Abstract
Melatonin, an emphatic endogenous molecule exerts protective effects either via activation of G-protein coupled receptors (Melatonin receptors, MTR 1–3), tumor necrosis factor receptor (TNFR), toll like receptors (TLRS), nuclear receptors (NRS) or by directly scavenging the free radicals. MTRs are extensively expressed in the heart as well as in the coronary vasculature. Accumulating evidences have indicated the existence of a strong correlation between reduction in the circulating level of melatonin and precipitation of heart attack. Apparently, melatonin exhibits cardioprotective effects via modulating inextricably interlinked pathways including modulation of mitochondrial metabolism, mitochondrial permeability transition pore formation, nitric oxide release, autophagy, generation of inflammatory cytokines, regulation of calcium transporters, reactive oxygen species, glycosaminoglycans, collagen accumulation, and regulation of apoptosis. Convincingly, this review shall describe the various signaling pathways involved in salvaging the heart against ischemia-reperfusion injury.
Keywords: Melatonin, Heart, Cardiovascular, Ischemia-reperfusion injury, Anti-oxidant
1. Introduction
Cardiovascular disorders (CVDs) are the leading cause of mortality among various individuals across the globe (Mc Namara and Jackson, 2019). Although, various revascularization techniques have come into existence to tackle conditions like infarction, yet most of the interventions themselves predispose the individuals to myocardial reperfusion injury. Collectively, prolonged ischemia in situations identical to infarction and thereafter reperfusion result in ischemia-reperfusion injury (IRI) (Han et al., 2019). Hence, the development of an emphatic treatment strategy for alleviating cardiovascular disorders is the need of the hour. Apparently, the exogenous delivery of drugs has somehow achieved the desired effects but also have a lot of shortcomings. For instance, the delivery of angiotensin-converting enzyme inhibitors/bradykinin in patients undergoing cardiac surgery may increase the incidence of adverse events including hypotension or cough in the patients (Rouleau et al., 2008; Wei et al., 2004). Therefore, to overcome the current situation it is essential to test the substances that exist endogenously in our system. Among them, melatonin has drawn considerable attention due to its innate anti-oxidant potential (Mukherjee et al., 2010; Yang et al., 2019). In fact, melatonin is undergoing various clinical trials for alleviating cardiac surgery-induced IRI (Gogenur et al., 2014; Green et al., 2014; Shafiei et al., 2018).
Melatonin is a neurohormone that plays predominant role in maintaining circadian rhythm (CR) in the body (Lochner et al., 2018). A plethora of studies have shown its anti-oxidant activity (Mukherjee et al., 2015; Yang et al., 2019; Aslan et al., 2020) and is also known to mitigate IRI in a variety of organs including heart, brain, liver, kidney (Yeung et al., 2015; Yu et al., 2017b; Aslan et al., 2020), lungs, intestine (Lochner et al., 2018). Cumulating preclinical and clinical evidence has indicated the protective role of melatonin in modulating hypertension (Simko et al., 2018), pulmonary hypertension, heart failure (Hung et al., 2017; Wang et al., 2018), atherosclerosis (Dominguez-Rodriguez et al., 2005), arrhythmia and IRI (Shafiei et al., 2018), (Aslan et al., 2020). In fact, the melatonin-dependent cardioprotective effects against functional disorders including arrhythmia are possibly mediated due to its ability to modulate oxidative stress (Szarszoi et al., 2001; Dobsak et al., 2003; Diez et al., 2009) as free radicals significantly contribute to the precipitation of reperfusion-induced arrhythmias (Vazan et al., 2005). Melatonin is known to exhibit cytoprotective effects either via activation of G-protein coupled membrane receptors (GPRCs), (Han et al., 2019), tumor necrosis factor receptor (TNFR), toll like receptors (TLRs) (Nduhirabandi et al., 2016) or nuclear retinoic acid receptor-related orphan receptors (RORs) (He et al., 2016). Apart from this, melatonin can also exert receptor independent effects due to its innate ability to scavenge free radicals (Mukherjee et al., 2010; Yang et al., 2019). Our review shall describe the role of melatonin in reducing cardiac IRI and the possible signaling pathways involved in eliciting melatonin-dependent cardioprotection.
1.1. Physiological role of melatonin in the heart
The CR of an individual is maintained by the pineal hormone which is very sensitive to light. The sympathetic nervous system plays pivotal role in controlling the synthesis and release of this hormone in synchronization with the light and dark cycle (Sun et al., 2016). Apart from the pineal gland, melatonin is also produced by other organs including the heart. After biosynthesis, melatonin is instantaneously released into the circulatory system and outreaches various biological fluids including cerebrospinal fluid (Jiki et al., 2018). Melatonin receptors (MTRs) are exclusively expressed in the ventricles, aorta, coronary arteries and endothelial cells of cardiovascular system which has made them potential targets for treating CVDs (Zhou et al., 2018). Melatonin possesses the ability to scavenge free radicals and also induces the expression of antioxidant enzymes (superoxide dismutase (SOD) and glutathione peroxidase (GPx)) and nuclear factor-erythroid-2 (NF-E2)-related factor 2 (Nrf2) to produce indirect antioxidant effects Mukherjee et al. (2015); (Yu et al., 2018). It has been reported that physiological concentration of melatonin is important in reducing the oxidative damage and the level of inflammatory mediators in the heart (Sahna et al., 2002). Owing to its high hydrophilicity and high lipophilicity, melatonin crosses the cell and nuclear membrane easily and exerts its antioxidant effects in the cytoplasm and nucleus as well (Fu et al., 2020). Besides this, melatonin protects the cardiomyocytes by preserving mitochondrial energy metabolism, structure of mitochondria, stabilizes the mitochondrial membrane potential and inhibits the mitochondrial apoptosis (Fu et al., 2020).
