Abstract
Background
Parainfluenza virus (PIV) is a leading cause of acute respiratory illness (ARI) in children. However, few studies have characterized the clinical features and outcomes associated with PIV infections among young children in the Middle East.
Methods
We conducted hospital-based surveillance for ARI among children < 2 years of age in a large referral hospital in Amman, Jordan. We systematically collected clinical data and respiratory specimens for pathogen detection using reverse transcription polymerase chain reaction. We compared clinical features of PIV-associated ARI among individual serotypes 1, 2, 3, and 4 and among PIV infections compared with other viral ARI and ARI with no virus detected. We also compared the odds of supplemental oxygen use using logistic regression.
Results
PIV was detected in 221/3168 (7.0%) children hospitalized with ARI. PIV-3 was the most commonly detected serotype (125/221; 57%). Individual clinical features of PIV infections varied little by individual serotype, although admission diagnosis of ‘croup’ was only associated with PIV-1 and PIV-2. Children with PIV-associated ARI had lower frequency of cough (71% vs 83%; p < 0.001) and wheezing (53% vs 60% p < 0.001) than children with ARI associated with other viruses. We did not find a significant difference in supplemental oxygen use between children with PIV-associated infections (adjusted odds ratio [aOR] 1.12, 95% CI 0.66–1.89, p = 0.68) and infections in which no virus was detected.
Conclusions
PIV is frequently associated with ARI requiring hospitalization in young Jordanian children. Substantial overlap in clinical features may preclude distinguishing PIV infections from other viral infections at presentation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06001-1.
Keywords: Parainfluenza virus, Acute respiratory illness, Viral pneumonia, Young children, Jordan
Background
Acute respiratory illnesses (ARI) are a leading cause of morbidity and mortality among children globally, and respiratory viruses are increasingly recognized as important etiologies of severe ARI in young children [1, 2]. Parainfluenza virus (PIV) is commonly detected in ARI across all age groups, with highest prevalence typically occurring among young children [2–6]. Clinical features associated with PIV infections range from asymptomatic infection or mild upper respiratory disease to lower respiratory tract illness, with symptoms typically more severe among infants and young children than older children and adults [7–9].
Four distinct serotypes of PIV have been associated with human disease, and individual serotypes may vary with regards to certain clinical features. PIV-3 is the most prevalent serotype and is often associated with lower respiratory disease, including bronchiolitis and pneumonia, while PIV-1 and PIV-2 infections in children are often associated with laryngotracheobronchitis (“croup”) [10, 11]. Due to difficulties in isolation and typical association with milder disease, PIV-4 has often been excluded from many epidemiologic surveillance studies [12], although with advancements in molecular diagnosis, there is increasing evidence that PIV-4 may be associated with more severe, lower respiratory tract disease [2]. Individual PIV serotypes may also vary in terms of their seasonal patterns of detection. In temperate regions, peak PIV-2 and PIV-3 detections typically occur annually in the late spring (April–June) and autumn (October–November), while PIV-1 exhibits biennial peaks later in the year in odd years [11, 13], although serotype-specific seasonality information is scarce in many regions [14].
Despite the importance of PIV as a common etiology of severe respiratory illnesses in children, many studies of PIV infections in children have focused on those with pneumonia and have excluded children with other forms of ARI [12, 15]. We conducted a prospective study of ARI-associated hospitalizations among young children < 2 years of age over a three-year period in Amman, Jordan. We sought to define the clinical features of PIV infections in young children and to compare these among serotypes 1, 2, 3, and 4 as well as with ARI associated with other respiratory viruses.
Methods
Study design and setting
We enrolled children less than 2 years of age admitted with ARI (fever and/or respiratory symptoms) to Al-Bashir Government Hospital in Amman, Jordan from March 2010 to March 2013. Detailed characterization of the study population, setting, and design have been previously published [16, 17]. Briefly, children were eligible if they were admitted with fever and/or a respiratory symptom (e.g. cough, shortness of breath, wheezing) and one of the following admission diagnoses: ARI, apnea, asthma exacerbation, bronchiolitis, bronchopneumonia, croup, cystic fibrosis exacerbation, febrile seizure, fever without localizing signs, respiratory distress, pneumonia, pneumonitis, pertussis, pertussis-like cough, rule-out sepsis (evaluation of neonates with fever), upper respiratory infection (URI) and/or a lower respiratory tract illness. Lower respiratory tract illness (LRTI) was defined as an admission diagnosis of asthma, bronchiolitis, bronchopneumonia, pneumonia, respiratory distress, or wheezing and/or the presence of retractions/accessory muscle use or wheezing on examination [16]. Children with neutropenia secondary to chemotherapy and newborns who had never been discharged from the hospital after birth were excluded from the study. Active, year-round, hospital-based surveillance was conducted to recruit eligible children during 5 days of each week (Sunday-Thursday) within 48 h of hospital admission.
