Abstract
Fungal periprosthetic joint infections are an uncommon but potentially devastating complication of arthroplasty surgery. The concurrent presence of a coexistent bacterial pathogen—a so called “super-infection”—adds further complexity. With delays to definitive diagnosis and a large number of procedures before cure, the associated physical and psychological morbidity is considerable. Beyond this, the economic and resource burden can be substantial. This case report presents the successful rapid treatment of an atypical bacterial and fungal periprosthetic super-infection with two-stage revision surgery augmented with a commercially available dissolving calcium sulfate bead system permitting targeted local antifungal elution. While not the panacea for treatment, these beads provide another potentially useful tool in the atypical pathogen eradication armamentarium. Much research is still indicated to define the optimal care pathway for fungal periprosthetic super-infections.
Keywords: Fungal PJI, Super-infection, Calcium sulfate beads, Prosthetic joint infection
Introduction
Fungal periprosthetic joint infections (PJIs) are an uncommon but potentially devastating complication of arthroplasty surgery. Often difficult to diagnose, genuinely curative outcomes are challenging to achieve. The concurrent presence of a coexistent bacterial pathogen—a so called “super-infection”—adds further complexity. While immunocompromised hosts represent an at-risk demographic, the majority of such infections occur in patients without clear causative susceptibility. Lengthy delays to definitive diagnosis remain the norm, with most patients undergoing a large number of surgical procedures before presumptive cure. The associated physical and psychological patient morbidity is considerable, and the economic and resource burden to hospitals and health-care networks can be substantial.
Historically, fungal PJIs have been treated with often multiple debridements and staged component exchange, supplemented by prolonged courses of antifungals. In practice, the bioavailability and then penetration of such medications around in situ prostheses is poor. The use and dosing of many antifungals is also limited by recognized adverse events, including systemic organ toxicity. Most are not sufficiently heat stable so as to survive the exothermic stages of bone cement curing, and topical delivery, such as sprinkled dry powder leads to rapid dose dumping phenomena with little enduring effect.
The presented case deals with a chronic fungal periprosthetic superinfection around an in situ total knee arthroplasty (TKA), successfully and rapidly treated with conventional two-stage revision surgery with systemic antifungal therapy augmented through the use of a commercially available calcium sulfate dissolving bead system permitting targeted antifungal impregnation and prolonged local elution.
Case history
A 70-year old male was seen initially in the emergency department of a tertiary arthroplasty center in December of 2018 complaining of severe, sudden-onset, right knee pain and swelling and an inability to bear weight on the affected limb. He had an ipsilateral TKA and a history of multiple previous treatments for PJI. He reported subjective fevers and chills for the previous 48 hours. A single temperature of 38.3°C was captured on admission. His previous comorbidities included a mild traumatic brain injury, psoriasis, an occupationally acquired left-sided hearing deficit [1], a repaired umbilical hernia, and gout affecting only the great toes. He was not on antibiotics at the time of first review. Clinical assessment revealed a red and swollen, warm-to-touch, right knee with a well-healed midline surgical scar. His arc-of-movement was grossly limited to 20˚ (30-50˚). His serum lymphocyte count was within normal limits (1.9 × 109/L).
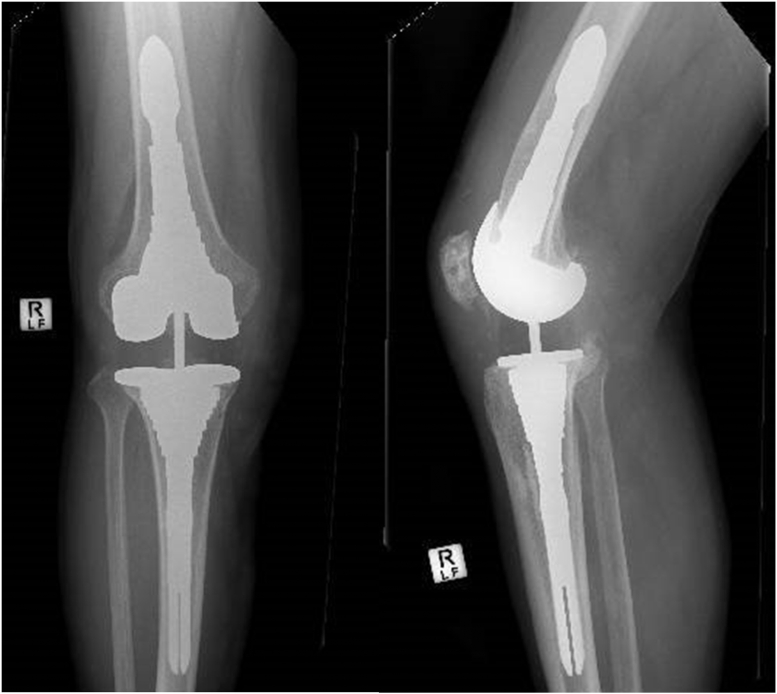
Screening blood work revealed a serum C-reactive protein level of 30.8 mg/dL and a white cell count of 17.3 × 109/L, with both a neutrophilia (14.4 × 109/L) and monocytosis (0.98 × 109/L). Plain radiographs demonstrated a stemmed, cruciate-retaining implant with patella resurfacing and hybrid fixation (Fig. 1), and 3-D metal artifact reduced computed tomography [2] suggested alignment parameters within accepted limits. Based on the presenting features and supportive blood results, a presumptive diagnosis of PJI was made.
