Abstract
Objectives:
Among adolescents with inflammatory bowel disease (IBD), nonadherence rates are 50 to 88% across medications. Improving education in adults with IBD has been shown to improve coping and adherence to treatment in adults with IBD. Therapeutic patient education (TPE) has been used in patients with chronic diseases to train patients in skills to support treatment adaptation and condition management. This study tested the feasibility and preliminary efficacy of a novel TPE intervention in adolescents with IBD.
Methods:
In this pilot, mixed-methods study, we evaluated the feasibility and preliminary efficacy of TPE with the IBD Pocket Guide on medication adherence, IBD knowledge, and transition readiness in adolescents ages 11 to 18 years. Medication adherence was monitored using a MedMinder Pill Dispensing system. Participants who were <90% adherent during a 4-week pre-intervention monitoring period were randomized to either a usual care group or an educational intervention (EI) group. Participants were followed for an additional 4 weeks after intervention.
Results:
Trends were found in the EI group indicating improved medication adherence and IBD knowledge compared with the usual care group, though differences between groups did not reach statistical significance. Qualitative data showed that participants perceived that they had improved knowledge after the educational intervention.
Conclusions:
Therapeutic patient education may be beneficial for improving patient medication adherence and IBD knowledge. Future directions include testing the effects of the intervention with a larger sample.
Keywords: adherence, educational intervention, medical knowledge, transition readiness
Nonadherence to medical treatment is a pervasive and significant problem in the treatment of pediatric inflammatory bowel disease (IBD). Studies have demonstrated nonadherence prevalence rates in pediatric IBD ranging from 50 to 88% (1–3). These data are alarming given that, for nonadherent patients, the risk of relapse is 5.5 times greater (4) and annual health care costs are 12.5% higher compared with adherent patients (5).
Educational interventions, which aim to increase patient knowledge about their disease, symptoms, medications, health care resources, and the consequences of nonadherence, represent 1 approach to targeting nonadherence. Among adults with IBD, educational interventions have been shown to increase disease-specific knowledge, enhance patient satisfaction with care, and lower the rate of missed medication doses (6,7). However, to our knowledge, no studies to date have examined the impact of an education-only intervention on adherence in adolescents with IBD. Educational interventions may be particularly salient for this population, as previous studies have documented deficits in adolescent knowledge related to insurance coverage, accessing providers, recognizing flare symptoms, and knowing when and how to refill prescriptions (8–10). Furthermore, adolescents must acquire the knowledge and skills needed to independently manage their condition in adulthood and successfully navigate the transition to adult health care. Adult providers have noted that transitioning adolescents are often ill-prepared to make this transition with only 23% of respondents stating that patients were adequately prepared (11). Accordingly, education may be a first step toward improving adherence, IBD knowledge, and transition readiness.
There are several barriers to providing patients with individually tailored, relevant, and timely education to support IBD management. Educating patients and parents about IBD can be time consuming, and there are often insufficient resources in the context of the clinic visit to provide patients with the necessary education and training. Therapeutic patient education (TPE) is an effective method of patient education, with most widespread application to date in Europe. TPE has been used in patients with chronic diseases, such as asthma, diabetes, obesity, and atopic dermatitis (12–14). TPE involves 4 steps: understanding the patient’s perspective, education, and acquisition of skills, and assessment following the TPE (12). TPE is a targeted intervention, which can be integrated into the medical care process. TPE is typically delivered in outpatient settings, with sessions lasting 20 to 30 minutes, and education does not need to be delivered by a physician.
The purpose of this pilot study was to examine the feasibility and preliminary efficacy of a therapeutic patient education intervention (EI) on adherence, IBD knowledge, and transition readiness. An educational tool, “The IBD Pocket Guide,” was developed specifically for this study. We hypothesized that participants in the EI group would demonstrate greater improvements in medication adherence, IBD knowledge, and transition readiness compared with participants in the usual care group.
METHODS
Participants
Participants were adolescents (11–18 years old) recruited from a gastroenterology clinic at a Midwestern children’s hospital. Inclusion/exclusion criteria were as follows: confirmed IBD diagnosis (ie, Crohn disease, ulcerative colitis, or indeterminate colitis), prescribed at least 1 daily oral medication for the control of IBD (ie, steroid, thiopurine, or aminosalicylate), no significant developmental disorders or serious mental illness, and not currently enrolled in another intervention targeting adherence.
