Abstract
An 80-year-old man was admitted due to biliary stricture with autoimmune pancreatitis. Although radiographical examinations suggested Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC), punched biopsies from the bile duct revealed adenocarcinoma. In the resected specimen, abundant N-terminus of Forkhead box P3 (Foxp3)-positive cells were localized in cholangiocarcinoma (CCA) tissue, while IgG4-positive cells were spread around the entire bile duct. Therefore, the case was diagnosed with IgG4-SC accompanied by CCA, not sporadic CCA. We herein report an informative case wherein IgG4-positive cells were abundant in CCA tissue and Foxp3 immunohistochemical staining allowed us to determine that this case had two entities.
Keywords: autoimmune pancreatitis, immunoglobulin G4-related sclerosing cholangitis, cholangiocarcinoma, forkhead box P3, immunoglobulin G4-related disease
Introduction
Immunoglobulin (Ig) G4-related sclerosing cholangitis (IgG4-SC) is a common presentation of IgG4-related disease (IgG4-RD), which is a chronic relapsing multi-organ fibro-inflammatory syndrome of presumed autoimmune etiology (1). IgG4-SC is distinct from primary sclerosing cholangitis (PSC) with regard to its histologic features, treatment strategy and prognosis. The concept of IgG4-SC has been prevailing since the diagnostic criteria of IgG4-SC were established (2, 3). IgG4-SC is classified into four types depending on the radiologic pattern on a cholangiogram. Type 1 IgG4-SC involves stenosis only in the lower part of the common bile duct (CBD) and thus should be differentiated from chronic pancreatitis, pancreatic cancer, and cholangiocarcinoma (CCA) (4). Among all IgG4-SC types, type 1 IgG4-SC is most frequently associated with type 1 autoimmune pancreatitis (AIP) (5).
CCA is a lethal biliary tract cancer with suboptimal treatment outcomes. The overall incidence of CCA has been increasing in Western countries (6, 7). The survival of patients with CCA is poor, with a 5-year survival rate of <10%, due to the typical advanced stage at presentation and limited treatment options (8). Although surgical resection and liver transplantation are potentially curative for select patients, most patients are not eligible for those options. In addition, the current standard chemotherapy with gemcitabine and cisplatin does not achieve a long-term survival (9).
Radiographic images sometimes fail to distinguish between IgG4-SC and CCA, both of which can present with bile duct stricture (4). Furthermore, the histologic diagnosis of coexistent CCA and IgG4-SC is far more challenging than the diagnosis of solitary IgG4-SC or CCA. Distal CCA, in addition to gallbladder cancer, is often accompanied by significant IgG4 reactions, so IgG4-positive plasmacyte infiltration is not always the histological hallmark of IgG4-SC (10, 11).
We herein report a case of IgG4-SC associated with CCA and AIP. This is an informative case that helps us recognize the challenge of establishing a diagnosis of lower CBD stricture associated with AIP, shedding light on the N-terminus of Forkhead box P3 (Foxp3) as a useful marker for distinguishing sporadic CCA from CCA accompanied by IgG4-SC (10, 11).
Case Report
An 80-year-old man with suspected AIP was referred to our hospital in 2011 for a further evaluation and treatment. His medical history included appendicitis at five years old. He presented to another hospital with a chief complaint of lower abdominal pain. His serum IgG4 concentration was 1,370 mg/dL (normal range 4.8-105 mg/dL). Computed tomography (CT) revealed dilated CBD and swelling of pancreas. Endoscopic retrograde cholangiopancreatography (ERCP) showed a stricture of main pancreatic duct (MPD). A diagnosis of AIP was established, and the patient was started on nafamostat mesilate. Three months later, his liver panel was found to be abnormal, so he was referred to our hospital.
His labs at presentation to our institution were as follows: aspartate transaminase 263 IU/dL (10-40 IU/dL), alanine aminotransferase 191 IU/dL (4-44 IU/dL), alkaline phosphatase 8,940 IU/dL (103-355 IU/dL), γ-glutamyl transpeptidase 631 IU/dL (16-84 IU/dL), amylase 77 IU/dL (37-114 IU/dL), lipase, 84 IU/dL (11-59 IU/dL), total bilirubin 18.24 mg/dL (0.22-1.20 mg/dL) and direct bilirubin 14.21 mg/dL (0.05-0.30 mg/dL). Serum total IgG, IgG4, IgA and IgM levels were 3,806 mg/dL (870-1,700 mg/dL), 1,370 mg/dL, 235 mg/dL (110-410 mg/dL) and 73 mg/dL (35-220 mg/dL), respectively. Serum levels of carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9 and DUPAN-2 were 2.7 ng/dL (<5.0 ng/dL), 98 U/mL (<37 U/mL) and 180 U/mL (<150 U/mL), respectively.
