Summary
The recently enriched genomic history of Indigenous groups in the Americas is still meager concerning continental Central America. Here, we report ten pre-Hispanic (plus two early colonial) genomes and 84 genome-wide profiles from seven groups presently living in Panama. Our analyses reveal that pre-Hispanic demographic events contributed to the extensive genetic structure currently seen in the area, which is also characterized by a distinctive Isthmo-Colombian Indigenous component. This component drives these populations on a specific variability axis and derives from the local admixture of different ancestries of northern North American origin(s). Two of these ancestries were differentially associated to Pleistocene Indigenous groups that also moved into South America, leaving heterogenous genetic footprints. An additional Pleistocene ancestry was brought by a still unsampled population of the Isthmus (UPopI) that remained restricted to the Isthmian area, expanded locally during the early Holocene, and left genomic traces up to the present day.
Keywords: archaeogenomics, genomic variation, population genetics, archaeology, anthropology and history, ancient and modern DNA, indigenous Americans, Isthmian populations, Central America, Panama
Graphical abstract
Highlights
-
•
Ancient Isthmian genomes address anthropological questions on pre-contact burials
-
•
The comparison with modern Panamanians highlights genomic structure on the Isthmus
-
•
A genomic component drives the Isthmian groups on a distinctive variability axis
-
•
A previously unknown Pleistocene ancestry identified in the Isthmo-Colombian area
Pre-contact and modern genomes from Panama highlight the distinctiveness of the Isthmo-Colombian area; detail number, source, and impact of Indigenous American genomic ancestries at the continental level; and explain complex pre-Hispanic burials.
Introduction
Archaeological and genetic evidence suggests that the peopling of sub-Arctic America started from Beringia before, during, and immediately after late Glacial times (Achilli et al., 2018; Ardelean et al., 2020; Becerra-Valdivia and Higham, 2020; Braje et al., 2017; Skoglund and Reich, 2016; Waters, 2019; Yu et al., 2020). Initial settlement attempts were followed by a more widespread peopling that reached southern South America as early as ∼15 thousand years ago (kya) (Dillehay et al., 2017). Recent studies of ancient and modern genomes describe a complex scenario prior to European contact with multiple migrations from Beringia, as initially suggested by mitochondrial DNA (mtDNA) data (Achilli et al., 2013; Brandini et al., 2018; Gómez-Carballa et al., 2018; Llamas et al., 2016; Perego et al., 2009; Perego et al., 2010; Tamm et al., 2007) as well as demographic spreads and admixture events along the two continents (Flegontov et al., 2019; Moreno-Mayar et al., 2018; Posth et al., 2018; Scheib et al., 2018; Schroeder et al., 2018). The great majority of ancestries in early Native Americans (NAs, here used to indicate Indigenous groups) derive from an ancestral Beringian population(s) that differentiated sometime between ∼22 and ∼18 kya and likely exhibited genetic sub-structure that may explain the initial late Glacial migration(s) as well as the spread of the so-called UPopA (unknown population in the Americas) whose legacy reappears in Central America ∼8.7 kya, leaving signs in the gene pool of the Mixe (Moreno-Mayar et al., 2018). In unglaciated eastern Beringia/northern North America, the first peoples split into two branches called Northern NA (NNA, or ANC-B) and Southern NA (SNA, or ANC-A). The most ancient representatives of SNA are individuals who were living on both sides of the Rocky Mountains more than 10 kya: the Clovis-associated Anzick-1 and the Spirit Cave individuals associated with Western Stemmed technology. Ancient individuals carrying SNA ancestries crossed the Panama land bridge and entered South America. Their fast spread along the southern continent is evidenced by the earliest archaeological human presence in the Southern Cone at 14.6 kya and by ancient human genomes dating more than 9 kya on both sides of the continent: at Cuncaicha (Peru) and Los Rieles (Chile) on the Pacific and Lapa do Santo and Lagoa Santa (Brazil) on the Atlantic. Another UPop (UPopY) with Australasian ancestry may have contributed to the early peopling of South America as recognized in one sample from the Lagoa Santa site and in some Amazonian groups that experienced isolation events (e.g., Surui and Karitiana) (Moreno-Mayar et al., 2018; Skoglund et al., 2015).
However, the demographic dynamics underlying many of these events, before and after European contact, are still uncharacterized, especially at the regional level (Fernandes et al., 2020; Lindo et al., 2017; Nakatsuka et al., 2020; Nägele et al., 2020). The Panamanian isthmus lies between the Atlantic and Pacific oceans and connects the two American continents. It was the only land bridge during the initial peopling of South America and has remained a crossroads of goods, technologies, ideas, and peoples throughout history, including more recent colonial times (Cooke, 2005; Cooke et al., 2019; Hernández Mora et al., 2021). In light of Panama’s geographic location, the archaeogenomic study of its past can reveal its demographic history, including movements between North and South America.
Ethics and community engagement
This study involves international collaborative efforts that bring together archaeologists, geneticists, historians, anthropologists, and computer engineers to incorporate existing knowledge with genomic information about pre-Hispanic as well as modern Indigenous individuals from the Isthmus of Panama. It was possible with the support of local authorities and Indigenous peoples of Panama and centrally involved local co-authors of the present paper (J.R., T.M., M.T., J.M.P., R.C., J.M., and J.G.M.) with years of experience in the Isthmo-Colombian region. Samples from the ancient individuals were collected for the ArtEmpire European Research Council (ERC) project (Consolidator Grant CoG-2014 no. 648535) in collaboration with the Patronato Panamá Viejo (PaPV) as established by the Convenio Específico de colaboración entre el Patronato Panamá Viejo de la República de Panamá y la Universidad Pablo de Olavide, de Sevilla, España, signed on January 20th, 2016. Excavations were undertaken with the permission of the Republic of Panama’s Instituto Nacional de Cultura, Dirección Nacional de Patrimonio Histórico (DNPH), Resolución DNPH no. 139-16 of November 11th, 2016, and resolución DNPH no. 006-18 of January 8th, 2018. Selected samples from bone and teeth were exported to Pavia (Italy) and Mannheim (Germany) in accordance with the Permission of the Republic of Panama’s Instituto Nacional de Cultura, Dirección Nacional de Patrimonio Histórico, Resolución no. 080-17 DNPH of April 19th, 2017, and resolución no. 304-18 DNPH of September 26th, 2018. Even though no ties to the pre-Hispanic inhabitants have been stated, we are engaging local Indigenous communities to present the information from this study and to seek their views. In general, the project has been designed to maximize opportunities for public engagement, as testified by ongoing meetings with local interest groups to discuss research design and findingst. In order to increase positive social impact, some information on the ancient samples are publicly available in the ArtEmpire’s database, translated into Spanish to increase accessibility (https://artempire.cica.es/) (Aram et al., 2020), and a documentary also has been released (https://www.youtube.com/watch?v=5BmxppS4oks).
The collection of the modern Indigenous samples was approved by the Comité de Bioética de la investigación del Instituto Conmemorativo Gorgas and undertaken by the Instituto Conmemorativo Gorgas de Estudios de la Salud (ICGES, Gorgas Memorial Institute for Health Studies) of Panama. The ICGES explained the project to community leaders in their native languages and sent biological samples to the Department of Biology and Biotechnology of the University of Pavia for DNA extraction and analysis in agreement with the memorandum of understanding (written in English and Spanish) signed on August 9th, 2016. All experimental procedures and individual written informed consent forms were also reviewed and approved by the Ethics Committee for Clinical Experimentation of the University of Pavia, Board minutes of October 5th, 2010, and April 11th, 2013. We are particularly grateful to and acknowledge the ancient and modern people who shared their DNAs.
Panama: archaeology and history
Paleoecological and archaeological data point to a continuous human inhabitation of the Isthmo-Colombian area from approximately 16 kya (Cooke et al., 2013; Ranere and Cooke, 2020). Clear evidence for the cultivation of domesticated plants, including maize (Zea mays), manioc (Manihot esculenta), and squash (Cucurbita moschata), dates back to more than 8–4.5 kya (Linares, 1977a, 1977b; Linares and Ranere, 1980; Linares et al., 1975; Piperno, 2011; Ranere and Cooke, 2020), while Panama’s first pottery (Monagrillo ware) appeared about 4.5 kya (Martín et al., 2015, 2016). By 3 kya, the area’s western region possesses all the characteristics of a coherent historical unit (Greater Chiriquí, which extended into present-day Costa Rica), while this consensus is not available for the central and eastern regions, often termed Greater Coclé and Greater Darién (see specific section in STAR Methods for further details).
From approximately 500 BCE to 1,500 CE, relations among neighbors oscillated between cooperation driven by exchange and trade and conflict over land and resources. Although polities often called chiefdoms expanded and contracted, there is no evidence for aggressive empire building as in Mexico or the Andes (Helms, 2014). On the eve of the Spanish invasion, historians and most archaeologists agree that much of the central and eastern Isthmus was inhabited by Indigenous polities that spoke languages in the Nuclear Chibchan family (with variants of languages in the Chocoan family probably also spoken on the Pacific side) and used the “language of Cueva” as a lingua franca (either a trade language or a group of vernaculars) in a linguistically complex region, much as the Huëtar did in Costa Rica (Cooke 2016; Costenla, 2012; Romoli, 1987). Based on partial and fragmentary data, historians have ventured estimates of the pre-Hispanic population in those areas where the language of Cueva was spoken at European contact from 130,000 to 240,000 people and archaeologists have identified specific villages capable of sustaining up to 2,400 inhabitants (Cooke, 2005; Cooke et al., 2019; Romoli, 1987). After nearly one millennium of less destructive war and trade among neighbors, European incursions provoked a rapid decline in the region’s Indigenous populations. However, not all Indigenous groups experienced simultaneous demographic decline. Historical records suggest that an expansion among the Guna followed the reduction of other Indigenous groups (Castillero Calvo, 2017).
Panama: Genetics
To date uniparental systems have been examined to assess the genetic history of Panama: mtDNA data identified specific lineages predating the Clovis technological horizon (13.2 kya), while the comparison with Y chromosome data revealed a sex bias during the colonial period consistent with “more native men perishing or being deprived of reproductive rights than women” (Grugni et al., 2015; Perego et al., 2012). Similar to mtDNA data, patterns of regional genetic continuity in some Indigenous American (IA) communities have been inferred from the analysis of nuclear genomes from continental Central America (Reich et al., 2012), but without ancient DNA (aDNA) data from the Isthmian land bridge.
To refine the human genetic history of the Isthmus, for the first time we have directly tested and analyzed autosomal markers of both pre-Hispanic human remains and contemporary Indigenous groups from Panama. Twenty ancient individuals (13 of them pre-Hispanic and seven colonial) were sampled from seven different archaeological excavations along the Pacific coast of Panama City, located from the residential area of Coco del Mar to the remnants of Old Panama’s Cathedral in Panamá Viejo, an area of pre-Hispanic inhabitation and the site of the colonial city from 1,519 to 1,671 CE (Hernández Mora et al., 2021). Modern sampling for a total of 84 individuals, 76 self-identified as associated with five different Indigenous groups plus four self-designated “Moreno” and four self-identified “Mestizo” individuals (Figure 1A; Table S1), took place in Panama City as well as in the provinces and Indigenous territories.
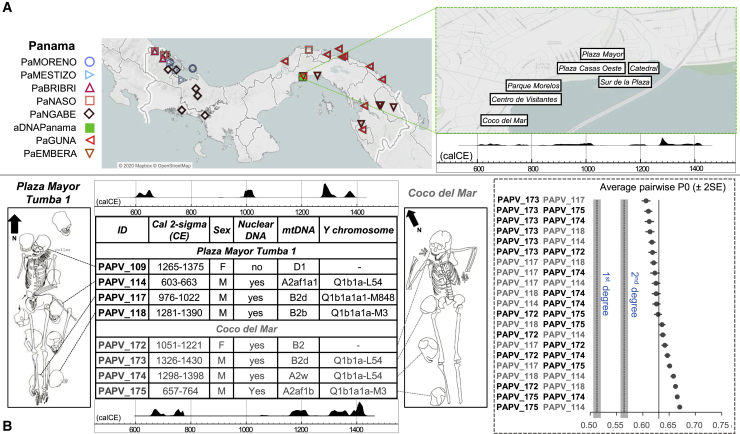
Figure 1.
Geographic locations and time ranges of modern and ancient individuals sampled
(A) Map showing the geographic origin of the Isthmian individuals sampled; the inset represents the locations of the archaeological excavations.
(B) Schematic drawings of Tumba 1 in the Plaza Mayor site of Panamá Viejo and the burial at Coco del Mar. The table reports mtDNA and Y chromosome haplogroup affiliations, molecular sex determination, and 14C-calibrated dates (CE). The sum distributions of all ages combined are shown, separately for the two sites, above (Tumba 1) and below (Coco del Mar) the table. Calibration dataset was IntCal20. Calibration software was OxCal 4.4.2. The inset on the right shows no kinship relationships (values extracted from Table S2 with error bars indicating 2-fold Standard Errors, SE) among individuals buried together in Plaza Mayor Tumba 1 and Coco del Mar. IDs (and additional information) are indicated in black and gray, respectively.
Results
Although the tropical environment and the proximity of the excavation sites to the ocean, with recurrent flooding, challenge the possibility of DNA preservation, we were able to obtain some of the first reliable aDNA data from the Isthmus. Starting from the initial collection of samples from 20 ancient individuals, we eventually assembled ancient low-coverage (≥0.01X) genomes from 12 unrelated individuals (one female and eleven males), including ten from pre-Hispanic times (radiocarbon dated from 603 to 1,430 CE). Molecular decay analyses demonstrated the poor preservation of endogenous DNA, but error rate and validation tests confirmed the reliability of the retrieved genomic data (Table S2). In order to characterize the genetics of Isthmian individuals with the greatest possible spatial range and temporal depth, the 12 ancient genomes were compared with genome-wide data from 74 unrelated modern Panamanians and to available modern and ancient data by assembling different datasets (STAR Methods; Table S3).
Uniparental lineages of pre-Hispanic Panamanians
The evaluation of uniparental markers revealed the presence of the “pan-American” mtDNA haplogroups A2 and B2 in the pre-Hispanic samples, while two haplogroups, H1j1a and L2a1c2a, typical of Europeans and sub-Saharan Africans, respectively, were identified in the samples taken from colonial ancient individuals (Figure S1A; Table S1).
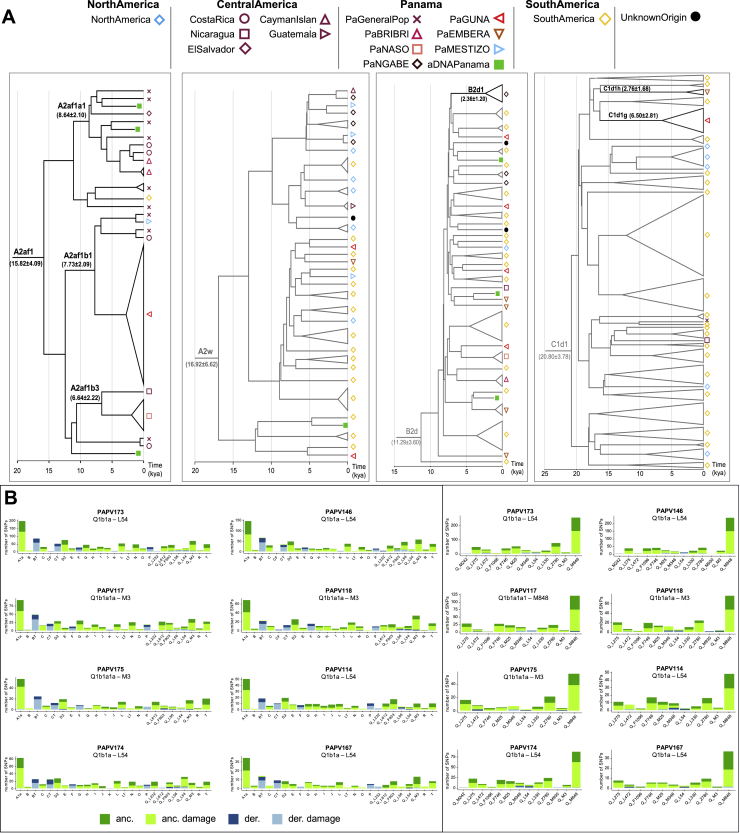
Figure S1.
Analyses of uniparental markers, related to Figure 5B and Table S1
(A) Schematic phylogenetic trees of the four major mtDNA haplogroups identified in the Isthmian area. The Bayesian phylogenetic trees, rooted on an L2c2 mitogenome from a “Moreno” individual, include all modern mitogenomes belonging to the four major haplogroups of modern and ancient Isthmian individuals (A2af1, A2w, B2d, C1d1). Black lines highlight branches specific to IA from the Isthmo-Colombian area. The Bayesian age (mean value with standard deviation) is shown for relevant branches. (B) Ancient Y chromosome classification. SNPs for each macro-haplogroup present in Poznik et al., 2016. In the right panel, SNPs for each sub-haplogroup Q in Grugni et al., 2019 and Pinotti et al., 2019. Different colors refer to the allele status (green: ancestral; blue: derived), while different shades indicate the aDNA possible damage. Haplogroup nomenclature as in ISOGG 2019.
The most represented mtDNA haplogroup of pre-Hispanic Panamanians, A2af1, was previously identified (as A2af) at high frequencies among present-day Panamanians, mainly in the Comarca of Guna Yala (Perego et al., 2012). It is characterized by the so-called “Huëtar deletion,” a peculiar 6-bp control-region deletion initially detected in the Chibchan-speaking Huëtar from Costa Rica (Santos et al., 1994).
The eight pre-contact Y chromosomes are positive for the L54 marker, which characterizes all the Indigenous American branches of haplogroup Q (Figure S1B). Two individuals (PAPV118, PAPV175) were further sub-classified as Q1b1a1a-M3 and one (PAPV117) as Q1b1a1a1-M848, the most frequent haplogroups among Indigenous peoples of the Americas (Grugni et al., 2019; Pinotti et al., 2019).
Archaeological and anthropological significance of two burials in Panamá Viejo and Coco del Mar
An initial evaluation of the ancient low-coverage genomes made it possible to address long-standing anthropological and archaeological questions regarding the possible genetic relationships among individuals buried together. These cases included the ten human remains (one adult, nearly complete, female skeleton with nine adult male skulls beneath and around her) recovered from a pre-Hispanic burial denominated Tumba 1 underneath the Plaza Mayor of Panamá Viejo, and a similar burial at Coco del Mar (approximately 1 km to the west of Panamá Viejo), where a female skeleton was found accompanied by three male crania (Figure 1B). Crania interred with prestigious individuals have been interpreted as evidence either of ancestor veneration or of human sacrifice with the ostentation of trophy heads (Mendizabal, 2004; Smith-Guzmán and Cooke, 2018). Arguments in either case draw on presumed (recent or ancestral) tribal and biological relationships. Using genome-wide data, we can now exclude any genetic relatedness among the individuals. Moreover, the two females exhibit different mtDNA haplogroups (D1 and B2, respectively) with respect to the surrounding male crania (A2af1a1, B2b, and B2d in Tumba 1; A2af1b, A2w, and B2d in Coco del Mar).
In combination with these genetic results, radiocarbon dates obtained for the pre-Hispanic individuals sampled from Panamá Viejo and Coco del Mar point toward a more complex and nuanced interpretation. The two female figures, PAPV109 (1265-1375 CE) and PAPV172 (1051-1221 CE), were interred with crania dated from 603 to 1,390 CE (2 sigma) in the first case and from 657 to 1,430 CE (2 sigma) in the second (Figure 1B). Hence, skulls spanning over 700 years, including the area’s earliest and latest pre-Hispanic remains recovered to date, accompanied each of the main individuals. Seven of the skulls that accompanied PAPV109 belonged to individuals who pre-dated her by hundreds of years, and the other two were roughly contemporary. One of the crania buried with PAPV172 belonged to a male individual who lived roughly 500 years before her, and the other two to male individuals deceased and buried over the subsequent 300 years. Drawing upon on Cueva as well as Guna ethnography (Castillero Calvo, 2017: pp. 26, 87, 281, 282, 476–478; Fernández de Oviedo, 1853: Vol. 2, pp. 125–154; Fortis, 2013), crania kept for hundreds of years or even deposited after the main interment probably pertained to enemy chiefs whose death in battle guaranteed their spirits’ eternal repose. Their skulls may have provided sorcerers and healers, in this case female seers or tequina, a gateway to knowledge about enemies as well as the afterlife. These women entered the next world with the tools of their trade (the skulls), like other individuals interred in Panamá Viejo (a “musician” poised as if playing her instrument, and a seated adolescent with flint blades and stingray tail barbs) or mentioned in Spanish chronicles (farmers buried with corn) (Fernández de Oviedo, 1853: pp. 125–154). The heads of prestigious enemies obtained in warfare would have facilitated the seers’ access to their strength and knowledge as well as their ability to communicate with other worlds. The different mtDNA lineages of these individuals might also support their origins from various pre-Hispanic groups, since we have found significant differences in the haplogroup distribution among the modern Indigenous populations analyzed here (p value < 0.0001). Although the literature contains reference to wives, slaves, and loyal servants sacrificed with their chiefs (Fernández de Oviedo, 1853; Romoli, 1987), these burials illustrate a different practice. Their arrangement stands out among a great diversity of pre-Hispanic interments over a wide zone of the Panamanian Pacific, some of these including offerings in local pottery, metal, lapidary, shell, and bone work, and many burial modes, including bones deposited in urns or bundles (Martín, 2002a, 2002b). PAPV172’s head was found beneath a ceramic offering, while five ceramic pots with offerings accompanied PAPV109, who also wore a necklace fashioned from thorny oyster shells (Spondylus spp.). Within the variety of pre-Hispanic burial patterns observed to date, individuals PAPV109 and PAPV172 appear unique on a local as well as a regional level.
