Abstract
Background
Men who have sex with men (MSM) are at high risk for human papillomavirus (HPV) infection and HPV-related anal cancer. Although a safe and effective vaccine is available to prevent HPV infection, HPV vaccine uptake among young MSM remains low.
Purpose
This pilot randomized controlled trial tested the acceptability, feasibility, and preliminary efficacy of a text messaging-based HPV vaccination intervention for young sexual minority men.
Methods
In 2018, unvaccinated sexual minority men aged 18–25 years were recruited from Chicago to participate in a 9 month sexual health program called txt2protect. Participants (N = 150) were randomized to the intervention or control condition. Intervention condition messages focused primarily on HPV vaccination, with only a brief mention of other sexual health practices (e.g., condom use and HIV testing), while control condition messages focused on a variety of sexual health practices with only a brief mention of HPV vaccination. Participants received daily text messages for the first 3 weeks and monthly text messages for the remaining ~8 months of the trial. Participants completed surveys at baseline and 3 week and 9 month follow-ups.
Results
Participants reported high satisfaction with the intervention. Although trial retention was high (with over 88% completing the 9 month survey), the study fell short of meeting its recruitment goal. HPV vaccine series initiation was significantly higher among intervention participants (19.4%) compared to control participants (6.6%), odds ratio = 3.43, 95% confidence interval: 1.17, 10.08.
Conclusions
Findings suggest that txt2protect is an acceptable and potentially promising intervention for increasing HPV vaccine initiation among young sexual minority men.
Clinical Trial Registration
Keywords: Human papillomavirus vaccination, Cancer prevention, Men who have sex with men, Mobile health (mHealth) intervention
Sexual minority men who participated in a text messaging intervention were more likely to initiate the HPV vaccine series compared to men in the control group.
Human papillomavirus (HPV) is a sexually transmitted infection that causes genital warts and different types of cancer, including cervical, anal, and oropharyngeal cancer [1]. Men who have sex with men (MSM) are at high risk for HPV infection and HPV-related anal cancer [1, 2]. The incidence of HPV-related anal cancer is estimated to be nearly 20 times higher among MSM relative to heterosexual men and even higher among HIV-positive MSM [3, 4]. HPV vaccination is a highly effective primary prevention strategy for reducing HPV infection and HPV-related cancers [5, 6]. Routine HPV vaccination is currently recommended for all 11 and 12 year old adolescents with “catch-up” vaccination advised for all persons through age 26 who did not receive the vaccine when they were younger [7]. When the present study was conducted, the three-dose HPV vaccine series was specifically recommended for all men through age 26 who identify as gay or bisexual, are attracted to men, or have sex with men [8]. Nevertheless, current estimates indicate that less than 40% of young MSM have received one or more doses of HPV vaccine [9–12]. Low rates of uptake coupled with the high burden of HPV-related disease point to the critical need for effective interventions to increase HPV vaccination among young sexual minority men.
Although promising strategies are beginning to emerge [13–16], few evidence-based interventions promoting HPV vaccination among young sexual minority men are currently available. A recent pilot trial assessing the effects of Outsmart HPV—a web-based intervention designed to increase HPV vaccination among young gay and bisexual men—demonstrated that the intervention was highly acceptable to participants and led to significant increases in HPV vaccine uptake [15, 16]. Other research with young MSM has suggested keen interest in the use of mobile technology for facilitating sexual health and HPV vaccination [13, 14]. Indeed, mobile health (mHealth) interventions may be an especially effective strategy for reaching and engaging young sexual minority populations in preventive health behavior [17–19]. Text messaging-based interventions have been identified as a particularly effective approach for promoting health behavior [20, 21], including vaccination [22]. Furthermore, unlike apps that often require extensive development and testing, interventions delivered via text messaging are relatively low cost, easy to adapt for different subgroups, and, perhaps most importantly, involve a platform that is widely used and accepted by young adults [21, 23, 24].
The purpose of the present study was to test the acceptability, feasibility, and preliminary efficacy of a text messaging-based HPV vaccination intervention for young sexual minority men in a pilot randomized controlled trial (RCT). We used the Information, Motivation and Behavioral Skills (IMB) model [25, 26] to guide intervention development. Furthermore, to ensure that the intervention was tailored to meet the unique needs of young sexual minority men, we conducted extensive formative research with the target population prior to the trial [27, 28]. Young sexual minority men from Chicago were recruited for the RCT in 2018 and were randomly assigned to the intervention or control condition. Text messages in the intervention condition focused primarily on HPV vaccination, with only a brief mention of other sexual health practices (e.g., condom use, pre-exposure prophylaxis [PrEP], and HIV testing), while text messages in the control condition focused on a variety of sexual health practices with only a brief mention of HPV vaccination. We hypothesized that, compared to control arm participants, a higher proportion of intervention arm participants would receive the first dose of the three-dose HPV vaccine series by the end of the trial. We also conducted exploratory mediation analyses to identify potential psychosocial mechanisms underlying intervention effects.
Methods
Participants and Procedure
Participants were invited to enroll in a 9 month text messaging-based sexual health program called “txt2protect” (t2p). Interested participants were directed to an online screening survey that assessed eligibility criteria. To be eligible, individuals had to: (a) be 18–25 years old, (b) be assigned male sex at birth and have a male gender identity, (c) self-identify as gay, bisexual, or queer, be physically attracted to men, or ever have had sex with a man, (d) be able to read and understand English, (e) live in the Chicago area and plan to live there for the next 9 months, (f) be the exclusive owner of a cell phone, (g) have used text messaging for at least 6 months, (h) plan to have the same phone number for the next 9 months, (i) have an unlimited text messaging plan, and (j) have not received any HPV vaccine doses. Using the Illinois Comprehensive Automated Immunization Registry Exchange (I-CARE), we attempted to confirm HPV vaccination status for individuals who reported receiving no doses or did not know their HPV vaccination status; however, not all individuals were found in the registry. Recruitment sources included social media sites (e.g., Facebook, Instagram, and Twitter), online dating applications for MSM, and a local participant registry for sexual minority individuals interested in translational behavioral research [29]. The study was advertised as an online sexual health program tailored for young sexual minority men. Sample recruitment advertisements are provided in Supplementary Figs. 1 and 2. Study enrollment took place from January through early September 2018.