CT revealed a low-density mass lesion measuring 15 mm in the lower bile duct with biliary dilation proximal to the mass and diffuse enlargement of the pancreatic body and tail with a capsule-like rim surrounding the pancreas (Fig. 1). Magnetic resonance cholangiopancreatography (MRCP) revealed a stricture in the lower bile duct with dilation proximal to the narrowing, in addition to diffuse pancreatic swelling and irregular MPD narrowing (Fig. 2). Endoscopic ultrasound (EUS) showed a mass lesion measuring 20 mm in the lower bile duct and swelling of the whole pancreas without MPD interruption (Fig. 3). ERCP showed irregular narrowing of the MPD from the pancreas head to the tail and biliary stricture in the lower bile duct with entire dilation proximal to the stricture (Fig. 4).
Figure 1.
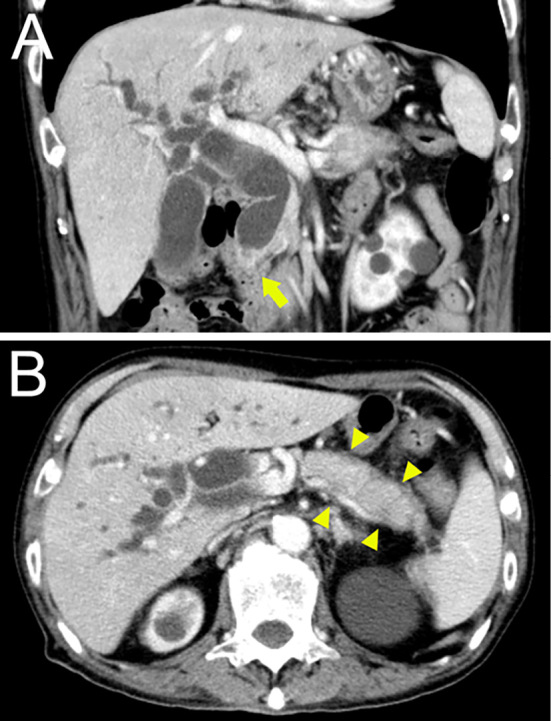
CT images revealing a low-density mass lesion measuring 15 mm in diameter at the pancreatic head (orange arrow) with bile duct dilation proximal to the mass lesion (A) and diffuse enlargement and a surrounding low-density line of the pancreatic body and tail that was considered a capsule-like rim (B).
Figure 2.
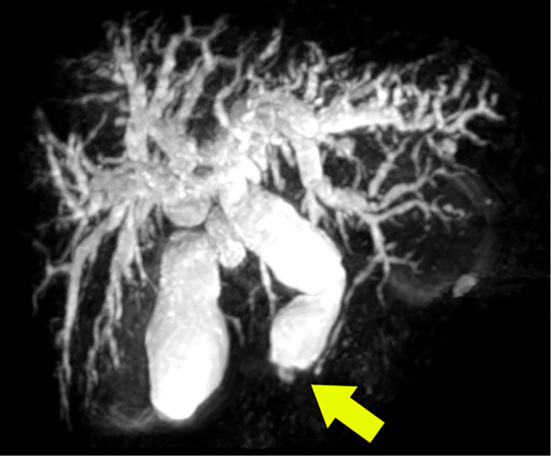
MRCP image revealing enlargement of the gallbladder and bile duct dilation proximal to the stricture at the lower bile duct, diffuse pancreatic swelling and irregular main pancreatic duct narrowing.
Figure 3.