Procedures
Potential participants meeting eligibility criteria were identified via chart review and were contacted during a gastroenterology clinic appointment or via recruitment letter with follow-up telephone call. Following informed consent, participants received an electronic pill box to monitor medication adherence. Participant adherence was monitored for 4 weeks before randomization; this baseline monitoring period is referred to as the “run-in phase.” We used 4 weeks of adherence monitoring to determine eligibility for randomization in order to account for any initial reactivity that may have occurred with electronic monitoring (15–17). Participants with average adherence below 90% over the 4-week run-in phase were randomly assigned to either the EI or usual care groups using a simple randomization procedure to eliminate imbalance of participants across the groups. Participants with 90% or greater average adherence during the run-in phase were not randomized and their participation was discontinued. Randomized participants completed measures assessing IBD knowledge and transition readiness at the baseline visit before receiving the intervention.
Intervention
Participants randomized to the EI group met individually with the educator for a 30-minute educational intervention session. Educational content was delivered using the IBD Pocket Guide. This guide was created in collaboration with pediatric IBD specialists, psychologists, social workers, pharmacists, and parents of patients with IBD. The IBD Pocket Guide provides an overview of gastrointestinal (GI) function and anatomy, answers to common questions about IBD, information about GI procedures, and information on common medications (including pictures and size representations) as well as reasons why taking medication is important. Additionally, the guide provides tips for adherence promotion, transition readiness, and information on where to obtain additional resources on IBD and self-management. The guide can also be personalized for each participant with space for the participant to write in answers to questions about their disease (See Supplemental Digital Content, http://links.lww.com/MPG/B674, which includes select pages from the IBD Pocket Guide).
The educational principles of the intervention were consistent across all patients to ensure maintenance of treatment integrity and fidelity. In order to tailor treatment to individual patient and family needs, the specific targets of each intervention varied based on developmental considerations, medication regimens, patient beliefs, and unique disease-specific problems. General topics covered with each patient included the following: etiology of disease, anatomy, information on procedures, imaging, nutrition, vaccines, and medications to avoid. In addition, the educator reviewed each chart before the intervention in order to provide individually tailored information, such as location of disease based on pathology and imaging. The IBD Pocket Guide was personalized to discuss pertinent information for each patient, including medications, pharmacy, and physician’s name and contact information. Participants in the usual care group were offered the educational intervention after the final assessment. All study procedures were approved by the Institutional Review Board.
Follow-up Visit
The IBD knowledge and transition readiness questionnaires were re-administered to participants in both the EI and UC groups 4 weeks after the baseline visit (ie, 4 weeks after the intervention for the EI group) to monitor for knowledge retention and transition readiness skills, respectively. We chose to administer questionnaires 4 weeks after the intervention in order to evaluate whether any knowledge gained from the intervention was used to improve transition readiness skills (eg, refilling a prescription, scheduling an appointment). Patients in the EI group also completed a qualitative interview regarding the educational intervention including the IBD Pocket Guide. All interviews were conducted by the primary investigator for internal consistency.
Measures
Randomized participants completed measures assessing IBD knowledge and transition readiness at baseline (week 5) and again at 4 weeks following the educational intervention (for the EI group) or 4 weeks after baseline assessment (for the usual care group). Measures of adherence, IBD knowledge, and transition readiness were selected on the basis of relevance and use in prior pediatric adherence and disease education studies.
Adherence
Participant adherence to medication was monitored during the run-in phase using the MedMinder Pill Dispensing System. Participants were instructed to use the MedMinder system to store and dispense their medications, similar to a weekly pill box. The MedMinder system recorded the date and time medications were dispensed, and data were transmitted via cellular signal to the research team. Pill counts were also conducted as a secondary measure of adherence. A multi-method assessment approach was chosen to assess adherence in order to compensate for the limitations of each method, thus providing more accurate assessment of the primary end point for this study. Participants randomized following the 4-week run-in phase continued to use the MedMinder system for the study duration. Adherence rates were calculated by dividing the number of actual openings over the number of expected openings based on prescribed regimen for the 4-week run-in period and the 4-week postrandomization period (ie, postintervention for EI group and postbaseline visit for the UC group).
