Abstract
Patients with atypical hemolytic uremic syndrome (aHUS) associated with a C3 p.Ile1157Thr mutation show a relatively high renal survival and low mortality rates, but renal histopathological findings after recurrence have been rarely reported. A 30-year-old man with a C3 p.Ile1157Thr mutation experienced a third recurrence of thrombotic microangiopathies with neurological and gastrointestinal disorders. A renal biopsy performed during the recovery phase of acute kidney injury revealed collapsed glomeruli and arteriolar vacuolization. Approximately 10% of glomeruli were globally sclerotic, despite the absence of arterio-/arteriolo-sclerosis. These findings suggest substantial progression of irreversible injuries in multiple organs, including kidneys, which occurs in aHUS patients with repeated thrombotic microangiopathies.
Keywords: atypical hemolytic uremic syndrome, C3 gene mutation, renal histopathology, thrombotic microangiopathy
Introduction
Atypical hemolytic uremic syndrome (aHUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury (AKI) and is induced by the overactivation of the alternative pathway of the complement system (1). A variety of mutations in genes coding for complement factor H (CFH), complement factor I, complement factor B, complement C3, membrane cofactor protein (MCP), and thrombomodulin have been identified as pathogenic factors that trigger the activation of the complement cascade.
C3 gene mutations are primarily located in the binding regions of C3b that interact with CFH, MCP, and complement receptor 1, which inactivate C3b (2). These mutations result in dysregulation of the alternative complement pathway. Generally, the prognosis of patients with C3 gene mutations is poor; patients typically develop severe symptoms impacting multiple organs, including the kidney. One-half to two-thirds of patients progress to end-stage renal disease within the first year of the initial clinical presentation.
Interestingly, C3 gene mutations in aHUS patients have been more frequently identified in Japan (31%) than in Western countries (2-8%) (3). However, Japanese patients with C3 gene mutations show a better prognosis than those in Western countries, with the p.Ile1157Thr mutation being the most frequently identified (3, 4). This mutation is located at the interface between C3b and the short consensus repeat (SCR) 1-4 of CFH and restricts the binding of C3b to SCR1-4 of CFH (5). Although aHUS associated with the C3 p.Ile1157Thr mutation is likely to relapse, the renal survival is relatively high, and mortality rates are low (3, 4). However, the histopathological findings of the kidney in recurrent aHUS have rarely been reported.
We herein report a case of aHUS with a C3 p.Ile1157Thr mutation that underwent a renal biopsy during the recovery phase of AKI induced by aHUS.
Case Report
A 30-year-old Japanese man presented with a fever and sore throat, followed by abdominal pain and diarrhea. He had previously experienced thrombotic microangiopathy (TMA) on three occasions. The patient had no family history of TMA.
At eight years old, he had suffered the first TMA-associated symptoms, including AKI. He had been diagnosed with HUS because verotoxin was detected and received treatment, including transfusion of plasma and platelets together with antibiotics. The peak serum creatinine concentration (sCr) in the first episode was 1.6 mg/dL. At 26 and 28 years old, he presented with clinical features of TMA and AKI again, with peak sCr values of 1.6 mg/dL and 2.7 mg/dL, respectively. In the third episode, he underwent plasma infusion. In each episode, his renal function subsequently returned to the normal range (sCr 0.9 mg/dL).
On an examination, his vital signs were as follows: body temperature (39.7℃), blood pressure (102/52 mmHg), and heart rate (96 bpm). The patient was conscious and lucid. His tonsils were inflamed, red, and swollen. There were no particular findings of interest in other parts of the body. Laboratory findings confirmed hemolytic anemia, thrombocytopenia, and a reduced renal function with microscopic hematuria and proteinuria (Table). Hypocomplementemia was not identified. Computed tomography of the neck, chest, and abdomen detected no apparent evidence of tumors or infections other than tonsillitis. Streptococcus pyogenes was identified from his tonsils, but cultures from his stool, blood, and urine were negative. Acute tonsillitis was initially treated with ceftriaxone and then changed to ampicillin after the pathogen was detected (Fig. 1).
Table.
