Abstract
The T Cell Project was the largest prospective trial to explore the incidence, treatment patterns, and outcomes for T cell lymphomas. The rare subtypes of T cell lymphomas, including hepatosplenic T cell lymphoma (HSTCL), enteropathy associated T cell lymphoma (EATL), and peripheral gamma delta T cell lymphomas (PGDTCLs) are poorly represented in most studies and there is little data regarding treatment patterns. We report results from 115 patients with hepatosplenic (n = 31), enteropathy associated (n = 65), and PGDTCLs (n = 19). While anthracycline regimens were most commonly used as first line therapy, response rates ranged from 20%−40% and were suboptimal for all groups. Autologous stem cell transplantation was performed as a consolidation in first remission in a small number of patients (33% of HSTCL, 7% of EATL, and 12% of PGDTCL), and four patients with HSTCL underwent allogeneic stem cell transplantation in first remission. The progression free survival at 3 years ranged from 28%−40% for these rare subtypes, and the overall survival at 3 years was most favorable for PGDTCL (70%). These data highlight the need for novel treatment approaches for rare subtypes of T cell lymphomas and for their inclusion in clinical trials.
1 |. INTRODUCTION
Current treatment strategies for mature or peripheral T-cell lymphomas (PTCL) patients are largely unsatisfactory and outcomes for patients with rare subtypes have not been well described. The International T Cell Lymphoma Project (ITCP) reported results from retrospective study of 1314 cases of T cell lymphomas from 22 worldwide centers. There, tissue biopsies and clinical information were reviewed, and this was the first comprehensive study of the incidence and outcomes for patients with common and rare subtypes of T cell lymphoma.1 In this review of patients from Asia, Europe, and North America, the incidence of hepatosplenic T cell lymphoma (HSTCL) was 1.4% and enteropathy associated T cell lymphoma (EATL) was 4.7%. The peripheral gamma delta T cell lymphomas (PGDTC)s were not identified as a unique category, and therefore not reported in the ITCP.
More recently, the T Cell Project (TCProject) initiated the first prospective world-wide study of patients with aggressive T cell lymphomas to determine incidence, treatment patterns, outcomes, and prognostic factors.2 Of 1553 patients registered by 74 institutions world-wide in the T cell Project, baseline data, information on first line treatment, response to initial therapy, time to relapse and salvage treatment were available. Here we review clinical features and treatment outcomes for pts with rare subtypes of T cell lymphoma, including HSTCL, EATL, and PGDTCL (PGDT) from this prospective database.
2 |. METHODS
The T Cell Project (NCT01142674) began in September 2006 as a prospective registry of patients with PTCL-NOS, angioimmunoblastic T cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL) and all of the rarer subtypes of nodal and extranodal aggressive histologies of PTCL.
Data collection was accomplished via electronic case report forms using a dedicated website (www.tcellproject2.org) with adoption of the proper technology to ensure protection of the data of individual subjects in web communications.
The study was conducted in compliance with the Helsinki Declaration, was approved by the appropriate research Ethics Committees/Institutional Review Boards and required each patient to consent in written prior to registration. Most of the cases from the T Cell Project (NCT01110733) underwent a central review of initial diagnosis, as per protocol.
For this analysis, we searched the database for patients with the diagnoses of EATL, HSTCL, and PGDTCL and identified 115 cases who met the criteria: HSTCL (N = 31), EATL (N = 65), and PGDTCL (N = 19). Central pathology review confirmed the diagnosis in all cases. Data for prognostic factors, initial and salvage therapies, and outcomes (OS, PFS) were extracted and are reported. Data were analyzed using Fisher’s exact test to identify associations between categorical variables. Two-tailed P values <.05 were considered statistically significant. The OS and the PFS distributions were calculated using the Kaplan-Meier method and time-to-event distributions were compared using the log-rank test (univariate regression).
3 |. RESULTS
Of 1695 cases entered in the T cell Project database, 38 were excluded based on central review, 95 were excluded for missing consent, eight were excluded for local diagnosis review, one was excluded for being in relapse, and 1553 were available for this review. As shown in Table 1, the overall incidence of HSTCL was 2% (31 cases), EATL was 4.2% (65 cases), and PGDTCL was 1.2% (19 cases) for a total of 115 cases identified. Of EATL,39% were from Europe, while HSTCL was slightly more prevalent in the USA (44%) vs Europe (25%). PGDTCL was found in USA and Europe, with no cases in South America and two cases in Asia. The median age was 52 (range 18–86). The majority of patients were under age 60.
TABLE 1.