Al-Bashir Hospital is the major government-run referral center that serves Amman (estimated population > 2 million). The hospital provided care for an estimated 50–60% of children residing in Amman during the study period [16]. During the study period, 11,230 of the 17,557 children (64%) admitted to the pediatric wards were under 2 years of age. Written informed consent was obtained from parents or guardians of children prior to enrollment and initiation of study activities. The institutional review boards of the University of Jordan, the Jordanian Ministry of Health, and Vanderbilt University approved the study. All methods were performed in accordance with relevant guidelines and regulations.
Data collection
After informed consent, trained local research staff interviewed parents/legal guardians in Arabic using a standardized questionnaire that included Arabic and English translations to record demographic, clinical, and socioeconomic data, including the presence of underlying medical conditions (UMCs) and household smoke (cigarette and/or hookah pipe) exposure. After hospital discharge, charts were abstracted for the following: antibiotic use, blood culture results, chest radiography results, oxygen use, intensive care unit (ICU) admission, mechanical ventilation (MV), length of stay (LOS) in the hospital, and status at discharge. All data were entered into a standardized, secure Research Electronic Data Capture (REDCap) database (Vanderbilt University, Nashville, Tennessee, USA). We performed data quality checks on a minimum of 10% of the charts and verified data from all case report forms after entry. The following were considered UMCs: diabetes, heart disease, Down syndrome, kidney disease, sickle cell disease, cystic fibrosis, cancer, genetic/metabolic, cerebral palsy, neurological, mental retardation/developmental delay, seizure disorder, chronic diarrhea (> 2 weeks), gastroesophageal reflux disease, immunodeficiency, asthma/reactive airway disease, or liver disease.
Sample collection and testing
Nasal and throat swabs were collected from enrolled subjects and combined in transport medium (M4RT, Remel, USA), aliquoted into MagMAX Lysis/Binding Solution Concentrate (Life Technologies, USA), snap-frozen and then stored at − 80 °C. Aliquots were shipped on dry ice to Nashville, Tennessee, USA, and were tested by individual single-plex RT-PCR for 16 viruses: respiratory syncytial virus (RSV); human rhinovirus (HRV); human metapneumovirus (HMPV); influenza (influenza) A, B and C; parainfluenza virus (PIV) 1, 2, 3, and 4; human adenovirus (AdV), human coronaviruses (HCoV) HKU1, OC43, 229E, and NL63, and Middle East respiratory syndrome coronavirus (MERS-CoV) [18].
Statistical analysis
We compared common clinical features and seasonality of detection in patients with PIV-1, PIV-2, PIV-3 and PIV-4-associated respiratory illness, as defined by detection of each PIV serotype from a respiratory sample by PCR, including co-detections of other respiratory viruses. Next, we compared features of PIV-only respiratory illness, excluding co-detections with other viruses, to ARI associated with detection of other viruses (sole or co-detection of hMPV, RSV, AdV, MERS-CoV, influenza, or HRV), and ARI with no virus detected. These categories were mutually exclusive. Chi-square and two sample t-tests allowing unequal variances were used to compare categorical and continuous variables, respectively, between groups.
Linear regression with robust standard errors was used to evaluate the association between ARI etiologic category (PIV, other viruses, no virus) and time to hospital discharge (hospital length of stay [LOS] measured in hours). Model covariates included age, sex, breastfeeding status, antibiotic use prior to admission, duration of symptoms prior to admission, smoke exposure, and presence of at least one UMC. Logistic regression with robust standard errors was used to evaluate the association between ARI etiologic category (PIV, other viruses, no virus) and the need for supplemental oxygen as a surrogate indicator of illness severity, using the same covariates as in the linear regression model. All analyses were conducted using statistical software StataIC 16.0 (StatCorp LLC, College Station, TX). For all statistical tests, we used a nominal significance level of α = 0.05.
Results
PIV-associated acute respiratory illness
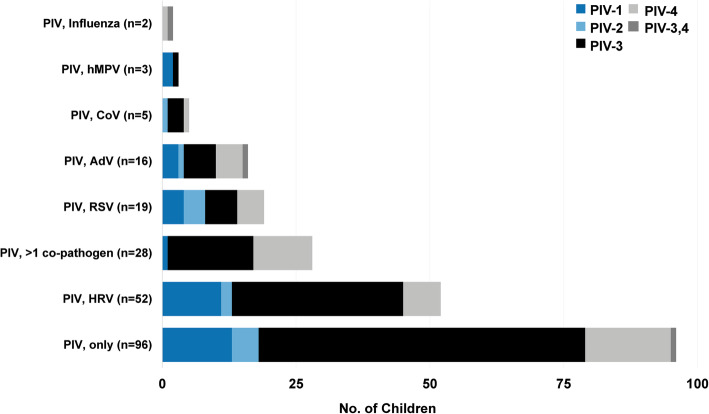
Among 3168 children admitted with ARI and included in this study, PIV was detected by nasal/throat swab PCR in 221 (7.0%; Supplementary Figure 1). Other viruses were commonly co-detected with PIV (125/221, 57%; Fig. 1); most commonly HRV (24%), RSV (9%), and adenovirus (7%). PIV-3 was the most commonly detected of the PIV serotypes (128/221, 58%, Table 1), followed by PIV-4 (49/221; 22%), PIV-1 (34/221; 15%), and PIV-2 (13/221; 6%). PIV-3 and PIV-4 were codetected in 3 children. Three of 95 children with PIV infection from whom a blood culture was collected had a positive blood culture result [Staphylococcus aureus (22 day old with discharge diagnosis of sepsis; n = 1), Candida spp. (40 day old with discharge diagnoses of pneumonia and sepsis; n = 1), Micrococcus spp. (suspected contaminant; n = 1)].