Laboratory Data on Admission.
| Blood | ||||||
| Value | Normal range | Value | Normal range | |||
| WBC count, /μL | 12,600 | 3,300–8,600 | IgG, mg/dL | 887 | 861–1,747 | |
| RBC count, /×106 μL | 3.62 | 4.35–5.55 | IgA, mg/dL | 147 | 93–393 | |
| Schistocyte count, % | 2.9 | IgM, mg/dL | 101 | 33–183 | ||
| Hemoglobin, g/dL | 10.6 | 13.7–16.8 | Haptoglobin, mg/dL | 22 | 66–218 | |
| Platelet count, ×103 /μL | 24 | 158–348 | Antinuclear antibody | negative | ||
| AST, U/L | 43 | 13–30 | MPO-ANCA, U/mL | <1.0 | ||
| ALT, U/L | 14 | 10–42 | PR3-ANCA, U/mL | <1.0 | ||
| LDH, U/L | 1,312 | 124–222 | Anti-DNA antibody | negative | ||
| Total bilirubin, mg/dL | 2.8 | 0.4–1.5 | Anti-Scl70 antibody | negative | ||
| Alkaline phosphatase, IU/L | 195 | 106–322 | Anti-centromere antibody | negative | ||
| Total protein, g/dL | 6.3 | 6.6–8.1 | Anti-cardiolipin-β2-glycoprotein I complex antibody, U/mL | negative | ||
| Albumin, g/dL | 3.9 | 4.1–4.5 | Anti-Epstein-Barr virus nuclear antigen antibody | 10 (+) | <10 | |
| BUN, mg/dL | 36 | 8–20 | Anti-cytomegalovirus IgM | negative | ||
| Creatinine, mg/dL | 4.01 | 0.65–1.07 | Anti-varicella-zoster virus IgM | negative | ||
| Uric acid, mg/dL | 8.7 | 3.7–7.0 | Anti-varicella-zoster virus IgG | 54.7 (+) | <2 | |
| Sodium, mEq/L | 132 | 138–145 | Anti-human immunodeficiency virus antigen/antibody | negative | ||
| Potassium, mEq/L | 3.5 | 3.6–4.8 | Hepatitis B virus surface antigen | negative | ||
| Chloride, mEq/L | 99 | 101–108 | Anti-hepatitis C virus antibody | negative | ||
| Calcium, mg/dL | 8.1 | 8.8–10.1 | PT-INR | 1 | ||
| CRP, mg/dL | 18.32 | <0.14 | aPTT, sec | 33.4 | 24–36 | |
| C3, mg/dL | 89 | 73–138 | Fibrinogen, mg/dL | 459 | 150–400 | |
| C4, mg/dL | 26 | 11–31 | Direct Coombs | negative | ||
| CH50, U/mL | 49.4 | 30–50 | Indirect Coombs | negative | ||
| Urine | ||||||
| Value | Value | |||||
| pH | 6 | Occult blood | 3+ | |||
| Specific gravity | 1.009 | Urinary protein creatinine ratio, g/gCr | 1.94 | |||
| Protein | 2+ | RBC, /high power field | 5–9 | |||
| Glucose | negative | WBC, /high power field | 1–4 | |||
AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, BUN: blood urea nitrogen, C: complement, CRP: C-reactive protein, Ig: immunoglobin, MPO/PR3-ANCA: myeloperoxidase/proteinase 3-anti-neutrophil cytoplasmic antibody, PT-INR: prothrombin time-international normalized ratio, aPTT: activated partial thromboplastin time: RBC: red blood cell, WBC: white blood cell
Figure 1.
Clinical course. CNS: central nervous system, CRP: C-reactive protein, GI: gastrointestinal, LDH: lactate dehydrogenase
Although therapies with antibiotics and plasma transfusion improved the hemolytic anemia, thrombocytopenia, as well as pyrexia and inflammation of the tonsils, the patient showed central nervous system (CNS) symptoms, including headache, seizure, and visual disorder from day 5 and mild hypertension (blood pressure of 150-165/70-90 mmHg) from day 8 of hospitalization.
Magnetic resonance imaging of the brain on day 8 revealed posterior reversible encephalopathy syndrome. Although his sCr rapidly increased to 7.4 mg/dL on day 5, the values tended to decrease subsequently. Microscopic hematuria, abdominal and CNS disorders finally disappeared by day 10. Hypertension was treated with 20 mg/day of oral sustained-release nifedipine from day 8 to 12, and then his blood pressure returned to normal without antihypertensive agents.
