Abstract
Background
Bisphenol A (BPA) is a plasticizer with high production and ubiquitous usage in polycarbonate plastics and epoxy resins. The association between prenatal or postnatal exposure to BPA and childhood wheeze/asthma has not been well established. Our study aimed to provide further justification for the current studies.
Methods
Studies were searched from PubMed, Web of Science, Scopus and Embase from inception until Sep 15, 2020. Meta-analysis was performed to calculate pooled adjusted odds ratios (aOR). The methodological quality of included studies was assessed by using the Newcastle Ottawa Scale (NOS).
Results
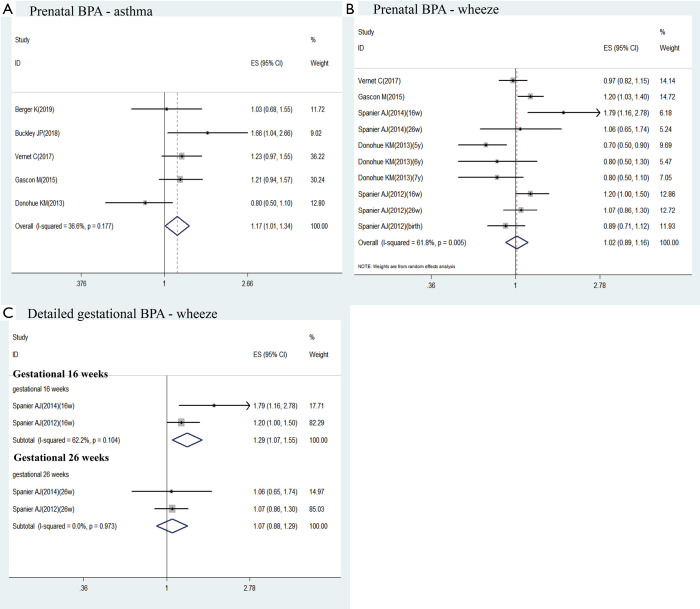
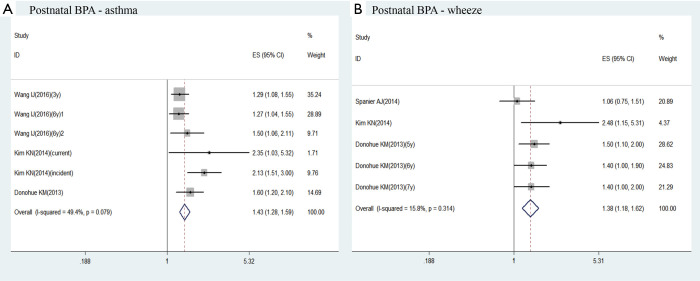
Of 2,814 screened articles, 9 studies with 3,885 participants were included in the final analysis. When all studies were pooled, postnatal exposure to BPA was associated with a higher risk of childhood asthma (aOR =1.43; 95% CI: 1.28–1.59) or childhood wheeze (aOR =1.38; 95% CI: 1.18–1.62). Prenatal exposure to BPA had a small but significant increased risk of childhood asthma (aOR =1.17; 95% CI: 1.01–1.34). An increased risk of childhood wheeze was related to prenatal exposure to BPA at 16 weeks’ gestation (aOR =1.29; 95% CI: 1.07–1.55), but not at 26 weeks’ gestation (aOR =1.07; 95% CI: 0.88–1.29) nor at random-time gestation (aOR =1.02; 95% CI: 0.89–1.16).
Conclusions
Prenatal and postnatal exposure to BPA was related to an increased risk of childhood asthma. However, only postnatal and early gestational exposure (at 16 weeks) to BPA could induce the risk of childhood wheeze, but not late gestational exposure (at 26 weeks).
Keywords: Bisphenol A (BPA), asthma, wheeze, children, meta-analysis
Introduction
Asthma is one of the most significant pediatric diseases in the world (1,2). Its prevalence in children has increased dramatically over a relatively short period of time, which is suspected to be associated with the expanded urbanization and industrialization (2-5). Given that increase of asthma shares an approximately similar timeframe with widespread use of industrial chemicals, some researchers have hypothesized that industrial chemicals may be significant contributors to the rising trend of pediatric asthma (6).
Bisphenol A (BPA), a critical endocrine disrupting chemical, has gained a lot of attention recently for its ubiquitous exposure (7). It is produced in large quantities and used in manufacture of polycarbonate plastics (toys, water bottles, dental sealants, et al.) or epoxy resins (coating the insides of cans for beverages and food) (8,9). International biomonitoring evidences show that there is higher BPA exposure in children than in adults and BPA exposure affects more than 90% of all children in America, Asia, Europe and Australia (10). The continuous daily BPA exposure, numerous BPA sources and various BPA exposure routes (mouth, skin and inhalation) cannot be ignored, although it is at low-level concentration in human body and is rapidly metabolized and excreted (7,10,11).
Prenatal and postnatal BPA exposure should be paid more attention to because the high dietary intake and long-term indoors time of pregnancy women/young children and hand to mouth behaviors for food consumption of infants and toddlers (10). Moreover, the immaturity of children’s lungs and immune systems might make irreversible, deleterious and long-lasting impact on allergic manifestation later in life (12).
Some animal evidences have confirmed that BPA had the immunomodulatory ability to influence the balance of Th1 and Th2 immune responses by increasing IL4 and reducing IFN-γ, IL10 (13-16). However, clinical studies which investigated the association between prenatal or postnatal exposure to BPA and childhood wheeze/asthma have inconsistent results (17-25). Therefore, our systemic review and meta-analysis aimed to provide further justification for the current studies.