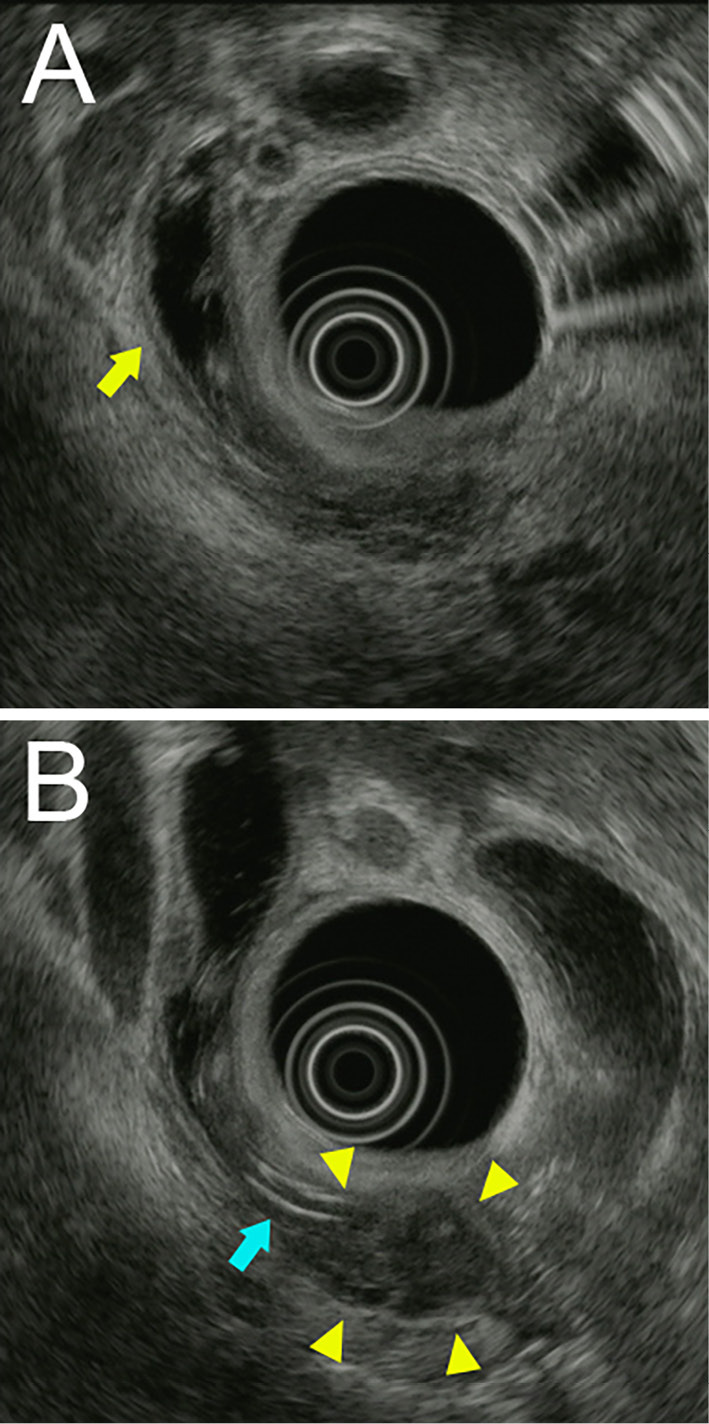
Images of endoscopic ultrasound showing intrahepatic and CBD dilation, a bile duct tube stent (blue arrow) and a low-echoic lesion measuring 15 mm in the pancreatic head (yellow arrow).
Figure 4.
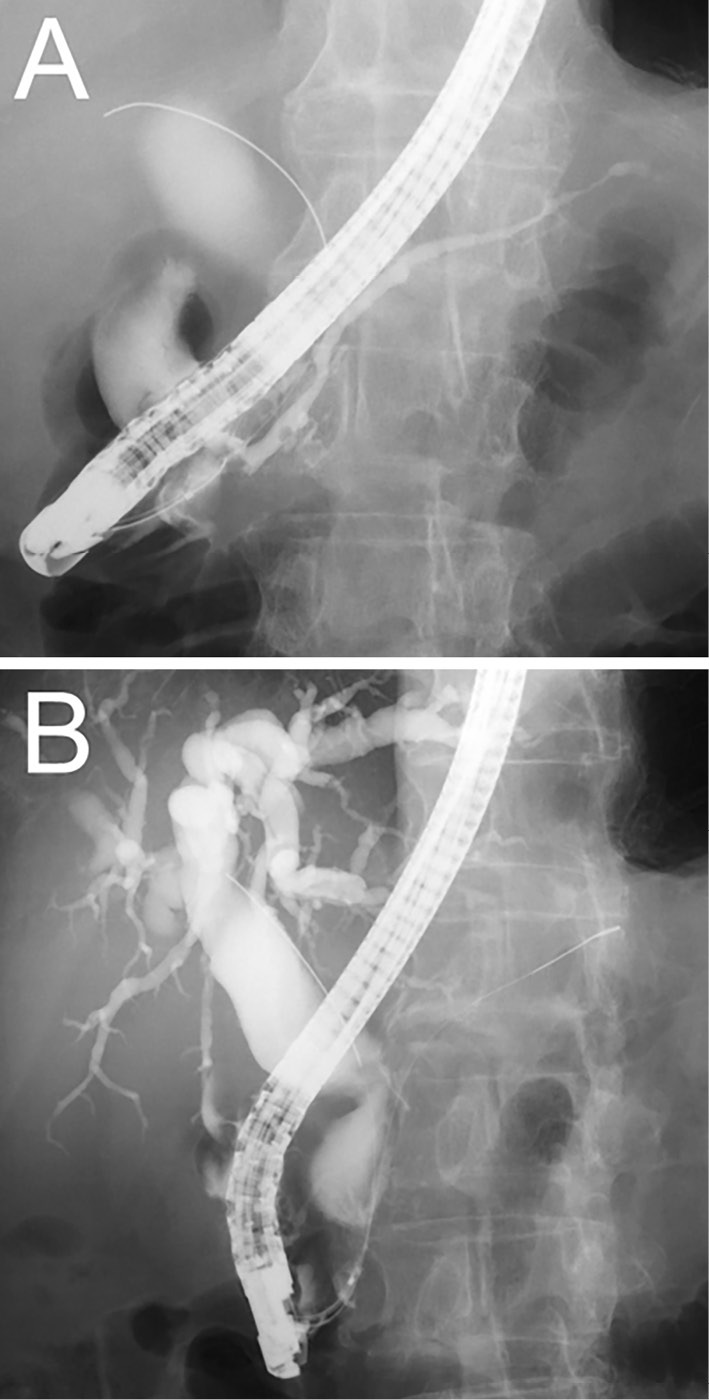
ERCP image showing irregular narrowing of the main pancreatic duct (A) and biliary stricture of the lower bile duct with entire dilation proximal to the stricture (B).
