihoods, Food Security and Sociocultural Significance. [Google Scholar]
- Kumar R., Jain S., Kumar S.S., Sethi K., Kumar S.S., Tripathi B.N. Impact estimation of animal trypanosomosis (surra) on livestock productivity in India using simulation model: current and future perspective. Vet. Parasitol. Reg. Stud. Rep. 2017;10:1–12. doi: 10.1016/j.vprsr.2017.06.008. [DOI] [PubMed] [Google Scholar]
- *Mutua E., De Haan N., Tumusiime D., Jost C., Bett B. A qualitative study on gendered barriers to livestock vaccine uptake in kenya and uganda and their implications on rift valley fever control. Vaccines. 2019;7:1–22. doi: 10.3390/vaccines7030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *OIE/FAO . 2015. Global Strategy for the Control and Eradication PPR. [Google Scholar]
- Okumu T.A., John N.M., Wabacha J.K., Tsuma V., VanLeeuwen J. Seroprevalence of antibodies for bovine viral diarrhoea virus, Brucella abortus and Neospora caninum, and their roles in the incidence of abortion/foetal loss in dairy cattle herds in Nakuru District, Kenya. BMC Vet. Res. 2019;15:95. doi: 10.1186/s12917-019-1842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olobatoke R., Mobayo E., Mathuthu M. Evaluation of local chicken production in Kogi State of Nigeria. Int. J. Agric. Policy Res. 2015;3:377–381. [Google Scholar]
- *Peacock C. Goats - A pathway out of poverty. Small Rumin. Res. 2005;60:179–186. doi: 10.1016/j.smallrumres.2005.06.011. [DOI] [Google Scholar]
- *Perry B.D., Randolph T.F., McDermott J.J., Sones K.R., Thornton P.K. 2002. Investing in Animal Health Research to Alleviate Poverty. Nairobi, Kenya. [Google Scholar]
- *Roeder P., Rich K. Conquering the cattle plague: the global effort to eradicate rinderpest. In: Spielman D.J., Pandya-Lorch R., editors. Millions Fed: Proven Successes in Agricultural Development. International Food Policy Research Institute (IFPRI); Washington, D.C: 2009. pp. 109–116. [Google Scholar]
- *Rushton J., Bruce M., Bellet C., Torgerson P., Shaw A., Marsh T., Pigott D., Stone M., Pinto J., Mesenhowski S., Wood P. Initiation of global burden of animal diseases programme. Lancet. 2018;392:538–540. doi: 10.1016/S0140-6736(18)31472-7. [DOI] [PubMed] [Google Scholar]
- Satisha M.C., Tiwari R., Roy R. Performance of dairy animals in commercial dairy farms in Karnataka. Indian J. Dairy Sci. 2018;71:620–624. [Google Scholar]
- Seyoum Z., Terefe G., Ashenafi H. Farmers’ perception of impacts of bovine trypanosomosis and tsetse fly in selected districts in Baro-Akobo and Gojeb river basins, Southwestern Ethiopia. BMC Vet. Res. 2013;9:214. doi: 10.1186/1746-6148-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherikar A., Waskar V. Emerging zoonoses and social-economic impact in India - A review. Indian J. Anim. Sci. 2005;76:700–705. [Google Scholar]
- Singh B.B.B., Dhand N.K.K., Gill J.P.S.P.S. Economic losses occurring due to brucellosis in Indian livestock populations. Prev. Vet. Med. 2015;119:211–215. doi: 10.1016/j.prevetmed.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Sitawa R., Mbogoh S.G., Gathuma J.M., Kairu S. An evaluation of economic returns from east coast fever control through infection and treatment method at household level in Nandi and Uasin-Gishu counties of Kenya. Int. J. Agric. Policy Res. 2016;4:149–156. [Google Scholar]
- *Steverding D. The history of African trypanosomiasis. Parasit. Vectors. 2008;1:1–8. doi: 10.1186/1756-3305-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Tanzania Veterinary Association . 2018. Veterinary Profession as a Catalyst for Transformative Change of the Animal Industry. Arusha, Tanzania. [Google Scholar]
- *Webb M., Gibson J., Strutt A. The impact of diseases on international beef trade: market switching and persistent effects. Food Policy. 2018;75:93–108. doi: 10.1016/j.foodpol.2018.01.006. [DOI] [Google Scholar]
- *Wieland B., Kinati W., Mulema A.A. 2016. Sheep Are Like Fast-growing Cabbage: Gender Dimensions of Small Ruminant Health in Ethiopia. [Google Scholar]
Further reading
- Abbas B., Yuusif M.A., Nur H.M. Animal health constraints to livestock exports from the Horn of Africa. Rev. Sci. Tech. l’OIE. 2014;33:711–721. doi: 10.20506/rst.33.3.2314. [DOI] [PubMed] [Google Scholar]
- Abdilatif M.H., Onono J.O., Mutua F.K. Analysis of pastoralists’ perception on challenges and opportunities for sheep and goat production in Northern Kenya. Trop. Anim. Health Prod. 2018;50:1701–1710. doi: 10.1007/s11250-018-1613-8. [DOI] [PubMed] [Google Scholar]
- Abebe R., Hatiya H., Abera M., Megersa B., Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016;12:1–11. doi: 10.1186/s12917-016-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abera Z., Degefu H., Gari G., Kidane M. Sero-prevalence of lumpy skin disease in selected districts of West Wollega zone, Ethiopia. BMC Vet. Res. 2015;11:135. doi: 10.1186/s12917-015-0432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abera T., Bitew M., Gebre D., Mamo Y., Deneke Y., Nandi S. Bluetongue disease in small ruminants in south western Ethiopia: cross- sectional sero-epidemiological study. BMC Res. Notes. 2018;11:112. doi: 10.1186/s13104-018-3222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham-Oyiguh J., Adewumi M.O., Onoja A.B., Suleiman I., Sulaiman L.K., Ahmed S.J., Jagboro S.T. Seroprevalence of infectious bursal disease virus in local chickens in Udu Local Government Area of Delta state, South East Nigeria. J. Immunoass. Immunochem. 2015;36:398–404. doi: 10.1080/15321819.2014.973116. [DOI] [PubMed] [Google Scholar]
- Abunna F., Tilahun G., Megersa B., Regassa A., Kumsa B. Bovine cysticercosis in cattle slaughtered at Awassa municipal abattoir, Ethiopia: prevalence, cyst viability, distribution and its public health implication. Zoonoses Public Health. 2008;55:82–88. doi: 10.1111/j.1863-2378.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- Adam I.A., Abdalla M.A., Mohamed M.E., Aradaib I.E. Prevalence of bluetongue virus infection and associated risk factors among cattle in North Kordufan State, Western Sudan. BMC Vet. Res. 2014;10:94. doi: 10.1186/1746-6148-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addy F., Alakonya A., Wamae N., Magambo J., Mbae C., Mulinge E., Zeyhle E., Wassermann M., Kern P., Romig T. Prevalence and diversity of cystic echinococcosis in livestock in Maasailand, Kenya. Parasitol. Res. 2012;111:2289–2294. doi: 10.1007/s00436-012-3082-8. [DOI] [PubMed] [Google Scholar]
- Adedeji A.J., Dashe Y., Akanbi O.B., Woma T.Y., Jambol A.R., Adole J.A., Bolajoko M.B., Chima N., Asala O., Tekki I.S., Luka P., Okewole P. Co-infection of peste des petits ruminants and goatpox in a mixed flock of sheep and goats in Kanam, North Central Nigeria. Vet. Med. Sci. 2019;170 doi: 10.1002/vms3.170. vms3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjou Moumouni P.F., Aplogan G.L., Katahira H., Gao Y., Guo H., Efstratiou A., Jirapattharasate C., Wang G., Liu M., Ringo A.E., Umemiya-Shirafuji R., Suzuki H., Xuan X. Prevalence, risk factors, and genetic diversity of veterinary important tick-borne pathogens in cattle from Rhipicephalus microplus-invaded and non-invaded areas of Benin. Ticks Tick. Dis. 2018;9:450–464. doi: 10.1016/j.ttbdis.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Adombi C.M., Waqas A., Dundon W.G., Li S., Daojin Y., Kakpo L., Aplogan G.L., Diop M., Lo M.M., Silber R., Loitsch A., Diallo A. Peste des petits ruminants in Benin: persistence of a single virus genotype in the country for over 42 years. Transbound. Emerg. Dis. 2017;64:1037–1044. doi: 10.1111/tbed.12471. [DOI] [PubMed] [Google Scholar]
- Agegnehu A., Bogale B., Tesfaye S., Dagnachew S. Status of mange infestation in indigenous sheep and goats and their control practices in Wag-Himra zone, Ethiopia. J. Vet. Med. Anim. Heal. 2018;10:128–134. [Google Scholar]
- Ahir V.B., Roy A., Jhala M.K., Bhanderi B.B., Mathakiya R.A., Bhatt V.D., Padiya K.B., Jakhesara S.J., Koringa P.G., Joshi C.G. Genome sequence of Pasteurella multocida subsp. gallicida Anand1_poultry. J. Bacteriol. 2011;193:5604. doi: 10.1128/JB.05706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadu B., Lovelace C.E.A., Samui K.L., Mahan S. Some observations on the sero-prevalence of heartwater and tick infestation in Zambian goats. Onderstepoort J. Vet. Res. 2004;71:161–164. doi: 10.4102/ojvr.v71i2.279. [DOI] [PubMed] [Google Scholar]
- Akidarju M.S., Onyemaechi E.G., Dauda M.G. An assessment of some poultry management practices and disease recognition by poultry farmers in Maiduguri arid zone, Nigeria. Worlds Poult. Sci. J. 2010;66:285–296. doi: 10.1017/S0043933910000334. [DOI] [Google Scholar]
- Akinseye V.O., Adesokan H.K., Ogugua A.J., Adedoyin F.J., Otu P.I., Kwaghe A.V., Kolawole N.O., Okoro O.J., Agada C.A., Tade A.O., Faleke O.O., Okeke A.L., Akanbi I.M., Ibitoye M.M., Dipeolu M.O., Dale E.J., Lorraine P., Taylor A.V., Awosanya E.A., Cadmus E.O., Stack J.A., Cadmus S.I. Sero-epidemiological survey and risk factors associated with bovine brucellosis among slaughtered cattle in Nigeria. Onderstepoort J. Vet. Res. 2016;83 doi: 10.4102/ojvr.v83i1.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwuobu C.A., Ayling R.D., Chah K.F., Oboegbulem S.I. Studies into the prevalence of Mycoplasma species in small ruminants in Benue State, North-central Nigeria. Trop. Anim. Health Prod. 2014;46:1087–1092. doi: 10.1007/s11250-014-0613-6. [DOI] [PubMed] [Google Scholar]
- Alemayehu G., Leta S., Hailu B. Sero-prevalence of Contagious Bovine Pleuropneumonia (CBPP) in bulls originated from Borena pastoral area of Southern Ethiopia. Trop. Anim. Health Prod. 2015;47:983–987. doi: 10.1007/s11250-015-0820-9. [DOI] [PubMed] [Google Scholar]
- Alemu B., Gari G., Libeau G., Kwiatek O., Kidane M., Belayneh R., Siraw B., Wieland B., Asfaw W., Abdi R.D. Molecular detection and phylogenetic analysis of Peste des petits ruminants virus circulating in small ruminants in eastern Amhara region, Ethiopia. BMC Vet. Res. 2019;15:84. doi: 10.1186/s12917-019-1828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaji N., Babalobi O. Socio-cultural factors influencing transmission of Mycoplasma mycoides mycoides small colony in pastoral cattle herds of north-central Nigeria. Vom J. Vet. Sci. 2015;10:1–13. [Google Scholar]
- Alhaji N.B., Babalobi O.O. Qualitative and quantitative impacts assessment of contagious bovine pleuropneumonia in Fulani pastoral herds of North-central Nigeria: the associated socio-cultural factors. Prev. Vet. Med. 2016;128:124–134. doi: 10.1016/j.prevetmed.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Alhaji Nma Bida, Babalobi O.O. Sero-positivity and associated risk factors for contagious bovine pleuropneumonia under two cattle production systems in North Central Nigeria. Trop. Anim. Health Prod. 2016;48:311–320. doi: 10.1007/s11250-015-0952-y. [DOI] [PubMed] [Google Scholar]
- Alhaji N.B., Wungak Y.S., Bertu W.J. Serological survey of bovine brucellosis in Fulani nomadic cattle breeds (Bos indicus) of North-central Nigeria: potential risk factors and zoonotic implications. Acta Trop. 2016;153:28–35. doi: 10.1016/j.actatropica.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Alhaji N.B., Babalobi O.O., Wungak Y., Ularamu H.G. Participatory survey of Rift Valley fever in nomadic pastoral communities of North-central Nigeria: the associated risk pathways and factors. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alingu R.A., Muhanguzi D., MacLeod E., Waiswa C., Fyfe J. Bovine trypanosome species prevalence and farmers’ trypanosomiasis control methods in south-western Uganda. J. S. Afr. Vet. Assoc. 2014;85 doi: 10.4102/jsava.v85i1.1094. [DOI] [PubMed] [Google Scholar]
- Allan K.J., Halliday J.E.B., Moseley M., Carter R.W., Ahmed A., Goris M.G.A., Hartskeerl R.A., Keyyu J., Kibona T., Maro V.P., Maze M.J., Mmbaga B.T., Tarimo R., Crump J.A., Cleaveland S. Assessment of animal hosts of pathogenic Leptospira in northern Tanzania. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allepuz A., Stevenson M., Kivaria F., Berkvens D., Casal J., Picado A. Risk factors for foot-and-mouth disease in Tanzania, 2001–2006. Transbound. Emerg. Dis. 2015;62:127–136. doi: 10.1111/tbed.12087. [DOI] [PubMed] [Google Scholar]
- Almaw G., Zerihun A., Asfaw Y. Bovine mastitis and its association with selected risk factors in smallholder dairy farms in and around Bahir Dar, Ethiopia. Trop. Anim. Health Prod. 2008;40:427–432. doi: 10.1007/s11250-007-9115-0. [DOI] [PubMed] [Google Scholar]
- Almaw G., Duguma M., Wubetie A., Tuli G., Koran T. A contagious bovine pleuropneumonia outbreak on a research farm in Ethiopia, and its dynamics over an eight-month period. Rev. Sci. Tech. l’OIE. 2016;35:787–793. doi: 10.20506/rst.35.3.2569. [DOI] [PubMed] [Google Scholar]
- Ameni G., Erkihun A. Bovine tuberculosis on small-scale dairy farms in Adama Town, central Ethiopia, and farmer awareness of the disease. Rev. Sci. Tech. 2007;26:711–719. [PubMed] [Google Scholar]
- Ameni G., Tadesse K., Hailu E., Deresse Y., Medhin G., Aseffa A., Hewinson G., Vordermeier M., Berg S. Transmission of Mycobacterium tuberculosis between farmers and cattle in Central Ethiopia. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni G., Tafess K., Zewde A., Eguale T., Tilahun M., Hailu T., Sirak A., Salguero F.J., Berg S., Aseffa A., Hewinson R.G., Vordermeier H.M. Vaccination of calves with Mycobacterium bovis Bacillus calmette-guerin reduces the frequency and severity of lesions of bovine tuberculosis under a natural transmission setting in Ethiopia. Transbound. Emerg. Dis. 2018;65:96–104. doi: 10.1111/tbed.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenu K., Szonyi B., Grace D., Wieland B. Important knowledge gaps among pastoralists on causes and treatment of udder health problems in livestock in southern Ethiopia: results of qualitative investigation. BMC Vet. Res. 2017;13:303. doi: 10.1186/s12917-017-1222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angwech H., Nyeko J.H.P., Opiyo E.A., Okello-Onen J., Opiro R., Echodu R., Malinga G.M., Njahira M.N., Skilton R.A. Heterogeneity in the prevalence and intensity of bovine trypanosomiasis in the districts of Amuru and Nwoya, Northern Uganda. BMC Vet. Res. 2015;11:255. doi: 10.1186/s12917-015-0567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyango G., Mutua F., Kagera I., Andang’O P., Grace D., Lindahl J.F. A survey of aflatoxin M1 contamination in raw milk produced in urban and peri-urban areas of Kisumu County, Kenya. Infect. Ecol. Epidemiol. 2018;8 doi: 10.1080/20008686.2018.1547095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arowolo O.O., Banmeke T.O.A., Ajayi M.T. Assessment of the training needs of poultry farmers in Ovia and oredo local government areas of edo state, Nigeria. Afr. J. Livest. Extension. 2012;10:23–30. [Google Scholar]
- Asakura S., Makingi G., Kazwala R., Makita K. Brucellosis risk in urban and agro-pastoral areas in Tanzania. Ecohealth. 2018;15:41–51. doi: 10.1007/s10393-017-1308-z. [DOI] [PubMed] [Google Scholar]
- Asakura S., Makingi G., Kazwala R., Makita K. Herd-level risk factors associated with Brucella sero-positivity in cattle, and perception and behaviours on the disease control among agro-pastoralists in Tanzania. Acta Trop. 2018;187:99–107. doi: 10.1016/j.actatropica.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Ashenafi F., Teshale S., Agga G., Fikru R., Laikemariam Y. Distribution of brucellosis among small ruminants in the pastoral region of Afar, eastern Ethiopia. Rev. Sci. Tech. l’OIE. 2016;26:731–739. doi: 10.20506/rst.26.3.1781. [DOI] [PubMed] [Google Scholar]
- Asmare K., Megersa B., Denbarga Y., Abebe G., Taye A., Bekele J., Bekele T., Gelaye E., Zewdu E., Agonafir A., Ayelet G., Skjerve E. A study on seroprevalence of caprine brucellosis under three livestock production systems in southern and central Ethiopia. Trop. Anim. Health Prod. 2013;45:555–560. doi: 10.1007/s11250-012-0258-2. [DOI] [PubMed] [Google Scholar]
- Asmare K., Sibhat B., Ayelet G., Gebremedhin E.Z., Lidete K.A., Skjerve E. Serological evidence of Bovine herpesvirus-1, Bovine Viral Diarrhea virus and Schmallenberg v irus infections in relation to reproductive disorders in dairy cattle in Ethiopia. Acta Trop. 2018;178:236–241. doi: 10.1016/j.actatropica.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Assefa G.A., Kelkay M.Z. Goat pasteurellosis: serological analysis of circulating Pasteurella serotypes in Tanqua aberegelle and Kola Tembien Districts, Northern Ethiopia. BMC Res. Notes. 2018;11:485. doi: 10.1186/s13104-018-3606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa H., Mulate B., Nazir S., Alemayehu A. Cystic echinococcosis amongst small ruminants and humans in central Ethiopia. Onderstepoort J. Vet. Res. 2015;82:E1–7. doi: 10.4102/ojvr.v82i1.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assenga J.A., Matemba L.E., Muller S.K., Mhamphi G.G., Kazwala R.R. Predominant leptospiral serogroups circulating among humans, livestock and wildlife in Katavi-Rukwa ecosystem, Tanzania. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awa D.N., Adakal H., Luogbou N.D.D., Wachong K.H., Leinyuy I., Achukwi M.D. Cattle ticks in Cameroon: is rhipicephalus (Boophilus) microplus absent in Cameroon and the Central African region? Ticks Tick Borne. Dis. 2015;6:117–122. doi: 10.1016/j.ttbdis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Awad H., Al-Hamidhi S., El Hussein A.-R.M., Yousif Y.M.Z., Taha K.M., Salih D.A., Weir W., Babiker H.A. Theileria lestoquardi in Sudan is highly diverse and genetically distinct from that in Oman. Infect. Genet. Evol. 2018;62:46–52. doi: 10.1016/j.meegid.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Awah-Ndukum J., Mouiche M.M.M., Bayang H.N., Ngwa V.N., Assana E., Feussom K.J.M., Manchang T.K., Zoli P.A. Seroprevalence and associated risk factors of brucellosis among indigenous cattle in the Adamawa and north regions of Cameroon. Vet. Med. Int. 2018;2018:1–10. doi: 10.1155/2018/3468596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayebazibwe C., Tjørnehøj K., Mwiine F.N., Muwanika V.B., Okurut A.R.A., Siegismund H.R., Alexandersen S., Ademun Okurut A.R., Siegismund H.R., Alexandersen S. Patterns, risk factors and characteristics of reported and perceived foot-and-mouth disease (FMD) in Uganda. Trop. Anim. Health Prod. 2010;42:1547–1559. doi: 10.1007/s11250-010-9605-3. [DOI] [PubMed] [Google Scholar]
- Ayelet G., Mahapatra M., Gelaye E., Egziabher B.G., Rufeal T., Sahle M., Ferris N.P., Wadsworth J., Hutchings G.H., Knowles N.J. Genetic characterization of foot-and-mouth disease viruses, Ethiopia, 1981–2007. Emerg. Infect. Dis. 2009;15:1409–1417. doi: 10.3201/eid1509.090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayelet G., Haftu R., Jemberie S., Belay A., Gelaye E., Sibhat B., Skjerve E., Asmare K. Lumpy skin disease in cattle in central Ethiopia: outbreak investigation and isolation and molecular detection of the virus. Rev. Sci. Tech. 2014;33:877–887. doi: 10.20506/rst.33.3.2325. [DOI] [PubMed] [Google Scholar]
