Abstract
Cellular senescence is a state of stable cell cycle arrest associated with macromolecular alterations and secretion of pro‐inflammatory cytokines and molecules. Senescence‐associated phenotypes restrict damage propagation and activate immune responses, two essential processes involved in response to viral infections. However, excessive accumulation and persistence of senescent cells can become detrimental and promote pathology and dysfunctions. Various pharmacological interventions, including antiviral therapies, lead to aberrant and premature senescence. Here, we review the molecular mechanisms by which viral infections and antiviral therapy induce senescence. We highlight the importance of these processes in attenuating viral dissemination and damage propagation, but also how prematurely induced senescent cells can promote detrimental adverse effects in humans. We describe which sequelae due to viral infections and treatment can be partly due to excessive and aberrant senescence. Finally, we propose that pharmacological strategies which eliminate senescent cells or suppress their secretory phenotype could mitigate side effects and alleviate the onset of additional morbidities. These strategies can become extremely beneficial in patients recovering from viral infections or undergoing antiviral therapy.
Keywords: ageing, antiretroviral therapy, cellular senescence, immunosenescence, viral infection
Subject Categories: Autophagy & Cell Death; Immunology; Microbiology, Virology & Host Pathogen Interaction
This review describes how viral infections and antiviral therapy induce senescence, how this attenuates viral dissemination and damage propagation, and that prematurely induced senescence has adverse effects. Implications for COVID‐19 pathology are also discussed.
Glossary
- Aβ
Amyloid beta
- A‐SAAs
Acute‐phase serum amyloids
- ARDS
Acute respiratory disease syndrome
- AVTIS
antiviral therapy‐induced senescence
- ACE2
Angiotensin‐converting enzyme 2
- BMD
Bone mineral density
- BM‐MSCs
Bone marrow mesenchymal stem cells
- cGAS
Cyclic GMP‐AMP synthase
- cGAMP
Cyclin GMP‐AMP
- COVID‐19
Coronavirus disease 2019
- DDR
DNA damage response
- EBV
Epstein–Barr virus
- e‐NOS
Endothelial nitric oxide synthase
- HAD
HIV‐associated dementia
- HAND
HIV‐associated neurocognitive disorders
- HAART
Highly active antiretroviral therapy
- HBV
Hepatitis B
- HMCV
Human cytomegalovirus virus
- HPV
Human papillomavirus
- HRSV
Human respiratory syncytial virus
- HIV
Human immunodeficiency virus
- HUVEC
Human umbilical vein endothelial cells
- IKK
IκB kinase
- IFN‐β
Interferon‐β
- IRF3
Interferon regulatory factor 3
- KSHV
Kaposi sarcoma‐associated herpesvirus
- MEF
Mouse embryonic fibroblasts
- NAC
N‐acetyl‐L‐cysteine
- NO
Nitric oxide
- NRTIs
Nucleoside reverse transcriptase inhibitors
- NNRTIs
Non‐nucleoside reverse transcriptase inhibitors
- ROS
Reactive oxygen species
- SA‐β‐gal
Senescence‐associated β‐galactosidase
- SARS‐Cov‐2
Severe acute respiratory syndrome coronavirus 2
- SASP
Senescence‐associated secretory phenotype
- SCAPs
Senescent cell anti‐apoptotic pathways
- STING
Stimulator of interferon genes
- TBK1
TANK‐binding kinase 1
- TERT
Telomerase reverse transcriptase
- TLR2
Toll‐like receptor 2
- VCAM‐1
Vascular cell adhesion molecule 1
- VIS
Virus‐induced senescence
- VSV
Vesicular stomatitis virus
Introduction
The ongoing coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2), has highlighted how ageing and age‐associated disease, such as diabetes or cardiovascular disease, are primary leading causes for the development of severe symptoms and death (Jordan et al, 2020). Together with this, infections might predispose to develop long‐term health consequences and secondary morbidities. SARS‐CoV and MERS‐CoV have been associated with increase susceptibility to fibrotic disease.
Meta‐analyses have reported that human cytomegalovirus virus (HMCV), Epstein–Barr virus (EBV) or influenza can increase the risk of cardiovascular disease (Warren‐Gash et al, 2009; Wang et al, 2017), idiopathic pulmonary fibrosis (Sheng et al, 2020) and frailty (Wang et al, 2010). Although some infections such as human immunodeficiency virus (HIV) can now be treated with antiretroviral therapy, these individuals still suffer from an earlier onset of the same age‐associated diseases. Overall lifespan is still shorter than HIV‐negative individuals, suggesting that the HIV virus and/or antiretroviral medication are detrimentally modulating ageing and longevity (Smith et al, 2013). Identification of the mechanisms linking viral infection and antiretroviral therapy to age‐associated morbidities might represent an important predictor and potential target for the consequences of long‐term damages.
Cellular senescence as an antiviral response
Cellular senescence is a state of generally irreversible cell cycle arrest which cells can enter upon exposure to stress‐inducing stimuli (Calcinotto et al, 2019). Originally, cellular senescence was defined as the mechanism regulating the finite replicative lifespan of cultured cells, also known as replicative senescence (Hayflick, 1965), and the consequence of progressive telomere attrition and eventual unwinding of the telomere cap. The exposed telomere end is recognised by DNA damage response (DDR) proteins γ‐H2AX and 53BP1, which activate cell cycle inhibitors p16 and/or p21 to halt proliferation (Calcinotto et al, 2019). In recent years, it became evident that various events leading to genomic instability and DDR activation, such as activated oncogenes (Serrano et al, 1997) or direct genotoxic stress (Demaria et al, 2017), can prematurely induce senescence independently from telomere shortening. Besides the expression of cell cycle inhibitors and DNA damage proteins, senescent cells are characterised by increased lysosomal activity, exemplified by induction of the lysosomal enzyme senescence‐associated β‐galactosidase (SA‐β‐gal) (Dimri et al, 1995), and activation of a complex pro‐inflammatory phenotype, called the senescence‐associated secretory phenotype (SASP), which is typically mediated by NF‐kB activity (Coppé et al, 2008; Chien et al, 2011).
Innate immune signalling pathways, which are usually activated in response to invading pathogens, are also stimulated in senescent cells. Cyclic GMP‐AMP synthase (cGAS) is a DNA sensor which binds cytosolic viral DNA, upon which it becomes active and synthesises the nucleotide messenger cyclin GMP‐AMP (cGAMP). cGAMP translocates to the endoplasmic reticulum where it binds and activates stimulator of interferon genes (STING) (Sun et al, 2013; Motwani et al, 2019). As a result, STING moves to the Golgi apparatus and recruits TANK‐binding kinase 1 (TBK1) and IκB kinase (IKK) to phosphorylate interferon regulatory factor 3 (IRF3) and the NF‐kB inhibitor IκBα. Activated IRF3 localises into the nucleus and transcribes type I interferons including interferon‐β (IFN‐β), while phosphorylated IκBα allows NF‐kB to transcribe pro‐inflammatory molecules (Ishikawa et al, 2009; Corrales et al, 2017; Motwani et al, 2019).
Recent work has demonstrated a critical role for the cGAS‐STING pathway in senescence induction and maintenance of the SASP. Host‐derived cytoplasmic DNA can be observed in senescent mouse and human cells, and cGAS‐ or STING‐depleted cells could not be induced to senescence with DNA damaging stimuli and p16, p21 and the SASP could not be upregulated (Yang et al, 2017; Glück et al, 2017; Dou et al, 2017). Cytoplasmic DNA in senescent cells originates from the derepression of LINE‐1 retrotransposable elements mediated by upregulation in the FOXA1 transcription factor and a downregulation in RB (De Cecco et al, 2019). Cytoplasmic DNAses TREX1 and DNase2, which promote degradation of LINE‐1 elements, are also downregulated in senescent cells and dependent on loss of E2F activity. Accumulated LINE‐1 DNA is then recognised by cGAS to promote IFN‐β expression (Takahashi et al, 2018; De Cecco et al, 2019). FOXA1 also promotes senescence via transcriptional activation of p16 (Li et al, 2013). Silencing IFN‐β leads to a bypass of the growth arrest (Yu et al, 2015). Overall, these results suggest the cGAS‐STING pathway regulates both growth arrest and inflammation in senescent cells through IFN‐β activity. cGAS‐STING also regulates innate immunity in senescent cells through NF‐kB‐mediated transcription of Toll‐like receptor 2 (TLR2) and acute‐phase serum amyloids (A‐SAAs). A‐SAAs are proteins which are recognised by TLR2 to further activate NF‐kB and expression of pro‐inflammatory SASP factors in a positive feedback loop. Overexpressing TLR2 induces cell cycle arrest and SA‐β‐gal, while silencing blunts p16, p21 and SASP expression (Hari et al, 2019).