Inflammatory Bowel Disease Knowledge
Inflammatory bowel disease knowledge was assessed using the IBD Knowledge Inventory Device (The IBD-KID) (18). This 23-item measure was designed for and validated in a pediatric IBD population and has been shown to have adequate internal consistency (α = 0.75) and good test-retest reliability (α = 0.84) and construct validity. The IBD-KID was used in the present study to evaluate pre-post changes in overall knowledge (total score) and in 4 domains: GI anatomy, general IBD knowledge, medications, and nutrition. Scores range from 0 to 23 and higher scores indicate more items were answered correctly.
Transition Readiness
Transition readiness was assessed using the c(TRAQ) Version 5.0 (19). Participants rated their ability level in completing 20 disease self-management skills using a 5-point Likert scale ranging from 1 (No, I don’t know how) to 5 (Yes, I always do this). Total scores can range from 20 to 100. Although there is no clinical cutoff to indicate transition readiness, higher scores indicate greater independence with disease management skills and readiness for transition. The TRAQ has been widely used in pediatric IBD studies and has been shown to have excellent internal consistency (α = 0.93) (19). We evaluated pre-post changes in overall transition readiness (total score) as well as changes across 5 established domains: managing medications, appointment keeping, tracking health issues, talking with providers, and managing daily activities.
Disease Activity
Baseline disease activity was assessed using the 6-item Partial Harvey Bradshaw Index (PHBI) (20) for patients with Crohn disease and the 6-item Pediatric Ulcerative Colitis Activity Index (PUCAI) (21) for patients with ulcerative colitis. Disease activity was categorized based on previously published cutoffs (22). Participants with a score of 4 or lower on the PHBI and participants scoring less than 10 on the PUCAI were classified as “in remission.” In addition, the Physician Global Assessment (23,24) was taken from the last clinic visit to provide an additional measure of disease activity.
Analysis Plan
T-tests were used to examine differences between groups at baseline for the following variables: adherence rate during the 4-week run-in period, IBD knowledge (total score and 4 subdomains), and transition readiness (total score and 4 subdomains). The paired samples t-test was used to compare differences in average adherence rates (prerandomization and postrandomization) between the UC and EI groups. The Wilcoxon sign ranked test, which is the most commonly used nonparametric test for paired data, was used to evaluate differences in IBD knowledge and transition readiness (total scores and domain scores) between the EI and UC groups. Qualitative analysis of responses to the semistructured interview was conducted using Grounded Theory (25), which involves deriving categories directly from the interview data rather than from preconceived hypotheses, and responses were grouped into themes using the inductive approach (26).
RESULTS
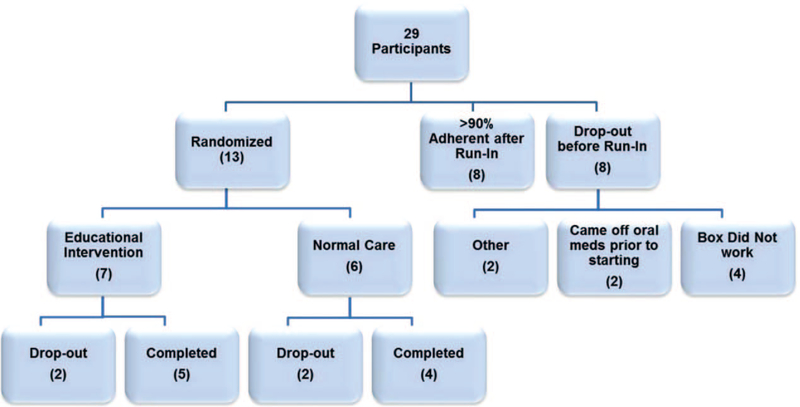
Twenty-nine patients were consented, and 21 participants completed the 4-week run-in adherence monitoring phase using the MedMinder pill box. Of those, 8 participants had 90% or greater average adherence over the run-in phase, and therefore, did not qualify for the intervention phase of the study. The remaining 13 participants were randomized to either the EI group (n = 7) or the UC group (n = 6). Five participants in the EI group completed the intervention and 4-week postintervention adherence assessment period, and 4 in the UC group completed the postrandomization adherence assessment period. See Figure 1 for recruitment and retention details.