Fig. 1.
Co-detection of parainfluenza viruses with other respiratory viruses
Table 1.
Characteristics of hospitalized children with PIV-1, PIV-2, PIV-3, and PIV-4 including co-detections with other pathogens
| PIV-1 (n = 34) No. (%) |
PIV-2 (n = 13) No. (%) |
PIV-3a (n = 125) No. (%) |
PIV-4a (n = 46) No. (%) |
p-value | |
|---|---|---|---|---|---|
| Female sex | 16 (47.1) | 6 (46.2) | 48 (38.4) | 21 (45.7) | 0.714 |
| Median age, months (IQR) | 9.0 (3.5–16.1) | 5.4 (2.6–8.6) | 3.2 (1.7–8.9) | 2.6 (1.7–9.1) | 0.003 |
| Age group | |||||
| < 6 months | 13 (38.2) | 7 (53.9) | 80 (64.0) | 29 (63.0) | 0.028 |
| 6 to < 12 months | 7 (20.6) | 5 (38.5) | 23 (18.4) | 8 (17.4) | |
| 12 to < 24 months | 14 (41.2) | 1 (7.7) | 22 (17.6) | 9 (19.6) | |
| Received influenza vaccine | 0 (0) | 0 (0) | 1 (1.0) | 0 (0) | 0.861 |
| Breastfed, any | 30 (88.2) | 13 (100) | 92 (73.6) | 37 (80.4) | 0.059 |
| Any underlying medical conditionb | 3 (8.8) | 2 (15.4) | 19 (15.2) | 6 (13.0) | 0.810 |
| Household smoke exposure | 23 (67.7) | 10 (76.9) | 95 (76.0) | 37 (80.4) | 0.620 |
| Symptom duration prior to admission, days (IQR) | 3.5 (2–5) | 2 (2–4) | 3 (2–5) | 2 (1–5) | 0.253 |
| Antibiotics prior to admission | 17 (50.0) | 3 (23.1) | 42 (33.6) | 19 (41.3) | 0.210 |
| Antibiotics during hospitalization | 33 (97.1) | 9 (69.2) | 115 (92.0) | 44 (95.7) | 0.010 |
| Clinical presentation | |||||
| Fever/feverishc | 21 (61.8) | 9 (69.2) | 77 (61.6) | 27 (58.7) | 0.923 |
| Coughc | 30 (88.2) | 10 (76.9) | 90 (72.0) | 36 (78.3) | 0.258 |
| Shortness of breathc | 23 (67.7) | 9 (69.2) | 55 (44.0) | 28 (60.9) | 0.045 |
| Flaring/Retractionsd | 14 (41.2) | 5 (38.5) | 36 (28.8) | 15 (32.6) | 0.541 |
| Retractions, onlyd | 2 (5.9) | 1 (7.7) | 4 (3.2) | 4 (8.7) | 0.492 |
| Wheezingd | 20 (58.8) | 6 (46.2) | 62 (49.6) | 22 (47.8) | 0.750 |
| Admission diagnosis | |||||
| Reactive airway disease | 3 (8.8) | 1 (7.7) | 4 (3.2) | 1 (2.2) | 0.387 |
| Bronchiolitis | 6 (17.7) | 3 (23.1) | 14 (11.2) | 10 (21.7) | 0.275 |
| Bronchopneumonia | 14 (41.2) | 4 (30.8) | 48 (38.4) | 13 (28.3) | 0.563 |
| Croup | 2 (5.9) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0.010 |
| Febrile seizure | 2 (5.9) | 1 (7.7) | 4 (3.2) | 1 (2.2) | 0.697 |
| Pneumonia | 4 (11.8) | 0 (0.0) | 12 (9.6) | 5 (10.9) | 0.649 |
| Pertussis-like cough | 0 (0.0) | 0 (0.0) | 9 (7.2) | 4 (8.7) | 0.269 |
| Rule-out sepsis | 5 (14.7) | 3 (23.1) | 40 (32.0) | 11 (23.9) | 0.211 |
| Classified as LRTI | 28 (82.4) | 9 (69.2) | 84 (67.2) | 31 (67.4) | 0.382 |
| Chest radiograph | |||||
| Abnormal | 23/31 (74.2) | 8/11 (72.7) | 78/116 (67.2) | 28/44 (63.4) | 0.785 |
| Required supplemental oxygen | 11 (32.4) | 2 (15.4) | 35 (28.0) | 14 (30.4) | 0.695 |
| Required ICU admission | 5 (14.7) | 0 (0.0) | 9 (7.2) | 3 (6.5) | 0.318 |
| Required mechanical ventilation | 2 (5.9) | 0 (0.0) | 5 (4.0) | 0 (0.0) | 0.393 |
| Length of stay (days), median (IQR) | 3 (2–6) | 5 (3–8) | 6 (3–8) | 5 (3–8) | 0.161 |
| Codetected with ≥1 other virus | 21 (61.8) | 8 (61.5) | 64 (51.2) | 30 (65.2) | 0.338 |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 0.431 |
a Individual serotypes detected and distinguished by PCR; excludes 3 cases of codetection of PIV-3 and PIV-4
+ p < 0.05 considered statistically significant, indicated by bold text
b UMC defined as at least one of the following: diabetes, heart disease, Down syndrome, kidney disease, sickle cell disease, cystic fibrosis, cancer, genetic/metabolic, cerebral palsy, neurological, mental retardation/developmental delay, seizure disorder, chronic diarrhea (eg, > 2 weeks), gastroesophageal reflux disease, immunodeficiency, asthma/reactive airway disease, liver disease
c Reported by parent/legal guardian
d Collected through clinical exam
Seasonality of PIV detections
While PIV detections occurred throughout the year during our study, peak PIV detections in 2011 occurred in November–December (Fig. 2), while in 2012 a earlier peak occurred in July in addition to the winter (November 2012 to January 2013) peak. PIV detections in the spring-summer months were primarily contributed by PIV-3. The majority of PIV-1 detections occurred in 2011.
