Abstract
The Developing Countries Vaccine Manufacturers' Network held its 21st Annual General Meeting virtually in November 2020 given the COVID-19 pandemic. Vaccine manufacturing experts, leaders from local and global public health organizations and multilateral organizations, through diverse presentations, questions and answers, focused on the pandemic and the response of vaccine manufacturers where many are engaged in research and development and production agreements. The pandemic is expanding rapidly which makes the global availability and equitable access to safe and effective COVID-19 vaccines critical. Strategies put in place include the establishment of the Access to COVID-19 Tools Accelerator (ACT-A) within which the COVAX facility aims to distribute 2 billion COVID-19 vaccine doses by the end of 2021, with procurement mechanisms already being established. At the same time, regulatory authorities have emergency use authorizations aimed at the rapid approval of safe and effective vaccines, with a push for harmonization in regulatory approaches being advocated. The Meeting was also apprised of other innovations being developed for vaccines including multi-array patches for easier vaccine application, increased heat stability for mitigating cold chain and storage challenges, the barcoding of primary packaging for enhancing vaccine traceability, and gathering data for decision-making. Over time, these innovations will facilitate the widespread availability and equitable access of vaccines including those addressing epidemics and pandemics. In addition, a number of manufacturers described technologies they have for accelerating vaccine manufacturing and supply globally.
Overall, there was agreement that manufacturers from developing countries play a critical role in the global research, development and supply of vaccines for a healthy future, with increasing collaboration and partnering between them a growing strength.
1. Introduction
The Developing Countries Vaccine Manufacturers' Network (DCMVN) held its 21st Annual General Meeting 2020 from 3rd to 5th November 2020; the first being held on a virtual platform, due to the pandemic declared by the World Health Organization (WHO) on 11th March 2020 [1]. The discussions focused on vaccines against SARS-CoV-2 virus to prevent the COVID-19 disease. Over 380 participants from 36 countries, including leaders from industry, research institutions and global health organizations, joined the online deliberations and agreed on the importance of vaccine developers and manufacturers in rapidly bringing to the world safe and effective vaccines to end the COVID-19 pandemic.
DCVMN President, S. Prasad, welcomed attendees, acknowledging their engagements in sharing views and knowledge on vaccines for fighting infectious diseases and achieving a healthy future. He reiterated that the 41 member companies of the Network jointly supply over 3.5 billion doses of vaccines annually [2], including multivalent vaccines, with a collective manufacturing capacity of over 4 billion doses. Trusted partnerships with WHO and other stakeholders are essential for the success of public health advancements.
2. The magnitude of the pandemic
Dr. Tedros Adhanom Ghebreyesus (WHO) reported on the global 2020 pandemic, with over 50 million cases since January 2020 and over 1.3 million deaths, calling for the speedy development and equitable access of vaccines against COVID-19, commending the COVAX Facility, aimed at distributing 2 billion doses of COVID-19 vaccines by end 2021 to countries that join the facility. He highlighted the essential role of the vaccine industry of developing countries to accelerate manufacturing, while ensuring that routine vaccines’ supply continues to protect millions of lives every year. The global crisis caused by COVID-19 must be a catalyst for building a healthier, safer, fairer and more sustainable world, he concluded.
J. Barbosa (Pan American Health Organization, PAHO) shared information on the magnitude of COVID-19 disease in the Americas, which bears nearly half of the global toll of disease and over half of global mortality [3]. Many distinct patterns of COVID-19 transmission have been observed in the countries of North, Central and South America. COVID-19 has had an impact on immunization programmes, exemplified by a 24% decrease in MMR1 vaccination regionally in the second quarter of the 2020, compared to the same period in 2019. PAHO is supporting countries around 9 strategic pillars, including coordination, planning and monitoring, risk communication and community engagement, case management and investigation, surveillance, operations and logistics. PAHO has encouraged countries to participate in the COVAX Facility arrangements (see below statements by S.Berkley), 27 having already signed commitment agreements. From 42 countries/territories in the Americas, 10 are eligible for Advanced Market Commitment (AMC) support, and 32 are self-financing. A well-coordinated effort will ensure timely access to prevention measures and vaccines in fighting the pandemic, and maintain public health gains achieved in decades of high immunization coverage in the Americas.
3. Strategy responses for accelerated equitable access to COVID-19 and other future vaccines
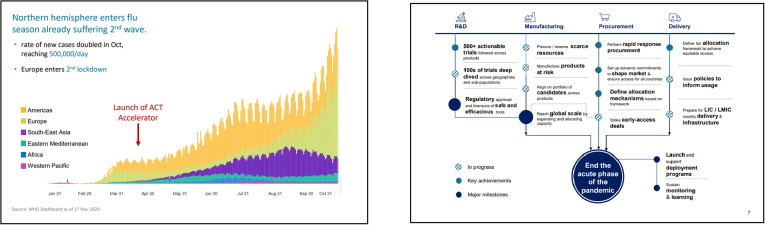
B. Aylward (WHO) elaborated on the Access to COVID-19 Tools Accelerator (ACT-A) plans, progress and challenges. The ACT-A, created in April 2020 [4], aims to rapidly reduce the risk of severe disease and end the acute phase of the pandemic, with two major objectives: 1) accelerate development of new diagnostics, treatments & vaccines, and 2) achieve equitable global access to all COVID-19 tools on a timely manner which is crucial for impact (Fig. 1 ). ACT-A achievements so far include access to 120 million low-cost diagnostics, the rollout of 100 million doses of dexamethasone for low- and middle- income countries (LMICs) and 186 countries expressing interest in the COVAX facility procurement mechanism. An allocation mechanism to provide initial doses of vaccine to high risk populations and up to 20% of the population in COVAX economies has been established. The total operational costs of the ACT-A was estimated at around 38 billion US dollars over 5 years, which is considered moderate compared to the cost of disease spread and the economic losses of up to several trillion US dollars. Challenges include funding and political will, and timely access to efficacious vaccines against COVID-19.
Fig. 1.
ACT-A goal: to rapidly reduce risk of severe disease to end the acute phase of pandemic. Courtesy of B. Aylward, WHO. Legend: ACT-A’s uses parallel workstreams to accelerate the rapid achievement of its goals. A) Context and magnitude of disease burden globally. Only 10 months into the pandemic, nearly 50 million people known to have been infected and 1.2 million people have died to COVID-19. Recent second wave in many European countries have exacerbated the magnitude of the pandemic. B) Covid-19 infection, depending on transmission, has outcomes as asymptomatic and mild disease (80–90% of detected infections), or severe disease (10–20% of detected infections) with hospitalization, that led to health, social and economic disruption in most countries. Major agencies (WHO, GAVI, CEPI, UNITAID, Wellcome Trust, World Bank, The Global Fund, FIND) are collaborating to coordinate the global response, together with other stakeholders, including DCVMN. Commitments of the initiative by end 2021 include 500 million diagnostic tests, 254 million courses of therapy and 2 billion doses of Covid-19 vaccines delivered to developing countries, although access to these tools is hampered by resources and infrastructure. Research, manufacturing, procurement and delivery are being prepared in parallel, to save time to access, and ensure efficient allocation of resources.