We present the following article/case in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/jtd-20-1550).
Methods
Our systemic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISRM) (26).
Data sources and search strategy
We performed a systematic search by using some databases including PubMed, Web of Science, Scopus and Embase from the databases’ inceptions until Sep 15, 2020. The following search items were applied in the search for eligible studies: (“bisphenol A” or “BPA” or “endocrine disrupting chemical” or “endocrine disrupting compounds” or “endocrine disruptors” or “EDCs”) and (“prenatal” or “maternal” or “postnatal”) and (“asthma” or “wheeze” or “wheezing”) and (“offspring” or “children” or “childhood” or “child” or “infant” or “infancy”). Reference lists of identified articles were scanned to avoid omission.
Study selection
All studies that fulfilled the following inclusion criteria were considered: (I) study investigation: the association between prenatal or postnatal exposure to BPA and the risk of childhood asthma or wheeze. (II) Study data: results should be reported as adjusted odds ratio (aOR) or adjusted relative risk (aRR) or adjusted hazard ratio (aHR) with the corresponding 95% confidence intervals (95% CIs). (III) Study language: only articles written in English. (IV) Studies type: original articles. The exclusion criteria were studies with no available data for outcome measures.
Data extraction and quality assessment
Two reviewers (M Wu and Q Weng) reviewed all included studies and extracted crucial information by using a data extraction form independently. The information included author, country, sample size, enrolling period, exposure detection, outcome measure, asthma assessment, pregnancy trimester, point estimate, results adjustment and so on. Quality of the included studies was assessed by Newcastle Ottawa Scale (NOS) (27). We regarded total scores of 0 to 3, 4 to 6, 7 to 9 as low, moderate, and high quality, respectively. A star assessment system was applied to evaluate the quality according to NOS.
Statistical analysis
We used aOR and corresponding 95% CIs for meta-analysis to assess the association between prenatal or postnatal exposure to BPA and the risk of childhood asthma or wheeze. Heterogeneity between studies was identified by the I2 statistic. We assigned I2 values of 25%, 50%, and 75% for low, moderate, and high heterogeneity, respectively. Random-effect meta-analysis was performed to calculate a pooled aOR if I2>50% otherwise fixed-effect was used. When there was high heterogeneity, sensitivity analysis would be conducted to find out which study contributed to the largest heterogeneity. Egger’s test and Begg’s test were performed to evaluate potential publication bias. All analyses were conducted by StataSE12.0. A P value <0.05 could help make conclusion that the result was statistically significant.
Results
Eligible studies and characteristics
There were totally 2,814 studies identified from databases. Of these, the first screening excluded 2,047 duplications and 730 studies based on title and/or abstract, leaving 37 studies for full-text review. Finally, we found that nine studies satisfied inclusion criteria for meta-analysis. The detailed steps of the study selection process are shown in Figure 1. Detailed characteristics of the included studies were demonstrated in Tables 1,2. All included studies were cohort studies. Urine samples were used for BPA exposure detection. Asthma or wheeze measure was identified mainly on the basis of questionnaires. Five studies (17-21) merely focused on prenatal exposure and two studies (22,23) only talked about postnatal exposure. Others (totally 2 studies) paid attention to both prenatal and postnatal exposure (24,25). Of the 9 studies, Wang et al. (22), Kim et al. (23), Donohue et al. (24) and the 2 studies from Spanier et al. (21,25) conducted several time-point exposure measurements, which offered us more data to do meta-analysis. The methodological quality of the 9 studies were assessed according to the NOS tool (Table S1). Furthermore, we also summarized the limitations of each included studies (Table S2).
Figure 1.
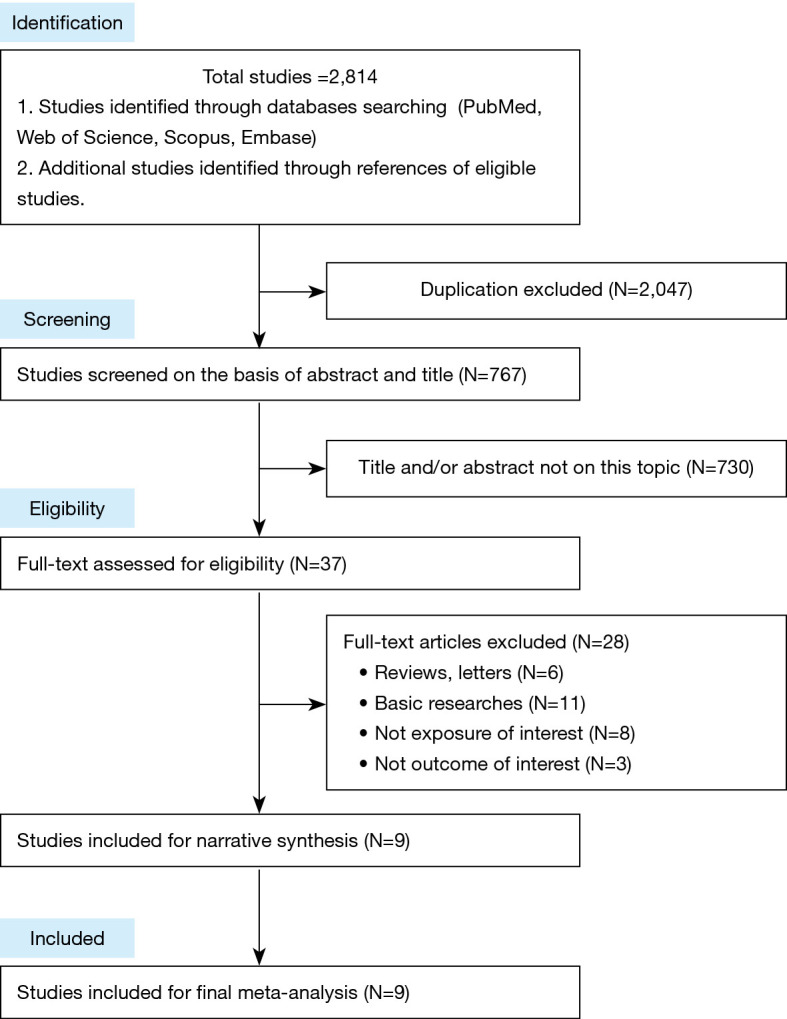
Flow diagram for search strategy.
