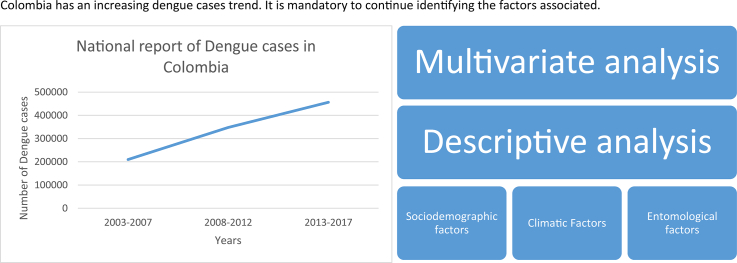
Abstract
According to the World Health Organization, dengue is a neglected tropical disease. Latin America, specifically Colombia is in alert regarding this arbovirosis as there was a spike in the number of reported dengue cases at the beginning of 2019. Although there has been a worldwide decrease in the number of reported dengue cases, Colombia has shown a growing trend over the past few years. This study performed a Poisson multilevel analysis with mixed effects on STATA® version 16 and R to assess sociodemographic, climatic, and entomological factors that may influence the occurrence of dengue in three municipalities for the period 2010–2015. Information on dengue cases and their sociodemographic variables was collected from the National Public Health Surveillance System (SIVIGILA) records. For climatic variables (temperature, relative humidity, and precipitation), we used the information registered by the weather stations located in the study area, which are managed by the Instituto de Hidrologia, Meteorologia y Estudios Ambientales (IDEAM) or the Corporación Autónoma Regional (CAR). The entomological variables (house index, container index, and Breteau index) were provided by the Health office of the Cundinamarca department. SIVIGILA reported 1921 dengue cases and 56 severe dengue cases in the three municipalities; of them, three died. One out of four cases occurred in rural areas. The age category most affected was adulthood, and there were no statistical differences in the number of cases between sexes. The Poisson multilevel analysis with the best fit model explained the presentation of cases were temperature, relative humidity, precipitation, childhood, live in urban area and the contributory healthcare system. The temperature had the biggest influence on the presentation of dengue cases in this region between 2010 and 2015.
Keywords: Dengue, Poisson multilevel analysis, Climate, Temperature, Tropical zone
Graphical abstract
Highlights
-
•
Climate, urban residency, healthcare affiliation, childhood are key factors for dengue.
-
•
Multilevel analysis identified temperature as the most important factor.
-
•
25% of dengue cases in this study were in rural areas.
-
•
Our research shows climate factors that could increase dengue cases probability.
-
•
Climate change trend could increase arbovirosis cases.
1. Introduction
Neglected tropical diseases include all the infections that affect populations with a high level of inequities and low access to basic utilities.These circumstances have an impact on the quality of life and the presence of a constant risk of getting sick related to being more exposed and having fewer protection barriers or behaviors than others [27,67]. Dengue is included in this category.
Dengue is a vector-borne disease of high incidence around the world; it is caused by dengue virus (DENV), a member of the Flaviviridae family, Flavivirus genus, with four serotypes (DENV 1–4), which are transmitted by the bite of Aedes aegypti (L), its principal vector, and also Aedes albopictus (S) [68]. All serotypes and both vectors are currently present in Colombia. Although in Colombia the transmission of dengue via A. albopictus has not been proven, there are reports of this species' natural infection with DENV-1 and DENV-2 in Valle del Cauca [40] and with DENV-2 in Medellin, Antioquia [22].
Weak monitoring systems for the vector and the disease, climate change, tourism, globalization, and migration have allowed this arbovirosis and other diseases transmitted by A. aegypti to gain worldwide prominence [62]. The disease burden report from the year 2010 showed 390 million dengue cases, of which 96 million cases exhibited clinical signs and symptoms [7]. In 2015, the Americas reported 2.35 million dengue cases and the region frequently reported a high cumulative incidence [69]. In Colombia, according to the cases reported by the National Public Health Surveillance System (SIVIGILA), when comparing the disease occurrence from the 1990s with those from 2000 to 2009 and 2010–2016, increases of 1.7 and 2.5 times, respectively [51]. This confirms that dengue is a prevalent disease that requires focused attention.
The environmental (altitude, temperature, humidity, and precipitation); social (population growth, unplanned urbanization, poor drinking water supply, inadequate solid waste disposal, population migration, poverty, and poor community participation and education on these topics); and entomological (presence of A. aegypti in 90% of the country) factors [50], as well as the poor execution of vector control programs, have led to this disease remaining a relevant issue for the national and international public health [25,28,55,61].
Global warming has changed the climate of the regions and even the biological and ecological characteristics of the vectors [37,63]. These changes have led to adaptations in A. aegypti [32] to a higher altitude, being found at 2302 m above sea level (m.a.s.l.), with the isolation of the flavivirus at 1984 m.a.s.l., in Colombia [55]. On the other hand, the endemic transmission of dengue in urban areas with high infestation of A. aegypti, water storage and irregular supply services, and high mobility between urban-rural settlements, provide the vector and the disease adequate conditions to spread in rural areas [26,48]. In Bolivia, this vector has been found at 2550 m.a.s.l. [13], and there are records confirming its presence in 78.8% (197/250) of all countries worldwide, but only 74.1% of these countries have reported cases of an arbovirosis that can be transmitted via this vector, such as dengue, Zika, chikungunya, and yellow fever, among others [35]. Additionally, new clinical descriptions in populations after infection with certain arboviruses like chronic arthritis [70], sexual transmission of Zika virus [71], microcephaly [33], and Guillain-Barré syndrome [8]), are factors that suggest the pertinence of permanent epidemiological and entomological monitoring of both the disease and the vector [28]. This study assessed sociodemographic, climatic, and entomological factors that may influence the occurrence of dengue in three municipalities of the central region of Colombia for the period 2010–2015.