2. Clinical reports
Melatonin, a hormone, is not only responsible for maintaining CR but is also known to confer protection to the heart against sustained IRI (Lochner et al., 2006). Ample reports have shed light on the existence of reciprocal relationship between reduced circulating levels of melatonin and increased incidence of acute cardiovascular event such as heart attack (Dominguez-Rodriguez et al., 2005), (Dwaich et al., 2016; McMullan et al., 2017). Acute myocardial infarction (MI) is associated with a nocturnal serum melatonin deficit as well as increased oxidative stress in patients, suggesting that melatonin is partly depleted during the dark phase, which can scavenge the free radicals formed during acute MI (Domínguez-Rodríguez et al., 2002). Patients suffering from MI show high levels of oxidized LDL but lower level of circulating melatonin. This also corroborates the existence of a reciprocal relationship between heart attack and melatonin levels in the serum (Dominguez-Rodriguez et al., 2005). This is further supported by the fact that elevation in the pre-operative plasma levels of melatonin lead to a decline in the markers of injury and inflammation (Intercellular adhesin molecule 1, Interleukin (IL)-8, Troponin I (TnI)) of IRI. Zaslavskaia et al. reported that melatonin treatment for twenty days produced remarkable anti-ischemic as well as anti-anginal effect and normalized the increased oxidative stress in the individuals suffering from CVDs. Owing to the anti-ischemic action of melatonin the authors proposed that melatonin can be used either as a monotherapy or as a supplementary drug for the treatment of cardiovascular diseases including hypertension (Zaslavskaia et al., 2010). McMullan et al. also reported that the individuals suffering from CVDs were found to have significantly lower melatonin levels at night. After analyzing a total of 209 cases of fatal and non-fatal myocardial infarction among women, the authors found that reduced melatonin secretion was linked with a more risk of myocardial infarction in women with high body mass index (McMullan et al., 2017). Interestingly, it was found that melatonin supplementation remarkably reduced myocardial IRI associated with coronary artery bypass graft surgery in a total of forty five patients by improving the ejection fraction and reducing cardiac TnI, IL-1β, and caspase-3 enzyme in the plasma. This indicated that melatonin can attenuate cardiac surgery associated IRI (Dwaich et al., 2016). Apart from this, acute MI and dilated cardiomyopathy patients also had lower circulating melatonin levels in comparison to the control subjects that could be correlated with myocardial injury and cardiac output in dilated cardiomyopathy patients (Misaka et al., 2019). On the contrary, few authors have reported that intravenous or intracoronary melatonin administration prior to undergoing primary percutaneous coronary intervention/revascularization failed to improve the myocardial salvage index in patients with ST segment elevation myocardial infarction (Ekelof et al., 2016), (Dominguez-Rodriguez et al., 2017).
3. Research findings
There have been numerous studies indicating the protective role of melatonin in rodent model of ischemic heart disease as well as supplementation benefits in human cardiovascular health (Szarszoi et al., 2001; Sun et al., 2016; Yu et al., 2018; Han et al., 2019). Owing to the ability to scavenge free radicals, melatonin can curb the oxidative damage of various key molecules as well as reduce apoptosis of the cells subjected to sustained ischemia and subsequent reperfusion (Kashimoto et al., 1999; Dobsak et al., 2003). Previous studies have indicated that MI remarkably increases the synthesis of melatonin in the pineal gland indicating the pivotal role of endogenous melatonin in shielding the heart against MI. Also, the expression of melatonin-1 and melatonin-2 receptors (MTR1 and MTR2) was up-regulated in the heart after inducing MI (Sallinen et al., 2007). The possible signaling pathways involved in mediating the protective effects are:
3.1. Glycosaminoglycans and collagen
Glycosaminoglycans and collagen are components of extra-cellular matrix and are key players of wound healing (Drobnik et al., 2008, 2011). Melatonin administration reduced the level of glycosaminoglycan within the scar but caused an increase in glycosaminoglycan content in the myofibroblast cultures. Apparently, the reduction in glycosaminoglycan content could not be correlated to direct action of the hormone on the myofibroblasts enclosing the infarction scar; the authors hypothesized that the changes in glycosaminoglycan content might be via an indirect action including changes in regulatory process or reduced inflammatory reaction in the infarcted area (Drobnik et al., 2011). Interestingly, Drobnik et al. reported that melatonin administration to pinealectomized rats increased the expression of α1 (I) as well as α1 (III) procollagen genes. But, melatonin did not influence the expression of collagen gene in cultured myofibroblasts (Drobnik et al., 2010). Seemingly, Ciosek and Drobnik reported the crucial role of melatonin in modulating hypothalamo-neurohypophysial system in infarcted rat hearts. Infarcted rats had considerably higher levels of vasopressin and oxytocin in the plasma. But, melatonin limited the release of these neurohormones in the rats. In pinealectomized rats, myocardial infarction inhibited vasopressin secretion but augmented oxytocin release. However, substitution with melatonin in the pinealectomized rats inhibited oxytocin secretion and stimulated vasopressin release (Ciosek and Drobnik, 2012). A previous study indicated that melatonin administration to the rats augmented accumulation of collagen in the heart scar. Melatonin treatment also raised accumulation of collagen in the cultured myofibroblasts. Melatonin-dependent increase in collagen content in the infarcted heart may possibly improve the tensile strength of the scar and retard the development of associated complications (Drobnik et al., 2008, 2013a). It has also been reported that melatonin administration raised glycosaminoglycan content remote to the infarcted area of the heart (fibroblasts) and in the fibroblast culture to improve the mechanics in the heart and these effects were abolished in the presence of luzindole (MT1 and MT2 membrane receptor blockers) (Drobnik et al., 2013b).
3.2. Vasodilation
Nitric oxide (NO) appears to attenuate IRI due to its inherent vasodilatory effect and its ability to reduce neutrophil infiltration (Genade et al., 2008). Genade and co-workers explored the potential of melatonin to exert the anti-adrenergic effects on isolated perfused rat hearts. Melatonin remarkably reduced both forskolin and isoproterenol induced cAMP production and these effects were abolished in the presence of luzindole, L-NAME (nitric oxide synthase inhibitor) and ODQ (a guanylyl cyclase inhibitor). Melatonin-dependent cardioprotective effects were accompanied with PKB/AKT activation and reduced activation of p-38 mitogen-activated protein kinases (p38MAPK) (the pro-apoptotic kinase) during early reperfusion. This indicates that melatonin-dependent anti-adrenergic effects are elicited via activation of NOS and guanylyl cyclase (Genade et al., 2008).
Intrauterine growth restriction (IUGR) is characterized with impairment of cardiac function in children and is even associated with short as well as long-term ailments. Tare et al. found that antenatal melatonin treatment significantly reduced infarct size in single umbilical artery ligated fetuses subjected to sustained IRI. Melatonin pretreatment considerably enhanced fetal oxygenation, contractile function and coronary flow. Melatonin treatment restored endothelium-dependent NO bioavailability and reduced the stiffening of the coronary arteries subjected to IUGR. Even in the presence of L-NAME, melatonin exposure led to indomethacin (COX-inhibitor) sensitive vasodilation possibly mediated via production of prostanoids (Tare et al., 2014). It has been also demonstrated that melatonin administration in the chick embryo commencing at 13th day of incubation improved cardiac systolic function, cardiac contractility and endothelial function. This was possibly due to reduction in oxidative stress, and restoration of expression of vascular endothelial growth factor, and ultimately increased NO bioavailability (Itani et al., 2016). Altogether, this indicates that NO plays a vital role in modulating IRI due to its vasodilatory action such that it reduces endothelial dysfunction (Fig. 1).