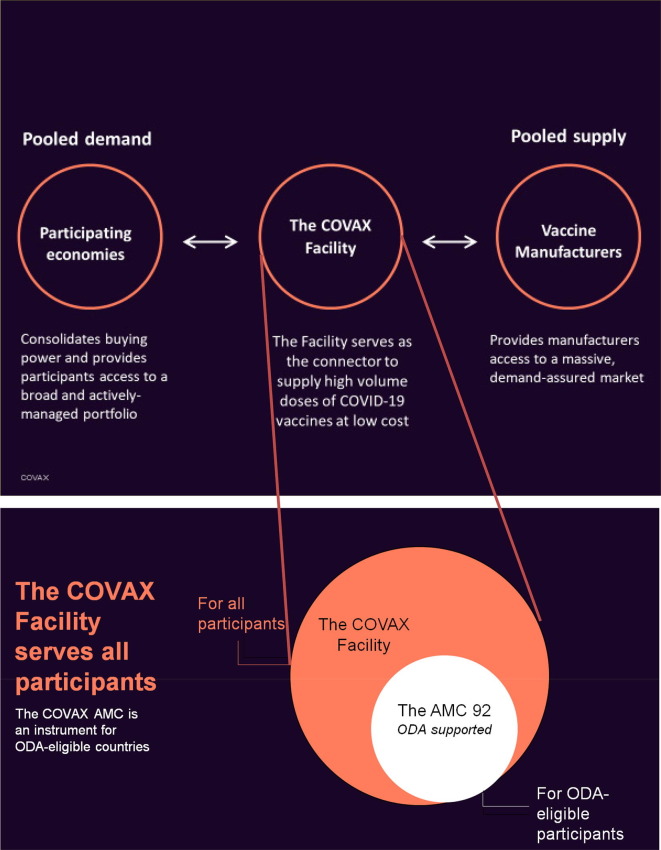
S. Berkley (Gavi, The Vaccine Alliance) emphasized the critical role of the COVAX Facility as a new multilateral mechanism to support the largest actively managed portfolio of COVID-19 vaccine candidates globally and end the acute phase of the pandemic. The Facility serves as the connector to supply high-volume vaccines at low cost, based on the principles of pooled demand from participating countries, while pooling supply from participating manufacturers (Fig. 2 ). It includes 97 self-financing economies as well as the donor-funded Advance Market Commitment (AMC) which supports 92 ODA-eligible economies2 . The Facility will ensure that the vaccines undergo rigorous evaluation by WHO or stringent regulatory assessment and aims to ship the first vaccines in early 2021. COVAX represents lower risks and lower costs for the global economy in distributing COVID-19 vaccines around the world.
Fig. 2.
COVAX Facility focused on transparency, global access and impact. Legend: Participating countries/economies will receive support from UNICEF and the PAHO Revolving Fund for procurement, financing and cold chain infrastructure for delivery, though ultra-cold-chain infrastructure is still under discussion. The facility provides two models of legally binding agreements: a committed upfront payment agreement and an optional agreement, where higher upfront payments with opt out options for vaccines. The manufacturers include the CEPI funded 9 vaccines portfolio (CEPI portfolio: see Fig. 3 (cf. https://cepi.net/research_dev/our-portfolio/). The list of COVAX participating economies and AMC eligible economies, as of December 2020, is available at https://www.gavi.org/sites/default/files/covid/pr/COVAX_CA_COIP_List_COVAX_PR_15-12.pdf.
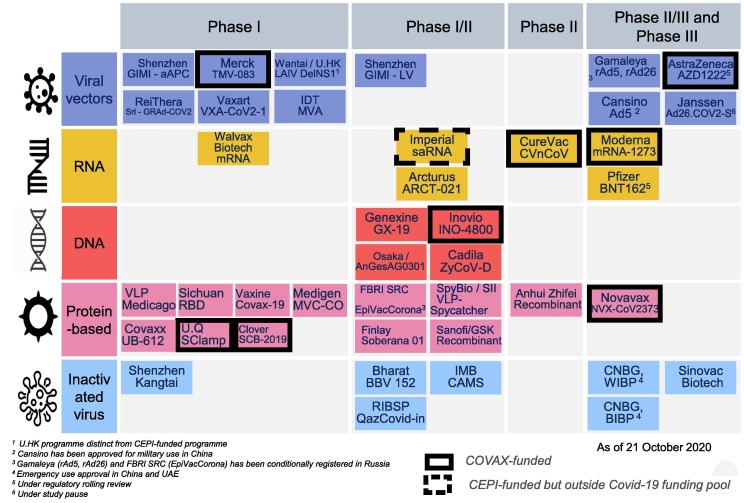
R. Hatchett (CEPI3 ) presented the COVID-19 vaccines development and manufacturing landscape, focusing on three criteria: speed, scalability and access. New technologies for rapid response vaccine platforms have emerged, such as mRNA-based vaccines (Fig. 3 ). The expanding of manufacturing capacity was also emphasized, CEPI having conducted a survey among 52 manufacturers globally and identified capacity for 10 billion doses, including two billion doses excess capacity, without compromising other critical production capacity. As examples, CEPI signed capacity-reservation agreements with SK Bioscience and Green Cross Pharma in the Republic of Korea and introduced manufacturers to other partners, resulting in six agreements for technology transfer which includes the Serum Institute of India. Long-term support is being envisaged, as it is prognosticated that COVID-19 might become endemic. In this regard, India’s Ministry of Science and Technology, through its Department of Biotechnology and in conjunction with CEPI, has set up an office in India, named IndCEPI, which funds activities specifically with Indian manufacturers. An increasing number of countries have joined CEPI to address epidemic risks, as partners for global collective efforts against epidemics and pandemics. He reiterated the desire in collaborating with DCVMN to promote collective global health security.
Fig. 3.