Fig. 2.
Detection of parainfluenza virus by month and year among Jordanian children admitted with acute respiratory illness from March 2010 to March 2013
Clinical features of PIV-1, PIV-2, PIV-3, and PIV-4 infections
The clinical features of infections associated with individual PIV serotypes, inclusive of co-detections with other viruses, are displayed in Table 1. The median age of hospitalized children with PIV-3 and PIV-4 detections was significantly lower than those with PIV-1 or PIV-2 (Table 1). The proportion of children with an underlying medical condition was similar among children with infection associated with each of the PIV serotypes. Greater than 90% of children with PIV-1, PIV-3, and PIV-4 infections received antibiotics during hospitalization, compared to only 69% of those hospitalized with PIV-2 associated infections (Table 1). The clinical symptoms of PIV infections did not vary significantly according to individual serotype. Bronchopneumonia, rule-out sepsis, and bronchiolitis were the most common admission diagnoses assigned to PIV 1–4 infections; an admission diagnosis of “croup” was only assigned to admissions associated with PIV-1 or PIV-2, while “pertussis-like cough” was assigned only to PIV-3 and PIV-4 associated admissions. Only 2/13 (15%) children with PIV-2-associated infections required supplemental oxygen, compared to 32, 28, and 30% of children with PIV-1, PIV-3, and PIV-4-associated infections, respectively, however this difference was not statistically significant (p = 0.695). The clinical features of infections associated with individual PIV serotypes, excluding co-detections with other viruses, are displayed in Supplementary Table 1.
Features of PIV-associated infections compared to other viral infections
After exclusion of infections in which PIV was codetected with other respiratory viruses, the clinical features of children with PIV-only associated ARI compared to other respiratory viruses or no virus detection are displayed in Table 2. Compared to both groups, PIV-only children had higher mean age (Table 2). Children with PIV had a higher frequency of fever (63%) than those with ARI associated with other viruses (53%; p < 0.001), but they had a lower frequency of nasal flaring/chest retractions (33% vs. 46%, p < 0.001) and wheezing (53% vs. 60%, p < 0.001). A higher frequency of children with PIV-only and other virus-associated ARI met the LRTI definition, had longer symptom onset prior to admission, and had higher occurrence of cough, nasal flaring/chest retractions, and wheezing compared to children with no virus detected. The most common admission diagnoses assigned to PIV-only group in descending order were bronchopneumonia, rule-out sepsis, pneumonia, and bronchiolitis while bronchopneumonia, bronchiolitis, rule-out sepsis, pneumonia were the most common admission diagnoses for any PIV detection (Tables 1 and 2). The frequency of croup as an admission diagnosis was higher in children with PIV-only compared to the other groups, while the frequencies of bronchiolitis, bronchopneumonia, and pneumonia were higher among the PIV-only and other virus group compared to the children in whom no virus was detected. In unadjusted comparisons, fewer children with PIV-associated infection required supplemental oxygen (23%) or ICU admission (6%) than those with ARI associated with other viruses (35 and 9%, respectively; p < 0.001 and p = 0.048).
Table 2.