Table 1. General characteristics of included studies.
| Author (year) | Country | Sample | Enrolling period | Exposure detection | Outcome measure | Asthma/wheeze assessment | Pregnancy trimester | Effect size | NOS score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Period | |||||||||
| Berger K (2019) (17) | California, USA | 329 | 1999–2000 | Urine | Prenatal | Asthma | Questionnaires | NA | aOR | 8 |
| Buckley JP (2018) (18) | New York, USA | 404 | 1998–2002 | Urine | Prenatal | Asthma | Questionnaires | NA | aOR | 8 |
| Vernet C (2017) (19) | Nancy/Poitiers, France | 587 | NA | Urine | Prenatal | Asthma wheeze | Questionnaires | NA | aHR | 8 |
| Wang IJ (2016) (22) | Taiwan, China | 453 | NA | Urine | Postnatal | Asthma | Questionnaires | NA | aOR | 8 |
| Gascon M (2015) (20) | Catalonia, Spain | 654 | 2004–2008 | Urine | Prenatal | Asthma wheeze | Questionnaires | NA | aRR | 8 |
| Spanier AJ (2014) (25) | Ohio, USA | 398 | 2003–2006 | Urine | Prenatal postnatal |
Wheeze | Questionnaires | 16 weeks 26 weeks |
aOR | 8 |
| Kim KN (2014) (23) | Seoul, Korea | 127 | 2005–2009 | Urine | Postnatal | Asthma wheeze | Questionnaires | NA | aOR | 8 |
| Donohue KM (2013) (24) | New York,USA | 568 | 1998–2006 | Urine | Prenatal postnatal | Asthma wheeze | Wheeze by questionnaires at 5,6,7y and asthma by physicians once between 5y and 12y. | NA | aOR | 9 |
| Spanier AJ (2012) (21) | Ohio, USA | 365 | 2003–2006 | Urine serum | Prenatal | Asthma wheeze | Questionnaires and ige levels | 16 weeks; 26 weeks |
aOR | 8 |
All studies were designed as cohort studies. BPA, Bisphenol A; NA, not available; aOR, adjusted odds ratio; aRR, adjusted rate ratio; aHR, adjusted hazard ratio; NOS, the New Castle-Ottawa Scale for cohort studies.
Table 2. Detailed characteristics of included studies.
| Author | Exposure measures | Questionnairemeasure time | Wheeze | Asthma | Adjustment |
|---|---|---|---|---|---|
| Prenatal | |||||
| Berger K (2019) (17) | Maternal urinary samples: at 16 and 26 weeks’ gestation | Children’ age at 7 years | NA | aOR, 1.03 (0.68–1.55) | Maternal age, parity, household income as a proportion of poverty at baseline, child’s family history of asthma, maternal education, monocarboxyisooctyl phthalate, propyl paraben, 2,4-dichlorophenol |
| Buckley JP (2018) (18) | Maternal urinary samples at third trimester (31.5±5.1 weeks) | Children’ age between 6 and 7 years | NA | aOR,1.66 (1.04–2.66); boys: aOR,3.04 (1.38–6.68); girls: aOR, 0.94 (0.48–1.84) | Creatinine, maternal age, race/ethnicity, pre-pregnancy body mass index, education, marital status, type of home ownership, smoking during pregnancy, person in household with asthma, person in household with allergies, number of occupants in the home, pets in the home, age at follow-up, and child’s sex |
| Vernet C (2017) (19) | Maternal urinary samples: once during 23–29 gestational weeks | Children’ ages from 8 months until 5 years | Only male offspring aHR, 0.97 (0.82–1.15) | Only male offspring; aHR, 1.23 (0.97–1.55) | Center, residence area, parental history of asthma or allergies, maternal ethnicity, maximal parental education level, maternal or passive smoking during pregnancy, postnatal passive smoking, older siblings, and child care |
| Gascon M (2015) (20) | Maternal urinary samples: at 12 and 32 weeks’ gestation | Children’ ages from birth until 7y | From birth until 7y: aRR, 1.20 (1.03–1.40) | At 7y: aRR, 1.21 (0.94–1.57) | Maternal education, number of siblings and maternal smoking during pregnancy |
| Spanier AJ (2014) (25) | Maternal urinary samples: at 16 and 26 weeks’ gestation | Children’ ages from birth until 5 years every 6 months | BPA (16 weeks): aOR, 1.79 (1.16–2.78); BPA (26 weeks): aOR, 1.06 (0.65–1.74); BPA (every 10-fold increase); aOR, 1.55 (0.91–2.63) | NA | Prenatal tobacco exposure, season, breastfeeding history, family history of asthma, family history of allergy, child eczema, child allergy, birth weight, maternal parity, pet ownership, and cockroach exposure |