COVID-19 vaccine development landscape: five main technologies and 44 candidates in human clinical trials. Courtesy of M. Saville. Legend: The COVAX R&D&M portfolio consists of 9 candidate vaccines in clinical development. As presented on 05th November, from 44 known COVID-19 candidate vaccines in clinical trials, the COVAX R&D portfolio consists of 9 candidate vaccines in clinical development supported by CEPI (boxes with black frame), whereby 3 have reached phase 3 trials. These candidates, based on multiple technology platforms and diversified across geographies contribute to minimize the risks of hampering global distribution by export and controls of countries hosting manufacturing. Cf. https://cepi.net/research_dev/our-portfolio/
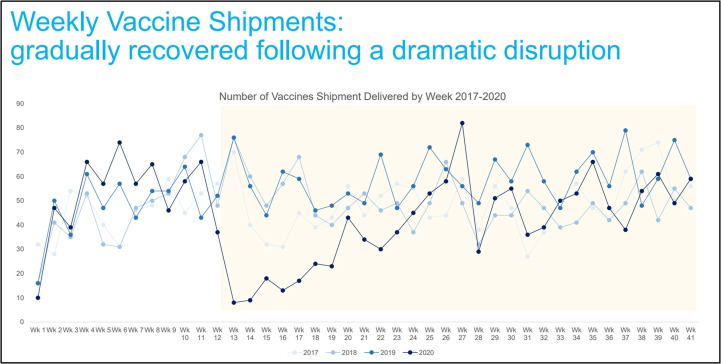
E. Kadili (UNICEF) discussed the impact of the pandemic on vaccine supply, immunization services and coverage, and the unprecedented challenges to launch COVID-19 vaccines’ globally including the need for countries readiness to implement vaccination without delay. UNICEF is engaged in the implementation of the COVAX Facility and serves as co-lead as Procurement Coordinator, aiming to secure 2 billion doses for delivery in 2021 for 189 participants. Procurement is implemented through a rolling tender of 12 weeks, with awards being made in three phases - immediate supply, supply in the first half-2021 and supply in the second half-2021 inviting all manufacturers to participate. Criteria for contracting include speed to market, volume availability, affordability, a balanced portfolio of products, meeting regulatory milestones of WHO Emergency Use Listing (EUL) [5] and/or authorization by Stringent Regulatory Authority (SRA). UNICEF will monitor supply and demand to inform the allocation and delivery to countries, supporting country capacity to receive and deploy vaccines, including funding needs, registration requirements, transportation, storage and distribution are planned and in place, and there is wide public vaccination. This is a complex and concerted effort at global, regional and national level (Fig. 4 ).
Fig. 4.
Number of UNICEF weekly vaccine shipments in 2020. Courtesy of E. Kadili. Legend: A disruption in airfreight capacity triggered a steep decrease in the volume of vaccine shipments in early April, recovering after May to reach average volumes in October 2020, as compared with shipment numbers in previous years (2017–2019).
A. Zaidi (BMGF) provided a brief summary of the Foundation’s four major engagements for COVID-19 response to address global needs: accelerating disease detection, protecting the most vulnerable, leveraging product development with partners, and fostering economic empowerment to limit the damage during this pandemic particularly in low-and middle-income countries. The Foundation has committed over 350 million US dollars to support the global response to COVID-19, in addition to maintaining its commitments to efforts in malaria, TB and global education. The Foundation is also supporting the ACT-A, to mitigate the risks of unbalances in the procurement of vaccines that consequently may hamper LMIC access to lifesaving vaccines. Whereas wealthy countries have funded research and development and secured access for domestic populations through bilateral advanced purchase agreements [6], DCVMN members’ expertise and capabilities are needed for supply for LMICs (Fig. 5 ).
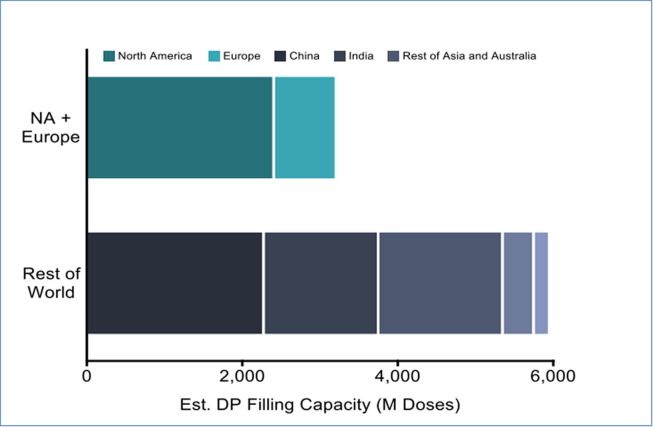
Fig. 5.
Expertise and capabilities play a pivotal role in enabling COVID-19 access. Courtesy of A. Zaidi. Legend: Mathematical modelling suggests that an equitable distribution of the first 2 billion doses of an 80% effective COVID-19 vaccine could avert nearly twice as many deaths, as opposed to an exclusive distribution to wealthy countries The global distribution of available excess fill/finish capacity highlights the potential for DCVM partnerships to ensure global supply and access beyond the US and Europe. Note: Where companies do not provide data 10% availability assumed; Source: Drug Product CEPI BMGF PATH CHAI Database (as of 21 May 2020). Cf. https://ww2.gatesfoundation.org/ideas/articles/coronavirus-vaccine-strategy-bill-gates.
N. Gilbert (PATH) highlighted the contributions of vaccine manufacturers in developing and supplying vaccines equitably to fight this pandemic, which is putting at risk the progress made over decades against other major infectious diseases. One key to equity is mitigating the risks, and for this PATH supports the COVAX Facility, to maximize vaccine supply and distribution and ensure that all countries are prepared to deliver the vaccines, when available, while maintaining healthcare interventions. PATH pursues vaccines’ development to protect against 18 different diseases, through partnerships that have resulted in safe and effective vaccines against meningitis, rotavirus and pneumonia, through supporting clinical development, solving manufacturing issues or identifying regulatory pathways. PATH works with countries on vaccine access and introduction policies. It also helps finding solutions to keep manufacturers sustainable, understanding market limitations, incentives and policies that can ensure vaccine accessibility and affordability. Science indicates that more epidemics and pandemics will emerge requiring preparedness to response quickly and equitably to protect everyone.
A. Bernaert (World Economic Forum) discussed the need for global allocation mechanisms for existing manufacturing capacity, and at-risk scale-up mechanisms in periods of pandemic. From the many emerging COVID-19 candidate vaccines, 10 have advanced to clinical studies. In this context, a flurry of bilateral agreements was made between vaccine innovators and large manufacturers, pledging their capacity to deliver vaccines to a limited number of nations. The Forum is a strong proponent of the COVAX Facility while currently recognizing an imbalance as the Facility’s pledges to LMICs is around 600 million doses for 3.5 billion people. Several candidate vaccines released in early 2021 may be driven by limited volumes and previously-signed bilateral advanced purchase agreements causing LMICs to access vaccines later. This inequity enhances the need to promote mechanisms that increase manufacturing and filling capacity, while reducing the financial risk for manufacturers. This warrants having manufacturing at scale, with balanced geographical distribution, a commitment to global supply and the capacity to respond in future pandemics.
4. COVID-19 vaccines research and development
Vaccine manufacturing companies have moved with unprecedented speed thanks to thousands of people in many countries who have volunteered to participate in clinical trials. In total there are more than 200 candidate vaccines reported4 : 48 being tested on humans, and about ten have reached phase 3 trials. Of these, two are advanced mRNA candidates by Pfizer/BioNTech [7], and Moderna [8]. Another is being developed by Oxford University and AstraZeneca [9], in collaboration with emerging manufacturers in Argentina, Mexico, Brazil, China, Korea and India. Other candidates include inactivated virus vaccines from Sinovac Biotech [10] in collaboration with Butantan, two from Sinopharm [11] and one from Bharat Biotech which is entering phase 3 trials in India in November 2020 [12]. CanSino Biologics [13] is well advanced with its vaccine development as is Johnson & Johnson [14], in collaboration with Biological E [15]. A vaccine produced in Russia entered phase 3 in October 2020 [16].