Evidence of virus‐induced senescence (VIS)
Although a small relatively number of studies are available, mounting evidence suggests the activation of senescence responses upon viral infections. Human respiratory syncytial virus (HRSV) was shown to induce senescence in A549 lung cancer cells and HEp‐2 epithelial laryngeal carcinoma cells in culture, as well as mouse epithelial lung cells in vivo (Martínez et al, 2016). Similarly, measles virus and HCMV induced senescence in normal human lung fibroblasts (Noris et al, 2002; Chuprin et al, 2013), while influenza A virus subtype H7N9 induced senescence in Neuro2a mouse neuroblast cells (Yan et al, 2017). Infected cells upregulated various senescence markers including SA‐β‐gal, p16, p21 and pro‐inflammatory SASP molecules IL‐6 and IL‐8 (Noris et al, 2002; Chuprin et al, 2013; Martínez et al, 2016; Yan et al, 2017). VIS IMR‐90 fibroblasts also upregulated MICA and ULBP2 ligands (Chuprin et al, 2013), which were previously found to be important for modulating NK mediated cell killing of senescent cells (Sagiv et al, 2016). HIV proteins Tat and Nef induce the same markers of senescence (SA‐β‐gal, p21, IL‐6 and IL‐8) in human microglia and bone marrow mesenchymal stem cells (BM‐MSCs) (Beaupere et al, 2015; Chen et al, 2018; Thangaraj et al, 2021). A recent study demonstrated that Tat protein induces senescence in microglial cells by increasing reactive oxygen species (ROS) levels through downregulation of SIRT3, a mitochondrial NAD+‐dependent deacetylase responsible for maintaining oxidative stress (Thangaraj et al, 2021).
Potential mechanisms behind VIS are illustrated in Fig 1. A DDR could be detected in VIS cells and reported to be caused by an increase in mitochondrial ROS; treating infected cells with the ROS inhibitor N‐acetyl‐L‐cysteine (NAC) was sufficient to prevent senescence induction (Beaupere et al, 2015; Martínez et al, 2016; Chen et al, 2018). It is possible that oxidative stress in VIS cells also leads to activation of cGAS‐STING pathways as previously mentioned (FOXA1 activation, LINE‐1 depression, etc.). The mechanism of ROS production in VIS cells remains poorly understood, although it has been reported that some viruses including influenza and HRSV stimulate upregulation of ROS‐generating enzymes including NADPH oxidases and xanthine oxidase (Khomich et al, 2018).
Figure 1. Potential mechanisms behind VIS in response to viral infections.
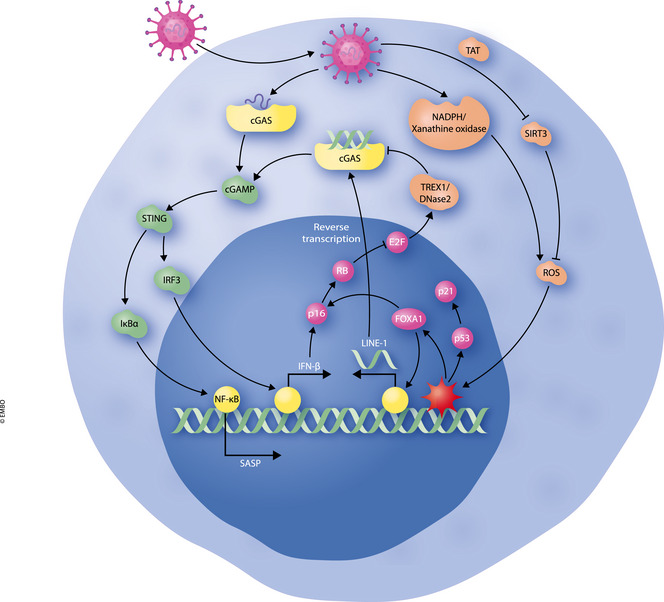
Viral entry into cells results in release of viral DNA into the host cytoplasm. Viral DNA may be recognised by cGAS, resulting in activation of the cGAS‐STING immune signalling pathway and downstream activation of NF‐κB and p‐IRF3. NF‐κB transcribes pro‐inflammatory SASP genes while p‐IRF3 can induce transcription of IFN‐β. IFN‐β can activate p16 and p21 tumour suppressors to induce growth arrest. Viruses may themselves induce activation of NADPH and xanthine oxidases, resulting in ROS accumulation. ROS can induce DNA damage and expression of FOXA1 to induce p16 transcription of LINE‐1 elements. LINE‐1 retrotransposons are reverse transcribed, and DNA elements are recognised by cGAS. E2F inhibition by RB in senescent cells also results in downregulation of TREX1 and DNase2, allowing DNA fragments to accumulate in the cytoplasm.
Interestingly, senescent cells have themselves been shown to inhibit virulence. Lower titres of vesicular stomatitis virus (VSV) are obtained when infected into mouse embryonic fibroblasts (MEFs) induced to senescence with the chemotherapeutic bleomycin, compared to non‐senescent MEFs. These results could be repeated with bleomycin‐treated A549 and HRASV12‐expressing MCF7 breast cancer cells (Baz‐Martínez et al, 2016). Similar results were independently observed with dengue virus and replicative senescent human umbilical vein endothelial (HUVEC) cells (Abubakar et al, 2014). In addition, senescence seems to control viral replication in vivo. When VSV was intranasally administered into mice, titres could not be obtained in lungs from mice treated intratracheally with bleomycin, a method to promote lung‐specific senescence (Aoshiba et al, 2003). NK and dendritic cell infiltrates were also found in these animals, suggesting a role for the SASP in mediating immune‐mediated viral clearance (Baz‐Martínez et al, 2016).
Overall, these results suggest innate immune pathways enforce both growth arrest to inhibit viral replication and prevent further spread of pathogens, as well as expression of SASP molecules and NK cell ligands to promote immunosurveillance and clearance of infected cells. It is possible that senescence is an evolutionary mechanism to promote antiviral defence. This also ponders the question on whether oncoviruses are tumorigenic because they have evolved mechanisms to bypass the antiviral properties of senescent cells, in order to increase their infection efficiency.
Evidence of senescence evasion by oncoviruses
Human papillomavirus (HPV) infections most notably lead to the development of cervical cancer. E6 and E7 are two gene products expressed by the virus which are reported to interact with a range of targets to promote various aspects of tumorigenesis including inflammation, invasion and apoptotic resistance. However, E6 and E7 are also known to interfere with the p53 and pRB pathways, respectively, to destabilise senescence (Estêvão et al, 2019). E6 targets p53 by first binding to the E3 ubiquitin ligase UBE3A (this interaction is known as E6‐AP). E6‐AP subsequently ubiquitinates p53 to initiate its proteasomal‐mediated degradation (Scheffner et al, 1990; Thomas et al, 1999). E7 binds to pRB, preventing the tumour suppressor from repressing E2F‐mediated transcription of genes required for the G1‐S transition in the cell cycle (Münger et al, 1989; Gonzalez et al, 2001). Repressing E6 or E7 in human cervical cancer cells can induce senescence through subsequent activation of p53 or p16/RB pathways (DeFilippis et al, 2003; Hall & Alexander, 2003).
Chronic hepatitis B (HBV) infections can lead to the development of hepatocellular carcinoma. HBx is one gene product expressed by HBV and is reported to be an important promoter of oncogenesis (Liang, 2009), possibly through HBx‐mediated suppression of senescence in infected cells. Hbx has been reported to bypass senescence induced by oncogenic RAS, most likely via reducing expression of p16, p53 and p21 tumour suppressors (Oishi et al, 2007). Another study demonstrated that HBx bypasses senescence in HepG2 liver cancer cells by inducing methylation of the p16 promoter, preventing binding of Ets1 and Ets2 transcription factors (Kim et al, 2010).
Kaposi sarcoma‐associated herpesvirus (KSHV) can lead to the development of Kaposi’s sarcoma. KSHV encodes LANA which inhibits senescence by binding to p53 to inhibit its transcriptional activity (Friborg et al, 1999), or to pRB to allow E2F to transcribe genes required for cell cycle progression (Radkov et al, 2000). Two other KSHV gene products, v‐cyclin and v‐FLIP, have been shown to synergistically coordinate senescence bypass and tumorigenesis. v‐cyclin is a homolog of mammalian cyclin D, whose expression by KSHV results in cell cycle dysregulation and senescence in fibroblasts, similar to what observed for RAS‐induced senescence. However, v‐FLIP can bypass v‐cyclin‐mediated senescence via inhibition of autophagy (Leidal et al, 2012). Since autophagy has been reported to be important for the generation of cytoplasmic DNA in senescent cells (Ivanov et al, 2013), a possibility is that v‐FLIP inhibits the formation of cytoplasmic DNA fragments in KSHV‐infected cells and prevents the subsequent engagement of the cGAS‐STING signalling pathway.
The Epstein–Barr virus (EBV) most typically infects B lymphocytes, inducing their transformation into lymphoblastic leukaemic cells. The oncogenic potential of EBV results from its expression of LMP1, a viral protein which tethers to cell membrane of infected cells and induces the activation of proliferative and anti‐apoptotic pathways (Young & Rickinson, 2004). However, LMP1 can suppress RAS‐induced and replicative senescence by reducing promoter activity of p16 (Yang et al, 2000) mainly by mediating the export of the Ets2 transcription from the nucleus into the cytoplasm (Ohtani et al, 2003). It should be noted that these findings were obtained in fibroblasts and need to be recapitulated in B cells.
Cellular senescence in response to antiviral therapy
Certain viral infections can be treated with the use of antiviral medicines. Interestingly, some of these drugs have been reported to be capable of inducing antiviral therapy‐induced senescence (AVTIS) in mammalian cells in vitro. The majority of these studies have been carried out with antiretrovirals typically used against HIV: (i) entry inhibitors, (ii) nucleoside reverse transcriptase inhibitors, (iii) non‐nucleoside reverse transcriptase inhibitors, (iv) integrase inhibitors and (v) protease inhibitors. HIV patients are typically administered drug combinations from these various categories in highly active antiretroviral therapy (HAART) in order to target multiple stages in a HIV’s life cycle (De Clercq & Li, 2016). However, most of these investigations have used single agents, rather than combinations of drugs typically used in HAART. This may underestimate the possible senescence‐inducing effect of these compounds in HIV patients. To our knowledge, only reverse transcriptase and protease inhibitors have been shown so far to have senescence‐inducing properties (Fig 2).