Characteristics of hospitalized children with PIV-associated respiratory illness compared to other-viral and virus-negative respiratory illness
| PIV only* (n = 96) No. (%) |
Other virus, not PIV (n = 2413) No. (%) |
No virus (n = 534) No. (%) |
p-value+ | |
|---|---|---|---|---|
| Female sex | 39 (40.6) | 954 (39.5) | 209 (39.1) | 0.960 |
| Median age, months (IQR) | 4.2 (1.7–10.8) | 3.8 (1.8–8.5) | 2.3 (1.1–7.6) | < 0.001 |
| Age group | ||||
| < 6 months | 54 (56.3) | 1544 (64.0) | 382 (71.5) | 0.001 |
| 6 to < 12 months | 19 (19.8) | 499 (20.7) | 79 (14.8) | |
| 12 to < 24 months | 23 (24.0) | 370 (15.3) | 73 (13.7) | |
| Received influenza vaccine | 0 (0.0) | 5 (0.21) | 1 (0.2) | 0.264 |
| Breastfed | 78 (81.3) | 2034 (84.3) | 453 (84.8) | 0.674 |
| Any underlying medical conditiona | 12 (12.5) | 269 (11.2) | 75 (14.0) | 0.164 |
| Asthma/reactive airway disease | 1 (1.0) | 65 (2.7) | 10 (1.9) | 0.285 |
| Household smoke exposure | 74 (77.1) | 1851 (76.7) | 406 (76.0) | 0.939 |
| Symptom duration prior to admission | 3 (1.5–4) | 3 (2–5) | 2 (1–3) | 0.002 |
| Antibiotics during hospitalization | 89 (92.7) | 2193 (90.9) | 489 (91.6) | 0.746 |
| Clinical presentation | ||||
| Fever/feverishb | 60 (62.5) | 1279 (53.0) | 347 (65.0) | < 0.001 |
| Coughb | 68 (70.8) | 1990 (82.5) | 207 (38.8) | < 0.001 |
| Shortness of breathb | 48 (50.0) | 1543 (64.0) | 172 (32.2) | < 0.001 |
| Flaring/Retractionsc | 32 (33.3) | 1099 (45.5) | 121 (22.7) | < 0.001 |
| Retractions, onlyc | 5 (5.2) | 240 (10.0) | 30 (5.6) | 0.003 |
| Wheezingc | 51 (53.1) | 1455 (60.3) | 189 (35.4) | < 0.001 |
| Admission diagnosis | ||||
| Reactive airway disease | 4 (4.2) | 123 (5.1) | 14 (2.6) | 0.047 |
| Bronchiolitis | 10 (10.4) | 483 (20.0) | 31 (5.8) | < 0.001 |
| Bronchopneumonia | 34 (35.4) | 818 (33.9) | 121 (22.7) | < 0.001 |
| Croup | 2 (2.1) | 6 (0.3) | 1 (0.2) | 0.005 |
| Febrile seizure | 5 (5.2) | 47 (2.0) | 28 (5.2) | < 0.001 |
| Pneumonia | 14 (14.6) | 338 (14.0) | 34 (6.4) | < 0.001 |
| Pneumonitis | 0 (0.0) | 1 (0.04) | 1 (0.19) | 0.477 |
| Pertussis-like cough | 6 (6.3) | 182 (7.5) | 22 (4.1) | 0.018 |
| Rule-out sepsis | 27 (28.1) | 559 (23.2) | 282 (52.8) | < 0.001 |
| Classified as LRTI | 68 (70.8) | 1878 (77.8) | 230 (43.1) | < 0.001 |
| Chest radiograph | ||||
| Abnormal | 60/90 (66.7) | 1734/2302 (75.3) | 203/454 (44.7) | < 0.001 |
| Required supplemental oxygen | 22 (22.9) | 829/2384 (34.8) | 122/532 (22.9) | < 0.001 |
| Required ICU admission | 6 (6.3) | 205 (8.5) | 62 (11.6) | 0.048 |
| Required mechanical ventilation | 3 (3.1) | 82/2383 (3.4) | 22/532 (4.1) | 0.717 |
| Length of stay (days), median (IQR) | 5 (3–8) | 5 (3–7) | 5 (3–8) | 0.154 |
| Death | 0 (0.0) | 21 (1.0) | 9 (2.0) | 0.140 |
* One child with both PIV 3 and PIV 4 serotypes is included in the PIV only group
+ p < 0.05 considered statistically significant, indicated by bold text
a UMC defined as at least one of the following: diabetes, heart disease, Down syndrome, kidney disease, sickle cell disease, cystic fibrosis, cancer, genetic/metabolic, cerebral palsy, neurological, mental retardation/developmental delay, seizure disorder, chronic diarrhea (eg, > 2 weeks), gastroesophageal reflux disease, immunodeficiency, asthma/reactive airway disease, liver disease
b Reported by parent/legal guardian
c Collected through clinical exam
Multivariable analysis: hospital length of stay
We used linear regression to compare the mean length of hospital stay between children with PIV-associated and other viral ARI compared to ARI in which no viruses were detected (reference), adjusting for age, sex, breastfeeding status, antibiotic use prior to admission, duration of symptoms prior to admission, smoke exposure, and presence of at least one UMC. Based on this model, we estimated the mean hospital length of stay to be 0.51 days longer in the PIV group compared to those with no viruses detected (95% confidence interval [CI] -0.50, 1.52, p = 0.32). Further, we estimated the mean hospital length of stay to be 0.30 days shorter for ARI associated with other viruses compared to ARI in which no viruses were detected (95% CI -0.70, 0.10, p = 0.14).