FIGURE 1.
Participant study flow diagram.
The mean age of randomized participants was 14.9 (SD = 1.9), 44% were girls and 78% were Caucasian. Sixty-seven percentage of participants had Crohn disease and 33% had ulcerative colitis. All randomized participants had inactive disease at baseline and remained in remission throughout the study.
Baseline Characteristics
Average adherence rate for the 4-week run-in baseline period was 85.68 (SD = 2.51) for the EI group and 82.40 (SD = 5.32) for the usual care group. An independent samples t-test revealed no significant differences on average adherence over the 4-week run-in period between the EI group and usual care group (P = 0.26). There were no significant differences on average IBD knowledge total scores (IBD-KID) at baseline between the UC (M = 12.25, SD = 3.30) and EI groups (M = 11.4, SD = 2.19; P = 0.68). Similarly, there were no significant differences on transition readiness scores (TRAQ) between the UC and EI groups. Baseline characteristics, including IBD knowledge and transition readiness total and domain scores for both groups, are presented in Table 1.
TABLE 1.
Baseline characteristics for usual care and educational intervention groups
| Usual care |
Educational intervention |
||
|---|---|---|---|
| Measure | Mean (SD) | Mean (SD) | P value |
| Adherence | 85.68 (2.51) | 82.40 (5.32) | 0.26 |
| IBD Knowledge Total Score (IBD-KID) | 12.25 (3.30) | 11.40 (2.19) | 0.68 |
| GI anatomy | 1.50 (.58) | 1.00 (.71) | 0.67 |
| General IBD Knowledge | 7.75 (2.1) | 8.00 (2.12) | 0.86 |
| Medications | 2.50 (.58) | 1.40 (.55) | 0.03 |
| Nutrition | .50 (1.0) | 1.00 (1.00) | 0.48 |
| Transition Readiness Total Score (TRAQ) | 62.50 (18.88) | 62.00 (12.67) | 0.97 |
| Managing medications | 15.25 (5.90) | 14.40 (3.72) | 0.73 |
| Appointment keeping | 16.25 (9.60) | 17.40 (7.09) | 0.90 |
| Tracking health issues | 10.00 (3.16) | 9.20 (3.96) | 0.81 |
| Talking with Providers | 9.00 (1.15) | 9.40 (.89) | 0.58 |
| Managing daily activities | 12.00 (1.41) | 11.60 (2.19) | 0.54 |
Intervention Effects on Adherence, Inflammatory Bowel Disease Knowledge, and Transition Readiness
Our primary aim was to examine the effect of therapeutic patient education with the IBD Pocket Guide on medication adherence in adolescents with IBD compared with a usual care group. We used a paired sample t-test to examine the difference in rates of adherence from baseline (run-in phase) to 1 month postrandomization (Table 2). For the EI group, average adherence over the 4 weeks following intervention was slightly higher (M = 86.04, SD = 9.65) compared with baseline. Adherence in the usual care group over the postrandomization period was lower (M = 67.1, SD = 29.82) compared with baseline adherence. Differences between groups were not statistically significant (P = 0.24; Cohen d = 0.85; 95% CI −2.51 to 80).
TABLE 2.
Difference in average adherence rates (pre- and postrandomization) between usual care and educational intervention groups
| Group | Mean | Standard deviation | Difference between EI and UC | P value | Cohen d effect size |
|---|---|---|---|---|---|
| Usual care, n=4 | −15.3 | 25.34 | 15.66 | 0.24 | 0.85 |
| Educational intervention, n=5 | 0.36 | 10.28 | |||
Adherence was calculated by averaging adherence rates (actual number of openings recorded with the MedMinder system divided by expected number of openings based on prescribed regimen) for each adherence period (ie, 4-week run in and 4-week postrandomization) for EI and UC groups. EI = educational intervention; UC = usual care.