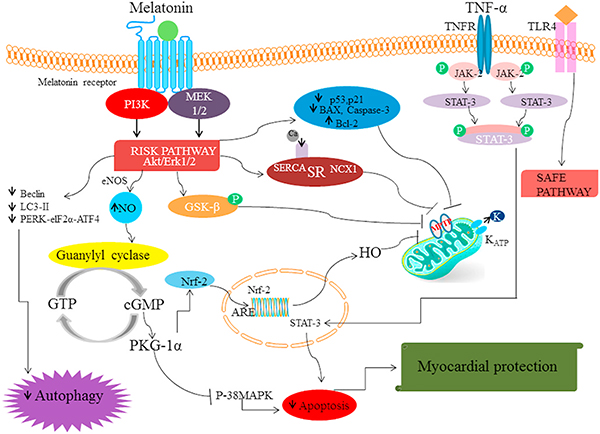
Fig. 1.
The binding of MTR agonist to G-protein coupled receptors (Melatonin 1, 2) leads to the activation of Akt/Erk pathway (RISK pathway). This leads to reduction in the levels of proteins including beclin-1, LC3-II, PERK-eIF2α-ATF4 which are markers of autophagy. Also, the activation of RISK pathway increases the generation of NO via endothelial nitric oxide synthase. The increase in NO activates guanylyl cyclase enzyme which increases the production of cGMP (cyclic guanosine monophosphate) which in turn activates PKG-1α which inhibits p-38 MAPK (p-38 mitogen-activated protein kinases) and subsequent apoptosis. The activation of PKG-1α (Protein Kinase G-1α) also facilitates nuclear translocation of Nrf-2 (Nuclear factor erythroid 2-related factor 2) and triggers the transcription of genes with ARE sequence including haem oxygenase (HO) to curb MPTP (mitochondrial permeability transition pore) formation. Meanwhile, the activation of RISK pathway augments GSK-β (glycogen synthase kinase-3) phosphorylation which halts mPTP formation. Moreover, melatonin administration increased the expression of calcium handling proteins sarcoendoplasmic reticulum SERCA2a and sodium-calcium exchanger to reduce Ca2+ overloading and aid storage of calcium in the SR. Apart from this, melatonin modulates the expression of apoptotic proteins such it reduces the level of Bax, p53, p21, caspase-3 but increases Bcl-2 level to promote cardiomyocyte survival. Furthermore, melatonin can activate the SAFE pathway either via activation of TNFR or TLR4 which subsequently increases STAT transcription to provide myocardial protection.
3.3. Role of melatonin in proliferation, apoptosis and cytoprotection
3.3.1. AMPK-PGC1α-SIRT3/SIRT-1
Silent information regulator 1 (SIRT-1), a member of sirtuin protein family is NAD dependent histone deacetylase which protects the heart against IRI by reducing stress-induced apoptosis via modulating the expression of pro-survival and pro-apoptotic molecules (Han et al., 2017). Another member of sirtuin family protein, SIRT-3 is a major mitochondrial protein deacetylase that considerably reduces apoptosis and necrosis in the heart upon stimulation (Yu et al., 2017b). Yu et al. reported that type 1 diabetes significantly down-regulated AMPK phosphorylation and decreased the expression of peroxisome proliferator-activated receptor-γ coactivator (PGC-1α), SIRT3 and SOD2 in the myocardium and made them more susceptible to myocardial injury. However, melatonin treatment attenuated IRI in type 1 diabetic rats by augmenting mitochondrial SOD activity, ATP production and oxidative phosphorylation complex (II, III and IV), decreasing myocardial apoptosis, mitochondrial malondialdehyde (MDA) and H2O2 generation. Also, melatonin increased AMPK phosphorylation, increased PGC-1α, SIRT3, SOD2, NRF1 and mitochondrial transcription factor A (TFAM) expressions which are known to provide beneficial effects to heart during stressful conditions. However, these effects were abrogated in the presence of Compound C (a specific AMPK signaling blocker). Apart from this, it was seen that SIRT3 siRNA transfection in the cells blunted the protective effects of melatonin without altering ratio of p-AMPK/AMPK and PGC-1α expression. Altogether, it was found that melatonin improved the myocardial function in diabetic rat hearts via reducing mitochondrial oxidative stress and increasing its biogenesis via modulating AMPK-PGC1α-SIRT3 signaling (Yu et al., 2017b).
Also, melatonin elicited cardioprotective effects in the isolated rat hearts in terms of improving functional contractility, reducing infarct size, apoptotic index, serum CK and LDH release, up-regulating the expression of SIRT1, Bcl-2 (anti-apoptotic protein) and down-regulating the expression of apoptotic markers Bax, caspase-3 and cleaved caspase-3. Melatonin treatment also resulted in the reduction of myocardial superoxide generation, gp91-phox expression, MDA level, and increased myocardium SOD level, which indicate that the IRI-induced oxidative stress was significantly attenuated. However, these protective effects were blocked by EX527 (SIRT-1 inhibitor) or luzindole, indicating that SIRT1 signaling and MTR may be specifically involved in these effects (Yu et al., 2014). Han et al. reported the protective effect of melatonin on adipose-derived mesenchymal stem cells in promoting the survival of these cells in the infarcted heart. In-vivo and in-vitro study revealed that melatonin markedly reduced inflammation, oxidative stress, and apoptosis in the infarcted heart. Melatonin treatment led to enhancement of SIRT-1 signaling, which was associated with the increase in the expression of Bcl-2, and reduction in the expression of Ac-FoxO 1 (Forkhead box protein), Ac-p53 (tumor suppressor protein), Ac–NF–ΚB, and Bax (pro-apoptotic protein). Hence, melatonin played a vital role in bolstering adipose-derived mesenchymal stem cell therapy for the protection against MI possibly via enhancing the survival of these through SIRT-1 signaling (Han et al., 2016) (Fig. 2).
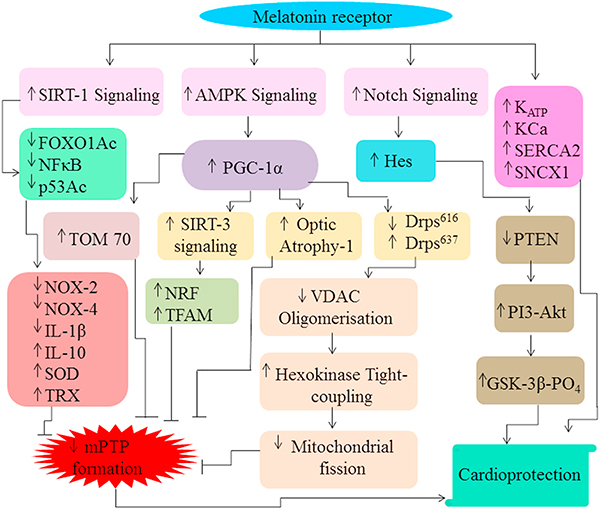
Fig. 2.