Figure 2. Cellular pathways involved in AVTIS in response to HIV antiretroviral compounds.
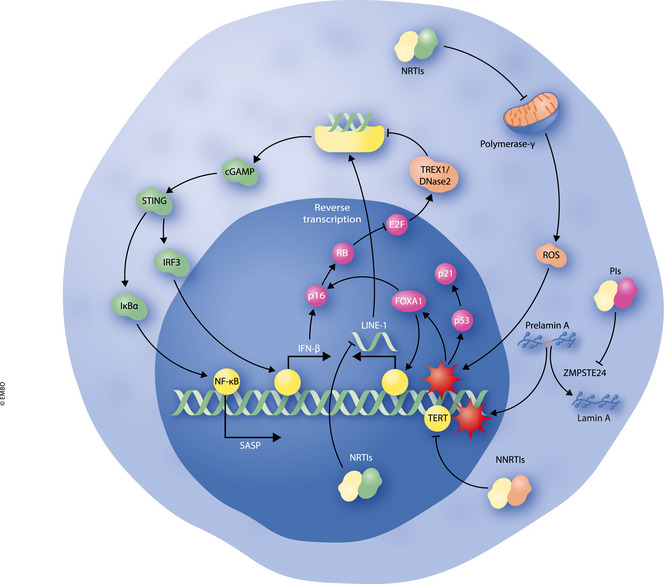
Protease inhibitors (PIs) inhibit the activity of ZMPSTE24, resulting in an accumulation of prelamin A. Prelamin A induces DNA damage at telomeres and induction of senescence by mechanisms described in the text. Nucleoside reverse transcriptase inhibitors (NRTIs) induce mitochondrial ROS and DNA damage through inhibition of polymerase‐γ, although NRTIs may also inhibit the SASP through repression of LINE‐1 elements. Non‐nucleoside reverse transcriptase inhibitors (NNRTIs) may induce senescence via inhibition of TERT, resulting in shortened telomeres and DNA damage.
Nucleoside reverse transcriptase inhibitors (NRTIs)
As HIV is a retrovirus, it must transcribe its RNA genome into DNA before being integrated into the cellular genome. HIV encodes a reverse transcriptase enzyme, transcribed from the pol gene, to carry out this essential step (Hu & Hughes, 2012). NRTIs competitively bind the reverse transcriptase enzyme at the catalytic site, thus inhibiting its enzymatic function (de Béthune, 2010). NRTIs stavudine and zidovudine as single agents induce various markers of senescence in human fibroblasts including SA‐β‐gal, p16 and p21, as well as a reduction in the number of proliferating cells. Levels of p16 and p21 are also higher in abdominal fat from HIV patients treated with HAART regiments that contained these two drugs, indicating they can induce AVTIS in vivo (Caron et al, 2008). The number of SA‐β‐gal‐positive cells is also increased in zidovudine‐treated human aortic endothelial cells (Chen et al, 2019). NRTIs tenofovir and emtricitabine induce AVTIS in human lung and cardiac fibroblasts as well as HUVECs when administered together, as judged by the increase in number of SA‐β‐gal‐positive cells, p16 and p21, as well as by elevated expression of various pro‐inflammatory SASP genes (Nacarelli et al, 2016; Cohen et al, 2018). Combination treatment of the NRTIs abacavir and lamivudine can induce SA‐β‐gal and high IL‐6 secretion in cultured microglia (Cohen et al, 2017). Interestingly, these two drugs could not induce senescence as single agents in fibroblasts in vitro, and HIV patients treated only with lamivudine do not display increased p16 or p21 expression in abdominal fat (Caron et al, 2008).
Interestingly, the NRTIs lamivudine and stavudine has also been shown to repress the SASP as they can inhibit the activation of LINE‐1 elements. Importantly, these drugs improve various aspects of mouse health lifespan where the SASP plays a detrimental role such as skeletal atrophy, bone density and muscle mass (De Cecco et al, 2019; Simon et al, 2019). Further work is clearly required to delineate the role of NRTIs in senescence and ageing. It is possible that the effect of NRTIs on senescence and the SASP is required on a range of factors including cell type, dosage or whether drugs are administered together or as lone agents.
Nevertheless, when AVTIS could be induced in cells in vitro, mitochondrial ROS levels were increased (Caron et al, 2008; Nacarelli et al, 2016; Cohen et al, 2017; Cohen et al, 2018; Chen et al, 2019), indicating NRTIs induce oxidative stress as a by‐product. NRTIs‐mediated ROS induction might by due to direct inhibition of mitochondrial enzymes or polymerase‐γ, an enzyme responsible for replication and repair of mitochondrial DNA (Smith et al, 2017).
Non‐nucleoside reverse transcriptase inhibitors
Non‐nucleoside reverse transcriptase inhibitors (NNRTIs) function in a similar manner to NRTIs to inhibit HIV replication. The major difference is that they bind the enzyme in a non‐competitive manner at an allosteric site, inducing a conformational change in the enzyme that results in reduced catalytic activity (de Béthune, 2010). NNRTIs nevirapine and efavirenz have been reported to induce AVTIS in vitro. However, these studies have only used immortalised cancer or stem cell lines, making it difficult to assess if NNRTI‐mediated senescence is solely due to inhibition of telomerase reverse transcriptase (TERT). Treatment with NNRTI induced SA‐β‐gal, reduced number of cycling cells, upregulation in p16 or p21, and presence of a DDR (Stefanidis et al, 2008; Fang & Beland, 2013; Jin et al, 2016; Hecht et al, 2018). Whether NNRTIs can induce AVTIS in primary cells is a question that should be investigated.
Protease inhibitors
Newly synthesised HIV DNA becomes integrated into the host genome by the HIV integrase enzyme, where it is then transcribed to synthesise HIV mRNA. This product is then translated by the host machinery to form an immature polyprotein. HIV protease cleaves at specific sites to yield newly matured HIV reverse transcriptase and integrase products and a subsequent restart of the viral replication cycle. Protease inhibitors interfere with this essential step (Lv et al, 2015). Protease inhibitors lopinavir and atazanavir independently induce SA‐β‐gal, p16 and p21, as well as lamin B1 loss in human BM‐MSCs. Although ritonavir, another protease inhibitor currently used as a booster of other retrovirals, is not sufficient to induce AVTIS in these cells, its administration with lopinavir and atazanavir synergised to promote senescence‐inducing capabilities (Hernandez‐Vallejo et al, 2013). A lopinavir/ritonavir combination also induced these markers in human arterial endothelial cells as well as production of IL‐6 and IL‐8, indicating presence of a pro‐inflammatory SASP (Lefèvre et al, 2010; Auclair et al, 2014; Afonso et al, 2017). Finally, the protease inhibitors nelfinavir and indinavir could individually induce AVTIS in human fibroblasts as shown by elevated p16 and p21 levels, SA‐β‐gal activity and reduced proliferative rates (Caron et al, 2007).
A potential mechanistic explanation of the senescence‐inducing potential of protease is accumulation of prelamin A. Prelamin A is the precursor of the nuclear lamina protein lamin A and undergoes a series of post‐translational modifications to produce the mature protein. One of these steps involves the conversion of farnesyl‐prelamin A to lamin A by the zinc metalloprotease ZMPSTE24 (Clarke, 2007). Prelamin A accumulation is observed in AVTIS cells induced by protease inhibitors but senescence induction was less efficient when cells were also treated with protein farnesylation inhibitors pravastatin or zoledronic acid (Caron et al, 2007; Lefèvre et al, 2010; Hernandez‐Vallejo et al, 2013; Auclair et al, 2014; Bonello‐Palot et al, 2014; Afonso et al, 2017). Therefore, AVTIS induction by protease inhibitors is dependent upon immature processing of lamin A. Lopinavir and atazanavir inhibit the activity of ZMPSTE24 as an off target, which explains how prelamin A can accumulate (Coffinier et al, 2007). It is speculated this off target effect occurs because ZMPSTE24 and HIV protease share sequence and conformation in regards to their enzymatic site (Clarke, 2007). Interestingly, the protease inhibitor darunavir does not inhibit ZMPSTE24 (Coffinier et al, 2008), which may explains why this drug does not induce senescence in human arterial endothelial cells (Auclair et al, 2014).
It should be noted that de novo lamin A mutations are commonly associated with people suffering from Hutchinson–Gilford progeria syndrome. This mutation results in the formation of a splice site and an in‐frame deletion of 50 amino acids near the C‐terminus. However, this abnormal product called progerin is not processed by ZMPSTE24, resulting in the accumulation of an immature farnesylated product (Gonzalo et al, 2017). Progerin can induce telomere‐associated DNA damage, leading to activation of a DDR and entry into senescence (Benson et al, 2010). It is therefore possible that, in cells treated with protease inhibitors, accumulated prelamin A can also induce senescence via this mechanism.
Viral infections, antiretroviral therapy and immunosenescence: a role for cellular senescence?