The effects of the intervention on IBD knowledge in adolescents with IBD compared with the usual care group were also examined. Average IBD knowledge scores increased in both groups. Rates of improvement, however, did not significantly differ between groups. Changes in IBD knowledge by domains were also examined (Table 3). There was improvement in the mean rank scores (MRS) in the EI group over the usual care group on GI Anatomy (MRS, 5.8 vs 4, P = 0.39), General IBD knowledge (MRS, 5.6 vs 4.25, P = 0.52) and medication knowledge (MRS 6.1 vs 3.6, P = 0.18), but a worsening of scores on questions related to nutrition (MRS 4.2 vs 6, P = 0.39). Differences between groups were not statistically significant.
TABLE 3.
Differences in inflammatory bowel disease knowledge and transition readiness between usual care and educational intervention groups
| Comparison scores Wilcoxon Mean Rank Score |
|||
|---|---|---|---|
| Category | Usual care | Educational intervention | P value |
| IBD knowledge (IBD-KID) | |||
| GI anatomy | 4.0 | 5.8 | 0.39 |
| General IBD knowledge | 4.3 | 5.6 | 0.52 |
| Medications | 3.6 | 6.1 | 0.18 |
| Nutrition | 6.0 | 4.2 | 0.39 |
| Transition readiness (TRAQ) | |||
| Managing medications | 5.3 | 4.8 | 0.90 |
| Appointment keeping | 5.0 | 5.0 | 0.99 |
| Tracking health issues | 4.4 | 5.5 | 0.52 |
| Talking with providers | 5.2 | 4.8 | 0.74 |
| Managing daily activities | 4.5 | 5.4 | 0.62 |
IBD-KID = IBD Knowledge Inventory Device; TRAQ = transition readiness scores.
Next, we examined transition readiness with the TRAQ from baseline to 4 weeks postintervention in the EI group or 4 weeks after baseline assessment for the usual care group. We examined the following domains of transition readiness: “Managing Medications,” “Appointment Keeping,” “Tracking Health Issues,” “Talking with Providers,” and “Managing Daily Activities” (Table 3). MRS were calculated for each domain for the EI and usual care groups. The EI group showed greater improvement in skills regarding tracking health issues over the usual care group (MRS 5.5 vs 4.4), but differences between groups were not statistically significant (P = 0.52).
Feasibility of the Education Intervention
At the time of the final assessment, participants in the EI group and their parents were asked to give feedback on the educational intervention via a semi-structured interview. The first theme that emerged was perceived improvement in knowledge after the educational session.
“I learned things I didn’t know before like where my Crohn’s was- I didn’t know it was outside my small intestine”.
“I thought the education session was helpful. (I) learned more from that than at the doctor’s office”.
“We had questions we didn’t know to ask”.
The second theme identified was usefulness of the IBD Pocket Guide following the educational intervention.
“I have looked at the guide since being home”.
“It will be helpful to me because I am doing a project to look at the genetics of UC”.
However, neither the parents nor the participants thought that the educational intervention changed their adherence or feelings of control over the disease.
Patient: “(The gain of new knowledge) didn’t change my feelings of control over disease.”
Parent: “I think it is a teenager thing- they think it won’t affect them down the road.”
“The education session did not change my motivation.”
DISCUSSION
In this pilot study, we tested the feasibility of a single session one-to-one therapeutic patient education session with the IBD Pocket Guide and examined preliminary efficacy of the educational intervention on medication adherence, IBD knowledge and transition readiness compared with a usual care control group. The educational intervention was well-accepted by participants. Participants and their families found the educational intervention helpful and felt that they learned new knowledge and skills from the therapeutic session. They also found the IBD Pocket Guide to be useful and a helpful resource to have at home. If efficacy of the intervention with the IBD Pocket Guide is supported in future larger scale trials, then this inexpensive guide could be implemented in the clinic setting with a nurse educator or social worker after diagnosis to help with IBD education and treatment planning. For instance, the pill size representations may help a patient to choose one over another. Overall, this educational guide could serve as a resource to support families in making educated care decisions in collaboration with their medical team.