Endoscopic sphincterotomy was performed. Punched biopsies were obtained from the stricture of the lower bile duct, and a plastic stent (8.5 Fr, 7 cm) was placed for biliary drainage. A pathological examination of the biopsy specimens revealed well-differentiated adenocarcinoma. However, IgG4-positive plasma cells could not be evaluated due to the scarcity of plasma cells. The patient was diagnosed with CCA accompanied by AIP according to the international consensus diagnostic criteria (12). No distant metastases (including lymph nodes) were radiographically detected, so the patient underwent pancreatoduodenectomy as a radical treatment.
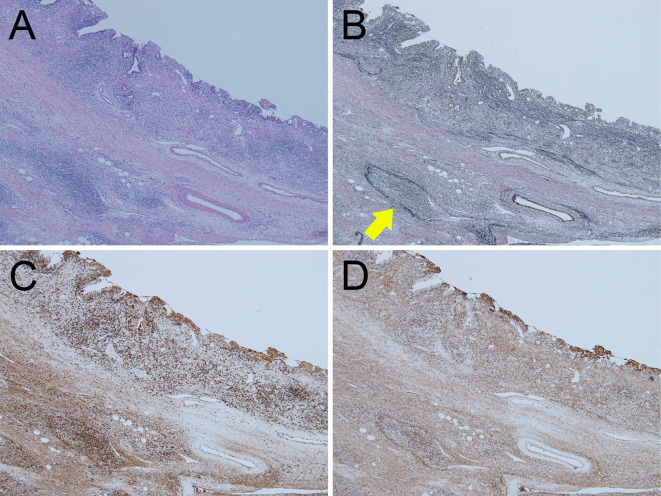
A gross examination revealed tumor extension from the lower CBD to the duodenal ampulla (Fig. 5). A pathologic examination showed moderately differentiated tubular adenocarcinoma invading the duodenal mucosa and the pancreas parenchyma [pT3N0M0, stage IIB in the Union of International Cancer Control (UICC) TNM classification]. Abundant IgG4-positive plasma cells were seen from the upper edge of the resected bile duct to the duodenal ampulla, including the surrounding area of the tumor (Fig. 6A, D), whereas fewer IgG4-positive plasma cells were seen in the tumor tissue. The dense infiltration of plasma cells and lymphocyte cells with fibrosis were shown in the distal bile duct, especially in the subepithelium and around the peribiliary glands (Fig. 6C). Although no storiform fibrosis was found around the bile duct, the obliterative phlebitis was detected in the subepithelium of the lower bile duct by Elastica van Gieson staining (Fig. 6B). The whole pathological features were consistent with IgG4-SC. In the pancreas, pancreatic tissue was mostly replaced by fibrotic tissue with plasmacytes and lymphocytes infiltrating around the pancreatic ducts. Immunohistochemical staining (IHC) showed the number of IgG-positive cells and IgG4-positive cells to be approximately 140 and 120 cells/high-power field, respectively (IgG/IgG4 ratio of 85.7%) (Fig. 7A-C). Based on these findings, a diagnosis of IgG4-SC with AIP was made.
Figure 5.