Eligible participants received a text message with the link to the online consent form and baseline survey. After providing consent, participants were randomized using a 1:1 allocation ratio to the intervention or control condition and were blind to arm allocation. Participants received daily text messages for the first 3 weeks of the study (Phase 1) and then transitioned to receiving monthly text messages for the remaining ~8 months of the trial (Phase 2). During Phase 1, participants were sent 10–12 messages per day grouped into three batches (e.g., 3–4 messages delivered around 10:00 am, 2:00 pm, and 6:00 pm). This number of messages was deemed acceptable in previous research with a similar population [30]. During Phase 2, participants received anywhere from five to eight messages on a given day about once per month. To assess change in HPV vaccination status during Phase 2, participants were sent three "pop-up" surveys spread evenly throughout the ~8 month period. For participants in the intervention arm, the pop-up surveys assessed whether they had received any doses of HPV vaccine since starting the trial. For participants in the control arm, the pop-up surveys did not assess HPV vaccination. Rather, participants were asked various questions about the program (e.g., “Since you started t2p, have you accessed the t2p website?”). Participants completed follow-up surveys at 3 weeks (Phase 1 survey) and 9 months (Phase 2 survey). Participants could earn up to $75 in gift cards for completing surveys. If an intervention arm participant reported receiving a dose of HPV vaccine on the Phase 1 survey or any pop-up survey, the participant subsequently received messages encouraging series completion. The university institutional review board approved the study. The trial was registered at ClinicalTrials.gov (NCT02994108).
Intervention and Control Conditions
The intervention was informed by theory [25, 26], existing research [28, 31–35], and extensive input from young sexual minority men. To develop the intervention, we followed a five-step formative approach [30], in which we: (a) conducted qualitative interviews [27] and a survey to inform intervention content, (b) drafted initial messages, (c) refined messages through an iterative structured process using three online “content advisory teams,” (d) tested software functionality and message delivery, and (e) beta tested the Phase 1 protocol with 20 participants randomly assigned to intervention or control. Beta test participants were asked to complete the baseline survey, receive the first 3 weeks of messages, provide weekly feedback, and complete the Phase 1 survey. Young sexual minority men from Chicago who met similar eligibility criteria for the RCT served as participants for steps a, c, and e.
Messages in the intervention and control conditions included similar content but differed in emphasis. Although the intervention condition addressed sexual health practices beyond HPV vaccination, such as condom use, PrEP, and HIV testing, content centered on HPV vaccination and provided in-depth information, motivation, and behavioral skills to foster vaccine uptake. Each week of Phase 1 reflected a different IMB model component. In contrast, the control condition addressed a variety of sexual health practices while providing only basic information about HPV vaccination [30, 36]. See Table 1 for details about message content and structure for each condition, theoretical constructs targeted in the intervention condition, and sample messages. To equate for attention, messages in the intervention and control conditions were similar in number and length. Text messages were supplemented with a supporting website tailored to condition. For example, the intervention condition website included essential information about HPV and contact information for local clinics providing HPV vaccine.
Table 1.
Message content and structure for the txt2protect intervention and control conditions
| Intervention | Control | |
|---|---|---|
| Phase 1: Week 1 | Information: the basics | HIV/STI facts |
| • Basic information about HIV and STIs • Details about HPV vaccination (safety, efficacy, and dosing) • Vaccine recommendation from study team physician • Details about how and where to get the first dose Constructs: Knowledge, Attitudes, Susceptibility, Severity |
• Basic information about HIV and STIs • Prevalence, transmission, symptoms, health consequences, and current treatments |
|
| Sample message | “This program has been designed to help you prevent HPV infection and its health consequences. Tomorrow we’ll talk about steps you can take to protect yourself.” | “Although there are many STIs we could review, we’re going to focus on the five most common STIs: HPV, chlamydia, gonorrhea, genital herpes, and syphilis.” |
| Phase 1: Week 2 | Motivation: overcoming barriers | Prevention and testing |
| • Overcoming common perceived barriers (HPV-related misinformation and lack of provider recommendation) • Norms for HPV vaccination • Reasons young MSM have decided to get vaccinated Constructs: Attitudes, Norms, Susceptibility, Regret |
• Using condoms correctly and overcoming barriers • Vaccines to prevent STIs (Hepatitis A and B and HPV) • Pre-exposure prophylaxis (PrEP) • STI and HIV testing |
|
| Sample message | “The next two days we’ll review reasons some young men haven’t received the HPV vaccine and ways to overcome those barriers.” | “To wrap up Week 2, let’s talk about STI and HIV testing. Getting tested for STIs and HIV is an essential step for maintaining sexual health.” |
| Phase 1: Week 3 | Behavioral skills: next steps | Healthy relationships |
| • Vaccine cost and health insurance issues • List of clinics offering HPV vaccine, search tool for local pharmacies (Health Map Vaccine Finder) [50] • Talking with a provider/parent about the HPV vaccine or one’s sexual identity • Action plan for getting vaccinated [51] Constructs: Perceived behavioral control, Self-efficacy |
• Tips for good communication • Signs of an unhealthy relationship • Meeting both partners’ health, emotional, and sexual needs |
|
| Sample message | “Last week we mentioned you might need to advocate for your own health if a doctor doesn’t raise the issue. So, how can you bring up the HPV vaccine to a doctor?” | “A healthy relationship is one that meets both partners’ health, emotional, and sexual needs. What this means may change over time as you grow as a person.” |
| Phase 2 | Reinforced Phase 1 content to encourage continued program engagement via “booster” messages | |
| Sample message | “If you’ve been wanting to get vaccinated but are having trouble finding the time, consider a walk-in clinic or pharmacy.” | “Taking PrEP daily can reduce risk of getting HIV from sex by more than 90%. Using both PrEP and condoms can provide even greater protection.” |
HPV human papillomavirus; MSM men who have sex with men; STI sexually transmitted infection.