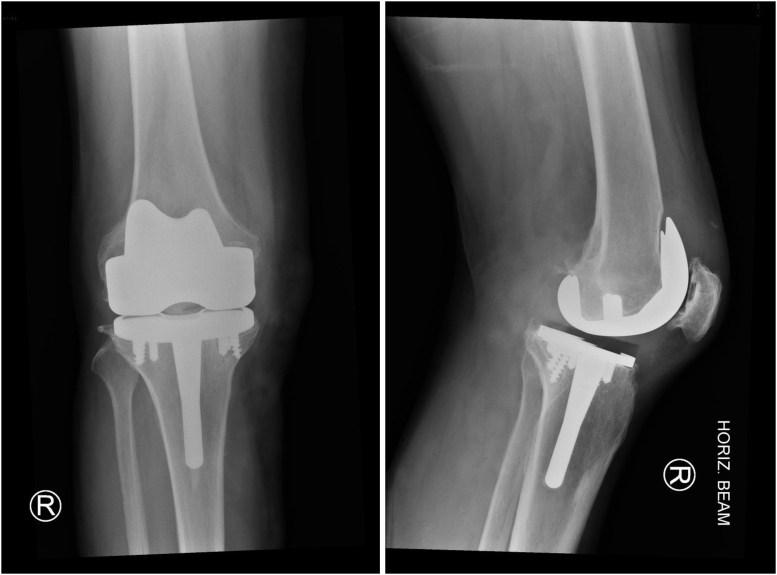
Figure 1.
Index TKA. Advantim (Wright medical, Arlington, TN).
Supplementary history revealed a primary TKA for osteoarthritis (Fig. 2), with a noted 20˚ preoperative fixed flexion deformity, just over 5.5 years earlier. His recovery period was complicated by persistent wound ooze and an early return to theater <6 weeks from the index surgery for an open washout, with component retention. Specimens collected at the time (early 2013) grew a methicillin-sensitive Staphylococcus aureus (S. aureus). A second [ie, debridement, antibiotics and implant retention (DAIR)] procedure was performed 3 months later (mid 2013), and culture specimens were positive for both broadly sensitive S. aureus and Escherichia coli (E. coli). The patient was managed with prolonged IV (ceftriaxone) and then oral antibiotic therapy (cephalexin and ciprofloxacin) under infectious diseases (ID) specialist guidance before being ceased some 3.5 years later (ie, late 2016). The patient revealed a sentiment that the knee “had never been right” and had failed to meet his pain and performance expectations.
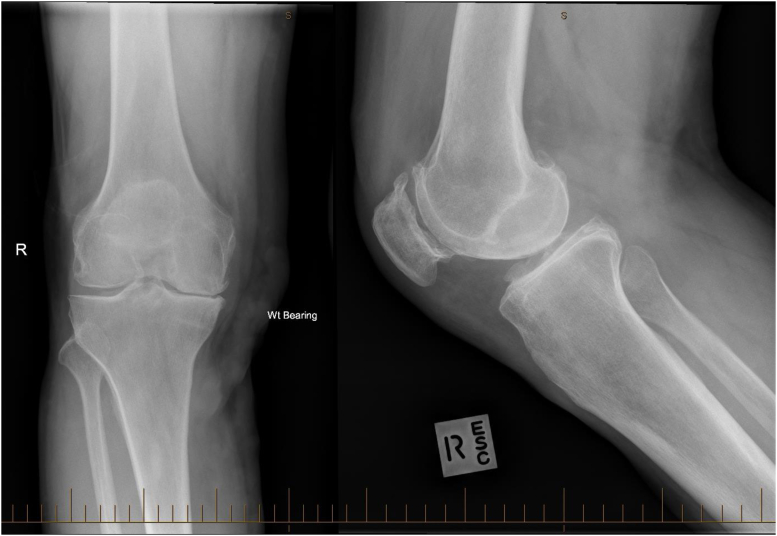
Figure 2.
Preindex surgery native knee showing tricompartmental osteoarthritis and varus limb alignment.