- Baby J., Mani R.S., Abraham S.S., Thankappan A.T., Pillai P.M., Anand A.M., Madhusudana S.N., Ramachandran J., Sreekumar S. Natural rabies infection in a domestic fowl (Gallus domesticus): a report from India. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan V., Saravanan P., Sen A., Rajak K.K., Bhanuprakash V., Krishnamoorthy P., Singh R.K. Sero-epidemiological study of peste des petits ruminants in sheep and goats in India between 2003 and 2009. Rev. Sci. Tech. 2011;30:889–896. doi: 10.20506/rst.30.3.2087. [DOI] [PubMed] [Google Scholar]
- Balamurugan V., Das S., Raju D.S.N., Chakravarty I., Nagalingam M., Hemadri D., Govindaraj G., Ibotombi Singh N., Ltu K., Devi M., Sharma K., Gajendragad M.R., Rahman H. Prevalence of peste des petits ruminants in goats in North-East India. VirusDisease. 2014;25:488–492. doi: 10.1007/s13337-014-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluka S.A. Economic effects of foot and mouth disease outbreaks along the cattle marketing chain in Uganda. Vet. World. 2016;9:544–553. doi: 10.14202/vetworld.2016.544-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangar Y.C., Dohare A.K., Kolekar D.V., Avhad S.R., Khan T.A. Seasonal variation in morbidity pattern in cattle by log- linear model approach. J. Appl. Anim. Res. 2015;43:283–286. doi: 10.1080/09712119.2014.963100. [DOI] [Google Scholar]
- Bangar Yogesh Chandrakant, Singh B., Dohare A.K., Verma M.R. A systematic review and meta-analysis of prevalence of subclinical mastitis in dairy cows in India. Trop. Anim. Health Prod. 2015;47:291–297. doi: 10.1007/s11250-014-0718-y. [DOI] [PubMed] [Google Scholar]
- Banumathi B., Vaseeharan B., Rajasekar P., Prabhu N.M., Ramasamy P., Murugan K., Canale A., Benelli G. Exploitation of chemical, herbal and nanoformulated acaricides to control the cattle tick, Rhipicephalus (Boophilus) microplus - A review. Vet. Parasitol. 2017;244:102–110. doi: 10.1016/j.vetpar.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Belgrad J.P., Rahman M.A., Abdullah M.S., Rashid M.H., Sayeed M.A., Anwer M.S., Hoque M.A. Newcastle disease sero and viro-prevalence in rural poultry in Chittagong, Bangladesh. Prev. Vet. Med. 2018;160:18–25. doi: 10.1016/j.prevetmed.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L., Koney E.B.M., Dogbey O., Walker A.R. Ehrlichia ruminantium seroprevalence in domestic ruminants in Ghana; I. Longitudinal survey in the Greater Accra Region. Vet. Microbiol. 2004;100:175–188. doi: 10.1016/j.vetmic.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bertram M.R., Bravo de Rueda C., Garabed R., Dickmu Jumbo S., Moritz M., Pauszek S., Abdoulkadiri S., Rodriguez L.L., Arzt J. Molecular epidemiology of foot-and-Mouth disease virus in the context of transboundary animal movement in the far north region of Cameroon. Front. Vet. Sci. 2018;5 doi: 10.3389/fvets.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertu W.J., Ducrotoy M.J., Muñoz P.M., Mick V., Zúñiga- Ripa A., Bryssinckx W., Kwaga J.K.P.P., Kabir J., Welburn S.C., Moriyón I., Ocholi R.A. Phenotypic and genotypic characterization of Brucella strains isolated from autochthonous livestock reveals the dominance of B. abortus biovar 3a in Nigeria. Vet. Microbiol. 2015;180:103–108. doi: 10.1016/j.vetmic.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Bessell P., Kushwaha P., Mosha R., Woolley R., Al-Riyami L., Gammon N. Assessing the impact of a novel strategy for delivering animal health interventions to smallholder farmers. Prev. Vet. Med. 2017;147:108–116. doi: 10.1016/j.prevetmed.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Bessell P.R., Sargison N.D., Mirende K., Dash R., Prasad S., Al-Riyami L., Gammon N., Stuke K., Woolley R., Barbaruah M., Wambura P. The impact of anthelmintic drugs on weight gain of smallholder goats in subtropical regions. Prev. Vet. Med. 2018;159:72–81. doi: 10.1016/j.prevetmed.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett B., Jost C., Allport R., Mariner J. Using participatory epidemiological techniques to estimate the relative incidence and impact on livelihoods of livestock diseases amongst nomadic pastoralists in Turkana South District, Kenya. Prev. Vet. Med. 2009;90:194–203. doi: 10.1016/j.prevetmed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Bett B., Mariner J.C., Kihu S., Swai E.S., Jost C.C., Njogu G., Nzietchueng S., Kihu S., Bett B., Njogu G., Swai E.S., Mariner J.C. Epidemiological assessment of the Rift Valley fever outbreak in Kenya and Tanzania in 2006 and 2007. Am. J. Trop. Med. Hyg. 2010;83:65–72. doi: 10.4269/ajtmh.2010.09-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettridge J.M., Lynch S.E., Brena M.C., Melese K., Dessie T., Terfa Z.G., Desta T.T., Rushton S., Hanotte O., Kaiser P., Wigley P., Christley R.M. Infection-interactions in Ethiopian village chickens. Prev. Vet. Med. 2014;117:358–366. doi: 10.1016/j.prevetmed.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene T.J., Eshetu A., Abdu A., Wondimu E., Beyi A.F., Tufa T.B., Ibrahim S., Revie C.W. Assisting differential clinical diagnosis of cattle diseases using smartphone-based technology in low resource settings: a pilot study. BMC Vet. Res. 2017;13:323. doi: 10.1186/s12917-017-1249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanuprakash V., Hosamani M., Singh R.K. Prospects of control and eradication of capripox from the Indian subcontinent: a perspective. Antiviral Res. 2011;91:225–232. doi: 10.1016/j.antiviral.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Bhatt P.R., Pandya K.B., Patel U.D., Patel H.B., Modi C.M. Survey on ethnoveterinary practices around Junagadh, Gujarat, India. Indian J. Pharm. Sci. 2019;81 [Google Scholar]
- Biffa D., Bogale A., Godfroid J., Skjerve E. Factors associated with severity of bovine tuberculosis in Ethiopian cattle. Trop. Anim. Health Prod. 2012;44:991–998. doi: 10.1007/s11250-011-0031-y. [DOI] [PubMed] [Google Scholar]
- Biswas P., Christensen J., Ahmed S., Barua H., Das A., Rahman M., Giasuddin M., Habib M., Hannan A., Debnath N. Mortality rate and clinical features of highly pathogenic avian influenza in naturally infected chickens in Bangladesh. Sci. Tech. Rev. Off. Int. des Epizoot. 2011;30:871–878. doi: 10.20506/rst.30.3.2080. [DOI] [PubMed] [Google Scholar]
- Blomström A.-L., Scharin I., Stenberg H., Figueiredo J., Nhambirre O., Abilio A., Berg M., Fafetine J. Seroprevalence of Rift Valley fever virus in sheep and goats in Zambézia, Mozambique. Infect. Ecol. Epidemiol. 2016;6:31343. doi: 10.3402/iee.v6.31343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J., Hogerwerf L., Germeraad E.A., Roest H.I.J., Faye- Joof T., Jeng M., Nwakanma D., Secka A., Stegeman A., Goossens B., Wegmüller R., van der Sande M.A.B., van der Hoek W., Secka O. Coxiella burnetii (Q fever) prevalence in associated populations of humans and small ruminants in the Gambia. Trop. Med. Int. Health. 2017;22:323–331. doi: 10.1111/tmi.12827. [DOI] [PubMed] [Google Scholar]
- Borah B., Deka P., Sharma K., Baro S., Hazarika A.K., Das C., Garam G.B., Boro P., Ltu K. Isolation, identification and retrospective study of foot-and-mouth disease virus from affected Mithun (Bos frontalis) in north-eastern India. Transbound. Emerg. Dis. 2018;65:e63–e69. doi: 10.1111/tbed.12678. [DOI] [PubMed] [Google Scholar]
- Boukary A.R., Saegerman C., Abatih E., Fretin D., Alambédji Bada R., De Deken R., Harouna H.A., Yenikoye A., Thys E. Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of Niger. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussini H., Lamien C.E., Nacoulma O.G., Kaboré A., Poda G., Viljoen G. Prevalence of Rift Valley fever in domestic ruminants in the central and northern regions of Burkina Faso. Rev. Sci. Tech. 2014;33:893–901. doi: 10.20506/rst.33.3.2327. [DOI] [PubMed] [Google Scholar]
- Brar P., Nanda A. Postpartum ovarian activity in South Asian Zebu cattle. Reprod. Domest. Anim. 2008;43:207–212. doi: 10.1111/j.1439-0531.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- Brookes V.J., Gill G.S., Singh B.B., Sandhu B.S., Dhand N.K., Aulakh R.S., Ward M.P. Challenges to human rabies elimination highlighted following a rabies outbreak in bovines and a human in Punjab, India. Zoonoses Public Health. 2019;66:325–336. doi: 10.1111/zph.12568. [DOI] [PubMed] [Google Scholar]
- Bugeza J., Muwonge A., Munyeme M., Lasuba P., Godfroid J., Kankya C. Seroprevalence of bovine brucellosis and associated risk factors in Nakasongola district, Uganda. Trop. Anim. Health Prod. 2018 doi: 10.1007/s11250-018-1631-6. [DOI] [PubMed] [Google Scholar]
- Byarugaba D.K., Mugimba K.K., Omony J.B., Okitwi M., Wanyana A., Otim M.O., Kirunda H., Nakavuma J.L., Teillaud A., Paul M.C., Ducatez M.F. High pathogenicity and low genetic evolution of avian paramyxovirus type I (Newcastle disease virus) isolated from live bird markets in Uganda. Virol. J. 2014;11:173. doi: 10.1186/1743-422X-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byaruhanga C., Oosthuizen M.C., Collins N.E., Knobel D. Using participatory epidemiology to investigate management options and relative importance of tick-borne diseases amongst transhumant zebu cattle in Karamoja Region, Uganda. Prev. Vet. Med. 2015;122:287–297. doi: 10.1016/j.prevetmed.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Campbell Z., Marsh T., Mpolya E., Thumbi S., Palmer G. Newcastle disease vaccine adoption by smallholder households in Tanzania: identifying determinants and barriers. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell Z., Otieno L., Shirima G., Marsh T., Palmer G. Drivers of vaccination preferences to protect a low-value livestock resource: willingness to pay for Newcastle disease vaccines by smallholder households. Vaccine. 2019;37:11–18. doi: 10.1016/j.vaccine.2018.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.J., Kracalik I.T., Ross N., Alexander K.A., Hugh- Jones M.E., Fegan M., Elkin B.T., Epp T., Shury T.K., Zhang W., Bagirova M., Getz W.M., Blackburn J.K. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat. Microbiol. 2019 doi: 10.1038/s41564-019-0435-4. [DOI] [PubMed] [Google Scholar]
- Catalano D., Biasibetti E., Lynen G., Di Giulio G., De Meneghi D., Tomassone L., Valenza F., Capucchio M.T. “Ormilo disease” a disorder of zebu cattle in Tanzania: bovine cerebral theileriosis or new protozoan disease? Trop. Anim. Health Prod. 2015;47:895–901. doi: 10.1007/s11250-015-0805-8. [DOI] [PubMed] [Google Scholar]
- Catley A., Irungu P., Simiyu K., Dadye J., Mwakio W., Kiragu J., Nyamwaro S.O. Participatory investigations of bovine trypanosomiasis in Tana river District, Kenya. Med. Vet. Entomol. 2002;16:55–66. doi: 10.1046/j.0269-283x.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- Catley A., Chibunda R.T.T., Ranga E., Makungu S., Magayane F.T.T., Magoma G., Madege M.J.J., Vosloo W. Participatory diagnosis of a heat-intolerance syndrome in cattle in Tanzania and association with foot-and-mouth disease. Prev. Vet. Med. 2004;65:17–30. doi: 10.1016/j.prevetmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Catley A., Admassu B., Bekele G., Abebe D. Livestock mortality in pastoralist herds in Ethiopia and implications for drought response. Disasters. 2014;38:500–516. doi: 10.1111/disa.12060. [DOI] [PubMed] [Google Scholar]
- Chah J., M.Obi U.P., Ndofor-Foleng H.M. Management practices and perceived training needs of small ruminant farmers in Anambra State, Nigeria. African J. Agric. Res. 2013;8:2713–2721. doi: 10.5897/AJAR2013.7209. [DOI] [Google Scholar]
- Chaka H., Aboset G., Garoma A., Gumi B., Thys E. Cross-sectional survey of brucellosis and associated risk factors in the livestock–wildlife interface area of Nechisar National Park, Ethiopia. Trop. Anim. Health Prod. 2018;50:1041–1049. doi: 10.1007/s11250-018-1528-4. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Dhama K., Tiwari R., Iqbal Yatoo M., Khurana S.K., Khandia R., Munjal A., Munuswamy P., Kumar M.A., Singh M., Singh R., Gupta V.K., Chaicumpa W. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population -a review. Vet. Q. 2019:1–30. doi: 10.1080/01652176.2019.1642546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang’A J.S., Robertson L.J., Mtambo M.M.A., Mdegela R.H., Løken T., Reksen O., Chang’a J.S., Robertson L.J., Mtambo M.M.A., Mdegela R.H., Løken T., Reksen O. Unexpected results from large-scale cryptosporidiosis screening study in calves in Tanzania. Ann. Trop. Med. Parasitol. 2011;105:513–519. doi: 10.1179/2047773211Y.0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatikobo P., Choga T., Ncube C., Mutambara J. Participatory diagnosis and prioritization of constraints to cattle production in some smallholder farming areas of Zimbabwe. Prev. Vet. Med. 2013;109:327–333. doi: 10.1016/j.prevetmed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Chauhan H.C., Lambade P.S., Sen A., Dadawala A.I., Ranaware P.B., Chandel B., Joshi D.V., Patel S.S., Pankaj K., Shah N.M., Kher H.N. The use of pathological and histopathological techniques in the diagnosis of peste des petits ruminants in India. Vet. Italy. 2011;47:41–47. [PubMed] [Google Scholar]
- Chauhan H.C., Patel B.K., Bhagat A.G., Patel M.V., Patel S.I., Raval S.H., Panchasara H.H., Shrimali M.D., Patel A.C., Chandel B.S. Comparison of molecular and microscopic technique for detection of Theileria annulata from the field cases of cattle. Vet. World. 2015;8:1370–1374. doi: 10.14202/vetworld.2015.1370-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazya R., Muma J.B., Mwacalimba K.K., Karimuribo E., Mkandawire E., Simuunza M. A qualitative assessment of the risk of introducing peste des petits ruminants into Northern Zambia from Tanzania. Vet. Med. Int. 2014;2014:1–10. doi: 10.1155/2014/202618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhetri B.K., Perez A.M., Thurmond M.C. Factors associated with spatial clustering of foot-and-mouth disease in Nepal. Trop. Anim. Health Prod. 2010;42 doi: 10.1007/s11250-010-9573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikerema S.M., Pfukenyi D.M., Matope G., Bhebhe E. Temporal and spatial distribution of cattle anthrax outbreaks in Zimbabwe between 1967 and 2006. Trop. Anim. Health Prod. 2012;44:63–70. doi: 10.1007/s11250-011-9888-z. [DOI] [PubMed] [Google Scholar]
- Chikerema S.M., Matope G., Pfukenyi D.M. Awareness and attitude toward zoonoses with particular reference to Anthrax Among cattle owners in selected rural communities of Zimbabwe. Vector-Borne Zoonotic Dis. 2013;13:243–249. doi: 10.1089/vbz.2011.0916. [DOI] [PubMed] [Google Scholar]
- Chimana H.M., Muma J.B., Samui K.L., Hangombe B.M., Munyeme M., Matope G., Phiri A.M., Godfroid J., Skjerve E., Tryland M. A comparative study of the seroprevalence of brucellosis in commercial and small-scale mixed dairy-beef cattle enterprises of Lusaka province and Chibombo district, Zambia. Trop. Anim. Health Prod. 2010;42:1541–1545. doi: 10.1007/s11250-010-9604-4. [DOI] [PubMed] [Google Scholar]
- Choudhary V., Garg S., Chourasia R., Hasnani J.J., Patel P.V., Shah T.M., Bhatt V.D., Mohapatra A., Blake D.P., Joshi C.G. Transcriptome analysis of the adult rumen fluke Paramphistomum cervi following next generation sequencing. Gene. 2015;570:64–70. doi: 10.1016/j.gene.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Cleaveland S., Shaw D.J., Mfinanga S.G., Shirima G., Kazwala R.R., Eblate E., Sharp M. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis (Edinb) 2007;87:30–43. doi: 10.1016/j.tube.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Clifford D.L., Kazwala R.R., Sadiki H., Roug A., Muse E.A., Coppolillo P.C., Mazet J.A.K. Tuberculosis infection in wildlife from the Ruaha ecosystem Tanzania: implications for wildlife, domestic animals, and human health. Epidemiol. Infect. 2013;141:1371–1381. doi: 10.1017/S0950268813000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J.L., Monje F., Asiimwe-Karimu G., Amuguni H.J., Odoch T. A one Health, participatory epidemiology assessment of anthrax (Bacillus anthracis) management in Western Uganda. Soc. Sci. Med. 2015;129:44–50. doi: 10.1016/j.socscimed.2014.07.037. [DOI] [PubMed] [Google Scholar]
- Craighead L., Meyer A., Chengat B., Musallam I., Akakpo J., Kone P., Guitian J., Häsler B. Brucellosis in West and Central Africa: a review of the current situation in a changing landscape of dairy cattle systems. Acta Trop. 2018;179:96–108. doi: 10.1016/j.actatropica.2017.12.026. [DOI] [PubMed] [Google Scholar]
- Dahmani M., Sambou M., Scandola P., Raoult D., Fenollar F., Mediannikov O. Bartonella bovis and Candidatus Bartonella davousti in cattle from Senegal. Comp. Immunol. Microbiol. Infect. Dis. 2017;50:63–69. doi: 10.1016/j.cimid.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Dalis J.S., Kazeem H.M., Kwaga J.K.P., Kwanashie C.N., Yakubu B., Owolodun O.A., Jambol A.R. Molecular characterization of dermatophytes isolated from cattle in Plateau State, Nigeria. Vet. Microbiol. 2018;219:212–218. doi: 10.1016/j.vetmic.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Dar P.A., Hajam I.A., Suryanarayana V.S., Kishore S., Kondabattula G. Kinetics of cytokine expression in bovine PBMCs and whole blood after in vitro stimulation with foot-and-mouth disease virus (FMDV) antigen. Cytokine. 2015;72:58–62. doi: 10.1016/j.cyto.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Dar M.A., Ahmed R., Urwat U., Ahmad S.M., Dar P.A., Kushoo Z.A., Dar T.A., Mumtaz P.T., Bhat S.A., Amin U., Shabir N., Bhat H.F., Shah R.A., Ganai N.A., Heidari M. Expression kinetics of natural resistance associated macrophage protein (NRAMP) genes in Salmonella typhimurium-infected chicken. BMC Vet. Res. 2018;14:180. doi: 10.1186/s12917-018-1510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar M.A., Urwat U., Ahmad S.M., Ahmad R., Kashoo Z.A., Dar T.A., Bhat S.A., Mumtaz P.T., Shabir N., Shah R.A., Heidari M. Gene expression and antibody response in chicken against Salmonella typhimurium challenge. Poult. Sci. 2019;98:2008–2013. doi: 10.3382/ps/pey560. [DOI] [PubMed] [Google Scholar]
- Dean A.S., Fournié G., Kulo A.E., Boukaya G.A., Schelling E., Bonfoh B. Potential risk of regional disease spread in West Africa through cross-border cattle trade. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb R., Kumar A., Chakraborty S., Verma A., Tiwari R., Dhama K., Singh U., Kumar S. Trends in diagnosis and treatment of bovine mastitis: a review. Pak. J. Biol. Sci. 2013;16:1653–1661. doi: 10.3923/pjbs.2013.1653.1661. [DOI] [PubMed] [Google Scholar]
- Debele G., Guru M., Hundessa F., Duguma M. Assessment of farmers’ management practices and factors affecting goats’ production system in Adami Tulu Jido Kombolcha district of East Shawa Zone, Ethiopia. Agric. Biol. J. North Am. 2013;4:520–526. [Google Scholar]