Multivariable analysis: supplemental oxygen use
We did not find sufficient evidence of a difference in supplemental oxygen use between children with PIV-associated infections (reference) adjusted odds ratio and infections in which no virus was detected (adjusted odds ratio [aOR] 0.90, 95% CI 0.53–1.53, p = 0.70; Fig. 3). Infections with viruses other than PIV and the presence of an underlying medical condition were associated with significantly increased odds of supplemental oxygen use (aOR 1.79, 95% CI 1.09–2.92, p = 0.02 and aOR 2.08, 95% CI 1.62–2.67, p < 0.001, respectively).
Fig. 3.
Association between PIV-associated infections, other infections, and supplemental oxygen use among young Jordanian children admitted with acute respiratory illness
Discussion
While RSV [16] and HMPV [19] have previously been recognized as important etiologies of ARI associated with hospitalization in young Jordanian children, our findings underscore the importance of PIV as an etiology of ARI and LRTI requiring hospitalization in young children in Jordan. We identified a higher frequency of fever and a lower frequency of nasal flaring/retractions and wheezing in children with PIV compared to other ARI in unadjusted analyses, and significantly lower odds of supplemental oxygen use in children with PIV compared to other viral infections. However, there was substantial overlap in the presenting signs and symptoms of PIV infections and those of other viral etiologies, limiting the ability to distinguish these etiologies clinically at the time of presentation. We also found substantial overlap in the clinical features of infections associated with individual PIV serotypes but substantial variability in the seasonality of individual PIV serotype detections. PIV-1 and PIV-2 were the only PIV serotypes associated with an admission diagnosis of croup, consistent with literature that these serotypes are the most common pathogens associated with laryngotrachobronchitis [8]. In addition, our report is among the first to include clinical and epidemiological information regarding PIV-4 infections in young children in the Middle East.
In our study, PIV was detected in 7% of children who presented with both fever and/or respiratory symptoms. Recent reports have focused on the role of PIV in respiratory illnesses in children, but many of these have focused on severe illnesses including pneumonia. For example, in the multicenter Etiology of Pneumonia in the Community (EPIC) study in the United States, PIV infection was detected in 6.6% of cases of radiographically-confirmed pneumonia in hospitalized children, and these infections were associated with similar severity as infections with other respiratory viruses [12]. In the international Pneumonia Etiology for Child Health (PERCH) study, PIV (including PIV-4) was detected in 12% of pneumonia cases and 5.9% of controls, and PIV was among the five most common pathogens in five of the seven study sites, which did not include the Middle East [2, 15]. Our results were consistent with this study, reporting that PIV was the fifth most commonly detected virus after RSV, HRV, AdV, and HMPV [17]. Although our study was limited to a single center, we uniquely included the full spectrum of respiratory conditions associated with PIV, such as laryngotracheobronchitis and bronchiolitis, in a setting in which household smoke exposure, an established risk factor associated with acute respiratory illnesses and respiratory outcomes in children, [20, 21] was relatively high [12].
We observed the majority of PIV-1 detections were in 2011, an odd year, which is consistent with prior reports of seasonal detections of PIV-1 from United States, in which PIV-1 often exhibits biennial peaks [11, 22]. In 2012, the earlier peak was dominated by PIV-3, consistent with other studies that report that PIV-3 primarily circulates annually during the summer months [11, 22]. We did not see predominant circulation of PIV-2 during even years although detection of few cases of PIV-2 in our hospitalized cohort limited seasonality assessment for this serotype, for which many milder infections managed in the outpatient setting may not have been captured. Few studies have reported the seasonal patterns of detection of PIV-4; we primarily observed detections in the late autumn and winter months in our cohort.
Our study has several strengths, including active surveillance and comprehensive respiratory viral testing. The study also has several limitations. Lower respiratory tract specimens were infrequently available. Healthy controls were not included. Although PIV is thought to be infrequently detected in asymptomatic young children [23], it has been detected more frequently among healthy controls in other studies [2]. Frequent co-detections of PIV with other respiratory viruses limited our ability to compare sole detections of PIV to other viruses and to compare the clinical features of individual PIV serotypes with robust conclusions.
Conclusions
In summary, PIV infections were common in children hospitalized with ARI. There were few distinguishing clinical features at presentation, yet several differences in seasonal detection patterns observed, according to PIV serotype. No clinical characteristics were identified that could reliably distinguish PIV from ARI caused by other viruses at presentation, although PIV-associated ARI were less frequently associated with supplemental oxygen use. Our findings suggest that all four PIV serotypes are important etiologies of ARI associated with hospitalization in young Jordanian children. Such surveillance may provide more conclusive characterization of the epidemiology of PIV in the Middle East to support the need for evaluation of future vaccines and antivirals in this region [24, 25].
Supplementary Information
Acknowledgments
We thank the patients who consented for their children to participate. We also would like to thank Al-Bashir Hospital and Jordan University for their collaboration in this surveillance project and our research recruiters: Hanan Amin, Amani Altaber, Hana’a Khalaf, Isra’aKharbat, Darin Yasin, Shireen Issa, and Nurse Sabah Gharbli, and the doctors at Al-Bashir Hospital.