Distribution of patients in T Cell Project
| N | % | |
|---|---|---|
| PTCL-NOS | 549 | 35.4 |
| AITL | 283 | 18 |
| ALCL, ALK− | 235 | 15.1 |
| ALCL, ALK+ | 131 | 8.4 |
| NKTCL | 166 | 11 |
| Hepatosplenic | 31 | 2.0 |
| Enteropathy-type | 65 | 4.2 |
| Subcutaneous panniculitis-like | 24 | 1.5 |
| Peripheral gamma-delta | 19 | 1.2 |
| Unclassifiable T-cell | 50 | 3.2 |
| Total | 1553 | 100.0 |
Prognostic factors for each disease entity are shown in Table 2. For patients with HSTCL, half were over age 50, 58% were stage IV disease and only 15% had bone marrow involvement. For patients with EATL, only 4% were over age 60, and the majority had high IPI and PIT scores. Patients with PGDTCL had a low incidence of bone marrow involvement (20%) and, interestingly, most had a high PIT score. Note, LDH was elevated in patients with EATL, and not elevated in most patients with HSTCL or PGDTCL.
TABLE 2.
Prognostic factors by disease
| Peripheral Gamma Delta (n = 19) | Hepatosplenic (N = 31) | Enteropathy type (N = 65) | P value | |
|---|---|---|---|---|
| Age >60 | 6 (33%) | 16 (51%) | 3 (4%) | |
| B symptoms | 8 (44%) | 9 (30%) | 50 (78%) | .032 |
| Stage III-IV | 16 (83%) | 18 (58%) | 65 (100%) | .082 |
| BM involvement | 4 (20%) | 5 (15%) | 56 (86%) | .025 |
| IPI intermediate/high | 6 (33%) | 19 (60%) | 65 (100%) | .027 |
| PIT intermediate/high | 14 (75%) | 12 (38%) | 47 (72%) | .67 |
| LDH elevated | 5 (28%) | 7 (24%) | 52 (80%) | .33 |
Treatment regimens were determined by the treating physician according to local or institutional standards. Of the 115 patients with locked treatment records analyzed in this dataset, therapy data was available on 93 (24 with HSTCL, 53 with EATL, 16 with PCGDT), Table 3. Anthracycline based regimens were used in most patients with EATL (97%) and peripheral gamma delta lymphoma (100%), but only in 60% of patients with HSTCL. Overall response rates to front line chemotherapy were suboptimal for all groups. Complete responses were observed in only 25% of patients with PGDTCL, and in 40% and 30% of hepatosplenic T cell and EATL respectively.
TABLE 3.
First line therapy and outcomes
| Peripheral Gamma Delta (n = 19) | Hepatosplenic (N = 31) | Enteropathy type (N = 65) | |
|---|---|---|---|
| Chemo with anthracycline | 100% | 60% | 97% |
| Chemo without anthracycline | 0 | 40% | 3% |
| CR/CRu to first line therapy | 25% | 40% | 30% |
| Auto consolidation therapy | 12.5% | 33% | 7.5% |
| OS median | 47 months | 13 months | 11 months |
| 3-year OS | 72% | 40% | 30% |
| PFS median | 14 months; 95% CI 6–21 | 11 months; 95% CI 8–14 | 7 months; 95% CI 4–10 |
| Three-year PFS | 33% | 40% | 28% |
Nine patients had a stem cell transplant in first remission as a consolidation. Autologous stem cell transplantation was used as a consolidation in one patient with PGDTCL, three with EATL, and one with HSTCL. Four patients with HSTCL had allogeneic stem cell transplant in first remission. Eleven patients had a stem cell transplant in the salvage setting (three HSTCL, seven EATL, one PGDTCL).