2. Materials and methods
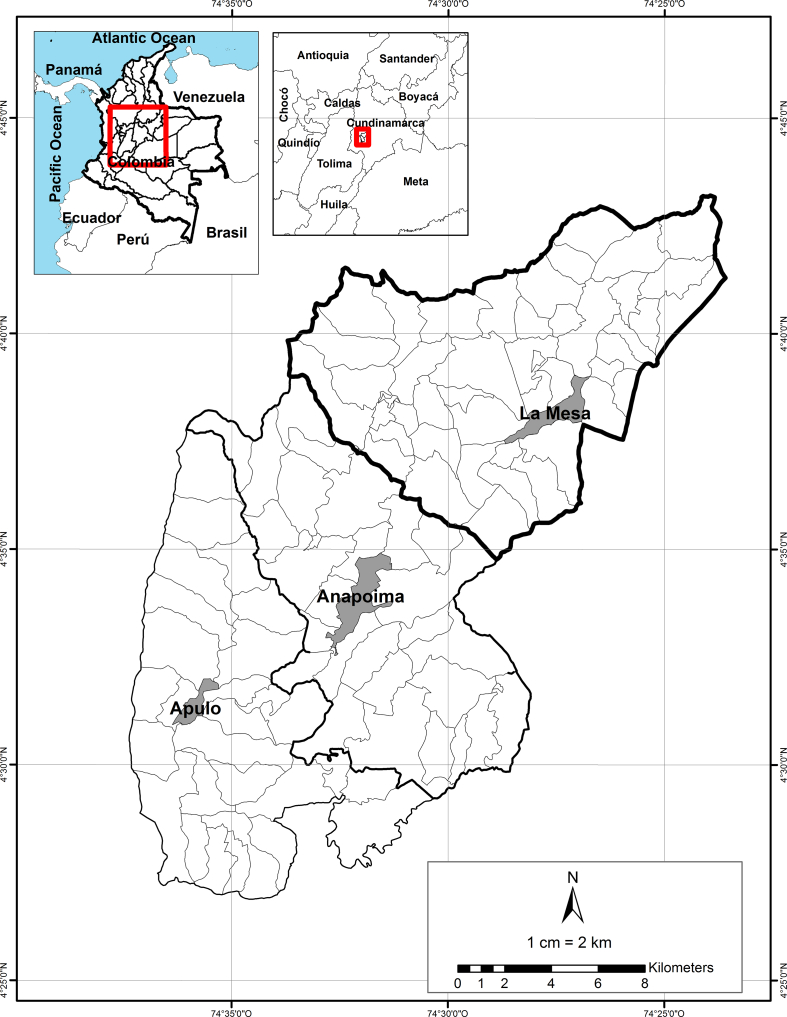
The municipalities of La Mesa, Anapoima, and Apulo are located in the southwest area of the department of Cundinamarca, 69, 87, and 100.8 km away from Bogota, respectively. These municipalities were recognized as tourist destinations due to their mild climate and closeness to the capital. Additionally, the region has reported a high number of dengue cases.
La Mesa is located at 4°37′49” N and 74°27′45” W. It has a population of 30,250 and is 1200 m.a.s.l.; it has an area of 148 km2 and an average temperature of 22 °C [3]. Anapoima is located at 4°33′01” N and 74°32′10” W. It has a population of 13,928; it is 710 m.a.s.l. and has an average temperature of 28 °C [2]. Apulo is located at 4°31′15” N and 74°35′55” W. It has a population of 8162; it is 420 m.a.s.l. and has an average temperature of 28 °C [47](Fig. 1).
Fig. 1.
Location of the municipalities of La Mesa, Anapoima, and Apulo (Colombia).
2.1. Sample
The present study included 100% of the population with a diagnosis of dengue [dengue or classical dengue (A90X) and dengue hemorrhagic fever (A91X)] that had visited the municipalities of La Mesa, Anapoima and Apulo, and were reported to SIVIGILA between 2010 and 2015. Sociodemographic data were provided by SIVIGILA; this system has the official records from Colombia. The study used age category defined by Ministerio de Salud y Protección Social of Colombia: early childhood (<5 years), childhood (6–13 years), youth (14–26 years), adulthood (27–59 years), and elderly (>60 years) [46]. Colombia's healthcare system is based on the assurance of individuals according to their payment capacity: health attention for people without payment capacity is subsidized by the State (subsidized healthcare), while people who work and receive a salary contribute to the healthcare system according to their income (contributory healthcare). Nonetheless, both categories have access to the same health services [16,57]. Individuals who stated that their home was outside the downtown of the three municipalities were defined as living in a rural area. In addition, if the rural settlement, neighborhood or place of residence was out of the borders of the three municipalities people were defined as tourists, otherwise they were resident.
The analyzed database from SIVIGILA was delivered by the Health office of the Cundinamarca department, the cases registered were without name or identification to comply with the national laws on personal data protection [43,53].
2.2. Entomological variables
The Health office of the Cundinamarca department provided the entomological indexes [house index (HI), container index (CI), and Breteau index (BI)] of the three municipalities for the period 2010–2015. The metodology used for measurement of A. aegypti entomological index are based on the national guidelines of Ministerio de Salud y Protección Social and Panamerican Health Organization [45].
2.3. Climatic variables
Climatological information was provided by the weather stations from Instituto de Hidrología, Meteorología y estudios Ambientales (IDEAM) or the Corporación Autónoma Regional (CAR) located in the study area. The requested climatological variables were precipitation (P), temperature (T), and relative humidity (RH). The climatic variables were calculated every month in three municipalities using the average of the data collected during that month from the region's weather stations records. Data analysis took average daily and monthly of each variable. Monthly average is calculated the average of the daily values for each variable.
We used linear regression and cross-multiplication to complete the time series analysis according to the correlation found between the measures of each station [20,38].