Despite his complex history and a high index of suspicion for active joint infection, the patient initially refused to consent to open surgery (DAIR or staged revision)—against strong medical recommendation and at odds with current international guidelines for the management of peri-prosthetic infection [3]. Instead, he sought medical review from the ID specialist who had managed his care previously and wished to resume suppressive antibiotic therapy. After a 2-day delay—and with worsening serum markers—the patient accepted an arthroscopic washout of his knee with the goal of basic source containment through reduction of the local infective burden and solid tissue specimen collection, noting that this is not a recognized treatment approach with curative intent. Frank pus was noted within the joint cavity. Collected solid tissue specimens grew E. coli and Group B Streptococcus. As per the current rendition of the The MusculoSkeletal Infection Society criteria (2018) [3], correlation of the preoperative workup yielded a score of >6, suggesting active infection. Thereafter, the patient consented for a planned two-stage revision TKA. The formal first stage was performed 7 days after initial presentation by an experienced, fellowship trained arthroplasty surgeon. Intraoperative findings included macroscopic evidence suggestive of chronic infection with frank pus, fibrinous stranding, and gross inflammatory synovium. A large bony void was noted at the anterior femoral margin, immediately associated with the anterior flange of the index femoral component. Multiple solid tissue specimens were collected from both deep soft tissue and bony origins for routine pathology and microscopy with extended (14 days) cultures, gram stain, fungal analyses, and metallosis grading [4]—using a standardized local sampling convention. Intraoperative frozen sections were not collected. After specimen collection and peri-articular debridement, the wound was sequentially irrigated with 5% aqueous betadine (povidone-iodine) solution (for 8 minutes) and then 3% hydrogen peroxide, with interspersed sterile saline lavage. A total of 12 liters of lavage fluid was used. An articulating, metal-backed, fully cemented cruciate-retaining implant spacer was used (NexGen; Zimmer Biomet, Warsaw, IN), using an oversized femoral component and a metal-backed tibial tray with a long, undersized, tibial stem to facilitate a thick eluting cement mantle. Two packets of Copal G + V (Heraeus Medical; Hanau, Germany) medium-viscosity bone cement were used. One gram of vancomycin and 1 g of gentamycin were added to each packet. A first-stage revision mixing technique was performed (ie, bowl mixed by hand) to increase the cement porosity to optimize the elution characteristics.
The procedure was otherwise uneventful. A negative pressure skin dressing was applied for optimized wound care. Early postoperative radiographs are shown in Figure 3. Using a surgeon-specific Enhanced Recovery After Surgery protocol and standard rehabilitation pathway, the patient stood and mobilized independently the day of surgery and commenced formal, albeit gentle, physiotherapist-guided range-of-motion (ROM) activities. No restrictions to weight bearing or active ROM were imposed.
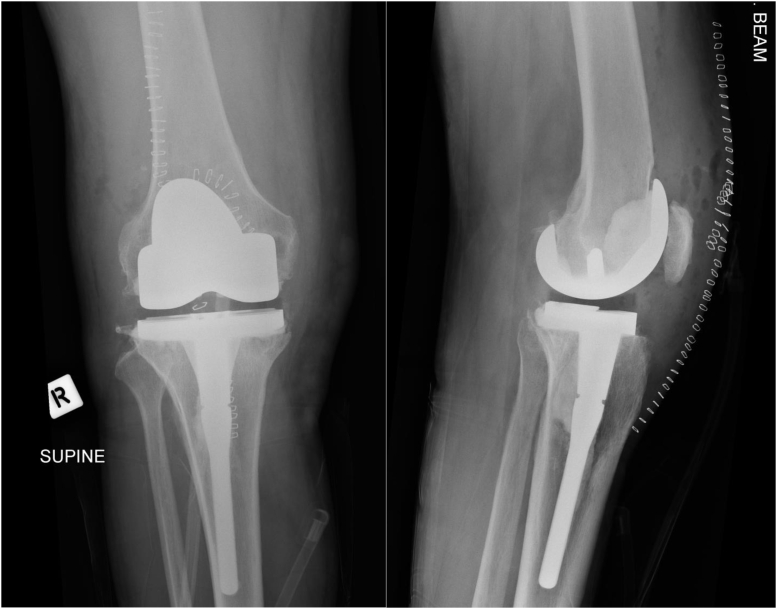
Figure 3.
Mobile eluting spacer first-stage revision TKA. NexGen CR (Zimmer Biomet, Warsaw, IN).
Specimen analysis subsequently confirmed Group B Streptococcus (6 of 6 specimens) and suggested fungal co-infection with Aspergillus fumigatus (3 of 6 specimens). There were concerns raised by the treating ID service that the latter result may have represented an operative field contaminant [5]. Lengthy direct conversations were had with the patient regarding the finding, including the ramifications and potential morbidity associated with prolonged IV and oral systemic antifungal therapy [6]. With a nearly healed midline surgical wound, the patient elected to undergo a repeat arthroscopic biopsy for confirmatory solid tissue analysis. Nine such specimens were collected under sterile conditions with 7 of 9 subsequently being deemed positive and confirming the underlying concurrent bacterial and fungal PJIs.
A repeat first-stage procedure was performed including removal of all in situ implant materials and cement mantles, complete open synovectomy, and wound lavage with sequential betadine and hydrogen peroxide. New implants were inserted of a similar nature to the previous procedure, using Copal G + V cement with 150 mg of powdered voriconazole per mix, again creating thick, porous eluting mantles. In addition, 2 packets of dissolving Stimulan (Biocomposites, Staffordshire, England) calcium sulfate solid bead mixes with 200 mg of added voriconazole, 1 g of gentamycin, 1 g of vancomycin, and a third packet of Stimulan as a semi-viscous paste (with the same additives) were added to the intra-articular and subfascial spaces and the cavity underlying the anterior femoral bony defect (Fig. 4). Beads were prepared using the manufacturer-recommended back-table technique to a “medium” 4.8-mm size. The wound was closed in layers without the use of a drain. A routine dressing combination was applied [ie, Hypafix (Essity, BSN Medical, Inc, Charlotte, NC) strips plus overlay IV 3000 (Smith and Nephew, London, UK)]. The early postoperative recovery was uneventful.