S. Gilbert (Oxford University) described the development of its SARS-CoV-2 vaccine, based on a non-replicating simian adenoviral-vectored vaccine platform expressing the nCoV-19 spike protein (ChAdOx1 nCoV-19) and designed to elicit immune responses, including in immunocompromised subjects. Similar existing veterinarian vaccines against bovine coronavirus successfully protect animals [17]. Four pre-clinical studies demonstrated immune response, safety and protection against challenges [18]. A Phase 1/2, single-blind, randomized controlled trial, with 1077 participants, showed an acceptable safety profile and antibody responses [19] up to 56 days after vaccination with increased IgG and neutralizing antibodies titres. The vaccine was well tolerated with reduced reactogenicity after the second dose. Ongoing phase 3 trials to evaluate vaccine efficacy include adults over 18 years in the United Kingdom, Brazil, South Africa and the USA. Future plans will include safety and immunogenicity trials in children, pregnant women and HIV+ populations. If licenced, the vaccine will be produced, by multiple manufacturers globally and distributed, once safety and efficacy is demonstrated [20]. Long-term post-marketing monitoring for waning antibodies is planned.
B. Pierce (Future Vaccine Manufacturing Research Hub5 ), reported on advancements of the Hub, a major academic consortium and DCVMN collaborator, in new vaccines for developing countries, where population growth drives the need for innovative technologies. The Hub has supported the development of a COVID-19 self-amplifying RNA vaccine, encoding the stabilized spike (S) glycoprotein of SARS-CoV-2 virus, delivered by liposomes formulation, and triggering neutralizing antibody responses in mice [21]. Safety and immunogenicity studies are ongoing in phase 1/2 human trials since June 2020, to support efficacy studies, subject to funding availability. RNA is a versatile, flexible and rapid tool with interest beyond COVID-19 vaccines. The Hub more broadly focuses on 4 technologies [22]: GMMA, baculovirus, modified yeast and RNA platforms. One example of successful efforts is the immunogenicity elicited by baculovirus expression of chikungunya proteins in pre-clinical studies. Another example is the elucidation of the binding domain of SARS-CoV-2 spike protein [23] that may impact therapies for COVID-19 infection. Quality-by-design approaches were highlighted for innovations in supply chain efficiency with the Hub providing technical consultancy support and a quality control hands-on training for manufacturers across vaccines for nine different disease areas.
R. Ella (Bharat Biotech) mentioned four COVID-19 candidate vaccines under development but focused on the clinical trials of a whole inactivated COVID-19 vaccine lead candidate. The whole virion inactivated approach, using a Vero cell manufacturing platform, produced in a BSL-3 facility, relies on 15 years of extensive clinical development successes and 300 million of doses, produced by Bharat Biotech on this technology platform. The inactivated COVID-19 vaccine has undergone challenge studies in rhesus macaques [24] and hamsters [25], with good immunogenicity and protection. The Phase 1 and Phase 2 double-blind human trials, using an adjuvanted inactivated candidate vaccine with 375 participants 18–55 years and 380 participants 12–65 years, respectively, across 11 hospitals in India, assessed safety, reactogenicity, tolerability and immunogenicity, including neutralizing titres. The company has initiated a Phase 3 trial in 25 centres around India, with a 2-dose regimen 28 days apart with 26 thousand participants.
W. Meng (Sinovac) presented an overview of cross-continent collaboration on the development, manufacture and commercialization of vaccines for COVID-19 and infectious diseases with significant unmet need. Development of a Vero-cell based inactivated COVID-19 vaccine began in January 2020 and provided partial or complete protection in a challenge study with primates [26], as compared to other approaches. Phase 1/2 randomized, double-blinded clinical trials in China, with 600 subjects, achieved high sero-positivity rates and a good safety profile. Given the prevailing low level of COVID-19 cases in China and the limited production capacity of the company, Phase 3 trials were started in July in collaboration with Butantan, in Brazil, and in August with Bio Farma, in Indonesia, leading to local manufacturing agreements. This has demonstrated effective collaboration between DCVMN members to overcome manufacturing challenges. Paediatric studies will be considered in the future.
M. Reers (Biological E) described the company's COVID-19 vaccine developments, which includes an agreement to manufacture and fill up to 500 million doses in support of Johnson & Johnson’s adenovirus based vaccine candidate [27] for global access. Another partnership is with the Baylor College of Medicine, Texas, to develop and commercialize a vaccine based on the receptor-binding domain of the spike (S) protein [28] expressed in the Pichia pastoris-recombinant platform already used to make large scale hepatitis B vaccine. The monthly capacity of the adjuvanted recombinant vaccine is not less than 100 million doses. Pre-clinical studies in mice resulting in high seroconversion rates have been followed by a Phase 1/2 human trial in India, testing 4 formulations leading to a final selection of the antigen adjuvanted in Alum/CpG. A follow-on Phase 3 trial in India was designed to assess the safety and immunogenicity of the selected formulation. In parallel, global Phase 3 trial is planned in Asia, Latin America, Europe and Canada to assess efficacy. Future assessment of efficacy is being considered for paediatric use and for a booster dose.
M. Joshi (Serum Institute of India) discussed challenges in the manufacture of COVID-19 vaccines, from a viewpoint of the world's largest vaccine manufacturer which supplies around 1.5 billion doses annually to 170 countries worldwide. It currently has collaborations in four distinct COVID-19 candidate vaccines across diverse manufacturing technology platforms, including the Oxford University candidate (see above). Preparedness for filling in single and multi-dose vials, with and without preservatives, as well as in pre-filled syringes has been secured [29]. Another candidate, derived from nanoparticles technology and antigen from Sars-CoV-2 spike (S) protein, combined with saponin-based Matrix-M adjuvant, has entered phase 3 trials in the UK. Challenges include the alignment of upstream and downstream processing units to handle large volumes, process validations and regulatory engagement. Supply chain challenges include establishing large-scale cold storage for stockpiling, distinct labelling requirements across countries, transportation bottlenecks, tracking vaccine supply and ensuring post marketing surveillance.
M. Li (CNBG) reviewed the COVID-19 vaccine research and development landscape in China, where 24 companies are developing 25 candidate vaccines: 10 based on recombinant protein vaccines, 7 inactivated vaccines, 5 nucleic acid vaccines and 3 vector based vaccines. Four candidates have reached Phase 3 trials. CNBG has two inactivated vaccines candidates in Phase 3 trials since June 2020, using the whole virus antigen, to provide broad protection and ensure high levels of safety as it is a large-scale widely-proven platform. Preclinical evaluations have achieved immune response in diverse animals by different dosages and schedules. In Phase 1 and Phase 2 trials, a two-dose immunization with 4 μg vaccine achieved higher neutralizing antibody titres than a single 8 μg dose [30]. Phase 3 randomized, double-blind placebo controlled trials are currently underway, recruiting 45,000 subjects in Middle-East and Latin American countries with manufacturing capacity set at 300 million doses annually in two BSL 3 certified facilities.