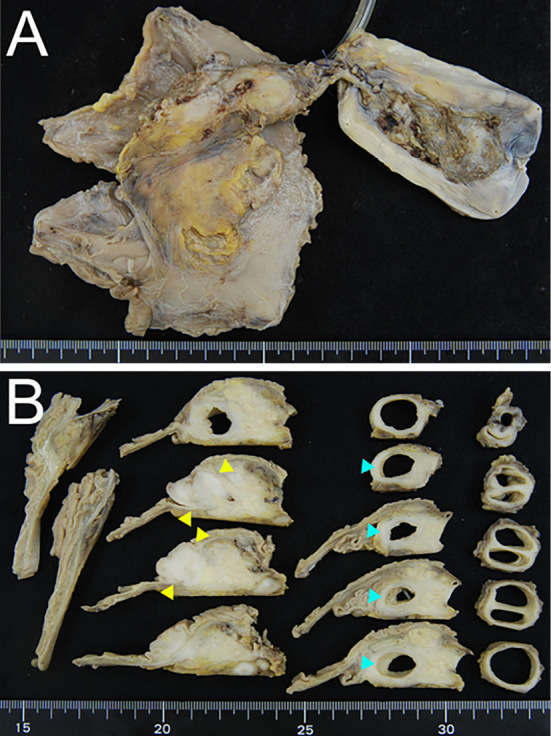
A resected specimen fixed by formalin. The yellow arrow depicts the tumor in the lower bile duct.
Figure 6.
Pathological pictures of the IgG4-SC lesion in the CBD on Hematoxylin and Eosin staining (A), Elastica van Gieson straining (B) and IHC for IgG (C) and IgG4 (D). Obliterative phlebitis is shown in B (yellow arrow). Original magnification, ×40.
Figure 7.
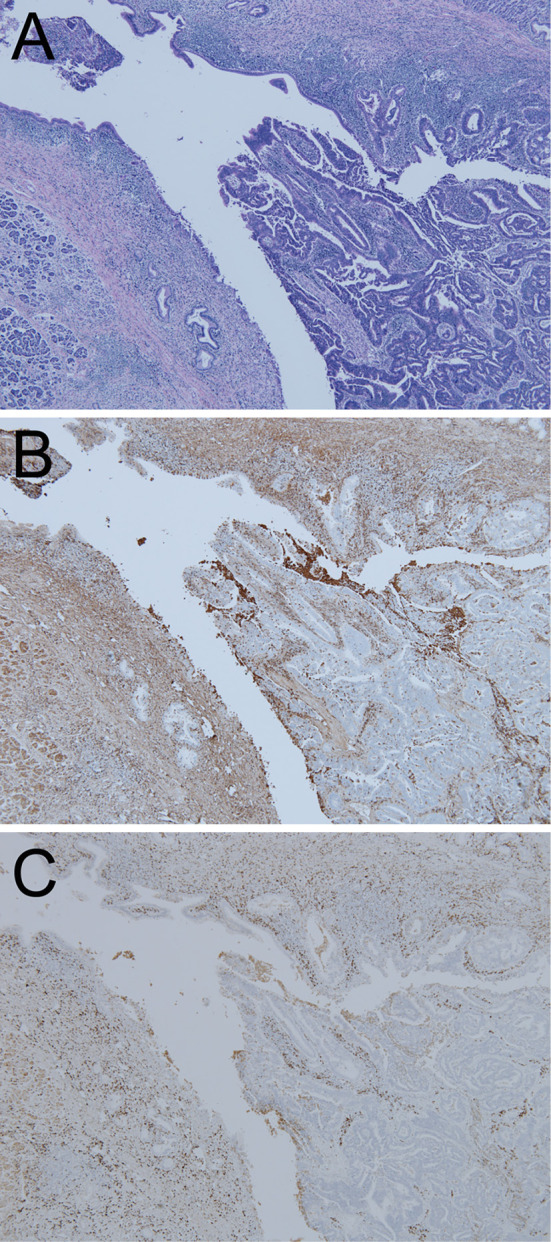
Pathological pictures of CCA in the lower CBD on Hematoxylin and Eosin staining (A) and IHC for IgG (B) and IgG4 (C). Original magnification, ×40.