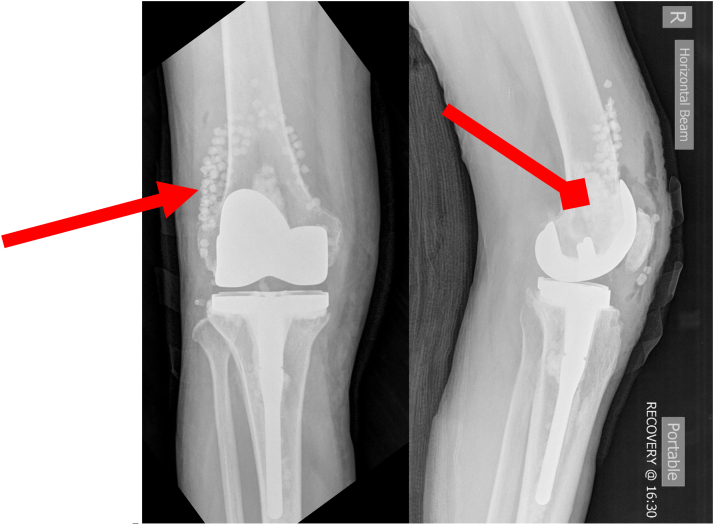
Figure 4.
Repeat first-stage revision with polyethylene exchange and the addition of Stimulan (Biocomposites, Staffordshire, England) eluting calcium phosphate beads (triangular arrow) and intrameduallary paste (diamond arrow). NexGen CR (Zimmer Biomet, Warsaw, IN) implant construct.
At 10 weeks after repeat first-stage procedure, under ID guidance and with normalized blood and clinical parameters, antibiotic and antifungal therapy was withdrawn. Three weeks later, a repeat arthroscopic solid tissue biopsy was performed, and specimens subjected to the same testing regime as previously were collected. No live pathogens were seen, and no growth was demonstrated on extended cultures. At 15 weeks after repeat first-stage procedure, the definitive second-stage operation was performed (Figure 5). While uncemented in the diaphyseal and metaphyseal zones, the subarticular zones were cemented using off-the-shelf Copal bone cement (G&V) with no additional inclusions. By postoperative day 45, the patient’s inflammatory markers had returned to within normal limits (C-reactive protein = 0.68 mg/dL), and his white cell indices equally remained normal. The patient was seen at 2 and 8 weeks, then 3 and 6 months postoperatively in the hospital outpatient setting. He had no persistent wound issues, no local or systemic infective features, his pain was minimal and not requiring supplemental regular analgesia, and his active ROM was good. He reported no enduring functional limitations, other than a discomfort associated with kneeling directly onto the operative knee (noting that he had also not been able to achieve this after his index TKA). He was last seen at the 12 months after second-stage revision mark, and he continues to do well being actively engaged in his desired life pursuits and has no pain. Still off antibiotics, he remains objectively infection free and is very happy with his end result. His most recent documented active ROM was 5 - 110°. His 1-year Forgotten Joint Score and Oxford Knee Scores were 75 and 47, respectively. We will continue to monitor him serially into the future.
Figure 5.
Definitive (2nd stage) TKA construct. Uncemented Attune Revision System RP CRS (DePuy Synthes; Raynham, MA, USA).
Discussion
Fungal PJIs are a relatively infrequent [7,8], albeit potentially devastating, complication of joint replacement surgery [9] reported to account for just 1.45–3.1% of all confirmed infections involving primary TKAs or THAs [[10], [11], [12]]. The rate has been proposed to be higher in the re-revision setting. Previous investigations have suggested that Candida albicans represents the most commonly encountered periprosthetic fungal infection of the knee (80–88% of cases) [8,[13], [14], [15]], with non-Candida species PJIs considered relatively rare [9]. While “classic” descriptions have inferred an underlying immunocompromised host requirement, recent review of the literature did not support this in most cases [16].