Activation of MTR enhances SIRT-1 signaling which subsequently deacetylases FOXO1, P53 and decreases NFκB activity. This eventually results in reduction in the expression of NOX-2, NOX-4, IL-1β but augmentation in IL-10, SOD, TRX activity. Also, melatonin boosts AMPK signaling which results in an increase in the level of PGC-1α. PGC-1α further modulates the expression of TOM70, optic atrophy-1, Drps637, Drps616 and increases SIRT-3 signaling which ultimately reduce MPTP formation. Increased SIRT-3 signaling increases the expression of nuclear factor erythroid 2-related factor 2 and mitochondrial transcription factor A (TFAM) to abate MPTP opening. Moreover, the alteration in the level of Drps637, Drps616 expression reduces VDAC1 oligomerization, disassociation of hexokinase 2 to reduce mitochondrial fission which leads to MPTP formation. Apart from this, increased Notch signaling up-regulates Hes which further decreases phosphatase and tensin homolog (PTEN) levels. PTEN further increases PI3/Akt signaling to escalate GSK-β (glycogen synthase kinase-3) phosphorylation to induce cardioprotective effects.
3.3.2. RISK pathway, SERCA, ERK1
Various authors have reported the role activation of reperfusion injury salvage kinase (RISK), survivor activating factor enhancement (SAFE) pathway and the interplay between them in mediating melatonin-dependent cytoprotective effects (Nduhirabandi et al., 2014). Nduhirabandi et al. reported that chronic melatonin treatment for three/six weeks to high calorie fed rats considerably reduced infarct size when subjected to sustained IRI. The protective effect was possibly due to increased myocardial STAT-3, PKB/AKT, ERK42/44 and GSK-3β phosphorylation at the time of reperfusion. Thus, melatonin administration to obese or insulin resistant rats decreased insulin resistance and provided protection against myocardial IRI via modulating AkKT/ERK/STAT signaling (Nduhirabandi et al., 2014).
It is manifested that short-term activation of TLR signaling also exhibits cardioprotective responses against IRI (Topkara et al., 2011). The authors have shown that melatonin administration led to reduction in myocardial infarct size and this protection was ameliorated in the presence of TAK242 (TLR4 signaling inhibitor). Melatonin treatment facilitated the pre-ischemic activation of STAT3 in the mitochondria that was abolished with TAK242. Moreover, stimulation of TLR4 using lipopolysaccharide reduced myocardial infarct size in the wild hearts but could not provide protection in TNFR2α -deficient or cardiomyocyte-specific STAT3 knock-out mice hearts. This indicated that melatonin provides cardioprotection via activation of TLR4 which subsequently stimulate the SAFE pathway (Nduhirabandi et al., 2016). Lamont and co-workers reported that red wine supplementation reduced the infarct size in rats and mice that was reversed in the presence of luzindole. However, red wine or melatonin administration did not protect TNFR2 deficient or cardiomyocyte specific signal transducer and activator of transcription 3 (STAT3) knock-out mice. This indicated that the melatonin present in red wine confers cardioprotection against IRI via activation of MTR and modulation of TNF-α and STAT3, the main players of the SAFE pathway (Lamont et al., 2011, 2015) (Fig. 1).
Prolonged ischemia and subsequent reperfusion (30 min left coronary artery ischemia followed by 120 min reperfusion) significantly increased heart-to-body weight ratio, ventricular hypertrophy and hematocrit value in chronically hypoxic rats. MDA level, LDH release and infarct size of the isolated hearts were significantly elevated in the vehicle-treated hypoxic rats but not in the melatonin-treated rats. Also, IRI remarkably increased the resting calcium levels and induced calcium overload in the cardiomyocytes. Moreover, there was significant decline in the sarcoendoplasmic reticulum (SR) calcium content, reduction in amplitude and decay time of calcium transients indicating impairment in contractility and re-uptake of SR calcium. Apart from this, calcium handling proteins SR-Ca2+ ATPase (SERCA) were markedly reduced in the hypoxic rat hearts. However, melatonin treatment significantly preserved SERCA expression and improved the handling of Ca2+ ions. Thus, melatonin exerted cardioprotective action against chronic hypoxia-induced myocardial injury via preserving the expression of calcium handling proteins in SR of cardiomyocytes (Yeung et al., 2008). Nduhirabandi et al. reported that melatonin treatment decreased the gain in body weight, visceral adiposity, insulin, triglyceride levels and thiobarbituric acid reactive substances in high calorie-induced diabetic rats. Also, melatonin reduced the infarct size and improved the functional recovery of high calorie-induced diabetic rat hearts. The hearts from melatonin-treated rats had increased activation of PKB/AKT, ERK42/44 but reduced activation of p38 MAPK at the time of reperfusion. Thus, chronic treatment with melatonin prevented the development of metabolic abnormalities induced by high calorie diet and shielded the heart against IRI (Nduhirabandi et al., 2011). Hu et al. reported that in-vitro melatonin administration led to protective effects against hypoxia/reoxygenation injury in cardiomyocytes via ERK1 activation. However, the beneficial effects were significantly abrogated in the presence of PD98059 (ERK1 inhibitor). Further, the authors have shown that melatonin treatment inhibited cardiomyocyte apoptosis and improved actin filament organization in the cardiomyocytes even when exposed to hypoxia-reoxygenation that was reversed by PD98059. Apart from this, melatonin reduced calcium overload, IP3R expression but promoted SERCA2a expression via ERK1 pathway in cardiomyocytes against hypoxia-reoxygenation and these effects were reversed by PD98059. Therefore, melatonin-dependent cardioprotection against IRI is partly mediated via modulating IP3R and SERCA2a in order to maintain calcium homeostasis intracellularly via activation of ERK1 (Hu et al., 2018). Kong et al. also reported that melatonin administration significantly reduced the calcium overload-induced increase in infarct size, caspase-3 release, LDH release and Cyt c levels (Kong et al., 2017).