Measures
Participants completed surveys at baseline, end of Phase 1 (3 week follow-up), and end of Phase 2 (9 month follow-up). The baseline survey assessed demographics and relevant background characteristics (e.g., health insurance status and HIV testing history). HPV vaccination was assessed on both the screener and baseline survey to ensure that no one who had previously been vaccinated was accidentally enrolled. The Phase 1 survey assessed initial program acceptability [37, 38], intervention exposure (i.e., number of texts read during Phase 1: 1 = almost none to 6 = all of them), and sexual health behavior in the past 3 weeks (i.e., condom use, HIV testing, PrEP use, and HPV vaccination) [28, 39]. We also assessed several psychosocial constructs that were expected to be affected by the intervention and, thus, could serve as potential mediators of intervention effects. These psychosocial constructs reflected the different components of the IMB model. The information component was represented via HPV-related knowledge [28, 40]. The motivation component was represented by constructs tapping into personal (i.e., attitudes toward HPV vaccination, perceived susceptibility to HPV-related outcomes, and perceived severity of HPV-related outcomes) and social motivation to get vaccinated (e.g., subjective norms for HPV vaccination) [28, 34, 35]. Although it is not a formal construct of the IMB model, we also assessed anticipated regret, as this construct may play an important role in MSM’s decisions to receive the HPV vaccine [28, 33] and has been identified as a potentially important predictor of health behavior [41]. Finally, the behavioral skills component was represented via perceived behavioral control and self-efficacy for HPV vaccination [28, 34, 35]. Attitudes, subjective norms, perceived behavioral control, self-efficacy, and intentions were assessed for each sexual health behavior addressed in the program (i.e., condom use, HIV testing, PrEP use, and HPV vaccination); however, in the current paper, we only report constructs relevant to HPV vaccination. The Phase 2 survey assessed full program acceptability [37, 38], number of texts read throughout the full program, and sexual health behavior in the past 9 months [28, 39].
Primary outcomes were intervention feasibility, acceptability, and preliminary efficacy (i.e., HPV vaccine initiation). Secondary outcomes were psychosocial constructs assessed at 3 week follow-up (e.g., attitudes and self-efficacy). Intervention feasibility was evaluated by recruitment and retention rates, with the goal of 80% retention by the end of the trial. Intervention acceptability was assessed at both 3 week and 9 month follow-ups with 11 and 12 closed-ended items, respectively (e.g., “I learned things in t2p that will help me make good decisions for my health.” 1 = strongly disagree to 5 = strongly agree), and open-ended items (i.e., “What did you like about the t2p program?”) adapted from previous research [37, 38]. After reverse scoring, closed-ended items were combined to create an acceptability score for both 3 week and 9 month follow-ups. Our goal was to achieve acceptability scores ≥4. Responses to the open-ended acceptability questions were coded by two raters for common themes concerning likes and dislikes about the program. Intervention efficacy was evaluated by comparing the number of intervention versus control arm participants who reported receiving at least one dose of HPV vaccine by the end of the trial. Participants who reported receiving a dose of HPV vaccine on the Phase 1 survey or any pop-up survey (intervention condition only) but did not complete the Phase 2 survey were counted as having initiated the series. We attempted to verify all reports of HPV vaccination in I-CARE.
Statistical Analysis
We conducted a power analysis to estimate the required sample size based on two-sided α = .05, 20% attrition, and the hypothesis that 18%–21% of intervention arm versus 6%–8% of control arm participants would receive their first dose of HPV vaccine [21, 42, 43]. The analysis indicated >80% power to detect hypothesized effects by enrolling 230 participants per arm. No interim outcome analyses were performed.
We calculated descriptive statistics for sample characteristics among participants in the intervention and control conditions (Table 2). To assess whether randomization was successful, we used t-tests and chi-square analyses to compare participants across conditions. Recruitment and retention rates are described in a CONSORT flow diagram (Fig. 1). t-tests were used to compare intervention exposure and acceptability scores at 3 week and 9 month follow-ups by condition. Primary themes from the open-ended acceptability questions for intervention participants are reported in Table 3. Interrater reliability was calculated with Cohen’s kappa [44]. Intervention efficacy, as indicated by the receipt of ≥1 dose of HPV vaccine, was assessed with logistic regression for all participants who were randomized and did not withdraw from the study. Using t-tests, we examined the effects of the intervention on psychosocial constructs assessed at 3 week follow-up. Using the PROCESS macro (version 3) [45], we conducted a series of exploratory mediation analyses to examine whether any of these constructs mediated the effect of the intervention on HPV vaccine uptake. Analyses were conducted using SPSS (version 26; IBM Corp., Armonk, NY).
Table 2.