Abbreviations
- AdV
Human adenovirus
- aOR
Adjusted odds ratio
- ARI
Acute respiratory illness
- CI
Confidence interval
- EPIC
Etiology of Pneumonia in the Community
- HCoV
Human coronaviruses
- HMPV
Human metapneumovirus
- HRV
Human rhinovirus
- ICU
Intensive care unit
- LOS
Length of stay
- LRTI
Lower respiratory tract illness
- MERS-CoV
Middle East respiratory syndrome coronavirus
- MV
Mechanical ventilation
- PERCH
Pneumonia Etiology for Child Health
- PIV
Parainfluenza virus
- REDCap
Research Electronic Data Capture
- RSV
Respiratory syncytial virus
- URI
Upper respiratory infection
- UMCs
Underlying medical conditions
Authors’ contributions
All authors read and approved the final manuscript. L.M.H. was the lead author, wrote the main manuscript text, and lead in study design. D.A.R. was provided edits to manuscript drafts, helped interpret data, and performed formal analysis, and developed tables and figures. A.J.S. provided edits to manuscript, provided assistance to analytical design, and interpretation. W.G. provided edits to manuscript and curated data and double checked analyses. Z.H. assisted with study design, reviewing and editing the manuscript. V.P. provided input on study design and reviewed and edited the manuscript. H.R. curated the data and provided edits to the manuscript. R.H. performed laboratory analyses and edits to the manuscript. C.G.P. performed laboratory analyses and edits to the manuscript. J.V.W. was one of the leads for the methodology of the study and provided edits to the manuscript. S.F. was one of the leads with project administration and investigation and provided edits to the manuscript. A.S. provided assistance to the investigation to the study and edits to the manuscript. N.K.B. also was a lead for the methodology and supervision of the study and helped with the review and editing of the manuscript. N.B.H. was the lead of the study’s methodology, project administration, study design, supervision, and provided edits to the manuscript.
Funding
Dr. Howard is supported by the National Institutes of Health under award number 1K23AI141621 and Danielle Rankin is supported by the National Institutes of Health under award number TL1TR002244. This work was supported by UBS Optimus Foundation, National Institutes of Health: R01AI085062, and the CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was given ethical approval by the University of Jordan, the Jordanian Ministry of Health, and Vanderbilt University. Informed consent was obtained from parents and/or the guardians of the children enrolled in this study.
Consent for publication
Not applicable.
Competing interests
Natasha Halasa, MD, MPH receives grant support from Sanofi, Quidel, and speaker compensation from an education grant supported by Genentech. Sanofi also donated vaccines and influenza antibody testing for influenza vaccine trial. John Williams, MD is on the Scientific Advisory Board for Quidel, Independent Data Safely Monitoring Committee for GlaxoSmithKline, and Scientific Advisory Board for ID Connect. All of the other authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Najwa Khuri-Bulos and Natasha B. Halasa denotes co-senior author.
References
- 1.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pneumonia Etiology Research for Child Health Study G. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019. [DOI] [PMC free article] [PubMed]
- 3.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD, et al. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23(11 Suppl):S188–S192. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 4.Iwane MK, Edwards KM, Szilagyi PG, Walker FJ, Griffin MR, Weinberg GA, Coulen C, Poehling KA, Shone LP, Balter S, Hall CB, Erdman DD, Wooten K, Schwartz B, for the New Vaccine Surveillance Network Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113(6):1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg GA, Hall CB, Iwane MK, Poehling KA, Edwards KM, Griffin MR, Staat MA, Curns AT, Erdman DD, Szilagyi PG, New Vaccine Surveillance Network Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154(5):694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Budge PJ, Griffin MR, Edwards KM, Williams JV, Verastegui H, Hartinger SM, Johnson M, Klemenc JM, Zhu Y, Gil AI, Lanata CF, Grijalva CG, RESPIRA-PERU Group A household-based study of acute viral respiratory illnesses in Andean children. Pediatr Infect Dis J. 2014;33(5):443–447. doi: 10.1097/INF.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branche AR, Falsey AR. Parainfluenza Virus Infection. Semin Respir Crit Care Med. 2016;37(4):538–554. doi: 10.1055/s-0036-1584798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003;16(2):242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maykowski P, Smithgall M, Zachariah P, Oberhardt M, Vargas C, Reed C, Demmer RT, Stockwell MS, Saiman L. Seasonality and clinical impact of human parainfluenza viruses. Influenza Other Respir Viruses. 2018;12(6):706–716. doi: 10.1111/irv.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker TA, Khurana S, Tilden SJ. Viral respiratory infections. Pediatr Clin N Am. 1994;41(6):1365–1381. doi: 10.1016/S0031-3955(16)38876-9. [DOI] [PubMed] [Google Scholar]