Concomitant infection with 2 or more phenotypically diverse pathogens—widely referred to as a “super-infection” [5]—is sadly not an infrequently encountered situation [5,8,[17], [18], [19]]. While polymicrobial bacterial infections are the most prevalent dual-infection type involving in situ joint replacements, given a lack of routine formal testing [20]—complicated by the organisms themselves often being difficult to isolate [5,21]—the true incidence of fungal PJIs overlaying a concurrent bacterial infection is likely to be greater than that conventionally reported. A high index of suspicion is required such that these important synergistic infective processes are not overlooked [16]. While the mean time to onset of symptoms for a fungal PJI after index surgery is reported to be 27.2 months [9], the average time to definitive diagnosis is a further 7.5–20 months thereafter [13,22]. These infections are recognized as being difficult to diagnose and treat [8,20,23], with patients having endured an average of 2.7 operations before definitive pathogen identification [13,23]. Once diagnosed, there exist no universal guidelines for the targeted management of fungal PJIs [9,13,20,24,25]. Despite a raft of varied approaches, fungal PJIs are associated with poorer outcomes [12] vs comparable bacterial infections; clearance (ie, cure) rates are lower [7] with far higher secondary failure rates [18,23], conversions to resection arthroplasties [7,23], amputation rates [17], and associated mortality [7,16]. When success has been (semi-)consistently achieved, most previous authors attribute a need for long-term systemic antifungal therapy [7,9,16], with a mean interstage period of just over 8 months [7]. Contemporary systemic antifungal therapies themselves are associated with not insignificant adverse effect profiles [6] and require diligent monitoring—courses should be directed by an experienced ID specialist. While two-stage approaches using antifungal-impregnated cemented constructs [13,24] appear to achieve the most predictable results [8,9] and currently exist as the accepted standard of care [8], intrinsic patient comorbidity burden [7] and regional variation in fungal isolate isotypes [26] may also influence endpoint treatment success. Most authors agree that a DAIR-type procedure is unlikely to be effective in definitively managing an established fungal infection [8,12,17], and while some have suggested (strongly) that a one-stage procedure is destined to fail [8,12], others have reported arguably acceptable successes with this approach [25,27,28].
Several features regarding our case highlight it as atypical, even for an uncommonly recognized condition. First, most reported bacterial and fungal super-infections have been a combination of fungus and Staphlycoccal species [5]—our patient was diagnosed with a concurrent Streptococcal infection. Similarly, while Candida albicans is by far the most commonly encountered fungal PJI [24]—representing nearing 90% of cases—our patient was infected with the far less prevalent Trichocomaceae, Aspergillus fumigatus. Thus we present a relatively unusual bacterial and fungal combination, to our knowledge, not previously reported in the arthroplasty literature. Despite intensive investigation, our patient was deemed globally immunocompetent. While fluconazole and amphotericin remain the most frequently used antifungals in septic arthroplasty [9,16,29], emerging evidence suggests that voriconazole may indeed be a more efficacious option, possibly with an improved adverse effect profile [23]. On these grounds, voriconazole was selected as the primary topical and systemic antifungal agent for this patient and was prepared with measured doses applied to both the peri-implant cement mantles and as slow-release eluting and dissolving beads. This likely contributed to the rapid eradication achieved. While detailed pharmacokinetic and material property information for voriconazole fall outside the scope of this article, summary information is included in Table 1.
Table 1.
| Voriconazole | |
|---|---|
| Description | Broad spectrum antifungal agent |
| Mechanism of action | Inhibits the fungal enzyme 14a-sterol demethylase, a critical step in ergosterol biosynthesis. |
| Metabolism pathway | Metabolized in the liver primarily by cytochrome P450 (CYP) 2C19, CYP2C9, and CYP3A4 play limited roles. The primary metabolite is voriconazole N-oxide, which has no antifungal activity. |
| Clearance | Primarily dependent on hepatic metabolism. |
| Excretion | Most (80%) of the drug is excreted in the urine, exclusively as metabolites. |
| Volume of distribution | 4.6 L/kg |
| Common adverse events | Visual disturbances, nausea & vomiting, skin rashes, and elevated liver enzyme levels |
| Drug monitoring (serum) | Target levels 1.0-5.5 mcg/mL Trough levels above 6 mcg/mL (and especially >10 mcg/mL) have been associated with toxicity in several reports. Trough levels below 1 mcg/mL have been associated with suboptimal response in several reports. |
| Heat stability | Accelerated metabolite degradation >60°Ca |
| Cautions | Metabolism may be altered by co-administration of drugs that metabolically induce or inhibit CYP2C19 or by genetic polymorphisms that affect enzyme activity. |
Demonstrated in vitro.
The described dissolving calcium sulfate delivery matrix system used has been available in the UK since 1998, the United States since 2000, the Australian setting since 2005, and in Canada since 2013. Its unique dissolution characteristics permit a more predictable and sustained elution of a broad range of powdered antimicrobial agents. Unlike direct wound bed sprinkling of dry antibiotics [25,27,30,31], the dissolving bead system avoids acute and often systemically overwhelming dose dumping [32] and permits a more sustained and predictable elution profile [33,34]. Equally, while a potentially large volume of mixed antibiotic is likely trapped within the matrix of eluting cement compounds and is not released, as only the surface elements and superficial antibiotics within direct contact of the surface through interconnecting porosity are released. By contrast, through progressive complete dissolution, the bead system allows sequential release of the entirety of the mixed additives [34]. Of critical importance in this case, and with wide potential application in other comparable settings, the absence of an exothermic reaction associated with standard cement curing permits the calcium sulfate beads to greatly broaden the safe additive portfolio beyond the limited grouping of conventional heat stable agents. This system was considered an ideal media for local, high-dose, sustained voriconazole delivery.