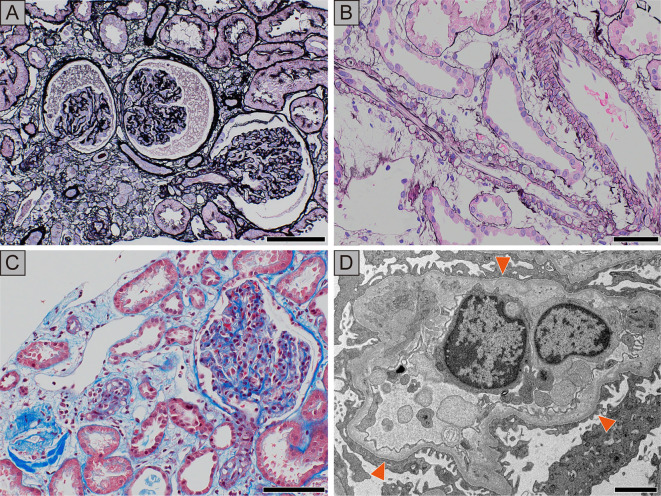
A percutaneous renal biopsy was performed on day 9, during the recovery phase of AKI when his sCr was still elevated (4.0 mg/dL). The specimen contained 52 glomeruli, 6 of which were globally sclerotic. Most of the glomeruli were collapsed (Fig. 2A). Neither crescent formation nor fibrin thrombosis was identified in the glomeruli. There was focal tubular atrophy and interstitial edema with the infiltration of lymphocytes. Although no sclerotic changes or fibrin thrombi were observed in the renal microvessels, vacuole formation was observed in the wall of the interlobular arteries and arterioles, and some sclerosed glomeruli were associated with arterioles showing vacuolization. (Fig. 2B, C). An immunofluorescence analysis revealed negative staining with immunoglobulin G (IgG), IgM, IgA, C3, and C1q in the glomeruli. Electron microscopy demonstrated mild endothelial injury that was evidenced by enlarged endothelial cells, subendothelial lucency, and focal podocyte effacement. Electron-dense deposits were not identified (Fig. 2D).
Figure 2.
Findings of renal biopsy. A: The glomeruli are collapsed, and wrinkling of the capillary tuft can be seen. Focal tubular atrophy and interstitial edema are noted. Periodic acid silver methenamine-Hematoxylin and Eosin (PASM-H&E) staining, scale bar=100 μm. B: Myocyte intracytoplasmic vacuoles are visualized in the intralobular arteries and arterioles. PASM-H&E staining, scale bar=50 μm. C: Masson’s trichrome staining demonstrates vacuolization of the arterioles, while thrombosis is not apparent. Scale bar=100 μm. D: The ultrastructural analysis shows swelling of endothelial cells and widening of the subendothelial area (arrowheads) filled by electron-lucent material with wrinkling of the glomerular basement membrane and mesangial interposition. Focal podocyte foot process effacement is seen around these lesions. Electron-dense deposits are not observed. Scale bar=2.5 μm.
Given his normal serum ADAMTS13 activity and negative studies associated with TMA, including stool culture, O-157 lipopolysaccharide (LPS) antigen or antibody, and other possible causes, such as drugs, malignancies, collagen diseases, and viral infection (Table), he was clinically suspected of having aHUS. The administration of antibiotics and plasma transfusion for six days effectively improved his clinical symptoms; therefore, additional therapies, such as plasma exchange or the administration of eculizumab, were not applied.
Later, a heterozygous C3 p.Ile1157Thr mutation was detected, which led to the definitive diagnosis of aHUS associated with a C3 gene mutation. Increased plasma Ba, C5a, and sC5b-9 [2,838.6 ng/mL (normal range: 275.6-685.2), 24.12 ng/mL (0.20-15.62), and 581.4 ng/mL (37.0-260.6), respectively] on day 4 were consistent with the diagnosis. A genetic analysis also detected heterozygous CFH p.Tyr1058His and p.Val1060Leu, which were previously reported in aHUS patients (6). However, the pathogenicity of these variants is unknown, and they were classified as likely benign variants in a recent study (7). The renal function and levels of Ba, C5a, and sC5b-9 returned to the normal ranges at 4 and 8 months, respectively. The latest renal data at 2 years' follow-up showed an sCr of 0.76 mg/dL, estimated glomerular filtration rate of 96 mL/min/1.73 m2, and urinary protein creatinine ratio of 0.04 g/gCr.