Deciphering genomic variation on the Isthmus
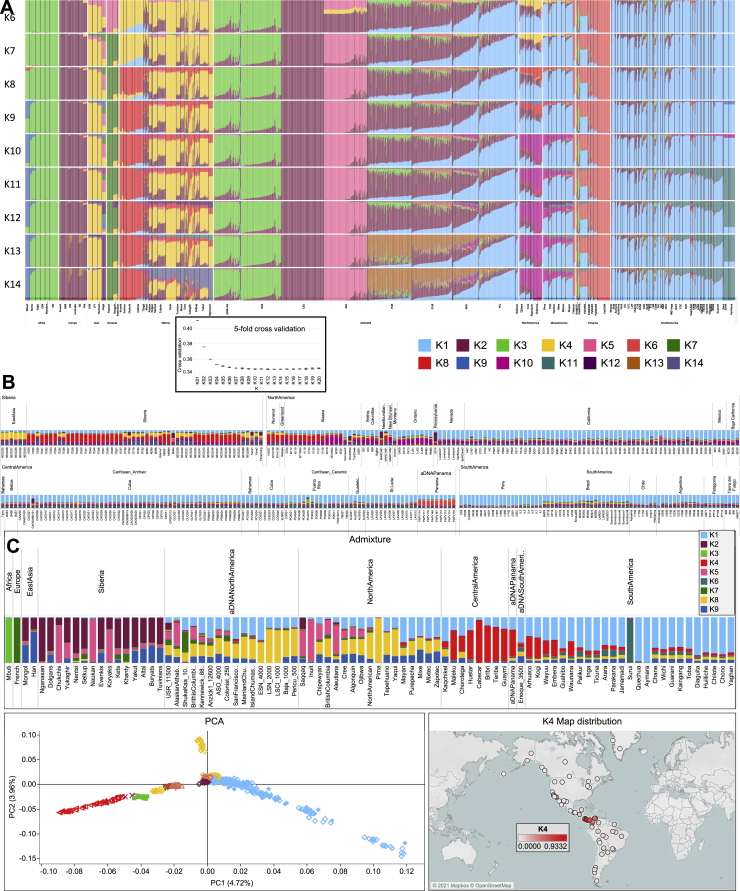
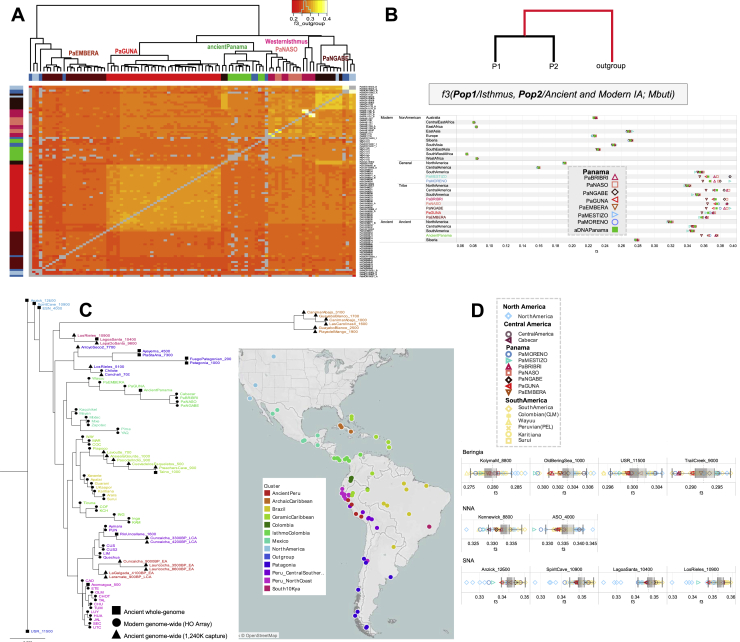
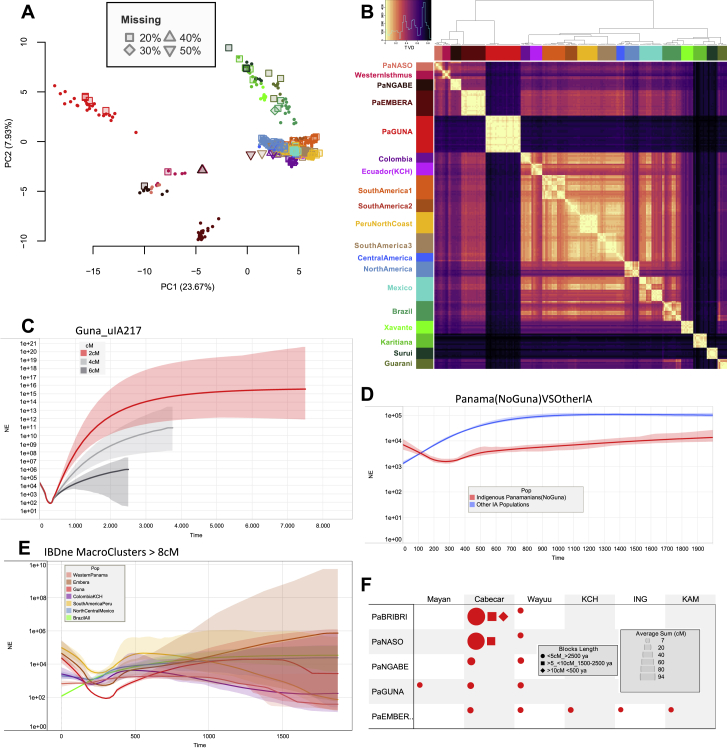
The analyses conducted in this study facilitate a microgeographic and diachronic assessment of Indigenous autosomal variation in this strategic region. The characterization of the pre-colonial genetic histories is clouded by the impacts of colonization. This is evident in the distinctive genetic profiles that differentiate the current gene pool of the Panamanian groups as obtained by the ADMIXTURE analyses (Figures 2A, S2A, and S2B). The two groups that experienced a history of admixture, the self-identified “Moreno” and “Mestizo,” reveal large proportions of their genomes not derived from Indigenous peoples of the Americas. Both show a comparable proportion of ancestry predominant in Europeans (K2) and the component common to Africans (K3) is more prevalent in the “Moreno” group; the “Mestizo” are characterized by a component also identified in Asians (K4). The coexistence of different continental genomic ancestries is common in the Americas, due to complex admixture that started during the colonial period (Ongaro et al., 2019). In Panama, where European colonization began in 1,502 CE, this is particularly evident in the “Mestizo,” but it is also revealed by individuals who self-identified as Indigenous and genealogically unadmixed, showing variable amounts of African and European ancestries in their genomes, with the lowest average values in the Guna, followed by the Ngäbe.
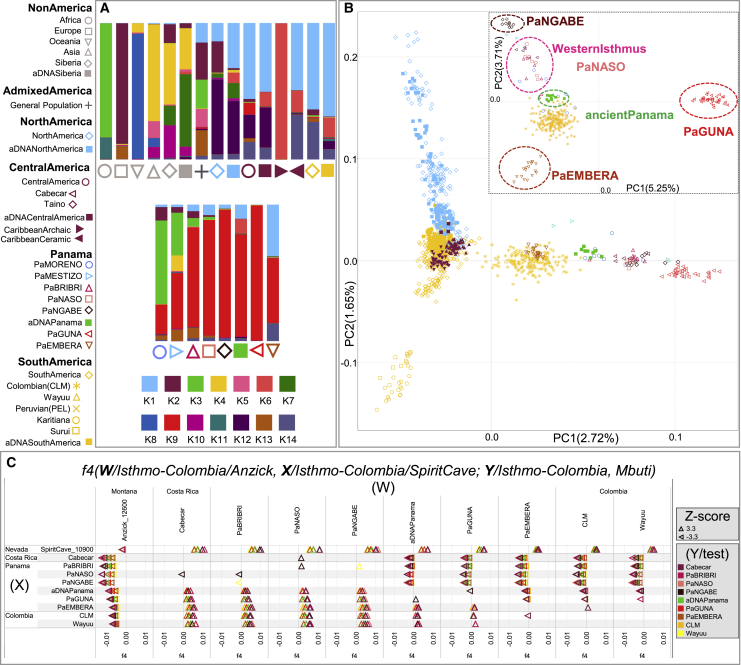
Figure 2.
Overview of the genetic structure of ancient and modern Isthmian groups
(A) ADMIXTURE plot for K = 14; each bar shows the average ancestry proportion of individuals within the same group considering the rWD1560 dataset plus the American and Siberian ancient individuals.
(B) Indigenous American (IA) PCA analysis including the mIA417 dataset and ancient genomes projected onto uIA217 variability. The inset shows a specific Isthmo-Colombian PCA.
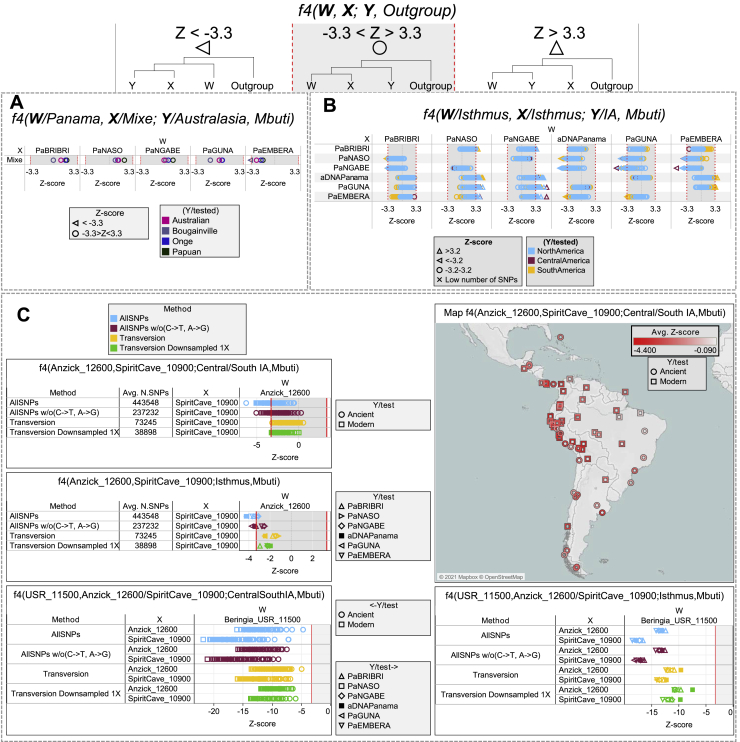
(C) f4 statistics in the form f4 (W/Isthmo-Colombia/Anzick, X/Isthmo-Colombia/SpiritCave; Y/Isthmo-Colombia, Mbuti) considering the uIA89 and mIA417 datasets plus ancient Isthmian individuals (all SNPs). The f4 values are reported in abscissa. Each tested population (Y) is shown (with triangles pointing to X or W population) only when the initial conformation of the tree is rejected (p value ∼ 0.001, for Z scores > |3.3|), thus visually pointing to the closer population (X or W) in each comparison. A legend for symbols used in the paper is reported on the top left.
Figure S2.
Worldwide ADMIXTURE plot on modern and ancient individuals, related to Figure 2
(A) ADMIXTURE analyses were performed from K1 to K20 on the modern rWD1560 dataset, even if only profiles from K6 to K14 are displayed. The inset shows the boxplot of 5-fold cross validation (CV) values for Ks from 1 to 20 after 10 runs. The median (most typical) values were plotted indicating 25th and 75th percentiles (dark and light gray, respectively) and arms extending 1.5 times the IQR (interquartile range). (B) ADMIXTURE analysis projecting ancient Siberian and American individuals on the modern worldwide variability. (C) ADMIXTURE plot and PCA performed on a comparative dataset (genotyped with Illumina chips) from Scheib et al., 2018 that includes the following Chibchan-speaking populations: Arhuaco and Kogi from Colombia; Guaymi, Cabecar, Teribe, Bribri, Huetar and Maleku from Costa Rica (Table S3). The K4 distribution map is also shown.
The modern and ancient Isthmian individuals are also characterized by a specific Indigenous component, which has been identified considering only modern individuals (K6, Figure S2A) as well as with the addition of ancient individuals (K9, Figure 2A). This component drives the Isthmo-Colombian axis, depicted by the first (main) component in the principal-component analysis (PCA) of Indigenous groups (Figure 2B), which includes ancient and modern Panamanians together with the Cabécar from southern Costa Rica and two populations from northern Colombia (the Wayuu and the admixed Columbia [CLM]). The Indigenous groups from the pre-colonial Greater Chiriquí cultural area form two closely related western clusters (one with the Ngäbe and the other including Bribri, Naso, and Cabécar). The pre-Hispanic individuals group together in the middle of the Isthmo-Colombian genetic landscape and create a distinct branch in the outgroup f3 statistics hierarchical tree, together with a few self-identified “Moreno” (Figure S3A), suggesting the integration of pre-Hispanic individuals into the forming multi-cultural colonial groups. The genetic closeness of the pre-Hispanic individuals is possibly expected when considering the geographic proximity of the archaeological excavations but less expected when taking into account the radiocarbon dates, from 603 to 1,430 CE, thus revealing a genetic continuity for almost one thousand years (Table S1). The modern Indigenous populations from the Greater Darién area of cultural influence to the east of the region (Guna, Emberá, and northern Colombians) create distinct groups in the PCA plot, well separated from the Greater Chiriquí populations in the west.
Figure S3.
f3 statistics involving Isthmian individuals, related to Figures 2, 3, and 6
(A) Heatmap based on outgroup f3-statistics. The shared drift among the Panamanian individuals was analyzed considering those included in uIA217 plus ancient individuals and masked data in mIA417. Color intensity is inversely proportional to the shared ancestry among individuals, which was used to build the dendrogram. (B) Outgroup f3 statistics where ancient and modern Isthmian groups (Pop1) were compared to worldwide populations (Pop2) including non-American groups in the rWD1560 dataset, all populations in the mIA417 and uIA217 datasets and all ancient individuals. All comparisons have a Z score > 32.912. The average f3 value for each population is reported in abscissa. (C) The neighbor-joining tree is built using the inverse values derived from the outgroup f3 statistics on all Central and South American populations pairs plus Anzick-1, Early San Nicolas (ESN), Spirit Cave and USR-1. The latter is considered as an outgroup in the tree. We retained only populations with more than 30K overlapping SNPs and significant Z scores (> 3.3) in all comparisons. The map shows the geographic distribution of the populations, which are colored according to their genetic proximity in the tree. (D) We also analyzed the shared genetic history of modern IA populations (included in mIA417 and uIA89) against ancient reference genomes from Beringia and the Americas (representative of the NNA and SNA ancestries). Boxplots in gray help to visualize the distribution of f3 values in each comparison, indicating the median (most typical) value, 25th and 75th percentiles (dark and light gray, respectively), and arms extending 1.5 times the IQR (interquartile range). The dotted line indicates the f3 average value.
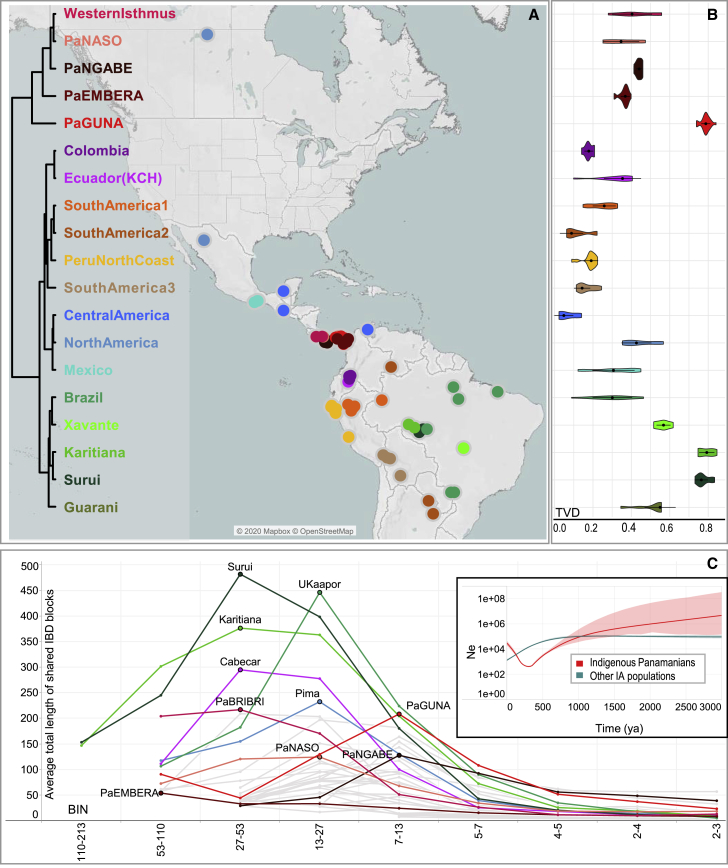
The details of this genetic sub-structure (and heterogeneity) on the Isthmus became apparent by analyzing the nearly unadmixed Indigenous haplotypes (uIA217 dataset) with fineSTRUCTURE (Figures 3A and S4A). Among the five Isthmian genetic clusters, four are specific to Indigenous Panamanian groups (PaNASO, PaNGABE, PaEMBERA, PaGUNA), while the Bribri individuals form a separate cluster (here called Western Isthmus) with the Cabécar from Costa Rica. The latter branch, together with Naso and Ngäbe, forms a macro-group that might be associated with the geographic region of the pre-colonial Greater Chiriquí cultural area. Genetically distinct are the Emberá and the Guna, suggesting a wider genetic variation in the Greater Darién cultural region. The Guna also show the highest level of similarity in intra- and inter-cluster comparisons (Figures 3B and S4B), analogous only to two Indigenous groups that experienced isolation events, the Amazonian Surui and Karitiana, and preserved an ancient Australasian-related ancestry, the so-called UPopY (Moreno-Mayar et al., 2018; Skoglund et al., 2015). We formally looked for UPopY variants in the Isthmus with f4 statistics in the form f4 (W/Panama, X/Mixe; Y/Australasia, Mbuti) without finding any significant sign of admixture or gene flow (Figure S5A). The same statistics were used to formally test the average correlation in allele frequency differences (mixture of ancestries) within the Isthmo-Colombian area (Figure 2C). This analysis provides statistical support to the genetic interactions (Z score often < |3|) in the western Isthmian area, eventually extended to Cabécar, Naso, and Ngäbe. On the other hand, it reveals close relationships among the Emberá and northern Colombians (CLM and Wayuu). Finally, the pre-Hispanic communities inhabiting the Pacific coast in the area of Panama City cluster together with the Guna compared with other Isthmo-Colombian populations (Z score < |3|), except for the Ngäbe.
Figure 3.
Population genetic structure as revealed by haplotype analysis of modern Panamanian and IA populations
(A) fineSTRUCTURE unrooted dendrogram showing the 19 identified Indigenous clusters and the geographic distributions of the individuals in the nearly unadmixed IA (uIA217) dataset.
(B) Violin plot showing cluster self-copy lengths (fragments copied from members of their own cluster) in the uIA217 dataset; higher values are for more isolated groups.
(C) Density of the intrapopulation average total length of shared IBD blocks, considering nine bins of IBD lengths in the Panamanian and non-Panamanian Indigenous groups of the uIA217 dataset. The inset shows the estimation of changes in effective population size (Ne) over time based on IBD segments with a minimum threshold of 2 cM (even if estimates older than ∼1,000 years should be considered with caution) and considering a generation time of 25 years.
Figure S4.
Haplotype-based analyses and estimates of effective population size variation over time, related to Figure 3
(A) The PCA was built using copying vectors inferred using a modified version of ChromoPainter that allows for the presence of missing data. The masked individuals (rmIA311) have been projected on the variability of the nearly unadmixed individuals (uIA217) regardless of the level of missing data. The unadmixed individuals are indicated with full filled dots while the masked ones are represented by different shapes, according to the percentage of missing SNPs. The colors refer to the clusters (donors) of Figure 3A. (B) Heatmap based on individual TVD (Total Variation Distance) values. Dendrogram branches are colored according to 19 clusters (Figure 3A). The TVD was compared both among and within clusters. Lighter colors (lower TVD values) in the matrix mean similarity, while darker colors (higher TVD values) indicate heterogeneity.
(C) We used RefinedIBD on inferred IBD length to estimate variations in the effective population size (Ne, on a log scale y axis) over time. The x-axes show the time before the present as years ago (ya) considering a generation time of 25 years and the colored regions show 95% bootstrap confidence intervals (CI). The analyses were limited to the last ~2,000 years, due to the wide variance of exponentially distributed IBD fragments and were performed on different datasets. The Guna group was evaluated considering different IBD thresholds (2 cM, 4 cM and 6 cM). (D) We double-checked the trend presented in the inset of Figure 3C without the Guna. (E) We also compared the IBDne of the Panamanian macro-clusters with the others identified in Figure 3A, Western Panama: Western Isthmus, PaNASO, PaNGABE; Emberà: PaEMBERA; Guna: PaGUNA; Colombia KCH: Colombia, Ecuador (KCH); South America Peru: SouthAmerica1, SouthAmerica2, Peru North Coast, SouthAmerica3; North Central Mexico: Central America, North America, Mexico; Brazil all: Brazil, Xavante, Karitiana, Surui, Guarani. (F) Visualization of the average of summed IBD lengths shared between modern Panamanians and other IA populations in each paired comparison, with identified IBD blocks in the range of 1–5 cM (oldest), 5–10 cM, and over 10 cM (youngest). Shape sizes are proportional to mean values; only those pairs sharing at least two blocks > 5 cM and four < 5 cM are plotted.
Figure S5.
f4 statistics involving Isthmian individuals, related to Figures 2 and 4
(A) f4 statistics in which Panamanian populations (W) were compared to Mixe (X), typically used to reveal UPopY among IA, and to four Australasian populations (Y). (B) Isthmian groups (W) were compared to each other (X) testing for other IA populations (Y, colored according to their geographic location) to test through the Z score whether a given Isthmian group carries excess of a specific IA ancestry. (C) To specifically test the differential relationships of Isthmian and other Central/South IA groups with Anzick-1 and Spirit Cave, we ran the f4 -statistics in the form f4 (Anzick1, Spirit Cave; Central and South IA, Mbuti) and reported the average Z score on a map. The Isthmian populations were also tested separately. In the lower part of the panel, we verified the same relationships in the form f4 (USR, Anzick1/Spirit Cave; Central and South IA, Mbuti), using USR as outgroup to the Central and South IA populations (again the Isthmian populations were tested separately, plot on the lower right). The datasets uIA89, mIA417 and ancient individuals were used considering different sets of variants.
Deciphering genomic connections outside the Isthmus
Previous studies (Gnecchi-Ruscone et al., 2019; Moreno-Estrada et al., 2013; Reich et al., 2012) have already provided hints of genetic patterns in the Isthmo-Colombian region, eventually extended to other groups that speak Chibchan languages. Here, we first confirmed that the previously discussed Isthmian component is also detectable in additional Chibchan-speaking populations genotyped with a different array (Arhuaco and Kogi from Colombia; Guaymí, Cabécar, Teribe, Bribri, Huëtar, and Maleku from Costa Rica) and that its highest legacy can be detected in the western Isthmian land bridge consisting of the present-day countries of Panama and Costa Rica (Figure S2C). We have now detailed patterns of genomic variation of the area’s core population(s), represented by pre-Hispanic Isthmian individuals and modern Panamanians, underlining their unique features in the Americas’ genetic landscape.
The f-statistics tests not only detected higher levels of shared genetic history between ancient and present-day groups of the Isthmian area extended to Costa Rica (Cabécar) and northern Colombia (CLM and Wayuu) in comparison with other ancient and modern populations (Figures 4 and S3B) but also revealed different levels of shared ancestries in the Isthmian groups (Figure S5B). The same pattern has been detected in the neighbor-joining tree (based on outgroup f3 statistics and rooted with an ancient Beringian genome; Figure S3C) of ancient and modern IA groups. This graph identifies an early Isthmo-Colombian branch with different sub-branches. This and the additional IA branches (with a geographic pattern) largely overlap with the haplotype-based clusters identified in the American-wide uIA217 dataset (Figure 3A), where in the absence of data from ancient individuals, the distinctiveness of the Isthmo-Colombian area in the modern America genomic structure remains evident.
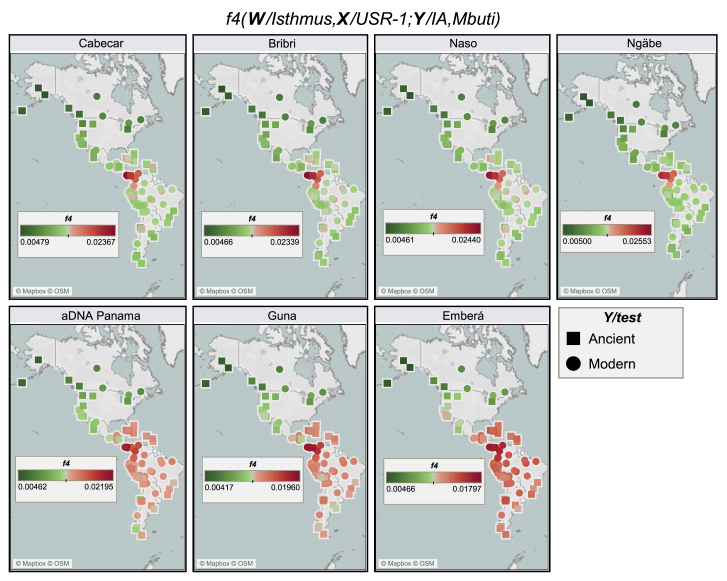
Figure 4.