For this patient, we added 200 mg of voriconazole to each calcium sulfate bead mix for periarticular application. While this had been selected on the basis of pathogen sensitivities and its general risk profile, the dosing regime represented anecdotal experience or expert opinion. In the absence of an established precedent, we undertook a process of second-daily serum voriconazole level and hepatorenal function testing for 14 postoperative days, then at the 4-, 6-, and 12-week marks. Serum levels remained below 5.0 mcg/mL at each sampling timepoint. In retrospect, it would have appeared that a less rigorous sampling regime may have been appropriate. In the absence of a strong evidence base, we would suggest that dosing parameters for voriconazole, including optimal, maximal and minimum inhibitory concentration levels used in eluting calcium sulfate beads should be considered in future research.
While likely of considerable benefit in the treatment of this singular, complex patient, dissolving calcium sulfate systems are equally not without their recognized shortcomings and complications. While the differing constitutional properties between available preparations influence elution characteristics, the slow dissolution of the calcium substrate transiently raises the local tissue oncotic pressures. The resultant extravascular fluid shift can often contribute to either deep seroma formation or persistent wound ooze [34] and potentially sterile sinus formation. These latter elements carry concerns regarding wound healing and the counter-productive risk of infection ingress. Less common concerns regarding heterotrophic ossification [34], and renal or parathyroid dysfunction secondary to systemic hypercalcaemia [33,34], also hold case-by-case potential and may warrant dedicated surveillance. Equally, while we report success in the treatment of an atypical fungal and bacterial super-infection in a single patient example, others have reported poorer results with these agents in differing contexts. For example, the earlier work by Flierl et al. (2017) [33] suggested no benefit through addition of antibiotic-impregnated calcium sulfate beads in the management of acute hip and knee PJIs managed through irrigation and debridement alone with component retention, citing a high early failure rate. The authors themselves recognized the small, retrospective nature of their study and the intrinsically poor success rate of this approach with washout and implant retention in the setting of PJI—a key consideration rehighlighted in a later systematic review on the topic [34]. Nonetheless, this highlights that dissolving calcium sulfate beads may not be a panacea in all infective cases and their use should be considered contextually on a case-by-case basis.
Our expectations with curative intent for this patient were derived crudely from the findings of the limited body of published literature available at the time. While we had hoped for cure in the order of 6-12 months [13], the patient had been counseled regarding the potential for a far prolonged treatment course. The very real possibility of outright treatment failure was also extensively discussed, as were the reported outcomes of amputation [17] and death [7,16]. The patient was surveyed regularly with great scrutiny with both clinical and serum monitoring after the first-stage procedure. While our relatively short curative timeframe of just 15 weeks between stages was substantially less than most other reported works [7,13,29], the early article of Hwang et al. (2012) [35] reported safe second-stage procedures performed on average just 10 weeks after the previous first-stage, athough this was in the setting of isolated fungal infection.
Fungal PJIs remain mercifully an uncommon event [24] plagued with difficulties in timely definitive diagnosis [5,21] and, to date, limited effective curative treatment options [8,20,23]. From the limited published body of knowledge, most reported cases present with pain and local swelling [8], although neither are in any way specific for fungal infections. Kuiper et al. (2013) [8] suggested that perhaps radiographic signs of interface loosening may appear and progress more rapidly in the setting of a fungal vs bacterial PJI, but their review was small with only 164 total cases included—only 8 from their own cohort. Most consistently, authors internationally suggest that infected joints that have undergone a high previous number of operations [13,23] and those poorly responsive to bacterial pathogen-appropriate treatment [5,26] should be considered for potential super-infection. In essence, in the absence of a reliable high-sensitivity and high-specificity, minimally invasive and timely screening test, and with fungal analysis testing not routine nor universally available [20], a high index of clinical suspicion [5] may remain one of the most critical diagnostic elements.
While the recent publication from South Korea by Lee et al. (2019) [36] reported spectacular success in the eradication of fungal infections from around prosthetic hips and knees (16/16) using echinocandins [15,37], these results have yet to be replicated elsewhere and require further exploration. Importantly when considering Lee’s work, these infections did not have concurrent bacterial coinfection [36], and 9 of 16 (56.3%) patients were treated with resection arthroplasty. Driven by high contemporary patient expectations for enduring functional capacity [38], the latter may not be routinely feasible nor palatable in many Western settings.
Summary
The presented case herein describes the uncommon, but increasingly encountered, circumstance of a fungally based PJI super-infection around an in situ TKA. While historically curative treatment of such infections has been a challenging—and often unrewarding—endeavor, the successes seen for this patient highlight a potentially useful and novel treatment approach that may help other surgeons yield improved results in the management of this often devastating postoperative condition. The dissolving calcium sulfate bead or paste delivery system offers a new treatment adjunct for difficult infective cases, including fungal pathogens, and may in time prove valuable in improving eradication rates and safely reducing necessary treatment timeframes. The possibility of expanding the safe additive inventory beyond the limited battery of heat stable antibiotics alone certainly has exciting potential. Further work is clearly required in the application of this product to add to the growing body of accepted scientific knowledge in its use, to reinforce safety profiles and to better define primary indications for use and best practice application methods.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
Informed Patient Consent: The patient provided consent for their de-identified information to be published in this case report.