Aged individuals are more prone to suffer from infections as the consequence of a general functional decline of innate and adaptive immunity—a phenomenon termed “immunosenescence” (Aw et al, 2007). Immunosenescence is generally associated with decreased ratio of CD4+ to CD8+ and of naïve to memory T cells, reduced total number of phagocytes and of antibody‐producing B cells and diminished NK cells cytotoxicity. Various intrinsic and extrinsic factors are reported to influence immunosenescence including genetics, hormones, nutrition, physical activity, but also chronic viral infections (Aiello et al, 2019).
HIV‐infected individuals, even in children and young adults, display an immunosenescent phenotype (Chiappini et al, 2018). Patients display decreased ratios of CD4+/CD8+ T cells, as well decreased ratios of naïve/effector memory T or B cells (Mansoor et al, 2009; Díaz et al, 2012; Sainz et al, 2013; Rinaldi et al, 2017). Many of these studies included patients undergoing HAART therapy but studies have determined whether HAART can solely modulate immunosenescence. NRTIs and NNRTIs may inhibit TERT activity in hematopoietic stem cells, which could diminish their differentiating capacity and limit the generation of new immune cells. Therefore, it is difficult to delineate to what extent the HIV virus or antiretroviral drugs are contributing to the immunosenescent phenotype.
It is currently debated whether immunosenescent cells in the aged environment should be defined senescent per se. T‐cell telomere length declines with age (Rufer et al, 1999), suggesting they have undergone replicative senescence from repetitive divisions. p16 levels also positively associate with age in this cell type (Liu et al, 2009). Interestingly, certain hallmarks of cellular senescence are also found in T cells from patients with HIV (Effros et al, 1996; Wolthers et al, 1996; Palmer et al, 1997; Nelson et al, 2012; Pereira Ribeiro et al, 2016). Telomere lengths of CD8+ T cells are shorter in HIV patients compared to healthy individuals, but this observation was not found in CD4+ T cells. These finds were also independent on whether HIV patients were on antiretroviral therapy (Effros et al, 1996; Wolthers et al, 1996; Palmer et al, 1997). However, p16 levels are increased in CD4+, but not CD8+, T cells in HIV patients untreated with HAART, compared to uninfected individuals (Nelson et al, 2012; Pereira Ribeiro et al, 2016). As HIV is only capable of infecting CD4+ T cells (Wilen et al, 2012), it is possible that the virus directly induces VIS in these cells. CD8+ T cells can still proliferate resulting in an overall reduction in the CD4+/CD8+ ratio.
HAART‐treated HIV patients displayed p16 levels comparable to uninfected controls in T cells (Nelson et al, 2012; Pereira Ribeiro et al, 2016). However, CD4+/CD8+ ratios are still low in patients receiving HAART (Serrano‐Villar et al, 2014). Although HAART may limit viral replication in CD4+ T lymphocytes and limit ageing of these cells, they may promote immunosenescence through other aspects, e.g. by exhausting the hematopoietic stem cell pool, as mentioned previously (section 2). Since senescent cells are capable of inducing paracrine senescence via the SASP (Acosta et al, 2013), it is possible that AVTIS induction in somatic cells in vivo promote senescence‐like phenotypes in immune cells.
p16+ T cells are likely to have a reduced immune capacity as specific deletion of p16 in these cells enhances immune function in aged mice (Liu et al, 2011). Therefore, one mechanism by which viruses may induce the premature onset of various age‐related diseases is by compromising the immune‐mediated clearance of senescent cells. Mice engineered with an impaired capacity to eliminate senescent cells display increased natural accumulation of senescent cells, premature signs of ageing and shortened lifespans compared to wild‐type animals (Ovadya et al, 2018).
Viral infections, antiretroviral therapy and age‐associated diseases
The natural accumulation of senescent cells in mammals during ageing contributes to the development of various age‐associated diseases (Baker et al, 2016; Calcinotto et al, 2019; Borghesan et al, 2020). Although immunosenescence may play a role in the development of these diseases, as previously mentioned, this section will discuss mechanisms behind how VIS and AVTIS could be linked to premature ageing in HIV patients (Fig 3).
Figure 3. Implications of cellular senescence in age‐related diseases commonly associated with HIV patients undergoing HAART therapy.
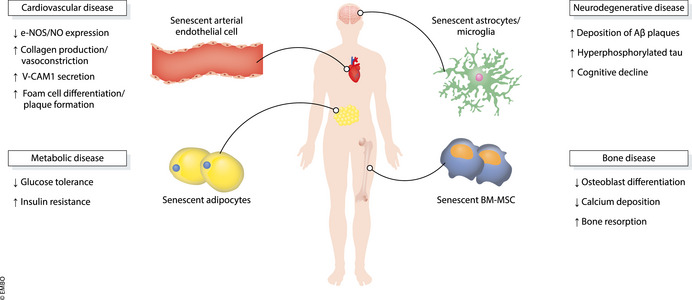
Potential mechanisms behind VIS and AVTIS in age‐associated diseases in HIV patients. See text for further details.
Bone disease
Decreased bone mineral density (BMD), otherwise known as osteopenia, is associated with increased age in humans. Low BMD results from impaired bone mineralisation and decreased bone strength. This results in a greater chance of suffering a bone fracture, otherwise known as osteoporosis. Bone structure is mediated by a tightly controlled balance between bone synthesis by osteoblasts and bone resorption by osteoclasts. However, this balance shifts towards bone resorption in osteoporotic patients (Tu et al, 2018). In aged mice, the SASP promotes decreased BMD from inhibition of osteoblasts‐mediated bone mineralisation and stimulation of osteoclast differentiation from progenitor cells (Farr et al, 2017).
HIV patients are consistently reported to display reduced BMD and increased risk of developing osteoporosis, although there is some divergence as to whether this phenomenon occurs due to the HIV retrovirus or protease inhibitor therapy (Tebas et al, 2000; Bruera et al, 2003; Amiel et al, 2004; Brown & Qaqish, 2006). These conflicts are possibly reconciled by reports that find both HIV proteins Tat and Nef and protease inhibitors lopinavir and atazanavir are capable of inducing senescence in BM‐MSCs. Importantly, senescent BM‐MSCs do not be effectively differentiated into osteoblasts and deposit reduced levels of calcium in vitro (Hernandez‐Vallejo et al, 2013; Beaupere et al, 2015). Overall, these results suggest that VIS and AVTIS of BM‐MSC in HIV patients compromise their osteoblastic potential, leading to reduced BMD and increased risk of osteoporosis.
Cardiovascular disease
Atherosclerosis is an age‐associated vascular disease characterised by the formation of plaques in arterial vessels, which can lead to the development of various cardiovascular disorders including myocardial infarction, stroke and sudden cardiac death (Tabas et al, 2015). Endothelial dysfunction plays a critical role in disease development. Although a healthy endothelium maintains vascular homeostasis through regulated secretion of various dilators such as nitric oxide (NO), endothelial cells in an atherosclerotic lesion produce less NO, resulting in vascular smooth muscle cell proliferation, collagen production and vasoconstriction. Importantly, a fraction of dysfunctional endothelial cells in atherosclerotic plaques is senescent (Minamino et al, 2002) and secretes vascular cell adhesion molecule 1 (VCAM‐1) to recruit and differentiate monocytes into foam cell macrophages (Davignon & Ganz, 2004; Childs et al, 2018). These foamy macrophages also display senescence‐associated features and secrete VCAM‐1 to further promote monocyte differentiation and plaque formation. Matrix metalloproteinase secretion from these cells also trigger plaque degradation (Childs et al, 2016).
HIV patients have been found to have a higher risk of cardiovascular events, which was even higher in patients treated with protease inhibitors (Friis‐Moller et al, 2007; Islam et al, 2012; Shah et al, 2018). Protease inhibitors are reported to induce senescence in human arterial endothelial cells in vitro. Importantly, these senescent cells displayed reduced expression of endothelial nitric oxide synthase (e‐NOS), an enzyme responsible for NO synthesis, and increased secretion of VCAM‐1 (Lefèvre et al, 2010; Auclair et al, 2014; Afonso et al, 2017). Therefore, it is possible that protease inhibitor administration in HIV patients induces atherosclerosis through induction of vascular endothelial cell senescence, which leads to dysregulated endothelial health and foam cell formation. The HIV virus may also independently contribute to cardiovascular risk as HIV proteins gp120 and Tat can induce senescence in aortic endothelial cells (Hijmans et al, 2018).
Metabolic disease
Cohort studies have found that the incidence of type II diabetes is increased in individuals with HIV compared to the general population, but exposure to antiretroviral therapy is reported to drive this association, rather than HIV infection per se. Results were also independent on age or whether patients were obese, suggesting traditional risk factors are not involved (Tripathi et al, 2014; Samad et al, 2017; Hernandez‐Romieu et al, 2017). Senescence is reported to detrimentally influence diabetes by inducing adipose tissue dysfunction. In mice, excessive caloric intake results in an accumulation of reactive oxygen species in adipose tissue and increased expression of various senescence and inflammatory markers such as p21, TNF and CCL2 (Minamino et al, 2009). Senescent cells in adipose tissue contribute to metabolic dysfunction as they induce impairments in glucose homeostasis and insulin sensitivity (Palmer et al, 2019), although precise mechanisms are currently unknown. To our knowledge, no evidence yet exists on whether antiretroviral compounds can induce senescence in adipocytes in vitro, but it has been demonstrated that protease inhibitors and NRTIs can induce p16 and p21 expression in human adipose tissue (Caron et al, 2007, 2008). When mice fed on a high‐fat diet were also administered with the NNRTI efavirenz and NRTI emtricitabine, there was a decrease in glucose tolerance and increase in insulin resistance (Pepin et al, 2018). Insulin sensitivity was also decreased in healthy individuals treated with the NRTI stavudine (Fleischman et al, 2007). Overall, these results suggest that AVTIS induction in adipose tissue induces metabolic dysfunction and onset of diabetes in HIV patients, but the exact role of senescence in promoting metabolic disease in this context remains to be further investigated.