5. Regulatory approaches, alignment and preparedness for future vaccines
M. Saville (CEPI) elaborated on regulatory challenges manufacturers face for COVID-19 vaccine development in the context of the ACT-A COVAX [31]. For rapid access, the US FDA has detailed an emergency use authorization (EUA) [32], [33] and the EMA has considered a conditional marketing authorization [34] as the most likely pathways. WHO has two mechanisms: emergency use listing (EUL) as well as its pre-qualification (PQ) assessment. Stability data and shelf life are critical for post-approval changes. CEPI and WHO are co-chairing a regulatory advisory group with representatives of regulatory authorities from around the world to provide regulatory guidance and promote harmonized and streamlined processes wherever feasible [35], issuing Q&A on regulating COVID-19 vaccines [36]. Challenges include acceptance of EUL/PQ, use of standard labels and barcoding, and reliance on robust manufacturing controls (CMC). COVAX enables manufacturers and experts to find solutions through a regulatory advisory group and SWAT groups.
C. Rodriguez (WHO) provided an overview of the regulatory alignment and authorization of vaccines under EUL and PQ aimed at optimizing the access and availability to safe, efficacious, quality-assured COVID-19 products [37] (Fig. 6 ). The WHO EUL was principally put in place for use during a public health emergency of international concern [38]. Aligning the regulation of vaccines allows countries to make decisions on vaccine use based on an independent assessment (e.g. EMA), a recommendation from a regional network (e.g. EAC), a reference NRA that has done independent assessment or reliance on WHO EUL or PQ. Labelling, for which WHO is developing model for single-language generic versions, may require agreements and waiver from legal national requirements in order to facilitate fill and finish operations of manufacturers. Manufacturers are encouraged to consult the WHO working position on labelling and barcodes for more details [39].
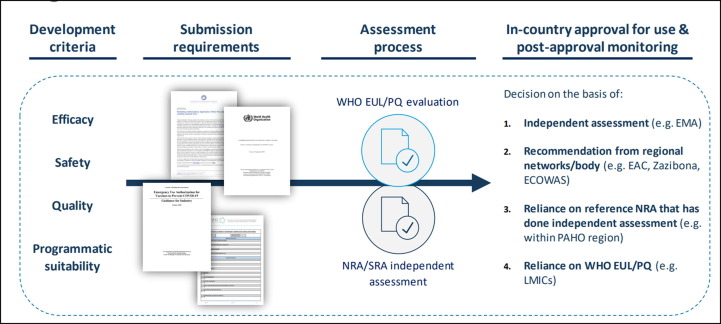
Fig. 6.
Overview of WHO’s end-to-end process for aligning the regulation and authorization of vaccines under EUL/PQ. Courtesy of C. Rodriguez. Legend: WHO has a road map for aligning the regulatory processes that impact access to COVID-19 vaccines, which has been ongoing since February 2020. Submissions of vaccines must address product efficacy, safety, quality and programme suitability in order to secure either WHO pre-qualification or an emergency use listing. Countries are able to make regulatory decisions based on WHO prequalification or emergency use listing or on assessments by independent or regional bodies.
S. Azatyan (WHO) described WHO's efforts in the harmonization of regulatory approaches in African countries where essential medical products are not always readily available or accessible, thus contributing to inequities in health and life expectancy. Regulatory convergence and harmonization would have the potential of saving lives, as well as reduce the administrative burden on regulators and enable quicker access to more affordable products [40]. The African Medicines Regulatory Harmonization (AMRH) initiative was launched in 2009, as a Consortium of International Partners [41] to respond to the challenges in harmonizing medicines regulation in African countries. The focus is on common technical documents, processes and shared information systems among regional economic communities, including joint activities of assessing dossiers and carrying out GMP inspections, as well as setting up the African Vaccine Regulators Forum, facilitating clinical studies (Fig. 7 ). In addition, an African COVID-19 Task Force was established to help achieve timely access to the COVID-19 tools.
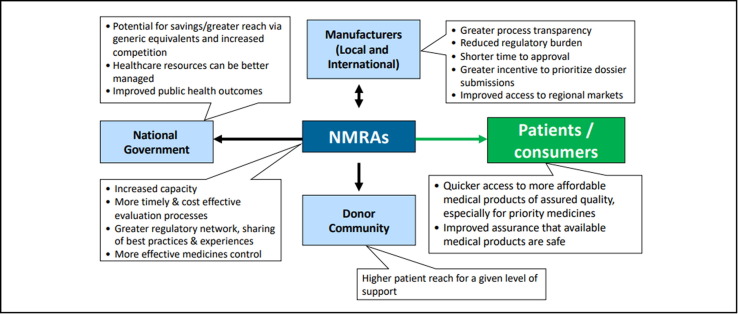
Fig. 7.
Rationale for Regulatory Convergence and Harmonization in African countries. Courtesy of S. Azatyan. Legend: About 30% of national regulatory authorities have limited capacity to perform core regulatory functions. Regulatory convergence and harmonization can help such authorities access increased capacity and secure savings enabling health care resources to be better managed. Harmonization through a regulatory network can more easily share best practices with improved outcomes. There are also benefits for manufacturers by reducing their regulatory burden with shorter times to approval at lower cost, as well as being able to improve their access to regional markets such as is the case with the African region.
C. von Hunolstein (Instituto Superiore di Sanità) provided an update of the improvement of legacy vaccines release testing taking the case of the pertussis serological potency test (PSPT). Currently, the Kendrick challenge test [42] is the only internationally-agreed test for the batch release of whole-cell (wP) containing vaccines and an alternative test is needed to mitigate some of the issues such as high variability, animal welfare and biohazard risks. The PSPT is based on in vitro testing of humoral response against a wide range of surface antigens in mice and guinea pigs immunized with wP [43]. A multi-laboratory study underway, with NIIMBL support and coordinated by DCVMN, with the participation of 8 manufacturers and 3 national control laboratories, is aimed at supporting the in-house validation of the PSPT, in reducing the variability of the potency testing and the number of animals used for routine batch release of wP containing vaccines. Benefits include the potential to reduce testing costs and use the same serological test to assess the potency of many wP combination vaccines, leading to the harmonization of testing by emerging country regulators and paving the way to the international adoption of this test for DTP-containing vaccines globally.
6. Other vaccine innovation strategies
B. Giersing and M. Menozzi (WHO-GAVI) communicated the outcomes of the Vaccine Innovative Prioritization Strategy (VIPS), established in 2018, as a long-term vision to accelerate innovations for current or future vaccines. Three innovations have been prioritized among 24 innovations: microarray patches (MAPs) [44], heat stable formulations including the controlled temperature chain (CTC) [45], and barcodes on primary vaccine packaging [46](Fig. 8 ). MAPs can address major immunization barriers and have broad applicability across different vaccines. Heat stability is considered highest priority by countries, while reducing vaccine wastage. Bar codes is a requirement for COVID-19 vaccines on secondary packaging, while primary package bar codes will enable tracking through supply chains, accelerating the adoption of electronic record keeping, and monitoring AEFIs more accurately. In synergy these three areas could have significant impact in improving vaccination globally. The VIPS 5-year action plan will focus on accelerating product development and uptake and engaging with stakeholders to address the challenges of lack of commercial incentive, given the lack of data on programmatic impact and unclear demand. These three areas could improve vaccination globally, raising immunization coverage and equity in LMICs. Notably, the COVID-19 pandemic may help catalyze investments in these innovations going forward.