- de Bronsvoort B.M.C., Thumbi S.M., Poole E.J., Kiara H., Tosas Auguet O., Handel I.G., Jennings A., Conradie I., Mbole-Kariuki M.N., Toye P.G., Hanotte O., Coetzer J., Woolhouse M.E. Design and descriptive epidemiology of the Infectious Diseases of East African Livestock (IDEAL) project, a longitudinal calf cohort study in western Kenya. BMC Vet. Res. 2013;9:171. doi: 10.1186/1746-6148-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeke B., Jenberie S., Tesfaye B., Ayelet G., Yami M., Lamien C.E., Gelaye E. Investigation of Marek’s disease virus from chickens in central Ethiopia. Trop. Anim. Health Prod. 2017;49:403–408. doi: 10.1007/s11250-016-1208-1. [DOI] [PubMed] [Google Scholar]
- Dhikusooka M.T., Ayebazibwe C., Namatovu A., Belsham G.J., Siegismund H.R., Wekesa S.N., Balinda S.N., Muwanika V.B., Tjørnehøj K. Unrecognized circulation of SAT 1 foot-and-mouth disease virus in cattle herds around Queen Elizabeth National Park in Uganda. BMC Vet. Res. 2016;12:5. doi: 10.1186/s12917-015-0616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossa L.H., Wollny C., Gauly M. Smallholders’ perceptions of goat farming in southern Benin and opportunities for improvement. Trop. Anim. Health Prod. 2007;39:49–57. doi: 10.1007/s11250-006-4440-2. [DOI] [PubMed] [Google Scholar]
- Duguma A., Abera S., Zewdie W., Belina D., Haro G. Status of bovine tuberculosis and its zoonotic implications in Borana zone, Southern Ethiopia. Trop. Anim. Health Prod. 2017;49:445–450. doi: 10.1007/s11250-016-1213-4. [DOI] [PubMed] [Google Scholar]
- Dutta T.K., Roychoudhury P., Bandyopadhyay S., Wani S.A., Hussain I. Detection and characterization of Shiga toxin producing Escherichia coli (STEC) and enteropathogenic Escherichia coli (EPEC) in poultry birds with diarrhoea. Indian J. Med. Res. 2011;133:541–545. [PMC free article] [PubMed] [Google Scholar]
- Edao B.M., Hailegebreal G., Berg S., Zewude A., Zeleke Y., Sori T., Almaw G., Whatmore A.M., Ameni G., Wood J.L.N. Brucellosis in the Addis Ababa dairy cattle: the myths and the realities. BMC Vet. Res. 2018;14:396. doi: 10.1186/s12917-018-1709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehizibolo D.O., Haegeman A., De Vleeschauwer A.R., Umoh J.U., Kazeem H.M., Okolocha E.C., Van Borm S., De Clercq K. Detection and molecular characterization of foot and mouth disease viruses from outbreaks in some states of Northern Nigeria 2013–2015. Transbound. Emerg. Dis. 2017;64:1979–1990. doi: 10.1111/tbed.12602. [DOI] [PubMed] [Google Scholar]
- Ehizibolo D.O., De Vleeschauwer A.R., Haegeman A., Lefebvre D., Nwosuh C.I., Umoh J.U., Okolocha E.C., Kazeem H.M., Van Borm S., De Clercq K. Serological and molecular epidemiology of foot‐and‐mouth disease viruses in agro‐pastoralist livestock herds in the kachia grazing reserve, Nigeria. Transbound. Emerg. Dis. 2019 doi: 10.1111/tbed.13182. tbed.13182. [DOI] [PubMed] [Google Scholar]
- Elbrissi A., Sabeil Y.A., Khalifa K.A., Enan K., Khair O.M., El Hussein A.M. Isolation, identification and differentiation of Campylobacter spp. using multiplex PCR assay from goats in Khartoum State, Sudan. Trop. Anim. Health Prod. 2017;49:575–581. doi: 10.1007/s11250-017-1231-x. [DOI] [PubMed] [Google Scholar]
- Elelu N. Epidemiological risk factors of knowledge and preventive practice regarding avian influenza among poultry farmers and live bird traders in Ikorodu, Lagos State, Nigeria. Int. J. Vet. Sci. Med. 2017;5:47–52. doi: 10.1016/j.ijvsm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elelu N., Ambali A., Coles G.C., Eisler M.C. Cross-sectional study of Fasciola gigantica and other trematode infections of cattle in Edu Local Government Area, Kwara State, north-central Nigeria. Parasit. Vectors. 2016;9:470. doi: 10.1186/s13071-016-1737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwelu I.A., Ezeuko E.L., Machebe N.S. Challenges of smallholder sheep and goat keeping in rural communities of Aguata Agricultural Zone of Anambra State, Nigeria. Indian J. Anim. Res. 2015;49:373. doi: 10.5958/0976-0555.2015.00131.4. [DOI] [Google Scholar]
- Esemu S.N., Besong W.O., Ndip R.N., Ndip L.M. Prevalence of Ehrlichia ruminantium in adult Amblyomma variegatum collected from cattle in Cameroon. Exp. Appl. Acarol. 2013;59:377–387. doi: 10.1007/s10493-012-9599-9. [DOI] [PubMed] [Google Scholar]
- Eshetu L., Yigezu L., Asfaw Y. A study on contagious caprine pleuropneumonia (CCPP) in goats at an export oriented abattoir, Debrezeit, Ethiopia. Trop. Anim. Health Prod. 2007;39:427–432. doi: 10.1007/s11250-007-9041-1. [DOI] [PubMed] [Google Scholar]
- Ezama A., Gonzalez J.-P., Majalija S., Bajunirwe F. Assessing short evolution brucellosis in a highly brucella endemic cattle keeping population of Western Uganda: a complementary use of Rose Bengal test and IgM rapid diagnostic test. BMC Public Health. 2018;18:315. doi: 10.1186/s12889-018-5228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafetine J., Neves L., Thompson P.N., Paweska J.T., Rutten V.P.M.G., Coetzer J.A.W. Serological evidence of rift valley fever virus circulation in sheep and goats in Zambézia Province, Mozambique. PLoS Negl. Trop. Dis. 2013;7:e2065. doi: 10.1371/journal.pntd.0002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafetine J.M., Coetzee P., Mubemba B., Nhambirre O., Neves L., Coetzer J.A.W., Venter E.H. Rift valley fever outbreak in livestock, Mozambique, 2014. Emerg. Infect. Dis. 2016;22:2165–2167. doi: 10.3201/eid2212.160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandamu P., Thys E., Duchateau L., Berkvens D. Perception of cattle farmers of the efficacy of east coast fever immunization in Southern Zambia. Trop. Anim. Health Prod. 2006;38:9–16. doi: 10.1007/s11250-006-4341-4. [DOI] [PubMed] [Google Scholar]
- Faris D., Yilkal A., Berhe G., Kelay B. Seroprevalence and sero-conversion after vaccination against Peste des Petits Ruminants in sheep and goats from Awash Fentale District, Afar, Ethiopia. Prev. Vet. Med. 2012;103:157–162. doi: 10.1016/j.prevetmed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Farougou S., Gagara M., Mensah G.A. Prevalence of peste des petits ruminants in the arid zone in the Republic of Niger. Onderstepoort J. Vet. Res. 2013;80 doi: 10.4102/ojvr.v80i1.544. [DOI] [PubMed] [Google Scholar]
- Fasina F.O., Sirdar M.M., Bisschop S.P.R. The financial cost implications of the highly pathogenic notifiable avian influenza H5N1 in Nigeria. Onderstepoort J. Vet. Res. 2008;75:39–46. doi: 10.4102/ojvr.v75i1.86. [DOI] [PubMed] [Google Scholar]
- Fenteng D.E., Ampofo W., Afari E., Wurapa F., Aryee M., Koney E., Yebuah N., Chima J., Awumbila B. Avian influenza surveillance in domestic poultry and wild bird-Tema Metropolis, Ghana, 2010. J. Commonw. Vet. Assoc. 2011;27:158–167. [Google Scholar]
- Fentie T., Abebe B., Kassa T. Small-scale family poultry production in north Gondar: characteristics, productivity and constraints. Livest. Res. Rural Dev. 2013:25. [Google Scholar]
- Fentie T., Fenta N., Leta S., Molla W., Ayele B., Teshome Y., Nigatu S., Assefa A. Sero-prevalence, risk factors and distribution of sheep and goat pox in Amhara Region, Ethiopia. BMC Vet. Res. 2017;13:385. doi: 10.1186/s12917-017-1312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetene T., Kebede N. Bovine tuberculosis of cattle in three districts of northwestern Ethiopia. Trop. Anim. Health Prod. 2009;41:273–277. doi: 10.1007/s11250-008-9186-6. [DOI] [PubMed] [Google Scholar]
- Fuller T.L., Ducatez M.F., Njabo K.Y., Couacy-Hymann E., Chasar A., Aplogan G.L., Lao S., Awoume F., Téhou A., Langeois Q., Krauss S., Smith T.B. Avian influenza surveillance in Central and West Africa, 2010-2014. Epidemiol. Infect. 2015;143:2205–2212. doi: 10.1017/S0950268814003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachohi J.M.M., Ngumi P.N.N., Kitala P.M.M., Skilton R.A.A. Estimating seroprevalence and variation to four tick-borne infections and determination of associated risk factors in cattle under traditional mixed farming system in Mbeere District, Kenya. Prev. Vet. Med. 2010;95:208–223. doi: 10.1016/j.prevetmed.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Gachohi J.M., Njenga M.K., Kitala P., Bett B. Modelling vaccination strategies against rift valley fever in livestock in Kenya. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakuya D.W., Mulei C.M., Wekesa S.B. Use of ethnoveterinary remedies in the management of foot and mouth disease lesions in a dairy herd. Afr. J. Tradit. Complement. Altern. Med. AJTCAM. 2011;8:165–169. doi: 10.4314/ajtcam.v8i2.63204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakuya F., Ombui J., Heukelbach J., Maingi N., Muchemi G., Ogara W., Mijele D., Alasaad S. Knowledge of mange among Masai pastoralists in Kenya. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gari G., Warer-Szkuta A., Grosbois V., Jacquiet P., Roger F. Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiol. Infect. 2010;138:1657–1666. doi: 10.1017/S0950268810000506. [DOI] [PubMed] [Google Scholar]
- Gari G., Bonnet P., Roger F., Waret-Szkuta A. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev. Vet. Med. 2011;102:274–283. doi: 10.1016/j.prevetmed.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Gari G., Grosbois V., Waret-Szkuta A., Babiuk S., Jacquiet P., Roger F. Lumpy skin disease in Ethiopia: seroprevalence study across different agro-climate zones. Acta Trop. 2012;123:101–106. doi: 10.1016/j.actatropica.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Gari G., Abie G., Gizaw D., Wubete A., Kidane M., Asgedom H., Bayissa B., Ayelet G., Oura C.A.L., Roger F., Tuppurainen E.S.M. Evaluation of the safety, immunogenicity and efficacy of three capripoxvirus vaccine strains against lumpy skin disease virus. Vaccine. 2015;33:3256–3261. doi: 10.1016/j.vaccine.2015.01.035. [DOI] [PubMed] [Google Scholar]
- Gebremedhin E., Agonafir A., Tessema T., Tilahun G., Medhin G., Vitale M., Marco V., Cox E., Vercruysse J., Dorny P. Seroepidemiological study of ovine toxoplasmosis in east and west shewa zones of oromia regional state, Central Ethiopia. BMC Vet. Res. 2013;9:117. doi: 10.1186/1746-6148-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin E., Tesfamaryam G., Yunus H., Duguma R., Tilahun G., Di Marco V., Vitale M. Seroepidemiology of Toxoplasma gondii infection in free-range chickens (Gallus domesticus) of Central Ethiopia. Epidemiol. Infect. 2015;143:608–617. doi: 10.1017/S0950268814000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geidam Y.A., Ayi V.K., Umar I.I., Sunday J., Musa D., Goni B., Mwapu D.N. Participatory disease surveillance in the detection of trans-boundary animal diseases (TADS) in Borno State of arid north-eastern Nigeria. Bull. Anim. Health Prod. Afr. 2013;61:231–239. [Google Scholar]
- Gelaye E., Mekonnen G., Jenberie S., Ayelet G. Detection of sheep-associated malignant catarrhal fever from clinical cases in Ethiopian cattle. Rev. Sci. Tech. l’OIE. 2013;32:851–856. doi: 10.20506/rst.32.2.2211. [DOI] [PubMed] [Google Scholar]
- Gelaye E., Belay A., Ayelet G., Jenberie S., Yami M., Loitsch A., Tuppurainen E., Grabherr R., Diallo A., Lamien C.E. Capripox disease in Ethiopia: genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antiviral Res. 2015;119:28–35. doi: 10.1016/j.antiviral.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Germeraad E.A., Hogerwerf L., Faye-Joof T., Goossens B., van der Hoek W., Jeng M., Lamin M., Manneh I.L., Nwakanma D., Roest H.I.J., Secka A., Stegeman A., Wegmüller R., van der Sande M.A.B., Secka O. Low seroprevalence of brucellosis in humans and small ruminants in the Gambia. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getaneh A.M., Gebremedhin E.Z. Meta-analysis of the prevalence of mastitis and associated risk factors in dairy cattle in Ethiopia. Trop. Anim. Health Prod. 2017;49:697–705. doi: 10.1007/s11250-017-1246-3. [DOI] [PubMed] [Google Scholar]
- Getaw A., Beyene D., Ayana D., Megersa B., Abunna F. Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopia. Acta Trop. 2010;113:221–225. doi: 10.1016/j.actatropica.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Giday M., Teklehaymanot T. Ethnobotanical study of plants used in management of livestock health problems by Afar people of Ada’ar District, Afar Regional State, Ethiopia. J. Ethnobiol. Ethnomed. 2013;9:8. doi: 10.1186/1746-4269-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girmay G., Arega B., Berkvens D., Altaye S.Z., Muleta G. Community-based tsetse fly control minimizes the effect of trypanosomosis on livestock in Metekel zone, Ethiopia. Trop. Anim. Health Prod. 2018;50:621–627. doi: 10.1007/s11250-017-1478-2. [DOI] [PubMed] [Google Scholar]
- Gitonga P.N., Gachene C.K., Njoroge E., Thumbi S.M. Small ruminant husbandry practices amongst Kajiado and Marsabit pastoralists and their effects on Peste des petits ruminants control strategies. Livest. Res. Rural Dev. 2016;28 [Google Scholar]
- Gomo C., Kanonhuwa K., Godobo F., Tada O., Makuza S.M. Temporal and spatial distribution of lumpy skin disease (LSD) outbreaks in Mashonaland West Province of Zimbabwe from 2000 to 2013. Trop. Anim. Health Prod. 2017;49:509–514. doi: 10.1007/s11250-017-1222-y. [DOI] [PubMed] [Google Scholar]
- Gorna K., Houndjè E., Romey A., Relmy A., Blaise-Boisseau S., Kpodékon M., Saegerman C., Moutou F., Zientara S., Bakkali Kassimi L. First isolation and molecular characterization of foot-and- mouth disease virus in Benin. Vet. Microbiol. 2014;171:175–181. doi: 10.1016/j.vetmic.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Govindaraj G.N., Roy G., Mohanty B.S., Balamurugan V., Pandey A.K., Sharma V., Patel A., Mehra M., Pandey S.K., Roy P. Evaluation of effectiveness of Mass Vaccination Campaign against Peste des petits ruminants in Chhattisgarh state. India. Transbound. Emerg. Dis. 2019;66:1349–1359. doi: 10.1111/tbed.13163. [DOI] [PubMed] [Google Scholar]
- Gowda R.N.S. An epidemiological study of sheep pox infection in Karnataka state, India. OIE Rev. Sci. Tech. 2005;24:909–920. doi: 10.20506/rst.24.3.1621. [DOI] [PubMed] [Google Scholar]
- Gururaj K., Pawaiya R.S., Gangwar N.K., Mishra A.K., Singh D.D., Andani D., Paul S., Sharma N., Shivasharanappa N., Rahal A., Chaturvedi V.K., Kumar A., Sharma D.K. Comparative molecular characterization and phylogenetic analysis of cerebral and non-cerebral coenurosis in Indian goats. Vet. Parasitol. Reg. Stud. Rep. 2019;15 doi: 10.1016/j.vprsr.2019.100266. [DOI] [PubMed] [Google Scholar]
- Gutiérrez- Expósito D., Ferre I., Ortega-Mora L.M., Álvarez-García G. Advances in the diagnosis of bovine besnoitiosis: current options and applications for control. Int. J. Parasitol. 2017;47:737–751. doi: 10.1016/j.ijpara.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Habitu T., Areda D., Muwonge A., Tessema G.T., Skjerve E., Gebrehiwot T. Prevalence and risk factors analysis of bovine tuberculosis in cattle raised in mixed crop-livestock farming system in Tigray region, Ethiopia. Transbound. Emerg. Dis. 2019;66:488–496. doi: 10.1111/tbed.13050. [DOI] [PubMed] [Google Scholar]
- Habte A., Ibrahim N. Prevalence of Haemonchus contortus infection in sheep slaughtered at Jimma town municipal abattoir, Ethiopia. Trop. Anim. Health Prod. 2018;50:1865–1870. doi: 10.1007/s11250-018-1637-0. [DOI] [PubMed] [Google Scholar]
- Habte T., Amare A., Bettridge J., Collins M., Robert C., Wigley P. 2017. Guide to Chicken Health and Management in Ethiopia. [Google Scholar]
- Hailu B., Tolosa T., Gari G., Teklue T., Beyene B. Estimated prevalence and risk factors associated with clinical Lumpy skin disease in north-eastern Ethiopia. Prev. Vet. Med. 2014;115:64–68. doi: 10.1016/j.prevetmed.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Hailu B., Alemayehu G., Sied N. Participatory epidemiological studies of major trade constraint diseases of goats in selected districts of Afar region. J. Biol. Agric. Healthc. 2015;5:140–148. [Google Scholar]
- Hamill L., Picozzi K., Fyfe J., von Wissmann B., Wastling S., Wardrop N., Selby R., Acup C.A., Bardosh K.L., Muhanguzi D., Kabasa J.D., Waiswa C., Welburn S.C. Evaluating the impact of targeting livestock for the prevention of human and animal trypanosomiasis, at village level, in districts newly affected with T. b. rhodesiense in Uganda. Infect. Dis. Poverty. 2017;6:16. doi: 10.1186/s40249-016-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami P., Lancelot R., Lesnoff M. Modelling the dynamics of post-vaccination immunity rate in a population of Sahelian sheep after a vaccination campaign against peste des petits ruminants virus. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan O.A., Ahlm C., Sang R., Evander M. The 2007 rift valley fever outbreak in Sudan. PLoS Negl. Trop. Dis. 2011;5:e1229. doi: 10.1371/journal.pntd.0001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy Profitós J.M., Moritz M., Garabed R.B. What to do with chronically sick animals? Pastoralists’ management strategies in the far north region of Cameroon. Pastoralism. 2013;3:1–11. doi: 10.1186/2041-7136-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector E., Elelu N., Ferrolho J., Couto J., Sanches G., Antunes S., Domingos A., Eisler M. PCR detection of Ehrlichia ruminantium and Babesia bigemina in cattle from Kwara State, Nigeria: unexpected absence of infection. Parasitol. Res. 2019;118:1025–1029. doi: 10.1007/s00436-019-06204-1. [DOI] [PubMed] [Google Scholar]
- Hegde R., Gomes A.R., Muniyellappa H.K., Byregowda S.M., Giridhar P., Renukaprasad C. A short note on peste des petits ruminants in Karnataka, India. Rev. Sci. Tech. 2009;28:1031–1035. doi: 10.20506/rst.28.3.1945. [DOI] [PubMed] [Google Scholar]
- Henning J., Bett B., Okike I., Abdu P., Perry B. Incidence of highly pathogenic avian influenza H5N1 in Nigeria, 2005–2008. Transbound. Emerg. Dis. 2013;60:222–230. doi: 10.1111/j.1865-1682.2012.01331.x. [DOI] [PubMed] [Google Scholar]
- Hiko A., Agga G.E. First-time detection of mycobacterium species from goats in Ethiopia. Trop. Anim. Health Prod. 2011;43:133–139. doi: 10.1007/s11250-010-9665-4. [DOI] [PubMed] [Google Scholar]
- Hinsu A.T., Thakkar J.R., Koringa P.G., Vrba V., Jakhesara S.J., Psifidi A., Guitian J., Tomley F.M., Rank D.N., Raman M., Joshi C.G., Blake D.P. Illumina next generation sequencing for the analysis of eimeria populations in commercial broilers and indigenous chickens. Front. Vet. Sci. 2018;5:176. doi: 10.3389/fvets.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hørning G., Rasmussen S., Permin A., Bisgaard M. Investigations on the influence of helminth parasites on vaccination of chickens against Newcastle disease virus under village conditions. Trop. Anim. Health Prod. 2003;35:415–424. doi: 10.1023/a:1025863412078. [DOI] [PubMed] [Google Scholar]
- Hota A., Biswal S., Sahoo N., Venkatesan G., Arya S., Kumar A., Ramakrishnan M.A., Pandey A.B., Rout M. Seroprevalence of Capripoxvirus infection in sheep and goats among different agro-climatic zones of Odisha, India. Vet. World. 2018;11:66–70. doi: 10.14202/vetworld.2018.66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove T., Lind P., Mukaratirwa S. Seroprevalence of Toxoplasma gondii infection in goats and sheep in Zimbabwe. Onderstepoort J. Vet. Res. 2005;72:267–272. doi: 10.4102/ojvr.v72i4.181. [DOI] [PubMed] [Google Scholar]