| Donohue KM (2013) (24) | Maternal urinary samples at third trimester (31.5±5.1 gestational weeks) | Children’ ages at 5, 6 and 7 years | Wheeze (5y): aOR, 0.7 (0.5–0.9); wheeze (6y): aOR, 0.8 (0.5–1.3) wheeze (7y): aOR, 0.8 (0.5–1.1) | aOR, 0.8 (0.5–1.1) | Maternal history of asthma, sex, race/ethnicity, prenatal and postnatal environmental tobacco smoke exposure, and urine specific gravity. Models for asthma were additionally controlled for the child’s age at the time of evaluation because this assessment was performed once per child between ages 5 and 12 years |
| Spanier AJ (2012) (21) | Maternal urine and serum samples: at enrollment (15.9±1.9 weeks/gestation), 26 gestational weeks, and birth | Children’ ages from birth until 3 years every 6 months | BPA(16 weeks) aOR,1.2(1.0, 1.5) BPA(26 weeks) aOR,1.07(0.86,1.3) BPA(at birth) aOR,0.89(0.71,1.12) |
NA | Maternal education, race/ethnicity, occupation, income, house volume, health insurance, prenatal tobacco exposure, health insurance status, prenatal tobacco exposure, season, history and duration of breast-feeding, family history of asthma, family history of allergy, child eczema, child allergy, neonatal characteristics, pet ownership and cockroach exposure |
| Postnatal | |||||
| Wang IJ (2016) (22) | Children urinary BPA glucuronide levels: at 3 and 6 years | Children’ ages at 3 and 6 years | NA | BPAG(3y) with: asthma(3y): aOR, 1.29 (1.08–1.55); asthma(6y): aOR, 1.27 (1.04–1.55); BPAG(6y) with: asthma(6y): aOR, 1.50 (1.06–2.11) | Urine creatinine, maternal age, maternal education, maternal history of atopy, breast feeding and ETS exposure |
| Spanier AJ (2014) (25) | Urine samples of annual child visits | Children’ ages from birth until 5 years every 6 months | Concurrent wheeze: aOR, 1.06 (0.75–1.51); future wheeze: aOR, 1.08 (0.65–1.78) | NA | Prenatal tobacco exposure, season, breastfeeding history, family history of asthma, family history of allergy, child eczema, child allergy, birth weight, maternal parity, pet ownership and cockroach exposure |
| Kim KN (2014) (23) | Children urinary BPA: between 11 and 12 years | Along with exposure measure | aOR, 2.48 (1.15–5.31) | Current asthma: aOR, 2.35 (1.03–5.32); incident asthma: aHR, 2.13 (1.51–3.00) | Gender, parental asthma history, fetal and environmental tobacco smoke exposure, pet ownership and grade at enrollment |
| Donohue KM (2013) (24) | Children urinary samples at 3, 5 and 7 years | Children’ ages at 5, 6 and 7 years | BPA(mean) with: wheeze(5y): aOR, 1.5 (1.1–2.0); wheeze(6y): aOR, 1.4 (1.0–1.9); wheeze(7y): aOR, 1.4 (1.0–2.0) | BPA(mean): aOR, 1.6 (1.2–2.1) | Maternal history of asthma, sex, race/ethnicity, environmental tobacco smoke exposure, and urine specific gravity. Models for asthma were additionally controlled for child’s age at the time of evaluation because this assessment was performed once per child between ages 5 and 12 years |
Donohue KM (2013) (24) and Spanier AJ (2014) (25) investigated both prenatal and postnatal exposure to BPA and childhood asthma/wheeze. Thus, their results were summarized and shown in different subgroup respectively. BPA, Bisphenol A; NA, not available; aOR, adjusted odds ratio; aRR, adjusted rate ratio; aHR, adjusted hazard ratio.
Results of meta-analysis
Prenatal exposure to BPA and childhood asthma
Of these studies identified, 5 reported the association between prenatal exposure to BPA and childhood asthma (17-20,24). Meta-analysis result showed that prenatal exposure to BPA was associated with an increased risk of childhood asthma by using fixed-effects model (aOR =1.17, 95% CI, 1.01–1.34; Figure 2A). Low heterogeneity was observed (I2=36.6%; P=0.177; Figure 2A).
Figure 2.
Effect sizes in the meta-analysis on prenatal exposure to BPA and children asthma and wheeze. (A) Meta-analysis on prenatal exposure to BPA and children asthma. (B) Meta-analysis on prenatal exposure to BPA and children wheeze. (C) Meta-analysis on detailed gestational exposure to BPA and children wheeze. Note: (I) two studies (21,25) investigated different exposure detection time was shown as Spanier AJ (2014) (16 w), Spanier AJ (2014) (26 w) and Spanier (2012) (16 w), Spanier (2012) (26 w), Spanier (2012) (birth). (II) One study (24) investigated different end-point childhood ages was shown as Donohue KM (2013) (5 y), Donohue KM (2013) (6 y) and Donohue KM (2013) (7 y).