We used one-way analysis of variance (ANOVA) to compare the data from the monthly and annual average values of the climatic variables. Additionally, we categorized the climatic variables by taking the extreme value of each variable and used the values found in the 33rd and 66th percentiles of those ranges as cut-off points. For the temperature, we set the ranges as low, medium, and high (<24 °C, 24 °C–27.3 °C, and > 27.3 °C, respectively), and for precipitation, we set the seasons as low, medium, and high (<128 mm; 128–253 mm and > 253 mm, respectively); furthermore, RH was set as low, medium, and high (<64.8%, 64.8%–83.3%, and > 83.3%, respectively).
2.4. Data analysis
We performed a bivariate and multilevel analyses with STATA® version 16 and R, using as the axes for analyses the climatic, entomological, and sociodemographic variables linked to dengue in the municipalities of Anapoima, La Mesa, and Apulo during 2010–2015.
3. Results
3.1. Entomological variables
The entomological indexes used in this study were HI, CI and BI. A BI value >5 indicated a risk of dengue transmission [39]. With the exception of Anapoima during 2011 and Apulo during 2012, all the BI values were above the reference value. When HI equal to the BI value, it means that all positive houses only have a positive container; as observed in Anapoima during 2011 and in Apulo during 2015 (Table 1).
Table 1.
Infestation indexes for Aedes aegypti in the municipalities of Apulo, Anapoima, and La Mesa (Cundinamarca), Colombia.
| Apulo |
Anapoima |
La Mesa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Infestation indexes | House % | Container % | Breteau | House % | Container % | Breteau | House % | Container % | Breteau |
| 2010 | 6.60 | 2 | 7 | 14.3 | 9 | 15 | 21.8 | 10 | 22 |
| 2011 | 8.6 | 6 | 9 | 3 | 10 | 3 | 14.5 | 8 | 15 |
| 2012 | 5 | 2 | 4 | 8 | 8 | 9 | 14.4 | 6.61 | 15 |
| 2013 | 8.23 | 2.27 | 8.56 | 12.9 | 6 | 14 | 15 | 9.24 | 17 |
| 2014 | 9.70 | 3.36 | 10 | 12.3 | 4.52 | 15 | 9.5 | 4 | 10 |
| 2015 | 10 | 3.5 | 10 | 9.80 | 5 | 23 | 4 | 2 | 7 |
The situation presented in Apulo during 2012, where the HI is 5 and the BI is 4, is not possible because the lowest value the BI can have is equal to the HI, as mentioned. When the BI value is higher than the HI value, it means that one or more of the inspected houses may have more than one positive container; this situation occurred in all of the municipalities in the rest of the years of analysis. It is worth noting that in Anapoima during 2015, the BI value was the highest in relation to the HI than in the other municipalities in the analyzed year range (9.80 and 23, respectively), which indicates multifocality; that is, some houses have more than one positive container, which would indicate a higher risk factor for dengue transmission.
3.2. Climatic variables
The variations of the climatic variables in three municipalities could be seen in fig. 2 (Supplementary 1).
3.2.1. Precipitation (P)
The three municipalities between 2010 and 2015 shows a bimodal dynamic with two periods of high precipitation during April–May and October–November and two periods of low precipitation during June–September and December–January.
3.2.2. Relative humidity (RH)
The monthly average RH in the three municipalities between 2010 and 2015 is related to precipitation, with two periods of high RH during November (80%–83%) and April (78%–85%) and two periods of low RH during September (62%–73%) and January–March (70%–81%).
3.2.3. Temperature (T)
We used mean temperature. During 2010–2015, the months with the lowest average temperature were May (22.6 °C–26.6 °C) and November (22.7 °C–24.2 °C) and the month with the highest average temperature was September (24.3 °C–27.3 °C). La Mesa was the municipality with the lowest monthly average temperature (23 °C–24 °C), followed by Anapoima (24 °C–27 °C) and Apulo with the highest average values (27 °C–28 °C). The temperature in the three municipalities ranged between 20.7 °C and 30.7 °C.
The one-way ANOVA of the monthly and annual average values of the climatic variables confirmed that the only different variable among the three municipalities was temperature [F(2,213) = 69.951; p < 0.05 and F(2,15) = 14.05; p < 0.05, respectively], supporting the hypothesis that the difference in the number of cases among the municipalities is attributable to temperature.
The categorization of the climatic variables using the two established cut-off points (the 33rd and 66th percentiles) generated 27 climate combinations in the three municipalities. We added up the months where the climate combinations were repeated in the three municipalities to verify the number of dengue cases by climate; we concluded that 5 of the 27 generated combinations (R, T, and RH) comprised 49.98% of the dengue cases. Moreover, the climate combination with high precipitation, low temperature, and medium RH registered an average of 20 dengue cases per month. This climate is seldom present in the study area, during the study just happened 0.9% of the overall time (Table 2 and Supplementary 2). The climate combinations were not included in the multilevel analysis (Supplementary 2, Table 6).
Table 2.
The five primary climate combinations (R, T, and RH) according to the average number of cases per month and the absolute frequency of dengue cases in the three municipalities between 2010 and 2015.
| Precipitation | Temperature | Relative humidity | Dengue cases1 (%) | Months2 (%) | Average number of cases per month |
|---|---|---|---|---|---|
| High | Low | Medium | 40 (2.0) | 2 (0.9) | 20 |
| Medium | Low | High | 179 (9.1) | 10 (4.6) | 17.9 |
| Medium | Low | Medium | 231 (11.7) | 15 (6.9) | 15.4 |
| Low | Medium | Low | 208 (10.5) | 15 (6.9) | 13.8 |
| Low | Low | Medium | 330 (16.7) | 26 (12.0) | 12.7 |
| Total | 988 (49.98) | 68 (31.48) | N/A3 | ||
Total number of dengue (with or without warning signs) and severe dengue cases in these municipalities between 2010 and 2015 = 1977 cases.
Number of months where the three categories of climatic variables are repeated within the 6 analyzed years.
N/A = Not applicable.