Heatmaps based on f4 statistics to compare the differential relationships between the Isthmian groups and other ancient/modern IAs
f4 statistics in the form f4 (W/Isthmus, X/USR-1; Y/Ancient and Modern IA, Mbuti). Each tested population (Y) is represented by squares (ancient) and circles (modern) with colors proportional to f4 values; Z score always >3.3.
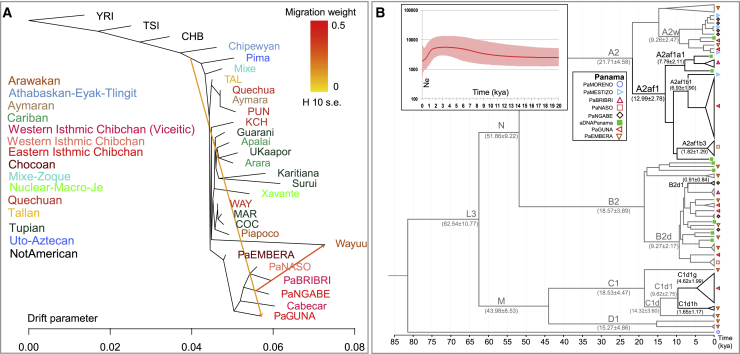
A pre-Hispanic origin of the Isthmo-Colombian distinctiveness is suggested by our analysis of effective population size based on the identical-by-descent (IBD) segments. A reduction in the population size of the Panamanian groups probably began during pre-colonial times (∼1 kya; inset of Figure 3C), thus before the average time of the other IA population groups taken together. This trend is mainly driven by the Guna, who on the other hand show unreliable population size (Ne) values when considering short fragments (<8 cM, Figure S4C). Therefore, we repeated this analysis without the Guna, confirming an earlier beginning of the Ne reduction in the Panamanian IA groups that became steeper in colonial times (Figure S4D). Finally, Ne estimates based on IBD longer (and more reliable) fragments (>8 cM) allowed us to confirm this trend for the Guna (Figure S4E). Three factors may have contributed to this demographic pattern: (1) a decrease in the population size that started before European contact; (2) a less intensive impact of European colonialism, as also suggested by the lower peaks of IBD bins for most Panamanian groups; and (3) an earlier and steeper demographic recovery after contact than in other IA populations, especially evident in Guna and Ngäbe who show an enrichment of shorter IBD segments, around 7–13 Mb, and then a fast decrease of IBD blocks (Figure 3C). All explanations are plausible and not mutually exclusive. Before European contact, cultural changes in the Panama Bay region may have accompanied migrations and demographic shifts (see “Panama: archaeology and history” and, in the STAR Methods section, “Insights into pre-Hispanic Panama”). This possibility finds support from other genetic analyses that showed a high level of genomic drift shared by the Isthmian Indigenous groups, including the ancient individuals sampled, on both allele frequencies and haplotypes (see PCA plots and all the trees), as well as from the comparison of the IBD fragments shared among the Panamanian groups and with other IA populations (Figure S4F). The latter analysis reveals ancient interactions (from at least 2,500 years ago) within the Isthmo-Colombian area, much stronger and temporally extended among the western populations (Cabécar, Bribri, and Naso) currently living in the geographic region associated with the pre-colonial Greater Chiriquí cultural area. On the eastern part of the Isthmus, the Guna also show a number of short (older) blocks shared with the Maya from Mexico, while the Emberá share shorter blocks with South American populations. Thus, the former probably received ancient genetic inputs from the north, while the latter admixed with external southern sources. The Guna also show a direct connection with the ancestors of North and Central American populations in the TreeMix maximum likelihood (ML) tree when two migration edges (gene flows) are added (Figure 5A). Such an ancient legacy is also confirmed by the Panamanian mtDNA tree. The most-represented haplogroups among ancient and modern Panamanian mitogenomes belong to the four main pan-American founding lineages (A2, B2, C1, and D1; Figure 5B). We also identified four Isthmo-specific sub-branches, the most represented one (A2af1) is dated at 15.82 ± 4.09 kya (Figure S1A). Finally, the Bayesian skyline plot (BSP) of Panamanian mtDNAs shows an increase in population size starting in the early Holocene (∼10 kya) (inset of Figure 5B).
Figure 5.
Schematic phylogenetic trees based on genome-wide and mtDNA data
(A) Inferred maximum likelihood tree built with TreeMix on the unadmixed dataset uIA89 allowing two admixture edges (migration events). Population groups are colored according to linguistic/geographic affiliation. Horizontal branch lengths are proportional to the amount of genetic drift that has occurred on the branch. Migration arrows are colored according to their weight.
(B) Bayesian phylogenetic tree of ancient and modern mitogenomes from Panama belonging to IA founding haplogroups. It was rooted on an L2c2 mitogenome from a “Moreno” individual. The Bayesian age (mean value with standard deviation) is shown for relevant branches. Black lines highlight Isthmo-Colombian-specific branches. The inset shows the Bayesian skyline plot (BSP), based on complete mitogenomes, displaying changes in the effective Ne through time considering a generation time of 25 years.
A previously undescribed ancestry among ancient Indigenous peoples of the Americas?
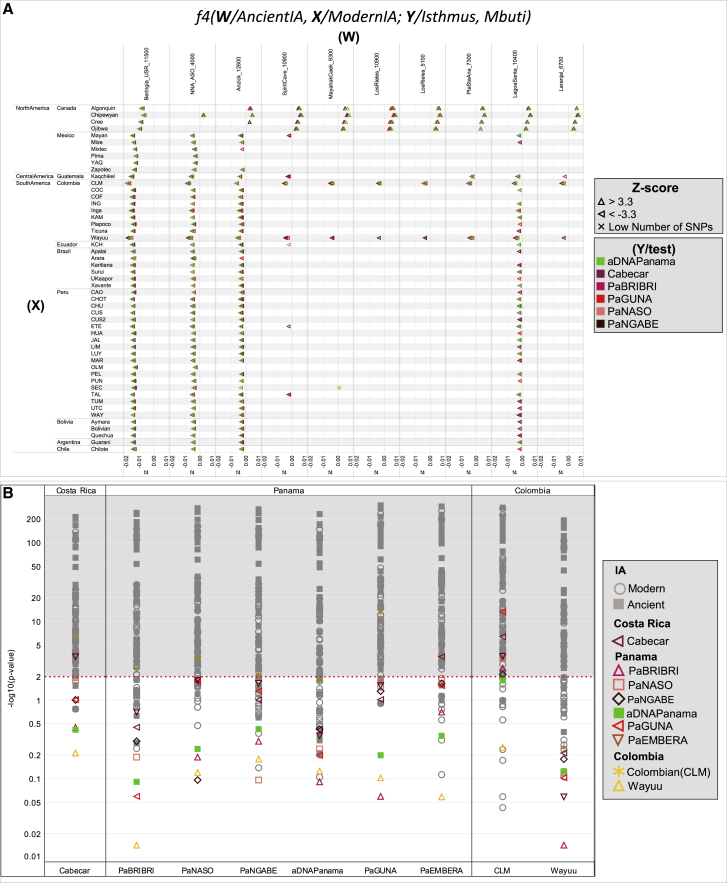
To further understand the peculiarities of the Isthmo-Colombian populations within the context of the most updated archaeogenomic scenario of non-Arctic America (see Introduction), we used f4 statistics, which controls for possible biases deriving from population-specific drift, to compare ancient individuals and contemporary Indigenous groups to the individuals we sampled. As expected, the Isthmus shows an excess of allele sharing with modern and ancient Indigenous populations from Central and South America when compared with Ancestral Beringia (USR-1, Upward Sun River, Alaska, ∼11.5 kya) and NNA (represented by ASO, Ancient Southwestern Ontario, ∼4.2 kya) genomes, but this picture is more intricate when dealing with the SNA-related ancient genomes (Table S4). The affinities between the (Y/tested) Isthmian populations and other Indigenous (X) groups are significantly stronger in relation to Anzick-1 (Montana, ∼12.8 kya) than to Spirit Cave (Nevada, ∼10.9 kya) (W individuals; Figure 6A), suggesting that Isthmian populations are related to Spirit Cave as much as to other Indigenous groups, while Anzick-1 is an outgroup to them. We directly tested the relationships of Isthmian and other Central/South American populations with Anzick-1 and Spirit Cave, highlighting a differential trend that becomes significant with a higher molecular resolution power, i.e., more single-nucleotide polymorphisms (SNPs) (Figures 2C and S5C). Moreover, the Isthmus seems more closely related to Spirit Cave than to Anzick-1 in comparison with Ancestral Beringia.
Figure 6.
f4 statistic tests on Isthmian and other IA groups and minimum number of ancestral sources
(A) f4 statistics in the form f4 (W/Ancient IA, X/Modern IA; Y/Isthmus, Mbuti) on uIA89, mIA417, and ancient genomes considering only transversions. Only sub-groups of meaningful ancient genomes were considered (see Table S4 for comparisons with the entire ancient dataset). The Emberá group was excluded due to a significant degree of admixture detected in the individuals. Each tested population (Y) is shown (with triangles pointing to X or W population) only when the initial conformation of the tree is rejected (p ~ 0.001, when the Z score is >|3.3|), thus visually pointing to the closer population (W or X) in each comparison.
(B) We used qpWave to compare (in pairs) ancient Panama and present-day Isthmian groups with all IA populations (considering rank 1). Outgroups were kept to the minimum and chosen to represent different IA ancestries identified here (STAR Methods) and in other papers. Rank 1 refers to a model in which all paired populations fit as derived from two ancestral sources, relative to the outgroups. A p value > 0.01 (< 2 in −log10 scale, dotted red line) means pairs that could be explained by a single independent source.
Such genomic differences are confirmed when moving southward in Central America (Table S4) and particularly for the early ancient genomes excavated on the southern continent. The Pacific coast populations (Los Rieles, Chile, ∼10.9 kya) exhibit greater affinity to Spirit Cave, while the ancient genomes from the Atlantic side show the same pattern as Anzick-1 when considering individuals older than ∼7 kya (Lagoa Santa, Brazil, ∼10.4 kya). These distinctive signals persisted up to about 7 kya, when they were probably erased by a major population turnover in South America (Moreno-Mayar et al., 2018; Posth et al., 2018), facilitated by a widespread population decline due to mid-Holocene climate changes (Riris and Arroyo-Kalin, 2019).
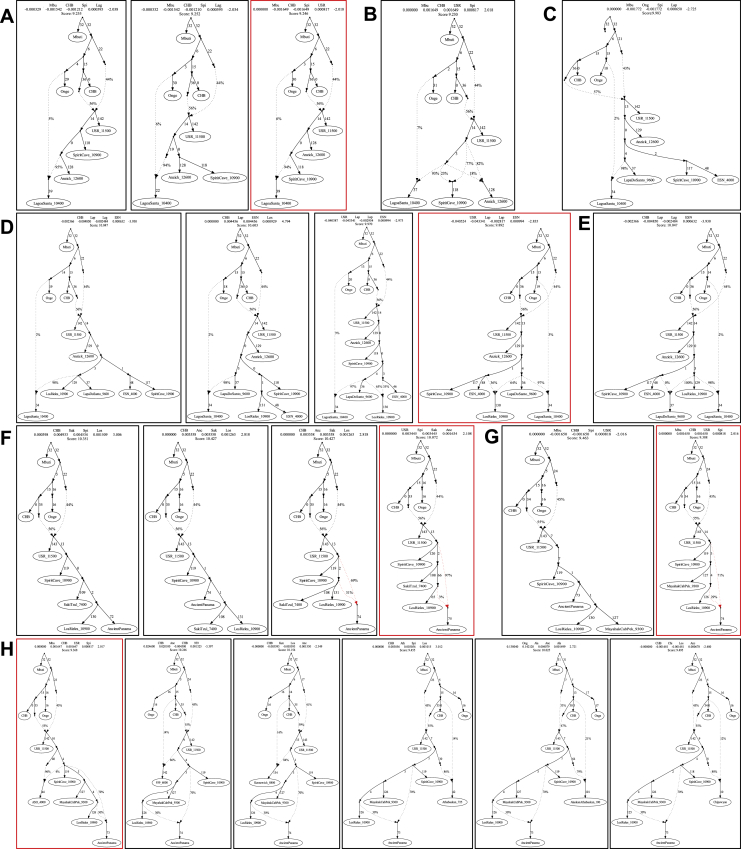
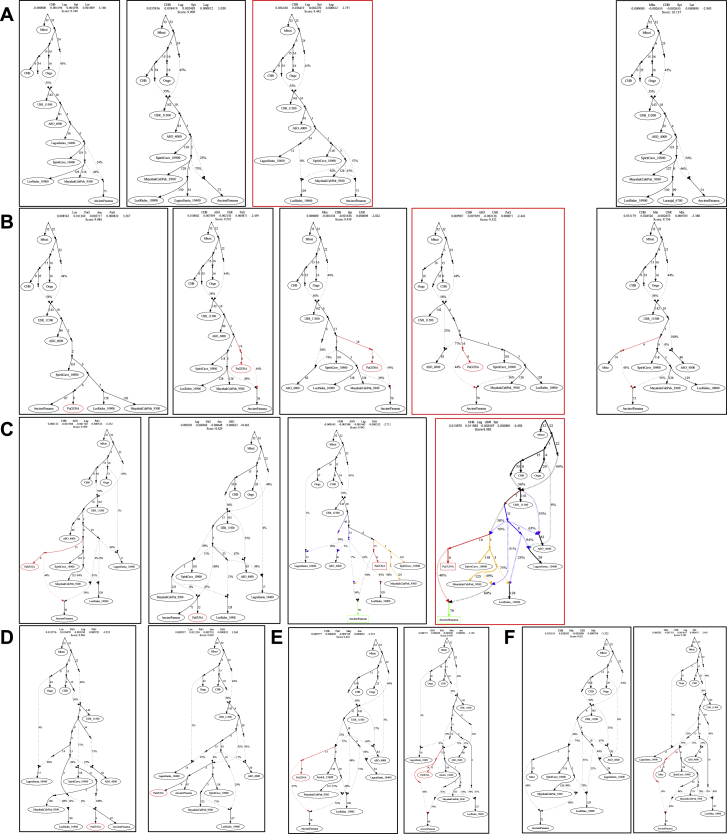
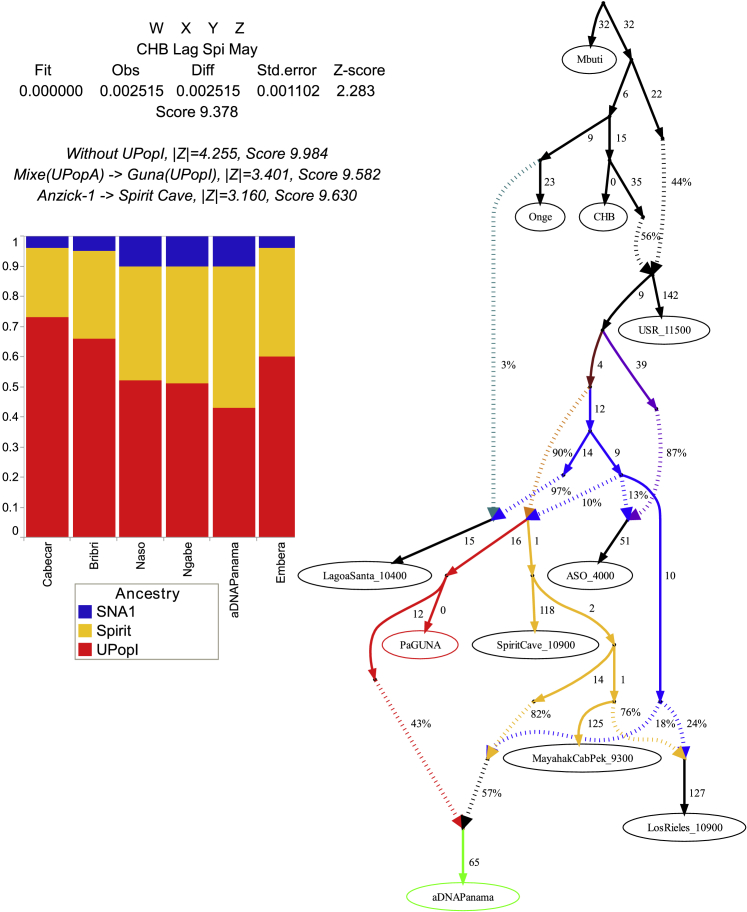
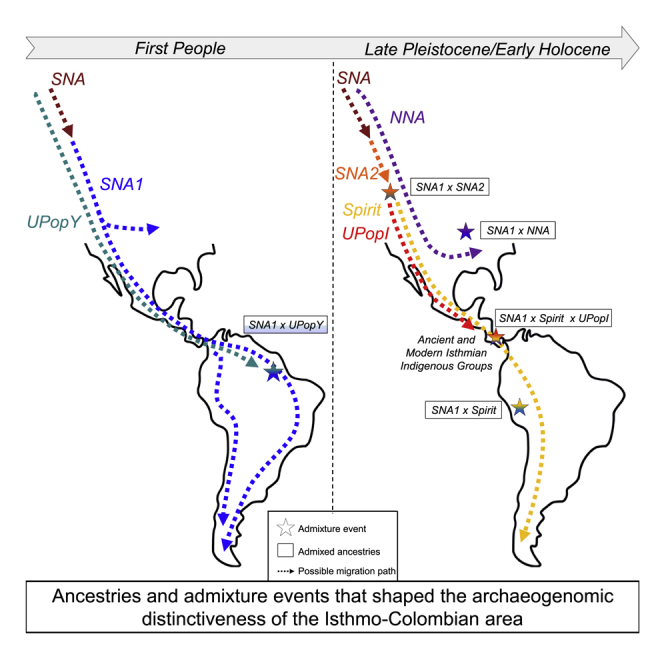
The results of previous analyses revealed that Isthmian and non-Isthmian IA populations are differentially related to available Pleistocene individuals, suggesting the contribution of different sources. To test whether the Isthmian and non-Isthmian groups derived from the same or distinct ancestral populations, we used qpWave (Patterson et al., 2012), which estimates the minimum number of sources necessary to explain the observed genetic composition of population groups. Significance values are consistent with pairs of Isthmian and non-Isthmian groups deriving from at least two separate streams of ancestry, as attested by rank 1 p value < 0.01 in most comparisons, especially for the Guna (Figure 6B). This finding demonstrates that the distinctiveness of the Isthmo-Colombian area cannot be explained by genetic drift alone, as recently inferred in other population contexts (Nägele et al., 2020). The Guna also show lower values (mostly <25th percentile) of shared genetic history with ancient genomes representative of well-known Indigenous ancestries than the average of the one shared by other IA populations (Figure S3D). Therefore, we modeled admixture graphs looking for the most plausible origin of the ancestral source(s) of the Isthmian component (Figures S6 and S7; see also the specific section of STAR Methods for further details). The best supported topology successfully tested the hypothesis that the ancestral gene pool of the Isthmo-Colombian area, here represented by pre-Hispanic Panamanians, derives from a local admixture between different ancestral components (Figure 7). One derives from the differential mixture of two ancestries, SNA1 and SNA2, that in turn stem from an ancestral SNA source. This scenario is strongly suggestive of the first split between SNA and NNA occurring in Beringia, thus further north than generally proposed (Waters, 2019). The NNA ancient individual in Figure 7, ASO (∼4.2 kya), results from an admixture between NNA and SNA1. We could not identify an unadmixed proxy for the NNA ancestry among the available modern and ancient individuals (Figure S6H), but NNA does not seem to be involved in shaping the Isthmian genomic pool. Founding populations carrying the SNA1 ancestry probably took part in an early peopling of the double continent passing through the Isthmus and leaving signals on both sides of South America, as attested by two of the most ancient genomes, Lagoa Santa in Brazil (∼10.4 kya) and Los Rieles in Chile (∼10.9 kya). The former also confirms a few traces of UPopY, the UPop of Australasian ancestry, which was previously proposed to have contributed to the early peopling of South America (Moreno-Mayar et al., 2018; Skoglund et al., 2015). On the other hand, only Los Rieles shows significant inputs of the SNA2 ancestry, which moved later or slower than SNA1 through the Americas, admixing multiple times with the first settlers along the way, as demonstrated by the ancient admixed genomes of Spirit Cave in North America and Mayahak Cab Pek (Belize, ∼9.3 kya) and the ancient Isthmians (this study) in Central America. Once SNA2 reached South America, it probably left a stronger contribution on the Pacific side, as suggested by Los Rieles and supported by the differential pattern depicted by the f4 statistics (Table S4). However, to fully explain the genetic variation of pre-Hispanic Panama, we need to consider an additional ancestry: an ancestral unsampled population of the Isthmus (UPopI; Figure 7), which is still unrepresented in the ancient dataset, but left its strongest traces in the contemporary Guna. UPopI parallels the Spirit Cave branch of SNA2, testifying to a long-shared drift between the pre-Hispanic Guna and the ancient Isthmian (including pre-Hispanic) individuals sampled. This best-fitting model was also checked without considering UPopI and replacing Spirit Cave with Anzick-1 and Guna with Mixe, previously used to identify UPopA (Moreno-Mayar et al., 2018), without finding any statistically supported graph (Figures S7D–S7F). Therefore, it is supported that Anzick-1 and Spirit Cave might represent different ancestries and UPopI is a still unsampled population distinct from UPopA. UPopI likely originated in the north during the late Pleistocene, as attested by the age (∼15 kya, Figure S1A) of the entire mtDNA haplogroup A2af1 that probably represents its mitochondrial legacy, and expanded more than 10 kya in the Isthmo-Colombian area (according to mitogenome data; inset of Figure 5B). These ancestries left different traces in pre-Hispanic Isthmian individuals and contemporary Panamanian groups (Figure 7), and only the presence of UPopI provides significant support for our final model. It also explains the ancestral component, already seen in the ADMIXTURE analyses, which is geographically restricted to the Isthmo-Colombian area and prevalent in the Guna, where it was probably maintained by a high level of isolation. Finally, this specific ancestry could explain the “archaeogenomic distinctiveness” of the Isthmo-Colombian area within the genomic landscape of IA groups.
Figure S6.
Admixture graphs modeling ancient SNA and NNA genomes and ancient Isthmians, related to Figure 7
(A) Basal tree with three of the most ancient SNA genomes available. The best fitting topology, highlighted in red, was initially tested by (B) considering an early admixture between the northern American SNA genomes, then extended by adding in turn: (C) Lapa do Santos and ESN; (D) Los Rieles, tested as either unadmixed or admixed, and then checking Lapa do Santos as admixed (E); ancient Isthmians together with other ancient Central American genomes, i.e., (F) Saki Tzul and (G) Mayahak Cab Pek; (H) NNA genomes, from the left to right, ASO, 939, Kennewick, Athabaskan_725, Athabaskan_100 and Chipewyan. The best fitting topologies are highlighted in red. See the legend of Figure 7 for further details.
Figure S7.
Admixture graph modeling Panama’s genetic history linked to ancient SNA and NNA genomes, related to Figure 7
Possible extensions of the best trees in Figure S6 by linking Ancient Isthmians to (A) Lagoa Santa and then testing Laranjal instead of Lagoa Santa (rightmost graph). Finally, we modeled Guna as representative of UPopI (B-C). The best tree topology is similar to the one in Figure 7, but with multiple splits from the SNA1 node. This tree as well as the final one (Figure 7) were checked multiple times: (D) considering Mixe (UPopA) instead of Guna (UPopI), also in the rightmost tree of the panel B; (E) replacing Spirit Cave with Anzick-1; (F) without UPopI or without admixture between UPopI and other SNA ancestries. The best fitting topologies are highlighted in red. See the legend of Figure 7 for further details.
Figure 7.