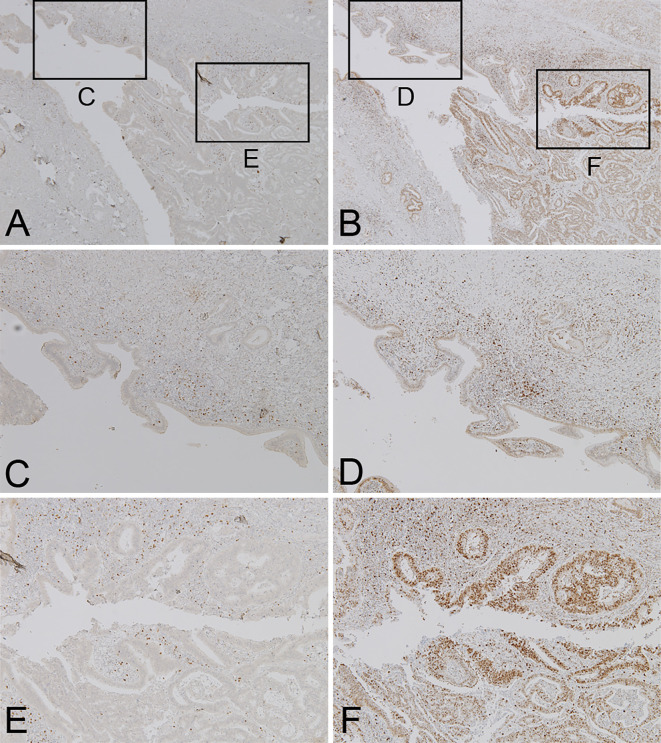
Interestingly, IHC using the C-terminus of the Foxp3 antibody (mouse monoclonal; 5 μg/mL; Abcam, Tokyo, Japan) showed that only mononuclear cells, which were consistent with regulatory T-cells (Treg), were positive in both the epithelia in the CCA and IgG4-SC region (Table, Fig. 8A, C, E). In contrast, affluent N-termini of Foxp3-positive cells (rat monoclonal, 2.5 μg/mL; eBioscience, San Diego, USA) were seen in the CCA epithelia with a low number of positive cells in the epithelia of the IgG4-SC region (Table, Fig. 8B, D, F).
Table.
Comparison of IHC Status between IgG4-SC and CCA in the Present Case.
| C-terminus of Foxp3 IHC | N-terminus of Foxp3 IHC | IgG4 IHC | |
|---|---|---|---|
| IgG4-SC | + Monocyte |
+ Monocyte |
+++ Plasmacyte |
| CCA | + Monocyte |
+++ Epithelium and monocyte |
+++ Plasmacyte |
Lower columns in cells show locations where IHCs were stained.
+: weakly postive,+++: strongly positive, IHC: immunohistochemical staining, IgG4-SC: immunogroblin G4-related sclerosing cholangitis, CCA: cholangiocarcinoma, Foxp3: Forkhead box P3
Figure 8.
Pathological pictures of the IgG4-SC and CCA areas on IHC for the C-terminus of Foxp3 (A, C, E) and the N-terminus of Foxp3 (B, D, F). (C-F) magnified pictures of IgG4-SC (C, D) and CCA (E, F) areas in A, B. Original magnification, ×40 (A, B) and ×100 (C-F).
After the surgery, steroids were not introduced due to a lack of CBD obstruction. Six months after surgery, CT detected a liver lesion consistent with CCA metastasis. The patient was started on chemotherapy with tegafur, gimeracil and oteracil (S-1). Although it was initially effective, the patient had disease recurrence 13 months after the initiation of chemotherapy and unfortunately died 22 months after the surgery. The serum IgG4 level did not change throughout the clinical course.
Discussion
Our patient had radiographical findings suggestive of AIP (sausage-shaped pancreas, capsule-like rim, and narrowing of MPD), with concomitant stricture in the lower common bile duct anticipating coexistent type 1 IgG4-SC (before CCA diagnosis). A transpapillary biopsy cannot confirm the diagnosis of IgG4-SC even after IgG4 immunostaining; however, it does confirm cancer existence in some cases (13). Indeed, in the present case, the patient had adenocarcinoma in the lower bile duct causing biliary stricture, which was proven by a transpapillary biopsy.
Although AIP can be accompanied by a pancreatic cancer (14), the tumor in our patient originated from the lower common bile duct (rather than the pancreas) as proven histologically and invaded the adjacent pancreatic parenchyma. Abundant lymphoplasmacystic infiltration and fibrosis of the bile duct in our patient involved the bile duct from the upper edge of dissection to the duodenal papilla, excluding the part of the stricture forming the wall thickening observed by EUS in the non-stricture area of the bile duct. Those findings support the presence of IgG4-SC in our patient in addition to CCA (13). Obliterative phlebitis in the subepithelium and abundant IgG4-positive cells in all layers of the bile duct were also important clues. Along with the presence of AIP and significant elevation of serum IgG4 level, our patient met the diagnostic criteria of IgG4-SC (2).
This case made us contemplate whether the patient had de novo malignancy or IgG4-SC complicated by malignancy. The infiltration of IgG4-positive cells cannot be used to differentiate between the two entities, since distal CCA often causes significant IgG4 reactions, similar to IgG4-SC (15). In fact, a substantial proportion of biliary tract cancers (with the exception of intrahepatic CCA) exhibit marked infiltration of IgG4-positive cells (10). Therefore, the infiltration of IgG4-positive cells as a diagnostic criterion of IgG4-SC (1, 2, 15) can only be counted if malignant neoplasm does not exist and cannot be used as a diagnostic criterion if IgG4-SC is accompanied by CCA, as in our patient.