Baseline sample characteristics for participants in the intervention and control condition
| Intervention (n = 72) n (%) |
Control (n = 76) n (%) |
|
|---|---|---|
| Demographic characteristics | ||
| Age (years), M (SD) | 22.78 (2.03) | 23.06 (2.39) |
| Race | ||
| American Indian | 1 (1) | 1 (1) |
| Asian | 4 (6) | 7 (9) |
| Black or African American | 13 (18) | 18 (24) |
| White | 42 (58) | 38 (50) |
| Multiracial | 3 (4) | 5 (7) |
| Unknown | 9 (13) | 7 (9) |
| Latino | ||
| No | 45 (63) | 56 (74) |
| Yes | 27 (38) | 20 (26) |
| Sexual orientation | ||
| Gay | 53 (74) | 57 (75) |
| Bisexual | 17 (24) | 15 (20) |
| Other (e.g., queer and pansexual) | 2 (3) | 4 (5) |
| Education level | ||
| Some high school/high school degree/GED | 23 (32) | 19 (26) |
| Some college or trade school certificate | 27 (37) | 29 (39) |
| College degree | 15 (21) | 16 (21) |
| Some graduate school/ graduate degree | 7 (10) | 11 (15) |
| Annual family income | ||
| <$20,000 | 10 (15) | 15 (21) |
| $20,000–$39,999 | 18 (28) | 17 (24) |
| $40,000–$59,999 | 11 (17) | 13 (18) |
| $60,000–$79,999 | 15 (23) | 10 (14) |
| ≥$80,000 | 11 (17) | 16 (23) |
| Relationship status | ||
| Married/domestic partnership | 2 (3) | 1 (1) |
| Serious relationship | 15 (21) | 23 (30) |
| Casually dating | 20 (28) | 22 (29) |
| Not currently dating | 35 (49) | 30 (40) |
| Location in Chicago | ||
| North side | 36 (50) | 28 (37) |
| West side | 10 (14) | 9 (12) |
| South side | 10 (14) | 14 (18) |
| Suburbs | 16 (22) | 25 (33) |
| Health care and sexual health characteristics | ||
| Health insurance | ||
| None | 11 (16) | 10 (13) |
| Parents’ insurance | 27 (38) | 35 (47) |
| Personal insurance | 33 (47) | 30 (40) |
| Visited health care provider past year | ||
| No | 21 (29) | 25 (33) |
| Yes | 51 (71) | 50 (67) |
| Have primary care provider | ||
| No | 23 (37) | 28 (44) |
| Yes | 40 (64) | 35 (56) |
| Provider recommended HPV vaccine | ||
| No | 51 (72) | 53 (73) |
| Yes | 20 (28) | 20 (27) |
| Ever had anal sex with a man | ||
| No | 7 (10) | 13 (17) |
| Yes | 65 (90) | 63 (83) |
| Ever tested for HIV | ||
| No | 26 (36) | 21 (28) |
| Yes | 46 (64) | 54 (72) |
Percentages may not sum to 100 due to rounding error. t-tests and chi-square analyses were used to compare intervention and control arm participants on sample characteristics. No significant differences were observed.
GED general education development diploma; HPV human papillomavirus; SD standard deviation.
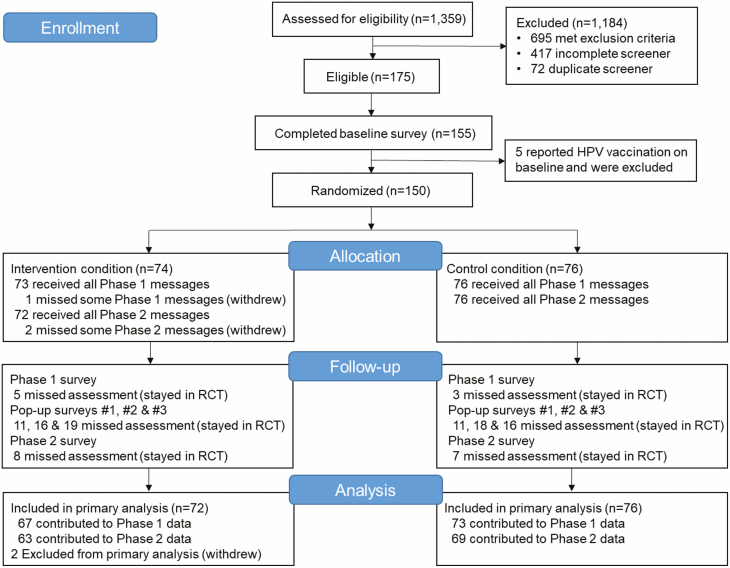
Fig. 1.
Consort flow diagram for the txt2protect pilot randomized controlled trial.
Table 3.
Acceptability of the txt2protect HPV vaccination intervention: themes from open-ended feedback and illustrative quotes
| n (%)a | Illustrative quotes | |
|---|---|---|
| Acceptability at 3 week follow-up | ||
| Likesb | ||
| Informative content | 53 (76) | Lots of useful information that I didn’t know before, brought up sensitive topics in a respectful and appropriate way |
| Convenience | 9 (13) | The convenience of short bursts of information sent to my phone as text messages that I would prioritize reading—as compared to a long article or something, reading a little bit everyday helps out. |
| Message quality | 13 (19) | I really liked the Q&A setup of the texts. They were super informative and I learned a lot. |
| Other | 14 (20) | I like that it provided constant information and reminders. It always had me thinking about the topic. |
| Dislikes/suggestions for improvementc | ||
| Nothing/not applicable | 29 (41) | Nothing |
| Message deliveryd | 34 (49) | When the texts came at a bad time (while I was working or in a meeting) I would forget to review them until then next batch came through. If I could choose when I would receive them it might be easier. |
| Concerns about content | 5 (7) | Some of the info felt like it could’ve been condensed. |
| Other | 3 (4) | Nothing, some might learn better from adding visuals. |
| Acceptability at 9 month follow-up | ||
| Likesb | ||
| Informative content | 46 (70) | I thought t2p was very informative. I learned a lot about all STDs, specifically the statistics surrounding each one. I appreciated the friendly yet objective and nonjudgmental tone of the messages. |
| Convenience | 4 (6) | It was easy to keep up with. |
| Consistency/frequency | 6 (9) | The pace and pacing of it. It’s short and factual knowledge delivered effectively. |
| Other | 21 (32) | I liked that it gave me reminders on when to schedule my HPV vaccines and held me accountable. |
| Dislikes/suggestions for improvementc | ||
| Nothing/not applicable | 22 (33) | I can’t really think of any negatives. |
| Message deliveryd | 30 (46) | It was harder to stay engaged in the program once it switched to messaging every few weeks as opposed to every day. |
| Concerns about content | 3 (5) | Because I am in a monogamous relationship, I felt like some info didn’t apply to me, or assumed that I had multiple sexual partners. While this could’ve applied better to me at other times in my life, it didn’t always feel relevant now. |
| Surveys | 3 (5) | Some of the forms could be tedious. |
| Other | 10 (15) | I would not change much. Maybe just more communication/reminders in the latter half of the project. |
Open-ended responses were provided by 70 participants at 3 week follow-up and 66 participants at 9 month follow-up. The table is limited to responses from intervention condition participants.
aNumber (percentage) of participants in the intervention condition whose comment reflected this theme. Comments were assigned multiple codes as necessary.
bCommon themes in response to the question: “What did you like about t2p?”
cCommon themes in response to the questions: “What did you dislike about t2p? What could be improved?”
dCode reflects concerns or suggestions related to the quantity, frequency, or timing of messages.