- Howell A., Mugisha L., Davies J., LaCourse E., Claridge J., Williams D.J., Kelly- Hope L., Betson M., Kabatereine N.B., Stothard J. Bovine fasciolosis at increasing altitudes: parasitological and malacological sampling on the slopes of Mount Elgon, Uganda. Parasit. Vectors. 2012;5:196. doi: 10.1186/1756-3305-5-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howson E.L.A., Armson B., Lyons N.A., Chepkwony E., Kasanga C.J., Kandusi S., Ndusilo N., Yamazaki W., Gizaw D., Cleaveland S., Lembo T., Rauh R., Nelson W.M., Wood B.A., Mioulet V., King D.P., Fowler V.L. Direct detection and characterization of foot-and-mouth disease virus in East Africa using a field-ready real-time PCR platform. Transbound. Emerg. Dis. 2018;65:221–231. doi: 10.1111/tbed.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton S., Bettridge J., Christley R., Habte T., Ganapathy K. Detection of infectious bronchitis virus 793B, avian metapneumovirus, Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Ethiopia. Trop. Anim. Health Prod. 2017;49:317–322. doi: 10.1007/s11250-016-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A.M.E., Elfahal A.M., El Hussein A.R.M. First report of Neospora caninum infection in cattle in Sudan. Trop. Anim. Health Prod. 2012;44:769–772. doi: 10.1007/s11250-011-9963-5. [DOI] [PubMed] [Google Scholar]
- Iqbal Yatoo M., Raffiq Parray O., Tauseef Bashir S., Ahmed Bhat R., Gopalakrishnan A., Karthik K., Dhama K., Vir Singh S. Contagious caprine pleuropneumonia - a comprehensive review. Vet. Q. 2019;39:1–25. doi: 10.1080/01652176.2019.1580826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraguha B., Hamudikuwanda H., Mushonga B. Bovine mastitis prevalence and associated risk factors in dairy cows in Nyagatare District, Rwanda. J. S. Afr. Vet. Assoc. 2015;86:1228. doi: 10.4102/jsava.v86i1.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac C., Ohiolei J.A.A., Ebhodaghe F., Igbinosa I.B., Eze A.A.A. Animal African Trypanosomiasis in Nigeria: a long way from elimination/eradication. Acta Trop. 2017;176:323–331. doi: 10.1016/j.actatropica.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Ishag O.M., Saeed I.K., Ali Y.H. Peste des petits ruminants outbreaks in White Nile State, Sudan. Onderstepoort J. Vet. Res. 2015;82:E1–4. doi: 10.4102/ojvr.v82i1.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.A., Khatun M.M., Werre S.R., Sriranganathan N., Boyle S.M. A review of Brucella seroprevalence among humans and animals in Bangladesh with special emphasis on epidemiology, risk factors and control opportunities. Vet. Microbiol. 2013;166:317–326. doi: 10.1016/j.vetmic.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Jackson D.S., Nydam D.V., Altier C. Prevalence and risk factors for brucellosis in domestic yak Bos grunniens and their herders in a transhumant pastoralist system of Dolpo, Nepal. Prev. Vet. Med. 2014;113:47–58. doi: 10.1016/j.prevetmed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Jagadeeswary V., Reddy M.S., Satyanarayan K. Ethno-veterinary practices used by tribals of Chittoor district, Andhra Pradesh, India. Indian J. Anim. Res. 2014;48:251. doi: 10.5958/j.0976-0555.48.3.054. [DOI] [Google Scholar]
- Jaiswal A.K., Sudan V., Kumar P., Srivastava A., Shanker D. Bovine hypodermosis in indigenous cattle herd and its successful therapeutic management. J. Parasit. Dis. 2016;40:166–168. doi: 10.1007/s12639-014-0470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakhesara S.J., Prasad V.V.S.P., Pal J.K., Jhala M.K., Prajapati K.S., Joshi C.G. Pathotypic and sequence characterization of newcastle disease viruses from vaccinated chickens reveals circulation of genotype II, IV and XIII and in India. Transbound. Emerg. Dis. 2016;63:523–539. doi: 10.1111/tbed.12294. [DOI] [PubMed] [Google Scholar]
- Jemberu W.T., Mourits M.C.M., Woldehanna T., Hogeveen H. Economic impact of foot and mouth disease outbreaks on smallholder farmers in Ethiopia. Prev. Vet. Med. 2014;116:26–36. doi: 10.1016/j.prevetmed.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Jemberu W., Mourits M., Hogeveen H. Farmers’ intentions to implement foot and mouth disease control measures in Ethiopia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemberu W.T., Mourits M.C.M., Sahle M., Siraw B., Vernooij J.C.M., Hogeveen H. Epidemiology of foot and mouth disease in Ethiopia: a retrospective analysis of district level outbreaks, 2007–2012. Transbound. Emerg. Dis. 2016;63:e246–e259. doi: 10.1111/tbed.12338. [DOI] [PubMed] [Google Scholar]
- Jemberu Wudu T., Mourits M., Rushton J., Hogeveen H. Cost-benefit analysis of foot and mouth disease control in Ethiopia. Prev. Vet. Med. 2016;132:67–82. doi: 10.1016/j.prevetmed.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Jenberie S., Lynch S.E., Kebede F., Christley R.M., Gelaye E., Negussie H., Asmare K., Ayelet G. Genetic characterisation of infectious bursal disease virus isolates in Ethiopia. Acta Trop. 2014;130:39–43. doi: 10.1016/j.actatropica.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenbreie S., Ayelet G., Gelaye E., Kebede F., Lynch S.E., Negussie H. Infectious bursal disease: seroprevalence and associated risk factors in major poultry rearing areas of Ethiopia. Trop. Anim. Health Prod. 2012;45:75–79. doi: 10.1007/s11250-012-0176-3. [DOI] [PubMed] [Google Scholar]
- Jibril A.H., Umoh J.U., Kabir J., Gashua M.M., Bello M.B. Application of participatory epidemiology techniques to investigate Newcastle disease among rural farmers in Zamfara state, Nigeria. J. Appl. Poult. Res. 2015;24:233–239. doi: 10.3382/japr/pfv012. [DOI] [Google Scholar]
- Kabi F., Masembe C., Muwanika V., Kirunda H., Negrini R. Geographic distribution of non-clinical Theileria parva infection among indigenous cattle populations in contrasting agro-ecological zones of Uganda: implications for control strategies. Parasit. Vectors. 2014;7:414. doi: 10.1186/1756-3305-7-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagira J.M., Kanyari P.W.N. Questionnaire survey on urban and peri-urban livestock farming practices and disease control in Kisumu municipality, Kenya. J. S. Afr. Vet. Assoc. 2012;81:82–86. doi: 10.4102/jsava.v81i2.110. [DOI] [PubMed] [Google Scholar]
- Kairu-Wanyoike S.W., Kaitibie S., Heffernan C., Taylor N.M., Gitau G.K., Kiara H., McKeever D. Willingness to pay for contagious bovine pleuropneumonia vaccination in Narok South District of Kenya. Prev. Vet. Med. 2014;115:130–142. doi: 10.1016/j.prevetmed.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakandelwa C., Siwila J., Nalubamba K.S., Muma J.B., Phiri I.G.K. Prevalence of Giardia in dairy cattle in Lusaka and Chilanga districts, Zambia. Vet. Parasitol. 2016;215:114–116. doi: 10.1016/j.vetpar.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Kalleshamurthy T., Shekar R., Niranjanamurthy H.H., Natesan K., Shome B.R., Bambal R.G., Sairiwal L., Barbuddhe S.B., Sahare A., Kilari S., Rahman H., Shome R. Assessment of fluorescence polarization assay: a candid diagnostic tool in Brucella abortus strain 19 vaccinated areas. Microbiol. Immunol. 2018;62:694–701. doi: 10.1111/1348-0421.12654. [DOI] [PubMed] [Google Scholar]
- Kamani J., Apanaskevich D.A., Gutiérrez R., Nachum- Biala Y., Baneth G., Harrus S. Morphological and molecular identification of Rhipicephalus (Boophilus) microplus in Nigeria, West Africa: a threat to livestock health. Exp. Appl. Acarol. 2017;73:283–296. doi: 10.1007/s10493-017-0177-z. [DOI] [PubMed] [Google Scholar]
- Kamboyi H.K., Garine‐Wichatitsky M., Hang’ombe M.B., Munyeme M. Risk mapping and eco‐anthropogenic assessment of anthrax in the upper Zambezi basin. Vet. Med. Sci. 2019:168. doi: 10.1002/vms3.168. vms3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanani A., Dabhi S., Patel Y., Chandra V., Kumar O.R.V., Shome R. Seroprevalence of brucellosis in small ruminants in organized and unorganized sectors of Gujarat state, India. Vet. World. 2018;11:1030–1036. doi: 10.14202/vetworld.2018.1030-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang’ethe E.K., Lang’a K.A. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr. Health Sci. 2009;9:218–226. [PMC free article] [PubMed] [Google Scholar]
- Kanouté Y.B., Gragnon B.G., Schindler C., Bonfoh B., Schelling E. Epidemiology of brucellosis, Q Fever and Rift Valley Fever at the human and livestock interface in northern Côte d’Ivoire. Acta Trop. 2017;165:66–75. doi: 10.1016/j.actatropica.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Kant N., Kulshreshtha P., Singh R., Mal A., Dwivedi A., Ahuja R., Mehra R., Tehlan M., Ahmed P., Kaushik S., Shipra Kumar S., Mohammad A., Shukla S., Singh D., Bhatnagar R. A study to identify the practices of the buffalo keepers which inadvertently lead to the spread of brucellosis in Delhi. BMC Vet. Res. 2018;14:329. doi: 10.1186/s12917-018-1670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimuribo E.D., Fitzpatrick J.L., Bell C.E., Swai E.S., Kambarage D.M., Ogden N.H., Bryant M.J., French N.P. Clinical and subclinical mastitis in smallholder dairy farms in Tanzania: risk, intervention and knowledge transfer. Prev. Vet. Med. 2006;74:84–98. doi: 10.1016/j.prevetmed.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Kasanga C.J., Sallu R., Kivaria F., Mkama M., Masambu J., Yongolo M., Das S., Mpelumbe-Ngeleja C., Wambura P.N., King D.P., Rweyemamu M.M. Foot-and-mouth disease virus serotypes detected in Tanzania from 2003 to 2010: conjectured status and future prospects. Onderstepoort J. Vet. Res. 2012;79 doi: 10.4102/ojvr.v79i2.462. [DOI] [PubMed] [Google Scholar]
- Kasanga C.J., Yamaguchi T., Munang’andu H.M., Ohya K., Fukushi H. Molecular epidemiology of infectious bursal disease virus in Zambia. J. S. Afr. Vet. Assoc. 2013;84 doi: 10.4102/jsava.v84i1.908. [DOI] [PubMed] [Google Scholar]
- Kasanga C.J., Wadsworth J., Mpelumbe- Ngeleja C.A.R., Sallu R., Kivaria F., Wambura P.N., Yongolo M.G.S., Rweyemamu M.M., Knowles N.J., King D.P. Molecular characterization of foot-and-Mouth disease viruses collected in Tanzania Between 1967 and 2009. Transbound. Emerg. Dis. 2015;62:e19–e29. doi: 10.1111/tbed.12200. [DOI] [PubMed] [Google Scholar]
- Kassaye E., Moser I., Woldemeskel M. Epidemiological study on clinical bovine dermatophilosis in northern Ethiopia. Tierarztl. Wochenschr. 2003;110:422–425. [PubMed] [Google Scholar]
- Kassian E.N., Simuunza M.C., Silayo R.S., Moonga L., Ndebe J., Sugimoto C., Namangala B. Prevalence and risk factors of bovine trypanosomosis in Kilwa district, Lindi region of southern Tanzania. Vet. Parasitol. Reg. Stud. Rep. 2017;9:1–5. doi: 10.1016/j.vprsr.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Katale B.Z., Mbugi E.V., Karimuribo E.D., Keyyu J.D., Kendall S., Kibiki G.S., Godfrey- Faussett P., Michel A.L., Kazwala R.R., van Helden P., Matee M.I. Prevalence and risk factors for infection of bovine tuberculosis in indigenous cattle in the Serengeti ecosystem, Tanzania. BMC Vet. Res. 2013;9:267. doi: 10.1186/1746-6148-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiravan G., Thirunavukkarasu, Michaelraj P. Willingness to pay for annual health care services in small ruminants: the case of South India. J. Appl. Sci. 2007;7:2361–2365. [Google Scholar]
- Kazoora H.B., Majalija S., Kiwanuka N., Kaneene J.B. Prevalence of Mycobacterium bovis skin positivity and associated risk factors in cattle from Western Uganda. Trop. Anim. Health Prod. 2014;46:1383–1390. doi: 10.1007/s11250-014-0650-1. [DOI] [PubMed] [Google Scholar]
- Kelly R.F., Hamman S.M., Morgan K.L., Nkongho E.F., Ngwa V.N., Tanya V., Andu W.N., Sander M., Ndip L., Handel I.G., Mazeri S., Muwonge A., de Bronsvoort B.M.C. Knowledge of bovine tuberculosis, cattle husbandry and dairy practices amongst pastoralists and small-scale dairy farmers in Cameroon. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T.R., Bunn D.A., Joshi N.P., Grooms D., Devkota D., Devkota N.R., Paudel L.N., Roug A., Wolking D.J., Mazet J.A.K. Awareness and practices relating to zoonotic diseases among smallholder farmers in Nepal. Ecohealth. 2018;15:656–669. doi: 10.1007/s10393-018-1343-4. [DOI] [PubMed] [Google Scholar]
- Kelly R.F., Mazeri S., Hartley C., Hamman S.M., Ngu Ngwa V., Nkongho E.F., Tanya V., Sander M., Ndip L., Morgan K.L., Muwonge A., Handel I., de Bronsvoort B.M.C., Williams D.J.L. Assessing the performance of a Fasciola gigantica serum antibody ELISA to estimate prevalence in cattle in Cameroon. BMC Vet. Res. 2019;15:8. doi: 10.1186/s12917-018-1762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemal J., Sibhat B., Abraham A., Terefe Y., Tulu K.T., Welay K., Getahun N. Bovine tuberculosis in eastern Ethiopia: prevalence, risk factors and its public health importance. BMC Infect. Dis. 2019;19:39. doi: 10.1186/s12879-018-3628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerario I.I., Simuunza M.C., Chenyambuga S.W., Koski M., Hwang S.-G., Muleya W. Prevalence and risk factors associated with Theileria parva infection in cattle in three regions of Tanzania. Trop. Anim. Health Prod. 2017;49:1613–1621. doi: 10.1007/s11250-017-1367-8. [DOI] [PubMed] [Google Scholar]
- Kerfua S.D., Shirima G., Kusiluka L., Ayebazibwe C., Martin E., Arinaitwe E., Cleaveland S., Haydon D.T. Low topotype diversity of recent foot-and-mouth disease virus serotypes O and A from districts located along the Uganda and Tanzania border. J. Vet. Sci. 2019;20:e4. doi: 10.4142/jvs.2019.20.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N., Jhala M.K., Joshi C.G. Genetic characterization of Indian peste des petits ruminants virus (PPRV) by sequencing and phylogenetic analysis of fusion protein and nucleoprotein gene segments. Res. Vet. Sci. 2008;85:176–183. doi: 10.1016/j.rvsc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Keyyu J., Kyvsgaard N., Kassuku A., Willingham A. Worm control practices and anthelmintic usage in traditional and dairy cattle farms in the southern highlands of Tanzania. Vet. Parasitol. 2003;114:51–61. doi: 10.1016/S0304-4017(03)00111-0. [DOI] [PubMed] [Google Scholar]
- Khan M.H., Manoj K., Pramod S. Reproductive disorders in dairy cattle under semi-intensive system of rearing in North-Eastern India. Vet. World. 2008;9:512–518. doi: 10.14202/vetworld.2016.512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihu S.M., Gachohi J.M., Ndungu E.K., Gitao G.C., Bebora L.C., John N.M., Wairire G.G., Maingi N., Wahome R.G., Ireri R. Sero-epidemiology of Peste des petits ruminants virus infection in Turkana County, Kenya. BMC Vet. Res. 2015;11:87. doi: 10.1186/s12917-015-0401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.Y., Kwak Y.S., Kim J.Y., Nam S.-H., Lee I.-Y., Mduma S., Keyyu J., Fyumagwa R., Yong T.-S. Prevalence of tick-borne pathogens from ticks collected from cattle and wild animals in Tanzania in 2012. Korean J. Parasitol. 2018;56:305–308. doi: 10.3347/kjp.2018.56.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimaro E.G., Mor S.M., Gwakisa P., Toribio J.-A. Seasonal occurrence of Theileria parva infection and management practices amongst Maasai pastoralist communities in Monduli District, Northern Tanzania. Vet. Parasitol. 2017;246:43–52. doi: 10.1016/j.vetpar.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Kimaro E.G., Toribio J.-A.L.M.L., Mor S.M. Climate change and cattle vector-borne diseases: use of participatory epidemiology to investigate experiences in pastoral communities in Northern Tanzania. Prev. Vet. Med. 2017;147:79–89. doi: 10.1016/j.prevetmed.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Kimaro E.G., Toribio J.-A.L.M.L., Gwakisa P., Mor S.M. Occurrence of trypanosome infections in cattle in relation to season, livestock movement and management practices of Maasai pastoralists in Northern Tanzania. Vet. Parasitol. Reg. Stud. Rep. 2018;12:91–98. doi: 10.1016/j.vprsr.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Kipronoh A.K., Ombui J.N., Kiara H.K., Binepal Y.S., Gitonga E., Wesonga H.O. Prevalence of contagious caprine pleuro-pneumonia in pastoral flocks of goats in the Rift Valley region of Kenya. Trop. Anim. Health Prod. 2016;48:151–155. doi: 10.1007/s11250-015-0934-0. [DOI] [PubMed] [Google Scholar]
- Kipronoh K.A., Ombui J.N., Binepal Y.S., Wesonga H.O., Gitonga E.K., Thuranira E., Kiara H.K. Risk factors associated with contagious caprine pleuro-pneumonia in goats in pastoral areas in the Rift Valley region of Kenya. Prev. Vet. Med. 2016;132:107–112. doi: 10.1016/j.prevetmed.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Kivaria F.M. Estimated direct economic costs associated with tick-borne diseases on cattle in Tanzania. Trop. Anim. Health Prod. 2006;38:291–299. doi: 10.1007/s11250-006-4181-2. [DOI] [PubMed] [Google Scholar]
- Kivaria F.M., Noordhuizen J.P.T.M., Kapaga A.M. Risk indicators associated with subclinical mastitis in smallholder dairy cows in Tanzania. Trop. Anim. Health Prod. 2004;36:581–592. doi: 10.1023/b:trop.0000040935.87175.bb. [DOI] [PubMed] [Google Scholar]
- Kivaria F.M., Noordhuizen J.P.T.M., Msami H.M. Risk factors associated with the incidence rate of clinical mastitis in smallholder dairy cows in the Dar es Salaam region of Tanzania. Vet. J. 2007;173:623–629. doi: 10.1016/j.tvjl.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Koka H., Sang R., Kutima H.L., Musila L. Coxiella burnetii detected in tick samples from pastoral communities in Kenya. Biomed. Res. Int. 2018;2018:1–5. doi: 10.1155/2018/8158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komba E.V.G., Mkupasi E.M., Mwesiga G.K., Mbyuzi A.O., Busagwe Z., Mzula A., Lupindu A.M., Nzalawahe J. Occurrence of helminths and coccidia in apparently healthy free range local chickens slaughtered at Morogoro live bird market. Tanzania Vet. J. 2013;28:55–61. [Google Scholar]
- Kouakou A.V., Kouakou V., Kouakou C., Godji P., Kouassi A.L., Krou H.A., Langeois Q., Webby R.J., Ducatez M.F., Couacy-Hymann E. Prevalence of Newcastle disease virus and infectious bronchitis virus in avian influenza negative birds from live bird markets and backyard and commercial farms in Ivory-Coast. Res. Vet. Sci. 2015;102:83–88. doi: 10.1016/j.rvsc.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouam M.K., Tchouankui H.N., Ngapagna A.N. Epidemiological features of highly pathogenic avian influenza in Cameroon. Vet. Med. Int. 2019;2019:1–5. doi: 10.1155/2019/3796369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracalik I.T., Kenu E., Ayamdooh E.N., Allegye-Cudjoe E., Polkuu P.N., Frimpong J.A., Nyarko K.M., Bower W.A., Traxler R., Blackburn J.K. Modeling the environmental suitability of anthrax in Ghana and estimating populations at risk: implications for vaccination and control. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulangara V., Joseph A., Thrithamarassery N., Sivasailam A., Kalappurackal L., Mattappillil S., Syam R., Mapranath S. Epidemiology of bovine viral diarrhoea among tropical small holder dairy units in Kerala, India. Trop. Anim. Health Prod. 2015;47:575–579. doi: 10.1007/s11250-015-0766-y. [DOI] [PubMed] [Google Scholar]