Fig. 8.
Three innovations have been prioritised by VIPS, which could also facilitate RI catch up/ recovery from COVID-19. Courtesy of B. Giesing and M. Menozzi-Arnaud. Legend: Microarray Patches: Potential to address most immunisation barriers identified by countries and applicable to several vaccines with broad applicability across the life course and outbreak response. Thermostability: Top priority by countries with synergies with other innovations, i.e. MAPs, VVM-Tis, dual chamber delivery devices, SDIs. (SDI = solid dose implant; VVM-Ti: Combined Vaccine Vial Monitor and Threshold Indicator). Barcodes on primary packaging: Greater accuracy in tracking at lower levels of distribution and accelerating the transition to electronic record keeping.
T. Wilmansyah (Bio-Farma) presented details on a pilot study the company is conducting on a track and trace system facilitated by the barcoding of primary packaging (vaccine vials). The pilot study has been successfully tested on 6000 individuals in three districts/catchment areas in one provincial level distribution area. A principal driver in this traceability innovation was to mitigate counterfeiting of vaccines [46]. A unique identifier, including the Global Trade Item Number (GTIN), batch/lot number, expiry date and serial number, on a GS1 data matrix barcode was used. The barcode is laser printed on the vial label and verified by an inspection camera before cartoning. The barcode is scanned at all intermediate points between the manufacturer and the end user, with smartphones used at point of vaccination (Fig. 9 ). Bio-Farma designed two software applications, Bio-tracking to update vaccine usage and Bio-detect to follow vaccine movement through the health system, continuously updating the database through the internet which is also accessible by the National Regulatory Agency.
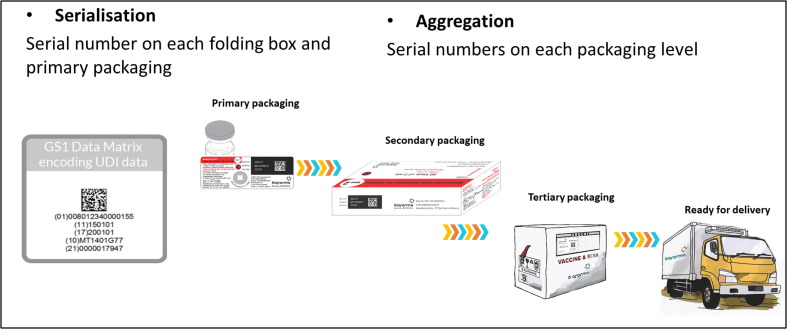
Fig. 9.
The Serialization and Aggregation of traceability. Courtesy of T. Wilmansyah Legend: Vaccine serialization enables traceability which authenticates the products and supports any recalls that may occur. Serialization leads to each vaccine vial or primary packaging to have a unique serial along with other key data such as the GTIN, lot number and expiry date. This enables tracking an individual vial from manufacturer through to final use. Secondary and tertiary packaging have corresponding serialized barcoding that match the barcodes on the primary packaging. Such barcoding is critical for mitigating the risk of counterfeiting. The safety and security of COVID-19 vaccines puts greater focus on the need for traceability through barcoding technology.
C. Mehta and B. Evans (Clinton Health Access Initiative), discussed data for decision making and the development of new vaccines. The Global Vaccine Market Model (GVMM), a privately owned, continuously updated demand and supply database, was introduced and is accessible free of charge to DCVMN members. Data on countries’ income, Gavi policies and country eligibility status, public demand on all vaccines for the next decade by country and partial information for campaign, outbreak and stockpile vaccines are available. On new vaccine development, emerging vaccine manufacturers have become key suppliers and developers for LMICs. Through a survey conducted in collaboration with DCVMN, four main challenges for emerging vaccine manufacturers were identified: constrained financing, low access to in-licensing and joint-venture opportunities, regulatory hurdles and lack of clearer commercial cases. Potential solutions included increasing funding for vaccine development, creating new networking platforms for partnerships, creating a centralized scientific regulatory support function and generating clearer demand forecast scenarios for novel vaccines.
J. Kim (IVI) highlighted the Institute's role, as a non-profit OECD-recognized international organization, in developing new vaccines for saving lives and building a healthier world, while benefitting from collaboration with infectious disease and vaccine research institutes. Its vision encompasses field epidemiology surveillance and gathering evidence; laboratory research and discovery; vaccine development and clinical trials; vaccine registration and last mile delivery, with a focus on vaccines of limited commercial potential for high-burden infectious diseases relevant to low- and middle-income countries. In addition to pre-licensure studies of cholera, chikungunya and schistosomiasis vaccines and post-licensure studies of typhoid and human papilloma virus vaccines, IVI is also currently supporting clinical trials for two COVID-19 vaccines to evaluate safety, tolerability and immunogenicity.
7. Technologies to accelerate vaccine manufacturing and supply globally
T. Prusik (Temptime/Zebra) provided updates on new vaccine vial monitors (VVMs) [47] in support of SARS-COV-2 vaccine delivery, highlighting the importance of vaccine storage and stability in ensuring quality, particularly given the varying challenges current COVID-19 vaccines pose. VVMs for less stable novel vaccine platforms, that require ultra-cold chain, were introduced in addition to new VVM types for short shelf life vaccines that can be stored at 2–8 degrees Celsius. Temptime’s preparedness for VVM supply in the pandemic indicated current demand is approximately 600 million VVMs each year and, while capacity is not a constraint, expansion would require appropriate lead times for scheduling.
J. Cheng (Merck Group) drew attention to the lessons learned from the COVID-19 pandemic, with the shifting paradigm of vaccine process development and manufacturing to address global health challenges. The need to establish multiple platforms to accelerate development was stressed, using the Jenner Institutes’ adenovirus vector technology as a success case study [48]. Next generation manufacturing process concepts were highlighted, illustrating their advantages in scale flexibility and time to market. Emphasis was also put on decentralizing manufacturing and strengthening capabilities to increase the total global vaccine production capacity for pandemic preparedness.
M. Lundgren (Cytiva) discussed the production of viral vector vaccines for pandemic use through a wide range of clinical grade vaccine manufacturing solutions, including adenovirus vector process development. An end-to-end process has been developed and is currently being implemented by manufacturers that are working on adenoviral-based COVID-19 vaccines6 . Recognizing the risk of upfront investment in new facilities, Cytiva’s enterprise solution allows manufacturers to delay capital investments and mitigate risk. A flex factory bio-manufacturing platform for the rapid production of viral vectors was illustrated, offering all the necessary automation that is needed under good manufacturing practices (GMP) and available with short lead times.
T. Kram (Rommelag) described the benefits of Blow-fill-Seal (BFS) technologies for injectable vaccines7 , specifically to lower the cost per dose delivered to GAVI countries. A compact auto-disable device technology was introduced which can be applied to COVID-19 vaccines. The Rommelag biologics site in Switzerland is prepared to conduct clinical and technical trials, in addition to having the capability to be a commercial production site.