Prenatal exposure to BPA and childhood wheeze
Five studies reported the association between prenatal exposure to BPA and childhood wheeze (19-21,24,25). There were two studies investigated different exposure detection time as following: Spanier AJ [2014] at 16/26 weeks (25) and Spanier [2012] at 16/26weeks/at birth (21). And Donohue KM investigated different end-point outcome at 5, 6 and 7 years (24). Since heterogeneity was moderate, we used random-effect rather than fixed-effect model to do the meta-analysis (I2=61.8%, P=0.005; Figure 2B). However, no significant association was found between prenatal exposure to BPA and childhood wheeze (aOR =1.02; 95% CI: 0.89–1.16; Figure 2B).
Gestational-week exposure to BPA and childhood wheeze
As the gestation period was too long to be vulnerable to BPA exposure, we made a further subgroup meta-analysis in the association between different gestational-week BPA exposure and childhood wheeze. Two studies collected maternal urinary BPA concentration at two exposure time points (gestational 16 and 26 weeks) during pregnancy (21,25). As the results shown, an increased risk of childhood wheeze was related to prenatal exposure to BPA at 16 weeks’ gestation (aOR =1.29; 95% CI: 1.07–1.55; I2=62.2%, P=0.104; Figure 2C), but not at 26 weeks’ gestation (aOR =1.07; 95% CI: 0.88–1.29; I2=0%, P=0.973; Figure 2C).
Postnatal exposure to BPA and childhood asthma
There were 3 studies investigated the association between the postnatal exposure to BPA and childhood asthma (22-24). Kim observed two kinds of childhood asthma outcomes (incident asthma and current asthma) (23). And Wang surveyed the relationship between postnatal exposure to BPA at 3 years and childhood asthma at 3 or 6 years, which was showed as Wang (3y) and Wang (6y)1 in Figure 3A (22). Moreover, he also investigated the relation between postnatal exposure to BPA at 6 years and childhood asthma at 6 years, which was showed as Wang (6y)2 in Figure 3A (22). Our result demonstrated that postnatal exposure to BPA exposure is a risk factor to childhood asthma (aOR =1.43; 95% CI: 1.28–1.59; Figure 3A). The statistical heterogeneity was moderate (I2=49.4%, P=0.079; Figure 3A).
Figure 3.
Effect sizes in the meta-analysis on postnatal exposure to BPA and children asthma and wheeze. (A) Meta-analysis on postnatal exposure to BPA and children asthma. (B) Meta-analysis on postnatal exposure to BPA and children wheeze. Note: (I) reference: (22). Wang IJ (2016) (3 y): postnatal exposure to BPA at 3 years and childhood asthma at 3 years. Wang IJ (2016) (6 y)1: postnatal exposure to BPA at 3 years and childhood asthma at 6 years. Wang IJ (2016) (6 y)2: postnatal exposure to BPA at 6 years and childhood asthma at 6 years. (II) Kim KN (23) observed two kinds of childhood asthma outcomes (incident asthma and current asthma) was shown as Kim KN (2014) (current) and Kim KN (2014) (incident). (III) Donohue KM (24) investigated different end-point childhood ages was shown as Donohue KM (2013) (5 y), Donohue KM (2013) (6 y) and Donohue KM (2013) (7 y).
Postnatal exposure to BPA and childhood wheeze
Three studies surveyed the relation between the postnatal exposure to BPA and the risk of childhood wheeze (23-25). Among them, Donohue KM investigated different end-point outcome at 5, 6 and 7 years (24). According to the meta-analysis results, postnatal exposure to BPA was associated with a higher risk of childhood wheeze (OR =1.38; 95% CI: 1.18–1.62; Figure 3B). And heterogeneity was low (I2=15.8%, P=0.314; Figure 3B).
Sensitivity analysis
The subgroup meta-analysis with I2 high than 50% was analyzed with random-effect model and was further done with sensitivity analysis to find out the source of heterogeneity. However, we failed to find any obvious studies contributing to high heterogeneity.
Publication bias
We used Egger’s test and Begg’s test to assess the publication bias, whose P value of each meta-analysis group were exhibited in Table S3. The figures of all the Begg’s test were showed in Figure S1. The results convinced us that there was no publication bias in our meta-analysis (P>0.05).
Discussion
To the best of our knowledge, this meta-analysis provides the first quantitative estimates of the association between prenatal or postnatal exposure to BPA and childhood wheeze/asthma. Our meta-analysis of 9 included studies (3,885 participants) shows that postnatal exposure to BPA was associated with a higher risk of childhood asthma and wheeze. Prenatal exposure to BPA had a small but significant increased risk of childhood asthma. An increased risk of childhood wheeze was related to prenatal exposure to BPA at 16 weeks’ gestation, but not at 26 weeks’ gestation nor at random-time gestation.
BPA is a synthetic environmental chemical with small molecular weight (228 Da) and high lipophilicity, which makes it pass through human epithelial barrier much more easily (28). BPA exposure is ubiquitous for its high production and wide application. Dietary and non-dietary (dermal absorption, inhalation and sublingual absorption) sources could contribute to total daily exposure in human. Moreover, residual BPA concentration can’t be ignored, which has been reported in the range of 1–140 mg/kg in polycarbonate plastics generally (29).