Admixture graph modeling ancestries and affinities of Isthmian groups in America
Best fitting f-statistics-based admixture graph optimized using qpGraph. We modeled the genetic history of ancient Isthmian individuals and the Guna directly linked to ancient IA genomes representative of the SNA ancestries. At the top, we show the f4 statistics with the worst Z score after optimizing the model. Statistics on alternative models are also listed (see Figure S7 for further details). Numbers to the right of solid edges represent optimized drift parameters and percentages to the right of dashed edges represent admixture proportions. Different colors indicate the specific ancestries discussed in the text. The bar chart shows different ancestry proportions in ancient and modern Isthmian groups (except for Guna) estimated with qpGraph on the final tree (Z score always < |2.5|).
Discussion
Our work enriches the IA genomic database with autosomal data from cultural groups of Panama and ten low-coverage pre-Hispanic genomes obtained from human remains excavated in the tropical area of Panama City on the Pacific Ocean. The ancient genetic profiles from Panamá Viejo and Coco del Mar sites, radiocarbon dated from 603 to 1,430 CE, confirm similarities in the gene pool of this pre-Hispanic population(s), suggesting common origins. The diachronic comparison with population groups presently living in Panama allowed us to identify genetic similarities with the Guna and Ngäbe and suggestive connections with some admixed individuals, implying that, in the wake of the conquest, there was extensive gene flow. A genetic sub-structure has been identified in the entire Isthmo-Colombian region, with a macro-group encompassing the Cabécar, Bribri, Naso, and Ngäbe, who currently live in the pre-colonial Greater Chiriquí cultural area. A wider genetic variation characterizes eastern Panama, here represented by pre-Hispanic individuals from Panamá Viejo and Coco del Mar plus Guna, Emberá, and northern Colombians. Our analyses suggest that pre-Hispanic demographic changes and isolation events, evident in the Guna, contributed to create the genetic structure currently seen in the region. Moreover, through allele frequency analyses and haplotype-based reconstructions, we describe the presence of a specific axis of Indigenous genetic variation in the Americas, which is typical in the Isthmo-Colombian area and possibly extended to other Chibchan-speaking groups. This component was present not only among pre-Hispanic Isthmian individuals but also strongly characterizes current Panamanian groups, particularly the Guna, surviving both pre-colonial demographic fluctuations and the genetic bottleneck (and admixture) caused by colonialism.
The detection of this component has an impact that expands far beyond the Isthmo-Colombian area and the ancestry of its past and current inhabitants. The following clues point to the scenario that it arose in the late Pleistocene: (1) the pre-Clovis age of the Isthmian-specific mtDNA haplogroup A2af1, (2) the internal structure that emerges when only the Indigenous genome-wide variation is analyzed, (3) the longer shared genetic history among Isthmo-Colombian populations with respect to other Indigenous populations, and (4) the differential relationships with Pleistocene individuals from North America. Next, to identify its ancestral source(s), we built a statistically significant model that explains this Isthmo-Colombian component as a local admixture of different ancestries of northern North American origin. At least two SNA ancestries, SNA1 and SNA2, differentially associated to available Pleistocene genomes, should be considered, as well as an additional Isthmian-specific ancestry. The latter requires the contribution of UPopI, which stemmed from the same source (SNA2) that contributed to the pre-Clovis groups with Western Stemmed technologies associated with Spirit Cave and, according to mitogenome data, expanded within the Isthmo-Colombian area during the early Holocene.
The ancestral admixtures described here were probably bound to now-submerged archeological sites on the Pacific coast of the Isthmus. Nevertheless, the genomes of the pre-Hispanic individuals from Panamá Viejo and Coco del Mar attest to these events, and the site of Vampiros-1 (initially named Cueva de los Vampiros), the only Pleistocene site on the lower Isthmian land bridge that contains cultural, but not human, skeletal remains, provides further archeological support. Vampiros-1 shows evidence of both Clovis and Fluted Fishtail Point lithic traditions indicating that hunter-gatherers of extra-Isthmian origin were on the lower Isthmus 13.2–11.7 kya with specific composite weaponry and cutting/scraping tools (Ranere and Cooke, 2020). Our model also fits well with recent archeological records from both sides of the Isthmo-Colombian area. Archeological findings in southern North America report early peopling as far south as central northern Mexico around the Last Glacial Maximum (LGM) (Ardelean et al., 2020) and more widespread settlements in warmer pre-Clovis times (14.7–12.9 kya) (Becerra-Valdivia and Higham, 2020). The cultural heterogeneity observed among the oldest reliable pre-Clovis archaeological sites of South America (dated 15.1–14.0 kya) along the Pacific coastal zone (Huaca Pietra in Central Andes; Monte Verde II in South Andes) (Dillehay et al., 2017) and in the Pampas (Arroyo Seco 2) (Politis et al., 2016) can be explained considering a deeper chronological time (between 16.6 and 15.1 kya) for the Isthmian crossing that led to the initial peopling of South America (Prates et al., 2020).
The preservation effect of an ancient legacy in “outlier populations,” such as the Guna, recalls that of Sardinians and Basques in Europe (Achilli et al., 2004; Chiang et al., 2018; Novembre et al., 2008; Olivieri et al., 2017; Palencia-Madrid et al., 2017). In the European context, Sardinians maintained the most evident traces of the early European Neolithic farmers (Lazaridis et al., 2014, 2016; Raveane et al., 2019). Among Indigenous peoples, some Amazonian groups, which match the very high internal similarities of the Guna, have preserved the specific ancestry of a UPop linked to Australasia (Moreno-Mayar et al., 2018; Skoglund et al., 2015). In the Isthmian context, demographic events connected to both pre-Hispanic and colonial times contributed to differentially retain and further shape the IA ancestries.
Limitations of study
The models reported in this study are based on 12 ancient low-coverage genomes from two archeological sites and genome-wide data from 74 unrelated modern Panamanians. These data provide a suggestive baseline for future interdisciplinary studies on the Isthmo-Colombian crossroads. High-coverage genomic data, from a wider time frame (since early Holocene to colonial times) and other archeological sites (across the entire Isthmo-Colombian area), as well as whole genomes from present-day individuals, are needed to continue to refine the region’s genetic history. This additional work, with more statistical power and higher molecular resolution, will be essential to further detail the genetic patterns (and ancestries) that we have identified in the Panamanian population(s) and to reconstruct variation in population sizes over time.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Ancient skeletal element | This study | PAPV26 |
| Ancient skeletal element | This study | PAPV27 |
| Ancient skeletal element | This study | PAPV52 |
| Ancient skeletal element | This study | PAPV53 |
| Ancient skeletal element | This study | PAPV57 |
| Ancient skeletal element | This study | PAPV61 |
| Ancient skeletal element | This study | PAPV93 |
| Ancient skeletal element | This study | PAPV106 |
| Ancient skeletal element | This study | PAPV109 |
| Ancient skeletal element | This study | PAPV114 |
| Ancient skeletal element | This study | PAPV117 |
| Ancient skeletal element | This study | PAPV118 |
| Ancient skeletal element | This study | PAPV137 |
| Ancient skeletal element | This study | PAPV146_FE |
| Ancient skeletal element | This study | PAPV146_TP |
| Ancient skeletal element | This study | PAPV156 |
| Ancient skeletal element | This study | PAPV167 |
| Ancient skeletal element | This study | PAPV172 |
| Ancient skeletal element | This study | PAPV173 |
| Ancient skeletal element | This study | PAPV174 |
| Ancient skeletal element | This study | PAPV175 |
| Chemicals, peptides, and recombinant proteins | ||
| NEBNext Multiplex Oligos for Illumina (Index Primers Set 1) | New England Biolabs | E7335L |
| NEBNext Multiplex Oligos for Illumina (Index Primers Set 2) | New England Biolabs | E7500L |
| NEBNext Multiplex Oligos for Illumina (Index Primers Set 3) | New England Biolabs | E7710L |
| NEBNext Multiplex Oligos for Illumina (Index Primers Set 4) | New England Biolabs | E7730L |
| NEBNext Multiplex Oligos for Illumina (Dual Index Primers Set 1) | New England Biolabs | E7600 |
| NEBNext Multiplex Oligos for Illumina (Methylated Adaptor, Index Primers Set 1) | New England Biolabs | E7535L |
| NEBNext Multiplex Oligos for Illumina (96 Index Primers) | New England Biolabs | E6609L |
| NEBNext High-Fidelity 2X PCR Master Mix | New England Biolabs | M0541L |
| Phusion High-Fidelity PCR Master Mix with HF Buffer | New England Biolabs | M0531L |
| Blunt-TA Ligase Master Mix | New England Biolabs | M0367 |
| Buffer PE (concentrate, 100 ml) | QIAGEN | 19065 |
| Buffer EB (250 ml) | QIAGEN | 19086 |
| TE buffer, pH 7.6, RNase free (250 ml) | Carlo Erba | LJ62285AK |
| Tween∗ 20 | Carlo Erba | ABP337-500 |
| Sodium Acetate | Carlo Erba | 478166 |
| Nuclease-free water | Promega | P1193 |
| Bleach 100% | Aurogene | AU1125 |
| Isopropanol | Carlo Erba | 415154 |
| EDTA 0.5 M | Carlo Erba | LJ62786AK |
| Proteinase K | Merck | 3115852001 |
| 10% N-lauryl sarcosine | Merck | 8147150100 |
| Guanidine hydrochloride | Carlo Erba | A120232500 |
| GoTaq(R) Long PCR Master Mix | Promega | M4021 |
| Critical commercial assays | ||
| Axiom Genome-Wide Human Origins 1 Array | Thermo Fisher Scientific | 901853 |
| NEBNext Ultra DNA Library Prep Kit for Illumina | NEB-Euroclone | BE7645S |
| MinElute Reaction Cleanup Kit | QIAGEN | 28206 |
| Qubit dsDNA HS Assay Kit | Thermo Fisher Scientific | Q32851 |
| MiSeq Reagent Kit v2 | Illumina | MS-102-2002 |
| Nextera XT DNA Library Preparation Kit | Illumina | FC-131-1096 |
| Maxwell RSC Stabilized Saliva DNA Kit | Promega | AS1630 |
| Software and algorithms | ||
| OxCal | Ramsey and Lee, 2013 | https://c14.arch.ox.ac.uk/oxcal.html |
| CutAdapt | Martin, 2011 | https://github.com/marcelm/cutadapt |
| FastQC | Andrews, 2010 | https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ |
| BWA | Li and Durbin, 2010 | http://bio-bwa.sourceforge.net/ |
| Picard MarkDuplicates | http://broadinstitute.github.io/picard | http://broadinstitute.github.io/picard |
| MapDamage2.0 | Jónsson et al., 2013 | https://ginolhac.github.io/mapDamage/ |
| ANGSD | Korneliussen et al., 2014 | https://github.com/ANGSD/angsd |
| READ | Monroy Kuhn et al., 2018 | https://bitbucket.org/tguenther/read/src/master/ |
| KING | Manichaikul et al., 2010 | http://people.virginia.edu/∼wc9c/KING/ |
| PLINK1.9 | Purcell et al., 2007 | https://www.cog-genomics.org/plink/2.0/ |
| ADMIXTURE | Alexander et al., 2009 | http://dalexander.github.io/admixture/index.html |
| RFMix | Maples et al., 2013 | https://sites.google.com/site/rfmixlocalancestryinference/ |
| CircularMapper | Peltzer et al., 2016 | https://github.com/apeltzer/CircularMapper |
| SAMtools | Li et al., 2009 | http://samtools.sourceforge.net/ |
| BCFtools | Li et al., 2009 | http://samtools.github.io/bcftools/bcftools.html |
| VCFtools | Danecek et al., 2011 | http://vcftools.sourceforge.net/ |
| HaploGrep2 | Weissensteiner et al., 2016 | https://github.com/seppinho/haplogrep-cmd |
| GATK | McKenna et al., 2010 | https://gatk.broadinstitute.org/hc/en-us |
| BEAST | Bouckaert et al., 2019 | http://beast.community/ |
| Tracer | Rambaut et al., 2018 | http://tree.bio.ed.ac.uk/software/tracer/ |
| FigTree | http://tree.bio.ed.ac.uk/software/figtree/ | http://tree.bio.ed.ac.uk/software/figtree/ |
| Yhaplo | Poznik, 2016 | https://github.com/23andMe/yhaplo |
| EIGENSOFT | Patterson et al., 2006 | https://github.com/DReichLab/EIG |
| CLUMPAK | Kopelman et al., 2015 | http://clumpak.tau.ac.il/ |
| DISTRUCT | Rosenberg, 2004 | https://rosenberglab.stanford.edu/distruct.html |
| AdmixTools | Patterson et al., 2012 | https://github.com/DReichLab/AdmixTools |
| TreeMix | Pickrell and Pritchard, 2012 | https://bitbucket.org/nygcresearch/treemix/wiki/Home |
| qpGraph | Patterson et al., 2012 | https://github.com/DReichLab/AdmixTools |
| SHAPEITv2 | Delaneau et al., 2011 | https://mathgen.stats.ox.ac.uk/genetics_software/shapeit/shapeit.html |
| CHROMOPAINTERv2 | Lawson et al., 2012 | http://www.paintmychromosomes.com/ |
| fineSTRUCTURE | Lawson et al., 2012 | http://www.paintmychromosomes.com/ |
| Refined-IBD | Browning and Browning, 2013 | http://faculty.washington.edu/browning/refined-ibd.html |
| IBDne | Browning et al., 2018 | http://faculty.washington.edu/browning/ibdne.html |
| ContaminationX | Moreno-Mayar et al., 2020 | https://github.com/sapfo/contaminationX |
| ContamMix | Fu et al., 2013 | - |
| Schmutzi | Renaud et al., 2015 | https://github.com/grenaud/schmutzi |
| Rx | Mittnik et al., 2016 | Mittnik et al., 2016 |
| Qpwave | Reich et al., 2012 | https://github.com/DReichLab/AdmixTools |
| Deposited data | ||
| Mitogenomes of Modern Individuals | GenBank | MW467798-MW467881 |
| Genotype Data of Modern Individuals | Mendeley Data | https://doi.org/10.17632/d45xg84bcj.1 |
| Sequencing Data of Ancient Individuals | European Nucleotide Archive | PRJEB42372 |
See specific file.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Alessandro Achilli (alessandro.achilli@unipv.it).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The accession number for the ancient DNA sequencing data reported in this paper is ENA: PRJEB42372. Modern genotype data have been deposited to Mendeley Data: https://doi.org/10.17632/d45xg84bcj.1. The accession numbers for modern complete mitogenomes reported in this paper are GeneBank: MW467798-MW467881. Scripts used to infer Y chromosome aDNA haplogroups are available on GitHub (https://github.com/raveancic/aDNAYchromosome), all the other scripts used for analysis and plots are available upon request.
Experimental model and subject details
Insights into pre-Hispanic Panama: details on archaeology, history, and linguistics
From ca. 4.5 kya agriculture along Panama’s Pacific watershed was complemented by fishing in rivers and estuaries, and catching birds, large snakes and iguanas (Iguanidae spp.), and hunting mammals with body masses < 55 kg on the offshore Pearl Island archipelago (Cooke et al., 2016). Some of these, e.g., white-tailed deer (Odocoileus virginianus), raccoons (Procyon lotor) and rodents including agouti (Dasyprocta punctata) and paca (Cuniculus paca), forage in human trash and eat crops in gardens and fields (Cooke et al., 2007, 2008; Martínez-Polanco et al., 2020). On Pedro Gonzalez (Pearl Islands), a very small deer (Mazama sp.) (7-10 kg) was extirpated by hunting. Dolphins (Tursiops and Delphinus) were consumed 6.2-5.6 kya and were possibly killed when beached (Cooke et al., 2016). Later, with the establishment of villages ca 4-2 kya, community activities diversified, especially regarding the exchange of goods. Hunting strategies now included communal drives in Pacific wooded savannas. Deer meat was stored salted and dried and then served at special feasts (Martínez-Polanco and Cooke, 2019).
Inhabitation of the Caribbean side of the Central American Isthmus (Costa Rica) began in the Late Pleistocene (13.4-12 kya) (Ranere and Cooke, 2020). In the very humid central Caribbean Panama, human activities date back to 5.9 kya when groups crossed the Central Cordillera to collect food and materials not available on the opposite side, e.g., embalming agents.
On the central Caribbean, maize is found in rock shelters with earth ovens from about 3.5 kya. Materials analysis demonstrates that Monagrillo pottery found here was manufactured in the central Pacific watershed (Griggs, 2005; Iizuka et al., 2014). One site in the coastal lowlands of Coclé province (Zapotal, PR-32), which used Monagrillo pottery, consisted of dwellings stratified within a shell-bearing midden accumulated between 4.3 and 3.2 kya. Zapotal has the characteristics of a small village. There are very large numbers of edge-ground cobbles here, which were used at many 8-3 kya sites in Panama for grinding several plant foods including maize and manioc. The abundance of small fish (< 300 g live weight) that were taken at Zapotal points to the use of tidal traps and weirs in the nearby estuary in order to maximize biomasses (Zohar and Cooke, 2019).
By 3 kya notable differences had arisen in the material culture of a western region (Greater Chiriquí; Chiriquí and Bocas del Toro provinces) and a central region (Greater Coclé; Coclé, Azuero Peninsula and Veraguas provinces). Greater Chiriquí shows material culture, art, genes and language that are broadly consistent (Ulloa, 2017), in distinction to the Greater Coclé or the even more diverse, but less studied, Greater Darién.
Unique ceremonial and religious precincts stand out in both the Chiriquí and Coclé cultural areas, although they are markedly different: Barriles in highland Chiriquí (Linares et al., 1975), and the twin sites of Sitio Conte and El Caño in the Pacific Coclé lowlands. The ceremonial site at Barriles consists of low platforms, boulder petroglyphs, urn burials, large statues depicting one man sitting on another’s shoulders–in an apparent display of social dominance–and an enormous maize-grinding stone (metate) showing explicit iconographic connections between maize and human fertility (Linares et al., 1975). Maize, beans (Phaseolus spp,), and palm and tree fruits characterize the samples of carbonized plant remains in the middens in domestic areas (Dickau, 2010; Smith, 1980).
The Barriles ceremonial precinct seems to have served many communities located between 1,000 and 2,300 m above sea level in the shadow of the Barú volcano, which last erupted 0.5 kya (Holmberg, 2007). It is inferred that Barriles was the initial settlement of a cultural group that first entered this highland zone from elsewhere ca 2.8 kya. Friction led to fission. A sector of the colonizing population moved to Cerro Punta and henceforth maintained vacillating relations with its ancestors (“peace, trade, war”). Communion among the entire descent group was not severed. Periodically, perhaps annually, festivals were held at ancestral Barriles. The feats of the founders and supernatural helpers were celebrated. Large quantities of alcoholic beverages (e.g., maize and palm sap) were likely brewed.
The well-studied heritage of Greater Coclé, which reached its apogee at the great ceremonial and burial precincts of Sitio Conte and El Caño 1.5–0.95 kya (Lothrop, 1937, 1942; Mayo Torné, 2015), confirms a continuity of iconography and symbolism on decorated pottery from 2.5 kya until two decades after Spanish conquest in this central region. But whether this area was also linguistically united cannot yet be determined.
Cultural geography becomes even more complex in the Greater Darién area extending from the El Valle Pleistocene volcano to eastern Darién. Historians and most archaeologists agree that by 1,500 CE the eastern region, and possibly much of the central area, was inhabited by speakers of the Cueva language. Historian Kathleen Romoli and linguists Jacob Loewen and Adolfo Costenla have proposed for many years that the group of settlements that at the time of Spanish conquest spoke the “language of Cueva” were not an “ethnic group” but, rather, a collection of settlements that shared the Cueva language as a lingua franca. These three researchers also argue that some polities in fact spoke variants of languages in the Chocoan family, especially those on the Pacific side of the Isthmus (Costenla, 2012; Loewen, 1963; Romoli, 1987). Chibchan languages probably derive from a proto-language that coalesced about 10 kya (O’Connor and Muysken, 2014) in a “core area” on the lower Central American Isthmus (southern Costa Rica and western central Panama). Ever since Barrantes et al. (1990) conducted an isozyme-based study of modern Central American Indigenous polities that spoke languages in the Nuclear Chibchan family (Costenla, 2012), it has been apparent that this population coalesced very early in the Holocene and at the onset of agriculture gradually experienced in situ fission and fusion.
Ceramics found in eastern Panama point to greater proximity to the Greater Coclé tradition than a putative Gran Darién cultural sphere among the peoples who inhabited this region from 200 BCE to 1200 CE. Recent findings on the Pearl Islands archipelago confirm the expansion of the ceramic style known as Cubitá, as well as molded and incised variations of the Conte style, both from the central region of Gran Coclé in the gulf of Panamá (Martín et al., 2016). Biese had already suggested that this expansion reached Panamá Viejo, where he reported examples of the Conte style excavated around Puente del Rey, toward the north of the site (Biese, 1964).
At the Miraflores site on the Banks of the Bayano River (Cooke, 1976; Oyuela-Calcedo and Raymond, 1998), 670–1015 CE and 700–1030 CE according to the latest 2 sigma calibrations (Martín et al., 2016), only one piece made in the region exhibited painted decorations with obvious influences from Greater Coclé: a plate with a tripod pedestal with the effigy of a monkey (Cooke, 1998). The same pattern of cultural replacement is documented on the Pearl Islands archipelago, where the islands’ Fifth Ceramic Horizon is identified from 750 through 1350 CE (Martín et al., 2016). This late ceramic configuration, which features incised decoration and molding procedures different from its immediate precedents, is that which archaeologists normally associate with Greater Darien, the region that sixteenth-century Spanish chroniclers described as populated by communities that spoke the “Cueva language” from Urabá to the eastern slope of the El Valle volcano (Martín, 2002b; Romoli, 1987).
Martín et al. (2016) argue that the discrepancy between the Greater Darien and Greater Coclé cultural areas, whose geographical extension may have shifted over time, could derive from a change in the population inhabiting the Pearl Islands archipelago: the group using Cubitá ceramics and Conte variations may have ceded before the entry of a population with a very different ceramic tradition from the Darien region and related to northwest Colombia. Another hypothesis (Cooke, 1998; Martín and Sánchez, 2007; Sánchez-Herrera and Cooke, 2000) relates the changes observed to a reorganization of the commercial routes and exchanges from 500 CE, which intensified after 800-900 CE, with the introduction of metallurgy to the isthmus and the replacement of shell artifacts with those of gold to represent high social status. From at least the beginning of the common era through the end of its first millennia, the dispersion of Cubitá ceramics allude to the fact that the Pearl Islands archipelago, the Azuero Peninsula and the central coast of the bay of Panama participated in the same sphere of social interaction. This relationship changed completely in the subsequent period, and until European contact.
The uneven impact of European colonization and the upheaval it induced, in addition to subsequent migrations, show that comprehensive and detailed studies of this time period need to be completed in this region to help trace population histories of Indigenous groups before and after 1500 CE. Although goods also crossed the isthmus in pre-colonial times, the process intensified after the early sixteenth century, when the Spanish established settlements on both sides of the Isthmus to forge a highway for the global transit (and often forced mobility) of persons and goods between the Atlantic and Pacific Oceans.
Ancient Individuals and archaeological information
In order to assess change and continuity in the most radically transformed area of the Isthmus, the under-studied eastern region, 20 ancient individuals (21 specimens in total, sometimes referred as ancient Panamanians for the sake of clarity) were collected from seven different archaeological excavations (six of these within today’s Patronato Panamá Viejo, and one at nearby Coco del Mar) (Figure 1A; Table S1). Based on the style of the ceramics recovered, archaeologists consider the area related to contiguous contemporary settlements as part of an extended pre-Hispanic presence of Cueva-speakers within the Greater Darien cultural region (Martín, 2002b). This population coexisted and mixed with Spanish settlers from 1519 to 1541 Common Era (CE), as an Indigenous presence among Old Panama’s earliest Christian burials suggests. The excavations sampled took place in and near Panamá Viejo, the site of the colonial city from 1519 through 1671 CE, and an area of pre-Hispanic occupation before that.