Results
Sample characteristics are provided in Table 2. Characteristics did not differ by condition, indicating that randomization was successful. The average age at enrollment was 23 years. The sample included participants from diverse racial (54% White, 21% Black or African American, 7% Asian, and 5% multiracial) and ethnic (32% Latino) backgrounds. About three-quarters of participants identified as gay and only one participant was HIV-positive.
The flow diagram is outlined in Fig.1. Approximately 20% of individuals who completed the screener were eligible for the pilot RCT. Primary reasons for ineligibility included receiving ≥1 dose of HPV vaccine, being outside the age range, and not living in the Chicagoland area. During enrollment, 150 participants were randomly assigned to the intervention (n = 74) or control (n = 76) condition. Three participants withdrew from the RCT, one of whom provided permission to use his data up through his withdrawal at Month 3 and, thus, was included in the primary analysis. Trial retention was high and did not vary by condition at both the 3 week follow-up, 93% (67/72) versus 96% (73/76) in the intervention versus control condition, respectively, Χ2 (1, n = 148) = 0.65, p = .486, and the 9 month follow-up, 88% (63/72) versus 91% (69/76), Χ2 (1, n = 148) = 0.42, p = .601.
Self-reported exposure to intervention content was high and did not differ for intervention and control participants at 3 week (M = 5.27, standard deviation [SD] = 0.81 vs. M = 5.41, SD = 0.90, respectively, t(138) = 0.98, p = .327) or 9 month follow-up (M = 5.00, SD = 1.32 vs. M = 5.10, SD = 1.34, respectively, t(130) = 0.66, p = .663). Mean exposure scores indicated that most participants had read “almost all of the messages.” Mean acceptability scores were also high (>4 on a 5-point scale) and equivalent for intervention and control participants at both 3 week (M = 4.25, SD = 0.44 vs. M = 4.16, SD = 0.49, respectively, t(138) = 1.16, p = .249) and 9 month follow-ups (M = 4.22, SD = 0.51 vs. M = 4.18, SD = 0.50, respectively, t(129) = 0.42, p = .675). Descriptive statistics for the individual acceptability items assessed at 3 week and 9 month follow-ups are reported in Supplementary Table 1.
Interrater reliability for coded responses on what participants liked and disliked about the program ranged from moderate to strong (kappa = .64 to .80), suggesting high consistency across raters. Similar themes were raised by participants across conditions and at roughly the same frequency. Here, we focus specifically on feedback from intervention arm participants. See Table 3 for percentages of participants mentioning each theme and illustrative quotes. Most participants were enthusiastic about Phase 1 content and commented on its personal relevance and usefulness. Participants also liked the quality of the messages (e.g., their clarity and respectful tone) and the general convenience of the program (e.g., receiving small chunks of information via text messaging). When sharing what they disliked or thought could be improved, 40% of participants said “nothing.” Dislikes centered primarily on concerns about the quantity, frequency, or timing of the messages. For example, some participants disliked receiving sensitive content during the middle of the day when they were often at work or school. Likes and dislikes at the end of the program echoed many of the same themes observed at the initial follow-up. Positive aspects of the overall program included its informative content, convenience, and consistency. Negative aspects again centered on message delivery issues, although one third of the participants reported no concerns at the end of the RCT. Several participants voiced concern about the decrease in the quantity and frequency of messages sent during Phase 2 compared to Phase 1 and shared that they would have preferred more even distribution of content throughout the program.
By the end of the 9 month trial, a significantly higher number of participants in the intervention condition initiated the HPV vaccine series (n = 14; 19.4%) compared to participants in the control condition (n = 5; 6.6%). Odds of initiating the series were over three times higher among intervention versus control participants, odds ratio (OR) = 3.43 (95% confidence interval [CI]: 1.17, 10.08), p = .025. Three participants (two in the intervention condition and one in the control condition) reported completing the three-dose series at 9 month follow-up, OR = 1.14 (95% CI: 0.08, 16.95), p = .923.
To identify the components of the txt2protect intervention that may have been responsible for differences in vaccine uptake, we examined the effects of the intervention on psychosocial constructs assessed at 3 week follow-up. Descriptive statistics and t-test results are reported in Table 4. After exposure to Phase 1 of the program, participants in the intervention (vs. control) condition reported more favorable attitudes toward HPV vaccination and higher intentions to receive the HPV vaccine in the next 9 months. Exposure to intervention (vs. control) messages also resulted in higher perceived susceptibility to HPV-related outcomes, higher anticipated regret if one declined HPV vaccination and later developed an HPV-related disease, and higher self-efficacy to get vaccinated for HPV, although these effects were only trending (p < .10). No intervention effects were observed for HPV-specific knowledge, perceived severity, subjective norms, or perceived behavioral control.
Table 4.