Discussion
We encountered a case of recurrent aHUS associated with a heterozygous C3 p.Ile1157Thr mutation, in which the renal histopathology was evaluated during the recovery phase of AKI. A renal biopsy revealed collapsed glomeruli, glomerular endothelial damage, and vacuolization of microarteries. Although a normal renal function was regained following treatment in every recurrent episode, the renal biopsy findings revealed that approximately 10% of the glomeruli were globally sclerotic, despite the absence of arterio- and arteriolo-sclerosis. These renal histopathological findings suggest substantial renal damage resulting from repeated vascular injuries associated with aHUS.
The present case was clinically diagnosed with aHUS based on microangiopathic hemolytic anemia, thrombocytopenia, and AKI in the absence of decreased ADAMTS13 levels, verotoxin, or any other possible causes of secondary TMA. The diagnosis was subsequently confirmed by a genetic analysis, which revealed a heterozygous C3 p.Ile1157Thr mutation, a well-known pathogenic mutation that causes overactivation of the complement pathway (3). The Ba, C5a, and sC5b-9 levels, which had been significantly elevated, were ameliorated after the treatment, further indicating that acute activation of the alternative complement pathway played a pathogenic role in this case (8).
Renal biopsy findings revealed glomerular endothelial damage and collapsed glomeruli. We could find no reason other than aHUS for the endothelial damage. Notably, vacuole formation was observed in the wall of the interlobular arteries and arterioles. Arteriolar vacuolization sometimes occurs in acute angiopathy of calcineurin inhibitors (CNIs); however, it is not specific to CNI toxicity (9). This lesion reflects direct toxic effects on the arterial wall or vasoconstriction or both (10), and extensive endothelial injury occasionally causes TMA (11). The complement activation product C5a can induce vasoconstriction in intrarenal arteries and arterioles (12-14). Therefore, the arteriolar vacuolization observed in our case might be a result of complement-mediated arterial damage. Although no remarkable thrombi were identified in the glomeruli or arteries in the renal tissue, thrombi are not always present in aHUS cases (15). One possible reason that no thrombosis was observed in the renal tissue specimens could be due to a sampling error; collapsed glomeruli implied stenosis or obstruction of the intrarenal arteries, which might not have been included in the specimen. Another possible reason is the timing of the renal biopsy, as the renal biopsy was performed four days after the sCr levels peaked in the recovery phase of AKI; therefore, modest thrombosis might have already disappeared. In the present case, the findings in the renal microvasculature were mild, which may be reflective of the better renal outcomes observed in cases of aHUS with a C3 p.Ile1157Thr mutation than in those with other causes, which frequently display severe TMA lesions.
The present case showed CNS involvement, including a headache, seizure, and visual disorders, which are extremely common in aHUS patients (16). In a Turkish registry of pediatric aHUS patients, seizure and visual loss were observed in 20.7% and 2.3% of patients, respectively (17). Magnetic resonance imaging of the brain revealed posterior reversible encephalopathy syndrome, which is sometimes observed in aHUS patients (17). The patient also presented with gastrointestinal disorders including abdominal pain and diarrhea, which are commonly involved in aHUS (16). These findings suggest that complement-mediated microvascular injuries might have occurred in multiple organs in addition to the kidney in the present patient.
In our case, treatment with plasma transfusion and antibiotics effectively improved the symptoms associated with TMA. Most cases of aHUS with a C3 p.Ile1157Thr mutation respond to plasma therapy, such as plasma transfusion and plasma exchange, or preserved therapy at the initial onset (3). Cases with frequent recurrence may be treated with eculizumab, a humanized monoclonal antibody to C5. Thus, eculizumab may be an effective treatment for highly recurrent aHUS associated with a C3 p.Ile1157Thr mutation. In fact, it has been reported that an aHUS patient with a C3 p.Ile1157Thr mutation achieved successful remission with eculizumab but suffered recurrence four months after eculizumab was discontinued (18). Eculizumab may be a preferable treatment for our case in order to reduce the risk of recurrent episodes of complement activation and further progression of the multiple organ injuries.
In summary, we reported a case of recurrent aHUS with a heterozygous C3 p.Ile1157Thr mutation. The coexistence of acute and chronic histopathological lesions of the kidney suggests substantial progression of irreversible injuries of multiple organs, including the kidneys, in patients with aHUS showing repeated clinical presentations of TMA. The present case suggested that multiple organ damage in aHUS can occur even if patients respond positively and appear to have recovered in response to the treatment.
The authors state that they have no Conflict of Interest (COI).