3.3.3. JAK/STAT3 pathway
Lan et al. reported that transplantation of rat hearts pre-treated with melatonin significantly reduced myocardial edema when evaluated on the basis of water content and wet/dry ratio. It was accompanied by reduction in inflammation in terms of decline in matrix metalloproteinase-9, IL-6, and TNF-α. Apart from this, oxidative stress was also reduced in terms of levels of MDA, cytochrome C (Cyt-C), activity of SOD and GPx, and expression of Nrf2 and NQO1. Myocardial apoptosis was also reduced in terms of increase in Bcl-2, decrease in bax and cleaved caspase-3. Furthermore, the involvement of JAK2/STAT3 signaling pathway was assessed by determining the levels of phosphorylated-JAK2 and phosphorylated-STAT3. However, it was seen that the protective effects were abolished in the presence of AG490 (JAK2/STAT3 inhibitor). This indicated that melatonin induced cardioprotective effects via the activation of the JAK2/STAT3 signaling system (Lan et al., 2019).
It has also been reported that melatonin provides protective effect in isolated rat hearts in terms of improvement in heart function, reduction in infarct size, apoptosis, LDH release, up-regulation of Bcl 2, and downregulation of Bax. However, these effects were abolished in the presence of AG490 and JAK2 silencing. Also, mitochondrial redox potential was preserved, SOD activity was increased, and mitochondrial hydrogen peroxide and MDA was decreased in the presence of melatonin. This indicated that melatonin pretreatment can ameliorate IRI by curtailing the mitochondrial oxidative damage via stimulating JAK2/STAT3 signaling pathway (Yang et al., 2013). Yu and co-workers reported that in-vivo as well as in-vitro melatonin treatment decreased myocardial apoptosis, oxidative stress and restored cardiac function. Also, melatonin administration enhanced phosphorylation of AKT, GSK-3β, ERK1/2 and STAT3 and decreased PERK-eIF2α-ATF4-dependent endoplasmic reticulum stress (ER stress). However, these effects were abrogated in the presence of LY294002 (AKTinhibitor), U0126 (ERK inhibitor) or AG490 (JAK inhibitor). Moreover, LY294002 and U0126 treatment ameliorated STAT3 phosphorylation while AG490 administration also decreased AKT and ERK1/2 phosphorylation indicating the existence of intercommunication between RISK and SAFE pathways in melatonin dependent cardioprotective effect. Thus, melatonin treatment reduced PERK/eIF2α/ATF4-mediated stress in the ER and apoptosis during myocardial IRI via enhancing intercommunication between RISK and SAFE pathways (Yu et al., 2016) (Fig. 1).
3.3.4. Notch1/Hes1/Akt signaling, ER stress
Previous studies in H9C2 cardiomyocytes as well as in isolated rat hearts showed that melatonin can give protection during ischemic conditions through inhibition of apoptosis and limitation of oxidative damage via modulation of Notch and Enhancer of split (HES) signaling (Yu et al., 2017a; Han et al., 2019). The Notch system actively participates in modulating cell proliferation as well as apoptosis in various tissues via cross-talking with inflammatory cytokines and growth factors (Yu et al., 2017a). Hes is one of the fundamental Notch targets’ which plays a critical role in cardiovascular signaling when subjected to prolonged ischemia and subsequent reperfusion (Wiese et al., 2010; Yu et al., 2017a; Han et al., 2019). It was reported that melatonin treatment increased the expression of Notch1, Notch intracellular domain (N1ICD), Hes1, pAkt/Akt ratio, Bcl-2 and reduced the expression of phosphatase and tensin homolog (PTEN), Bax (pro-apoptotic protein), and caspase-3 both in vivo and in vitro. However, melatonin-dependent protection was abolished in the presence of DAPT (Notch1 signaling inhibitor), luzindole (non-selective melatonin membrane receptor antagonist), as well as by knockdown of Notch1 and Hes1 using siRNA. Another study suggested that PTEN/AKT signaling pathway, a downstream target of Notch signaling plays important role in melatonin mediated cardioprotective effects (Yu et al., 2015). In accordance with the above study, melatonin treatment significantly reduced myocardial IRI via enhancing Notch1/HES1/AKT signaling and salvaged the intracellular thioredoxin system by up-regulating Notch1, N1ICD, Hes1, and p-AKT expressions, increasing thioredoxin activity, and down-regulating thioredoxin-interacting protein expression. However, these protective effects were abolished in the presence of luzindole, DAPT (a γ-secretase inhibitor), or LY294002 (a PI3-kinase/AKT inhibitor) indicating the involvement of MTRs and AKT signaling in mediating the protective effects. It was found that thioredoxin-interacting protein overexpression decreased thioredoxin activity as well as the cytoprotective effect of melatonin. In fact, inefficient Notch1 signaling exaggerated myocardial injury in acute hyperglycemic state. This indicated that melatonin treatment could overcome sustained IRI by restoring thioredoxin activity and reducing thioredoxin-interacting protein expression via enhancing Notch1/Hes1/AKT signaling (Yu et al., 2017a).
Recently, Han and co-workers reported that melatonin exhibits protective effects against IRI via activation of myocardial MTRs using in-vivo and in-vitro models. The authors used various approaches including MT1, MT2 gene silencing in-vivo, in-vitro MT1, MT2 overexpression to reveal the receptor that was responsible for providing cardioprotective effects. It was seen that MT-2, but not MT-1, was essentially up-regulated after myocardial IRI. Mechanistically, melatonin pretreatment reduced myocardial oxidative and nitrative stress, ER stress, mitochondrial injury, and myocardial apoptosis by increasing Notch 1, HES1 expression. These beneficial activities were absent in MT2-silenced heart. Apart from this, adeno-associated virus 9 mediated cardiomyocyte-specific overexpression of MT2 reduced myocardial injury. Thus, melatonin administration protected the primary cardiomyocytes against IRI via modulating melatonin/Notch1/Hes1 signaling (Han et al., 2019) (Fig. 2).
3.4. Mitochondria
3.4.1. Fusion/fission (AMPK/OPA-1)
Earlier studies have reported that melatonin regulates mitochondrial fusion/fission event through optic atrophy-1 protein expression (OPA-1). OPA-1 regulates mitochondrial fusion and mitophagy to maintain mitochondrial homeostasis under stressful conditions (Knowlton and Liu, 2015). It has been reported that cardiac IRI induces apoptosis and represses OPA-1 related mitochondrial fusion and mitophagy (Zhang et al., 2019). However, melatonin treatment lead to stabilization of OPA-1 and improved in vivo myocardial function as well as in vitro cardiomyocyte survival (Zhang et al., 2019). Cardiac-specific knockout of OPA-1 abrogated the favorable effects of melatonin on cardiomyocyte viability and mitochondrial homeostasis both in-vivo and in-vitro indicating that these protective effects were highly dependent on OPA-1-related mitochondrial fusion and mitophagy (Zhang et al., 2019). Apart from this, the authors have brought into notice that melatonin modulates OPA-1 stabilization through AMPK signaling, as blockade of AMPK signaling suppressed OPA-1 expression and the subsequent cardioprotective effects of melatonin. This suggests that melatonin exerts cardioprotective action against IRI via activating AMPK signaling and subsequent OPA-1 related mitochondrial fusion/mitophagy (Zhang et al., 2019). In corroboration with the above study, Ma and Dong reported that IRI curbed the mitochondrial fusion which is utmost necessary to sustain mitochondrial homeostasis (Ma et al., 2018a,b). Further, the authors have also shown that melatonin provides cardioprotection through Yap-Hippo pathway by regulating the expression of OPA-1 and Yap proteins and mitochondrial fusion (Ma and Dong, 2019).
