Abstract
This cross-sectional study examines whether race is associated with differences in 6-year lung cancer risk among patients eligible for US Preventive Services Task Force–recommended lung cancer screening.
Introduction
African American individuals at high risk of lung cancer may experience greater mortality benefit from annual lung cancer screening (LCS) compared with White individuals.1,2 However, African American individuals develop lung cancer with fewer pack-years of smoking and at younger ages than White individuals, and they are less often eligible for LCS when using United States Preventive Services Task Force (USPSTF) criteria.3 One approach to mitigate disparities in LCS eligibility is to use lung cancer risk prediction models to identify the patients with the highest risk.4 The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial modified logistic regression model for lung cancer prediction (PLCOm2012) may improve sensitivity in lung cancer detection, specifically among African American individuals, and potentially reduce underrepresentation in screening cohorts.5 This study aimed to identify differences in PLCOm2012 6-year lung cancer risk among USPSTF-eligible LCS patients.
Methods
The Thomas Jefferson University institutional review board approved this cross-sectional study and granted a waiver of informed consent because of the minimal risk nature of the research. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The Jefferson LCS Program is a centralized program at an urban, academic medical center. Sociodemographic and clinical data from patients screened from January 2018 to September 2020 were extracted from the LCS Registry. This included lung cancer risk using the PLCOm2012 model, calculated at entry into LCS, and diagnosis of screen-detected lung cancer.6 After excluding patients who self-reported races other than African American or White, risk quartiles were generated using median PLCOm2012 risk values for the screening and cancer cohorts. Descriptive statistics, independent t tests, χ2 tests, and Mann-Whitney U tests were performed using a P < .05 significance threshold. Statistical analyses were 2-sided and conducted using SPSS statistical software version 26 (IBM Corp) from October 5 to November 11, 2020.
Results
Among 1276 individuals in the screening cohort, the mean (SD) age was 64.25 (5.81) years, 545 (42.7%) were African American individuals, and 757 (59.3%) were female individuals (Table). There were significant differences in gender distribution (African American: 343 female individuals [62.9%] vs White: 414 female individuals [56.6%]; χ21 = 5.137; P = .02), smoking history (African American: 348 currently smoking [63.9%] vs White: 358 currently smoking [49.0%]; χ21 = 27.967; P < .001), education level (eg, African American: 243 high school graduates [44.6%] and 37 college graduates [6.8%] vs White: 287 high school graduates [39.3%] and 98 college graduates [13.4%]; χ26 = 31.500; P < .001), and insurance (eg, African American beneficiaries: 192 Medicaid or dual eligible [35.2%] and 138 private only [25.3%] vs White beneficiaries: 134 Medicaid or dual eligible [18.3%] and 243 private only [33.2%]; χ25 = 58.992; P < .001) among races. Among the 32 patients with screen-detected lung cancer in the cancer cohort, 14 (44%) were African American.
Table. Baseline Characteristics of African American and White Patients Undergoing Lung Cancer Screening.
| Characteristics | Patients, No. (%) | P value | |
|---|---|---|---|
| 1276 Patients in full sample | African American (n = 545) | White (n = 731) | |
| Age, mean (SD), y | 64.09 (5.70) | 64.37 (5.89) | .40 |