Appendix A. Supplementary data
References
- 1.Kurmis A.P., Apps S.A. Occupationally-acquired noise-induced hearing loss: a senseless workplace hazard. Int J Occup Med Environ Health. 2007;20(2):127. doi: 10.2478/v10001-007-0016-2. [DOI] [PubMed] [Google Scholar]
- 2.Kurmis A.P. The developing role of knee MRI in musculo-skeletal radiology: the progression to 3-D imaging. Radiographer. 2001;48(1):21. [Google Scholar]
- 3.Parvizi J., Tan T.L., Goswami K. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 4.Kurmis A.P., Herman A., McIntyre A., Masri B.A., Garbuz D.S. Pseudotumors and high grade ALVALs around total knee replacements identified at aseptic revision surgery: findings of a large-scale histologic review. J Arthroplasty. 2019;34:2434. doi: 10.1016/j.arth.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Matthews S., Sloan S., McCaffrey D., Ruiz A. Septic arthritis of the hip complicated by secondary fungal superinfection. J Orthop Case Rep. 2017;7(1):46. doi: 10.13107/jocr.2250-0685.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girois S.B., Chapuis F., Decullier E., Revol B.G.P. Adverse effects of antifungal therapies in invasive fungal infections: review and metanalysis. Eur J Clin Microbiol Infect Dis. 2006;25:138. doi: 10.1007/s10096-005-0080-0. [DOI] [PubMed] [Google Scholar]
- 7.Ueng S.W., Lee C.Y., Hu C.C., Hsieh P.H., Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471(9):3002. doi: 10.1007/s11999-013-3007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuiper J.W., van den Bekerom M.P., van der Stappen J., Nolte P.A., Colen S. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta Orthop. 2013;84(6):517. doi: 10.3109/17453674.2013.859422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koutserimpas C., Zervakis S.G., Maraki S. Non-albicans Candida prosthetic joint infections: a systematic review of treatment. World J Clin Cases. 2019;7(12):1430. doi: 10.12998/wjcc.v7.i12.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng H.M., Wang L.C., Chen J.Y. Microbiology analysis of periprothetic joint infection post total hip and knee arthroplasty of 9 centers in Beijing between 2014 and 2016. Zhonghua Wai Ke Za Zhi. 2019;57(8):596. doi: 10.3760/cma.j.issn.0529-5815.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Li Z.L., Hou Y.F., Zhang B.Q. Identifying common pathogens in periprosthetic joint infection and testing drug-resistance rate for different antibiotics: a prospective, single center study in Beijing. Orthop Surg. 2018;10(3):235. doi: 10.1111/os.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo F.C., Goswami K., Shohat N., Blevins K., Rondon A.J., Parvizi J. Two-stage exchange arthroplasty is a favorable treatment option upon diagnosis of a fungal periprosthetic joint infection. J Arthroplasty. 2018;33(11):3555. doi: 10.1016/j.arth.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q.J., Shen H., Zhang X.L. Staged reimplantation for the treatment of fungal peri-prosthetic joint infection following primary total knee arthroplasty. Orthop Traumatol Surg Res. 2015;101(2):151. doi: 10.1016/j.otsr.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Brown T.S., Petis S.M., Osmon D.R. Periprosthetic joint infection with fungal pathogens. J Arthroplasty. 2018;33(8):2605. doi: 10.1016/j.arth.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Cobo F., Rodriguez-Granger J., Sampedro A., Aliaga-Martinez L., Navarro-Mari J.M. Candida prosthetic joint infection. A review of treatment methods. J Bone Jt Infect. 2017;2(2):114. doi: 10.7150/jbji.17699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobo F., Rodríguez-Granger J., López E.M. Candida-induced prosthetic joint infection. A literature review including 72 cases and a case report. Infect Dis (Lond) 2017;49(2):81. doi: 10.1080/23744235.2016.1219456. [DOI] [PubMed] [Google Scholar]
- 17.Sidhu M.S., Cooper G., Jenkins N., Jeys L., Parry M., Stevenson J.D. Prosthetic fungal infections: poor prognosis with bacterial co-infection. Bone Joint J. 2019;101-B(5):582. doi: 10.1302/0301-620X.101B5.BJJ-2018-1202.R1. [DOI] [PubMed] [Google Scholar]
- 18.Theil C., Schmidt-Braekling T., Gosheger G., Idelevich E.A., Moellenbeck B., Dieckmann R. Fungal prosthetic joint infection in total hip or knee arthroplasty: a retrospective single-centre study of 26 cases. Bone Joint J. 2019;101-B(5):589. doi: 10.1302/0301-620X.101B5.BJJ-2018-1227.R2. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Y., Xuan Y.Y., Li G. Successful treatment for acute prosthetic joint infection due to MRSA and Candida albicans: a case report and literature review. Ther Clin Risk Manag. 