3.3. Sociodemographic variables
Between 2010 and 2015, SIVIGILA registered 1921 dengue cases (with or without warning signs) and 56 severe dengue cases in the three municipalities; of them, three died as consequence of the disease. The first months of the studied years showed an increase in the number of cases. Moreover, there were no statistically significant differences between the cases registered in both sexes (z = 1.026, p < 0.05), the most affected age category was adulthood, the contributory healthcare was the category with a higher number of dengue cases, tourists exhibited low infection rate (21.03%), and one out of every four dengue infections occurred in rural areas. (Table 3).
Table 3.
Sociodemographic variables of reported dengue cases in the three municipalities 2010–2015.
| SEX | Municipality |
||
|---|---|---|---|
| La Mesa | Anapoima | Apulo | |
| Male | 620 | 270 | 125 |
| Female | 568 | 267 | 127 |
| Age Category | |||
| Early childhood | 85 | 40 | 42 |
| Childhood | 211 | 82 | 60 |
| Youth | 309 | 126 | 54 |
| Adulthood | 411 | 203 | 73 |
| Elderly | 172 | 86 | 23 |
| Heathcare | |||
| Contributory | 728 | 340 | 131 |
| Subsidized | 361 | 163 | 105 |
| No data | 99 | 34 | 16 |
| Home Location | |||
| Urban area | 830 | 295 | 183 |
| Rural area | 273 | 160 | 65 |
| No data | 85 | 82 | 4 |
| Place of Residence | |||
| Resident of the study area | 972 | 384 | 204 |
| Tourist | 216 | 153 | 48 |
| Dengue Classification | |||
| Dengue with warning signs | 669 | 305 | 164 |
| Dengue without warning signs | 487 | 218 | 78 |
| Severe dengue | 32 | 14 | 10 |
| Deaths from dengue | 1 | 1 | 1 |
According to the guidelines, 59.2% of the cases were dengue with warning signs [49]. Furthermore, 42.2% of all patients were hospitalized, 50.5% (n = 410) of them during the first 3 days of the onset of symptoms. Of all the hospitalized patients, 45% were female and 55% were male. The age category with the highest frequency of hospitalizations was adulthood (29.9%), followed by youth (25.5%) and childhood (19.7%). 13.9% of people who did not have dengue-warning signs were hospitalized. Moreover, 26.6% of hospitalized patients attended late (five or more days with symptoms), and three out of four cases of late hospitalization showed warning signs during the consultation.
Tourists showed a higher percentage of warning signs than residents (68.8% vs. 56.6%); however, these people had a smaller proportion of hospitalization than residents (39.9% vs. 44.5%), with statistically significant differences in both comparisons (Z = −5.57 and Z = 4.78, p < 0.01, respectively).
3.4. Multilevel analysis with mixed effects
Several models were executed to assess the variables that best explained the presentation of cases in the three municipalities and the model that best fit was mixed – effects Poisson multilevel analysis having as group variable the municipality. The model assessed the variables T, RH, P, childhood as the age category, home in an urban area, and contributory healthcare system (Wald chi (6) = 1353.89, Prob > chi2 = 0.0000). An additional model was executed with the aforementioned variables, but childhood was substituted with adulthood as the age category, given that the latter stage comprised the highest proportion of dengue cases. This model had a prediction (Wald chi2 (6) = 1396.97, Prob > chi2 = 0.0000) (See Supplementary 3). Although both models were statistically significant, we opted for the first one. The model show that the variable with the highest predictive value was temperature between munipalities and it is the main reason in the difference of dengue cases of them. The entomological indexes were included in the multilevel analysis, but their inclusion did not improve the statistical prediction, possibly because we only had one value per year in each municipality.
4. Discussion
We performed a multilevel analysis that showed that the most important variable related to dengue cases in these municipalities was temperature. It has also been established in Singapore, Puerto Rico, and Brazil that temperature is one of the primary climatic factors that impact the presentation of dengue in these countries [21,41,52]. Its importance lies in the influence it has on the life cycle of A. aegypti. Currently, it is known that the adequate temperature for the reproduction and survival of this mosquito ranges between 22 °C and 30 °C; furthermore, the higher the temperature, the shorter its life-cycle development, changing from 22.42 days for the development of egg to adult to 11.64 days. The vector is more active during this short period, and it can breed two generations, whereas in colder climates it can only breed one generation during the same period. When we assessed the average temperature by month in the studied municipalities, the values were optimal for A. aegypti. Temperature does not only affect the biological characteristics of the vector, but also allows the virus to be more virulent, which influences the pathogens transmission of A. aegypti. This could explain why there was an increase in the number of dengue cases without an increase in the municipality's indexes [6], for example in Anapoima and La Mesa in 2013.
One in four cases of dengue reported in SIVIGILA were inhabitants of rural area. It could be explained by presence of A. aegypti in rural areas [48,60] and endemic dengue transmission in cities with high mobility between rural and urban areas [26]. In addition, we can infer that A. aegypti adaptations in rural areas, DENV vertical or transovarial transmission [23,65], and the presence of patients with asymptomatic dengue [15,18] contributed to this disease becoming endemic in these territories.
Epidemiological and entomological studies linked dengue with climate variability, associating a rise in dengue transmission with high temperatures, which causes an increased virulence of the different serotypes of this flavivirus [36,61]. Temperature also plays a key role in vector behavior because it improves the vector's ability to sting, increasing the disease transmission probability [10,34,36]. Moreover, we found ideal climate combinations for this arbovirosis that resulted in a high incidence. It would be important in future studies to verify whether these conditions are present in other regions of the country and whether these combinations show the same behavior with regard to dengue cases. The same combinations were not found in other studies.