| Gender | |||
| Female | 343 (62.9) | 414 (56.6) | .02 |
| Male | 202 (37.1) | 317 (43.4) | |
| Ethnicity | |||
| Hispanic/Latinx | 1 (0.2) | 48 (6.6) | <.001 |
| Smoking status | |||
| Current | 348 (63.9) | 358 (49.0) | <.001 |
| Former | 197 (36.1) | 373 (51.0) | |
| Pack-years, mean (SD), y | 48.71 (20.56) | 57.95 (27.51) | <.001 |
| Cigarettes, mean (SD) | 21.62 (8.45) | 25.89 (11.79) | <.001 |
| Smoking duration, mean (SD), y | 45.25 (7.15) | 44.81 (8.18) | .304 |
| Time since quitting, mean (SD), y | 5.16 (4.97) | 6.69 (5.45) | .001 |
| Time smoked, mean (SD), y | 45.25 (7.15) | 44.81 (8.18) | .30 |
| Personal history of cancer | 83 (15.6) | 161 (22.6) | .002 |
| Family history of lung cancer | 123 (22.6) | 167 (22.8) | .91 |
| COPD | 241 (44.2) | 292 (39.9) | .13 |
| Body mass index, mean (SD)a | 30.01 (7.27) | 29.11 (6.42) | .02 |
| Education | |||
| Less than high school diploma | 77 (14.1) | 85 (11.6) | <.001 |
| High school graduate | 243 (44.6) | 287 (39.3) | |
| Post high school training | 19 (3.5) | 28 (3.8) | |
| Some college | 113 (20.7) | 119 (16.3) | |
| College graduate | 37 (6.8) | 98 (13.4) | |
| Post graduate/professional degree | 19 (3.5) | 60 (8.2) | |
| Unknown | 37 (6.8) | 54 (7.4) | |
| Insurance status | |||
| Medicare only | 174 (31.9) | 240 (32.8) | <.001 |
| Medicaid/dual eligible | 192 (35.2) | 134 (18.3) | |
| Private only | 138 (25.3) | 243 (33.2) | |
| Medicare with supplement | 26 (4.8) | 69 (9.4) | |
| Private with supplement | 10 (1.8) | 24 (3.3) | |
| Other/none | 5 (0.9) | 21 (2.9) | |
| 32 Patients with screen-detected lung cancer | |||
| Characteristics | African American (n = 14) | White (n = 18) | |
| Age, mean (SD) | 64.86 (5.60) | 66.44 (5.87) | NA |
| Gender | |||
| Female | 12 (85.7) | 11 (61.1) | NA |
| Male | 2 (14.3) | 7 (38.9) | NA |
| Histology | NA | ||
| Adenocarcinoma | 11 (78.6) | 10 (55.6) | NA |
| Squamous cell carcinoma | 1 (7.1) | 3 (16.7) | NA |
| Small cell carcinoma | 0 (0.0) | 3 (16.7) | NA |
| Neuroendocrine carcinoma | 0 (0.0) | 1 (5.6) | NA |
| Non-small cell carcinoma | 2 (14.3) | 1 (5.6) | NA |
| Stage | |||
| I | 10 (71.4) | 9 (50.0) | NA |
| II | 1 (7.1) | 0 (0.0) | NA |
| III | 2 (14.3) | 4 (22.2) | NA |
| IV | 1 (7.1) | 2 (11.1) | NA |
| Limited stage small cell | 0 | 1 (5.6) | NA |
| Extensive stage small cell | 0 | 2 (11.1) | NA |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
In the screening cohort (median PLCOm2012 risk of lung cancer: 4.75%; interquartile range [IQR], 2.63%-8.47%), African American individuals had a significantly higher median risk of lung cancer compared with White individuals (median PLCOm2012 risk of lung cancer among African American individuals: 5.81%; IQR, 3.42%-9.79% vs White individuals: 4.10%; IQR, 2.14%-7.26%; P < .001) (Figure). When comparing among risk quartiles, 326 of African American individuals (59.8%) undergoing screening had a risk score in quartiles 3 and 4 compared with 311 White individuals (42.5%). However, in the cancer cohort (median PLCOm2012 risk: 7.23%; IQR, 2.37%-12.64%), White individuals had a higher median risk compared with African Americans (median PLCOm2012 risk of lung cancer among White individuals: 10.55%; IQR, 2.21%-16.16% vs African American individuals: 5.87%; IQR, 2.89%-7.88%; P = .18), but this result did not reach statistical significance. Moreover, 11 White individuals (61.1%) with screen-detected lung cancer had a risk score in quartiles 3 and 4, compared with just 5 African American individuals (35.7%).
Figure. Six-Year Lung Cancer Risk Among the Screening and Cancer Cohorts by Risk Quartile.