Neurodegenerative disease
The term HIV‐associated neurocognitive disorders (HAND) refers to a collection of neurological conditions which can occur in HIV patients. Although this condition is normally mild, some patients can develop a highly severe form of HAND known as HIV‐associated dementia (HAD), associated with extreme motor and cognitive symptoms typical of Alzheimer patients (Kaul, 2009). Moreover, brains from post‐mortem HIV patients display increased levels of deposited amyloid beta (Aβ) and hyperphosphorylated tau compared to age‐matched controls, suggesting pathological links exist between Alzheimer’s disease and HAD (Green et al, 2005; Anthony et al, 2006; Achim et al, 2009). Evidence accumulated in recent years has shown direct correlation exists between senescence in various brain cells and Alzheimer' disease. Increased p16 protein levels can be observed in astrocytes from Alzheimer patients compared to age‐matched controls (Bhat et al, 2012), while tau‐containing neurofibrillary tangles in neurons of Alzheimer patients also display increased expression of p16 mRNA, along with upregulation of an inflammatory transcriptome (Musi et al, 2018). Senescent astrocytes and microglia accumulate in a tau‐dependent mouse model of AD where they contribute to cognitive decline in these mice (Bussian et al, 2018; Zhang et al, 2019). The HIV virus has been reported to be capable of inducing senescence in human astrocytes and microglia in vitro, as well as rat astrocytes in vivo (Yu et al, 2017; Chen et al, 2018). This process is likely to be mediated by a Tat‐dependent downregulation of SIRT3, as the enzyme is found to be downregulated in the prefrontal cortex of HIV patients, along with an upregulation of p16 and p21 (Thangaraj et al, 2021). Therefore, HIV‐mediated senescence of these cells could lead to cognitive decline in HIV patients.
Conclusions and future questions
Potential roles for cellular senescence in COVID‐19 pathology
The biological links between viral infections and ageing are still poorly characterised (see also Box 1). However, cellular senescence could play a key role, either because of the engagement of antiviral defence pathways or because different types of somatic cells can be reengaged to enter senescence upon exposure to viral infection. Most experimental evidence is based on the correlation between HIV VIS and the premature onset of various age‐associated pathologies, but similar phenomena might well happen for other viruses, including SARS‐Cov‐2. This could be an important factor in how COVID‐19 manifests, especially in the lungs, and why older people suffer from higher mortality (illustrated in Fig 4). In young individuals, SARS‐Cov‐2 entry into lung alveolar cells may induce senescence and a SASP, in order to limit viral infection and trigger immune‐mediated clearance of infected cells. The SASP may additionally induce paracrine senescence in order to limit viral infection in neighbouring lung cells. However, inflammation may already be high in lungs of older individuals owing to an age‐associated increase of senescent cells. Moreover, immune‐mediated clearance of infected cells could also be compromised because of age‐associated immunosenescence. SARS‐Cov‐2 infection induces senescence in lung cells but increased presence of inflammatory factors and failure to clear infected cells could lead to a hyperinflammatory environment (also referred to as a cytokine storm) and the onset of acute respiratory disease syndrome (ARDS) (preprint: Evangelou et al, 2021). Approximately 70% of COVID‐19 deaths are reported to occur from ARDS with age being one of the most significant risk factors (Hojyo et al, 2020; Jordan et al, 2020).
Box 1. In need of answers.
Does senescence play a direct role in why HIV patients have shorter lifespans than people without HIV? To what extent does VIS or AVTIS play a role in each age‐associated disease?
Can immunosenescent cells be classified as “senescent?” Can these cells be targeted by senolytic compounds and improve immune function?
Can senolytics or SASP modulators be used as novel therapies for people suffering from both acute and chronic viral infections.
What is the role of senescence in COVID‐19 pathology and is there a link to why older patients have a higher chance of mortality?
Figure 4. Potential roles for senescence in COVID‐19 pathology in old individuals.
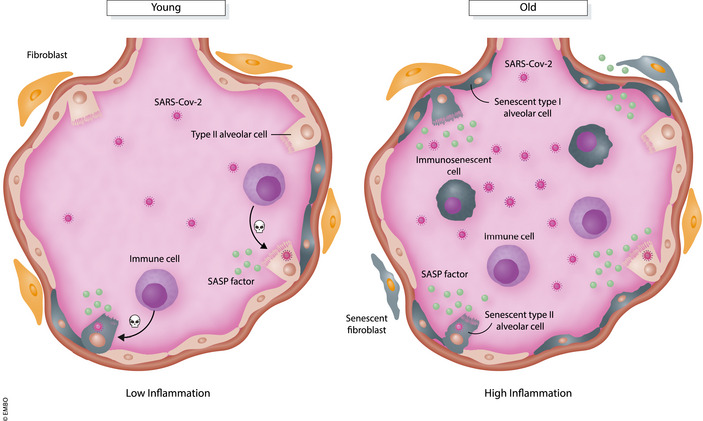
In young individuals, SARS‐Cov‐2 infection of lung alveolar cells may promote VIS and secretion of SASP molecules to promote paracrine senescence in neighbouring cells and immune‐mediated clearance of infected cells. However, immune‐mediated clearance is compromised due to immunosenescent cells in old individuals. SARS‐Cov‐2 infected cells, along with the presence of age‐associated senescent lung cells, secrete hyper levels of SASP factors leading to a cytokine storm and immunopathology.
Individuals who recover from SARS‐Cov‐2 display other damaging symptoms in various organs where senescence could play a detrimental role. For example, around 30% of SARS‐Cov‐2 patients in intensive care units display thrombotic complications (Klok et al, 2020), and senescence is reported to promote clotting via the secretion of certain SASP molecules (Wiley et al, 2019). Neurological and cardiovascular complications are also common observations in COVID‐19 patients (Ellul et al, 2020; Zheng et al, 2020). Since the receptor for SARS‐Cov‐2, angiotensin‐converting enzyme 2 (ACE2) is expressed in the brain and heart (Hamming et al, 2004), it is possible that viral‐mediated entry of these cells induces VIS and localised inflammation leading to tissue dysfunction.
Targeting cellular senescence and the SASP to alleviate viral pathologies
There is currently much interest for the discovery and characterisation of novel compounds which can either specifically induce cell death in senescent cells (senolytics) or inhibit the SASP (SASP modulators). This does pend the question on whether these drugs could also be therapeutic approach against the detrimental pro‐ageing sequelae of VIS or AVTIS in patients who suffer from HIV or other infections. Senolytics typically target senescent cell anti‐apoptotic pathways (SCAPs), survival pathways utilised by senescent cells to maintain their viability (Soto‐Gamez et al, 2019). ABT‐263 is one senolytic and functions to inhibit the BCL‐2 family of anti‐apoptotic proteins, which are typically upregulated in senescent cells (Zhu et al, 2016; Chang et al, 2016). Interestingly, ABT‐263 has been shown to sensitise retinal pigment epithelium cells and macrophages infected with a variety of virus types (influenza, herpes simplex, measles) to undergo cell death (Kakkola et al, 2013). However, this study did not analyse whether infected cells were senescent. The AKT pathway is another SCAP used by senescent cells to maintain survival. HSP90 inhibitors such as geldanamycin, or natural flavonoids such as quercetin, have been demonstrated to exert senolytic properties by interfering with this pathway (Zhu et al, 2015; Fuhrmann‐Stroissnigg et al, 2017; Kirkland et al, 2017). To our knowledge, there is no evidence on whether these senolytics can sensitise viral‐infected cells to die and studies are warranted to answer this.
SASP modulators are actively being trialled to reduce mortality in hospitalised COVID‐19 patients, owing to their potential in repressing the detrimental cytokine storm (Nehme et al, 2020). Some compounds are shown to be beneficial in improving mortality in severely ill patients, but more investigations are required to determine whether this occurs from any possible effect in repressing the SASP from senescent SARS‐Cov‐2 senescent cells in the lung. Glucocorticoids are a group of steroid hormones which have anti‐inflammatory properties by repressing IL‐6 secretion via inhibition of NF‐κB activity (Laberge et al, 2012). Dexamethasone is an approved glucocorticoid (Dexamethasone in Hospitalized Patients with Covid‐19—Preliminary Report, 2020) and has been demonstrated to blunt the SASP, albeit in models of senescent cancer cells (Ge et al, 2018; Buhl et al, 2019). Two inhibitors of the IL‐6 receptor, tocilizumab and sarilumab, have also recently been approved for use against the disease after promising clinical results in severely ill patients (Wise, 2020).