Nowadays, accumulating evidences have demonstrated that BPA exposure are associated with some adverse health outcome, such as obesity, hyperactivity and asthma (30,31). Our meta-analysis results provided further justification for the current studies and demonstrated that the prenatal and postnatal exposure to BPA were related with an increased risk of childhood asthma/wheeze. Until now, there are three potential mechanisms supporting the role of BPA exposure in pathological processes of asthma/wheeze. Firstly, BPA was reported to have immunomodulatory effects by increasing the production of proallergic Th2 cytokine and antigen-specific IgE (14,16,32), reducing the levels of IFN-r/IL-10/regulatory T CD41CD251 cells (14) and enhancing bronchial eosinophilic inflammation/allergic sensitization (6,16). Secondly, as an endocrine disrupting chemical, BPA also has the ability to enhance or inhibit the hormone signaling pathway by bounding to estrogen receptors (ERs), estrogen-related receptors (ERRs), toll-like receptors (TLRs) and others (33). It is acknowledged that ERs, ERRs and TLRs are expressed in most immune cells which allows BPA to act on immune systems. Activation of ERs was suggested to encourage the Th2 polarization with increased proallergic inflammatory cytokines, production of IgE in B cells and degranulation of mast cells (34,35). Lastly, some researches demonstrated that BPA-induced damage was related with the oxidative stress and mitochondrial dysfunction (36,37). And it is well known that the progress of asthma has a certain relationship with oxidative stress (38). Thus, BPA-causing oxidative stress might enhance the susceptibility to asthma to an extent. However, despite mechanisms mentioned above, whether results from laboratory rodent studies are applicable to human still remains unknown.
It is acknowledged that in early life, even subtle alterations can have the potential to alter normal human growth and development, and result in irreversible, deleterious and long-lasting changes later in life (12). Its exposure and adverse affection in children are more severe than adults due to the following factors (10). During the fetal period, placenta was unable to provide effective barrier against fetus exposure to BPA (39). Another notable factor was complex metabolism of BPA in the maternal-fetal unit. About 90% BPA that pregnant mice ingested after 24 hours was accumulated in the placental unit (40,41). Although free BPA (an active BPA) was conjugated as BPA-glucuronide (an inactive metabolite) in maternal rat liver, the conjugated BPA could be absorbed and deconjugated back to free BPA in placenta (42). Even worse, fetal hepatic detoxification systems was not mature enough to provide enzymes to metabolize free BPA to conjugated BPA. Thus, fetus was exposed to higher active BPA concentration. In regard to children, BPA are predicted to have higher concentration and longer retention time in children than adults (43). Firstly, higher requirement of dietary intake for growth and development makes children more vulnerable to BPA than adults because dietary exposure routes are the most important source of BPA (44). Besides, sucking, chewing and frequent hand-to-mouth action, special behaviors in infants and toddlers, can result in additional indirect ingestion sources of BPA when some BPA-containing products (plastics, pacifiers and toys) are placed in mouth (44). Secondly, degeneration of BPA-containing consumer products can release BPA into air, dust and contact surfaces, which makes BPA be a ubiquitous pollutant in daily living environment (45,46). Thus, dermal absorption is a critical non-dietary exposure route in neonates due to comparatively higher surface area to body mass ratio and immature skin barrier function. Besides, compared with skin, the respiratory tract has more mucosal surface and some chemicals can be absorbed by respiratory epithelium (47). Higher oxygen requirements per kilogram body weight, faster respiratory rate and long-term indoor time make children more susceptible to inhalation contamination. Thus, inhalation route may not be ignored for BPA exposure. Lastly, it is well established that free BPA is first-pass metabolized by liver via UDP glucuronosyltransferase enzymes family and is eliminated through kidney (48,49). However, hepatic detoxification systems have not yet been fully developed in infants and toddlers.
Nowadays, some governments have taken measures to reduce the BPA exposure in human. European Food Safety Authority (EFSA) sets the tolerable daily intake of BPA to be no more than 4 µg/kg/day in 2014. Most European countries have adopted such criteria and the regulatory restriction on the use of BPA in children feeding products is implemented in Canada, the United States, Japan and so on (50-52). But there are no surveillance biomonitoring researches to assess the BPA exposure before and after restriction implementation and whether current regulatory restriction could effectively reduce BPA exposure remains unknown. In addition, BPA analogues and derivatives, as BPA substitute, have been used increasingly in manufacture and advertised and marketed as “BPA free”, including bisphenol S, bisphenol B, and bisphenol F (53). Nevertheless, a systematic review demonstrated that these BPA substitute had similar property to BPA. Therefore, multiple bisphenol exposure should be noteworthy.
Several limitations should be acknowledged and the corresponding suggestions are given for future studies. First of all, some included studies only measured urine BPA exposure once. In consideration of its short half-time and rapid excretion, BPA concentration had better to be detected more than once and taken the average value (49,54). Second, it is imprecise to use maternal urine BPA concentration as prenatal BPA exposure levels because only 6% of BPA that pregnant mice ingested after 24 hours was excreted in maternal urine (40,41). However, it is difficult to gain amniotic fluid or fetal blood as exposure measurement although that is more precise. Third, most included studies used parent-reported asthma/wheeze data as outcome assessment which were depended on parent recall. Only Donohue assessed asthma by physicians and Spanier used questionnaires combined with experimental IgE levels to determine asthma or wheeze (21,24). Thus, more accurate outcome assessments such as combination of diverse measurement are recommended to take into consideration to avoid outcome misclassification. Fourth, due to the manufacture of BPA substitute, multiple bisphenol exposure might be potential confounders. Besides, other chemicals like phalates which also have the immunomodulatory properties can influence the results if not ruled out. Potential confounders should receive great attention from researchers for it may affect result accuracy to a large extent. Fifth, the timing of exposure and outcome measurement was inconsistent among all included studies which might contribute to the discrepancy between each studies and heterogeneity in our meta-analysis. Therefore, standardized criteria are required for future researches including measure method of exposure, outcome assessment and elaborate confounders. At last, some surveillance biomonitoring researches are needed to make sure whether regulatory restriction could reduce the BPA exposure effectively by assessing the general BPA exposure before and after regulatory restriction implementation.