- Kumaresan A., Bujarbaruah K.M., Pathak K.A., Chhetri B., Ahmed S.K., Haunshi S. Analysis of a village chicken production system and performance of improved dual purpose chickens under a subtropical hill agro-ecosystem in India. Trop. Anim. Health Prod. 2008;40:395–402. doi: 10.1007/s11250-007-9097-y. [DOI] [PubMed] [Google Scholar]
- Kumbhakar N.K., Sanyal P.K., Rawte D., Kumar D., Kerketta A.E., Pal S. Efficacy of pharmacokinetic interactions between piperonyl butoxide and albendazole against gastrointestinal nematodiasis in goats. J. Helminthol. 2016;90:624–629. doi: 10.1017/S0022149X15000930. [DOI] [PubMed] [Google Scholar]
- Kundave V.R., Patel A.K., Patel P.V., Hasnani J.J., Joshi C.G. Qualitative and quantitative assessment of Theileria annulata in cattle and buffaloes Polymerase Chain Reaction. Trop. Biomed. 2014;31:728–735. [PubMed] [Google Scholar]
- Kundave V.R., Patel A.K., Patel P.V., Hasnani J.J., Joshi C.G. Detection of theileriosis in cattle and buffaloes by polymerase chain reaction. J. Parasit. Dis. 2015;39:508–513. doi: 10.1007/s12639-013-0386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing G., Aragrande M., Canali M., Savic S., De Meneghi D. Control of cattle ticks and tick-borne diseases by acaricide in southern province of Zambia: a retrospective evaluation of animal health measures according to current one health concepts. Front. Public Health. 2018;6:45. doi: 10.3389/fpubh.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisser E.L.K., Kipanyula M.J., Msalya G., Mdegela R.H., Karimuribo E.D., Mwilawa A.J., Mwega E.D., Kusiluka L., Chenyambuga S.W. Tick burden and prevalence of Theileria parva infection in Tarime zebu cattle in the lake zone of Tanzania. Trop. Anim. Health Prod. 2014;46:1391–1396. doi: 10.1007/s11250-014-0651-0. [DOI] [PubMed] [Google Scholar]
- Laisser E.L.K., Chenyambuga S.W., Karimuribo E.D., Msalya G., Kipanyula M.J., Mwilawa A.J., Mdegela R.H., Kusiluka L.J.M. A review on prevalence, control measure, and tolerance of Tanzania shorthorn Zebu cattle to East Coast fever in Tanzania. Trop. Anim. Health Prod. 2017;49:813–822. doi: 10.1007/s11250-017-1266-z. [DOI] [PubMed] [Google Scholar]
- Lankester F., Russell G.C., Lugelo A., Ndabigaye A., Mnyambwa N., Keyyu J., Kazwala R., Grant D., Percival A., Deane D., Haig D.M., Cleaveland S. A field vaccine trial in Tanzania demonstrates partial protection against malignant catarrhal fever in cattle. Vaccine. 2016;34:831–838. doi: 10.1016/j.vaccine.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson P.S., Espira L., Grabow C., Wang C.A., Muloi D., Browne A.S., Deem S.L., Fèvre E.M., Foufopoulos J., Hardin R., Eisenberg J.N.S. The sero‐epidemiology of Coxiella burnetii (Q fever) across livestock species and herding contexts in Laikipia County, Kenya. Zoonoses Public Health. 2019;66:316–324. doi: 10.1111/zph.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasecka-Dykes L., Wright C., Di Nardo A., Logan G., Mioulet V., Jackson T., Tuthill T., Knowles N., King D. Full genome sequencing reveals new southern African territories genotypes bringing us closer to understanding true variability of foot-and-mouth disease virus in Africa. Viruses. 2018;10:192. doi: 10.3390/v10040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Mossaad E., Ibrahim A.M., Ismail A.A., Adjou Moumouni P.F., Liu M., Ringo A.E., Gao Y., Guo H., Li J., Efstratiou A., Musinguzi P., Angara T.E.E., Suganuma K., Inoue N., Xuan X. Detection and molecular characterization of tick-borne pathogens infecting sheep and goats in Blue Nile and West Kordofan states in Sudan. Ticks Tick. Dis. 2018;9:598–604. doi: 10.1016/j.ttbdis.2018.01.014. [DOI] [PubMed] [Google Scholar]
- Legesse Y., Asfaw Y., Sahle M., Ayelet G., Jenberie S., Negussie H. First confirmation of foot and mouth disease virus serotype SAT-1 in cattle and small ruminants in Ethiopia in 2007/08. Trop. Anim. Health Prod. 2013;45:1265–1267. doi: 10.1007/s11250-012-0339-2. [DOI] [PubMed] [Google Scholar]
- Legesse M., Medhin G., Bayissa M., Mamo G. Knowledge and perception of pastoral community members about brucellosis as a cause of abortion in animals and its zoonotic importance in Amibara district, Afar Region, Ethiopia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnoff M., Laval G., Bonnet P., Abdicho S., Workalemahu A., Kifle D., Peyraud A., Lancelot R., Thiaucourt F. Within-herd spread of contagious bovine pleuropneumonia in Ethiopian highlands. Prev. Vet. Med. 2004;64:27–40. doi: 10.1016/j.prevetmed.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Lindahl J.F., Young J., Wyatt A., Young M., Alders R., Bagnol B., Kibaya A., Grace D. Do vaccination interventions have effects? A study on how poultry vaccination interventions change smallholder farmer knowledge, attitudes, and practice in villages in Kenya and Tanzania. Trop. Anim. Health Prod. 2019;51:213–220. doi: 10.1007/s11250-018-1679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobago F., Woldemeskel M. An outbreak of Marek’s disease in chickens in central Ethiopia. Trop. Anim. Health Prod. 2004;36:397–406. doi: 10.1023/b:trop.0000026665.78878.f4. [DOI] [PubMed] [Google Scholar]
- Lopes P.H., Akweongo P., Wurapa F., Afari E., Sackey S., Ocansey D., Nyarko K.M. Bovine tuberculosis surveillance system evaluation, Greater-Accra region, Ghana, 2006–2011. Pan. Afr. Med. J. 2016;25 doi: 10.11604/pamj.supp.2016.25.1.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludi A., Ahmed Z., Pomeroy L.W., Pauszek S.J., Smoliga G.R., Moritz M., Dickmu S., Abdoulkadiri S., Arzt J., Garabed R., Rodriguez L.L. Serotype diversity of foot-and-mouth-disease virus in livestock without history of vaccination in the far north region of Cameroon. Transbound. Emerg. Dis. 2016;63:e27–e38. doi: 10.1111/tbed.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu L., Bettridge J., Christley R.M., Melese K., Blake D., Dessie T., Wigley P., Desta T.T., Hanotte O., Kaiser P., Terfa Z.G., Collins M., Lynch S.E. Prevalence and molecular characterisation of Eimeria species in Ethiopian village chickens. BMC Vet. Res. 2013;9:208. doi: 10.1186/1746-6148-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons N.A., Alexander N., Stärk K.D.C., Dulu T.D., Rushton J., Fine P.E.M. Impact of foot-and-mouth disease on mastitis and culling on a large-scale dairy farm in Kenya. Vet. Res. 2015;46:41. doi: 10.1186/s13567-015-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons N.A., Jemberu W.T., Chaka H., Salt J.S., Rushton J. Field-derived estimates of costs for Peste des Petits Ruminants vaccination in Ethiopia. Prev. Vet. Med. 2019;163:37–43. doi: 10.1016/j.prevetmed.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan S., Mor S.K., Jindal N., Joshi V.G., Ravishankar C., Singh V.K., Ravindran R., Sahoo N., Radzio-Basu J., Schilling M.A., McVey W.R., Mahajan N.K., Kapur V., Goyal S.M. Complete genome sequences of Newcastle disease virus isolates from backyard chickens in Northern India. Microbiol. Resour. Announc. 2019;8 doi: 10.1128/MRA.00467-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machila N., Wanyangu S.W., McDermott J., Welburn S.C., Maudlin I., Eisler M.C. Cattle owners’ perceptions of African bovine trypanosomiasis and its control in Busia and Kwale Districts of Kenya. Acta Trop. 2003;86:25–34. doi: 10.1016/S0001-706X(02)00288-7. [DOI] [PubMed] [Google Scholar]
- Madut N.A., Muwonge A., Nasinyama G.W., Muma J.B., Godfroid J., Jubara A.S., Muleme J., Kankya C. The sero-prevalence of brucellosis in cattle and their herders in Bahr el Ghazal region, South Sudan. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafimisebi T.E., Oguntade A.E., Fajemisin A.N., Aiyelari O.P. Local knowledge and socio- economic determinants of traditional medicines’ utilization in livestock health management in Southwest Nigeria. J. Ethnobiol. Ethnomed. 2012;8:2. doi: 10.1186/1746-4269-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafirakureva P., Saidi B., Mbanga J. Incidence and molecular characterisation of lumpy skin disease virus in Zimbabwe using the P32 gene. Trop. Anim. Health Prod. 2017;49:47–54. doi: 10.1007/s11250-016-1156-9. [DOI] [PubMed] [Google Scholar]
- Magona J.S.P., Olaho-Mukani W., Coleman P.G., Jonsson N.N., Welburn S.C., Eisler M.C., J.W. M A comparative study on the clinical, parasitological and molecular diagnosis of bovine trypanosomosis in Uganda. Onderstepoort J. Vet. Res. 2003;70:213–218. [PubMed] [Google Scholar]
- Magona J.W., Walubengo J., Odimin J.T. Acute haemorrhagic syndrome of bovine trypanosomosis in Uganda. Acta Trop. 2008;107:186–191. doi: 10.1016/j.actatropica.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Maitho T., Kinyua J.W. Factors and diseases influencing dairy goats production among small scale farmers in Laikipia East District, Kenya. Int. J. Livest. Res. 2015;5:43–48. [Google Scholar]
- Majekodunmi A., Fajinmi A., Dongkum C., Picozzi K., Thrusfield M., Welburn S. A longitudinal survey of African animal trypanosomiasis in domestic cattle on the Jos Plateau, Nigeria: prevalence, distribution and risk factors. Parasit. Vectors. 2013;6:239. doi: 10.1186/1756-3305-6-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majekodunmi A.O., Fajinmi A., Dongkum C., Picozzi K., MacLeod E., Thrusfield M.V., M Shaw A.P., Welburn S.C. Social factors affecting seasonal variation in bovine trypanosomiasis on the Jos Plateau, Nigeria. Parasit. Vectors. 2013;6:293. doi: 10.1186/1756-3305-6-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita K., Fèvre E.M., Waiswa C., Eisler M.C., Thrusfield M., Welburn S.C. Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Vet. Res. 2011;7:60. doi: 10.1186/1746-6148-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malak A.K., Mpoke L., Banak J., Muriuki S., Skilton R.A., Odongo D., Sunter J., Kiara H. Prevalence of livestock diseases and their impact on livelihoods in Central Equatoria State, southern Sudan. Prev. Vet. Med. 2012;104:216–223. doi: 10.1016/j.prevetmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Malik M., Verma H. Epidemiological aspect and major constraints in controlling haemorrhagic septicemia in dairy animals of Punjab. Indian J. Anim. Sci. 2018;88:1112–1117. [Google Scholar]
- Mamoudou A., Njanloga A., Hayatou A., Suh P.F., Achukwi M.D. Animal trypanosomosis in clinically healthy cattle of north Cameroon: epidemiological implications. Parasit. Vectors. 2016;9:206. doi: 10.1186/s13071-016-1498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjeet, Pander B.L., Sharma R., Dhaka S.S., Magotra A., Dev K. Evaluation of genetic and non-genetic factors on foot and mouth disease (FMD) virus vaccine-elicited immune response in Hardhenu (Bos taurus x Bos indicus) cattle. Trop. Anim. Health Prod. 2017;49:1689–1695. doi: 10.1007/s11250-017-1379-4. [DOI] [PubMed] [Google Scholar]
- Matiko M.K., Salekwa L.P., Kasanga C.J., Kimera S.I., Evander M., Nyangi W.P. Serological evidence of inter-epizootic/inter-epidemic circulation of Rift Valley fever virus in domestic cattle in Kyela and Morogoro, Tanzania. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matope G., Bhebhe E., Muma J.B., Oloya J., Madekurozwa R.L., Lund A., Skjerve E. Seroprevalence of brucellosis and its associated risk factors in cattle from smallholder dairy farms in Zimbabwe. Trop. Anim. Health Prod. 2011;43:975–982. doi: 10.1007/s11250-011-9794-4. [DOI] [PubMed] [Google Scholar]
- Matos C.A., Gonçalves L.R., de Souza Ramos I.A., Mendes N.S., Zanatto D.C.S., André M.R., Machado R.Z. Molecular detection and characterization of Ehrlichia ruminantium from cattle in Mozambique. Acta Trop. 2019;191:198–203. doi: 10.1016/j.actatropica.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Mazeri S., Scolamacchia F., Handel I.G., Morgan K.L., Tanya V.N., de Bronsvoort B.M.C. Risk factor analysis for antibodies to Brucella, Leptospira and C. Burnetii among cattle in the Adamawa Region of Cameroon: a cross- sectional study. Trop. Anim. Health Prod. 2013;45:617–623. doi: 10.1007/s11250-012-0268-0. [DOI] [PubMed] [Google Scholar]
- Mbengue M., Diallo A.A., Lo F.T., Lo M.M., Diop M., Seck P.S., Samb Y., Diouf M., Thiongane Y. Réémergence de la péripneumonie contagieuse bovine au Sénégal. Bull. la Société Pathol. Exot. 2013;106:212–215. doi: 10.1007/s13149-013-0298-5. [DOI] [PubMed] [Google Scholar]
- Mbuh J.V.V., Ndamukong K.J.N.J.N., Ntonifor N., Nforlem G.F.F. Parasites of sheep and goats and their prevalence in Bokova, a rural area of Buea Sub Division, Cameroon. Vet. Parasitol. 2008;156:350–352. doi: 10.1016/j.vetpar.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Mbyuzi A.O., Komba E.V.G., Kimera S.I., Kambarage D.M. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuro-pneumonia in goats and sheep in the Southern Zone of Tanzania. Prev. Vet. Med. 2014;116:138–144. doi: 10.1016/j.prevetmed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Meas S., Nakayama M., Usui T., Nakazato Y., Yasuda J., Ohashi K., Onuma M. Evidence for bovine immunodeficiency virus infection in cattle in Zambia. Jpn. J. Vet. Res. 2004;52:3–8. [PubMed] [Google Scholar]
- Mebrahtu K., Teshale S., Esatu W., Habte T., Gelaye E. Evaluation of spray and oral delivery of Newcastle disease I2 vaccine in chicken reared by smallholder farmers in central Ethiopia. BMC Vet. Res. 2018;14:48. doi: 10.1186/s12917-018-1355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena H., Ram H., Sahoo A., Rasool T. Livestock husbandry scenario at high altitude Kumaon Himalaya. Indian J. Anim. Sci. 2008;78:882–886. [Google Scholar]
- Mekonnen S.A., Koop G., Lam T.J.G.M., Hogeveen H. The intention of North-Western Ethiopian dairy farmers to control mastitis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekonnen S.A.A., Koop G., Melkie S.T.T., Getahun C.D.D., Hogeveen H., Lam T.J.G.M. Prevalence of subclinical mastitis and associated risk factors at cow and herd level in dairy farms in North-West Ethiopia. Prev. Vet. Med. 2017;145:23–31. doi: 10.1016/j.prevetmed.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Mekuriaw A., Bitew M., Gelaye E., Mamo B., Ayelet G. Infectious bursal disease: outbreak investigation, molecular characterization, and vaccine immunogenicity trial in Ethiopia. Trop. Anim. Health Prod. 2017;49:1295–1302. doi: 10.1007/s11250-017-1328-2. [DOI] [PubMed] [Google Scholar]
- Meskerem A. Major health constraints and ethno-veterinary practices of small scale and backyard chicken production in some selected regions of Ethiopia. Rev. Med. Vet. (Toulouse) 2017;168:63–71. [Google Scholar]
- Meunier N.V., Sebulime P., White R.G., Kock R. Wildlife-livestock interactions and risk areas for cross-species spread of bovine tuberculosis. Onderstepoort J. Vet. Res. 2017;84 doi: 10.4102/ojvr.v84i1.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Nakavuma J.L., Ssajjakambwe P., Vudriko P., Musisi N., Kaneene J.B. The prevalence of brucellosis in cattle, goats and humans in rural Uganda: a comparative study. Transbound. Emerg. Dis. 2016;63:e197–e210. doi: 10.1111/tbed.12332. [DOI] [PubMed] [Google Scholar]
- Mishra D., Sahu R., Mishra N., Behera A. Herbal treatment for common diseases in ruminants: an overview. J. Livest. Sci. 2015;6:36–43. [Google Scholar]
- Mkama M., Kasanga C.J., Sallu R., Ranga E., Yongolo M., Mulumba M., Rweyemamu M., Wambura P. Serosurveillance of foot-and-mouth disease virus in selected livestock-wildlife interface areas of Tanzania. Onderstepoort J. Vet. Res. 2014;81 doi: 10.4102/ojvr.v81i2.718. [DOI] [PubMed] [Google Scholar]
- Mlilo D., Mhlanga M., Mwembe R., Sisito G., Moyo B., Sibanda B. The epidemiology of malignant catarrhal fever (MCF) and contribution to cattle losses in farms around Rhodes Matopos National Park, Zimbabwe. Trop. Anim. Health Prod. 2015;47:989–994. doi: 10.1007/s11250-015-0821-8. [DOI] [PubMed] [Google Scholar]
- Mohamed S.B., Alagib A., AbdElkareim T.B., Hassan M.M., Johnson W.C., Hussein H.E., Taus N.S., Ueti M.W. Molecular detection and characterization of Theileria spp. infecting cattle in Sennar State, Sudan. Parasitol. Res. 2018;117:1271–1276. doi: 10.1007/s00436-018-5775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed S., Munissi J.J.E., Nyandoro S.S. Aflatoxin M1 in raw milk and aflatoxin B1 in feed from household cows in Singida, Tanzania. Food Addit. Contam. Part B Surveill. 2016;9:85–90. doi: 10.1080/19393210.2015.1137361. [DOI] [PubMed] [Google Scholar]
- Moiane I., Machado A., Santos N., Nhambir A., Inlamea O., Hattendorf J., Källenius G., Zinsstag J., Correia-Neves M. Prevalence of bovine tuberculosis and risk factor assessment in cattle in Rural Livestock Areas of Govuro District in the southeast of Mozambique. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla B., Delil F. Mapping of major diseases and devising prevention and control regimen to common diseases in cattle and shoats in Dassenech district of South Omo Zone, South-Western Ethiopia. Trop. Anim. Health Prod. 2015;47:45–51. doi: 10.1007/s11250-014-0681-7. [DOI] [PubMed] [Google Scholar]
- Molla W., de Jong M.C.M., Frankena K. Temporal and spatial distribution of lumpy skin disease outbreaks in Ethiopia in the period 2000 to 2015. BMC Vet. Res. 2017;13:310. doi: 10.1186/s12917-017-1247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla W., Frankena K., De Jong M.C.M. Transmission dynamics of lumpy skin disease in Ethiopia. Epidemiol. Infect. 2017;145:2856–2863. doi: 10.1017/S0950268817001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla Wassie, de Jong M.C.M.M., Gari G., Frankena K. Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Prev. Vet. Med. 2017;147:100–107. doi: 10.1016/j.prevetmed.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Molla Wassie, Frankena K., Gari G., de Jong M.C.M. Field study on the use of vaccination to control the occurrence of lumpy skin disease in Ethiopian cattle. Prev. Vet. Med. 2017;147:34–41. doi: 10.1016/j.prevetmed.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Molla W., Frankena K., Gari G., Kidane M., Shegu D., de Jong M.C.M. Seroprevalence and risk factors of lumpy skin disease in Ethiopia. Prev. Vet. Med. 2018;160:99–104. doi: 10.1016/j.prevetmed.2018.09.029. [DOI] [PubMed] [Google Scholar]
- Mondal D.B., Sarma K., Saravanan M. Upcoming of the integrated tick control program of ruminants with special emphasis on livestock farming system in India. Ticks Tick. Dis. 2013;4:1–10. doi: 10.1016/j.ttbdis.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Morakinyo O.A., Babalobi O.O. Southern African Society for Veterinary Epidemiology and Preventive Medicine. KZN Dolphin Coast; South Africa: 2013. Participatory appraisal of Peste des Petite Ruminants (PPR) outbreaks in Iseyin local government area of Oyo State, Nigeria; pp. 28–33. [Google Scholar]