T. Xu (Tofflon) focused on smart factory solutions for vaccine manufacturing across upstream, downstream, and fill & finish capabilities. Tofflon’s KUFill® integrated system was shared as an example citing recombinant and inactivated vaccine manufacturing8 . KUFill®, which is cGMP compliant, integrates systems and project management, with modular building interfaces and equipment operating with automation and in isolation.
V. Joseph (Biozeen) introduced an approach to steering COVID-19 vaccine manufacturing dynamics. Leveraging expertise across vaccine technology platforms such as live attenuated vaccines, inactivated vaccines, recombinant/subunit vaccines, in addition to CHO, Vero and insect cell lines Biozeen has optimized production flow and tailored it for COVID-19 vaccines. It has generalized manufacturing approaches for an adaptable universal design of hardware and software through flexibly using existing resources, and optimizing speed and cost to ensure high titres and yields in pilot scale production and supporting scalability for further clinical development.
A. Daruvala (Bioengineering) presented on considerations for adapting existing facilities to changing vaccine development demands. To minimize time and retrofitting costs, one could ideally use an existing cell-culture bioreactor for a cell-based vaccine, and a fermentor for microbial processes. Alternatively, a microbial fermentor can be more easily repurposed for use with cell cultures than the other way around. Key modifications may be required to the aeration, agitation and cleaning systems. Additionally, there are numerous design features that when planning a greenfield plant for multiple products should be considered, including self-modifiable software, a higher motor power input and range, larger aeration capacities, average vessel geometry and flexible interfaces to DSP among others.
M. Kühberger (GEA) described efficient and secure vaccine production using pharmaceutical separators. Self-desludging centrifuges operate with high precision, maximizing yields with only 3–5% of solids in the volume depending on the vaccine. A patented hydro-hermetic feed can result in gentle product handling, higher viability of cells and more efficient clarification. Furthermore, GEA’s laser-welded disk spacers help manufacturers reach the next level of pharmaceutical cleanability.
8. Conclusion
The COVID-19 crisis highlighted once more the importance of vaccines in protecting people against infectious diseases and enabled new pandemic response mechanisms, such as the ACT-A, COVAX Facility and WHO EUL that may become models for future responses to regional epidemics and global pandemics. Reported data on COVID-19 vaccine development emphasized the new pace and magnitude of vaccine development and supply to tackle the current pandemic. It also highlighted the importance of a variety of technology platforms and diversity of vaccine manufacturing capacity across geographies, to mitigate risks and accelerate the global fight against diseases ensuring a healthy future. COVID-19 will be a major focus of the workload of DCVMN members for the next months, or perhaps years, concluded P. Tippoo (Biovac), emphasizing the commitment to working jointly to overcome challenges and accelerate vaccine development, manufacturing and supply. He encouraged DCVMN members to develop more capabilities and to do more through partnerships. Vaccines are firmly recognized as key contribution to a healthy future for all.
Acknowledgments
Acknowledgements
We thank all the DCVMN Members for inspiring presentations and productive discussions (cf. https://www.dcvmn.org/Members-list-412). We are grateful for the generous availability of all speakers who graciously made themselves available for the meeting and contributed to its success. Furthermore, we would like to extend our gratitude to all colleagues who directly or indirectly contributed to the knowledge shared in this report, particularly Ms. S. Villasenor for support throughout the meeting and Dr. M. Dennehy for serving as moderator. We thank corporate partners for supporting DCVMN with unrestricted educational grants: Bioengineering, Biozeen, Cytiva, GEA Group, Gihon, InDevR, Merck Group, Rommelag, Syntegon, Temptime-Zebra Corporation, Tofflon, Univercells and ViroClinics. Some reported activities are funded by the Department of Health and Social Care using UK Aid funding and is managed by the Engineering and Physical Sciences Research Council (EPSRC, grant number: EP/R013764/1, note: the views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health and Social Care). This virtual meeting was partly supported by a grant from the Bill & Melinda Gates Foundation, United States, Grant no. OPP1204376.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Disclaimer: The authors alone are responsible for the statements and expressed in this article, which do not necessarily represent the views, decisions or policies of any mentioned institutions mentioned in this report, or with which the authors are affiliated.
Measles-Mumps-Rubella
CEPI = Coalition for Epidemic Preparedness Innovations, cf. www.cepi.net
References
- 1.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 2.Pagliusi S, et al. Emerging manufacturers’ engagements in the COVID −19 vaccine research, development and supply. Vaccine 2020 Jul; 2238(34):5418–5423. https://doi.org/10.1016/j.vaccine.2020.06.022. [DOI] [PMC free article] [PubMed]
- 3.COVID-19 CORONAVIRUS PANDEMIC updates. https://www.worldometers.info/coronavirus/.
- 4.WHO: The Access to COVID-19 Tools (ACT) Accelerator. Cf. https://www.who.int/initiatives/act-accelerator.
- 5.WHO, Emergency use listing procedure, version 9 January 2020, https://www.who.int/publications/m/item/emergency-use-listing-procedure.
- 6.Gross A, Bott I. How close is a coronavirus vaccine? Financial Times, SEPTEMBER 23 2020. https://www.ft.com/content/e5012891-58da-4a4f-8a05-182adf3ba0e2.
- 7.Pfizer and Biontech conclude phase 3 study of covid-19 vaccine candidate, meeting all primary efficacy endpoints. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine.
- 8.Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy.
- 9.ZD1222 vaccine met primary efficacy endpoint in preventing COVID-19 https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html.
- 10.Sinovac's COVID-19 vaccine induces quick immune response – study. https://www.reuters.com/article/uk-health-coronavirus-sinovac/sinovacs-covid-19-vaccine-induces-quick-immune-response-study-idUKKBN27X35I.
- 11.Sinopharm says may be able to make over 1 billion coronavirus vaccine doses in 2021 https://www.reuters.com/article/health-coronavirus-china-vaccine-int-idUSKBN2750WM.
- 12.Coronavirus, Bharat Biotech’s COVID-19 vaccine Covaxin enters phase-3 trials. https://www.thehindu.com/news/national/coronavirus-bharat-biotechs-covid-19-vaccine-covaxin-enters-phase-3-trials/article33107393.ece.
- 13.Zhu et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet vol. 396, page 479-488, AUGUST 15, 2020. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31605-6/fulltext. [DOI] [PMC free article] [PubMed]
- 14.Johnson & Johnson Initiates Second Global Phase 3 Clinical Trial of its Janssen COVID-19 Vaccine Candidate. https://www.jnj.com/johnson-johnson-initiates-second-global-phase-3-clinical-trial-of-its-janssen-covid-19-vaccine-candidate.
- 15.Indian drugmaker Biological E. to make substance used in J&J's potential COVID-19 vaccine. https://www.reuters.com/article/us-health-coronavirus-india-j-j-idUSKCN2591PI.
- 16.Logunov et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020; (published online Sept 4.) https://doi.org/10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed]
- 17.Calf-Guard Product information attachment (Pfizer) https://media.qcsupply.com/media/product_attachments/attachment_file/5/4/540463_Label.pdf.