1. Plaza Mayor (N = 4)
The burial site excavated in the Plaza Mayor, originally identified as Tumba 1 (Figure 1B), contained the remains of a female individual with a spondylus necklace and surrounded by offerings that included nine male crania. Scholars have awaited genetic research in order to test the hypothesized relationships regarding the individuals buried within this tomb since its discovery in 1996 (Mendizabal, 2004). DNA was successfully extracted from the main individual (PAPV109) and three of the nine skulls (PAPV114, 117 and 118) around her. PAPV109 did not meet quality standards to be included in autosomal analyses (Table S1).
2. Plaza Casas Oeste (N = 1)
In the Plaza Mayor’s Casas Oeste, the remains of 35 pre-Hispanic individuals were found in different positions, including extended burials, as well as urns and packages of bones. DNA of one of these individuals (PAPV137), radiocarbon dated 898-1014 (2 sigma), a male individual of at least 15 years at death, extended yet leaning toward the right side, with his skull pointed northwest, was extracted in this work.
3. Catedral (N = 5)
Human remains excavated from Old Panama’s Cathedral in Panamá Viejo (PAPV52, 53, 57) and its courtyard (PAPV61, 93) in 2000 reflect the African and European presence, as well as a mixed Indigenous inheritance, in the city from 1542 to 1671 CE (Hernández Mora et al., 2021).
4. Sur de la Plaza (N = 2)
Two post-contact individuals analyzed here were excavated from Sur de la Plaza (PAPV26, 27) and dated 1519-1541 CE, based on the historical and archaeological evidence.
5. Parque Morelos (N = 4)
Other pre-Hispanic burials were excavated from the Parque Morelos (roughly 1 km to the west of the Plaza Mayor) in 2001-2003. These excavations uncovered the remains of two pre-Columbian residential structures, including evidence of post-holes, pottery, grinding stones, seashell beads, fragments of three bone flutes and a frog-shaped gold pendant. Within a few meters from the residential structures, urns and “packages” with cranial and long bones belonging to children and adults (PAPV146 and 156, dated 776-966 and 1264-1289 CE, respectively, and the first one with DNA extracted from two different bones), were also recovered (Martín, 2002a, 2006). An additional individual was excavated in an extended primary burial (PAPV167, 779-985 CE, 2 sigma) some 50 m from the residential structures.
6. Centro de Visitantes (N = 1)
Another pre-Hispanic individual was extracted from an extended burial excavated by Juan Martín in 2001 at the Centro de Visitantes site, close to Parque Morelos, in the west part of Panamá Viejo (PAPV106), and dated 894-986 CE (2 sigma). Five ceramic offerings accompanied this female individual, of approximately 30-35 years at death.
7. Coco del Mar (N = 4) located outside Panamá Viejo
The additional site where pre-Hispanic human remains were uncovered in 2005 was in an adjacent residential area, Coco del Mar, one kilometer to the west of the Morelos statue. In this case, the burial is characterized by a particular conformation (Figure 1B), where a primary, female individual (PAPV172) was discovered with three male crania (PAPV173, 174, 175) and additional pottery as offerings (Martín et al., 2008), similar to that of Plaza Mayor’s Tumba 1.
Modern Individuals
In 2010, Indigenous polities encompassed about 12% of the 3.4 million inhabitants of Panama. The most numerous are individuals who identify as the Ngäbe (62.3%), Guna (19.3%) and Emberá (7.5%), followed by smaller polities such as the Chocoan-speaking Wounaan (the Noanomá of Spanish chronicles), and the Buglé, Bribri, and Naso Djërdi (formerly known as Teribe). The contemporary population also includes important numbers of self-identified Afro-Panamanians (“Morenos”) and individuals of mixed Hispano-Indigenous (“Mestizo”) ancestry.
Modern sampling took place in Panama City as well as in the provinces and Indigenous territories. A total of 84 biological samples were collected in Panama from healthy adult (> 18 years old) individuals, almost equally distributed between females and males (41 and 43, respectively), and belonging to different Indigenous groups using NorgenBiotek kits (Figure 1A; Table S1). During sample collection, genealogical information (for at least two generations), cultural affiliation, and spoken language were also gathered from each subject.
Method details
Collagen extraction and 14C-dating
The individuals PAPV106, PAPV109, PAPV114, PAPV117, PAPV118, PAPV137, PAPV146, PAPV156, PAPV167, PAPV172, PAPV173, PAPV174 and PAPV175 were analyzed by accelerator mass spectrometry (AMS) at Mannheim (Table S1) together with additional individuals from the same sites, i.e., PAPV110, PAPV111, PAPV112, PAPV113, PAPV115, PAPV116, PAPV139, and PAPV 169, dated between 990 and 1,385 CE (https://artempire.cica.es/archeo/list), with an overall time span from 603 to 1,430 CE.
Compact bone portions were cut and the surfaces removed. About 1 g of sample was placed in glass tubes, demineralized in 10 mL of 0.5 N HCl at initially 4°C and later at room temperature for 14 days, rinsed to neutrality and reacted with 10 mL of 0.1 M NaOH for 24 h at 4°C, rinsed again to neutrality and gelatinized in 4 mL of acidified H2O (pH 2-3) for 48 h at 75°C. Insoluble particles were separated using EZEE filter separators. Ultrafiltration (molecular mass > 30 kD) removed the short-chained collagen and concentrated the long-chained collagen, which was frozen and lyophilized.
The collagen was combusted and the relative amounts of carbon (C) and nitrogen (N) determined using an elemental analyzer (Elementar Inc., MicroCube). The produced CO2 was reduced to graphite using either a custom-made, semi-automated graphitization unit or a fully automated and commercially available unit (IonPLus Inc., AGE3). The resulting graphite powder was compressed into aluminum targets and subsequently analyzed using a MICADAS-type AMS-system (IonPlus Inc.) (Kromer et al., 2013).
The isotopic ratios 14C/12C and 13C/12C of samples, calibration standard (Oxalic Acid-II), blanks and control standards were measured simultaneously in the AMS. 14C-ages were corrected for isotopic fractionation to δ13C = −25‰ (Stuiver and Polach, 1977) using the 13C/12C AMS-values and calibrated using the dataset INTCAL13 (Reimer et al., 2013, 2020) and software SwissCal (L.Wacker, ETH-Zürich). Calibration graphs were generated using the software OxCal (Ramsey and Lee, 2013).
Ancient DNA processing
Different bones (femur, humerus and petrous bone) and teeth were available from ancient individuals for DNA extraction (Table S1), which was carried out in the dedicated clean rooms at the Carl R. Woese Institute for Genomic Biology, University of Illinois, following published protocols (Dabney et al., 2013; Lindo et al., 2017; Pinhasi et al., 2015; Scheib et al., 2018). Bones or teeth were soaked in sodium hypochlorite (bleach 100%) for 3 minutes to remove surface contamination, then washed three times with DNA-free ddH2O and once with isopropanol. Dried samples were placed in a DNA Crosslinker under UV. About 0.1 g of tooth or bone powder were drilled. The powder was incubated in 1 mL of 0.5 M EDTA with 60-100 μl of Proteinase K (33.3 mg/ml) and 50 μl of 10% N-lauryl sarcosine for 20-24 hours at 56°C. The digested samples were concentrated at ∼100 μl using Amicon centrifugal filter units. The DNA was purified using the MinElute Reaction Cleanup Kit (QIAGEN). The extracted DNA was quantified with Qubit dsDNA HS Assay Kit and tested through a PCR amplification of mtDNA control-region fragments (< 150 bps). Samples showing at least 5 ng of DNA and with at least one successful short mtDNA amplification were selected for shotgun sequencing.
Double-stranded DNA libraries were prepared starting from ∼55.5 μl of extracted DNA using the NEBNext® Ultra DNA Library Prep Kit for Illumina®. Adapters were used in a dilution of 1:20, which is recommended for low concentration DNA samples. Adaptor-ligated libraries were purified using the MinElute Reaction Cleanup Kit (QIAGEN) and then prepared for amplification, which was carried out in thermocyclers in the modern DNA laboratory. The MinElute Reaction Cleanup Kit (QIAGEN) was used to clean amplified libraries, whose quality was then checked on the E-Gel Precast Agarose Electrophoresis System and quantified on the Agilent 2100 Bioanalyzer Instrument with the High Sensitivity DNA kit.
Eventually, 21 DNA libraries (10 nM) were selected (two for the individual PAPV146) for Illumina sequencing on the HiSeq4000 (single-end 100 bp, for a total of 150-200M reads) at the Roy J. Carver Biotechnology Center of the University of Illinois.
Mitochondrial DNA capture was also performed at the University of Florence for four individuals (PAPV27, PAPV53, PAPV93, PAPV109), as in (Modi et al., 2020).
Modern DNA processing
After automated DNA extraction with Maxwell® RSC Instrument in the lab of Genomics of Human and Animal Populations at the University of Pavia, the samples were genotyped with the Affymetrix Human Origin 600K chip at the Institute of Healthcare Research in Santiago de Compostela (CEGEN).
As for the mitogenome sequencing, the entire mtDNA was amplified in two overlapping fragments (Modi et al., 2020). Libraries were prepared using the Illumina Nextera XT DNA preparation kit and sequenced at the National Neurological Institute C. Mondino in Pavia on an Illumina MiSeq instrument (paired-end reads 150x2).
Quantification and statistical analysis
Ancient data preparation for analysis
Illumina adapters were removed from raw data using CutAdapt (Martin, 2011). Trimmed FASTQ files were then checked with FastQC (Andrews, 2010). Taking into account the high number of K-mers that were found, mostly poli-A probably generated during the blunt-end phase, CutAdapt was run twice to remove these artifacts. Trimmed reads were mapped against the human reference genome hg19 build 37.1, as well as versus the revised Cambridge Reference Sequence (rCRS) in separate runs, using the algorithm aln of bwa v0.6.1 (Li and Durbin, 2010). The numerous duplicates, generated during library amplification, were removed with the tool MarkDuplicates of the Picard package (http://broadinstitute.github.io/picard).
Validation tests
Ancient DNA damage pattern. Damage patterns (Table S2) were analyzed with MapDamage2.0 (Jónsson et al., 2013). The molecular decay after death was calculated from the length of the reads, as in (Allentoft et al., 2012). We used the error rate estimate to confirm the antiquity of our reads (Table S2). It calculates the excess of derived alleles observed in an ancient genome (compared to a high-quality genome) taking into account that all the anatomically modern humans should have the same percentage of derived alleles. The error rate analysis was performed comparing our trimmed FASTQ with the “Error estimation” tool of ANGSD (Analysis of Next Generation Sequencing Data) (Korneliussen et al., 2014), using the chimp genome as an outgroup and the genome NA12778 (from the 1000 genomes project), as an error-free individual (Schroeder et al., 2018).
To double-check if an excess of damage and/or erroneous adapters’ trimming might affect the results of the subsequent analyses, we repeated two AncientPanama FASTQ trimming using more stringent criteria: removing 10 additional bases from both ends of each read; removing the adapters using a higher mismatch rate (up to 50%) than in the original pipeline (20%). These trimmed FASTQ were then mapped against the reference genome hg19 build 37.1. We repeated the specific Isthmo-Colombian PCA analysis including the Ancient Panamanian data trimmed with more stringent criteria finding consistent results with the original plot in Figure 2B.
Contamination tests
Chromosome X contamination. For each library derived from ancient male individuals we estimated nuclear contamination using the approach described in Moreno-Mayar et al. (2020), based on reads mapping to the X chromosome. This method relies on the fact that males are hemizygous for X-linked loci outside the pseudo-autosomal regions, making multiple alleles in these loci attributable to either errors or contaminations. These estimates can be used as proxy for nuclear contamination estimates in ancient male individuals.
We used ANGSD (Korneliussen et al., 2014) to estimate contamination on reads with mapping quality greater than 30 and base quality greater than 20. We considered at least 100 sites with depth greater than or equal to 2 (Nägele et al., 2020) matching the HapMap CEU allele frequencies (Altshuler et al., 2010) as potential contaminants, after excluding pseudo-autosomal regions on chromosome X. We then applied the tool presented in Moreno-Mayar et al. (2020) to estimate contamination using low-depth X chromosome data, setting to 1,000 the maximum number of jackknife samples used for estimating standard errors and considering the estimates from the Two-consensus method.
Mitochondrial DNA contamination. Jones’s method: Contamination in ancient mitochondrial sequences was first estimated by assessing the number of non-consensus base calls (with base quality greater than or equal to 20) at haplogroup diagnostic positions as a function of the total coverage for each of these sites (Jones et al., 2017).
contamMix: We also estimated mtDNA contamination in our data by using contamMix v. 1.0-10, which provides the maximum a posteriori probability of the consensus sequence being authentic (Fu et al., 2013). This method is based on the reconstructed mtDNA consensus to estimate contamination, which should not exceed 50% for contamMix to work. First, we built an mtDNA consensus sequence running ANGSD (Korneliussen et al., 2014) and using the parameters -doCounts 1 and -doFasta 2 (majority rule). We retained only reads with mapping quality higher than 30 and nucleotides with base quality greater than 20. Moreover, we filtered for sites with a minimum depth of 5X. Then, we remapped to the rebuilt consensus sequence only the reads that mapped uniquely to the mitochondrial reference sequence. We used mafft (Katoh et al., 2002; Katoh and Standley, 2013) to align our consensus sequence to a panel of 311 worldwide mtDNA sequences (Green et al., 2008), representing potential contaminant sequences. Finally, we used both the alignment and the remapped reads for contamination estimation with contamMix, running five independent chains for 50,000 iterations. The results were checked by monitoring the Gelman diagnostic (Gelman and Rubin, 1992) to confirm convergence.
Schmutzi: The third method to estimate mtDNA contamination was Schmutzi (Renaud et al., 2015), which jointly estimates present-day human contamination and reconstructs the endogenous mitochondrial sequence by considering both deamination patterns and fragment length distributions. Present-day human contamination was evaluated by an iterative likelihood method implemented in Schmutzi using a non-redundant database of 197 human mitogenomes available in the software package.
Molecular sex and kinship determination
The sex of each individual was determined using two computational approaches specific for low-coverage genomes, Ry (Skoglund et al., 2013) and Rx (Mittnik et al., 2016) (Table S2). The relationships between the ancient Isthmian individuals were verified using the tool READ with default parameters (Monroy Kuhn et al., 2018) (Table S2).
Checking for reference bias
The reference bias indicates an increased probability of detecting the alleles present in the reference sequence, especially when dealing with paleogenomic data affected by fragmentation and other post-mortem damage, often generating C to T and G to A transitions at 5′ and 3′ fragment ends (Type II damage) (Günther and Nettelblad, 2019). These characteristics might influence mapping scores in low-coverage ancient genomes, particularly for those reconstructed from very short reads, eventually leading to an artificial decrement of reads carrying alternative alleles, in comparison to high-coverage modern genomes. In order to verify that this issue did not affect our downstream analyses, we compared the alternative allele frequency distributions between modern and ancient individual data. We used the PLINK 2.0 toolset to calculate the average proportion of alternative alleles for each individual. This analysis was repeated considering all SNPs, all SNPs without C to T and G to A and only transversions without finding significant differences between the pseudo-haploid datasets (derived from ancient and masked individuals).
Modern data preparation for analysis
The raw data were initially checked with Affymetrix suite software, then the data from 81 individuals that passed the quality control (call rate > 98.5) (Raveane et al., 2019) were converted into PLINK files and retained for the kinship analyses to exclude putatively related individuals using KING (Manichaikul et al., 2010). Considering that Indigenous populations have a higher degree of relatedness due to long periods of isolation and endogamy, there was the need to evaluate a different threshold. For this reason, the 97.5-percentile of population IBD for each population was used as threshold for exclusion (Busby et al., 2015). For each pair of related individuals, the exclusion was based on the number of missing SNPs. A total of 74 individuals (out of the initial 84) were retained after quality and kinship filters.
Ancient comparative datasets
The 545,942 SNPs retained in the rWD1560-dataset (see “Reduced worldwide (rWD1560) dataset” section) were called on the ancient dataset that contained our 12 ancient Panamanian individuals merged with 241 ancient Siberian and American individuals with a minimum coverage of 0.01X (Table S3). The calling was performed for all individuals in one run using ANGSD (Korneliussen et al., 2014) with the haplocall 1 option, which picks a random read starting from an input set. In addition, to avoid possible biases due to low coverage data, we down-sampled all ancient genomes to 1X and 0.5X coverage using ANGSD with the -downSample option.
The ancient dataset was merged using PLINK 1.9 (Purcell et al., 2007) with our modern datasets and then filtered using–geno and–mind options set respectively to 0.60 and 0.98 (Scheib et al., 2018), keeping only individuals with at least 10,000 SNPs (Table S3) (Posth et al., 2018).
Modern comparative datasets
As detailed below, different comparative datasets were assembled: 1) a worldwide dataset of modern samples (rWD1560, 545,942 SNPs); 2) two datasets of (nearly) “unadmixed” Indigenous Americans (uIA217, 534,569 SNPs and uIA89 523,210 SNPs) obtained by removing individuals with signatures of non-Indigenous genetic contributions using different stringent criteria; and 3) a larger dataset of “admixed” individuals where only genetic fragments inherited from Indigenous individuals were retained, masking (removing) variants not belonging to haplotypes inherited by Indigenous groups (mIA417, 545,942 SNPs), to focus on pre-Hispanic interactions. The individuals used for the latter dataset were considered pseudo-haploid in allele frequency analyses, while in haplotype-based methods the individual chromosome pairs were jointly analyzed. Moreover, some of the samples with less than 50% Indigenous ancestry were removed (rmIA311).
1) Reduced worldwide (rWD1560) dataset
A first dataset of 4,939 modern individuals was built encompassing worldwide Affymetrix Human-Origins genotyped individuals and American whole-genome sequences from the literature considering a minimum threshold of 500K overlapping SNPs. This dataset was merged using PLINK 1.9 with our modern individuals and then filtered using–geno and–mind options set to 0.02. After excluding related individuals (see “Modern data preparation for analysis” section), the dataset was geographically restricted to 1,560 individuals (rWD1560, Table S3) including our modern Panamanians (74), all individuals from America (1,084) and Siberia (203), and the western Eurasian (61), African (73) and Australasian (65) populations that left a greater genomic impact on Indigenous Americans during colonial times (Chacón-Duque et al., 2018; Homburger et al., 2015; Montinaro et al., 2015; Ongaro et al., 2019).
2) “Nearly unadmixed” Indigenous American (uIA217 and uIA89) datasets
European colonialism and the African slave trade left a strong impact on the Indigenous American populations. Therefore, the analyses of pre-colonial genetic history might be strongly influenced by these components and it is very difficult to find non-admixed modern individuals, even within Indigenous groups. Thus, three different approaches (ADMIXTURE, Local Ancestry and f4) have been used to create a sub-set of individuals with the maximum possible Indigenous genetic component, to avoid signals altered by recent admixture events. Considering the inconsistency of the preliminary results obtained by using the three methods independently, a stepwise merging approach was preferred, retaining individuals classified as Indigenous (with the lowest content of non-Indigenous component) after each step.
1. ADMIXTURE. We extend the analyses on our rWD1560 dataset until K20. However, considering that K14 has the lowest cross-validation (CV) error (Figure S2A), we used K14 to identify the individuals that have more than 95% of Indigenous components (290 in total) (Alexander et al., 2009).
2. f4 statistics. In the second approach, we used f4 statistics in the following form: f4 (ancientIndigenous, X; Europe/Africa, Mbuti). Ancient Indigenous was composed by five high-coverage ancient genomes selected on the basis of country of origin and age (Table S3). The selected individuals (N = 305) were those with a Z score < |3| for both Europe and Africa.
3. Local ancestry (LA). Combining the positive results of ADMIXTURE and f4 statistics, we could retain 230 individuals to be selected for the Local Ancestry (LA) using the software RFMix (Maples et al., 2013). Among these, we selected 58 individuals to be used as ancestral source in LA analysis. The overall criteria used to select these 58 ancestral individuals were as follows: i) successfully passing the f4 filter (see “f4 statistics” section); ii) 100% IA in K14 (see “ADMIXTURE” section); iii) belonging to a population that best represents a specific IA component in the ADMIXTURE analysis (Figure S2A) for each K (until K20):
-
•
K1 6 Puno individuals (selected at K20 among the population with more K1)
-
•
K6 25 Guna individuals (selected at K14)
-
•
K10 2 Chipewyan individuals (selected at K14)
-
•
K11 10 Karitiana individuals (selected at K14)
-
•
K17 8 Surui individuals (selected at K18)
-
•
K20 7 Kichwa Orellana individuals (selected at K20)
We also used all African (73) and European (51) individuals (representing the respective ancestries). The Finns were excluded due to their known admixture with a central Asian population (Saag et al., 2019; Sikora et al., 2019). The entire dataset was screened for the LA of these selected individuals allowing us to identify 210 individuals showing less than 5% of non-IA ancestry (plus the 58 used as IA sources).
Merging all the positive results of these three independent analyses, we identified a restricted dataset with 217 almost unadmixed individuals (uIA217, Table S3). Moreover, more stringent criteria, i.e., < 1% African, < 2% European (Gnecchi-Ruscone et al., 2019) and Z < |2|, were used to select a second restricted dataset with only 89 almost unadmixed individuals (uIA89, Table S3).
3) Indigenous American dataset with masked haplotypes (mIA417)
The non-Indigenous component (> 5%) identified using LA was removed from each haplotype of the 417 individuals, not included in the almost unadmixed Indigenous dataset. This masked dataset mIA417 combined with uIA217 (or uIA89) creates an overall Indigenous dataset encompassing 634 (506) individuals.
Uniparental analyses
Ancient mitogenomes
Taking into account that the mitogenome sequence included in the whole hg19 human reference is different from the mtDNA reference sequence (rCRS) commonly used in phylogenetic studies on mtDNA, we have chosen rCRS for mapping the FASTQ data in our pipeline. Processed reads from shotgun (single-end) sequencing were aligned to the rCRS sequence (Andrews et al., 1999) with BWA v0.7.17 aln/samse algorithm (Li et al., 2009) and realigned with CircularMapper (Peltzer et al., 2016). Duplicate reads were removed with Picard MarkDuplicates (https://github.com/broadinstitute/picard) and BAM files were further processed with SAMtools (Li et al., 2009).
Raw paired-end reads derived from captured mitogenomes that overlapped for at least 11 bases were merged using ClipAndMerge v1.7.7 (Peltzer et al., 2016) and then processed as above (see “Ancient data preparation for analyses” section) with an additional step to remove the indexes used for multiplexing on the MiSeq sequencer. Clean reads were mapped to rCRS (Andrews et al., 1999) with BWA v0.7.17 mem algorithm and BAM files were filtered with Picard MarkDuplicates (https://github.com/broadinstitute/picard) to remove duplicates and with SAMtools (Li et al., 2009). The final mtDNA BAM of the four captured individuals were merged with the shotgun mtDNA BAM using SAMtools merge.
For all mitochondrial BAM files, only reads with minimum mapping and base quality of 30 and positions with a minimum depth of 1 were retained for downstream analyses. Eventually, we obtained 13 ancient mitogenomes with mtDNA genome coverage ≥ 0.99 (11 from pre- Hispanic individuals) (Table S1).