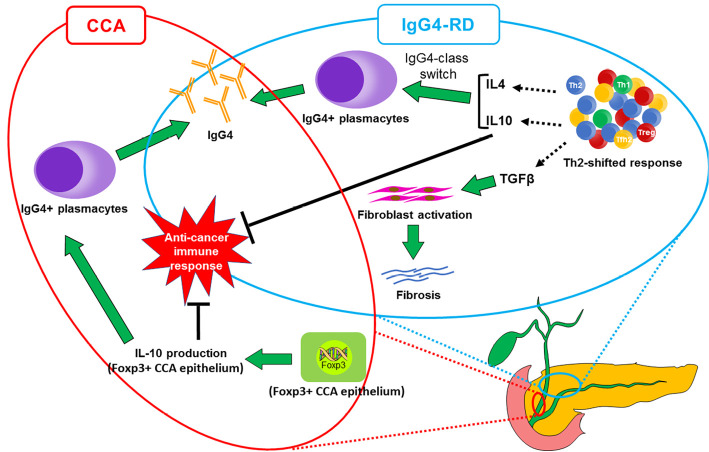
Foxp3 is the transcription factor involved in Treg differentiation (16). The existence of the C-terminus of Foxp3 can play a potential role in differentiating IgG4-SC from CCA (11). Foxp3, a member of the FOX protein family, is a protein involved in immune system responses (17) and appears to function as a master regulator of the regulatory pathway in the development and function of Treg cells (18). Harada et al. reported that the antibody against the N-terminus of Foxp3 highlighted carcinoma epithelia and Treg cells in 39% of CCA cases, while the antibody against the C-terminus of Foxp3 detected only Treg cells (10, 11). The discrepancy between antibodies against different antigenic sites of Foxp3 may account for the presence of Foxp3 splice variants in CCA epithelia. It lacks exon 3, causes a frameshift at the C-terminus, and creates a novel amino acid, which has been reported in a melanoma cell line (19). In addition, the number of IgG4-positive cells is significantly higher in Foxp3-positive CCA than in Foxp3-negative CCA (10, 11). Since Foxp3 expression is closely correlated with the expression of interleukin (IL)-10 in all Foxp3-positive cell lines (20), Foxp3-positive CCA cells can exert immunosuppressive effects similar to Treg cells via IL-10 production and possibly induce the differentiation of IgG4-positive plasma cells in biliary tract cancers (Fig. 9) (10, 11). However, IgG4 antibody in IgG4-RD, including IgG4-SC, was characteristically produced by a IgG4-class-switch in B cells after the activation of Treg cells, and also by follicular T-helper 2 cells via IL-10, IL-4, or IL-21 (1). This mechanism is not associated with IgG4 production in the Foxp3-positive epithelium (Fig. 9) (1, 21), although the numbers of Foxp3-positive and IL-10-positive Tregs were significantly higher in AIP and IgG4-SC patients than in patients with other pancreatic diseases (16, 22). In our patient, the N-terminus of Foxp3 was strongly positive in the CCA epithelia on IHC, indicating the presence of abundant IgG4-postive cells induced there. In contrast, C-terminus of Foxp3 was weakly positive at the point of CCA invasion and the surrounding tissue of CCA, suggesting that our case had IgG4-SC accompanied by CCA.
Figure 9.
Schematic illustration depicting the mechanistic difference in IgG4 production between Foxp3-producing CCA and IgG4-RD including IgG4-SC. Th2, helper T2.
Tumors modulate the inflammatory environment through the secretion of soluble growth factors and chemoattractants, which render inflammatory cells suppressive against anticancer T cell responses (23), and large numbers of tumor-infiltrating neutrophils, including Treg cells, reportedly suppress the antitumor immune response and indicate a poor prognosis in patients with cancers, including distal CCA (24). CCA cases with abundant IgG4-positive cells also have a poorer prognosis than those with few IgG4-positive cells (25). The presence of abundant Treg and IgG4-positive cells due to the surrounding IgG4-SC environment and Foxp3-positive CCA epithelia in our patient might have caused the aggressive tumor progression with a poor prognosis.