Descriptive statistics for psychosocial constructs assessed at 3 week follow-up
| Intervention (n = 67) M (SD) |
Control (n = 73) M (SD) |
p | Cohen’s d | |
|---|---|---|---|---|
| Information | ||||
| HPV-related knowledgea | 1.11 (0.40) | 1.00 (0.50) | .174 | .24 |
| Motivation | ||||
| Attitudesb | 4.51 (0.61) | 4.11 (0.87) | .002 | .53 |
| Subjective normsc | 4.10 (0.78) | 4.11 (0.94) | .964 | −.01 |
| Perceived susceptibilityd | 3.12 (1.00) | 2.78 (1.04) | .053 | .33 |
| Perceived severitye | 4.56 (0.49) | 4.66 (0.44) | .183 | −.21 |
| Anticipated regretf | 4.76 (0.60) | 4.50 (0.99) | .064 | .32 |
| Behavioral skills | ||||
| Perceived behavioral controlg | 4.42 (0.68) | 4.19 (1.07) | .147 | .26 |
| Self-efficacyh | 3.96 (0.98) | 3.67 (1.05) | .096 | .29 |
| Intentionsi | 4.16 (0.86) | 3.75 (1.06) | .013 | .42 |
All items were assessed on a 5-point scale, unless noted otherwise. Composite scores were computed for constructs with multiple items by taking the average of the items. Higher scores indicate higher levels of the construct. Independent samples t-tests were used to compare means by condition.
aAssessed with two questions (e.g., “HPV can cause anal cancer.”) interspersed among other true/false questions assessing HIV/STI knowledge. Participants received 1 point for each correct response and 0 points for incorrect and “do not know” responses. Points were summed to create a composite score that ranged from 0 to 2.
bAssessed with one item: “My attitude toward getting vaccinated for HPV in the next 9 months is...” 1 = very negative to 5 = very positive.
cAssessed with one item: “People who are important to me would want me to get vaccinated for HPV in the next 9 months.” 1 = strongly disagree to 5 = strongly agree.
dAssessed with four items (e.g., “If you do not get the HPV vaccine, what do you think are the chances that you will get HPV?” 1 = very unlikely to 5 = very likely).
eAssessed with four items (e.g., “How serious would each of the following be for you: If you developed warts on your penis or scrotum?” 1 = not at all serious to 5 = very serious).
fAssessed with two items (e.g., “How much regret would you feel if you decided not to get the HPV vaccine and later developed genital or anal warts?” 1 = none to 5 = quite a lot).
gAssessed with one item: “To what extent is whether or not you get vaccinated for HPV in the next 9 months under your control?” 1 = not at all to 5 = completely.
hAssessed with one item: “Assuming you wanted to, how hard or easy would it be for you to get vaccinated for HPV in the next 9 months?” 1 = very hard to 5 = very easy.
iAssessed with one item: “I intend to get vaccinated for HPV in the next 9 months.” 1 = strongly disagree to 5 = strongly agree.
Exploratory analyses examined whether any of the psychosocial constructs assessed at 3 week follow-up mediated effects of the intervention on HPV vaccine uptake. The only construct for which we observed significant support for mediation was behavioral intentions. Participants in the intervention (vs. control) condition reported higher intentions to receive the HPV vaccine at 3 week follow-up (a = .41, Bootstrap 95% CI: 0.09, 0.74) and, in turn, higher intentions were associated with greater likelihood of initiating the series at 9 month follow-up (b = .87, Bootstrap 95% CI: 0.08, 1.66). The bootstrap CI for the indirect effect (ab = .36) of intentions based on 10,000 samples was entirely above 0 (95% CI: 0.03, 1.25). However, it should be noted that the current study may not have been sufficiently powered to adequately test for mediation.
Discussion
Findings suggest that txt2protect is a highly acceptable and potentially promising mHealth intervention for increasing HPV vaccine uptake in young sexual minority men. Intervention arm participants had more than three times the odds of initiating the HPV vaccine series relative to control arm participants. Moreover, participants exposed to the intervention (vs. control) reported higher intentions to receive the HPV vaccine at the initial follow-up, and greater intentions, in turn, were associated with higher rates of initiation at the final follow-up. A strength of the study was its use of a rigorous control condition that included HPV vaccine-related content. Despite the similarity between the two conditions, we were able to detect the effects of the intervention on vaccine uptake. Findings suggest that young sexual minority men are willing to participate in sexual health programs delivered via text messaging and such programs may be an effective method for increasing initiation of the HPV vaccine series.
Results from the acceptability assessment indicated that participants were highly satisfied with the intervention, and levels of satisfaction were similar across treatment arms. The majority of intervention condition participants found program content to be highly informative, practical, and pertinent to their day-to-day lives. At the same time, participants expressed concerns about the volume of information that was delivered (particularly during Phase 1), as well as the frequency and timing of the messages. Based on this feedback, it will be important for future iterations to distribute content more evenly throughout the program and give participants more control over when and how often they receive text messages. To increase engagement, future iterations should also consider incorporating two-way messaging and including more images, videos, and links to additional resources. Once the txt2protect intervention has been further refined and subjected to additional testing, LGBTQ health centers and sexual health clinics might benefit from implementing the program.
Another goal of this study was to assess intervention feasibility as indicated by recruitment and retention. Although retention throughout the 9 month trial was high, we had difficulty reaching our recruitment goal in the allotted enrollment period. New restrictions on social media advertising (e.g., the inability to direct ads to individuals “interested in men” on Facebook) were implemented just prior to the RCT, creating unexpected challenges for recruitment. Additionally, the trial involved numerous eligibility criteria (e.g., have received no doses of HPV vaccine, identify as a sexual minority, be aged 18–25, meet all of the technology requirements, and live in Chicago), which may have hindered recruitment. Lastly, the most common reason for ineligibility was having already initiated or completed the HPV vaccine series. This may reflect recent successful HPV vaccination campaigns targeting the Chicagoland area [46], which have resulted in high rates of HPV vaccination in Chicago, relative to other areas of the country [47].
Although a higher proportion of participants in the intervention condition initiated the HPV vaccine series compared to participants in the control condition, overall initiation rates were relatively modest and lower than a similar pilot RCT that tested a web-based intervention (Outsmart HPV) for increasing HPV vaccine uptake among gay and bisexual men [15, 16]. Although the two RCTs had similar sample sizes and eligibility criteria, Outsmart HPV used a different advertising strategy, which may have affected participant enrollment [29]. Outsmart HPV was explicitly advertised as an HPV vaccination intervention, while txt2protect was advertised as a sexual health program. Thus, it is possible that Outsmart HPV recruited a larger proportion of men who were already considering HPV vaccination. It will be important for future research to assess whether the way an mHealth intervention is advertised affects interest and participation in the program.