Precipitation is a climatic variable that has always been associated with an increased number of dengue cases, mainly in Asia, where monsoons and wet seasons are the factors most linked to the increase in the occurrence of this disease [58,66]. In Colombia, the climatic variables of precipitation, RH, and temperature have been evaluated, but the results obtained in the different studies are not the same. For example, researchers in Medellin assessed the behavior of this disease with regard to climatic variables, and they found that the variable most linked to the presentation of this arbovirosis was precipitation with a delay of 20 weeks [54]. Another study conducted in Monteria found that precipitation, RH, and temperature are important when trying to explain the presentation of dengue cases in this municipality [11]. In addition, a study conducted in Medellin [1] assessed other factors affecting the occurrence of dengue such as the Oceanic Niño Index and the Multivariate ENSO Index, which are variables obtained from the behavior exhibited by El Niño–Southern Oscillation (ENSO). By analyzing the above mentioned indexes and standardizing the dengue cases in the city of Medellin, it was possible to prove that there is a delay of 6 or 7 months in the occurrence of cases after an increase in the temperature that affects these two indexes. Recently, a thesis assessed similar factors for the same period in several municipalities in Cundinamarca, which included La Mesa, Anapoima, and other towns with different altitudes. This analysis established that a rise of 1 °C increases the risk of dengue occurrence by 14% in the evaluated municipalities [64]. These findings coincide with the results obtained in this study and indicate that temperature is an important factor in the prevalence of dengue in the region.
Additionally, our study provides data regarding the presentation of dengue in rural areas, a piece of evidence that very few studies in Colombia and Latin America have been able to obtain [51]. Between 2010 and 2015, the National Health Institute of Colombia reported that, on average, 18.7% of all dengue cases occur in the rural areas of the country [4,5,[29], [30], [31],42]. These data are similar to those obtained in our study. Dengue cases have also been reported in rural areas in the rest of Latin America but lower prevalence, as recorded in Paraguay with 13.05% [9] and an observational study from Ecuador [17] confirmed that 17% of all dengue cases were found in rural areas. Furthermore, the presence of this disease in rural areas has been confirmed in Mexico, although the proportion is not mentioned [59]. The actual number of dengue cases in Colombian rural areas may be higher than the number recorded as people do not always attend healthcare centers, because the high cost of transport from their home to the hospital and/or the lack of permanent transport service.
After evaluating the population affected by dengue, we concluded that individuals with more complications were the residents of these municipalities. It could be due to had secondary dengue infections, which is normally associated with a severe symptomatic and clinical manifestation [19,24]. Therefore, local community education programs must be promoted to help people recognize warning signs and avoid complications in patients.
The regions with the highest number of dengue cases in the world are Southeast Asia, Africa, and Latin America. These territories have a high number of people with low income, primarily because they rely on an informal economy. Latin America has improved healthcare for this infection and has reduced its mortality, but this disease still shows a rising trend, as it has registered an increase of 8.6 times the number of cases compared with those in the 80s and the first years of the 21st century (2000–2007) [56]. The incidence rates in the three municipalities as well as in the country provide the evidence of a growing trend in the number of dengue cases, which corresponds with the region's trend. Brazil, Mexico, and Colombia currently have the largest disease burden in the region, comprising 70% of the reported dengue cases in the Caribbean and Latin American regions [44]. It proves to need continue improving monitoring systems in these countries and analyzing the causes behind the constant increase in the occurrence of this arbovirosis.
This disease continues to impose high costs on the Colombian healthcare system and bring economic losses to the people that suffer from it. In 2011, Colombia registered 30,694 dengue cases and an expenditure of 54 million dollars on dengue direct care; this figure is equivalent to 0.02% of the country's GDP for that year, but the disease was considered non-epidemic [12]. Other studies have proven that in Colombia, the disability-adjusted life years (DALY) caused by dengue can range between 765.4 and 1376.5 DALY per million inhabitants in endemic years and between 9845.9 and 13,584.2 DALY per million inhabitants in epidemic years [14]. Therefore, we recognize the importance of further monitoring of this disease and its primary vector, which is also a transmitter of other diseases such as Zika virus infection, chikungunya and yellow fever. These diseases are relevant to not only Latin American countries but also the entire world.
A limitation of the study is that we could only obtain an annual average value of A. aegypti infestation indexes for each municipality, which limits the analysis of these indicators. We requested more detailed information (monthly or quarterly infestation indexes) from both departmental and national administrations on numerous occasions, but we were unable to obtain the required information. Additionally, we did not verify the filled-out physical forms for dengue events in the municipalities nor were there attempts to fill in the gaps in variables or to correct erroneous information in the database provided by the Health office of Cundinamarca.
5. Conclusions
Although dengue is a multifactorial disease, this study was able to conclude that in the studied Colombian region, the variable that best explains the presentation of dengue is temperature. However, we found five optimal climate combinations for the vector that encourage the development of this arbovirosis in the individuals within the region.
It is important to continue implementing dengue prevention and control programs as well as to monitor this arbovirosis because it remains a public health issue that primarily affects the working age population, thus impacting the family and national economies. Promoting vector control measures and early diagnosis will reduce the cost incurred due to dengue-related complications and other cost overruns (deaths, DALY).
Author statement
Gustavo Ordoñez-Sierra: investigation, data curation, formal analysis, writing – original draft and writing – review & editing.
Diana Sarmiento-Senior: Conceptualization, methodology Formal analysis and writing – review & editing.
Juan Felipe Jaramillo: Conceptualization, methodology Formal analysis and writing – review & editing.
Paola Giraldo Conceptualization, methodology and investigation.
Alexandra Porras-Ramirez: Formal analysis and writing – review & editing.
Víctor Alberto Olano: Conceptualization, methodology, Formal analysis and writing – review & editing.
Funding
This research was supported by Universidad El Bosque (Colombia), Research call 2016 (PCI 8811).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank to the Universidad El Bosque, the Head director of La Mesa and Tocaima Hospitals, the Secretaries of Health of the studied municipalities and the Cundinamarca Governor's Office for the information provided. Jesus David Ramos supported the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100234.
Contributor Information
Gustavo Ordoñez-Sierra, Email: gordonezs@unbosque.edu.co.
Diana Sarmiento-Senior, Email: sarmientodiana@unbosque.edu.co.
Juan Felipe Jaramillo Gomez, Email: jjaramillo@unbosque.edu.co.