- 11.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990-2004. Clin Infect Dis. 2006;43(8):1016–1022. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- 12.Howard LM, Edwards KM, Zhu Y, Williams DJ, Self WH, Jain S, et al. Parainfluenza virus types 1-3 infections among children and adults hospitalized with community-acquired pneumonia. Clin Infect Dis. 2020. 10.1093/cid/ciaa973. [DOI] [PMC free article] [PubMed]
- 13.Wang F, Zhao LQ, Zhu RN, Deng J, Sun Y, Ding YX, Tian R, Qian Y. Parainfluenza virus types 1, 2, and 3 in pediatric patients with acute respiratory infections in Beijing during 2004 to 2012. Chin Med J. 2015;128(20):2726–2730. doi: 10.4103/0366-6999.167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, Moore DP, Nunes M, Rath B, Campbell H, Nair H, Acacio S, Alonso WJ, Antonio M, Ayora Talavera G, Badarch D, Baillie VL, Barrera-Badillo G, Bigogo G, Broor S, Bruden D, Buchy P, Byass P, Chipeta J, Clara W, Dang DA, de Freitas Lázaro Emediato CC, de Jong M, Díaz-Quiñonez JA, Do LAH, Fasce RA, Feng L, Ferson MJ, Gentile A, Gessner BD, Goswami D, Goyet S, Grijalva CG, Halasa N, Hellferscee O, Hessong D, Homaira N, Jara J, Kahn K, Khuri-Bulos N, Kotloff KL, Lanata CF, Lopez O, Lopez Bolaños MR, Lucero MG, Lucion F, Lupisan SP, Madhi SA, Mekgoe O, Moraleda C, Moyes J, Mulholland K, Munywoki PK, Naby F, Nguyen TH, Nicol MP, Nokes DJ, Noyola DE, Onozuka D, Palani N, Poovorawan Y, Rahman M, Ramaekers K, Romero C, Schlaudecker EP, Schweiger B, Seidenberg P, Simoes EAF, Singleton R, Sistla S, Sturm-Ramirez K, Suntronwong N, Sutanto A, Tapia MD, Thamthitiwat S, Thongpan I, Tillekeratne G, Tinoco YO, Treurnicht FK, Turner C, Turner P, van Doorn R, van Ranst M, Visseaux B, Waicharoen S, Wang J, Yoshida LM, Zar HJ. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7(8):e1031–e1e45. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 15.Feikin DR, Fu W, Park DE, Shi Q, Higdon MM, Baggett HC, et al. Is Higher Viral Load in the Upper Respiratory Tract Associated With Severe Pneumonia? Findings From the PERCH Study. Clin Infect Dis. 2017;64(suppl_3):S337–SS46. doi: 10.1093/cid/cix148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halasa N, Williams J, Faouri S, Shehabi A, Vermund SH, Wang L, Fonnesbeck C, Khuri-Bulos N. Natural history and epidemiology of respiratory syncytial virus infection in the Middle East: hospital surveillance for children under age two in Jordan. Vaccine. 2015;33(47):6479–6487. doi: 10.1016/j.vaccine.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khuri-Bulos N, Lawrence L, Piya B, Wang L, Fonnesbeck C, Faouri S, Shehabi A, Vermund SH, Williams JV, Halasa NB. Severe outcomes associated with respiratory viruses in newborns and infants: a prospective viral surveillance study in Jordan. BMJ Open. 2018;8(5):e021898. doi: 10.1136/bmjopen-2018-021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, Staat MA, Iwane M, Prill MM, Williams JV, New Vaccine Surveillance Network Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368(7):633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster JE, Khuri-Bulos N, Faouri S, Shehabi A, Johnson M, Wang L, Fonnesbeck C, Williams JV, Halasa N. Human Metapneumovirus infection in Jordanian children: epidemiology and risk factors for severe disease. Pediatr Infect Dis J. 2015;34(12):1335–1341. doi: 10.1097/INF.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuge Y, Qian H, Zheng X, Huang C, Zhang Y, Li B, Zhao Z, Deng Q, Yang X, Sun Y, Zhang X, Sundell J. Effects of parental smoking and indoor tobacco smoke exposure on respiratory outcomes in children. Sci Rep. 2020;10(1):4311. doi: 10.1038/s41598-020-60700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12(1):5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeGroote NP, Haynes AK, Taylor C, Killerby ME, Dahl RM, Mustaquim D, et al. Human parainfluenza virus circulation, United States, 2011-2019. J Clin Virol. 2020;124:104261. doi: 10.1016/j.jcv.2020.104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard LM, Johnson M, Williams JV, Zhu Y, Gil AI, Edwards KM, et al. Respiratory Viral Detections During Symptomatic and Asymptomatic Periods in Young Andean Children. Pediatr Infect Dis J. 2015. [DOI] [PMC free article] [PubMed]
- 24.Schmidt AC, Schaap-Nutt A, Bartlett EJ, Schomacker H, Boonyaratanakornkit J, Karron RA, Collins PL. Progress in the development of human parainfluenza virus vaccines. Expert Rev Respir Med. 2011;5(4):515–526. doi: 10.1586/ers.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chibanga VP, Dirr L, Guillon P, El-Deeb IM, Bailly B, Thomson RJ, et al. New antiviral approaches for human parainfluenza: inhibiting the haemagglutinin-neuraminidase. Antivir Res. 2019;167:89–97. doi: 10.1016/j.antiviral.2019.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.