Although uncommon, the coexistence of IgG4-SC and CCA has been previously reported (26-28). One report described a case of IgG4-SC accompanied by distal CCA (26), and the others described a case of IgG4-SC with intrahepatic CCA (27, 28). CCA was eventually found in all three cases, so CCA should always be considered if steroid therapy is ineffective for the treatment of IgG4-SC. Whether or not IgG4-SC is considered a risk factor for CCA, similar to PSC, is unclear. In PSC, the lymphocytic infiltration is more dominant superficially near the lumen of the distal bile duct and leads to erosion and damage of the surface epithelium. In IgG4-SC, however, the inflammation and fibrosis are observed in all layers of the distal CBD, and the surface epithelium is less badly damaged than the epithelium in PSC (26). The cancer risk in patients with IgG4-SC and AIP merits further investigation (1).
Our patient underwent pancreatoduodenectomy for his distal CCA; however, his cancer recurred. Recently, the United States Food and Drug Administration (FDA) approved the fibroblast growth factor receptor (FGFR) inhibitor pemigatinib for the treatment of CCA associated with FGFR-2 gene fusions or certain other rearrangements based on a phase II study (29). This is the first FDA-approved targeted therapy for CCA, and some improvement in the prognosis can hopefully be expected with the development of new therapeutic agents. Although IgG4-SC is usually treated by steroids (4), our patient was not treated with steroids due to a lack of symptoms.
In summary, we encountered a patient with IgG4-SC accompanied by distal CCA, which can be challenging to diagnose. Immunostaining of the C-terminus in Foxp3 can be useful for distinguishing sporadic CCA from CCA coexisting with IgG4-SC pathologically and can help determine treatment options.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Miyabe K, Zen Y, Cornell LD, et al. Gastrointestinal and extra-intestinal manifestations of IgG4-related disease. Gastroenterology 155: 990-1003 e1001, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Ohara H, Okazaki K, Tsubouchi H, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci 19: 536-542, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Kamisawa T, Nakazawa T, Tazuma S, et al. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci 26: 9-42, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakazawa T, Shimizu S, Naitoh I. IgG4-related sclerosing cholangitis. Semin Liver Dis 36: 216-228, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Ohara H, Nakazawa T, Kawa S, et al. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: a Japanese cohort. J Gastroenterol Hepatol 28: 1247-1251, 2013. [DOI] [PubMed] [Google Scholar]
- 6. von Hahn T, Ciesek S, Wegener G, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol 46: 1092-1098, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Yang JD, Kim B, Sanderson SO, et al. Biliary tract cancers in Olmsted County, Minnesota, 1976-2008. The American journal of gastroenterology 107: 1256-1262, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 145: 1215-1229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362: 1273-1281, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol 2014: 803876, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harada K, Shimoda S, Kimura Y, et al. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 56: 157-164, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 40: 352-358, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Naitoh I, Nakazawa T, Ohara H, et al. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol 44: 1147-1155, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Inoue H, Miyatani H, Sawada Y, et al. A case of pancreas cancer with autoimmune pancreatitis. Pancreas 33: 208-209, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25: 1181-1192, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Zen Y, Liberal R, Nakanuma Y, et al. Possible involvement of CCL1-CCR8 interaction in lymphocytic recruitment in IgG4-related sclerosing cholangitis. J Hepatol 59: 1059-1064, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27: 68-73, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057-1061, 2003. [DOI] [PubMed] [Google Scholar]
- 19. Ebert LM, Tan BS, Browning J, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res 68: 3001-3009, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Karanikas V, Speletas M, Zamanakou M, et al. Foxp3 expression in human cancer cells. J Transl Med 6: 19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ito F, Kamekura R, Yamamoto M, et al. IL-10(+) T follicular regulatory cells are associated with the pathogenesis of IgG4-related disease. Immunol Lett 207: 56-63, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Kusuda T, Uchida K, Miyoshi H, et al. Involvement of inducible costimulator- and interleukin 10-positive regulatory T cells in the development of IgG4-related autoimmune pancreatitis. Pancreas 40: 1120-1130, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13: 759-771, 2013. [DOI] [PubMed] [Google Scholar]
- 24. Kitano Y, Okabe H, Yamashita YI, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer 118: 171-180, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimura Y, Harada K, Nakanuma Y. Pathologic significance of immunoglobulin G4-positive plasma cells in extrahepatic cholangiocarcinoma. Hum Pathol 43: 2149-2156, 2012. [DOI] [PubMed] [Google Scholar]
- 26. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol 5: 181-185, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang YA, Shen XZ, Zhu JM, et al. Extensive metastatic cholangiocarcinoma associated with IgG4-related sclerosing cholangitis misdiagnosed as isolated IgG4-related sclerosing cholangitis: a case report and literature review. Medicine (Baltimore) 94: e2052, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Straub BK, Esposito I, Gotthardt D, et al. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch 458: 761-765, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 21: 671-684, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]