2018;14:1133. doi: 10.2147/TCRM.S165247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokarski A.T., O'Neil J., Deirmengian C.A., Ferguson J., Deirmengian G.K. The routine use of atypical cultures in presumed aseptic revisions is unnecessary. Clin Orthop Relat Res. 2013;471(10):3171. doi: 10.1007/s11999-013-2917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLawhorn A.S., Nawabi D.H., Ranawat A.S. Management of resistant, atypical and culture-negative periprosthetic joint infections after hip and knee arthroplasty. Open Orthop J. 2016;10:615. doi: 10.2174/1874325001610010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.K., Lee D.Y., Kang D.W., Ro D.H., Lee M.C., Han H.S. Efficacy of antifungal-impregnated cement spacer against chronic fungal periprosthetic joint infections after total knee arthroplasty. Knee. 2018;25(4):631. doi: 10.1016/j.knee.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Graw B., Woolson S., Huddleston J.I. Candida infection in total knee arthroplasty with successful reimplantation. J Knee Surg. 2010;23(3):169. doi: 10.1055/s-0030-1267470. [DOI] [PubMed] [Google Scholar]
- 24.Anagnostakos K., Kelm J., Schmitt E., Jung J. Fungal periprosthetic hip and knee joint infections clinical experience with a 2-stage treatment protocol. J Arthroplasty. 2012;27(2):293. doi: 10.1016/j.arth.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Ji B., Zhang X., Xu B., Guo W., Mu W., Cao L. Single-stage revision for chronic fungal periprosthetic joint infection: an average of 5 Years of follow-up. J Arthroplasty. 2017;32(8):2523. doi: 10.1016/j.arth.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Tsai Y., Chang C.H., Lin Y.C., Lee S.H., Hsieh P.H., Chang Y. Different microbiological profiles between hip and knee prosthetic joint infections. J Orthop Surg (Hong Kong) 2019;27(2) doi: 10.1177/2309499019847768. 2309499019847768. [DOI] [PubMed] [Google Scholar]
- 27.Zou C., Xu B.Y., Guo W.T., Mu W.B., Ji B.C., Cao L. Efficacy evaluation of one-stage revision combined with intra-articular injection of antifungal agents in the treatment of chronic periprosthetic fungal infection. Zhonghua Wai Ke Za Zhi. 2019;57(5):348. doi: 10.3760/cma.j.issn.0529-5815.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Klatte T.O., Kendoff D., Kamath A.F. Single-stage revision for fungal peri-prosthetic joint infection: a single-centre experience. Bone Joint J. 2014;96-B(4):492. doi: 10.1302/0301-620X.96B4.32179. [DOI] [PubMed] [Google Scholar]
- 29.Phelan D.M., Osmon D.R., Keating M.R., Hanssen A.D. Delayed reimplantation arthroplasty for candidal prosthetic joint infection: a report of 4 cases and review of the literature. Clin Infect Dis. 2002;34(7):930. doi: 10.1086/339212. [DOI] [PubMed] [Google Scholar]
- 30.Ishida W., Perdomo-Pantoja A., Elder B.D. Effects of intraoperative intrawound antibiotic administration on spinal fusion: a comparison of vancomycin and tobramycin in a rat model. J Bone Joint Surg Am. 2019;101(19):1741. doi: 10.2106/JBJS.18.00988. [DOI] [PubMed] [Google Scholar]
- 31.Bakhsheshian J., Dahdaleh N.S., Lam S.K., Savage J.W., Smith Z.A. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015;83(5):816. doi: 10.1016/j.wneu.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J.D., Nessler J.M., Horazdovsky R.D., Vang S., Thomas A.J., Marston S.B. Serum and wound vancomycin levels after intrawound administration in primary total joint arthroplasty. J Arthroplasty. 2017;32(3):924. doi: 10.1016/j.arth.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Flierl M.A., Culp B.M., Okroj K.T., Springer B.D., Levine B.R., Della Valle C.J. Poor outcomes of irrigation and debridement in acute periprosthetic joint infection with antibiotic-impregnated calcium sulfate beads. J Arthroplasty. 2017;32(8):2505. doi: 10.1016/j.arth.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 34.Abosala A., Ali M. The use of calcium sulphate beads in periprosthetic joint infection, a systematic review. J Bone Jt Infect. 2020;5(1):43. doi: 10.7150/jbji.41743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang B.H., Yoon J.Y., Nam C.H. Fungal peri-prosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94(5):656. doi: 10.1302/0301-620X.94B5.28125. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y.R., Kim H.J., Lee E.J., Sohn J.W., Kim M.J., Yoon Y.K. Prosthetic joint infections caused by Candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23. doi: 10.1007/s11046-018-0286-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y.R., Kim H.J., Lee E.J., Sohn J.W., Kim M.J., Yoon Y.K. Prosthetic joint infections caused by Candida species: a systematic review and a case series. Mycopathologia. 2019;184(1):23. doi: 10.1007/s11046-018-0286-1. [DOI] [PubMed] [Google Scholar]
- 38.Kurmis A.P. Thromboprophylaxis after total hip replacement. J Orthop Surg (Hong Kong) 2010;18(1):92. doi: 10.1177/230949901001800121. [DOI] [PubMed] [Google Scholar]
- 39.Andes D., Pascual A., Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrobial Agents Chemother. 2009;53(1):24. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams A.I.H., Gosmann G., Schneider P.H., Bergold A.M. LC stability studies of voriconazole and structural elucidation of its major degradation product. Chromatographia. 2009;69:115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.