Even with our small sample size, there were trends in the data suggesting improvement in IBD knowledge and adherence. The effect size for improved adherence following the educational intervention (Cohen d = 0.85) suggested that with more participants, there may be statistically significant improvement. This may show promise for future studies that wish to pursue educational interventions to improve the health and knowledge of adolescent patients with IBD. There was no effect found for the educational intervention on transition readiness. Baseline TRAQ scores indicated room for improvement on transition readiness across participants in both groups. Therefore, future studies to examine how to best improve transition-readiness are warranted.
Although the present study demonstrates preliminary feasibility of this novel educational intervention, there are several limitations. First, the sample size for this pilot study was small. Recruitment was impacted by the fact that many patients were not eligible for the study because they were not prescribed oral medications for the control of their IBD. Therefore, it will be important for future studies to find ways to objectively measure adherence to other types of IBD treatments, such as infliximab and adalimumab, in order to determine the intervention’s effectiveness among patients taking nonoral medications. Participant retention was impacted by difficulties related to the electronic pillbox, such as loss of cellular signal inside a participant’s home, which prevented the transmission of adherence data. In addition, several participants found the use of this monitoring system to be quite burdensome. One patient withdrew because “seeing the large box everyday reminded (them) of having IBD and it was too depressing.”
An additional limitation was that patients in the present study had relatively high rates of adherence at baseline, ranging from 76% to 89%. This impacted our ability to evaluate the effect of the intervention among individuals with more problematic adherence rates (ie, adherence below 75%). Our sample was also homogenous with regard to disease activity; all participants had quiescent disease throughout the study period. Future directions include testing the intervention among patients with more active disease and among those with poorer adherence. Finally, the qualitative interviews were all conducted by the principle investigator, which introduces the potential for both researcher and participant bias. Future iterations should examine feasibility and acceptability of the educational intervention using independent assessors and standardized feasibility and acceptability questionnaires.
An additional future direction for the study of the therapeutic patient education in this group of patients is to formalize a lesson plan. In the present study, the educational sessions were tailored to each patient in order to ensure that they received the information relative to their disease state. All sessions used the IBD Pocket Guide to give direction to the teaching. As participants and parents asked questions, each educational session varied to some degree. In addition, some topics were not covered with all patients because they were not pertinent for that participant (ie, 5-ASA use in a patient with small intestinal Crohn disease). By formalizing the educational session, this therapeutic patient education using the IBD Pocket Guide could be disseminated broadly. Finally, future studies should extend the follow-up assessment time to 6 or 12 months after therapeutic patient education in order to evaluate whether the concepts presented were retained and to determine the effect of the intervention on adherence trajectories.
In conclusion, this pilot study demonstrated the feasibility of a one-on-one therapeutic educational intervention and showed potential trends of improvement in IBD knowledge and medication adherence following the educational intervention. Future studies with larger groups of patients are needed to evaluate efficacy of this novel educational intervention. Patients and parents are eager to learn more about IBD, and this can easily be done using therapeutic patient education and the IBD Pocket Guide. As organizations are becoming more accountable for patient outcomes, this educational intervention could be used as a low-cost, high-impact method to improve patient adherence and outcomes.
Supplementary Material
What Is Known
Among adolescents with inflammatory bowel disease, nonadherence rates range from 50% to 88% across medications.
Therapeutic patient education helps patients acquire skills needed to manage their conditions and adapt treatments. In other conditions, therapeutic patient education has been shown to improve disease-specific knowledge and treatment adherence.
Therapeutic patient education has not been tested in adolescents with inflammatory bowel disease.
What Is New
Among adolescents with inflammatory bowel disease who received therapeutic patient education, trends indicated improvements in objectively measured medication adherence and inflammatory bowel disease knowledge compared with the usual care group.
Therapeutic patient education may be beneficial for improving adherence and inflammatory bowel disease knowledge in adolescents with inflammatory bowel disease. Larger-scale studies are needed to demonstrate efficacy.
Acknowledgments
This study was supported by a National Institutes of Health training grant awarded to the Cincinnati Children’s Hospital Medical Center Division of Pediatric Gastroenterology, Hepatology and Nutrition (T32 DK007727).