Two strategies were used to determine the haplotypes of these 13 mitogenomes. First, we performed variant calling with BCFtools (Li et al., 2009) and filtering the VCF files with VCFtools (Danecek et al., 2011). Haplotypes were refined by manually checking BAM files.
Then, to better define indels in our dataset, as there are diagnostic deletions for some Indigenous lineages, we realigned cleaned reads of our ancient individuals to modern Panamanian mitogenomes belonging to the same haplogroup. The alignment was performed with BWA 0.7.17 aln/samse algorithm (Li et al., 2009) and reads were realigned with CircularMapper (Peltzer et al., 2016). Consensus sequences were generated using the same filters as before and then compared to the rCRS to obtain final haplotypes.
We also reconstructed the consensus sequence for the four contaminated individuals. We used ANGSD (Korneliussen et al., 2014) applying the same filters as in Sánchez-Quinto et al. (2019).
Haplogroups classification, based on phylotree.org (mtDNA tree Build 17) (Van Oven, 2015), was assessed using the online tool HaploGrep2 (Weissensteiner et al., 2016).
Modern mitogenomes
After adaptors trimming paired-end reads were aligned to rCRS (Andrews et al., 1999) with BWA mem algorithm (Li et al., 2009). BAM files were filtered with Picard MarkDuplicates (https://github.com/broadinstitute/picard) and SAMtools (Li et al., 2009). Variant calling was performed with GATK HaplotypeCaller (McKenna et al., 2010) and mitochondrial haplotypes were also checked by manually inspecting BAM files. HaploGrep2 was used for haplogroup assignment (Table S1). We have also double-checked the final haplotypes for possible NUMTS without finding any problems. Four mitogenomes (PaGUN9659, PaGUN9671, PaNAS16050, and PaNGA1193) were obtained with Sanger sequencing and analyzed using Sequencher v5.0 (http://www.genecodes.com/).
MtDNA tree, dating, and demography
Phylogenetic tree and Bayesian Skyline Plot (BSP) were generated using BEAST v2.6.2 (Bouckaert et al., 2019). BEAST was also employed to calculate Bayesian age estimates. Radiocarbon dates of ancient individuals were used as priors. The L2c2 mitogenome from a Moreno individual (PaMOR16007) was included in the analyses as an outgroup. BEAST runs were performed with complete mtDNA sequences under the HKY substitution model (gamma-distributed rates plus invariant sites) with a fixed molecular clock as in (Brandini et al., 2018). We set the clock rate considering the ones published in Posth et al. (2016) and Soares et al. (2009). The chain length was established at 10,000,000 iterations with samples drawn every 1,000 Markov chain Monte Carlo (MCMC) steps, after a discarded burn-in of 10% steps (default value 0). Panama-specific haplogroups were set as monophyletic in the analyses. The same BEAST settings were used to: i) estimate the ages of haplogroups and (ii) evaluate population expansions in Panama through BSPs (Drummond et al., 2005) by including all Panamanian Indigenous mitogenomes analyzed in this study. BSPs were visualized in a plot using Tracer v1.7 (Rambaut et al., 2018) and converted into an excel graph by assuming a generation time of 25 years as in Brandini et al. (2018). The maximum clade credibility tree was determined using TreeAnnotator and visualized with FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Ancient Y chromosomes
The haplogroup classification of the eight ancient Y chromosomes (Table S1) was deducted from the aDNA aligned sequences by: i) extracting with bcftools all the positions belonging to the Y chromosome; ii) considering only the positions that matched the list of the SNPs belonging to the main branches of the phylogenetic tree present in Poznik (2016) after taking into account any possible aDNA damage (C/T– > T/C; G/A– > A/G) as in Fregel et al. (2018). To further sub-classify the ancient Y chromosomes the same workflow was performed by considering the list of 1,104 specific haplogroup Q SNPs reported by Grugni et al. (2019). All codes and pipeline for this part can be found at the link: https://github.com/raveancic/aDNAYchromosome.
Modern Y chromosomes
The Y chromosome haplogroup classification of the 43 modern male individuals was first inferred from genotyped files by using the script called Haplogroups.py in Yhaplo with Python3 (Poznik et al., 2016) (https://github.com/23andMe/yhaplo), using default parameters. Then, the obtained classification was confirmed by hierarchical analysis, as previously described (Battaglia et al., 2013), of the following Y chromosome haplogroup markers: M9, M242, M3, M89, YAP, M96, M304, M172, M241, M269, L23, S116. In addition, the M242 positive samples (Hg Q) were further sub-classified by typing the signature markers (M848, Z780, M925, Z5908, Y780, CTS2731) of the main Indigenous sub-haplogroups recently identified (Grugni et al., 2019; Pinotti et al., 2019). Haplogroup nomenclature is according to Grugni et al. (2019).
Population genetics analysis based on allele frequencies
Principal Component Analysis (PCA)
PCAs were performed using ‘smartpca’ program from the package EIGENSOFT v7.2.0 (Patterson et al., 2006). Ancient data, characterized by a large amount of missing data, were projected onto the modern variation with the lsqproject and autoshrink options. The same approach was used for the masked dataset (mIA417) that also shows a variable amount of missing data. Several PCAs were performed considering ancient and modern world-wide datasets and different sub-datasets. Those individuals showing peculiar outlier positions in the PCA plots were excluded from the downstream analyses (Tables S1 and S3).
ADMIXTURE clustering analysis
Different datasets (only modern and modern plus ancient individuals) were pruned with PLINK 1.9 (–indep-pairwise 200 25 0.4) and used to perform a biogeographical ancestry analysis with ADMIXTURE v.1.23 (Alexander et al., 2009). We performed ten independent unsupervised ADMIXTURE runs for each K, from K1 to K20, adding the –cv flag to identify the 5-fold cross-validation (CV) error for each K. The average cross-validation (cv) value for each K were plotted to select the model with highest likelihood. The software CLUMPAK (Kopelman et al., 2015) was used to combine different runs and to find the best alignment of the results across a range of K values with the tool DISTRUCT (Rosenberg, 2004).
The analysis on the rWD1560 dataset reveals well-defined structures and consistent trends, associated with a low CV value, until K14 (or even K20) suggesting that the observed pattern is not an artifact. In the ADMIXTURE plot it is possible to observe seven specific IA ancestries, some mirroring the PCA clusters (Figure 2B):
-
•
K1 that is widely distributed in all IA populations with high percentage in Puno, Aymara, Quechua and Paran-Cusco individuals, all speaking Andean languages.
-
•
K6 is modal in the Guna and highly represented in all the populations from Costa Rica to Panama, speaking Chibchan languages.
-
•
K10 is typical of the Chipewyan (speaking a Na-Dene language) with lower percentage in all populations from northern North America.
-
•
K11 is modal in Karitiana and Surui speaking Tupi (Equatorial-Tucanoan) languages.
-
•
K17 separates the Surui from Karitiana.
-
•
K15 is represented by the Pima and mostly present in Mexico, but also widely distributed in Central and Northern IA speaking groups.
-
•
K19, like K1, is widely distributed across the double continent and reaches the highest level in South America, particularly in the Andes Mountains (i.e., KCH).
Additional interesting components are K13 and K16 that are present in high percentages in Puerto Rico (PUR) and Colombia (CLM), respectively. They also can be observed in admixed American and European populations. The above-mentioned components are present in different proportions and differentially distributed among IA populations including the Panamanians, with the only notable exception of the Guna showing a specific component. The addition of 329 ancient Siberian and American individuals to the rWD1560 dataset confirms the already-discussed ancestries, revealing an additional K specific of Archaic Caribbean individuals (Figure 2A). A further ADMIXTURE analysis (Figure S2B) was performed projecting the ancient individuals on to the population structure from only modern individuals using the option -P and the .P file from the analysis in Figure S2A at K14.
Admixture tests (f statistics)
The f statistics was performed using EIGENSOFT v7.2 and AdmixTools v4.1 (Patterson et al., 2012). Outgroup f3 was used to highlight only the shared genetic history between individuals or populations relative to an outgroup (Peter, 2016). A high f3 value means more genetic history shared between the pair population analyzed. This method is less sensitive to lineage-specific genetic drift over the use of pairwise distance measures, such as Fst (Skoglund et al., 2015). A graphic explanation of the outgroup f3 is reported in Figure S3.
We analyzed the shared genetic history of modern IA populations (included in the mIA417 and uIA89 datasets) against some ancient reference genomes (aRG) from Siberia, Beringia, North America (representative of the NNA ancestry) and South America (representative of SNA). The Guna always show a shared genetic drift with the ancient reference individuals lower than the average f3 value (dotted line) of all modern IA groups. It could be also noticed that the average f3 value is higher in the comparison with Spirit Cave than with Anzick-1.
We also built a distance matrix using the inverse values derived from the outgroup f3 statistics on all Central and South American populations pairs plus Anzick-1, Early San Nicolas (ESN), Spirit Cave and USR-1 (as an outgroup). We retained only populations with more than 30K overlapping SNPs and significant Z scores (p value∼0.001, for Z scores > |3.3|) in all comparisons. This distance matrix was used to generate a neighbor joining tree (Figure S3C) with the program PHYLIP 3.6 (Nägele et al., 2020). The tree was visualized with FigTree 1.4.4 (https://github.com/rambaut/figtree).
The f4 statistics was eventually used to identify gene flows among different populations. The comparison was performed in the form f4(W, X; Y/test, Outgroup), as reported in the software documentation. A graphic explanation of the f4 statistics analyses is reported in Figure S5. In each figure showing a f4 statistics test the form is reported above the plot(s). Results from f4 analyses presented in Figure 2C display only tests in which the initial conformation of the tree is rejected (p∼0.001, Z score > 3.3 or < −3.3) (Moreno-Mayar et al., 2018), meaning that the investigated population (Y) has a significantly higher genetic affinity with W rather than with X if the f4 results are positive, the opposite when values are negative. If the results are not displayed, the proposed tree cannot be rejected and there are no significant preferential relationships between the test population (Y) and W or X. In Figure S5 the Z score is displayed in abscissa and the region where the tested tree cannot be rejected is highlighted in gray (see above).
In all f4 statistics, we considered a minimum threshold of 30K SNPs, the comparisons with less SNPs are highlighted with a specific symbol (X). Due to the low number of SNPs retained in multiple analyses, the following individuals were excluded: Baja_100, CuevadelPerico_2700, Enoque_3500, Kaillachuro_4000, LosIndios_600, Moraes_5800, SanFranciscoBay_25, ShukaKaa_10300, SoroMikayaPatjxa_6800 and Tibes_1200.
In particular, to specifically check for the relationships between Anzick-1 and Spirit Cave, with the Isthmian populations as well as with other ancient and modern IA individuals from Central and South America, we ran two f4statistics in the following forms f4 (Anzick-1, Spirit Cave; Isthmo, Mbuti) and f4 (Anzick-1, Spirit Cave; Central and South IA, Mbuti). The datasets uIA89, mIA417 and ancient individuals were used considering different sets of variants: all SNPs, all SNPs without C to T and G to A variants, only transversions and only transversion with ancient individuals’ coverage downsampled to a maximum of 1X (to avoid coverage biases).
We observed a clear pattern that becomes significant when increasing the number of SNPs. We further verified this pattern using a f4 statistics in the form f4 (USR-1, Anzick-1/Spirit Cave; Central and South IA, Mbuti) that confirmed a higher proximity to Spirit Cave in comparison to Anzick-1. The same pattern has been also observed in the outgroup f3 analyses (see above).
TreeMix
In order to obtain a maximum likelihood tree, we ran TreeMix (Pickrell and Pritchard, 2012) on the pruned dataset uIA89 using TSI, CHB and YRI (Tuscans, Chinese Han and Yoruba) as outgroups. The -noss and -global parameters were added considering zero to five admixture edges. The trees with the highest likelihood were selected after 1,000 runs (Gnecchi-Ruscone et al., 2019; Moreno-Mayar et al., 2018).
Ancestry modeling with qpWave
In order to verify that the Isthmo-Colombian ancestry (UPopI) is independent from other IA ancestries, we compared in pairs the Isthmian populations with all other modern and ancient IA populations using qpWave (Patterson et al., 2012) to test whether they were homogeneously related to a set of external outgroups. The outgroups were kept to the minimum and chosen to represent different IA ancestries identified here and in other papers:
-
•
Mbuti, Papuan, CHB, Malta_24000 and USR-1_11500 as non-IA sources (Nägele et al., 2020; Posth et al., 2018)
-
•
ASO_4000 and Chipewyan for NNA
-
•
LagoaSanta_10400 for SNA1
-
•
SpiritCave_10900 for SNA2
-
•
Mixe for UPopA
-
•
GuayaboBlanco_1700 for Archaic Caribbean
-
•
Ayayema_4500 for Patagonia
-
•
Aymara and KCH (K1 and K19 respectively in Figure S2A) representing modern South American populations.
We took in consideration the p value of “taildiff” for Rank1, a statistically significant p value (< 0.01) means that each compared pair could be explained by two sources. We observed that one ancestry is usually needed (p value > 0.01) to define pairs of Isthmo-Colombian populations, while pairs of Isthmian and non-Isthmian populations require two ancestries. This pattern is more evident in the Guna, the best representative of the Isthmo-Colombian component. This pattern confirms that a different ancestry, instead of only genetic drift by isolation, is needed to explain the distinctiveness of the Isthmo-Colombian populations.
Demographic modeling with qpGraph
We used qpGraph (Patterson et al., 2012), on a merged dataset of the uIA89, mIA417 and ancient individuals (considering only transversions), to reconstruct the best tree modeling the relationships between Isthmian populations and ancient Indigenous genomes. In our f4 statistics we noted a differential relationship between Isthmian groups and modern/ancient Indigenous Americans in comparison to the individuals older than 10 kya (Figure 6A; Table S4). Therefore, we modeled a basal tree with three of the most ancient available genomes of the SNA ancestry, an ancestry that certainly went through the Isthmus to reach South America. Our best tree revealed that the SNA dispersal involved a complex demographic pattern, with three possible ancestries (Figure S6A). To resolve the inferred zero-length internal branch, we tested all three possible split orders obtaining similar scores. Therefore, it might represent a very short branch that we cannot resolve with this dataset power (Lipson, 2020) and the three lineages, Anzick-1 (Montana, ∼12.6 kya), Lagoa Santa (Brazil, ∼10.4 kya; SNA1) and Spirit Cave (Nevada, ∼10.9 kya; SNA2) are statistically consistent with forming a trifurcation. The best fitting topology was tested by considering an early admixture between the SNA2 source and SNA1 obtaining a still supported model without zero-length branches (Figure S6B). To increase the resolution power and considering the results of previous analyses (f3- and f4-statistics), we added to this graph the captured and genotyped data from Lapa do Santos (Brazil, ∼9.6 kya) as SNA1 and the whole-genome sequences of ESN (California; ∼4-5 kya) as SNA2. This allowed us also to check for any bias due to sequencing methods. Even in this case the best fit tree confirms the trifurcation (Figure S6C). After this step, we added Los Rieles (Chile, ∼10.9 kya), the most ancient Pacific coast genome, which turned out to be better modeled as an admixture of SNA1 and SNA2 (|Z| = 2.835) than as non-admixed and considering only geographic origins (|Z| = 3.930) (Figure S6D). This finding confirms that both SNA1 and SNA2 reached South America and seems to indicate that the latter had a lower impact on the Atlantic side of South America. When Los Rieles is replaced by Lapa do Santos, the tree does not fit (|Z| = 3.930) (Figure S6E).
We then took into account that, in the f4 statistics (Figure 6A; Table S4), there is a significant allele sharing of the Isthmian populations with Spirit Cave and two Central American ancient genomes, Mayahak Cab Pek (Belize, ∼9.3 kya) and Saki Tzul (Belize, ∼7.4 kya), as well as a higher genetic proximity to Los Rieles relative to Lagoa Santa. Therefore, we attempted to model ancient Panama in relation to these ancient genomes (Figures S6F and S6G). Ancient Panama fits better when considering an admixture between the Central-South American branch of SNA2 and another ancestry parallel to SNA2 and shows the best score with Mayahak Cab Pek (Figure S6G). The additions of NNA individuals (Figure S6H), ASO (Ancient Southwestern Ontario, ∼4.2 kya), 939 (Lucy Island, British Columbia, ∼6.1 kya), Kennewick (Washington State, ∼8.8 kya), ancient Athabaskan (dated 100 and 725 ya), and modern Chipewyan, hold better when assuming some admixture events involving these genomes. These findings confirm that we cannot identify an unadmixed proxy for the NNA ancestry among ancient individuals. The best graph was obtained when including ASO as NNA and Mayahak Cab Pek as the central American ancient genome.
At this point, we further evaluated the relationship of Panama with SNA1 that we initially linked to Lagoa Santa (Figure S6A). As before, we started from basal admixture graphs without Guna and considered the conformation with ASO and Mayahak Cab Pek, based on previous results (Figure S6H). We first tested the tree without admixture in Los Rieles, placing Lagoa Santa as a parallel branch of SNA2, but the tree would fit only when considering Los Rieles as an admixture between SNA1 and SNA2 (|Z| = 2.896), the two early South American ancestries that we identified above (Figure S7A). It is worth mentioning, however, that the graph without admixture in Los Rieles became statistically significant (|Z| < 3) when Lagoa Santa was replaced with the younger Laranjal sample (Brazil, ∼6.8 kya) (Figure S7B). This confirms our f4 statistics (Figure 6A; Table S4) and the scenario of a widespread population turnover in South American during mid-Holocene, as previously suggested (Moreno-Mayar et al., 2018; Posth et al., 2018), a finding that also correlates with climate changes in the southern continent (Riris and Arroyo-Kalin, 2019). As for the Ancient Panamanians, they are a mix between the source of SNA2 (prior to Spirit Cave) and the admixture between SNA1 and SNA2 (Figure S7A).
Lastly, we assessed different admixture graphs also including the Guna, which was the best representative of the Isthmian-specific component in our previous analyses (Figure 2A). As for the ancient Isthmians, we first explored the link of Guna with SNA2 (i.e., Spirit Cave) and in this setting only the admixed model was supported, with the Guna group representing a still unsampled population of the Isthmus, UPopI (Figure S7B). To assess if UPopI might correspond to the previously identified UPopA, we replaced Guna with Mixe, previously used to identify UPopA (Moreno-Mayar et al., 2018). The resulting admixture graph was not statistically supported (|Z| > 3, the rightmost graph). Then, we linked Guna also to SNA1, obtaining the best Z score in the graph when UPopI was placed as a parallel ancestry to SNA1 and SNA2, all radiating from the same early SNA source (Figure S7C). The latter derives from an initial split of the early Indigenous group into SNA and NNA. In this scenario the ASO group is the result of an admixture between NNA and SNA1. It is therefore likely that the first split occurred further north and earlier than the diversification of SNA into SNA1 e SNA2. Finally, the same graph shows that when UPopI reached the Isthmian area, it admixed locally with population groups derived from both SNA1 and SNA2.
Taking into account the presence of a zero-length internal branch in the final tree with UPopI (Figure S7C) and the results obtained on the basal graph (Figure S6), we modeled a binary tree without zero-length branches when considering an initial migration of population with an early SNA ancestry (SNA1) and a later North American admixture between SNA1 and a different SNA branch (SNA2) (Figure 7). This admixture gave rise to two ancestries, one, related to Spirit Cave, that reached South America, leaving evident footprints on the Pacific coast, and another restricted to the Isthmo-Colombian area (UPopI) that is well represented in the Guna. The best topologies were also checked replacing Guna (UPopI) with Mixe (UPopA), Spirit Cave with Anzick-1, and without UPopI (Figures S7D–S7F), but no statistically supported graphs were found. The other Isthmian Indigenous groups were also tested (replacing ancient Isthmians) using this final model (Z score always < 2.5) to estimate the differential legacy of the three ancestries in present-day populations (inset of Figure 7).
Population genetics analysis based on reconstructed haplotypes
Phasing
Phased haplotypes were generated from the rWD1560 dataset using the Segmented Haplotype Estimation and Imputation tool SHAPEITv2 (Delaneau et al., 2011) and the HapMap37 human genome build 37 recombination map.
Local Ancestry and Masking
The local ancestry for genomic fragments in the American individuals was estimated using RFMix (Maples et al., 2013). As source populations, we used Bantu, Esan (ESN), Gambia (GWDwg), Mandenka, Mbuti and Yoruba (YRI) for Africa, Spanish (IBS), British (GBR), French, Icelandic and Tuscany (TSI) for Europe and Chipewyan, Kichwa Orellana, PaGUNA, Puno, Surui and Karitiana for Indigenous ancestry. We used “PopPhased,” “-n 5” and “–forward-backward” options as recommended in RFMix manual. Then, starting from RFMix output files, we built a PLINK file set in which the non-Indigenous SNPs were masked. The masking process was done with this rationale: if in the “Viterbi” output a particular SNP was not assigned to the Indigenous ancestry and if the probability of belonging to the Indigenous ancestry (reported in the “forwardbackward” output) was less than a threshold (< 0.9) that allele was set as missing. In this analysis we kept individuals as separated into the two phased haplotypes.
ChromoPainter
To obtain the painting profile of all the 217 individuals in the uIA217 dataset consisting in a matrix of ‘recipient’ individuals (rows) that appear as a mosaic of the ‘donors’ (columns), we processed the genomic information contained in phased data (haplotypes) through the use of inferential algorithms implemented in CHROMOPAINTERv2 (Lawson et al., 2012). Technically for this analysis the recombination (-n) and mutation (-m) parameters used were respectively 233.1352 and 0.00084 estimated on five randomly selected chromosomes (3, 7, 10, 18 and 22). Since the genetic variability among IA populations is low, we ran CHROMOPAINTER in two runs, one with standard parameters, the other adding the flag -k 50 (Gnecchi-Ruscone et al., 2019). No significant differences were observed between the two runs.
fineSTRUCTURE
The CHROMOPAINTER square (217 × 217 individuals) chunkcounts.out matrix was used as input file for fineSTRUCTURE in order to identify similar genetic clusters. We ran the software with three millions MCMC iterations thinned every 10,000 and preceded by one million burn in iterations: -x 1000000 ; -y 3000000 ; -z 10000 ; -t 1000000. The MCMC file (.xml) was used to build the tree structure using both the options –T1 and –T3, without major changes between the two methods.
Initially, we obtained 50 clusters in the final tree (data not shown). However, to obtain more robust genetic inferences the number of clusters was reduced to 19 considering the number of individuals in each cluster (less than five) and the Total Variation Distance (TVD < 0.03) as elimination criteria. TVD is an index that measures the similarity between copying vectors of the CHROMOPAINTER matrix (calculated on the chunklengths) (Leslie et al., 2015); lower values of TVD mean similarity, while higher values indicate heterogeneity.
Haplotype Analyses on ‘Masked’ Individuals
The masked haplotypes (mIA417) were initially filtered for the individuals that had a maximum of 50% of missing SNPs (considered as the mean of the summed missingness of the two haplotypes) and, among these individuals, we selected only those with at least 25% of SNPs retained in each haplotype. Eventually, we obtained a restricted dataset of 311 masked individuals (rmIA311). This dataset (rmIA311) was then converted in PLINK1.9 format and subsequently in a CHROMOPAINTERv2 input. To enable missing data in CHROMOPAINTERv2, we slightly modified CHROMOPAINTERv2 such that a recipient/target’s emission probability is set to 0 at missing (i.e., masked) SNPs when tabulating the expected number of segments matched to each “Donor” individual that the recipient is compared to. Therefore, in regions of high missingness the expected number of segments matched to each “Donor” will tend toward the prior, which assumes equal matching to all “Donor” individuals. However, in our application here we found that inference seems to be dominated by data at non-missing SNPs, where the usual CHROMOPAINTERv2 machinery is employed. In particular, we did not identify any correlation between the percentage of missing data (even when reaching 50%) and bad placements/outlier behaviors in the PCA created from the CHROMOPAINTER output projecting the ‘masked’ individuals (Figure S4A).