Future studies are warranted to determine whether senolytics or SASP modulators can induce death or repress pro‐inflammatory signalling in VIS and ATVIS cells respectively (see also Box 1). As well as benefiting patients suffering from acute viral infections like SARS‐Cov‐2, they may also be beneficial in alleviating onset of age‐associated symptoms in patients with chronic viral infections, such as HIV patients on antiretroviral therapy. Moreover, HIV patients treated with protease inhibitors may also benefit from treatment with pravastatin or zoledronic acid, as they may prevent senescence induced by accumulation of prelamin A. Phase 2 trials have reported that zoledronic acid prevents bone loss in HAART‐treated HIV patients (Ofotokun et al, 2016).
Conflict of interest
M.D. is advisor and shareholder of Cleara Biotech.
Acknowledgements
We thank the Demaria laboratory for fruitful discussions. This work was supported by grants from the Dutch cancer Foundation (KWF to M.D.) and from the Horizon 2020 (J. K. and M.D.).
EMBO Reports (2021) 22: e52243.
See the Glossary for abbreviations used in this article.
References
- Abubakar S, Shu MH, Johari J, Wong PF (2014) Senescence affects endothelial cells susceptibility to dengue virus infection. Int J Med Sci 11: 538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E (2009) Increased accumulation of intraneuronal amyloid β in HIV‐infected patients. J Neuroimmune Pharmacol 4: 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M et al (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso P, Auclair M, Caron‐Debarle M, Capeau J (2017) Impact of CCR5, integrase and protease inhibitors on human endothelial cell function, stress, inflammation and senescence. Antivir Ther 22: 645–657 [DOI] [PubMed] [Google Scholar]
- Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, Accardi G (2019) Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol 10: 2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel C, Ostertag A, Slama L, Baudoin C, N’Guyen T, Lajeunie E, Neit‐Ngeilh L, Rozenbaum W, De Vernejoul MC (2004) BMD is reduced in HIV‐infected men irrespective of treatment. J Bone Miner Res 19: 402–409 [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2006) Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus‐1 before and after the advent of highly active anti‐retroviral therapy. Acta Neuropathol 111: 529–538 [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Tsuji T, Nagai A (2003) Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J 22: 436–443 [DOI] [PubMed] [Google Scholar]
- Auclair M, Afonso P, Capel E, Caron‐Debarle M, Capeau J (2014) Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antivir Ther 19: 773–782 [DOI] [PubMed] [Google Scholar]
- Aw D, Silva AB, Palmer DB (2007) Immunosenescence: Emerging challenges for an ageing population. Immunology 120: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, A. Saltness R, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM (2016) Naturally occurring p16 Ink4a‐positive cells shorten healthy lifespan. Nature 530: 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz‐Martínez M, Da Silva‐Álvarez S, Rodríguez E, Guerra J, El Motiam A, Vidal A, Garciá‐Caballero T, González‐Barcia M, Sánchez L, Munõz‐Fontela C et al (2016) Cell senescence is an antiviral defense mechanism. Sci Rep 6: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaupere C, Garcia M, Larghero J, Fève B, Capeau J, Lagathu C (2015) The HIV proteins tat and nef promote human bone marrow mesenchymal stem cell senescence and alter osteoblastic differentiation. Aging Cell 14: 534–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson EK, Lee SW, Aaronson SA (2010) Role of progerin‐induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci 123: 2605–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Béthune MP (2010) Non‐nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV‐1 infection: a review of the last 20 years (1989–2009). Antiviral Res 85: 75–90 [DOI] [PubMed] [Google Scholar]
- Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C (2012) Astrocyte senescence as a component of Alzheimer’s disease. PLoS One 7: e45069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonello‐Palot N, Simoncini S, Robert S, Bourgeois P, Sabatier F, Levy N, Dignat‐George F, Badens C (2014) Prelamin A accumulation in endothelial cells induces premature senescence and functional impairment. Atherosclerosis 237: 45–52 [DOI] [PubMed] [Google Scholar]
- Borghesan M, Hoogaars WMH, Varela‐Eirin M, Talma N, Demaria M (2020) A senescence‐centric view of aging: implications for longevity and disease. Trends Cell Biol 30: 777–791 [DOI] [PubMed] [Google Scholar]
- Brown TT, Qaqish RB (2006) Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta‐analytic review. AIDS 20: 2165–2174 [DOI] [PubMed] [Google Scholar]
- Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J (2003) Decreased bone mineral density in HIV‐infected patients is independent of antiretroviral therapy. AIDS 17: 1917–1923 [DOI] [PubMed] [Google Scholar]
- Buhl JL, Selt F, Hielscher T, Guiho R, Ecker J, Sahm F, Ridinger J, Riehl D, Usta D, Ismer B et al (2019) The senescence‐associated secretory phenotype mediates oncogene‐induced senescence in pediatric pilocytic astrocytoma. Clin Cancer Res 25: 1851–1866 [DOI] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau‐dependent pathology and cognitive decline. Nature 562: 578–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A (2019) Cellular senescence: aging, cancer, and injury. Physiol Rev 99: 1047–1078 [DOI] [PubMed] [Google Scholar]
- Caron M, Auclair M, Donadille B, Béréziat V, Guerci B, Laville M, Narbonne H, Bodemer C, Lascols O, Capeau J et al (2007) Human lipodystrophies linked to mutations in A‐type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ 14: 1759–1767 [DOI] [PubMed] [Google Scholar]
- Caron M, Auclairt M, Vissian A, Vigouroux C, Capeau J (2008) Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir Ther 13: 27–38 [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W et al (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NC, Partridge AT, Tuzer F, Cohen J, Nacarelli T, Navas‐Martín S, Sell C, Torres C, Martín‐García J (2018) Induction of a senescence‐like phenotype in cultured human fetal microglia during HIV‐1 infection. J Gerontol A Biol Sci Med Sci 73: 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Hebert VY, Stadler K, Xue SY, Slaybaugh K, Luttrell‐Williams E, Glover MC, Krzywanski DM, Dugas TR (2019) Coenzyme Q10 alleviates chronic nucleoside reverse transcriptase inhibitor‐induced premature endothelial senescence. Cardiovasc Toxicol 19: 500–509 [DOI] [PubMed] [Google Scholar]
- Chiappini E, Bianconi M, Dalzini A, Petrara MR, Galli L, Giaquinto C, De Rossi A (2018) Accelerated aging in perinatally HIV‐infected children: clinical manifestations and pathogenetic mechanisms. Aging (Albany NY) 10: 3610–3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS et al (2011) Control of the senescence‐associated secretory phenotype by NF‐κB promotes senescence and enhances chemosensitivity. Genes Dev 25: 2125–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354: 472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, Li H, Van Deursen JM (2018) Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest 128: 1217–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuprin A, Gal H, Biron‐Shental T, Biran A, Amiel A, Rozenblatt S, Krizhanovsky V (2013) Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev 27: 2356–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SG (2007) HIV protease inhibitors and nuclear lamin processing: getting the right bells and whistles. Proc Natl Acad Sci USA 104: 13857–13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Hudon SE, Farber EA, Chang SY, Hrycyna CA, Young SG, Fong LG (2007) HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells. Proc Natl Acad Sci USA 104: 13432–13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Hudon SE, Lee R, Farber EA, Nobumori C, Miner JH, Andres DA, Spielmann HP, Hrycyna CA, Fong LG et al (2008) A potent HIV protease inhibitor, darunavir, does not inhibit ZMPSTE24 or lead to an accumulation of farnesyl‐prelamin a in cells. J Biol Chem 283: 9797–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, D’Agostino L, Wilson J, Tuzer F, Torres C (2017) Astrocyte senescence and metabolic changes in response to HIV antiretroviral therapy drugs. Front Aging Neurosci 9: 281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, D’Agostino L, Tuzer F, Torres C (2018) HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECs. Mech Ageing Dev 175: 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J‐P, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez P‐Y, Campisi J (2008) Senescence‐associated secretory phenotypes reveal cell‐nonautonomous functions of oncogenic RAS and the p53 Tumor suppressor. PLoS Biol 6: e301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Matson V, Flood B, Spranger S, Gajewski TF (2017) Innate immune signaling and regulation in cancer immunotherapy. Cell Res 27: 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J, Ganz P (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109: III‐27–III‐32 [DOI] [PubMed] [Google Scholar]
- De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke JD et al (2019) L1 drives IFN in senescent cells and promotes age‐associated inflammation. Nature 566: 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E, Li G (2016) Approved antiviral drugs over the past 50 years. Clin Microbiol Rev 29: 695–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis RA, Goodwin EC, Wu L, DiMaio D (2003) Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol 77: 1551–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM et al (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7: 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz L, Méndez‐Lagares G, Correa‐Rocha R, Pacheco YM, Ferrando‐Martínez S, Ruiz‐Mateos E, Del P‐B, León JA, Gurbindo MD, Isabel De José M et al (2012) Detectable viral load aggravates immunosenescence features of CD8 T‐cell subsets in vertically HIV‐infected children. J Acquir Immune Defic Syndr 60: 447–454 [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira‐Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc Natl Acad Sci USA 92: 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z et al (2017) Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550: 402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji KI, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV (1996) Shortened telomeres in the expanded CD28‐ CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 10: F17–F22 [DOI] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T (2020) Neurological associations of COVID‐19. Lancet Neurol 19: 767–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estêvão D, Costa NR, Gil da Costa RM, Medeiros R (2019) Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta – Gene Regul Mech 1862: 153–162 [DOI] [PubMed] [Google Scholar]