- 18.Van Doremalen et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020. https://www.nature.com/articles/s41586-020-2608-y. [DOI] [PMC free article] [PubMed]
- 19.Folegatti PM et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial, The Lancet, Vol 396, August 15, 2020, https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31604-4/fulltext. [DOI] [PMC free article] [PubMed]
- 20.Voyses M et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet, published December 08, 2020. doi:https://doi.org/10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed]
- 21.McKay et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. https://www.nature.com/articles/s41467-020-17409-9. [DOI] [PMC free article] [PubMed]
- 22.Kis, et al. Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol J. 2018 doi: 10.1002/biot.201800376. [DOI] [PubMed] [Google Scholar]
- 23.Toelzer et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science. 2020. 6;370(6517):725–730. doi: 10.1126/science.abd3255. [DOI] [PMC free article] [PubMed]
- 24.Yadaf et al. Remarkable immunogenicity and protective efficacy of BBV152, an inactivated SARS-CoV-2 vaccine in rhesus macaques. Nature Research 2020 https://www.researchsquare.com/article/rs-65715/v1. [DOI] [PMC free article] [PubMed]
- 25.Yadaf et al. Remarkable immunogenicity and protective efficacy of BBV152, an inactivated SARS-CoV-2 vaccine in rhesus macaques. Nature Research 2020 https://www.researchsquare.com/article/rs-65715/v1. [DOI] [PMC free article] [PubMed]
- 26.Gao Q. et al., Development of an inactivated vaccine candidate for SARS-CoV-2, Science, vol 369, issue 6499, pp.77-81, 03 Jul 2020, https://science.sciencemag.org/content/369/6499/77. [DOI] [PMC free article] [PubMed]
- 27.Blankenship K, With J&J's COVID-19 shot on tap, Indian vaccine maker ramps up manufacturing with Akorn deal, Fierce Pharma, August 17, 2020, https://www.fiercepharma.com/manufacturing/j-j-s-covid-19-shot-hand-indian-vaccine-maker-ramps-up-manufacturing-akorn-deal.
- 28.Chiu M, Baylor and Biological E Limited team up for a global COVID-19 vaccine, Baylor College of Medicine, Houston, Texas, August 13, 2020, https://www.bcm.edu/news/baylor-and-biological-e-limited-team-up-for-a-global-covid-19-vaccine.
- 29.May B. New collaboration boosts Serum Institute of India's COVID-19 vaccine production by 100 million doses, BioSpace, Sep 30, 2020, https://www.biospace.com/article/new-collab-boosts-serum-institute-of-india-s-production-of-covid-19-vaccine-doses-by-100-million/.
- 30.Xia S et al. Safety and immunogenicity of an inactivated SARS-CoV-2, BBIBP-CorV: a randomized, double-blind, placebo-controlled, phase 12 trial, The Lancet Infectious Diseases, October 15, 2020, https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30831-8/fulltext. [DOI] [PMC free article] [PubMed]
- 31.Kupferschmidt K. WHO unveils global plan to fairly distribute COVID-19 vaccine, but challenges await, Science, Sept. 21, 2020, https://www.sciencemag.org/news/2020/09/who-unveils-global-plan-fairly-distribute-covid-19-vaccine-challenges-await.
- 32.FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency, Guidance for Industry, Investigators, and Institutional Review Boards USA FDA industry guidance and https://www.fda.gov/media/136238/download.
- 33.Emergency Use Authorization for Vaccines to Prevent COVID-19 Guidance for Industry. October 2020. EUA guidance COVID-19 vaccines: development, evaluation, approval and monitoring. https://www.fda.gov/media/142749/download.
- 34.EMA Conditional marketing authorization. https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/conditional-marketing-authorisation.
- 35.CEPI, COVAX: The vaccines pillar of the access to COVID-19 tools (ACT) Accelerator Structure and Principles, 9 November 2020, https://cepi.net/wp-content/uploads/2020/11/COVAX_the-Vaccines-Pillar-of-the-Access-to-COVID-19-Tools-ACT-Accelerator.pdf
- 36.WHO, Frequently asked questions on regulation of COVID-19 vaccines : Q&As developed by the COVAX Regulatory Advisory Group (RAG) https://www.who.int/publications/m/item/frequently-asked-questions-on-regulation-of-covid-19-vaccines.
- 37.WHO, Considerations for evaluation of COVID-19 vaccines: points to consider for manufacturers of COVID-19 vaccines, Version 24 September 2020, https://www.who.int/publications/m/item/considerations-for-evaluation-of-covid-19-vaccines
- 38.WHO IHR Procedures concerning public health emergencies of international concern (PHEIC). https://www.who.int/ihr/procedures/pheic/en/.
- 39.WHO, Model packaging for COVID-19 vaccines – WHO working position. Version of 04th November 2020 https://www.who.int/teams/regulation-prequalification/eul/covid-19/covid-19-model-packaging.
- 40.Ndomondo-Sigonda, Ambali A. The African medicines regulatory harmonization initiative: rationale and benefits, Clin Pharmacol Ther. 2011 Feb; 89(2): 176-8, https://pubmed.ncbi.nlm.nih.gov/21252936/. [DOI] [PubMed]
- 41.African Union Commission, the Pan-African Parliament, the African Union Development Agency - New Partnership for Africa’s Development (AUDA-NEPAD), WHO, Bill and Melinda Gates Foundation (BMGF), UK DFID and the CHAI.
- 42.Xing, et al. Whole-cell pertussis vaccine potency assays: the Kendrick test and alternative assays. Expert Rev Vacc. 2014;13:1175–1182. doi: 10.1586/14760584.2014.939636. [DOI] [PubMed] [Google Scholar]
- 43.Von Hunolstein C., et al. Potency testing of whole cell pertussis vaccines evaluation of two serological methods for potency testing of whole cell pertussis vaccines. Pharmaeuropa Bio. 2008:7–18. [PubMed] [Google Scholar]
- 44.Peyraud N., Zehrung D., Jarrahian C., Frivold C., Orubu T., Giersing B. Potential use of microarray patches for vaccine delivery in low- and middle- income countries. Vaccine. 2019;37:4427–4434. doi: 10.1016/j.vaccine.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen D., Lorenson T., Bartholomew K., Villadiego S. Can thermostable vaccines help address cold-chain challenges? Results from stakeholder interviews in six low- and middle-income countries. Vaccine. 2016;34:899–904. doi: 10.1016/j.vaccine.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarrett et al. The role of manufacturers in the implementation of global traceability standards in the supply chain to combat vaccine counterfeiting and enhance safety monitoring. Vaccine 2020, available online 13 November 2020 https://www.sciencedirect.com/science/article/pii/S0264410X20314377?dgcid=rss_sd_all. [DOI] [PMC free article] [PubMed]
- 47.WHO: Getting started with vaccine vial monitors. 2002. Available at https://apps.who.int/iris/bitstream/handle/10665/67806/WHO_V-B_02.35_eng.pdf;jsessionid=7656E05A4255F3ED2B9F3DB34BE8F470?sequence=1
- 48.Fedosyuk, et al. Simian adenovirus vector production for early-phase clinical trials: A simple method applicable to multiple serotypes and using entirely disposable product-contact components. Vaccine. 2019;37(47):6951–6961. doi: 10.1016/j.vaccine.2019.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]