Identical by Descent (IBD) Analysis
The pattern of IBD sharing within each population of the uIA217 phased dataset was analyzed using Refined-IBD (Browning and Browning, 2013), which makes it possible to improve the accuracy and efficiency of identity by descent detection in population data, using default parameters. The average IBD-sharing was calculated for nine different bin categories corresponding to different degrees of relatedness (Gnecchi-Ruscone et al., 2019; Moreno Estrada et al., 2014). The total length of shared IBD was calculated for each bin, by considering all pairs of individuals within each population group. The summed length was then divided by the numbers of pairs in each population in order to obtain the average total length of intrapopulation IBD shared blocks for each population in the uIA217 dataset (y axis in Figure 3C).
In order to reconstruct the population dynamics, we applied IBDne on the uIA217 dataset, using both IBDseq, which does not require phased data, and Refined-IBD that uses phased data, using different minimum thresholds of IBD segment length (2 and 4 centimorgan, cM). Considering all the Panamanian individuals, lower confidence intervals were obtained when using windows of 2 cM. Moreover, the Ne obtained with Refined-IBD is more compatible with historical estimates of the area’s pre-colonial population size (see introduction). We observed the same trend in the ancestry-specific effective population size (asIBDne) from the 74 Panamanian individuals, masked for the IA component, following the pipeline presented by Browning et al. (2018), as reported in Ongaro et al. (2019). Therefore, Refined-IBD was applied using 2 cM windows for the comparison between Panamanian and non-Panamanian IA individuals present in the uIA217 dataset (inset Figure 3C). This analysis shows a decrease in the region’s population size that pre-dates the average values of other IA populations considered together. This decrease started in pre-Hispanic times (∼1100 ya) then became steeper in the early colonial period (∼500 ya). To retain more information, we used the three macro-clusters from the fineSTRUCTURE tree: Guna (30 individuals), Emberá (18 individuals) and Western Panama (20 individuals) encompassing Bribri, Naso and Ngäbe. The Guna group shows a peculiar trend when considering shorter IBD fragments < 6 cM (Figure S4C). However, even when the Guna were removed from the Panamanian / non-Panamanian comparison, the peculiar pre-Hispanic demographic contraction of the Panamanian populations was still detectable and with smaller confidence intervals (Figure S4D). The same approach was applied including all of the macro-clusters obtained with fineSTRUCTURE and considering a minimum threshold of 8 cM. This analysis confirmed the behavior of the Isthmian groups including the Guna (Figure S4E).
Dating Admixture Events with IBD Sharing
In order to date the admixture events between our Panamanian groups and other IA populations included in the uIA217 dataset, we calculated the IBD sharing segments using RefineIBD as performed by Liu et al. (2020). The IBD blocks were divided into three categories, based on their length (1-5 cM, 5-10 cM and over 10 cM), each roughly representing different time periods: 1,500-2,500 ya, 500-1,500 ya and < 500 ya (Liu et al., 2020; Ralph and Coop, 2013). We have calculated the mean of summed IBD lengths shared between population pairs for each length category. To reduce noise and false positives only the pairs that shared at least two blocks > 5 cM and four < 5 cM were considered.
Additional Notes
The text has been revised in order to minimize the use of “colonial language” and to avoid the connotation of people as mere samples or data. The adjective Indigenous has been preferred to Native American with only notable exceptions of Northern Native American (NNA) and Southern Native American (SNA) ancestries, which were used for consistency with previous papers.
Many figures in the paper have been created using various versions of the software Tableau (https://www.tableau.com/) or with different R packages.
Acknowledgments
We thank Nicole Smith-Guzmán for her suggestions and Sandra Kraus, Susanne Lindauer, Robin van Gyseghem, and Matthias Hänisch for processing and analyzing radiocarbon samples at the CEZA in Mannheim. We also thank the anonymous reviewers for their insightful comments. We are grateful to the Patronato Panamá Viejo, the Dirección Nacional del Patrimonio Histórico of the Instituto Nacional de Cultura, República de Panamá, and all volunteers who generously participated in this survey and made this research possible. This research received support from the following: ERC Horizon 2020 Consolidator Grant CoG-2014, no. 648535 (to B.A., J.R., I.H.-M., C.K., J.G.M., and A.A.); the University of Pavia–INROAd program (to A.A.); the Fondazione Cariplo project no. 2018-2045 (to A.O., A.T., and A.A.); the Italian Ministry of Education, University and Research (MIUR) for Progetti PRIN2017 20174BTC4R (to M.L. and A.A.), Dipartimenti di Eccellenza Program (2018–2022)–Department of Biology and Biotechnology “L. Spallanzani” University of Pavia (to A.O., A.T., O.S., and A.A.), and Department of Biology, University of Florence (to M.L. and D.C.); Italian Ministry of Health (Ricerca Corrente) and AIRC IG20109 (to A.R. and F.B.); and the National Research System-SENACYT, Panama (to J.M.P.). G.H. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (098386/Z/12/Z). M.R.C. thanks G. Lardo for his support. The aDNA and modern population genetics lab at the University of Tartu is supported by the European Union through the European Regional Development Fund (2014-2020.4.01.16-0030) (to C.L.S., L.P., L.O., F.M., and M.M.) and the Estonian Research Council (PRG243) (to C.L.S. and M.M.). The ERC grant CoG-2014 no. 648535 also provided (admittedly limited) funding for M.R.C. and the analyses undertaken by R.F.
Author contributions
J.R., T.M., I.H.-M., and J.G.M. excavated archaeological sites, provided or acquired ancient samples, and contextualized the archaeological findings. M.T. and U.A.P. performed modern sample collections supervised by J.M.P., J.M., O.S., and A.A. R.F. and C.K. produced and analyzed radiocarbon data. M.R.C., H.L., and A.M. performed aDNA laboratory work supervised by M.L., D.C., R.S.M., and A.A. M.R.C., A.R., and A.G.-C. produced modern genome-wide data supervised by A.S. and A.A. M.R.C., A.R., N.R.M., G.C., L.O., C.L.S., and M.L. analyzed ancient and modern genomic data supervised by F.M. and A.A. M.R.C., N.R.M., G.L., and G.S.G. produced and analyzed mitogenome sequences supervised by A.O., A.T., and A.A. M.R.C., A.R., G.C., and V.G. classified Y chromosome data supervised by O.S. G.H., F.B., C.C., L.P., and M.M. provided technical and computational resources. M.R.C., B.A., and A.A. wrote the original draft with major inputs from R.C., R.S.M., A.T., and J.G.M. All authors discussed the results and contributed to the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: March 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cell.2021.02.040.
Contributor Information
Marco Rosario Capodiferro, Email: marcorosario.capodiferro01@universitadipavia.it.
Alessandro Achilli, Email: alessandro.achilli@unipv.it.
Supplemental information
Ancient and modern Isthmian individuals analyzed in this study.
Post-mortem damage pattern, length distribution of reads, molecular sex determination and family relationships.
Published ancient and modern data used for comparison.
We tested the form f4 (W/Ancient IA, X/Modern IA; Y/Isthmus, Mbuti) on uIA89, mIA417 and ancient genomes from different geographic regions (considering only transversions): North America; Central America; South American Pacific Coast and South American Atlantic Coast (plus Anzick-1 and Spirit Cave). In the last comparison we tested ancient IA genome pairs in the form f4 (W/Ancient IA, X/Ancient IA; Y/Isthmus, Mbuti). See the legend of Figure 6A for further details.
References
- Achilli A., Rengo C., Magri C., Battaglia V., Olivieri A., Scozzari R., Cruciani F., Zeviani M., Briem E., Carelli V. The molecular dissection of mtDNA haplogroup H confirms that the Franco-Cantabrian glacial refuge was a major source for the European gene pool. Am. J. Hum. Genet. 2004;75:910–918. doi: 10.1086/425590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achilli A., Perego U.A., Lancioni H., Olivieri A., Gandini F., Hooshiar Kashani B., Battaglia V., Grugni V., Angerhofer N., Rogers M.P. Reconciling migration models to the Americas with the variation of North American native mitogenomes. Proc. Natl. Acad. Sci. USA. 2013;110:14308–14313. doi: 10.1073/pnas.1306290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achilli A., Olivieri A., Semino O., Torroni A. Ancient human genomes-keys to understanding our past. Science. 2018;360:964–965. doi: 10.1126/science.aat7257. [DOI] [PubMed] [Google Scholar]
- Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allentoft M.E., Collins M., Harker D., Haile J., Oskam C.L., Hale M.L., Campos P.F., Samaniego J.A., Gilbert M.T., Willerslev E. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proc. Biol. Sci. 2012;279:4724–4733. doi: 10.1098/rspb.2012.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler D.M., Gibbs R.A., Peltonen L., Altshuler D.M., Gibbs R.A., Peltonen L., Dermitzakis E., Schaffner S.F., Yu F., Peltonen L., International HapMap 3 Consortium Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data.https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ [Google Scholar]
- Andrews R.M., Kubacka I., Chinnery P.F., Lightowlers R.N., Turnbull D.M., Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- Aram B., López Fernández A., Muñiz Amian D. The integration of heterogeneous information from diverse disciplines regarding persons and goods. Digit. Scholarsh. Humanit. 2020:fqaa021. [Google Scholar]
- Ardelean C.F., Becerra-Valdivia L., Pedersen M.W., Schwenninger J.-L., Oviatt C.G., Macías-Quintero J.I., Arroyo-Cabrales J., Sikora M., Ocampo-Díaz Y.Z.E., Rubio-Cisneros I.I. Evidence of human occupation in Mexico around the Last Glacial Maximum. Nature. 2020;584:87–92. doi: 10.1038/s41586-020-2509-0. [DOI] [PubMed] [Google Scholar]
- Barrantes R., Smouse P.E., Mohrenweiser H.W., Gershowitz H., Azofeifa J., Arias T.D., Neel J.V. Microevolution in lower Central America: genetic characterization of the Chibcha-speaking groups of Costa Rica and Panama, and a consensus taxonomy based on genetic and linguistic affinity. Am. J. Hum. Genet. 1990;46:63–84. [PMC free article] [PubMed] [Google Scholar]
- Battaglia V., Grugni V., Perego U.A., Angerhofer N., Gomez-Palmieri J.E., Woodward S.R., Achilli A., Myres N., Torroni A., Semino O. The first peopling of South America: new evidence from Y-chromosome haplogroup Q. PLoS ONE. 2013;8:e71390. doi: 10.1371/journal.pone.0071390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Valdivia L., Higham T. The timing and effect of the earliest human arrivals in North America. Nature. 2020;584:93–97. doi: 10.1038/s41586-020-2491-6. [DOI] [PubMed] [Google Scholar]
- Biese L. The Prehistory of Panama Viejo. Bur. Am. Ethnol. 1964;191:1–52. [Google Scholar]
- Bouckaert R., Vaughan T.G., Barido-Sottani J., Duchêne S., Fourment M., Gavryushkina A., Heled J., Jones G., Kühnert D., De Maio N. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019;15:e1006650. doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braje T.J., Dillehay T.D., Erlandson J.M., Klein R.G., Rick T.C. Finding the first Americans. Science. 2017;358:592–594. doi: 10.1126/science.aao5473. [DOI] [PubMed] [Google Scholar]
- Brandini S., Bergamaschi P., Cerna M.F., Gandini F., Bastaroli F., Bertolini E., Cereda C., Ferretti L., Gómez-Carballa A., Battaglia V. The Paleo-Indian Entry into South America According to Mitogenomes. Mol. Biol. Evol. 2018;35:299–311. doi: 10.1093/molbev/msx267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning B.L., Browning S.R. Improving the accuracy and efficiency of identity-by-descent detection in population data. Genetics. 2013;194:459–471. doi: 10.1534/genetics.113.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning S.R., Browning B.L., Daviglus M.L., Durazo-Arvizu R.A., Schneiderman N., Kaplan R.C., Laurie C.C. Ancestry-specific recent effective population size in the Americas. PLoS Genet. 2018;14:e1007385. doi: 10.1371/journal.pgen.1007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby G.B.J., Hellenthal G., Montinaro F., Tofanelli S., Bulayeva K., Rudan I., Zemunik T., Hayward C., Toncheva D., Karachanak-Yankova S. The role of recent admixture in forming the contemporary West Eurasian genomic landscape. Curr. Biol. 2015;25:2878. doi: 10.1016/j.cub.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillero Calvo A. Editora Nova; 2017. Conquista, evangelización y resistencia: Triunfo o fracaso de la política indigenista? [Google Scholar]
- Chacón-Duque J.C., Adhikari K., Fuentes-Guajardo M., Mendoza-Revilla J., Acuña-Alonzo V., Barquera R., Quinto-Sánchez M., Gómez-Valdés J., Everardo Martínez P., Villamil-Ramírez H. Latin Americans show wide-spread Converso ancestry and imprint of local Native ancestry on physical appearance. Nat. Commun. 2018;9:5388. doi: 10.1038/s41467-018-07748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.W.K., Marcus J.H., Sidore C., Biddanda A., Al-Asadi H., Zoledziewska M., Pitzalis M., Busonero F., Maschio A., Pistis G. Genomic history of the Sardinian population. Nat. Genet. 2018;50:1426–1434. doi: 10.1038/s41588-018-0215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R.G. Centro de Investigaciones Antropológicas, Actas del IV Simposium Nacional de Antropología, Arqueología y Ethnohistoria de Panamá; 1976. Informe Sobre Excavaciones en el Sitio CHO-3, Miraflores, Río Bayano, Febrero 1973; p. 369. 342. [Google Scholar]
- Cooke R. Institute of Archeology, University of California, Los Angeles; 1998. Recent advances in the archaeology of the Northern Andes: In memory of Gerardo Reichel-Dolmatoff. [Google Scholar]
- Cooke R. Prehistory of Native Americans on the Central American land bridge: Colonization, dispersal, and divergence. J. Archaeol. Res. 2005;13:129–187. [Google Scholar]
- Cooke R.G. Editorial Universitaria Carlos Manuel Gasteazoro; 2016. Orígenes, dispersión y supervivencia de las sociedades originarias de la subregión istmeña de América: Una reseña en el marco de la historia profunda. In Memoria: Encuentro el Mar del Sur: 500 años después, una visión interdisciplinaria; pp. 25–53. [Google Scholar]
- Cooke R., Jiménez M., Ranere A.J. Influencia humanas sobre la vegetación y fauna de vertebrados de Panamá: actualización de datos arqueozoológicos y su relación con el paisaje antrópico durante la época precolombina. In: Santos-Granero F.E., Santos-Granero F., editors. Ecología y Evolución en los Trópicos. Smithsonian Tropical Research Institute; 2007. pp. 562–593. [Google Scholar]
- Cooke R., Jiménez M., Ranere A.J. Archaeozoology art documents and the life assemblage. In: Reitz E., Scarry C.M., Scudder S.J., editors. Case studies in environmental archaeology. Springer; 2008. pp. 95–121. [Google Scholar]
- Cooke R., Ranere A., Pearson G., Dickau R. Radiocarbon chronology of early human settlement on the Isthmus of Panama (13,000–7000 BP) with comments on cultural affinities, environments, subsistence, and technological change. Quat. Int. 2013;301:3–22. [Google Scholar]
- Cooke R.G., Wake T.A., Martínez-Polanco M.F., Jiménez-Acosta M., Bustamante F., Holst I., Lara-Kraudy A., Martín J.G., Redwood S. Exploitation of dolphins (Cetacea: Delphinidae) at a 6000 yr old Preceramic site in the Pearl Island archipelago, Panama. J. Archaeol. Sci. Rep. 2016;6:733–756. [Google Scholar]
- Cooke R.G., Sánchez H.L.A., Smith-Guzmán N., Lara-Kraudy A. Panamá prehispánico. In: Castillero-Calvo A., editor. La Nueva Historia General de Panamá, Vol. I-I. Editora Novo Art, S.A.; 2019. [Google Scholar]
- Costenla A. Chibchan languages. In: Campbell L., Grondona V., editors. The Indigenous languages of South America: A Comprehensive Guide. Mouton de Gruyter; 2012. [Google Scholar]
- Dabney J., Knapp M., Glocke I., Gansauge M.T., Weihmann A., Nickel B., Valdiosera C., García N., Pääbo S., Arsuaga J.L., Meyer M. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. USA. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaneau O., Marchini J., Zagury J.F. A linear complexity phasing method for thousands of genomes. Nat. Methods. 2011;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- Dickau R. Microbotanical and macrobotanical evidence of plant use and the transition to agriculture in Panama. In: VanDerwacker A.M., Peres T.M., editors. Integrating Zooarchaeology and Paleoethnobotany. Springer; 2010. pp. 99–134. [Google Scholar]
- Dillehay T.D., Goodbred S., Pino M., Vásquez Sánchez V.F., Tham T.R., Adovasio J., Collins M.B., Netherly P.J., Hastorf C.A., Chiou K.L. Simple technologies and diverse food strategies of the Late Pleistocene and Early Holocene at Huaca Prieta, Coastal Peru. Sci. Adv. 2017;3:e1602778. doi: 10.1126/sciadv.1602778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Rambaut A., Shapiro B., Pybus O.G. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 2005;22:1185–1192. doi: 10.1093/molbev/msi103. [DOI] [PubMed] [Google Scholar]
- Fernandes D.M., Sirak K.A., Ringbauer H., Sedig J., Rohland N., Cheronet O., Mah M., Mallick S., Olalde I., Culleton B.J. A genetic history of the pre-contact Caribbean. Nature. 2020;590:103–110. doi: 10.1038/s41586-020-03053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Oviedo G. la Real Academia de la Historia; 1853. Historia general y natural de las Indias, Vol. 2. [Google Scholar]
- Flegontov P., Altınışık N.E., Changmai P., Rohland N., Mallick S., Adamski N., Bolnick D.A., Broomandkhoshbacht N., Candilio F., Culleton B.J. Palaeo-Eskimo genetic ancestry and the peopling of Chukotka and North America. Nature. 2019;570:236–240. doi: 10.1038/s41586-019-1251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis P. University of Texas Press; 2013. Kuna art and shamanism: an ethnographic approach. [Google Scholar]
- Fregel R., Méndez F.L., Bokbot Y., Martín-Socas D., Camalich-Massieu M.D., Santana J., Morales J., Ávila-Arcos M.C., Underhill P.A., Shapiro B. Ancient genomes from North Africa evidence prehistoric migrations to the Maghreb from both the Levant and Europe. Proc. Natl. Acad. Sci. USA. 2018;115:6774–6779. doi: 10.1073/pnas.1800851115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Mittnik A., Johnson P.L.F., Bos K., Lari M., Bollongino R., Sun C., Giemsch L., Schmitz R., Burger J. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr. Biol. 2013;23:553–559. doi: 10.1016/j.cub.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A., Rubin D.B. Inference from iterative simulation using multiple sequences. Stat. Sci. 1992;7:457–472. [Google Scholar]
- Gnecchi-Ruscone G.A., Sarno S., De Fanti S., Gianvincenzo L., Giuliani C., Boattini A., Bortolini E., Di Corcia T., Sanchez Mellado C., Dàvila Francia T.J. Dissecting the pre-Columbian genomic ancestry of Native Americans along the Andes-Amazonia divide. Mol. Biol. Evol. 2019;36:1254–1269. doi: 10.1093/molbev/msz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Carballa A., Pardo-Seco J., Brandini S., Achilli A., Perego U.A., Coble M.D., Diegoli T.M., Álvarez-Iglesias V., Martinón-Torres F., Olivieri A. The peopling of South America and the trans-Andean gene flow of the first settlers. Genome Res. 2018;28:767–779. doi: 10.1101/gr.234674.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.E., Malaspinas A.-S., Krause J., Briggs A.W., Johnson P.L.F., Uhler C., Meyer M., Good J.M., Maricic T., Stenzel U. A complete Neandertal mitochondrial genome sequence determined by high-throughput sequencing. Cell. 2008;134:416–426. doi: 10.1016/j.cell.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs J.C. The University of Texas at Austin; 2005. The archaeology of central Caribbean Panama. [Google Scholar]
- Grugni V., Battaglia V., Perego U.A., Raveane A., Lancioni H., Olivieri A., Ferretti L., Woodward S.R., Pascale J.M., Cooke R. Exploring the Y chromosomal ancestry of modern Panamanians. PLoS ONE. 2015;10:e0144223. doi: 10.1371/journal.pone.0144223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grugni V., Raveane A., Ongaro L., Battaglia V., Trombetta B., Colombo G., Capodiferro M.R., Olivieri A., Achilli A., Perego U.A. Analysis of the human Y-chromosome haplogroup Q characterizes ancient population movements in Eurasia and the Americas. BMC Biol. 2019;17:3. doi: 10.1186/s12915-018-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther T., Nettelblad C. The presence and impact of reference bias on population genomic studies of prehistoric human populations. PLoS Genet. 2019;15:e1008302. doi: 10.1371/journal.pgen.1008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms M.W. University of Texas Press; 2014. Ancient Panama: Chiefs in Search of Power. [Google Scholar]
- Hernández Mora I., Martín J.G., Aram B. The first Cathedral on America’s Pacific coast. Hist. Archaeol. 2021 doi: 10.1007/s41636-020-00275-z. Published online January 4, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K. Beyond the catastrophe: The volcanic landscape of Baru, western Panama. In: Gallen E.J., Torrence R., editors. Living under the Shadow: Cultural Impacts of Volcanic Eruptions. Left Coast Press; 2007. pp. 274–297. [Google Scholar]
- Homburger J.R., Moreno-Estrada A., Gignoux C.R., Nelson D., Sanchez E., Ortiz-Tello P., Pons-Estel B.A., Acevedo-Vasquez E., Miranda P., Langefeld C.D. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka F., Cooke R.G., Frame L., Vandiver P.B. Inferring provenance, manufacturing technique, and firing temperatures of the Monagrillo ware (3520-1300 cal BC), Panama’s first pottery. In: Martinón-Torres M., editor. Craft and science: International perspectives on archaeological ceramics. Bloomsbury Qatar Foundation; 2014. [DOI] [Google Scholar]
- Jones E.R., Zarina G., Moiseyev V., Lightfoot E., Nigst P.R., Manica A., Pinhasi R., Bradley D.G. The Neolithic transition in the Baltic was not driven by admixture with early European farmers. Curr. Biol. 2017;27:576–582. doi: 10.1016/j.cub.2016.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsson H., Ginolhac A., Schubert M., Johnson P.L., Orlando L. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics. 2013;29:1682–1684. doi: 10.1093/bioinformatics/btt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman N.M., Mayzel J., Jakobsson M., Rosenberg N.A., Mayrose I. Clumpak: a program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015;15:1179–1191. doi: 10.1111/1755-0998.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen T.S., Albrechtsen A., Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinformatics. 2014;15:356. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer B., Lindauer S., Synal H.-A., Wacker L. MAMS–a new AMS facility at the Curt-Engelhorn-Centre for Achaeometry, Mannheim, Germany. Nucl. Instrum. Methods Phys. Res. B. 2013;294:11–13. [Google Scholar]