Limitations and Directions for Future Research
When interpreting the current findings, it is important to consider study limitations and directions for future research. Although the intervention led to higher vaccine uptake and differences on several putative mediators, behavioral intentions was the only construct for which significant evidence of mediation was found. Null effects may have reflected the lack of statistical power as the study sample size was relatively small. Pinpointing the mechanisms of the intervention is an important goal for future research.
Although the intervention appears to have encouraged the initiation of the HPV vaccine series, a relatively low number of participants completed the three-dose series during the assessment period. The current study was not designed to evaluate series completion and thus included a relatively short follow-up period. Although the recommended dosing schedule specifies the receipt of three doses over a 6 month period [8], research indicates that a significant percentage of patients take longer to complete the series [48]. Thus, because the series takes time to complete, the current study design did not allow for a sufficient evaluation of series completion. Future iterations of the program would benefit from including a longer assessment period to evaluate the effects of the intervention on series completion.
Other study limitations include the fact that HPV vaccination was self-reported and we were not able to verify all reported doses in the immunization registry. Nevertheless, it is important to note that previous research suggests a relatively high accuracy of self-reported HPV vaccination among young adults [49]. Only one HIV-positive participant enrolled in the trial; thus, the extent to which the current findings generalize to HIV-positive sexual minority men is unknown. Finally, as participant recruitment was limited to the Chicago area, the sample may not be representative of young sexual minority men across the USA.
Conclusions
Despite the high burden of HPV infection and relatively low rates of HPV vaccination among MSM, few HPV vaccination interventions have been developed for young sexual minority men. Findings from this pilot RCT suggest that txt2protect is an acceptable and potentially promising mHealth intervention for increasing HPV vaccine initiation among young sexual minority men. Additional research is needed to further refine and strengthen the intervention and identify strategies to expand its reach.
Supplementary Material
Acknowledgements:
This research was supported by the National Cancer Institute of the National Institutes of Health (R21CA208329). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge the support of the Third Coast Center for AIDS Research (P30AI117943) and Northwestern University Clinical and Translational Sciences Institute (UL1TR001422). M.A.G. was at the Department of Medical Social Sciences, Northwestern University, Chicago, Illinois, when portions of this research were conducted. Findings related to this manuscript were presented at the National LGBT Health Conference in Atlanta, GA in May 2019.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions
All authors made substantial contributions to this manuscript. M.A.G. was responsible for study design, intervention development, data analysis and interpretation, and drafting and revising the manuscript. B.M. and G.L.P.II contributed to study design, intervention development, data interpretation, and revising the manuscript. M.H. contributed to study design and intervention development. M.B. led intervention delivery with assistance from K.M. and S.C. and assisted with revising the manuscript. K.M. led participant recruitment and data collection and assisted with intervention development, data management, data coding, and revising the manuscript. S.C. led data management and contributed to data analysis. A.K.K. contributed to data collection and coding.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Moscicki AB, Palefsky JM. Human papillomavirus in men: An update. J Low Genit Tract Dis. 2011;15:231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glick SN, Feng Q, Popov V, Koutsky LA, Golden MR. High rates of incident and prevalent anal human papillomavirus infection among young men who have sex with men. J Infect Dis. 2014;209:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Machalek DA, Grulich AE, Jin F, Templeton DJ, Poynten IM. The epidemiology and natural history of anal human papillomavirus infection in men who have sex with men. Sex Health. 2012;9:527–537. [DOI] [PubMed] [Google Scholar]
- 4. Silverberg MJ, Lau B, Justice AC, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drolet M, Bénard É, Pérez N, Brisson M; HPV Vaccination Impact Study Group . Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet. 2019;394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Markowitz LE, Naleway AL, Lewis RM, et al. Declines in HPV vaccine type prevalence in women screened for cervical cancer in the United States: Evidence of direct and herd effects of vaccination. Vaccine. 2019;37:3918–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: Updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meites E, Kempe A, Markowitz LE. Use of a 2-Dose schedule for human papillomavirus vaccination—Updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–1408. [DOI] [PubMed] [Google Scholar]
- 9. Gorbach PM, Cook R, Gratzer B, et al. Human papillomavirus vaccination among young men who have sex with men and transgender women in 2 US cities, 2012-2014. Sex Transm Dis. 2017;44:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loretan C, Chamberlain AT, Sanchez T, Zlotorzynska M, Jones J. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliver SE, Hoots BE, Paz-Bailey G, et al. Increasing human papillomavirus vaccine coverage among men who have sex with men-national HIV behavioral surveillance, United States, 2014. J Acquir Immune Defic Syndr. 2017;75(suppl 3):S370–S374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srivastav A, O’Halloran A, Lu PJ, Williams WW, Hutchins SS. Vaccination differences among U.S. adults by their self-identified sexual orientation, National Health Interview Survey, 2013-2015. PLoS One. 2019;14:e0213431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fontenot HB, Fantasia HC, Vetters R, Zimet GD. Increasing HPV vaccination and eliminating barriers: Recommendations from young men who have sex with men. Vaccine. 2016;34:6209–6216. [DOI] [PubMed] [Google Scholar]
- 14. Fontenot HB, Rosenberger JG, McNair KT, Mayer KH, Zimet G.. Perspectives and preferences for a mobile health tool designed to facilitate HPV vaccination among young men who have sex with men. Hum Vaccin Immunother. 2019;15:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McRee AL, Shoben A, Bauermeister JA, Katz ML, Paskett ED, Reiter PL. Outsmart HPV: Acceptability and short-term effects of a web-based HPV vaccination intervention for young adult gay and bisexual men. Vaccine. 2018;36:8158–8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reiter PL, Katz ML, Bauermeister JA, Shoben AB, Paskett ED, McRee AL. Increasing human papillomavirus vaccination among young gay and bisexual men: A randomized pilot trial of the outsmart HPV intervention. LGBT Health. 2018;5:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mustanski B, Lyons T, Garcia SC. Internet use and sexual health of young men who have sex with men: A mixed-methods study. Arch Sex Behav. 2011;40:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noar SM, Black HG, Pierce LB. Efficacy of computer technology-based HIV prevention interventions: A meta-analysis. AIDS. 2009;23:107–115. [DOI] [PubMed] [Google Scholar]
- 19. Sullivan PS, Jones J, Kishore N, Stephenson R. The roles of technology in primary HIV prevention for men who have sex with men. Curr HIV/AIDS Rep. 2015;12:481–488. [DOI] [PubMed] [Google Scholar]
- 20. Armanasco AA, Miller YD, Fjeldsoe BS, Marshall AL. Preventive health behavior change text message interventions: A meta-analysis. Am J Prev Med. 2017;52:391–402. [DOI] [PubMed] [Google Scholar]
- 21. Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: A meta-analysis. Soc Sci Med. 2013;97:41–48. [DOI] [PubMed] [Google Scholar]
- 22. Francis DB, Cates JR, Wagner KPG, Zola T, Fitter JE, Coyne-Beasley T. Communication technologies to improve HPV vaccination initiation and completion: A systematic review. Patient Educ Couns. 2017;100:1280–1286. [DOI] [PubMed] [Google Scholar]
- 23. Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: A systematic review of reviews. Annu Rev Public Health. 2015;36:393–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pew Research Center: Texting is nearly universal among young adult cell phone owners. Available at https://www.pewresearch.org/fact-tank/2012/12/14/texting-is-nearly-universal-among-young-adult-cell-phone-owners/. Accessibility verified March 31, 2020.