- Evangelou K, Veroutis D, Foukas PG, Paschalaki K, Kittas C, Tzioufas AG, De Leval L, Vassilakos D, Barnes PJ, Gorgoulis VG (2021) Alveolar type II cells harbouring SARS‐CoV‐2 show senescence with a proinflammatory phenotype. bioRxiv 10.1101/2021.01.02.424917 [PREPRINT] [DOI] [Google Scholar]
- Fang JL, Beland FA (2013) Differential responses of human hepatocytes to the non‐nucleoside HIV‐1 reverse transcriptase inhibitor nevirapine. J Toxicol Sci 38: 741–752 [DOI] [PubMed] [Google Scholar]
- Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM et al (2017) Targeting cellular senescence prevents age‐related bone loss in mice. Nat Med 23: 1072–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman A, Johnsen S, Systrom DM, Hrovat M, Farrar CT, Frontera W, Fitch K, Thomas BJ, Torriani M, Côté HCF et al (2007) Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol – Endocrinol Metab 292: E1666–E1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friborg J, Kong WP, Hottlger MO, Nabel GJ (1999) p53 Inhibition by the LANA protein of KSHV protects against cell death. Nature 402: 889–894 [DOI] [PubMed] [Google Scholar]
- Friis‐Moller N, Reiss P, Sabin CA, Weber R, Monforte ADA, El‐Sadr W, Thiebaut R, De Wit S, Kirk O, Fontas E et al (2007) Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 356: 1723–1735 [DOI] [PubMed] [Google Scholar]
- Fuhrmann‐Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A et al (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8: 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Ke J, Xu N, Li H, Gong J, Li X, Song Y, Zhu H, Bai C (2018) Dexamethasone alleviates pemetrexed‐induced senescence in Non‐Small‐Cell Lung Cancer. Food Chem Toxicol 119: 86–97 [DOI] [PubMed] [Google Scholar]
- Glück S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A (2017) Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 19: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez SL, Stremlau M, He X, Basile JR, Münger K (2001) Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol 75: 7583–7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo S, Kreienkamp R, Askjaer P (2017) Hutchinson‐Gilford Progeria Syndrome: a premature aging disease caused by LMNA gene mutations. Ageing Res Rev 33: 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL (2005) Brain deposition of beta‐amyloid is a common pathologic feature in HIV positive patients. AIDS 19: 407–411 [DOI] [PubMed] [Google Scholar]
- Hall AHS, Alexander KA (2003) RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol 77: 6066–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari P, Millar FR, Tarrats N, Birch J, Quintanilla A, Rink CJ, Fernández‐Duran I, Muir M, Finch AJ, Brunton VG et al (2019) The innate immune sensor Toll‐like receptor 2 controls the senescence‐associated secretory phenotype. Sci Adv 5: eaaw0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 37: 614–636 [DOI] [PubMed] [Google Scholar]
- Hecht M, Harrer T, Körber V, Sarpong EO, Moser F, Fiebig N, Schwegler M, Stürzl M, Fietkau R, Distel LV (2018) Cytotoxic effect of efavirenz in BxPC‐3 pancreatic cancer cells is based on oxidative stress and is synergistic with ionizing radiation. Oncol Lett 15: 1728–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Romieu AC, Garg S, Rosenberg ES, Thompson‐Paul AM, Skarbinski J (2017) Is diabetes prevalence higher among HIV‐infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care 5: 304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Vallejo SJ, Beaupere C, Larghero J, Capeau J, Lagathu C (2013) HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marrow mesenchymal stem cells: beneficial effect of pravastatin. Aging Cell 12: 955–965 [DOI] [PubMed] [Google Scholar]
- Hijmans JG, Stockleman K, Reiakvam W, Levy MV, Brewster LM, Bammert TD, Greiner JJ, Connick E, DeSouza CA (2018) Effects of HIV‐1 gp120 and tat on endothelial cell sensescence and senescence‐associated microRNAs. Physiol Rep 6: e13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, Hirano T (2020) How COVID‐19 induces cytokine storm with high mortality. Inflamm Regen 40: 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WS, Hughes SH (2012) HIV‐1 reverse transcription. Cold Spring Harb Perspect Med 2: a006882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN (2009) STING regulates intracellular DNA‐mediated, type i interferon‐dependent innate immunity. Nature 461: 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam FM, Wu J, Jansson J, Wilson DP (2012) Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta‐analysis. HIV Med 13: 453–468 [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pawlikowski J, Manoharan I, Van TJ, Nelson DM, Singh Rai T, Shah PP, Hewitt G, Korolchuk VI, Passos JF et al (2013) Lysosome‐mediated processing of chromatin in senescence. J Cell Biol 202: 129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Grimmig B, Izzo J, Brown LAM, Hudson C, Smith AJ, Tan J, Bickford PC, Giunta B (2016) HIV non‐nucleoside reverse transcriptase inhibitor efavirenz reduces neural stem cell proliferation in vitro and in vivo . Cell Transplant 25: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RE, Adab P, Cheng KK (2020a) Covid‐19: Risk factors for severe disease and death. BMJ 368: m1198 [DOI] [PubMed] [Google Scholar]
- Kakkola L, Denisova OV, Tynell J, Viiliäinen J, Ysenbaert T, Matos RC, Nagaraj A, Öhman T, Kuivanen S, Paavilainen H et al (2013) Anticancer compound ABT‐263 accelerates apoptosis in virus‐infected cells and imbalances cytokine production and lowers survival rates of infected mice. Cell Death Dis 4: 742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M (2009) HIV‐1 associated dementia: Update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol 22: 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV (2018) Redox biology of respiratory viral infections. Viruses 10: 392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y‐J, Jung JK, Lee SY, Jang KL (2010) Hepatitis B virus X protein overcomes stress‐induced premature senescence by repressing p16INK4a expression via DNA methylation. Cancer Lett 288: 226–235 [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD (2017) The clinical potential of senolytic drugs. J Am Geriatr Soc 65: 2297–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 191: 145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PLJ, Liu S, Demaria M, Cong YS, Kapahi P et al (2012) Glucocorticoids suppress selected components of the senescence‐associated secretory phenotype. Aging Cell 11: 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre C, Auclair M, Boccara F, Bastard J‐P, Capeau J, Vigouroux C, Caron‐Debarle M (2010) Premature senescence of vascular cells is induced by HIV protease inhibitors. Arterioscler Thromb Vasc Biol 30: 2611–2620 [DOI] [PubMed] [Google Scholar]
- Leidal AM, Cyr DP, Hill RJ, Lee PWK, McCormick C (2012) Subversion of autophagy by Kaposi’s sarcoma‐associated herpesvirus impairs oncogene‐induced senescence. Cell Host Microbe 11: 167–180 [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang Y, Fu J, Han L, Xue L, Lv C, Wang P, Li G, Tong T (2013) FOXA1 mediates p16INK4a activation during cellular senescence. EMBO J 32: 858–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang TJ (2009) Hepatitis B: The virus and disease. Hepatology 49: S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE (2009) Expression of p16INK4a in peripheral blood T‐cells is a biomarker of human aging. Aging Cell 8: 439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Johnson SM, Fedoriw Y, Rogers AB, Yuan H, Krishnamurthy J, Sharpless NE (2011) Expression of p16INK4a prevents cancer and promotes aging in lymphocytes. Blood 117: 3257–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Chu Y, Wang Y (2015) HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS 7: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor N, Abel B, Scriba TJ, Hughes J, de Kock M, Tameris M, Mlenjeni S, Denation L, Little F, Gelderbloem S et al (2009) Significantly skewed memory CD8+ T cell subsets in HIV‐1 infected infants during the first year of life. Clin Immunol 130: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I, García‐Carpizo V, Guijarro T, García‐Gomez A, Navarro D, Aranda A, Zambrano A (2016) Induction of DNA double‐strand breaks and cellular senescence by human respiratory syncytial virus. Virulence 7: 427–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H et al (2009) A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15: 1082–1087 [DOI] [PubMed] [Google Scholar]
- Motwani M, Pesiridis S, Fitzgerald KA (2019) DNA sensing by the cGAS–STING pathway in health and disease. Nat Rev Genet 20: 657–674 [DOI] [PubMed] [Google Scholar]
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM (1989) Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 8: 4099–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17: 12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Sell C (2016) Mitochondrial stress induces cellular senescence in an mTORC1‐dependent manner. Free Radic Biol Med 95: 133–154 [DOI] [PubMed] [Google Scholar]