3.4.2. Mitophagy (KH2/PINK1/parkin pathway)
The binding of hexokinase-2 (HK2) to the heart mitochondria is associated with ensuing resistance against reperfusion injury. It is believed that during ischemia, HK2 dissociates from heart mitochondria, and the degree of this dissociation is interrelated with the size of infarct after reperfusion (Halestrap et al., 2015). Evidently, PINK1/Parkin-mediated mitophagy ceases the buildup of mitochondrial products that are toxic in nature and can result in cell death (Zhou et al., 2017). Zhou et al. explored the role of melatonin in providing cardioprotective effects via preventing the stimulation of PINK1/Parkin-mediated mitophagy as melatonin administration markedly reduced infarction, improved cardiac function, blood flow, and microcirculation perfusion defects. Melatonin treatment reduced the unbroken endothelial barrier, augmented endothelial nitric oxide synthase (NOS) expression, reduced inflammatory cell infiltration, and endothelial damage in the cardiac microcirculation endothelial cells. However, the beneficial effects of melatonin on microvasculature were abrogated in AMP-activated protein kinase α (AMPKα) deficient mice. In-vitro study revealed that IRI stimulates dynamin-related protein 1 (Drp-1)-dependent mitochondrial fission which causes voltage-dependent anion channel 1 (VDAC1) oligomerization, disassociation of HK2, opening of mitochondrial permeability transition pore (MPTP), up-regulation of PINK1/Parkin, and mitophagy-mediated death of cardiac microcirculation endothelial cells. But, melatonin treatment improved cardiac microcirculation endothelial cell survival via activating AMPKα, down-regulating phosphorylated-Drp1S616 and up-regulating phosphorylated-Drp1S637, which dampened Drp 1-induced mitochondrial fission. Furthermore, melatonin treatment suppressed mitochondrial fission by reducing VDAC1 oligomerization, inducing tight coupling of HK2 on the mitochondria such that it prevented MPTP opening, activation of PINK1/Parkin and eventually blocked mitophagy-mediated cellular death (Zhou et al., 2017) (Fig. 2).
3.4.3. Tom70 (mitochondrial ROS, mitochondrial injury)
The translocases of mitochondrial outer membrane (TOM) complex is a gate for permitting the entry of all mitochondrial pre-proteins and is known to exhibit cardioprotective effects (Pei et al., 2017). Pei and co-workers reported that sustained ischemia or hypoxia significantly reduced Tom70 expression in the cardiomyocytes. Furthermore, it was seen that Tom70 knock down exaggerated the myocardial injury and was associated with increased mitochondrial fragmentation and ROS formation. But, Tom70 overexpression ameliorated myocardial injury and restored mitochondrial integrity and reduced ROS production. Melatonin treatment increased the expression of PGC-1α and Tom70 expression in the ischemic myocardium that was significantly abrogated in the presence of luzindole. Also, it was seen that melatonin treatment failed to provide protective effects in the Tom70 deficient mice indicating their potential role in mediating cytoprotective effects via protecting mitochondrial integrity and reducing subsequent ROS generation (Pei et al., 2017) (Fig. 2).
3.4.4. ATP-sensitive potassium channels/Ca2+-dependent K+-channel
Activation of ATP-sensitive potassium channels (KATPs) and Ca2+-dependent K+-channels (KCas) play prominent role in modulating IRI (Bentzen et al., 2009; Aggarwal et al., 2017). KCas are highly sensitive to alterations in intracellular calcium or voltage variations in the membrane and play pivotal role in preserving the vascular tone (Bentzen et al., 2009). Stroethoff et al. explored the role of ramelteon (MTR agonist, a clinically approved drug for insomnia) in reducing the acute infarct size by drug-induced postconditioning. It was found that treatment with melatonin and ramelteon at the beginning of reperfusion remarkably reduced the infarct size. However, the cardioprotective effects were significantly abolished in the presence of luzindole (MTR antagonist). This possibly indicated that ramelteon postconditioning induced cardioprotection via activation of MRs (Stroethoff et al., 2018a). The same group of authors found that ramelteon dependent cardioprotective effects were abrogated in the presence of paxilline (KCa inhibitor), 5-hydroxydecanoate (mitochondrial KATP inhibitor), and luzindole (MR antagonist). This indicated that ramelteon produced cardioprotective effects via stimulating MRs and subsequent activation of mitochondrial KATP and KCa (Stroethoff et al., 2018b) (Figs. 1 and 2).
3.4.5. MPTP, cardiolipin peroxidation
The level of oxidative stress and inflammatory cytokines play an essential role in determining the fate of the cell after IRI. It is manifested that sustained accumulation of free radicals in the mitochondria can alter the metabolic pathway and make it more susceptible to mitochondrial permeability transition pore (MPTP) formation (Neri et al., 2015). Petrosillo et al. reported that melatonin treatment remarkably reduced the infarct size, LDH release and improved the functional recovery of hearts subjected to Langendorff heart reperfusion. Also, it was seen that the mitochondria which was isolated from melatonin-treated hearts had higher resistance to Ca2+ and was less sensitive to MPTP opening. Moreover, melatonin treatment prevented NAD+ release, cytochrome c (Cyt c) release from the mitochondria and IR-induced cardiolipin oxidation. Thus, melatonin protects the heart against IRI via inhibiting cardiolipin peroxidation and subsequently MPTP opening (Petrosillo et al., 2009). The same group of authors explored the role of aging (24 months old rats) in increasing the vulnerability of the hearts to IRI. The authors have shown that aging was accompanied with increased Ca2+-mediated MPTP opening, augmented release of Cyt c and oxidation of cardiolipin in comparison to the young animals (5 months old). However, melatonin treatment remarkably counteracted these deleterious effects. It was hypothesized that the augmented level of oxidized cardiolipin was possibly responsible for increasing the susceptibility to Ca2+-dependent MPTP opening and increased Cyt c release in the aged rat heart mitochondria. Altogether, melatonin treatment probably prevented the oxidation/depletion of cardiolipin to prevent subsequent opening of MPTP and Cyt c release (Petrosillo et al., 2010).