- Morgan K.L., Handel I.G., Tanya V.N., Hamman S.M., Nfon C., Bergman I.E., Malirat V., Sorensen K.J., de Bronsvoort B.M.C., de Bronsvoort B.M.C. Accuracy of herdsmen reporting versus serologic testing for estimating foot-and-mouth disease prevalence. Emerg. Infect. Dis. 2014;20:2048–2054. doi: 10.3201/eid2012.140931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moti Y., de Deken R., Thys E., van den Abbeele J., Duchateau L., Delespaux V. PCR and microsatellite analysis of diminazene aceturate resistance of bovine trypanosomes correlated to knowledge, attitude and practice of livestock keepers in South-Western Ethiopia. Acta Trop. 2015;146:45–52. doi: 10.1016/j.actatropica.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Motta P., Handel I.G., Rydevik G., Hamman S.M., Ngwa V.N., Tanya V.N., Morgan K.L., de Bronsvoort B.M.C., Porphyre T. Drivers of live cattle price in the livestock trading system of Central Cameroon. Front. Vet. Sci. 2018;4 doi: 10.3389/fvets.2017.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta P., Porphyre T., Handel I.G., Hamman S.M., Ngu Ngwa V., Tanya V.N., Morgan K.L., de Bronsvoort B.M.C. Characterizing livestock markets, primary diseases, and key management practices along the livestock supply chain in Cameroon. Front. Vet. Sci. 2019;6:101. doi: 10.3389/fvets.2019.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msami H. 2007. Poultry Sector Country Review: Tanzania. [Google Scholar]
- Msoffe P.L.M.M., Bunn D., Muhairwa A.P., Mtambo M.M.A.A., Mwamhehe H., Msago A., Mlozi M.R.S.S., Cardona C.J. Implementing poultry vaccination and biosecurity at the village level in Tanzania: a social strategy to promote health in free-range poultry populations. Trop. Anim. Health Prod. 2010;42:253–263. doi: 10.1007/s11250-009-9414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubamba C., Ramsay G., Abolnik C., Dautu G., Gummow B. A retrospective study and predictive modelling of Newcastle Disease trends among rural poultry of eastern Zambia. Prev. Vet. Med. 2016;133:97–107. doi: 10.1016/j.prevetmed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Muema J., Thumbi S.M., Obonyo M., Wanyoike S., Nanyingi M., Osoro E., Bitek A., Karanja S. Seroprevalence and factors associated with Coxiella burnetii infection in small ruminants in Baringo County, Kenya. Zoonoses Public Health. 2017;64:e31–e43. doi: 10.1111/zph.12342. [DOI] [PubMed] [Google Scholar]
- Mugabi K.N., Mugisha A., Ocaido M. Socio-economic factors influencing the use of acaricides on livestock: a case study of the pastoralist communities of Nakasongola District, Central Uganda. Trop. Anim. Health Prod. 2010;42:131–136. doi: 10.1007/s11250-009-9396-6. [DOI] [PubMed] [Google Scholar]
- Mugisha A., McLeod A., Percy R., Kyewalabye E. Strategies, effectiveness and rationale of vector-borne disease control in the pastoralist system of south-western Uganda. Trop. Anim. Health Prod. 2005;37:479–489. doi: 10.1007/s11250-005-2174-1. [DOI] [PubMed] [Google Scholar]
- Mugisha A., McLeod A., Percy R., Kyewalabye E. Socio-economic factors influencing control of vector-borne diseases in the pastoralist system of south western Uganda. Trop. Anim. Health Prod. 2008;40:287–297. doi: 10.1007/s11250-007-9093-2. [DOI] [PubMed] [Google Scholar]
- Mugizi D.R., Boqvist S., Nasinyama G.W., Waiswa C., Ikwap K., Rock K., Lindahl E., Magnusson U., Erume J. Prevalence of and factors associated with Brucella sero- positivity in cattle in urban and peri-urban Gulu and Soroti towns of Uganda. J. Vet. Med. Sci. 2015;77:557–564. doi: 10.1292/jvms.14-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhanguzi D., Okello W.O., Kabasa J.D., Waiswa C., Welburn S.C., Shaw A.P.M. Cost analysis of options for management of African Animal Trypanosomiasis using interventions targeted at cattle in Tororo District; south-eastern Uganda. Parasit. Vectors. 2015;8:387. doi: 10.1186/s13071-015-0998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhanguzi D., Mugenyi A., Bigirwa G., Kamusiime M., Kitibwa A., Akurut G.G., Ochwo S., Amanyire W., Okech S.G., Hattendorf J., Tweyongyere R. African animal trypanosomiasis as a constraint to livestock health and production in Karamoja region: a detailed qualitative and quantitative assessment. BMC Vet. Res. 2017;13:355. doi: 10.1186/s12917-017-1285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muleme M., Barigye R., Khaitsa M.L., Berry E., Wamono A.W., Ayebazibwe C. Effectiveness of vaccines and vaccination programs for the control of foot-and-mouth disease in Uganda, 2001–2010. Trop. Anim. Health Prod. 2012;45:35–43. doi: 10.1007/s11250-012-0254-6. [DOI] [PubMed] [Google Scholar]
- Mulugeta Y., Yacob H.T., Ashenafi H. Ectoparasites of small ruminants in three selected agro-ecological sites of Tigray Region, Ethiopia. Trop. Anim. Health Prod. 2010;42:1219–1224. doi: 10.1007/s11250-010-9551-0. [DOI] [PubMed] [Google Scholar]
- Muma J.B., Godfroid J., Samui K.L., Skjerve E. The role of Brucella infection in abortions among traditional cattle reared in proximity to wildlife on the Kafue flats of Zambia. Rev. Sci. Tech. 2007;26:721–730. [PubMed] [Google Scholar]
- Muma J.B., Syakalima M., Munyeme M., Zulu V.C., Simuunza M., Kurata M. Bovine tuberculosis and brucellosis in traditionally managed livestock in selected districts of southern province of Zambia. Vet. Med. Int. 2013;2013:1–7. doi: 10.1155/2013/730367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munang’andu H.M., Banda F., Chikampa W., Mutoloki S., Syakalima M., Munyeme M. Risk analysis of an anthrax outbreak in cattle and humans of Sesheke district of Western Zambia. Acta Trop. 2012;124:162–165. doi: 10.1016/j.actatropica.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Mungube E.O., Tenhagen B.A., Kassa T., Regassa F., Kyule M.N., Greiner M., Baumann M.P.O. Risk factors for dairy cow mastitis in the central highlands of Ethiopia. Trop. Anim. Health Prod. 2004;36:463–472. doi: 10.1023/b:trop.0000034999.08368.f3. [DOI] [PubMed] [Google Scholar]
- Munyeme M., Munang’andu H.M., Nambota A., Muma J.B., Phiri A.M., Nalubamba K.S. The Nexus between bovine tuberculosis and fasciolosis infections in cattle of the Kafue Basin Ecosystem in Zambia: implications on abattoir surveillance. Vet. Med. Int. 2012;2012:1–6. doi: 10.1155/2012/921869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguri G.R., McLeod A., McDermott J.J., Taylor N. The incidence of calf morbidity and mortality due to vector-borne infections in smallholder dairy farms in Kwale District, Kenya. Vet. Parasitol. 2005;130:305–315. doi: 10.1016/j.vetpar.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Murugeswari R., Valli C., Karunakaran R., Leela V., Pandian A.S.S. Prevalence and magnitude of acidosis sequelae to rice-based feeding regimen followed in Tamil Nadu, India. Vet. World. 2018;11:464–468. doi: 10.14202/vetworld.2018.464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa I., Ndahi M., Pam E., Okike A., Cyrile P., Saskia H., Jost C. Using participatory epidemiological techniques to establish rural based poultry disease profiles: practical field experience in the Jos Plateau, Nigeria. Worlds Poult. Sci. J. 2013;69:387–400. doi: 10.1017/S0043933913000378. [DOI] [Google Scholar]
- Musinguzi S., Suganuma K., Asada M., Laohasinnarong D., Sivakumar T., Yokoyama N., Namangala B., Sugimoto C., Suzuki Y., Xuan X., Inoue N. A PCR-based survey of animal African trypanosomosis and selected piroplasm parasites of cattle and goats in Zambia. J. Vet. Med. Sci. 2016;78:1819–1824. doi: 10.1292/jvms.16-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutebi F., Krücken J., Mencke N., Feldmeier H., von Samson- Himmelstjerna G., Waiswa C. Two severe cases of Tungiasis in goat kids in Uganda. J. Insect Sci. 2016;16:34. doi: 10.1093/jisesa/iew016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutinda W.U., Nyaga P.N., Mbuthia P.G., Bebora L.C., Muchemi G. Risk factors associated with infectious bursal disease vaccination failures in broiler farms in Kenya. Trop. Anim. Health Prod. 2014;46:603–608. doi: 10.1007/s11250-013-0533-x. [DOI] [PubMed] [Google Scholar]
- Mutua E.N., Bukachi S.A., Bett B.K., Estambale B.A., Nyamongo I.K. “We do not bury dead livestock like human beings”: community behaviors and risk of Rift Valley Fever virus infection in Baringo County, Kenya. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyobela J., Nkunika P.O.Y., Mwase E.T. Resistance status of ticks (Acari; Ixodidae) to amitraz and cypermethrin acaricides in Isoka District, Zambia. Trop. Anim. Health Prod. 2015;47:1599–1605. doi: 10.1007/s11250-015-0906-4. [DOI] [PubMed] [Google Scholar]
- Mwakapuja R.S., Makondo Z.E., Malakalinga J., Moser I., Kazwala R.R., Tanner M. Molecular characterization of Mycobacterium bovis isolates from pastoral livestock at Mikumi-Selous ecosystem in the eastern Tanzania. Tuberculosis. 2013;93:668–674. doi: 10.1016/j.tube.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Mwebe R., Nakavuma J., Moriyón I. Brucellosis seroprevalence in livestock in Uganda from 1998 to 2008: a retrospective study. Trop. Anim. Health Prod. 2011;43:603–608. doi: 10.1007/s11250-010-9739-3. [DOI] [PubMed] [Google Scholar]
- Mwenda R., Changula K., Hang’ombe B.M., Chidumayo N., Mangani A.S., Kaira T., Takada A., Mweene A.S., Simulundu E., Hang’ombe B.M., Chidumayo N., Mangani A.S., Kaira T., Takada A., Mweene A.S., Simulundu E., Hang’ombe B.M., Chidumayo N., Mangani A.S., Kaira T., Takada A., Mweene A.S., Simulundu E. Characterization of field infectious bursal disease viruses in Zambia: evidence of co-circulation of multiple genotypes with predominance of very virulent strains. Avian Pathol. 2018;47:300–313. doi: 10.1080/03079457.2018.1449941. [DOI] [PubMed] [Google Scholar]
- Nabukenya I., Rubaire-Akiiki C., Olila D., Ikwap K., Höglund J. Ethnopharmacological practices by livestock farmers in Uganda: survey experiences from Mpigi and Gulu districts. J. Ethnobiol. Ethnomed. 2014;10:9. doi: 10.1186/1746-4269-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namatovu A., Belsham G.J., Ayebazibwe C., Dhikusooka M.T., Wekesa S.N., Siegismund H.R., Muwanika V.B., Tjørnehøj K. Challenges for serology-based characterization of foot-and-Mouth disease outbreaks in endemic areas; identification of two separate lineages of serotype O FMDV in Uganda in 2011. Transbound. Emerg. Dis. 2015;62:522–534. doi: 10.1111/tbed.12170. [DOI] [PubMed] [Google Scholar]
- Namatovu Alice, Tjørnehøj K., Belsham G.J., Dhikusooka M.T., Wekesa S.N., Muwanika V.B., Siegismund H.R., Ayebazibwe C. Characterization of foot- and-mouth disease viruses (FMDVs) from Ugandan cattle outbreaks during 2012-2013: evidence for circulation of multiple serotypes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanyingi M.O., Muchemi G.M., Thumbi S.M., Ade F., Onyango C.O., Kiama S.G., Bett B. Seroepidemiological survey of rift valley fever virus in ruminants in Garissa, Kenya. Vector- Borne Zoonotic Dis. 2017;17:141–146. doi: 10.1089/vbz.2016.1988. [DOI] [PubMed] [Google Scholar]
- Nazki S., Wani S.A., Parveen R., Ahangar S.A., Kashoo Z.A., Hamid S., Dar Z.A., Dar T.A., Dar P.A. Isolation, molecular characterization and prevalence of Clostridium perfringens in sheep and goats of Kashmir Himalayas, India. Vet. World. 2017;10:1501–1507. doi: 10.14202/vetworld.2017.1501-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndengu M., de Garine-Wichatitsky M., Pfukenyi D., Tivapasi M., Mukamuri B., Matope G. Assessment of community awareness and risk perceptions of zoonotic causes of abortion in cattle at three selected livestock–wildlife interface areas of Zimbabwe. Epidemiol. Infect. 2017;145:1304–1319. doi: 10.1017/S0950268817000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndhlovu D.N., Masika P.J. Risk factors associated with clinical dermatophilosis in smallholder sector cattle herds of Zimbabwe at the Amblyomma variegatum and Amblyomma hebraeum interface. Trop. Anim. Health Prod. 2015;47:353–360. doi: 10.1007/s11250-014-0727-x. [DOI] [PubMed] [Google Scholar]
- Ndhlovu F., Ndhlovu D.N., Chikerema S.M., Masocha M., Nyagura M., Pfukenyi D.M. Spatiotemporal patterns of clinical bovine dermatophilosis in Zimbabwe 1995–2014. Onderstepoort J. Vet. Res. 2017;84 doi: 10.4102/ojvr.v84i1.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negusssie H., Kyule M.N., Yami M., Ayelet G., Jenberie T.S. Outbreak investigations and genetic characterization of foot-and-mouth disease virus in Ethiopia in 2008/2009. Trop. Anim. Health Prod. 2011;43:235–243. doi: 10.1007/s11250-010-9683-2. [DOI] [PubMed] [Google Scholar]
- Ngomtcho S.C.H., Weber J.S., Ngo Bum E., Gbem T.T., Kelm S., Achukwi M.D. Molecular screening of tsetse flies and cattle reveal different Trypanosoma species including T. grayi and T. theileri in northern Cameroon. Parasit. Vectors. 2017;10:631. doi: 10.1186/s13071-017-2540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngonyoka A., Gwakisa P.S., Estes A.B., Salekwa L.P., Nnko H.J., Hudson P.J., Cattadori I.M. Patterns of tsetse abundance and trypanosome infection rates among habitats of surveyed villages in Maasai steppe of northern Tanzania. Infect. Dis. Poverty. 2017;6:126. doi: 10.1186/s40249-017-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguhiu-Mwangi J., Mbithi P., Wabacha J., Mbuthia P. Retrospective study of foot conditions in dairy cows in urban and periurban areas of Kenya. Isr. J. Vet. Med. 2008;63:40–45. [Google Scholar]
- Nigussie Z., Mesfin T., Sertse T., Fulasa T.T., Regassa F., Tolosa Fulasa T., Regassa F. Seroepidemiological study of bovine viral diarrhea (BVD) in three agroecological zones in Ethiopia. Trop. Anim. Health Prod. 2010;42:319–321. doi: 10.1007/s11250-009-9445-1. [DOI] [PubMed] [Google Scholar]
- Njeru J., Wareth G., Melzer F., Henning K., Pletz M.W., Heller R., Neubauer H. Systematic review of brucellosis in Kenya: disease frequency in humans and animals and risk factors for human infection. BMC Public Health. 2016;16:853. doi: 10.1186/s12889-016-3532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuguna J.N., Gicheru M.M., Kamau L.M., Mbatha P.M. Incidence and knowledge of bovine brucellosis in Kahuro district, Murang’a County, Kenya. Trop. Anim. Health Prod. 2017;49:1035–1040. doi: 10.1007/s11250-017-1296-6. [DOI] [PubMed] [Google Scholar]
- Nkegbe E., Munkaila L., Odoom-Sam K. Regulatory compliance of small holder livestock farmers and herdsmen in the use of acaricides and gastrointestinal anthelmintics in sub-urban Accra, Ghana. Int. J. Dis. Disord. 2013;1:39–44. [Google Scholar]
- Nnko H.J., Gwakisa P.S., Ngonyoka A., Saigilu M., Ole-Neselle M., Kisoka W., Sindato C., Estes A. Pastoralists’ vulnerability to trypanosomiasis in Maasai Steppe. Ecohealth. 2017;14:718–731. doi: 10.1007/s10393-017-1275-4. [DOI] [PubMed] [Google Scholar]
- Noah E.Y., Kimera S.I., Kusiluka L.J.M., Wambura P. Abattoir surveillance demonstrates contagious bovine pleuropneumonia is widespread in Tanzania. Trop. Anim. Health Prod. 2015;47:1607–1613. doi: 10.1007/s11250-015-0907-3. [DOI] [PubMed] [Google Scholar]
- Nonga H.E., Karimuribo E.D. A retrospective survey of hydatidosis in livestock in Arusha, Tanzania, based on abattoir data during 2005–2007. Trop. Anim. Health Prod. 2009;41:1253–1257. doi: 10.1007/s11250-009-9308-9. [DOI] [PubMed] [Google Scholar]
- Ntirandekura J.-B., Matemba L.E., Kimera S.I., Muma J.B., Karimuribo E.D. Association of brucellosis with abortion prevalence in humans and animals in Africa: a review. Afr. J. Reprod. Health. 2018;22:120–136. doi: 10.29063/ajrh2018/v22i3.13. [DOI] [PubMed] [Google Scholar]
- Nuru A., Mamo G., Zewude A., Mulat Y., Yitayew G., Admasu A., Medhin G., Pieper R., Ameni G. Preliminary investigation of the transmission of tuberculosis between farmers and their cattle in smallholder farms in northwestern Ethiopia: a cross-sectional study. BMC Res. Notes. 2017;10:31. doi: 10.1186/s13104-016-2349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwankiti O.O., Ikeh E.I., Asala O., Seuberlich T. A pilot study for targeted surveillance of bovine spongiform encephalopathy in Nigeria. Transbound. Emerg. Dis. 2013;60:279–283. doi: 10.1111/j.1865-1682.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- Nyaguthii D.M., Armson B., Kitala P.M., Sanz-Bernardo B., Di Nardo A., Lyons N.A. Knowledge and risk factors for foot-and-mouth disease among small-scale dairy farmers in an endemic setting. Vet. Res. 2019;50:33. doi: 10.1186/s13567-019-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyakarahuka L., de St. Maurice A., Purpura L., Ervin E., Balinandi S., Tumusiime A., Kyondo J., Mulei S., Tusiime P., Lutwama J., Klena J.D., Brown S., Knust B., Rollin P.E., Nichol S.T., Shoemaker T.R., de St Maurice A., Purpura L., Ervin E., Balinandi S., Tumusiime A., Kyondo J., Mulei S., Tusiime P., Lutwama J., Klena J.D., Brown S., Knust B., Rollin P.E., Nichol S.T., Shoemaker T.R. Prevalence and risk factors of Rift Valley fever in humans and animals from Kabale district in Southwestern Uganda, 2016. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda S.S., Sakala M., Moonde L., Kayesa E., Fandamu P., Banda F., Sinkala Y. Prevalence of bovine fascioliasis and economic impact associated with liver condemnation in abattoirs in Mongu district of Zambia. BMC Vet. Res. 2019;15(21 January 2019) doi: 10.1186/s12917-019-1777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzalawahe J., Kassuku A.A., Stothard J., Coles G.C., Eisler M.C. Trematode infections in cattle in Arumeru District, Tanzania are associated with irrigation. Parasit. Vectors. 2014;7:107. doi: 10.1186/1756-3305-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzalawahe J., Kassuku A.A., Stothard J.R., Coles G.C., Eisler M.C. Associations between trematode infections in cattle and freshwater snails in highland and lowland areas of Iringa Rural District, Tanzania. Parasitology. 2015;142:1430–1439. doi: 10.1017/S0031182015000827. [DOI] [PubMed] [Google Scholar]
- Ocholi R.A., Kwaga J.K.P., Ajogi I., Bale J.O.O. Abortion due to Brucella abortus in sheep in Nigeria. Rev. Sci. Tech. 2005;24:973–979. [PubMed] [Google Scholar]
- Ochwo S., VanderWaal K., Munsey A., Ndekezi C., Mwebe R., Okurut A.R.A., Nantima N., Mwiine F.N. Spatial and temporal distribution of lumpy skin disease outbreaks in Uganda (2002–2016) BMC Vet. Res. 2018;14:174. doi: 10.1186/s12917-018-1503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoi A., Gathuma J.M., Gachuiri C.K., Omore A. Risk factors of gastrointestinal nematode parasite infections in small ruminants kept in smallholder mixed farms in Kenya. BMC Vet. Res. 2007;3:6. doi: 10.1186/1746-6148-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odongo D.O., Tiampati C.M., Mulinge E., Mbae C.K., Bishop R.P., Zeyhle E., Magambo J., Wasserman M., Kern P., Romig T. Prevalence and genotyping of Echinococcus granulosus in sheep in Narok County, Kenya. Parasitol. Res. 2018;117:2065–2073. doi: 10.1007/s00436-018-5889-4. [DOI] [PubMed] [Google Scholar]