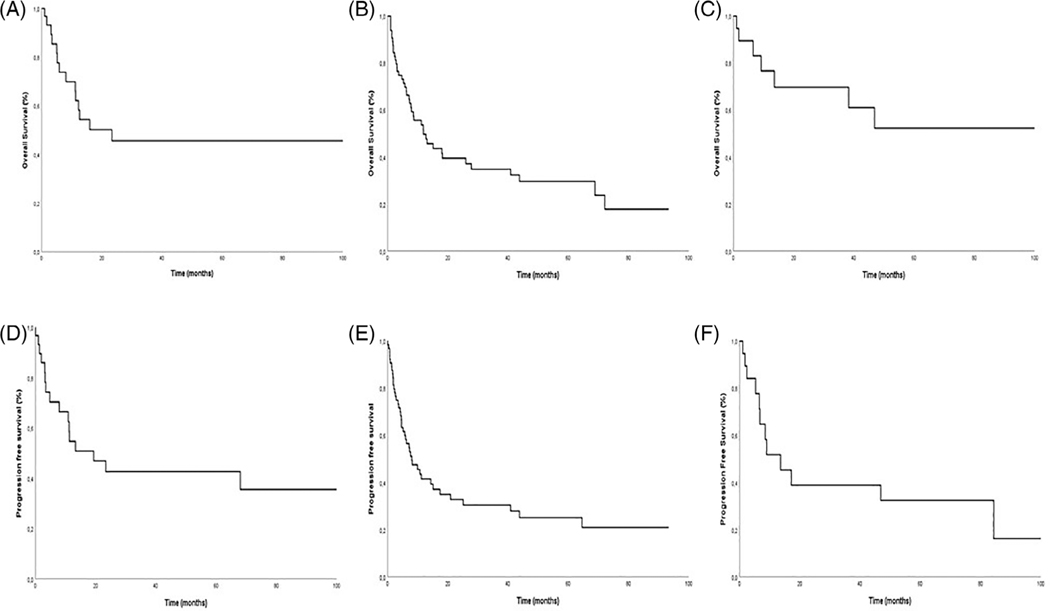
As shown in Figure 1, the median follow-up was 32 months for EATL, 64 months for HSTCL, and 77 months for PGDTCL. The median survival and progression free survival are shown in Table 3. Patients with PGDTCL had an overall favorable outcome, with a median survival not reached, 5-year OS of 51%, and median PFS of 14 months (95% CI 6–21). This is despite the fact that few of these patients underwent stem cell transplant. Patients with HSTCL had a median OS of 13 months with a 5-year OS of 40%, and a median PFS of 11 months (95% CI 8–14). Patients with EATL had a median OS of 12 months, 5-year OS of 30%, and a median PFS of only 7 months (95% CI 4–10).
FIGURE 1.
Survival and progression free survival. Survival and PFS for HSTCL (A,D), Enteropathy associated T cell lymphoma (B,E), and PGDTCL (C,F)
4 |. DISCUSSION
Hepatosplenic T cell lymphoma, EATL, and PGDTCLs are rare subtypes of aggressive T cell lymphomas and are under-represented in clinical trials, so there is little data available describing treatment approaches and outcomes in a real-world setting. In this cohort of patients from the TCP registry, outcomes using physician guided treatment regimens are described. When compared to retrospective data from the International T cell Project (ITCP), which represented a similar geographic distribution of cases, this prospective study showed that the incidence of these rare subtypes was similar. The incidence of EATL was 4.7% in ITCP and 4.3% in our TCP, and HSTCL was 1.4% in ITCP and 1.8% in TCP.1
When outcomes are compared between the retrospective ITCP data and our prospective data in TCP, the 5-year OS are similar or slightly improved for EATL (20% vs 30% respectively). However, they are better for HSTCLs (7% vs 40%), perhaps due to the recent increased use of stem cell transplantation as a consolidation in front line, or in the salvage setting. Similar to the ITCP, most of the patients in the current TCP registry were treated with anthracycline based chemotherapy regimens in the front line.
While most of our enteropathy associated T cell lymphoma EATL patients received anthracycline based therapy, a non- anthracycline approach, the Newcastle regimen (ifosfamide, etoposide, epirubicin alternating with intermediate dose methotrextate, followed by autologous stem cell transplant), has shown improved outcomes compared to CHOP, with a 5 year PFS of 52% and OS of 60%.3,4 The Nordic Lymphoma group has recently reported outcomes with CHOEP followed by ASCT with a 3 year PFS of 52% and OS of 47%. Of our 65 patients with EATL in TCP, only 30% responded to their front line chemotherapy, all had anthracycline based regimens, and only three had ASCT in first remission. Seven EATL patients underwent stem cell transplant after salvage therapy, two with allo and five with autoBMT. One allo and three auto patients have relapsed. Outcomes for the EATL patients reported here were inferior to those reported in the Newcastle study and support aggressive utilization of consolidation autologous stem cell transplantation in the first remission in appropriate patients.
For patients with HSTCL, there is no standard of care. Outcomes are poor with conventional chemotherapy approaches.5–8 Purine analogs have demonstrated activity and consolidation with allogeneic stem cell transplant has been curative in up to 41% of patients after a CHOP like regimen. In our TCP cohort, 60% of patients received an anthracycline regimen as initial therapy, 40% has a CR, and 33% went to transplant (one auto, four allo). An additional three patients had allotransplant as a salvage. The more favorable results for the hepatosplenic patients in TCP compared to those in retrospective series may be related to a wider use of non-anthracycline regimens in 40% and the success of allogeneic stem cell transplant as a consolidation in first remission or in a salvage situation.