- 25. Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111:455–474. [DOI] [PubMed] [Google Scholar]
- 26. Fisher WA. Understanding human papillomavirus vaccine uptake. Vaccine. 2012;30(suppl 5):F149–F156. [DOI] [PubMed] [Google Scholar]
- 27. Gerend MA, Madkins K, Crosby S, et al. A Qualitative analysis of young sexual minority men’s perspectives on human papillomavirus vaccination. LGBT Health. 2019;6:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerend MA, Madkins K, Phillips G II, Mustanski B. Predictors of human papillomavirus vaccination among young men who have sex with men. Sex Transm Dis. 2016;43:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reiter PL, Katz ML, Bauermeister JA, Shoben AB, Paskett ED, McRee AL. Recruiting young gay and bisexual men for a human papillomavirus vaccination intervention through social media: The effects of advertisement content. JMIR Public Health Surveill. 2017;3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ybarra ML, Prescott TL, Philips GL II, Bull SS, Parsons JT, Mustanski B. Iteratively developing an mHealth HIV prevention program for sexual minority adolescent men. AIDS Behav. 2016;20:1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Apaydin KZ, Fontenot HB, Shtasel D, et al. Facilitators of and barriers to HPV vaccination among sexual and gender minority patients at a Boston community health center. Vaccine. 2018;36:3868–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nadarzynski T, Smith H, Richardson D, Jones CJ, Llewellyn CD. Human papillomavirus and vaccine-related perceptions among men who have sex with men: A systematic review. Sex Transm Infect. 2014;90:515–523. [DOI] [PubMed] [Google Scholar]
- 33. Reiter PL, McRee AL, Katz ML, Paskett ED. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wheldon CW, Daley EM, Buhi ER, Nyitray AG, Giuliano AR. Health beliefs and attitudes associated with HPV vaccine intention among young gay and bisexual men in the Southeastern United States. Vaccine. 2011;29:8060–8065. [DOI] [PubMed] [Google Scholar]
- 35. Wheldon CW, Daley EM, Walsh-Buhi ER, Baldwin JA, Nyitray AG, Giuliano AR. An integrative theoretical framework for HPV vaccine promotion among male sexual minorities. Am J Mens Health. 2018;12:1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ybarra ML, Prescott TL, Phillips GL, et al. Pilot RCT results of an mHealth HIV prevention program for sexual minority male adolescents. Pediatrics. 2017;140(1):e20162999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mustanski B, Garofalo R, Monahan C, Gratzer B, Andrews R. Feasibility, acceptability, and preliminary efficacy of an online HIV prevention program for diverse young men who have sex with men: The keep it up! intervention. AIDS Behav. 2013;17:2999–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ybarra ML, Prescott T, Mustanski B, Parsons J, Bull SS. Feasibility, acceptability, and process indicators for Guy2Guy, an mHealth HIV prevention program for sexual minority adolescent boys. J Adolesc Health. 2019;65:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mustanski B, Starks T, Newcomb ME. Methods for the design and analysis of relationship and partner effects on sexual health. Arch Sex Behav. 2014;43:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalichman SC, Picciano JF, Roffman RA. Motivation to reduce HIV risk behaviors in the context of the Information, Motivation and Behavioral Skills (IMB) model of HIV prevention. J Health Psychol. 2008;13:680–689. [DOI] [PubMed] [Google Scholar]
- 41. Brewer NT, DeFrank JT, Gilkey MB. Anticipated regret and health behavior: A meta-analysis. Health Psychol. 2016;35:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gerend MA, Shepherd JE. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Ann Behav Med. 2012;44:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stockwell MS, Kharbanda EO, Martinez RA, et al. Text4Health: Impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health. 2012;102:e15–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb). 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 45. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. 2nd ed. New York, NY: The Guilford Press; 2018. [Google Scholar]
- 46. Choi N, Curtis CR, Loharikar A, et al. Successful use of interventions in combination to improve human papillomavirus vaccination coverage rates among adolescents—Chicago, 2013 to 2015. Acad Pediatr. 2018;18:S93–S100. [DOI] [PubMed] [Google Scholar]
- 47. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Widdice LE, Hoagland R, Callahan ST, et al. Caregiver and adolescent factors associated with delayed completion of the three-dose human papillomavirus vaccination series. Vaccine. 2018;36:1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rolnick SJ, Parker ED, Nordin JD, et al. Self-report compared to electronic medical record across eight adult vaccines: Do results vary by demographic factors? Vaccine. 2013;31:3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huston JE, Mekaru SR, Kluberg S, Brownstein JS. Searching the web for influenza vaccines: Healthmap vaccine finder. Am J Public Health. 2015;105:e134–e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.