Conclusions
Prenatal and postnatal exposure to BPA was related to an increased risk of childhood asthma. However, only postnatal and early gestational exposure (at 16 weeks) to BPA could induce the risk of childhood wheeze, but not late gestational exposure (at 26 weeks). Future studies with standardized criteria and larger sample sizes are warranted.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/jtd-20-1550
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1550). The authors have no conflicts of interest to declare.
References
- 1.Baldacci S, Maio S, Cerrai S, et al. Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respir Med 2015;109:1089-104. 10.1016/j.rmed.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics 2016;137:1-7. 10.1542/peds.2015-2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis 2014;18:1269-78. 10.5588/ijtld.14.0170 [DOI] [PubMed] [Google Scholar]
- 4.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief 2013;(121):1-8. [PubMed] [Google Scholar]
- 5.Wong GW, Chow CM. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr Pulmonol 2008;43:107-16. 10.1002/ppul.20755 [DOI] [PubMed] [Google Scholar]
- 6.Kwak ES, Just A, Whyatt R, et al. Phthalates, Pesticides, and Bisphenol-A Exposure and the Development of Nonoccupational Asthma and Allergies: How Valid Are the Links? Open Allergy J 2009;2:45-50. 10.2174/1874838400902010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention b. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January, 2017.
- 8.Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology 2006;226:79-89. 10.1016/j.tox.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 9.Dodson RE, Marcia N, Standley LJ, et al. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect 2012;120:935-43. 10.1289/ehp.1104052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healy BF, English KR, Jagals P, et al. Bisphenol A exposure pathways in early childhood: Reviewing the need for improved risk assessment models. J Expo Sci Environ Epidemiol 2015;25:544-56. 10.1038/jes.2015.49 [DOI] [PubMed] [Google Scholar]
- 11.Morgan MK, Jones PA, Calafat AM, et al. Assessing the quantitative relationships between preschool children's exposures to bisphenol A by route and urinary biomonitoring. Environ Sci Technol 2011;45:5309-16. 10.1021/es200537u [DOI] [PubMed] [Google Scholar]
- 12.Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect 2010;118:1155-64. 10.1289/ehp.0901856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MH CS, Kang BY, Park J, Lee CH, Hwang SY, et al. Enhanced interleukin-4 production in CD41 T cells and elevated immunoglobulin E levels in antigen-primed mice by bisphenol A and nonylphenol, endocrine disruptors: involvement of nuclear factor-AT and Ca21. Immunology 2003;109:76-86. 10.1046/j.1365-2567.2003.01631.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect 2008;116:514-9. 10.1289/ehp.10829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawai C, Anderson K, Walser-Kuntz D. Effect of bisphenol A on murine immune function: modulation of interferon-gamma, IgG2a, and disease symptoms in NZB X NZW F1 mice. Environ Health Perspect 2003;111:1883-7. 10.1289/ehp.6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Midoro-Horiuti T, Tiwari R, Watson CS, et al. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ Health Perspect 2010;118:273-7. 10.1289/ehp.0901259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger K, Eskenazi B, Balmes J, et al. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr Allergy Immunol 2019;30:36-46. 10.1111/pai.12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckley JP, Quiros-Alcala L, Teitelbaum SL, et al. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7years. Environ Int 2018;115:79-88. 10.1016/j.envint.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vernet C, Pin I, Giorgis-Allemand L, et al. In Utero Exposure to Select Phenols and Phthalates and Respiratory Health in Five-Year-Old Boys: A Prospective Study. Environ Health Perspect 2017;125:097006. 10.1289/EHP1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gascon M, Casas M, Morales E, et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol 2015;135:370-8. 10.1016/j.jaci.2014.09.030 [DOI] [PubMed] [Google Scholar]
- 21.Spanier AJ, Kahn RS, Kunselman AR, et al. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ Health Perspect 2012;120:916-20. 10.1289/ehp.1104175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang IJ, Chen CY, Bornehag CG. Bisphenol A exposure may increase the risk of development of atopic disorders in children. Int J Hyg Environ Health 2016;219:311-6. 10.1016/j.ijheh.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Kim KN, Kim JH, Kwon HJ, et al. Bisphenol A exposure and asthma development in school-age children: a longitudinal study. PLoS One 2014;9:e111383. 10.1371/journal.pone.0111383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donohue KM, Miller RL, Perzanowski MS, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 2013;131:736-42. 10.1016/j.jaci.2012.12.1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spanier AJ, Kahn RS, Kunselman AR, et al. Bisphenol a exposure and the development of wheeze and lung function in children through age 5 years. JAMA Pediatr 2014;168:1131-7. 10.1001/jamapediatrics.2014.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GA SB, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 28.Hormann AM, Vom Saal FS, Nagel SC, et al. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PLoS One 2014;9:e110509. 10.1371/journal.pone.0110509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoekstra EJ, Simoneau C. Release of bisphenol A from polycarbonate: a review. Crit Rev Food Sci Nutr 2013;53:386-402. 10.1080/10408398.2010.536919 [DOI] [PubMed] [Google Scholar]