Paola Giraldo, Email: paolacgiraldop@hotmail.com.
Alexandra Porras Ramírez, Email: rporrasalexandra@unbosque.edu.co.
Víctor Alberto Olano, Email: olanovictor@unbosque.edu.co.
Appendix A. Supplementary data
Supplementary 1: Graphics of the Behavior of climate variables in three municipalities.
Supplementary 2: The tables detail the information of the climatic combinations.
Supplementary 3: The models described in multilevel analysis section.
References
- 1.Acosta Cardona L.A. Nacional de Colombia; 2015. Evaluación de Factores Ambientales Y climáticos Como Elementos de Riesgo Asociados Con la Transmision del Dengue Y la Leishmaniasis a Diferentes Escalas Temporales Y Espaciales en Colombia. [Google Scholar]
- 2.Alcaldia de Anapoima . 2016. Plan de desarrollo del municipio de anapoima “vamos por la equidad social 2016–2019”. [Google Scholar]
- 3.Alcaldia de La Mesa Nuestro municipio. Información general de La Mesa. 2016. http://www.lamesa-cundinamarca.gov.co/informacion_general.shtml [WWW Document]. URL. (accessed 6.11.16)
- 4.Bello Pérez S.L. 2012. Comportamiento epidemiológico del Dengue en Colombia del año 2011. (Bogotá D.C) [Google Scholar]
- 5.Bello S.L. 2012. Comportamiento epidemiológico del Dengue en Colombia año 2010. (Bogotá D.C) [Google Scholar]
- 6.Beserra E.B., Castro F.P., Jr., de Santos J.W., dos Santos T. da S., Fernandes C.R.M. Biologia e exigências térmicas de Aedes aegypti (L.)(Diptera: Culicidae) provenientes de quatro regiões bioclimáticas da Paraíba. Neotrop. Entomol. 2006;35:853–860. doi: 10.1590/S1519-566X2006000600021. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao-Lormeau V.-M., Blake A., Mons S., Lastère S., Roche C., Vanhomwegen J., Dub T., Baudouin L., Teissier A., Larre P., Vial A.-L., Decam C., Choumet V., Halstead S.K., Willison H.J., Musset L., Manuguerra J.-C., Despres P., Fournier E., Mallet H.-P., Musso D., Fontanet A., Neil J., Ghawché F. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpinelli M., Picaguá E., Rovira C. Frecuencia de dengue durante el brote epidémico en individuos que acudieron al Instituto de Investigaciones en Ciencias de la Salud (IICS), desde Febrero. Inst. Investig. Cienc. Salud. 2009;5:15–20. [Google Scholar]
- 10.Carrington L.B., Armijos M.V., Lambrechts L., Scott T.W. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl. Trop. Dis. 2013;7:1–8. doi: 10.1371/journal.pntd.0002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassab A., Morales V., Mattar S. Factores climáticos y casos de Dengue en Montería, Colombia. 2003-2008. Rev. Salud Públ. 2011;13:115–128. doi: 10.1590/s0124-00642011000100010. [DOI] [PubMed] [Google Scholar]
- 12.Castañeda-Orjuela, C., Díaz, H., Alvis-Guzman, N., Olarte, A., Rodriguez, H., Camargo, G., De la Hoz-Restrepo, F., 2012. Burden of disease and economic impact of dengue and severe dengue in Colombia, 2011. Value Heal. Reg. Issues 1, 123–128. doi: 10.1016/J.VHRI.2012.09.014. [DOI] [PubMed]
- 13.Castillo-Quino R., Vallejo-Castro E., Camacho-Aliaga A.V., Quiñones-López A., Canelas-Urey H.I. Adaptación del mosquito Aedes aegypti a 2.550 m.s.n.m Cochabamba, Bolivia. Gac. Méd. Boliv. 2018;41:24–30. [Google Scholar]
- 14.Castro Rodríguez R., Carrasquilla G., Porras A., Galera-Gelvez K., Guillermo J., Yescas L., Rueda-Gallardo J.A. The burden of dengue and the financial cost to Colombia, 2010–2012. Am. J. Trop. Med. Hyg. 2016;94:1065–1072. doi: 10.4269/ajtmh.15-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chastel C. Eventual role of asymptomatic cases of dengue for the introduction and spread of dengue viruses in non-endemic regions. Front. Physiol. 2012;3:70. doi: 10.3389/fphys.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Congreso de la República . 1993. LEY 100 1993, 23 de diciembre de 1993. Diario oficial N° 41148. [Google Scholar]
- 17.Connors R. The American Journal of Tropical Medicine and Hygiene; New Orleans: 2008. Road Access Linked to Dengue Fever Risk in Rural Ecuador. [Google Scholar]
- 18.Duong V., Lambrechts L., Paul R.E., Ly S., Laya R.S., Long K.C., Huy R., Tarantola A., Scott T.W., Sakuntabhai A., Buchy P. Asymptomatic humans transmit dengue virus to mosquitoes. Proceed. Natl. Acad. Sci. U. S. A. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durán C.A., Lanza T., Plata J. Fisiopatología y diagnóstico del dengue. Rev. Med. Hondur. 2010;78:113–168. [Google Scholar]
- 20.Fries A., Rollenbeck R., Nauß T., Peters T., Bendix J. Near surface air humidity in a megadiverse Andean mountain ecosystem of southern Ecuador and its regionalization. Agric. For. Meteorol. 2012;152:17–30. doi: 10.1016/j.agrformet.2011.08.004. [DOI] [Google Scholar]