- Lawson D.J., Hellenthal G., Myers S., Falush D. Inference of population structure using dense haplotype data. PLoS Genet. 2012;8:e1002453. doi: 10.1371/journal.pgen.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis I., Patterson N., Mittnik A., Renaud G., Mallick S., Kirsanow K., Sudmant P.H., Schraiber J.G., Castellano S., Lipson M. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis I., Nadel D., Rollefson G., Merrett D.C., Rohland N., Mallick S., Fernandes D., Novak M., Gamarra B., Sirak K. Genomic insights into the origin of farming in the ancient Near East. Nature. 2016;536:419–424. doi: 10.1038/nature19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie S., Winney B., Hellenthal G., Davison D., Boumertit A., Day T., Hutnik K., Royrvik E.C., Cunliffe B., Lawson D.J., Wellcome Trust Case Control Consortium 2. International Multiple Sclerosis Genetics Consortium The fine-scale genetic structure of the British population. Nature. 2015;519:309–314. doi: 10.1038/nature14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., Subgroup G.P.D.P., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares O.F. Adaptive strategies in western Panama. World Archaeol. 1977;8:304–319. [Google Scholar]
- Linares O.F. Dumbarton Oaks; 1977. Ecology and the Arts in Ancient Panama: On the Development of Social Rank and Symbolism in the Central Provinces. [Google Scholar]
- Linares O.F., Ranere A.J. Peabody Museum of Archaeology and Ethnology, Harvard University; 1980. Adaptive radiations in prehistoric Panama. [Google Scholar]
- Linares O.F., Sheets P.D., Rosenthal E.J. Prehistoric agriculture in tropical highlands. Science. 1975;187:137–145. doi: 10.1126/science.187.4172.137. [DOI] [PubMed] [Google Scholar]
- Lindo J., Achilli A., Perego U.A., Archer D., Valdiosera C., Petzelt B., Mitchell J., Worl R., Dixon E.J., Fifield T.E. Ancient individuals from the North American Northwest Coast reveal 10,000 years of regional genetic continuity. Proc. Natl. Acad. Sci. USA. 2017;114:4093–4098. doi: 10.1073/pnas.1620410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson M. Applying f4 -statistics and admixture graphs: Theory and examples. Mol. Ecol. Resour. 2020;20:1658–1667. doi: 10.1111/1755-0998.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Duong N.T., Ton N.D., Van Phong N., Pakendorf B., Van Hai N., Stoneking M. Extensive ethnolinguistic diversity in Vietnam reflects multiple sources of genetic diversity. Mol. Biol. Evol. 2020;37:2503–2519. doi: 10.1093/molbev/msaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas B., Fehren-Schmitz L., Valverde G., Soubrier J., Mallick S., Rohland N., Nordenfelt S., Valdiosera C., Richards S.M., Rohrlach A. Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Sci. Adv. 2016;2:e1501385. doi: 10.1126/sciadv.1501385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen J.A. Chocó I: Introduction and bibliography. Int. J. Am. Linguist. 1963;29:239–263. [Google Scholar]
- Lothrop S.K. Peabody Museum of Archaeology and Ethnology, Harvard University; 1937. Coclé: an archaeological study of central Panama part 1. [Google Scholar]
- Lothrop S.K. Peabody Museum of Archaeology and Ethnology, Harvard University; 1942. Coclé: an archaeological study of central Panama part 2. [Google Scholar]
- Manichaikul A., Mychaleckyj J.C., Rich S.S., Daly K., Sale M., Chen W.M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples B.K., Gravel S., Kenny E.E., Bustamante C.D. RFMix: a discriminative modeling approach for rapid and robust local-ancestry inference. Am. J. Hum. Genet. 2013;93:278–288. doi: 10.1016/j.ajhg.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín J.G. Excavaciones Arqueológicas en el Parque Morelos (Panamá La Vieja) In: Rovira B., Martín J.G., editors. No. 2. Patronato Panamá Viejo; Panamá Viejo, Panama: 2020. pp. 203–229. (Arqueologóa de Panamá la Vieja: Avances de investigación). [Google Scholar]
- Martín J.G. Panamá la Vieja y el Gran Darién. In: Rovira B., Martín J.G., editors. No. 2. Patronato Panamá Viejo; Panamá Viejo, Panama: 2020. pp. 230–250. (Arqueología de Panamá la Vieja: avances de investigación). [Google Scholar]
- Martín J.G. Universidad de Huelva; 2006. Arqueología de Panamá La Vieja: del asentamiento prehispánico a la ciudad colonial. [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011;17:10–12. [Google Scholar]
- Martín J.G., Sánchez L.A. El istmo mediterráneo: intercambio, simbolismo y filiación social en la bahía de Panamá durante el período 500-1000 DC. Arqueología del área intermedia. 2007;7:113–122. [Google Scholar]
- Martín J.G., Kenzler A.Z., Mommsen H., Kottmann A. Gres: la sutil presencia alemana en el Panamá colonial. Canto Rodado: Revista especializada en patrimonio. 2008;3:65–94. [Google Scholar]
- Martín J.G., Mendizábal T., Schreg R., Cooke R.G., Piperno D. Pre-Columbian raised fields in Panama: First evidence. J. Archaeol. Sci. Rep. 2015;3:558–564. [Google Scholar]
- Martín J.G., Cooke R.G., Bustamante F., Holst I., Lara A., Redwood S. Ocupaciones prehispánicas en Isla Pedro González, Archipielago de Las Perlas, Panamá: Aproximación a una cronología con comentarios sobre las conexiones externas. Lat. Am. Antiq. 2016;27:378–396. [Google Scholar]
- Martínez-Polanco M.F., Cooke R.G. Zooarchaeological and taphonomical study of the white-tailed deer (Cervidae: Odocoileus virginianus Zimmerman 1780) at Sitio Sierra, a pre-Columbian village in Pacific Coclé province, Panama, with an evaluation of its role in feasts. Archaeol. Anthropol. Sci. 2019;11:5405–5422. [Google Scholar]
- Martínez-Polanco M.F., Ranere A., Cooke R.G. Following white-tailed deer to the hilltop: A zooarchaeological and taphonomic analysis of deer hunting at Cerro Mangote, a Late Preceramic (7800-4600 cal yr BP) site in central Pacific Panama. Quat. Int. 2020 doi: 10.1016/j.quaint.2020.06.003. Published online June 26, 2020. [DOI] [Google Scholar]
- Mayo Torné J. Fundación El Caño; 2015. Golden Warriors: the lords of Rio Grande in Panama. [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal T.E. University of London; 2004. Panama Viejo: an analysis of the construction of archaeological time in Eastern Panama. [Google Scholar]
- Mittnik A., Wang C.-C., Svoboda J., Krause J. A molecular approach to the sexing of the triple burial at the Upper Paleolithic Site of Dolní Věstonice. PLoS ONE. 2016;11:e0163019. doi: 10.1371/journal.pone.0163019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A., Lancioni H., Cardinali I., Capodiferro M.R., Rambaldi Migliore N., Hussein A., Strobl C., Bodner M., Schnaller L., Xavier C. The mitogenome portrait of Umbria in Central Italy as depicted by contemporary inhabitants and pre-Roman remains. Sci. Rep. 2020;10:10700. doi: 10.1038/s41598-020-67445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy Kuhn J.M., Jakobsson M., Günther T. Estimating genetic kin relationships in prehistoric populations. PLoS ONE. 2018;13:e0195491. doi: 10.1371/journal.pone.0195491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montinaro F., Busby G.B., Pascali V.L., Myers S., Hellenthal G., Capelli C. Unravelling the hidden ancestry of American admixed populations. Nat. Commun. 2015;6:6596. doi: 10.1038/ncomms7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Estrada A., Gravel S., Zakharia F., McCauley J.L., Byrnes J.K., Gignoux C.R., Ortiz-Tello P.A., Martínez R.J., Hedges D.J., Morris R.W. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013;9:e1003925. doi: 10.1371/journal.pgen.1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Estrada A., Gignoux C.R., Fernández-López J.C., Zakharia F., Sikora M., Contreras A.V., Acuña-Alonzo V., Sandoval K., Eng C., Romero-Hidalgo S. Human genetics. The genetics of Mexico recapitulates Native American substructure and affects biomedical traits. Science. 2014;344:1280–1285. doi: 10.1126/science.1251688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Mayar J.V., Vinner L., de Barros Damgaard P., de la Fuente C., Chan J., Spence J.P., Allentoft M.E., Vimala T., Racimo F., Pinotti T. Early human dispersals within the Americas. Science. 2018;362:eaav2621. doi: 10.1126/science.aav2621. [DOI] [PubMed] [Google Scholar]
- Moreno-Mayar J.V., Korneliussen T.S., Dalal J., Renaud G., Albrechtsen A., Nielsen R., Malaspinas A.-S. A likelihood method for estimating present-day human contamination in ancient male samples using low-depth X-chromosome data. Bioinformatics. 2020;36:828–841. doi: 10.1093/bioinformatics/btz660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele K., Posth C., Iraeta Orbegozo M., Chinique de Armas Y., Hernández Godoy S.T., González Herrera U.M., Nieves-Colón M.A., Sandoval-Velasco M., Mylopotamitaki D., Radzeviciute R. Genomic insights into the early peopling of the Caribbean. Science. 2020;369:456–460. doi: 10.1126/science.aba8697. [DOI] [PubMed] [Google Scholar]
- Nakatsuka N., Lazaridis I., Barbieri C., Skoglund P., Rohland N., Mallick S., Posth C., Harkins-Kinkaid K., Ferry M., Harney É. A paleogenomic reconstruction of the deep population history of the Andes. Cell. 2020;181:1131–1145.e21. doi: 10.1016/j.cell.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre J., Johnson T., Bryc K., Kutalik Z., Boyko A.R., Auton A., Indap A., King K.S., Bergmann S., Nelson M.R. Genes mirror geography within Europe. Nature. 2008;456:98–101. doi: 10.1038/nature07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor L., Muysken P. Cambridge University Press; 2014. The native languages of South America: Origins, development, typology. [Google Scholar]
- Olivieri A., Sidore C., Achilli A., Angius A., Posth C., Furtwängler A., Brandini S., Capodiferro M.R., Gandini F., Zoledziewska M. Mitogenome diversity in Sardinians: A genetic window onto an island’s past. Mol. Biol. Evol. 2017;34:1230–1239. doi: 10.1093/molbev/msx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongaro L., Scliar M.O., Flores R., Raveane A., Marnetto D., Sarno S., Gnecchi-Ruscone G.A., Alarcón-Riquelme M.E., Patin E., Wangkumhang P. The genomic impact of European colonization of the Americas. Curr. Biol. 2019;29:3974–3986.e4. doi: 10.1016/j.cub.2019.09.076. [DOI] [PubMed] [Google Scholar]
- Oyuela-Calcedo A., Raymond J.S. ISD; 1998. Recent Advances in the Archaeology of the Northern Andes: In Memory of Gerardo Reichel-Dolmatoff, Vol. 39. [Google Scholar]
- Palencia-Madrid L., Cardoso S., Keyser C., López-Quintana J.C., Guenaga-Lizasu A., de Pancorbo M.M. Ancient mitochondrial lineages support the prehistoric maternal root of Basques in Northern Iberian Peninsula. Eur. J. Hum. Genet. 2017;25:631–636. doi: 10.1038/ejhg.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N., Price A.L., Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N., Moorjani P., Luo Y., Mallick S., Rohland N., Zhan Y., Genschoreck T., Webster T., Reich D. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer A., Jäger G., Herbig A., Seitz A., Kniep C., Krause J., Nieselt K. EAGER: efficient ancient genome reconstruction. Genome Biol. 2016;17:60. doi: 10.1186/s13059-016-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego U.A., Achilli A., Angerhofer N., Accetturo M., Pala M., Olivieri A., Hooshiar Kashani B., Ritchie K.H., Scozzari R., Kong Q.P. Distinctive Paleo-Indian migration routes from Beringia marked by two rare mtDNA haplogroups. Curr. Biol. 2009;19:1–8. doi: 10.1016/j.cub.2008.11.058. [DOI] [PubMed] [Google Scholar]
- Perego U.A., Angerhofer N., Pala M., Olivieri A., Lancioni H., Hooshiar Kashani B., Carossa V., Ekins J.E., Gómez-Carballa A., Huber G. The initial peopling of the Americas: a growing number of founding mitochondrial genomes from Beringia. Genome Res. 2010;20:1174–1179. doi: 10.1101/gr.109231.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego U.A., Lancioni H., Tribaldos M., Angerhofer N., Ekins J.E., Olivieri A., Woodward S.R., Pascale J.M., Cooke R., Motta J., Achilli A. Decrypting the mitochondrial gene pool of modern Panamanians. PLoS ONE. 2012;7:e38337. doi: 10.1371/journal.pone.0038337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter B.M. Admixture, population structure, and F-statistics. Genetics. 2016;202:1485–1501. doi: 10.1534/genetics.115.183913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell J.K., Pritchard J.K. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 2012;8:e1002967. doi: 10.1371/journal.pgen.1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhasi R., Fernandes D., Sirak K., Novak M., Connell S., Alpaslan-Roodenberg S., Gerritsen F., Moiseyev V., Gromov A., Raczky P. Optimal ancient DNA yields from the inner ear part of the human petrous bone. PLoS ONE. 2015;10:e0129102. doi: 10.1371/journal.pone.0129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotti T., Bergström A., Geppert M., Bawn M., Ohasi D., Shi W., Lacerda D.R., Solli A., Norstedt J., Reed K. Y chromosome sequences reveal a short Beringian Standstill, rapid expansion, and early population structure of Native American founders. Curr. Biol. 2019;29:149–157.e143. doi: 10.1016/j.cub.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Piperno D.R. The origins of plant cultivation and domestication in the New World tropics: patterns, process, and new developments. Curr. Anthropol. 2011;52:S453–S470. [Google Scholar]
- Politis G.G., Gutiérrez M.A., Rafuse D.J., Blasi A. The Arrival of Homo sapiens into the Southern Cone at 14,000 Years Ago. PLoS ONE. 2016;11:e0162870. doi: 10.1371/journal.pone.0162870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posth C., Renaud G., Mittnik A., Drucker D.G., Rougier H., Cupillard C., Valentin F., Thevenet C., Furtwängler A., Wißing C. Pleistocene mitochondrial genomes suggest a single major dispersal of Non-Africans and a Late Glacial population turnover in Europe. Curr. Biol. 2016;26:827–833. doi: 10.1016/j.cub.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Posth C., Nakatsuka N., Lazaridis I., Skoglund P., Mallick S., Lamnidis T.C., Rohland N., Nägele K., Adamski N., Bertolini E. Reconstructing the deep population history of Central and South America. Cell. 2018;175:1185–1197.e22. doi: 10.1016/j.cell.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznik G.D. Identifying Y-chromosome haplogroups in arbitrarily large samples of sequenced or genotyped men. bioRxiv. 2016:088716. [Google Scholar]
- Poznik G.D., Xue Y., Mendez F.L., Willems T.F., Massaia A., Wilson Sayres M.A., Ayub Q., McCarthy S.A., Narechania A., Kashin S., 1000 Genomes Project Consortium Punctuated bursts in human male demography inferred from 1,244 worldwide Y-chromosome sequences. Nat. Genet. 2016;48:593–599. doi: 10.1038/ng.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prates L., Politis G.G., Perez S.I. Rapid radiation of humans in South America after the last glacial maximum: A radiocarbon-based study. PLoS ONE. 2020;15:e0236023. doi: 10.1371/journal.pone.0236023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Coop G. The geography of recent genetic ancestry across Europe. PLoS Biol. 2013;11:e1001555. doi: 10.1371/journal.pbio.1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C.B., Lee S. Recent and planned developments of the program OxCal. Radiocarbon. 2013;55:720–730. [Google Scholar]
- Ranere A.J., Cooke R.G. Late glacial and Early Holocene migrations, and Middle Holocene settlement on the lower isthmian land-bridge. Quat. Int. 2020 doi: 10.1016/j.quaint.2020.06.002. Published online June 26, 2020. [DOI] [Google Scholar]
- Raveane A., Aneli S., Montinaro F., Athanasiadis G., Barlera S., Birolo G., Boncoraglio G., Di Blasio A.M., Di Gaetano C., Pagani L. Population structure of modern-day Italians reveals patterns of ancient and archaic ancestries in Southern Europe. Sci. Adv. 2019;5:eaaw3492. doi: 10.1126/sciadv.aaw3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich D., Patterson N., Campbell D., Tandon A., Mazieres S., Ray N., Parra M.V., Rojas W., Duque C., Mesa N. Reconstructing Native American population history. Nature. 2012;488:370–374. doi: 10.1038/nature11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer P.J., Bard E., Bayliss A., Beck J.W., Blackwell P.G., Ramsey C.B., Buck C.E., Cheng H., Edwards R.L., Friedrich M. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- Reimer P.J., Austin W.E., Bard E., Bayliss A., Blackwell P.G., Ramsey C.B., Butzin M., Cheng H., Edwards R.L., Friedrich M. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP) Radiocarbon. 2020;62:725–757. [Google Scholar]
- Renaud G., Slon V., Duggan A.T., Kelso J. Schmutzi: estimation of contamination and endogenous mitochondrial consensus calling for ancient DNA. Genome Biol. 2015;16:224. doi: 10.1186/s13059-015-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riris P., Arroyo-Kalin M. Widespread population decline in South America correlates with mid-Holocene climate change. Sci. Rep. 2019;9:6850. doi: 10.1038/s41598-019-43086-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romoli K. Ediciones Tercer Mundo; 1987. Los de la lengua de Cueva. [Google Scholar]
- Rosenberg N.A. Dros. Inf. Serv.TRUCT: a program for the graphical display of population structure. Mol. Ecol. Notes. 2004;4:137–138. [Google Scholar]
- Saag L., Laneman M., Varul L., Malve M., Valk H., Razzak M.A., Shirobokov I.G., Khartanovich V.I., Mikhaylova E.R., Kushniarevich A. The arrival of Siberian ancestry connecting the Eastern Baltic to Uralic speakers further East. Curr. Biol. 2019;29:1701–1711.e1716. doi: 10.1016/j.cub.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Herrera L.A., Cooke R.G. Cubitá: un nuevo eslabón estilístico en la tradición cerámica del “Gran Coclé”, Panamá. Precolombart. 2000;3:5–20. [Google Scholar]
- Sánchez-Quinto F., Malmström H., Fraser M., Girdland-Flink L., Svensson E.M., Simões L.G., George R., Hollfelder N., Burenhult G., Noble G. Megalithic tombs in western and northern Neolithic Europe were linked to a kindred society. Proc. Natl. Acad. Sci. USA. 2019;116:9469–9474. doi: 10.1073/pnas.1818037116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Ward R.H., Barrantes R. mtDNA variation in the Chibcha Amerindian Huetar from Costa Rica. Hum. Biol. 1994;66:963–977. [PubMed] [Google Scholar]
- Scheib C.L., Li H., Desai T., Link V., Kendall C., Dewar G., Griffith P.W., Mörseburg A., Johnson J.R., Potter A. Ancient human parallel lineages within North America contributed to a coastal expansion. Science. 2018;360:1024–1027. doi: 10.1126/science.aar6851. [DOI] [PubMed] [Google Scholar]
- Schroeder H., Sikora M., Gopalakrishnan S., Cassidy L.M., Maisano Delser P., Sandoval Velasco M., Schraiber J.G., Rasmussen S., Homburger J.R., Ávila-Arcos M.C. Origins and genetic legacies of the Caribbean Taino. Proc. Natl. Acad. Sci. USA. 2018;115:2341–2346. doi: 10.1073/pnas.1716839115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M., Pitulko V.V., Sousa V.C., Allentoft M.E., Vinner L., Rasmussen S., Margaryan A., de Barros Damgaard P., de la Fuente C., Renaud G. The population history of northeastern Siberia since the Pleistocene. Nature. 2019;570:182–188. doi: 10.1038/s41586-019-1279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P., Reich D. A genomic view of the peopling of the Americas. Curr. Opin. Genet. Dev. 2016;41:27–35. doi: 10.1016/j.gde.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P., Storå J., Götherström A., Jakobsson M. Accurate sex identification of ancient human remains using DNA shotgun sequencing. J. Archaeol. Sci. 2013;40:4477–4482. [Google Scholar]
- Skoglund P., Mallick S., Bortolini M.C., Chennagiri N., Hünemeier T., Petzl-Erler M.L., Salzano F.M., Patterson N., Reich D. Genetic evidence for two founding populations of the Americas. Nature. 2015;525:104–108. doi: 10.1038/nature14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. Plant remains from the Volcán sites. In: Linares O.F., Raner A.J., editors. Adaptive Radiations in Prehistoric Panama. Peabody Museum of Archaeology and Ethnology, Harvard University; 1980. [Google Scholar]
- Smith-Guzmán N.E., Cooke R.G. Interpersonal violence at Playa Venado, Panama (550-850 AD): a reevaluation of the evidence. Lat. Am. Antiq. 2018;29:718–735. [Google Scholar]
- Soares P., Ermini L., Thomson N., Mormina M., Rito T., Röhl A., Salas A., Oppenheimer S., Macaulay V., Richards M.B. Correcting for purifying selection: an improved human mitochondrial molecular clock. Am. J. Hum. Genet. 2009;84:740–759. doi: 10.1016/j.ajhg.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuiver M., Polach H.A. Discussion reporting of 14 C data. Radiocarbon. 1977;19:355–363. [Google Scholar]
- Tamm E., Kivisild T., Reidla M., Metspalu M., Smith D.G., Mulligan C.J., Bravi C.M., Rickards O., Martinez-Labarga C., Khusnutdinova E.K. Beringian standstill and spread of Native American founders. PLoS ONE. 2007;2:e829. doi: 10.1371/journal.pone.0000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F.C. La Gran Chiriquí: Una historia cada vez más profunda. Canto Rodado. 2017;11:27–58. [Google Scholar]
- Van Oven M. PhyloTree Build 17: Growing the human mitochondrial DNA tree. Forensic Sci. Int. Genet. 2015;5:e392–e394. [Google Scholar]
- Waters M.R. Late Pleistocene exploration and settlement of the Americas by modern humans. Science. 2019;365:eaat5447. doi: 10.1126/science.aat5447. [DOI] [PubMed] [Google Scholar]
- Weissensteiner H., Pacher D., Kloss-Brandstätter A., Forer L., Specht G., Bandelt H.J., Kronenberg F., Salas A., Schönherr S. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016;44(W1):W58–W63. doi: 10.1093/nar/gkw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Spyrou M.A., Karapetian M., Shnaider S., Radzevičiūtė R., Nägele K., Neumann G.U., Penske S., Zech J., Lucas M. Paleolithic to Bronze Age Siberians reveal connections with First Americans and across Eurasia. Cell. 2020;181:1232–1245.e20. doi: 10.1016/j.cell.2020.04.037. [DOI] [PubMed] [Google Scholar]
- Zohar I., Cooke R. The role of dried fish: A taphonomical model of fish butchering and long-term preservation. J. Archaeol. Sci. Rep. 2019;26:101864. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ancient and modern Isthmian individuals analyzed in this study.
Post-mortem damage pattern, length distribution of reads, molecular sex determination and family relationships.
Published ancient and modern data used for comparison.
We tested the form f4 (W/Ancient IA, X/Modern IA; Y/Isthmus, Mbuti) on uIA89, mIA417 and ancient genomes from different geographic regions (considering only transversions): North America; Central America; South American Pacific Coast and South American Atlantic Coast (plus Anzick-1 and Spirit Cave). In the last comparison we tested ancient IA genome pairs in the form f4 (W/Ancient IA, X/Ancient IA; Y/Isthmus, Mbuti). See the legend of Figure 6A for further details.
Data Availability Statement
The accession number for the ancient DNA sequencing data reported in this paper is ENA: PRJEB42372. Modern genotype data have been deposited to Mendeley Data: https://doi.org/10.17632/d45xg84bcj.1. The accession numbers for modern complete mitogenomes reported in this paper are GeneBank: MW467798-MW467881. Scripts used to infer Y chromosome aDNA haplogroups are available on GitHub (https://github.com/raveancic/aDNAYchromosome), all the other scripts used for analysis and plots are available upon request.