Financial Support
The genetic analyses and complement analyses performed by the Japanese Association for Complement Research were supported by Alexion GK as a company-sponsored study.
Acknowledgement
We thank Dr. Yoshida Y, Dr. Kato H, and Dr. Nangaku M (Tokyo University, Tokyo, Japan), Dr. Miyata T and Dr. Uchida Y (National Cerebral and Cardiovascular Center, Suita, Japan) for their support with the genetic analyses and complement analyses. We also appreciate the support of the Japanese Association for Complement Research for the genetic analyses and complement analyses.
References
- 1. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676-1687, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Schramm EC, Roumenina LT, Rybkine T, et al. Mapping interactions between complement C3 and regulators using mutations in atypical hemolytic uremic syndrome. Blood 125: 2359-2369, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujisawa M, Kato H, Yoshida Y, et al. Clinical characteristics and genetic backgrounds of Japanese patients with atypical hemolytic uremic syndrome. Clin Exp Nephrol 22: 1088-1099, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsumoto T, Toyoda H, Amano K, et al. Clinical manifestation of patients with atypical hemolytic uremic syndrome with the C3 p.I1157T variation in the Kinki region of Japan. Clin Appl Thromb Hemost 24: 1301-1307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshida Y, Miyata T, Matsumoto M, et al. A novel quantitative hemolytic assay coupled with restriction fragment length polymorphisms analysis enabled early diagnosis of atypical hemolytic uremic syndrome and identified unique predisposing mutations in Japan. PLoS One 10: e0124655, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsumoto T, Fan X, Ishikawa E, et al. Analysis of patients with atypical hemolytic uremic syndrome treated at the Mie University Hospital: Concentration of C3 p.I1157T mutation. Int J Hematol 100: 437-442, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Osborne AJ, Breno M, Borsa NG, et al. Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J Immunol 200: 2464-2478, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pryzdial EL, Isenman DE. Alternative complement pathway activation fragment Ba binds to C3b. Evidence that formation of the factor B-C3b complex involves two discrete points of contact. J Biol Chem 262: 1519-1525, 1987. [PubMed] [Google Scholar]
- 9. Horike K, Takeda A, Yamaguchi Y, et al. Is arteriolar vacuolization a predictor of calcineurin inhibitor nephrotoxicity? Clin Transplant 25: 23-27, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Young BA, Burdmann EA, Johnson RJ, et al. Cyclosporine A induced arteriolopathy in a rat model of chronic cyclosporine nephropathy. Kidney Int 48: 431-438, 1995. [DOI] [PubMed] [Google Scholar]
- 11. Leal R, Tsapepas D, Crew RJ, Dube GK, Ratner L, Batal I. Pathology of calcineurin and mammalian target of rapamycin inhibitors in kidney transplantation. Kidney Int reports 3: 281-290, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen JA, Davies D, Linn BS, Snyderman R, Franklin L. Complement-mediated vasoconstriction and graft rejection. Circ Res 30: 332-340, 1972. [DOI] [PubMed] [Google Scholar]
- 13. Pelayo JC, Chenoweth DE, Hugli TE, Wilson CB, Blantz RC. Effects of the anaphylatoxin, C5a, on renal and glomerular hemodynamics in the rat. Kidney Int 30: 62-67, 1986. [DOI] [PubMed] [Google Scholar]
- 14. Gulbins E, Schlottmann K, Rauterberg EW, Steinhausen M. Effects of rC5a on the circulation of normal and split hydronephrotic rat kidneys. Am J Physiol 265: F96-F103, 1993. [DOI] [PubMed] [Google Scholar]
- 15. Goodship THJ, Cook HT, Fakhouri F, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 91: 539-551, 2017. [DOI] [PubMed] [Google Scholar]
- 16. Formeck C, Swiatecka-Urban A. Extra-renal manifestations of atypical hemolytic uremic syndrome. Pediatr Nephrol 34: 1337-1348, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fidan K, Göknar N, Gülhan B, et al. Extra-renal manifestations of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 33: 1395-1403, 2018. [DOI] [PubMed] [Google Scholar]
- 18. Toyoda H, Wada H, Miyata T, et al. Disease recurrence after early discontinuation of eculizumab in a patient with atypical hemolytic uremic syndrome with complement C3 I1157T mutation. J Pediatr Hematol Oncol 38: e137-e139, 2016. [DOI] [PubMed] [Google Scholar]