- Odugbo M.O., Ogunjumo S.O., Chukwukere S.C., Kumbish P.R., Musa A., Ekundayo S.O., Okewole P.A., Nwankpa N.D., Itodo A.E., Haruna G. The first report of Histophilus somni pneumonia in Nigerian dairy cattle. Vet. J. 2009;181:340–342. doi: 10.1016/j.tvjl.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ogendo A., Obonyo M., Wasswa P., Bitek A., Mbugua A., Thumbi S.M. Cryptosporidium infection in calves and the environment in Asembo, Western Kenya: 2015. Pan. Afr. Med. J. 2017;28 doi: 10.11604/pamj.supp.2017.28.1.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogugua A.J., Akinseye V.O., Cadmus E.O., Jolaoluwa Awosanya E.A., Alabi P.I., Idowu O.S., Akinade S.A., Dale E.J., Perrett L., Taylor A., Ignocio M., Cadmus S.I.B. Prevalence and risk factors associated with bovine brucellosis in herds under extensive production system in southwestern Nigeria. Trop. Anim. Health Prod. 2018;50:1573–1582. doi: 10.1007/s11250-018-1597-4. [DOI] [PubMed] [Google Scholar]
- Ogundiyi A.I., Bemji M.N., Adebambo O.A., Dipeolu M.A., Onagbesan O.M., James I.J., Osinowo O.A. Prevalence of mange among West African Dwarf sheep and goats and associated haematological and biochemical parameters. Trop. Anim. Health Prod. 2012;44:1263–1269. doi: 10.1007/s11250-011-0067-z. [DOI] [PubMed] [Google Scholar]
- Ohaga S.O., Kokwaro E.D., Ndiege I.O., Hassanali A., Saini R.K. Livestock farmers’ perception and epidemiology of bovine trypanosomosis in Kwale District, Kenya. Prev. Vet. Med. 2007;80:24–33. doi: 10.1016/j.prevetmed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Okuni J.B., Reinacher M., Loukopoulos P., Ojok L. Prevalence and spectrum of Johne’s disease lesions in cattle slaughtered at two abattoirs in Kampala, Uganda. Trop. Anim. Health Prod. 2013;45:1197–1202. doi: 10.1007/s11250-012-0346-3. [DOI] [PubMed] [Google Scholar]
- Oluwayelu D.O., Todd D., Ball N.W., Scott A.N.J., Oladele O.A., Emikpe B.O., Fagbohun O.A., Owoade A.A., Olaleye O.D. Isolation and preliminary characterization of chicken anemia virus from chickens in Nigeria. Avian Dis. 2005;49:446–450. doi: 10.1637/7339-020705R.1. [DOI] [PubMed] [Google Scholar]
- Oluwayelu D., Adebiyi A., Tomori O. Endemic and emerging arboviral diseases of livestock in Nigeria: a review. Parasit. Vectors. 2018;11:337. doi: 10.1186/s13071-018-2911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omony J.B., Wanyana A., Mugimba K.K., Kirunda H., Nakavuma J.L., Otim-Onapa M., Byarugaba D.K. Disparate thermostability profiles and HN gene domains of field isolates of Newcastle disease virus from live bird markets and waterfowl in Uganda. Virol. J. 2016;13:103. doi: 10.1186/s12985-016-0560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onono J.O., Wieland B., Rushton J. Estimation of impact of contagious bovine pleuropneumonia on pastoralists in Kenya. Prev. Vet. Med. 2014;115:122–129. doi: 10.1016/j.prevetmed.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Onzima R.B., Mukiibi R., Ampaire A., Benda K.K., Kanis E. Between-breed variations in resistance/resilience to gastrointestinal nematodes among indigenous goat breeds in Uganda. Trop. Anim. Health Prod. 2017;49:1763–1769. doi: 10.1007/s11250-017-1390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opara M.N., Ukpong U.M., Okoli I.C., Anosike J.C. Cysticercosis of slaughter cattle in southeastern Nigeria. Ann. N. Y. Acad. Sci. 2006;1081:339–346. doi: 10.1196/annals.1373.048. [DOI] [PubMed] [Google Scholar]
- Orono S.A., Gitao G.C., Mpatswenumugabo J.P., Chepkwony M., Mutisya C., Okoth E., de Bronsvoort B.M.C., Russell G.C., Nene V., Cook E.A.J. Field validation of clinical and laboratory diagnosis of wildebeest associated malignant catarrhal fever in cattle. BMC Vet. Res. 2019;15:69. doi: 10.1186/s12917-019-1818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Pelaez A., Pfeiffer D.U., Tempia S., Otieno F.T., Aden H.H., Costagli R. Risk mapping of Rinderpest sero-prevalence in Central and Southern Somalia based on spatial and network risk factors. BMC Vet. Res. 2010;6:22. doi: 10.1186/1746-6148-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani M.G., Ward M.P., Giasuddin M., Islam M.R., Kalam A. The spread of highly pathogenic avian influenza (subtype H5N1) clades in Bangladesh, 2010 and 2011. Prev. Vet. Med. 2014;114:21–27. doi: 10.1016/j.prevetmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Owolodun O.A., Yakubu B., Jambol A.R., Audu B.J., Dogonyaro B.B., Luka P.D. Further evidence for very virulent infectious bursal disease virus in vaccinated chickens in Nigeria. Trop. Anim. Health Prod. 2015;47:1437–1441. doi: 10.1007/s11250-015-0880-x. [DOI] [PubMed] [Google Scholar]
- Panda T., Mishra N. Indigenous knowledge on animal health care practices in Kendrapara District of Odisha, India. Int. Lett. Nat. Sci. 2016;53:10–27. doi: 10.18052/www.scipress.com/ILNS.53.10. [DOI] [Google Scholar]
- Pandey V., Nigam R., Jaiswal A.K., Sudan V., Singh R.K., Yadav P.K. Haemato-biochemical and oxidative status of buffaloes naturally infected with Trypanosoma evansi. Vet. Parasitol. 2015;212:118–122. doi: 10.1016/j.vetpar.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Pandey G.S., Simulundu E., Mwiinga D., Samui K.L., Mweene A.S., Kajihara M., Mangani A., Mwenda R., Ndebe J., Konnai S., Takada A. Clinical and subclinical bovine leukemia virus infection in a dairy cattle herd in Zambia. Arch. Virol. 2017;162:1051–1056. doi: 10.1007/s00705-016-3205-0. [DOI] [PubMed] [Google Scholar]
- Patil M.P., Nagvekar A.S., Ingole S.D., Bharucha S.V., Palve V.T. Somatic cell count and alkaline phosphatase activity in milk for evaluation of mastitis in buffalo. Vet. World. 2015;8:363–366. doi: 10.14202/vetworld.2015.363-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado A., Speybroeck N., Kivaria F., Mosha R.M., Sumaye R.D., Casal J., Berkvens D. Foot-and-Mouth Disease in Tanzania from 2001 to 2006. Transbound. Emerg. Dis. 2011;58:44–52. doi: 10.1111/j.1865-1682.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- Pomeroy L.W., Bjørnstad O.N., Kim H., Jumbo S.D., Abdoulkadiri S., Garabed R. Serotype-specific transmission and waning immunity of endemic foot-and-mouth disease virus in Cameroon. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Ramachandran N., Raju S. Mortality patterns in dairy animals under organized herd management conditions at Karnal India. Trop. Anim. Health Prod. 2004;36:645–654. doi: 10.1023/B:TROP.0000042855.58026.bd. [DOI] [PubMed] [Google Scholar]
- Priyadarshini A., Sarangi L., Palai T., Panda H., Mishra R., Behera P. Brucellosis in cattle and occupationally exposed human beings: a serosurvey in Odisha, India. J. Pure Appl. Microbiol. 2013;7:3255–3260. [Google Scholar]
- Rajeev M., Mutinda M., Ezenwa V.O. Pathogen exposure in cattle at the livestock-wildlife interface. Ecohealth. 2017;14:542–551. doi: 10.1007/s10393-017-1242-0. [DOI] [PubMed] [Google Scholar]
- Rajkhowa S., Rajkhowa C., Hazarika G.C. Prevalence of Cryptosporidium parvum in mithuns (Bos frontalis) from India. Vet. Parasitol. 2006;142:146–149. doi: 10.1016/j.vetpar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Rajkumar K., Bhattacharya A., David S., Balaji S.H., Hariharan R., Jayakumar M., Balaji N. Socio-demographic study on extent of knowledge, awareness, attitude, and risks of zoonotic diseases among livestock owners in Puducherry region. Vet. World. 2016;9:1018–1024. doi: 10.14202/vetworld.2016.1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranabijuli S., Mohapatra J.K., Pandey L.K., Rout M., Sanyal A., Dash B.B., Sarangi L.N., Panda H.K., Pattnaik B. Serological evidence of foot-and-mouth disease virus infection in randomly surveyed goat population of Orissa, India. Transbound. Emerg. Dis. 2010 doi: 10.1111/j.1865-1682.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- *Randolph T., Schelling E., Grace D., Nicholson C., Leroy J., Cole D., Demment M., Omore A., Zinsstag J., Ruel M. Invited Review: role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007;85:2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- Rao K.A., Rao K.S., Rao S., Ravi A., Anitha A. Ethnoveterinary practices in sheep of North coastal zone of Andhra Pradesh. Indian J. Small Rumin. 2011;17:252–253. [Google Scholar]
- Regassa F., Araya M. In vitro antimicrobial activity of Combretum molle (Combretaceae) against Staphylococcus aureus and Streptococcus agalactiae isolated from crossbred dairy cows with clinical mastitis. Trop. Anim. Health Prod. 2012;44:1169–1173. doi: 10.1007/s11250-011-0054-4. [DOI] [PubMed] [Google Scholar]
- Romha G., Gebru G., Asefa A., Mamo G. Epidemiology of Mycobacterium bovis and Mycobacterium tuberculosis in animals: transmission dynamics and control challenges of zoonotic TB in Ethiopia. Prev. Vet. Med. 2018;158:1–17. doi: 10.1016/j.prevetmed.2018.06.012. [DOI] [PubMed] [Google Scholar]
- Saka J.O., Adesehinwa A.O.K., Oyegbami A., Omole A.J., Gyoung Rae C., Young Joo S., ChongDae K., SungWoo K., IkSoo J. The effects of health management system on the growth of chicken small farm in southwest states of Nigeria. Korean J. Poult. Sci. 2017;44:225–233. [Google Scholar]
- Salih D.A., El Hussein A.M., Kyule M.N., Zessin K.-H., Ahmed J.S., Seitzer U. Determination of potential risk factors associated with Theileria annulata and Theileria parva infections of cattle in the Sudan. Parasitol. Res. 2007;101:1285–1288. doi: 10.1007/s00436-007-0634-4. [DOI] [PubMed] [Google Scholar]
- Sambo E., Bettridge J., Dessie T., Amare A., Habte T., Wigley P., Christley R.M. Participatory evaluation of chicken health and production constraints in Ethiopia. Prev. Vet. Med. 2015;118:117–127. doi: 10.1016/j.prevetmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel A., Nayak B., Paldurai A., Xiao S., Aplogan G.L., Awoume K.A., Webby R.J., Ducatez M.F., Collins P.L., Samal S.K. Phylogenetic and pathotypic characterization of Newcastle disease viruses circulating in West Africa and efficacy of a current vaccine. J. Clin. Microbiol. 2013;51:771–781. doi: 10.1128/JCM.02750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangma D.B., Manohara T. The role of Garo tribes of Meghalaya (India) in the conservation and management of medicinal plants diversity used in treating livestock diseases. Plant Sci. Today. 2018;5:155. doi: 10.14719/pst.2018.5.4.416. [DOI] [Google Scholar]
- Sanogo M., Abatih E., Thys E., Fretin D., Berkvens D., Saegerman C. Importance of identification and typing of Brucellae from West African cattle: a review. Vet. Microbiol. 2013;164:202–211. doi: 10.1016/j.vetmic.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Sarma K., Prasad H., Das G., Behera P., Behera S.K., Rajesh J.B., Borthakur S.K. Theileriasis in crossbred cows and its therapeutic management: first report from Lushai hill district of Mizoram. J. Parasit. Dis. 2016;40:605–610. doi: 10.1007/s12639-014-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyabarathi M., Jeyakumar S., Manimaran A., Jayaprakash G., Pushpadass H.A., Sivaram M., Ramesha K.P., Das D.N., Kataktalware M.A., Prakash M.A., Kumar R.D. Infrared thermography: a potential noninvasive tool to monitor udder health status in dairy cows. Vet. World. 2016;9:1075–1081. doi: 10.14202/vetworld.2016.1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonman L., Swai E.S. Herd- and animal-level risk factors for bovine leptospirosis in Tanga region of Tanzania. Trop. Anim. Health Prod. 2010;42:1565–1572. doi: 10.1007/s11250-010-9607-1. [DOI] [PubMed] [Google Scholar]
- Schoonman L.B., Wilsmore T., Swai E.S. Sero-epidemiological investigation of bovine toxoplasmosis in traditional and smallholder cattle production systems of Tanga Region, Tanzania. Trop. Anim. Health Prod. 2010;42:579–587. doi: 10.1007/s11250-009-9460-2. [DOI] [PubMed] [Google Scholar]
- Seck M.T., Bouyer J., Sall B., Bengaly Z., Vreysen M.J.B. The prevalence of African animal trypanosomoses and tsetse presence in Western Senegal. Parasite. 2010;17:257–265. doi: 10.1051/parasite/2010173257. [DOI] [PubMed] [Google Scholar]
- Shabani S.S., Ezekiel M.J., Mohamed M., Moshiro C.S. Knowledge, attitudes and practices on Rift Valley fever among agro pastoral communities in Kongwa and Kilombero districts, Tanzania. BMC Infect. Dis. 2015;15:363. doi: 10.1186/s12879-015-1099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K.K., Kshirsagar D.P., Kalyani I.H., Patel D.R., Vihol P.D., Patel J.M. Diagnosis of peste des petits ruminants infection in small ruminants through in-house developed Indirect ELISA: practical considerations. Vet. World. 2015;8:443–448. doi: 10.14202/vetworld.2015.443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G.K., Mahajan S., Matura R., Biswal J.K., Ranjan R., Subramaniam S., Misri J., Bambal R.G., Pattnaik B. Herd immunity against foot- and-Mouth disease under different vaccination practices in India. Transbound. Emerg. Dis. 2017;64:1133–1147. doi: 10.1111/tbed.12478. [DOI] [PubMed] [Google Scholar]
- Shiferaw T.J., Moses K., Manyahilishal K.E. Participatory appraisal of foot and mouth disease in the Afar pastoral area, northeast Ethiopia: implications for understanding disease ecology and control strategy. Trop. Anim. Health Prod. 2010;42:193–201. doi: 10.1007/s11250-009-9405-9. [DOI] [PubMed] [Google Scholar]
- Shirima G.M., Masola S.N., Malangu O.N., Schumaker B.A. Outbreak investigation and control case report of brucellosis: experience from livestock research centre, Mpwapwa, Tanzania. Onderstepoort J. Vet. Res. 2014;81 doi: 10.4102/ojvr.v81i1.818. [DOI] [PubMed] [Google Scholar]
- Shittu A., Abdullahi J., Jibril A., Mohammed A.A., Fasina F.O. Sub-clinical mastitis and associated risk factors on lactating cows in the Savannah Region of Nigeria. BMC Vet. Res. 2012;8:134. doi: 10.1186/1746-6148-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimali R.G., Patel M.D., Patel R.M. Comparative efficacy of anthelmintics and their effects on hemato-biochemical changes in fasciolosis of goats of South Gujarat. Vet. World. 2016;9:524–529. doi: 10.14202/vetworld.2016.524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyma K.P., Gupta J.P., Singh V. Breeding strategies for tick resistance in tropical cattle: a sustainable approach for tick control. J. Parasit. Dis. 2015;39:1–6. doi: 10.1007/s12639-013-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibhat B., Asmare K., Demissie K., Ayelet G., Mamo G., Ameni G. Bovine tuberculosis in Ethiopia: a systematic review and meta-analysis. Prev. Vet. Med. 2017;147:149–157. doi: 10.1016/j.prevetmed.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijapenda J.E., Komba E.V.G., Nonga H.E. Studies on seroprevalence and risk factors for occurrence of bovine brucellosis in cattle in Lindi District, Tanzania. Tanzania Vet. J. 2017;35:82–89. [Google Scholar]
- Simukoko H., Marcotty T., Vercruysse J., Van den Bossche P. Bovine trypanosomiasis risk in an endemic area on the eastern plateau of Zambia. Res. Vet. Sci. 2011;90:51–54. doi: 10.1016/j.rvsc.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Simulundu E., Mtine N., Kapalamula T.F., Kajihara M., Qiu Y., Ngoma J., Zulu V., Kwenda G., Chisanga C., Phiri I.K., Takada A., Mweene A.S. Genetic characterization of orf virus associated with an outbreak of severe orf in goats at a farm in Lusaka, Zambia (2015) Arch. Virol. 2017;162:2363–2367. doi: 10.1007/s00705-017-3352-y. [DOI] [PubMed] [Google Scholar]
- Simwango M., Ngonyoka A., Nnko H.J., Salekwa L.P., Ole- Neselle M., Kimera S.I., Gwakisa P.S. Molecular prevalence of trypanosome infections in cattle and tsetse flies in the Maasai Steppe, northern Tanzania. Parasit. Vectors. 2017;10:507. doi: 10.1186/s13071-017-2411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.V., Singh A.V., Singh R., Sharma S., Shukla N., Misra S., Singh P.K., Sohal J.S., Kumar H., Patil P.K., Misra P., Sandhu K.S. Sero-prevalence of Bovine Johne’s disease in buffaloes and cattle population of North India using indigenous ELISA kit based on native Mycobacterium avium subspecies paratuberculosis ‘Bison type’ genotype of goat origin. Comp. Immunol. Microbiol. Infect. Dis. 2008;31:419–433. doi: 10.1016/j.cimid.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Singh K., Chandel B.S., Chauhan H.C., Dadawala A., Singh S.V., Singh P.K. Efficacy of “indigenous vaccine” using native “Indian bison type” genotype of Mycobacterium avium subspecies paratuberculosis for the control of clinical Johne’s disease in an organized goat herd. Vet. Res. Commun. 2013;37:109–114. doi: 10.1007/s11259-013-9551-4. [DOI] [PubMed] [Google Scholar]
- Singh M., Dixit A., Roy A., Singh S. Analysis of prospects and problems of goat production in Bundelkhand region. Range Manag. Agrofor. 2014;35:163–168. [Google Scholar]
- Singh B.B., Kaur R., Gill G.S., Gill J.P.S., Soni R.K., Aulakh R.S. Knowledge, attitude and practices relating to zoonotic diseases among livestock farmers in Punjab, India. Acta Trop. 2019;189:15–21. doi: 10.1016/j.actatropica.2018.09.021. [DOI] [PubMed] [Google Scholar]
- Sitali D.C., Twambo M.C., Chisoni M., Bwalya M.J., Munyeme M. Lay perceptions, beliefs and practices linked to the persistence of anthrax outbreaks in cattle in the Western Province of Zambia. Onderstepoort J. Vet. Res. 2018;85 doi: 10.4102/ojvr.v85i1.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeck C.J., Adeyanju A.T., Owoade A.A., Couacy- Hymann E., Alkali B.R., Ottosson U., Muller C.P. Genetic diversity of newcastle disease virus in wild birds and pigeons in West Africa. Appl. Environ. Microbiol. 2013;79:7867–7874. doi: 10.1128/AEM.02716-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodjinou E., Henningsen A., Koudande O.D. Improving village poultry’s survival rate through community-based poultry health management: evidence from Benin. Trop. Anim. Health Prod. 2012;45:59–66. doi: 10.1007/s11250-012-0174-5. [DOI] [PubMed] [Google Scholar]
- Souley Kouato B., Thys E., Renault V., Abatih E., Marichatou H., Issa S., Saegerman C. Spatio-temporal patterns of foot-and-mouth disease transmission in cattle between 2007 and 2015 and quantitative assessment of the economic impact of the disease in Niger. Transbound. Emerg. Dis. 2018;65:1049–1066. doi: 10.1111/tbed.12845. [DOI] [PubMed] [Google Scholar]
- Specht E. Prevalence of bovine trypanosomosis in Central Mozambique from 2002 to 2005. Onderstepoort J. Vet. Res. 2008;75:73–81. doi: 10.1520/D0850-11.1. [DOI] [PubMed] [Google Scholar]
- Spiegel K.A., Havas K.A. The socioeconomic factors surrounding the initial emergence of peste des petits ruminants in Kenya, Uganda, and Tanzania from 2006 through 2008. Transbound. Emerg. Dis. 2019;66:627–633. doi: 10.1111/tbed.13116. [DOI] [PubMed] [Google Scholar]
- Squire S.A., Yang R., Robertson I., Ayi I., Squire D.S., Ryan U. Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Parasitol. Res. 2018;117:3183–3194. doi: 10.1007/s00436-018-6017-1. [DOI] [PubMed] [Google Scholar]
- Stothard J.R., Lockyer A.E., Kabatereine N.B., Tukahebwa E.M., Kazibwe F., Rollinson D., Fenwick A. Schistosoma bovis in western Uganda. J. Helminthol. 2004;78:281–284. doi: 10.1079/joh2004239. [DOI] [PubMed] [Google Scholar]
- Sudan V., Jaiswal A.K., Shanker D. Infection rates of Linguatula serrata nymphs in mesenteric lymph nodes from water buffaloes in North India. Vet. Parasitol. 2014;205:408–411. doi: 10.1016/j.vetpar.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Sudan V., Jaiswal A.K., Parashar R., Shanker D. A duplex PCR-based assay for simultaneous detection of Trypanosoma evansi and Theileria annulata infections in water buffaloes. Trop. Anim. Health Prod. 2015;47:915–919. doi: 10.1007/s11250-015-0808-5. [DOI] [PubMed] [Google Scholar]