- 21.Gomes A.F., Nobre A.A., Cruz O.G. Temporal analysis of the relationship between dengue and meteorological variables in the city of Rio de Janeiro, Brazil, 2001-2009. Cad. Saude Publ. 2012;28:2189–2197. doi: 10.1590/S0102-311X2012001100018. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Palacio A., Suaza-Vasco J., Castaño S., Triana O., Uribe S. Infección de Aedes albopictus (Skuse, 1984) con el genotipo asiático-americano de dengue serotipo 2 en Medellín, sugiere un posible papel como vector de dengue en Colombia. Biomédica. 2017;37:24. doi: 10.7705/biomedica.v33i4.836. [DOI] [PubMed] [Google Scholar]
- 23.Gutiérrez-Bugallo G., Rodriguez-Roche R., Díaz G., Vázquez A.A., Alvarez M., Rodríguez M., Bisset J.A., Guzmán M.G. First record of natural vertical transmission of dengue virus in Aedes aegypti from Cuba. Acta Trop. 2017;174:146–148. doi: 10.1016/j.actatropica.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Guzmán M.G., Vázquez S. Apuntes sobre el diagnóstico de laboratorio del virus dengue. Rev. Cubana Med. Trop. 2002;54:180–188. [PubMed] [Google Scholar]
- 25.Guzmán M.G., García G., Kourí G. El dengue y el dengue hemorrágico: prioridades de investigación. Rev. Panam. Salud Públ. 2006;19:204–215. doi: 10.1590/S1020-49892006000300015. [DOI] [PubMed] [Google Scholar]
- 26.Hayes C.G., Phillips I.A., Callahan J.D., Griebenow W.F., Hyams K.C., Shuenn-Juh W., Watts D.M. The epidemiology of dengue virus infection among urban, jungle, and rural populations in the Amazon region of Peru. Am. J. Trop. Med. Hyg. 1996;55:459–463. doi: 10.4269/ajtmh.1996.55.459. [DOI] [PubMed] [Google Scholar]
- 27.Hotez P.J., Bottazzi M.E., Franco-Paredes C., Ault S.K., Periago M.R. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl. Trop. Dis. 2008 doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Instituto Nacional de Salud (Colombia) Instituto Nacional de Salud; Bogotá D.C: 2012. Informe final del evento dengue, año 2012. [Google Scholar]
- 29.Instituto Nacional de Salud (Colombia) 2014. Informe del evento dengue año 2013. Bogotá D.C. [Google Scholar]
- 30.Instituto Nacional de Salud (Colombia) 2015. Boletin epidemiológico semanal numero 52 (2015). Bogotá D.C. [Google Scholar]
- 31.Instituto Nacional de Salud (Colombia) 2016. Informe del evento dengue, Colombia, hasta el decimo tercer periodo epidemiológico, 2015. Bogotá D.C. [Google Scholar]
- 32.Iwamura T., Guzman-Holst A., Murray K.A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-16010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahorgue Nunes M., Carlini C.R., Marinowic D., Kalil Neto F., Holmer Fiori H., Comerlato Scotta M., Ávila Zanella P.L., Bernardi Soder R., Costa da Costa J. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. J. Pediatr. 2016;92:230–240. doi: 10.1016/j.jped.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Lambrechts L., Paaijmans K.P., Fansiri T., Carrington L.B., Kramer L.D., Thomas M.B., Scott T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leta S., Beyene T.J., De Clercq E.M., Amenu K., Kraemer M.U.G., Revie C.W. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu-Helmersson J., Stenlund H., Wilder-Smith A., Rocklö J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.López-Latorre M.A., Neira M. Influencia del cambio climático en la biología de Aedes aegypti (Diptera: Culicidae) mosquito transmisor de arbovirosis humanas. Rev. Ecuat. Med. Cienc. Biol. 2016;37 [Google Scholar]
- 38.Luna Romero E., Lavado Casimiro W. Evaluación de métodos hidrológicos para lacompletación de datos faltantes de precipitación en estaciones de la cuenca Jetepeque. Perú. Rev. Tecnol. ESPOL - RTE. 2015;28:42–52. [Google Scholar]
- 39.Macdonald W.W. Aedes aegypti in Malaya. II. Larval and adult biology. Ann. Trop. Med. Parasitol. 1956;50:399–414. [PubMed] [Google Scholar]
- 40.Méndez F., Barreto M., Arias J.F., Rengifo G., Muñoz J., Burbano M.E., Parra B. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am. J. Trop. Med. Hyg. 2006;74:678–683. doi:74/4/678 [pii] [PubMed] [Google Scholar]
- 41.Méndez-Lázaro P., Muller-Karger F.E., Otis D., McCarthy M.J., Peña-Orellana M. Assessing climate variability effects on dengue incidence in San Juan, Puerto Rico. Int. J. Environ. Res. Public Health. 2014;11:9409–9428. doi: 10.3390/ijerph110909409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mercado Reyes M. 2015. Informe Final Dengue, Colombia, 2014. (Bogotá D.C) [Google Scholar]
- 43.Ministerio de comercio, industria y turismo . 2012. Implementación y cumplimiento de la ley 1581 del 2012, congreso. [Google Scholar]
- 44.Ministerio de Protección Social, Organización Panamericana de la Salud . 2010. Análisis y recomendaciones sobre evaluación de los estándares de atención, vigilancia, prevención y control del dengue en Colombia en el marco de la epidemia nacional año 2010. Convenio MPS-OPS N° 637. Bogota D.C. [Google Scholar]
- 45.Ministerio de Salud y Protección Social., n.d. Guía de Vigilancia entomológiac Y Control de Dengue. (Bogotá).
- 46.Ministerio de Salud y Protección Social (Colombia), n.d. Ciclo de Vida [WWW Document]. URL https://www.minsalud.gov.co/proteccionsocial/Paginas/cicloVida.aspx (accessed 8.2.19).