- Nehme J, Borghesan M, Mackedenski S, Bird TG, Demaria M (2020) Cellular senescence as a potential mediator of COVID‐19 severity in the elderly. Aging Cell 19: e13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JAE, Krishnamurthy J, Menezes P, Liu Y, Hudgens MG, Sharpless NE, Eron JJ (2012) Expression of p16INK4a as a biomarker of T‐cell aging in HIV‐infected patients prior to and during antiretroviral therapy. Aging Cell 11: 916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris E, Zannetti C, Demurtas A, Sinclair J, De Andrea M, Gariglio M, Landolfo S (2002) Cell cycle arrest by human cytomegalovirus 86‐kDa IE2 protein resembles premature senescence. J Virol 76: 12135–12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofotokun I, Titanji K, Lahiri CD, Vunnava A, Foster A, Sanford SE, Sheth AN, Lennox JL, Knezevic A, Ward L et al (2016) A Single‐dose zoledronic acid infusion prevents antiretroviral therapy‐induced bone loss in treatment‐naive HIV‐infected patients: a phase IIb trial. Clin Infect Dis 63: 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Brennan P, Gaubatz S, Sanij E, Hertzog P, Wolvetang E, Ghysdael J, Rowe M, Hara E (2003) Epstein‐Barr virus LMP1 blocks p16INK4a‐RB pathway by promoting nuclear export of E2F4/5. J Cell Biol 162: 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi N, Shilagardi K, Nakamoto Y, Honda M, Kaneko S, Murakami S (2007) Hepatitis B virus X protein overcomes oncogenic RAS‐induced senescence in human immortalized cells. Cancer Sci 98: 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A et al (2018) Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 9: 5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang‐Verzosa G et al (2019) Targeting senescent cells alleviates obesity‐induced metabolic dysfunction. Aging Cell 18: e12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LD, Weng NP, Levine BL, June CH, Lane HC, Hodes RJ (1997) Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV‐discordant monozygotic twins. J Exp Med 185: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin ME, Padgett LE, McDowell RE, Burg AR, Brahma MK, Holleman C, Kim T, Crossman D, Kutsch O, Tse HM et al (2018) Antiretroviral therapy potentiates high‐fat diet induced obesity and glucose intolerance. Mol Metab 12: 48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Ribeiro S, M. Milush J, Cunha‐Neto E, G. Kallas E, Kalil J, D. Passero LF, W. Hunt P, G. Deeks S, F. Nixon D, SenGupta D (2016) p16INK4a expression and immunologic aging in chronic HIV infection. PLoS One 11: e0166759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov SA, Kellam P, Boshoff C (2000) The latent nuclear antigen of Kaposi sarcoma‐associated herpesvirus targets the retinoblastoma‐E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med 6: 1121–1127 [DOI] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group (2020) Dexamethasone in hospitalized patients with Covid‐19 –preliminary report. N Engl J Med 384: 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi S, Pallikkuth S, George VK, de Armas LR, Pahwa R, Sanchez CM, Pallin MF, Pan L, Cotugno N, Dickinson G et al (2017) Paradoxical aging in HIV: immune senescence of B Cells is most prominent in young age. Aging (Albany NY) 9: 1307–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer N, Brümmendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM (1999) Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv A, Burton DGA, Moshayev Z, Vadai E, Wensveen F, Ben‐Dor S, Golani O, Polic B, Krizhanovsky V (2016) NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 8: 328–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz T, Serrano‐Villar S, Díaz L, Tome MIG, Gurbindo MD, De José MI, Mellado MJ, Ramos JT, Zamora J, Moreno S et al (2013) The CD4/CD8 ratio as a marker T‐cell activation, senescence and activation/exhaustion in treated HIV‐infected children and young adults. AIDS 27: 1513–1516 [DOI] [PubMed] [Google Scholar]
- Samad F, Harris M, Puskas CM, Ye M, Chia J, Chacko S, Bondy GP, Lima VD, Montaner JSG, Guillemi SA (2017) Incidence of diabetes mellitus and factors associated with its development in HIV‐positive patients over the age of 50. BMJ Open Diabetes Res Care 5: 457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136 [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16(INK4a). Cell 88: 593–602 [DOI] [PubMed] [Google Scholar]
- Serrano‐Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY et al (2014) HIV‐infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of Non‐AIDS morbidity and mortality. PLoS Pathog 10: e1004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ASV, Stelzle D, Ken Lee K, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W et al (2018) Global burden of atherosclerotic cardiovascular disease in people living with HIV systematic review and meta‐analysis. Circulation 138: 1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G, Chen P, Wei Y, Yue H, Chu J, Zhao J, Wang Y, Zhang W, Zhang HL (2020) Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta‐analysis. Chest 157: 1175–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Van Meter M, Ablaeva J, Ke Z, Gonzalez RS, Taguchi T, De Cecco M, Leonova KI, Kogan V, Helfand SL et al (2019) LINE1 derepression in aged wild‐type and SIRT6‐deficient mice drives inflammation. Cell Metab 29: 871–885.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, de Boer R, Brul S, Budovskaya Y, van der Spek H (2013) Premature and accelerated aging: HIV or HAART? Front Genet 3: 328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Tan JME, Jonker MJ, Jongejan A, Buissink T, Veldhuijzen S, Van Kampen AHC, Brul S, van der Spek H (2017) Beyond the polymerase‐γ theory: production of ROS as a mode of NRTI‐induced mitochondrial toxicity. PLoS One 12: e0187424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto‐Gamez A, Quax WJ, Demaria M (2019) Regulation of survival networks in senescent cells: from mechanisms to interventions. J Mol Biol 431: 2629–2643 [DOI] [PubMed] [Google Scholar]
- Stefanidis K, Loutradis D, Vassiliou LV, Anastasiadou V, Kiapekou E, Nikas V, Patris G, Vlachos G, Rodolakis A, Antsaklis A (2008) Nevirapine induces growth arrest and premature senescence in human cervical carcinoma cells. Gynecol Oncol 111: 344–349 [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP‐AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, García‐Cardeña G, Owens GK (2015) Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Loo TM, Okada R, Kamachi F, Watanabe Y, Wakita M, Watanabe S, Kawamoto S, Miyata K, Barber GN et al (2018) Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat Commun 9: 1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE (2000) Accelerated bone mineral loss in HIV‐infected patients receiving potent antiretroviral therapy. AIDS 14: F63–F67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj A, Chivero ET, Tripathi A, Singh S, Niu F, Guo ML, Pillai P, Periyasamy P, Buch S (2021) HIV TAT‐mediated microglial senescence: role of SIRT3‐dependent mitochondrial oxidative stress. Redox Biol 40: 101843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, David P, Banks L (1999) The role of the E6–p53 interaction in the molecular pathogenesis of HPV. Oncogene 18: 7690–7700 [DOI] [PubMed] [Google Scholar]
- Tripathi A, Liese AD, Jerrell JM, Zhang J, Rizvi AA, Albrecht H, Duffus WA (2014) Incidence of diabetes mellitus in a population‐based cohort of HIV‐infected and non‐HIV‐infected persons: the impact of clinical and therapeutic factors over time. Diabet Med 31: 1185–1193 [DOI] [PubMed] [Google Scholar]
- Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, Van K, Hyun D (2018) Osteoporosis: a review of treatment options. P T 43: 92–104 [PMC free article] [PubMed] [Google Scholar]
- Wang GC, Kao WHL, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD et al (2010) Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol 171: 1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peng G, Bai J, He B, Huang K, Hu X, Liu D (2017) Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): a meta‐analysis of prospective studies up to 2016. J Am Heart Assoc 6: e005025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren‐Gash C, Smeeth L, Hayward AC (2009) Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 9: 601–610 [DOI] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW (2012) HIV: cell binding and entry. Cold Spring Harb Perspect Med 2: a006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CD, Liu S, Limbad C, Zawadzka AM, Beck J, Demaria M, Artwood R, Alimirah F, Lopez‐Dominguez JA, Kuehnemann C et al (2019) SILAC analysis reveals increased secretion of hemostasis‐related factors by senescent cells. Cell Rep 28: 3329–3337.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J (2020) Covid‐19: critically ill patients treated with arthritis drug tocilizumab show improved outcomes, researchers report. BMJ 371: m4530 [DOI] [PubMed] [Google Scholar]
- Wolthers KC, Wisman GBA, Otto SA, De Roda Husman AM, Schaft N, De Wolf F, Goudsmit J, Coutinho RA, Van Der Zee AGJ, Meyaard L et al (1996) T cell telomere length in HIV‐1 infection: No evidence for increased CD4+ T cell turnover. Science 274: 1543–1547 [DOI] [PubMed] [Google Scholar]
- Yan Y, Du Y, Zheng H, Wang G, Li R, Chen J, Li K (2017) NS1 of H7N9 influenza A virus induces NO‐mediated cellular senescence in Neuro2a cells. Cell Physiol Biochem 43: 1369–1380 [DOI] [PubMed] [Google Scholar]
- Yang X, He Z, Xin B, Cao L (2000) LMP1 of Epstein‐Barr virus suppresses cellular senescence associated with the inhibition of p16(INK4a) expression. Oncogene 19: 2002–2013 [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Ren U, Chen Q, Chena ZJ (2017) CGAS is essential for cellular senescence. Proc Natl Acad Sci USA 114: E4612–E4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson AB (2004) Epstein‐Barr virus: 40 years on. Nat Rev Cancer 4: 757–768 [DOI] [PubMed] [Google Scholar]
- Yu Q, Katlinskaya YV, Carbone CJ, Zhao B, Katlinski KV, Zheng H, Guha M, Li N, Chen Q, Yang T et al (2015) DNA‐damage‐induced Type I interferon promotes senescence and inhibits stem cell function. Cell Rep 11: 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Narasipura SD, Richards MH, Hu XT, Yamamoto B, Al‐Harthi L (2017) HIV and drug abuse mediate astrocyte senescence in a β‐catenin‐dependent manner leading to neuronal toxicity. Aging Cell 16: 956–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M et al (2019) Senolytic therapy alleviates Aβ‐associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22: 719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, Xie X (2020) COVID‐19 and the cardiovascular system. Nat Rev Cardiol 175: 259–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M et al (2015) The achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Fuhrmann‐Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB et al (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors. Aging Cell 15: 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]