3.5. Autophagy (AMPK/mTOR)
Autophagy is a vital process that is responsible for removing the dysfunctional proteins as well as organelles and holds utmost importance in maintaining the normal structure and functioning of the heart (Wu et al., 2019). Chen et al. evaluated the effects of melatonin against IRI using in-vivo rat model and in-vitro neonatal cardiac microvascular endothelial cells. The authors found that in vivo melatonin pretreatment reduced the infarcted area, LDH release and improved the cardiac function to a large extent. However, melatonin-dependent beneficial effects against IRI were abolished in the presence of the AICAR (AMPK activator) and rapamycin (mTOR inhibitor). Furthermore, in vitro melatonin administration also increased the cell viability and reduced LDH release. Besides, melatonin treatment down-regulated beclin 1 and light chain 3-II (LC3-II) protein expression. Also, there was marked reduction in the formation of autophagosomes. This indicated that melatonin exerted protective effects in cardiac microvascular endothelial cells probably by inhibiting autophagy via the AMPK/mTOR pathway (Chen et al., 2018) (Fig. 1).
3.6. Oxidative stress (Nrf2; RIP3/MLK/Ca2+-calmodulin-dependent protein kinase II)
Increased oxidative stress is one of the main offenders for causing IRI-induced cellular dysfunction and ultimately death. Anti-oxidants are the chemicals which possess the ability to reduce tissue damage via keeping a check on the amount of reactive oxygen species (ROS) produced in a tissue (Zhou et al., 2018). Various authors have explored the anti-oxidant potential of melatonin to overcome the damage in the heart due the generation of free radicals (Mukherjee et al., 2015), (Yeung et al., 2015). He et al. investigated the role of retinoic acid receptor-related orphan receptor α (RORα) in reducing myocardial injury (He et al., 2016). The authors revealed that knock-out of this receptor significantly increased myocardial IRI in terms of increased MI, apoptosis, ER stress, oxidative stress and deteriorated contractile function. Nevertheless, overexpression of RORα in the cardiomyocytes made the cells less vulnerable to myocardial IRI. Thus, activation of this nuclear MTR can provide protection against cardiac IRI (He et al., 2016).
Nrf2 and haem oxygenase-1 (HO-1) signaling plays an imperative role in monitoring the cellular homeostasis in response to IR induced oxidative stress (Wang et al., 2016). Being a transcriptional factor, Nrf2 monitors the expression of various antioxidant genes and enzymes that are exclusively expressed in the cardiovascular system (Wang et al., 2016). Yu and co-workers reported that melatonin treatment shielded the cardiac function and decreased oxidative damage and myocardial apoptosis in diabetic rats. In addition, it was found that melatonin treatment augmented intracellular cGMP level, Protein Kinase G-1α (PKGIα) expression, p-VASP/VASP (vasodilator-stimulated phosphoprotein) ratio, MAPK signaling and myocardial Nrf2-HO-1 signaling. However, these effects were abolished in the presence of KT5823 (a selective inhibitor of PKG) or PKGIα siRNA. Moreover, the in-vitro study indicated that luzindole or 4P-PDOT (a selective MT2 receptor antagonist) abrogated the cytoprotective effect of melatonin. Also, luzindole and 4P-PDOT ameliorated the stimulatory action of melatonin on cGMP-PKGIα, MAPK signaling and its modulatory effect on Nrf2/HO-1 signaling. This study indicated that melatonin blunted diabetic IRI via activation of MT2 receptors and subsequent modulation of Nrf2-HO-1 and MAPK signaling to decrease myocardial apoptosis, oxidative stress and stabilize cardiac function (Yu et al., 2018) (Fig. 1).
Interestingly, Zhang and co-workers reported that exposure to ischemia and reperfusion not only led to significant increase in apoptosis and oxidative stress, but also enhanced Nrf2 expression within H9C2 cells. However, treatment with melatonin partially reversed the oxidative stress, apoptosis and promoted nuclear translocation of Nrf2. Also, it was found that Nrf2 gene silencing prohibited the beneficial effects of melatonin on H9C2 cells on being subjected to ischemia-reperfusion. This indicated that melatonin possibly protects H9C2 cells against IRI via decreasing apoptosis and oxidative stress through activation of the Nrf2 signaling pathway (Zhang et al., 2018). Further, Chen et al. reported that the cardioprotective effect of melatonin is not because of GPx-1 activity. It was seen that melatonin exhibited cardioprotection in GPx-1 deficient mice. There was considerable improvement in left ventricular end-diastolic pressure and significant decrease in infarct size as well as (LDH) release in the melatonin-treated GPx-1 deficient hearts (Chen et al., 2009). In contrast to the above reports, Schaefer et al. reported that addition of melatonin to physiological solution containing histidine-ketoglutarate-tryptophan attenuated the post-ischemic ROS burst but failed to improve the contractile dysfunction in the heart during reperfusion (Schaefer et al., 2019).
3.6.1. LDH release
Mukherjee and co-workers reported that treatment of rats with isoproterenol led to myocardial injury in terms of alteration in the activity of mitochondrial enzymes responsible for regulating to energy metabolism, increase in the level of oxidative stress, augmentation in level of the proteins regulating the progression of apoptosis (Mukherjee et al., 2010, 2012), upraise in the level of inflammatory markers, neutrophil infiltration (Patel et al., 2010). Also, changes were observed in the ECG pattern, including ST-segment elevation. Melatonin treatment considerably reduced the leakage of LDH, and cardio-specific LDH1 (cardiac injury biomarker) (Mukherjee et al., 2012), creatine kinase-MB, aspartate transaminase, alanine transaminase; increased glutathione content and decreased tissue lipid peroxidation in the rat hearts (Patel et al., 2010). Also, melatonin treatment improved morphology of the cardiac tissue as well as functioning of the heart (Mukherjee et al., 2010, 2012). There have been reports that administration of Neu-p11, melatonin agonist significantly decreased the levels of creatine kinase (CK), LDH in the cells indicating the preservation of cell membrane permeability (Yu et al., 2014). This indicates that melatonin-dependent cardioprotective effects were exhibited due to its ability to scavenge free radicals and reduction in the oxidative stress.