- 30.Kim KY, Lee E, Kim Y. The Association between Bisphenol A Exposure and Obesity in Children-A Systematic Review with Meta-Analysis. Int J Environ Res Public Health 2019;16:2521. 10.3390/ijerph16142521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochester JR, Bolden AL, Kwiatkowski CF. Prenatal exposure to bisphenol A and hyperactivity in children: a systematic review and meta-analysis. Environ Int 2018;114:343-56. 10.1016/j.envint.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 32.Clayton EM, Todd M, Dowd JB, et al. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003-2006. Environ Health Perspect 2011;119:390-6. 10.1289/ehp.1002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers JA, Metz L, Yong VW. Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol 2013;53:421-30. 10.1016/j.molimm.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 34.Cai Y, Zhou J, Webb DC. Estrogen stimulates Th2 cytokine production and regulates the compartmentalisation of eosinophils during allergen challenge in a mouse model of asthma. Int Arch Allergy Immunol 2012;158:252-60. 10.1159/000331437 [DOI] [PubMed] [Google Scholar]
- 35.Jing H, Wang Z, Chen Y. Effect of oestradiol on mast cell number and histamine level in the mammary glands of rat. Anat Histol Embryol 2012;41:170-6. 10.1111/j.1439-0264.2011.01120.x [DOI] [PubMed] [Google Scholar]
- 36.Hassan ZK, Elobeid MA, Virk P, et al. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev 2012;2012:194829. 10.1155/2012/194829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song S, Zhang L, Zhang H, et al. Perinatal BPA exposure induces hyperglycemia, oxidative stress and decreased adiponectin production in later life of male rat offspring. Int J Environ Res Public Health 2014;11:3728-42. 10.3390/ijerph110403728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahiner UM, Birben E, Erzurum S, et al. Oxidative stress in asthma: Part of the puzzle. Pediatr Allergy Immunol 2018;29:789-800. 10.1111/pai.12965 [DOI] [PubMed] [Google Scholar]
- 39.Balakrishnan B, Henare K, Thorstensen EB, et al. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol 2010;202:393.e1-7. 10.1016/j.ajog.2010.01.025 [DOI] [PubMed] [Google Scholar]
- 40.Zalko D, Soto AM, Dolo L, et al. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 2003;111:309-19. 10.1289/ehp.5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schonfelder G, Wittfoht W, Hopp H, et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect 2002;110:A703-7. 10.1289/ehp.021100703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishikawa M, Iwano H, Yanagisawa R, et al. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect 2010;118:1196-203. 10.1289/ehp.0901575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mielke H, Gundert-Remy U. Bisphenol A levels in blood depend on age and exposure. Toxicol Lett 2009;190:32-40. 10.1016/j.toxlet.2009.06.861 [DOI] [PubMed] [Google Scholar]
- 44.Landrigan P. Children are not little adults. In: Pronezuk-Garbino J. editor. Chidren’s Health and the Environment: A Global Perspective, 1st ed. World Health Organization: Geneva 2005:3-17. [Google Scholar]
- 45.Liao C, Liu F, Guo Y, et al. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 2012;46:9138-45. 10.1021/es302004w [DOI] [PubMed] [Google Scholar]
- 46.Wilson NK, Chuang JC, Lyu C, et al. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J Expo Anal Environ Epidemiol 2003;13:187-202. 10.1038/sj.jea.7500270 [DOI] [PubMed] [Google Scholar]
- 47.Haghi M, Ong HX, Traini D, et al. Across the pulmonary epithelial barrier: Integration of physicochemical properties and human cell models to study pulmonary drug formulations. Pharmacol Ther 2014;144:235-52. 10.1016/j.pharmthera.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 48.Taylor JA, Vom Saal FS, Welshons WV, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect 2011;119:422-30. 10.1289/ehp.1002514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teeguarden JG, Waechter JM, Jr, Clewell HJ, 3rd, et al. Evaluation of oral and intravenous route pharmacokinetics, plasma protein binding, and uterine tissue dose metrics of bisphenol A: a physiologically based pharmacokinetic approach. Toxicol Sci 2005;85:823-38. 10.1093/toxsci/kfi135 [DOI] [PubMed] [Google Scholar]
- 50.Health Canada. Bisphenol A. April 2013. Available online: http://healthycanadians.gc.ca/healthy-living-vie-saine/environment-environnement/home-maison/bisphenol_a-eng.php?ga=1.208995026.1960898991.1431095744. Accessed 10 May 2015.
- 51.United States Food and Drug Administration. Bisphenol A (BPA): Use in Food Contact Application. Updated Nov 2014. Available online: http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm064437.htm#summary. Accessed 9 February 2015.
- 52.Kawamura Y, Etoh M, Hirakawa Y, et al. Bisphenol A in domestic and imported canned foods in Japan. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2014;31:330-40. 10.1080/19440049.2013.874047 [DOI] [PubMed] [Google Scholar]
- 53.Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect 2015;123:643-50. 10.1289/ehp.1408989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkel W, Colnot T, Csanady GA, et al. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol 2002;15:1281-7. 10.1021/tx025548t [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as