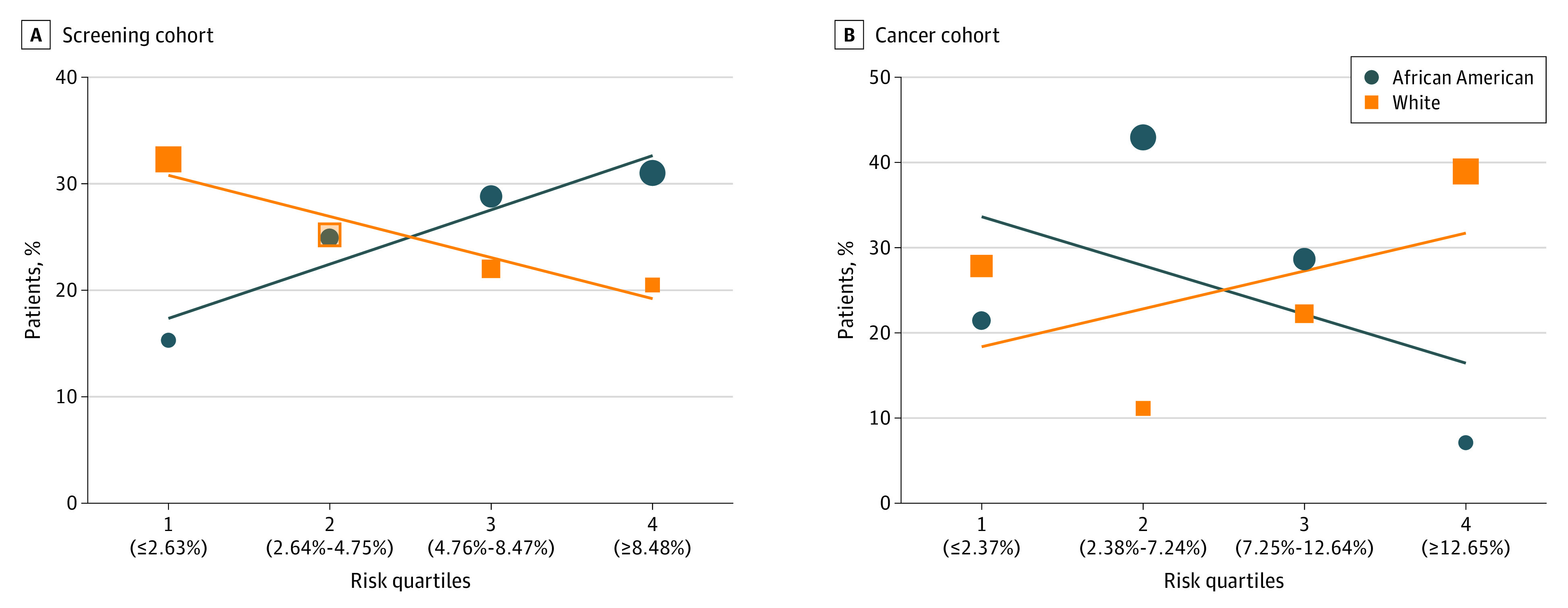
A, Percentage of patients who underwent lung cancer screening in each risk quartile by race. African American and White individuals have overlapping percentages, with 24.9% and 25.2% of each cohort in risk quartile 2. B, Percentage of patients who had a screen-detected lung cancer in each risk quartile by race. The size of each data icon is proportional to the number of patients in each risk quartile. Risk quartiles are determined by median PLCOm2012 risk values (with cutoffs shown in parentheses along the x-axis).
Discussion
To our knowledge, this study is the first to measure PLCOm2012 lung cancer risk among screened individuals and include more than 40% African American participants. We found that lung cancer risk scores were not aligned with lung cancer diagnoses in African American patients. In fact, African American individuals with screen-detected lung cancers were clustered predominantly in the lower risk quartiles. However, among White individuals undergoing screening, higher risk scores were associated with lung cancer diagnoses.
Our findings suggest that we should use caution in applying risk models to diverse populations, given that our current understanding of lung cancer risk is incomplete. Existing models have been derived from screening trials including 5% or fewer African American individuals and may not apply equitably to real-world screening participants.2,4 Although our study is limited by its single-center approach and short duration of follow-up, the racially diverse patient population allowed us to identify weaknesses in risk calculation. Determinants of health, including social constructs and environmental factors, may be critical moderators of lung cancer risk among underserved populations. Further research on comprehensive risk prediction for underrepresented racial and ethnic populations should prioritize diversity and focus on additional factors related to socioeconomic status, geographic variables, the environment, and exposure history.
References
- 1.Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the national lung screening trial. implications for widespread implementation. Am J Respir Crit Care Med. 2015;192(2):200-208. doi: 10.1164/rccm.201502-0259OC [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi: 10.1001/jamaoncol.2019.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300-2311. doi: 10.1001/jama.2016.6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. doi: 10.1016/j.jtho.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728-736. doi: 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]