- 47.Municipio Apulo Nuestro municipio. Apulo, Cundinamarca [WWW Document]. NA. URL. 2018. http://apulocundinamarca.micolombiadigital.gov.co/sites/apulocundinamarca/content/files/000001/41_simbolos.pdf (accessed 10.31.18)
- 48.Olano V.A. Aedes aegypti in rural areas: public health implications. Biomedica. 2016;36:169–173. doi: 10.7705/biomedica.v36i2.3374. [DOI] [PubMed] [Google Scholar]
- 49.Organización Panamericana de la Salud . Catalogación en la Fuente, Biblioteca Sede de la OPS; Washington D.C: 2015. Dengue. Guías Para la atención de Enfermos en la región de Las américas. [Google Scholar]
- 50.Padilla J.C., Rojas D.P., Saenz-Gómez R. 1era ed. Guias de impresion LTDA; Bogota D.C.: 2012. Dengue en Colombia. Epidemiologia de la reemergencia a la hiperendemia. [Google Scholar]
- 51.Padilla J.C., Lizarazo E., Murillo O.L., Mendigaña F.A., Pachón E., Vera M.J. Epidemiología de las principales enfermedades transmitidas por vectores en Colombia, 1990-2016. Biomédica. 2017;37:27–40. doi: 10.7705/biomedica.v37i0.3769. [DOI] [PubMed] [Google Scholar]
- 52.Pinto E., Coelho M., Oliver L., Massad E. The influence of climate variables on dengue in Singapore. Int. J. Environ. Health Res. 2011;21:415–426. doi: 10.1080/09603123.2011.572279. [DOI] [PubMed] [Google Scholar]
- 53.República de Colombia Ley Colombiana estatutaria 1581 de 2012. 2012. http://www.secretariasenado.gov.co/senado/basedoc/ley_1581_2012.html
- 54.Rúa-Uribe G.L., Suárez-Acosta C., Chauca J., Ventosilla P., Almanza R. Modelado del efecto de la variabilidad climática local sobre la transmisión de dengue en Medellín (Colombia) mediante análisis de series temporales. Biomédica. 2013;33:142–152. doi: 10.7705/biomedica.v33i0.1444. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-López F., González-Mazo A., Vélez-Mira A., Gómez G.F., Zuleta L., Uribe S., Vélez-Bernal I.D. Presencia de Aedes (stegomyia) aegypti (Linnaeus, 1762) y su infección natural con el virus del dengue en alturas no registradas para Colombia. Biomedica. 2016;36:303–308. doi: 10.7705/biomedica.v36i2.3301. [DOI] [PubMed] [Google Scholar]
- 56.San Martín J.L., Brathwaite O., Zambrano B., Solórzano J.O., Bouckenooghe A., Dayan G.H., Guzmán M.G. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am. J. Trop. Med. Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santa M., Salamanca M., Carlos J., Garzón E., Muñoz E.G., Lucía M., Reyes G., Adolfo G., Díaz B., Alonso R., Tamara V., Ángela L., Hermida G., Patricia E., Tovar G., Posada Sánchez M. 2011. Comisión de regulación en Salud. [Google Scholar]
- 58.Sirisena P.D.N.N., Noordeen F. Evolution of dengue in Sri Lanka-changes in the virus, vector, and climate. Int. J. Infect. Dis. 2014 doi: 10.1016/j.ijid.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Sosa Cabrera T.J., Santos Pérez M. Caracterización clínica y de laboratorio de un brote de dengue en un área rural de Campeche, México. Rev. Cubana Med. Trop. 2008;60:136–140. [Google Scholar]
- 60.Suárez M., Nelson M., Morales A., Archila L., Galvis E., Guzmán J., Dussán R. 1984. Aedes aegypti en áreas Rurales de Colombia. (Informe reunión sobre Aedes aegypti. Bogotá D.C) [Google Scholar]
- 61.Sutherst R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres A.H., García Vázquez E., Moral Escudero E., Herrero Martínez J.A., Gómez Gómez J., Segovia Hernández M. Infecciones víricas endémicas: dengue, fiebre del Nilo y otras viriasis. Rev. educa. Super. 2018 doi: 10.1016/j.med.2018.05.001. [DOI] [Google Scholar]
- 63.Trenberth K.E. Changes in precipitation with climate change. Clim. Res. 2011;47:123–138. doi: 10.3354/cr00953. [DOI] [Google Scholar]
- 64.Vásquez Rodríguez A.B. Universidad Nacional de Colombia; 2019. Factores geográficos, ecológicos Y sociodemográficos en la Ocurrencia de Dengue en Cundinamarca. [Google Scholar]
- 65.Velandia-Romero M.L., Olano V.A., Coronel-Ruiz C., Cabezas L., Calderón-Peláez M.A., Castellanos J.E., Matiz M.I. Dengue virus detection in Aedes aegypti larvae and pupae collected in rural areas of Anapoima, Cundinamarca, Colombia. Biomédica. 2017;37:193. doi: 10.7705/biomedica.v37i0.3584. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization . World Health Organization; Washington: 2009. Dengue. Guías para el diagnostico, tratamiento, prevención y control. [Google Scholar]
- 67.World Health Organization . 1st ed. 2011. Accelerating work to overcome theglobal imact of neglected tropical diseases. A roadmap for implementation. Ginebra. [Google Scholar]
- 68.World Health Organization Dengue y dengue grave. 2016. http://www.who.int/es/news-room/fact-sheets/detail/dengue-and-severe-dengue [WWW Document]. 5 may. URL. (accessed 6.20.05)
- 69.World Health Organization WHO|Dengue and severe dengue. 2017. https://apps.who.int/mediacentre/factsheets/fs117/en/index.html [WWW Document]. URL. (accessed 1.13.21)
- 70.Mateo L., Roure S. Chronic arthritis in chikungunya virus infection. Reumatol. Clínica (English Ed.) 2019;15:113–116. doi: 10.1016/j.reumae.2017.06.0022. [DOI] [PubMed] [Google Scholar]
- 71.D’Ortenzio E., Matheron S., de Lamballerie X., Hubert B., Piorkowski G., Maquart M., Descamps D., Damond F., Yazdanpanah Y., Leparc-Goffart I. Evidence of sexual transmission of Zika virus. N. Engl. J. Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1: Graphics of the Behavior of climate variables in three municipalities.
Supplementary 2: The tables detail the information of the climatic combinations.
Supplementary 3: The models described in multilevel analysis section.