PGDTCL is a disease of activated cytotoxic gamma-delta T cells which has a higher incidence in patients with autoimmune disorders.9 The paucity of data regarding treatment and outcomes is related to the fact that many series describing outcomes in this entity include both subcutaneous panniculitis-like T-cell lymphoma of alpha/beta phenotype, along with cases of PCGDTCL of the skin. The CHOP based regimens have been reported in many series, and stem cell transplantation has been shown to be associated with long term remissions in selected patients.10 Remissions are short-lived for patients who do not undergo consolidation therapy.11 In our patients from the TCP, the CR rate was low with anthracycline based chemotherapy, and progression free survival was 14 months. Due to the small number of patients in our study, it is difficult to compare these results to other series.
Overall, while initial therapeutic approaches with CHOP based or other anthracycline regimens is most frequently implemented for most patients with aggressive T cell lymphomas, these results from the TCP demonstrate that the CR rates for these rare subtypes are lower than those for nodal T cell lymphomas. In addition, while NCCN guidelines suggest that HDT be considered for consolidation in first remission for most aggressive T cell lymphomas except for ALK+ anaplastic large cell lymphomas, only a proportion of patients undergo HDT after initial therapy. In a report from the COMPLETE registry, a prospective outcomes registry of patients with aggressive T cell lymphomas in the United States, Park et al report that of 119 patients with nodal T cell lymphomas who achieved a CR to front line therapy, only 36 (30%) underwent consolidation ASCT.12 The use of autologous or allogeneic stem cell transplantation in HSTCLs and PGDTCLs has not been well defined in large clinical trials as it has been in the EATLs. A recent report from the American Society for Blood and Marrow Transplantation representing consensus opinion has recommended that transplant be considered in these rare subtypes in first remission 13
In summary, these data from the TCP highlight the relevance of registries for rare diseases. Information regarding treatment patterns for these disorders reveals that approaches are not standardized, especially with respect to the use of high dose therapy and transplant as a consolidation strategy. Further, adoption of standard anthracycline based chemotherapy regimens for most of the patients in TCP as in the retrospective ITCL project has not improved outcomes. This highlights the need to incorporate novel agents in the front line for these rare subtypes. Inclusion of rare subtypes with historically inferior outcomes as exploratory subsets in trials exploring novel agents should be encouraged.
Acknowledgments
Funding information
Allos pharmaceuticals; Associazione Angela Serra per la Ricerca sul Cancro; Associazione Italiana per la Ricerca sul Cancro; Fondazione Cassa di Risparmio di Modena; Fondazione Italiana Linfomi; NCI CCSG P30 CA008748; Spectrum Pharmaceuticals
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 2.Bellei M, Nabhan C, Pesce EA, et al. The value and relevance of the T cell lymphoma registries and International collaborations: the case of COMPLETE and the T-cell project. Curr Hematol Malig Rep. 2015; 10(4):448–455. [DOI] [PubMed] [Google Scholar]
- 3.Sieniawski M, Angamuthu N, Boyd K, et al. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood. 2010;115(18):3664–3670. [DOI] [PubMed] [Google Scholar]
- 4.Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep. 2011;6(4):231–240. [DOI] [PubMed] [Google Scholar]
- 5.Gowda L, Foss F. Hepatosplenic T-cell lymphomas. Cancer Treat Res. 2019;176:185–193. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan M, Lunning M. Hepatosplenic gamma-delta T-Cell Lymphoma: who is on your speed dial? J Oncol Pract. 2019;15(6):307–312. [DOI] [PubMed] [Google Scholar]
- 7.Yabe M, Miranda RN, Medeiros LJ. Hepatosplenic T-cell lymphoma: a review of clinicopathologic features, pathogenesis, and prognostic factors. Hum Pathol. 2018;74:5–16. [DOI] [PubMed] [Google Scholar]
- 8.Visnyei K, Grossbard ML, Shapira I. Hepatosplenic gammadelta T-cell lymphoma: an overview. Clin Lymphoma Myeloma Leuk. 2013;13(4): 360–369. [DOI] [PubMed] [Google Scholar]
- 9.Foppoli M, Ferreri AJ. Gamma-delta t-cell lymphomas. Eur J Haematol. 2015;94(3):206–218. [DOI] [PubMed] [Google Scholar]
- 10.Gibson JF, Alpdogan O, Subtil A, et al. Hematopoietic stem cell transplantation for primary cutaneous gammadelta T-cell lymphoma and refractory subcutaneous panniculitis-like T-cell lymphoma. J Am Acad Dermatol. 2015;72(6):1010–1015.e5. [DOI] [PubMed] [Google Scholar]
- 11.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101(9):3407–3412. [DOI] [PubMed] [Google Scholar]
- 12.Park SI, Horwitz SM, Foss FM, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019;125(9):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical practice recommendations on indication and timing of hematopoietic cell transplantation in mature T cell and NK/T cell lymphomas: an International collaborative effort on behalf of the guidelines committee of the american society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2017;23(11):1826–1838. [DOI] [PubMed] [Google Scholar]