- Sudan V., Singh S.K., Jaiswal A.K., Parashar R., Shanker D. First molecular evidence of the transplacental transmission of Theileria annulata. Trop. Anim. Health Prod. 2015;47:1213–1215. doi: 10.1007/s11250-015-0835-2. [DOI] [PubMed] [Google Scholar]
- Sudan V., Shanker D., Jaiswal A., Singh A., Pandey V. Standardization and validation of simple PCR, duplex PCR and RAPD in comparison to blood smear examination for diagnosing bovine tropical theileriosis. Biologicals. 2017;46:88–91. doi: 10.1016/j.biologicals.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Sulayeman M., Dawo F., Mammo B., Gizaw D., Shegu D. Isolation, molecular characterization and sero-prevalence study of foot-and- mouth disease virus circulating in central Ethiopia. BMC Vet. Res. 2018;14:110. doi: 10.1186/s12917-018-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman A., Bello M., Dzikwi A.A., Talba A.M., Grema H.A., Geidam Y.A. Serological prevalence of contagious bovine pleuropneumonia in agro-pastoral areas of Nigeria. Trop. Anim. Health Prod. 2015;47:1033–1042. doi: 10.1007/s11250-015-0824-5. [DOI] [PubMed] [Google Scholar]
- Suleiman A., Jackson E.L., Rushton J. Challenges of pastoral cattle production in a sub-humid zone of Nigeria. Trop. Anim. Health Prod. 2015;47:1177–1185. doi: 10.1007/s11250-015-0845-0. [DOI] [PubMed] [Google Scholar]
- Suleiman A., Jackson E., Rushton J. Perceptions, circumstances and motivators affecting the implementation of contagious bovine pleuropneumonia control programmes in Nigerian Fulani pastoral herds. Prev. Vet. Med. 2018;149:67–74. doi: 10.1016/j.prevetmed.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Sumaye R.D., Geubbels E., Mbeyela E., Berkvens D. Inter-epidemic transmission of rift valley fever in livestock in the Kilombero River Valley, Tanzania: a cross-sectional survey. PLoS Negl. Trop. Dis. 2013;7:e2356. doi: 10.1371/journal.pntd.0002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundufu A.J., Ansumana R., Bockarie A.S., Bangura U., Lamin J.M., Jacobsen K.H., Stenger D.A. Syndromic surveillance of peste des petits ruminants and other animal diseases in Koinadugu district, Sierra Leone, 2011- 2012. Trop. Anim. Health Prod. 2015;47:473–477. doi: 10.1007/s11250-014-0736-9. [DOI] [PubMed] [Google Scholar]
- Swai E.S., Karimuribo E.D., Ogden N.H., French N.P., Fitzpatrick J.L., Bryant M.J., Kambarage D.M. Seroprevalence estimation and risk factors for A. Marginale on smallholder dairy farms in Tanzania. Trop. Anim. Health Prod. 2005;37:599–610. doi: 10.1007/s11250-005-4307-y. [DOI] [PubMed] [Google Scholar]
- Swai E.S., Kessy M.J., Sanka P.N., Mtui P.F. A serological survey for infectious bursal disease virus antibodies in free-range village chickens in northern Tanzania. J. S. Afr. Vet. Assoc. 2011;82:32–35. doi: 10.4102/jsava.v82i1.30. [DOI] [PubMed] [Google Scholar]
- Swai E.S., Karimuribo E.D., Kambarage D.M. Risk factors for smallholder dairy cattle mortality in Tanzania. J. S. Afr. Vet. Assoc. 2012;81:241–246. doi: 10.4102/jsava.v81i4.155. [DOI] [PubMed] [Google Scholar]
- Swai E.S., Hayghaimo A.A., Hassan A.A., Mhina B.S. The slaughter of increased numbers of pregnant cows in Tanga abattoir, Tanzania: a cause for concern? Onderstepoort J. Vet. Res. 2015;82:E1–5. doi: 10.4102/ojvr.v82i1.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiswa S., Masocha M., Pfukenyi D.M., Dhliwayo S., Chikerema S.M. Long-term changes in the spatial distribution of lumpy skin disease hotspots in Zimbabwe. Trop. Anim. Health Prod. 2017;49:195–199. doi: 10.1007/s11250-016-1180-9. [DOI] [PubMed] [Google Scholar]
- Syakalima M., Simuunza M., Zulu V.C. Ethnoveterinary treatments for common cattle diseases in four districts of the Southern Province, Zambia. Vet. World. 2018;11:141–145. doi: 10.14202/vetworld.2018.141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla S., Sidimé Y., Sun Y., Doumbouya S., Cong Y. Seroprevalence investigation of bovine brucellosis in Macenta and Yomou, Guinea. Trop. Anim. Health Prod. 2014;46:1185–1191. doi: 10.1007/s11250-014-0625-2. [DOI] [PubMed] [Google Scholar]
- Taha K.M., Salih D.A., Ahmed B.M., Enan K.A., Ali A.M., Elhussein A.M. First confirmed report of outbreak of malignant ovine theileriosis among goats in Sudan. Parasitol. Res. 2011;109:1525–1527. doi: 10.1007/s00436-011-2428-y. [DOI] [PubMed] [Google Scholar]
- Taku A., Chhabra R., War B.A. Footrot on a sheep breeding farm in the Himalayan state of Jammu and Kashmir. Rev. Sci. Tech. 2010;29:671–675. doi: 10.20506/rst.29.3.2012. [DOI] [PubMed] [Google Scholar]
- Tarnagda Z., Yougbare I., Kam A., Tahita M.C., Ouedraogo J.B. Prevalence of infectious bronchitis and Newcastle disease virus among domestic and wild birds in H5N1 outbreaks areas. J. Infect. Dev. 2011;5:565–570. doi: 10.3855/jidc.1441. [DOI] [PubMed] [Google Scholar]
- Tebug S.F., Njunga G.R., Chagunda M.G.G., Mapemba J.P., Awah- Ndukum J., Wiedemann S. Risk, knowledge and preventive measures of smallholder dairy farmers in northern Malawi with regard to zoonotic brucellosis and bovine tuberculosis. Onderstepoort J. Vet. Res. 2014;81 doi: 10.4102/ojvr.v81i1.594. [DOI] [PubMed] [Google Scholar]
- Tedla M., Degefa K. Bacteriological study of calf colisepticemia in alage dairy farm, Southern Ethiopia. BMC Res. Notes. 2017;10:710. doi: 10.1186/s13104-017-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedla M., Mehari F., Kebede H. A cross-sectional survey and follow up study on major dairy health problems in large and small scale urban farms in Mekelle, Tigray, Ethiopia. BMC Res. Notes. 2018;11:236. doi: 10.1186/s13104-018-3347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekle T., Terefe G., Cherenet T., Ashenafi H., Akoda K.G., Teko-Agbo A., Van Den Abbeele J., Gari G., Clausen P.-H., Hoppenheit A., Mattioli R.C., Peter R., Marcotty T., Cecchi G., Delespaux V. Aberrant use and poor quality of trypanocides: a risk for drug resistance in south western Ethiopia. BMC Vet. Res. 2018;14:4. doi: 10.1186/s12917-017-1327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terefe Y., Girma S., Mekonnen N., Asrade B. Brucellosis and associated risk factors in dairy cattle of eastern Ethiopia. Trop. Anim. Health Prod. 2017;49:599–606. doi: 10.1007/s11250-017-1242-7. [DOI] [PubMed] [Google Scholar]
- Terfa Z.G.G., Garikipati S., Kassie G., Bettridge J.M.M., Christley R.M.M. Eliciting preferences for attributes of Newcastle disease vaccination programmes for village poultry in Ethiopia. Prev. Vet. Med. 2018;158:146–151. doi: 10.1016/j.prevetmed.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye G., Tsegaye W., Chanie M., Abinet F. Seroprevalence and associated risk factors of bovine brucellosis in Addis Ababa dairy farms. Trop. Anim. Health Prod. 2011;43:1001–1005. doi: 10.1007/s11250-011-9798-0. [DOI] [PubMed] [Google Scholar]
- Teshome D., Sori T., Sacchini F., Wieland B. Epidemiological investigations of contagious caprine pleuropneumonia in selected districts of Borana zone, Southern Oromia, Ethiopia. Trop. Anim. Health Prod. 2019;51:703–711. doi: 10.1007/s11250-018-1744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur R., Thakur D., Dogra P. Indigenous feeding practices for better reproductive performance in buffaloes in Himachal Pradesh, India. Buffalo Bull. 2018;37:361–368. [Google Scholar]
- Tibbo M., Mukasa-Mugerwa E., Woldemeskel M., Rege J.E.O. Risk factors for mortality associated with respiratory disease among Menz and Horro sheep in Ethiopia. Vet. J. 2003;165:276–287. doi: 10.1016/s1090-0233(02)00184-3. [DOI] [PubMed] [Google Scholar]
- Tolosa T., Verbeke J., Piepers S., Supré K., De Vliegher S. Risk factors associated with subclinical mastitis as detected by California mastitis Test in smallholder dairy farms in Jimma, Ethiopia using multilevel modelling. Prev. Vet. Med. 2013;112:68–75. doi: 10.1016/j.prevetmed.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Toye P.G., Batten C.A., Kiara H., Henstock M.R., Edwards L., Thumbi S., Poole E.J., Handel I.G., Bronsvoort B.Mde C., Hanotte O., Coetzer J.A.W., Woolhouse M.E.J., Oura C.A.L. Bluetongue and Epizootic Haemorrhagic Disease virus in local breeds of cattle in Kenya. Res. Vet. Sci. 2013;94:769–773. doi: 10.1016/j.rvsc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S.M., Thaker A.M., Joshi C.G., Sankhala L.N. Acephate immunotoxicity in White Leghorn cockerel chicks upon experimental exposure. Environ. Toxicol. Pharmacol. 2012;34:192–199. doi: 10.1016/j.etap.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Tschopp R., Hattendorf J., Roth F., Choudhoury A., Shaw A., Aseffa A., Zinsstag J. 2012. Cost Estimate of Bovine Tuberculosis to Ethiopia; pp. 249–268. [DOI] [PubMed] [Google Scholar]
- Tsegay A., Tuli G., Kassa T., Kebede N. Seroprevalence and risk factors of Brucellosis in small ruminants slaughtered at Debre Ziet and Modjo export abattoirs, Ethiopia. J. Infect. Dev. 2015;9:373–380. doi: 10.3855/jidc.4993. [DOI] [PubMed] [Google Scholar]
- Ularamu H.G., Ibu J.O., Wood B.A., Abenga J.N., Lazarus D.D., Wungak Y.S., Knowles N.J., Wadsworth J., Mioulet V., King D.P., Shamaki D., Adah M.I. Characterization of foot-and-Mouth disease viruses collected in Nigeria between 2007 and 2014: evidence for epidemiological links between west and East Africa. Transbound. Emerg. Dis. 2017;64:1867–1876. doi: 10.1111/tbed.12584. [DOI] [PubMed] [Google Scholar]
- Umeta Gurmesa. Participatory analysis of problems limiting goat production at selected districts of East Showa zone, Ethiopia. African J. Agric. Res. 2011;6 doi: 10.5897/AJAR11.314. [DOI] [Google Scholar]
- Usman I.S., Bzugu P.M., Pur J.T., Abdullahi A. Indigenous control methods for parasites among pastoralists communities in Adamawa state, Nigeria. J. Agric. Ext. 2017;21:109. doi: 10.4314/jae.v21i1.9. [DOI] [Google Scholar]
- VanderWaal K., Omondi G.P., Obanda V. Mixed-host aggregations and helminth parasite sharing in an East African wildlife–livestock system. Vet. Parasitol. 2014;205:224–232. doi: 10.1016/j.vetpar.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Vhoko K., Iannetti S., Burumu J., Ippoliti C., Bhebhe B., De Massis F. Estimating the prevalence of brucellosis in cattle in Zimbabwe from samples submitted to the central veterinary laboratory between 2010 and 2014. Vet. Ital. 2018;54:21–27. doi: 10.12834/VetIt.1111.6191.2. [DOI] [PubMed] [Google Scholar]
- Vijayasarathi M.K., Sreekumar C., Venkataramanan R., Raman M. Influence of sustained deworming pressure on the anthelmintic resistance status in strongyles of sheep under field conditions. Trop. Anim. Health Prod. 2016;48:1455–1462. doi: 10.1007/s11250-016-1117-3. [DOI] [PubMed] [Google Scholar]
- Vordermeier M., Ameni G., Berg S., Bishop R., Robertson B.D., Aseffa A., Hewinson R.G., Young D.B. The influence of cattle breed on susceptibility to bovine tuberculosis in Ethiopia. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:227–232. doi: 10.1016/j.cimid.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vudriko P., Okwee-Acai J., Tayebwa D.S., Byaruhanga J., Kakooza S., Wampande E., Omara R., Muhindo J.B., Tweyongyere R., Owiny D.O., Hatta T., Tsuji N., Umemiya-Shirafuji R., Xuan X., Kanameda M., Fujisaki K., Suzuki H. Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit. Vectors. 2016;9:4. doi: 10.1186/s13071-015-1278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vudriko P., Okwee-Acai J., Byaruhanga J., Tayebwa D.S., Omara R., Muhindo J.B., Lagu C., Umemiya-Shirafuji R., Xuan X., Suzuki H. Evidence-based tick acaricide resistance intervention strategy in Uganda: concept and feedback of farmers and stakeholders. Ticks Tick. Dis. 2018;9:254–265. doi: 10.1016/j.ttbdis.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Wambua L., Wambua P.N., Ramogo A.M., Mijele D., Otiende M.Y. Wildebeest-associated malignant catarrhal fever: perspectives for integrated control of a lymphoproliferative disease of cattle in sub-Saharan Africa. Arch. Virol. 2016;161:1–10. doi: 10.1007/s00705-015-2617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanyoike F., Rich K.M. An assessment of the regional and national socio-economic impacts of the 2007 rift valley fever outbreak in Kenya. Am. J. Trop. Med. Hyg. 2010;83:52–57. doi: 10.4269/ajtmh.2010.09-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardrop N.A., Thomas L.F., Cook E.A.J., de Glanville W.A., Atkinson P.M., Wamae C.N., Fèvre E.M. The sero-epidemiology of Coxiella burnetii in humans and cattle, Western Kenya: evidence from a cross-sectional study. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B.L., Diaw O.T., Seye M.M., Webster J.P., Rollinson D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: species barrier break down between ruminant and human schistosomes. PLoS Negl. Trop. Dis. 2013;7:e2110. doi: 10.1371/journal.pntd.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekesa S.N., Muwanika V.B., Siegismund H.R., Sangula A.K., Namatovu A., Dhikusooka M.T., Tjørnehøj K., Balinda S.N., Wadsworth J., Knowles N.J., Belsham G.J. Analysis of recent serotype O foot-and-mouth disease viruses from livestock in Kenya: evidence of four independently evolving lineages. Transbound. Emerg. Dis. 2015;62:305–314. doi: 10.1111/tbed.12152. [DOI] [PubMed] [Google Scholar]
- Welay G.M., Tedla D.G., Teklu G.G., Weldearegay S.K., Shibeshi M.B., Kidane H.H., Gebrezgiabher B.B., Abraha T.H. A preliminary survey of major diseases of ruminants and management practices in Western Tigray province, northern Ethiopia. BMC Vet. Res. 2018;14:293. doi: 10.1186/s12917-018-1621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weny G., Okwee-Acai J., Okech S.G., Tumwine G., Ndyanabo S., Abigaba S., Goldberg T.L. Prevalence and risk factors associated with hemoparasites in cattle and goats at the edge of Kibale National Park, Western Uganda. J. Parasitol. 2017;103:69–74. doi: 10.1645/16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesonga F.D., Wesongah J.O., Chemuliti J., Wanjala K., Munga L., Gitau P. Seroprevalence of Ehrlichia ruminantium (Heartwater) in small ruminants in a pastoral production system in Narok District, Kenya. Bull. Anim. Heal. Prod. Africa. 2006;54:23–33. [Google Scholar]
- Wesonga F.D., Gachohi J.M., Kitala P.M., Gathuma J.M., Njenga M.J. Seroprevalence of Anaplasma marginale and Babesia bigemina infections and associated risk factors in Machakos County, Kenya. Trop. Anim. Health Prod. 2017;49:265–272. doi: 10.1007/s11250-016-1187-2. [DOI] [PubMed] [Google Scholar]
- Whittington R., Donat K., Weber M.F., Kelton D., Nielsen S.S., Eisenberg S., Arrigoni N., Juste R., Sáez J.L., Dhand N., Santi A., Michel A., Barkema H., Kralik P., Kostoulas P., Citer L., Griffin F., Barwell R., Moreira M.A.S., Slana I., Koehler H., Singh S.V., Yoo H.S., Chávez-Gris G., Goodridge A., Ocepek M., Garrido J., Stevenson K., Collins M., Alonso B., Cirone K., Paolicchi F., Gavey L., Rahman M.T., de Marchin E., Van Praet W., Bauman C., Fecteau G., McKenna S., Salgado M., Fernández-Silva J., Dziedzinska R., Echeverría G., Seppänen J., Thibault V., Fridriksdottir V., Derakhshandeh A., Haghkhah M., Ruocco L., Kawaji S., Momotani E., Heuer C., Norton S., Cadmus S., Agdestein A., Kampen A., Szteyn J., Frössling J., Schwan E., Caldow G., Strain S., Carter M., Wells S., Munyeme M., Wolf R., Gurung R., Verdugo C., Fourichon C., Yamamoto T., Thapaliya S., Di Labio E., Ekgatat M., Gil A., Alesandre A.N., Piaggio J., Suanes A., de Waard J.H. Control of paratuberculosis: who, why and how. A review of 48 countries. BMC Vet. Res. 2019;15:198. doi: 10.1186/s12917-019-1943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemeskel M., Mersha G. Study on caprine and ovine dermatophilosis in Wollo, Northeast Ethiopia. Trop. Anim. Health Prod. 2010;42:41–44. doi: 10.1007/s11250-009-9383-y. [DOI] [PubMed] [Google Scholar]
- Wolff C., Boqvist S., Stǻhl K., Masembe C., Sternberg-Lewerin S., Ståhl K., Masembe C., Sternberg-Lewerin S. Biosecurity aspects of cattle production in Western Uganda, and associations with seroprevalence of brucellosis, salmonellosis and bovine viral diarrhoea. BMC Vet. Res. 2017;13(6 December 2017) doi: 10.1186/s12917-017-1306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Thrusfield M. Perceptions of zoonotic and animal diseases in the Van Gujjar community of North India. Prev. Vet. Med. 2016;123:143–153. doi: 10.1016/j.prevetmed.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Wu T., Perrings C. The live poultry trade and the spread of highly pathogenic avian influenza: regional differences between Europe, West Africa, and Southeast Asia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A., Panadero R., Katoch R., Godara R., Cabanelas E. Myiasis of domestic and wild ruminants caused by Hypodermatinae in the Mediterranean and Indian subcontinent. Vet. Parasitol. 2017;243:208–218. doi: 10.1016/j.vetpar.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Yamada S., Konnai S., Imamura S., Simuunza M., Chembensofu M., Chota A., Nambota A., Onuma M., Ohashi K. PCR-based detection of blood parasites in cattle and adult Rhipicephalus appendiculatus ticks. Vet. J. 2009;182:352–355. doi: 10.1016/j.tvjl.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Yang Z., Xu G., Reboud J., Ali S.A., Kaur G., McGiven J., Boby N., Gupta P.K., Chaudhuri P., Cooper J.M. Rapid veterinary diagnosis of bovine reproductive infectious diseases from semen using paper-origami DNA microfluidics. ACS Sens. 2018;3:403–409. doi: 10.1021/acssensors.7b00825. [DOI] [PubMed] [Google Scholar]
- Yasine A., Kumsa B., Hailu Y., Ayana D. Mites of sheep and goats in Oromia Zone of Amhara Region, North Eastern Ethiopia: species, prevalence and farmers awareness. BMC Vet. Res. 2015;11:122. doi: 10.1186/s12917-015-0433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yihunie A., Urga B., Alebie G. Prevalence and risk factors of bovine schistosomiasis in Northwestern Ethiopia. BMC Vet. Res. 2019;15:12. doi: 10.1186/s12917-018-1757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid U.N., Randhawa Swaran Singh, Hussain S.A., Randhawa Sarnarinder Singh, Mahajan V., Dua K. Claw lesions causing clinical lameness in lactating holstein frisian crossbred cows. Vet. Med. Int. 2014;2014 doi: 10.1155/2014/764689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúquete S.T., Coelho J., Rosa F., Vaz Y., Cassamá B., Padre L., Santos D., Basto A.P., Leitão A. Tick (Acari: ixodidae) infestations in cattle along Geba River basin in Guinea-Bissau. Ticks Tick. Dis. 2017;8:161–169. doi: 10.1016/j.ttbdis.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Zvinorova P.I., Halimani T.E., Muchadeyi F.C., Katsande S., Gusha J., Dzama K. Management and control of gastrointestinal nematodes in communal goat farms in Zimbabwe. Trop. Anim. Health Prod. 2017;49:361–367. doi: 10.1007/s11250-016-1200-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.