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Hommel KA, Davis CM, Baldassano RN. Medication adherence and quality of life in pediatric inflammatory bowel disease. J Pediatr Psychol 2008;33:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2009;15:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackner LM, Crandall WV. Oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2005;11:1006–12. [DOI] [PubMed] [Google Scholar]
- 4.Kane S, Huo D, Aikens J, et al. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med 2003;114:39–43. [DOI] [PubMed] [Google Scholar]
- 5.Higgins PDR, Rubin DT, Kaulback K, et al. Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther 2009;29:247–57. [DOI] [PubMed] [Google Scholar]
- 6.Reusch A, Weiland R, Gerlich C, et al. Self-management education for rehabilitation inpatients suffering from inflammatory bowel disease: a cluster-randomized controlled trial. Health Educ Res 2016;31: 782–91. [DOI] [PubMed] [Google Scholar]
- 7.Waters BM, Jensen L, Fedorak RN. Effects of formal education for patients with inflammatory bowel disease: a randomized controlled trial. Cana J Gastroenterol 2005;19:235–44Epub 2005/04/30. [DOI] [PubMed] [Google Scholar]
- 8.Fishman LN, Barendse RM, Hait E, et al. Self-management of older adolescents with inflammatory bowel disease: a pilot study of behavior and knowledge as prelude to transition. Clin Pediatr (Phila) 2010;49:1129–33. [DOI] [PubMed] [Google Scholar]
- 9.Fishman LN, Houtman D, van Groningen J, et al. Medication knowledge: an initial step in self-management for youth with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2011;53:641–5. [DOI] [PubMed] [Google Scholar]
- 10.Gumidyala AP, Plevinsky JM, Poulopoulos N, et al. What teens do not know can hurt them: an assessment of disease knowledge in adolescents and young adults with IBD. Inflamm Bowel Dis 2017;23:89–96. [DOI] [PubMed] [Google Scholar]
- 11.Wright EK, Williams J, Andrews JM, et al. Perspectives of paediatric and adult gastroenterologists on transfer and transition care of adolescents with inflammatory bowel disease. Intern Med J 2014;44:490–6. [DOI] [PubMed] [Google Scholar]
- 12.Barbarot S, Stalder JF. Therapeutic patient education in atopic eczema. Br J Dermatol 2014;170(Suppl 1):44–8. [DOI] [PubMed] [Google Scholar]
- 13.Lagger G, Pataky Z, Golay A. Efficacy of therapeutic patient education in chronic diseases and obesity. Patient Educ Couns 2010;79:283–6. [DOI] [PubMed] [Google Scholar]
- 14.Valinsky L, Mishali M, Endevelt R, et al. Reducing resistance to treatment, through group intervention, improves clinical measurements in patients with type 2 diabetes. BMC Endocr Disord 2013;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modi AC, Guilfoyle SM, Mann KA, et al. A pilot randomized controlled clinical trial to improve antiepileptic drug adherence in young children with epilepsy. Epilepsia 2016;57:e69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai AL, Gray E, Kurivial K, et al. The Allocation of Treatment Responsibility scale: a novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatr Transplant 2010;14:993–9. [DOI] [PubMed] [Google Scholar]
- 17.Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? J Clin Psychol Med Settings 2002;9:25–34. [Google Scholar]
- 18.Haaland D, Day A, Otley A. Development and validation of a pediatric IBD knowledge inventory device - the IBD-KID. J Pediatr Gastroenterol Nutr 2013;58:313–9. [DOI] [PubMed] [Google Scholar]
- 19.Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ–Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011;36:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz J, Grancher K, Kohn N, et al. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology 2000;119:895–902. [DOI] [PubMed] [Google Scholar]
- 21.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 22.Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016;14:348.e17–54.e17. [DOI] [PubMed] [Google Scholar]
- 23.Crandall WV, Margolis PA, Kappelman MD, et al. , ImproveCareNow Collaborative. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 2012;129: e1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr 1991;12:439–47. [PubMed] [Google Scholar]
- 25.Charmaz K, Belgrave L. Qualitative interviewing and grounded theory analysis. The SAGE Handbook of Interview Research: The Complexity of the Craft. Vol. 2. Thousand Oaks, CA: SAGE Publications, Inc; 2012:347–65. [Google Scholar]
- 26.Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Evaluation 2006;27:237–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.