3.6.2. SOD activity, MDA levels
Liu et al. reported that melatonin treatment abrogated myocardial apoptosis (by increasing Bcl-2 vs Bax expression), shielded the structural integrity of mitochondria in myocardial cells, enhanced ATP synthesis, GSH levels and decreased mitochondrial MDA and Ca2+ in the rat hearts subjected to sustained ischemic insult. Apart from this, melatonin promoted the functional recovery after the ischemic insult owing to its anti-oxidant activity (Liu et al., 2014). In fact, Neu-11, a melatonin agonist also exerted protective effects against hypoxia-reoxygenation injury in H9C2 myocardial cells through inhibition of cellular refractivity and apoptosis of myocardial cells. Also, SOD level was increased and MDA level was decreased in the cells which resulted in reduced lipid peroxidation and protection of mitochondria against IRI (Yu et al., 2014). In corroboration with the above study, Mukherjee and co-workers reported that in-vitro administration of melatonin protected the cardiac mitochondria against isoproterenol-induced cardiac injury in terms of restoration of succinate dehydrogenase activity in the mitochondria as well as other enzymes of the Kreb’s cycle and the respiratory chain. Also, melatonin lowered the oxidative stress and restored the mitochondrial membrane potential (Mukherjee et al., 2015). Liu et al. reported that melatonin administration down-regulated the expression of porimin (preoncosis protein), reduced the infarct size, and enhanced cell NAD+ content to provide myocardial protection by inhibiting MPTP opening and subsequent production of ROS (Liu et al., 2015).
Interestingly, Yeung et al. explored the role of melatonin in reducing obstructive sleep apnea (OSA)-induced mortality (Yeung et al., 2015). Obstructive sleep apnea is often accompanied with chronic intermittent hypoxia such that it causes mortality in patients suffering from ischemic heart disease (Yeung et al., 2015). Yeung et al. reported that melatonin administration reduced the systolic blood pressure, heart weight, and MDA levels, infarct size in rats after being subjected to chronic intermittent hypoxia (28 weeks) to mimic OSA. There was considerable decline in the expression of inflammatory mediators (TNF-α, IL-6, and COX-2) and fibrotic proteins (PC1 and TGF-β) after melatonin administration. Besides, melatonin treatment reduced Ca2+ overloading, increased SR-Ca 2+ content, the expression and activity of Ca2+ -handling proteins viz. SR calcium transport ATPase (SERCA2a) and sodium-calcium exchanger (NCX1), and the expressions of CAMKII and phosphorylated eNOS (ser 1177) (Fig. 2). Furthermore, melatonin ameliorated the activation of NADPH oxidase and subsequent generation of ROS but increased the expression of catalase and SOD. Thus, melatonin can be used as a prophylactic drug in patients suffering from OSA to confer protection against chronic intermittent hypoxia-induced exaggerated IRI (Yeung et al., 2015).
Yang et al. reported that administration of melatonin infusion into the para ventricular nucleus shielded the heart against IRI by modulating the oxidative stress and reducing the expression of inflammatory cytokines in rats. The rats exposed to IRI had greater infarct size, increase in left ventricular end-diastolic volume, and reduced left ventricular ejection fraction and left ventricular fractional shortening. Furthermore, IRI led to a significant increase in norepinephrine level in the plasma, heart, and para ventricular nucleus; higher levels of ROS in the para ventricular nucleus, increased NOX2, NOX4, IL-1β, NF-κB production and decreased IL-10, SOD activity. However, melatonin infusion in para ventricular nucleus reduced left ventricular end-diastolic volume, norepinephrine levels, NOX2, NOX4, IL-1β, the level of ROS, and NF-κB activity, increased IL-10 and SOD levels, and improved left ventricular ejection fraction, left ventricle fractional shortening. Thus, melatonin infusion attenuated sympathetic nerve activity and myocardial IRI by ameliorating oxidative stress and the level of inflammatory cytokines in the para ventricular nucleus of rats (Yang et al., 2019).
3.6.3. ROS formation, anti-oxidant properties, free radical scavenging
Hosseini et al. reported that melatonin post-conditioning, nicotinamide mononucleotide (NMN) pre-conditioning and their combination exerted significant cardioprotective effects in aged rats against IRI. It was seen that melatonin post-conditioning and NMN pre-conditioning led to improvement in the hemodynamic parameters, reduced the infarct size and LDH release in comparison to the control group. Moreover, the authors have indicated that pretreatment with NMN increased the window of cardioprotection by melatonin. All sorts of treatment decreased oxidative stress and ROS burst and preserved mitochondrial membrane potential and improved NAD+/NADH ratio. Interestingly, the combined therapy had more predominant effects on reduction of mitochondrial ROS and in the improvement of oxidative status and mitochondrial membrane potential in comparison to solo treatment. Thus, combination of melatonin and NMN can be a favorable treatment approach to ameliorate myocardial IRI damages in aged hearts (Hosseini et al., 2020). Recently, Aslan and co-workers have reported that post-conditioning and melatonin administration to non-pinealectomized rats led to significant reduction in the infarct size. However, post-conditioning did not induce protective effect in pinealectomized rats. But co-administration of melatonin along with post-conditioning provided the protective effects. Furthermore, a significant decline was found in the levels of uncoupling protein and an escalation in irisin and NFkB levels after exposure to IRI in pinealectomized rats. Post-conditioning, melatonin administration in non-pinealectomized and their co-administration in pinealectomized groups preserved the expression of all the genes almost near to normal levels. Thus, the authors concluded that post-conditioning and melatonin play crucial role in regulating energy metabolism, inflammation and in protecting the mitochondria against oxidative stress by modulating the UCP3, irisin, and NFkB levels (Aslan et al., 2020).
3.7. microRNA
3.7.1. miR-98 (allograft survival)
Micro RNA’s belong to a class of non-coding RNA which participate in regulating post-transcriptional expression of genes in infarcted hearts (Cai et al., 2016). Nowadays, transplantation of stem cells or cardiac progenitor cells into the infarcted heart has drawn a considerable attention as a therapeutic treatment approach for treating myocardial infarction (Ma et al., 2018a,b). Previous reports have indicated that melatonin treatment mitigated acute allograft rejection and prolonged graft survival (Jung et al., 2004). It is suggested that transplanted cells have relatively less survival rates because of increased oxidative stress in the ischemic heart tissue (Ma et al., 2018a,b). Supplementation of melatonin, being an endogenous antioxidant can protect the cells against oxidative damage. Ma et al. reported that in vitro melatonin administration ablated H2O2 induced reduction in proliferation and apoptosis of cardiac progenitor cells via reducing miR-98 (microRNA) levels. Furthermore, it was seen that in vivo transplantation of cardiac progenitor cells pretreated with melatonin displayed marked improvement in the functioning of post-infarct hearts in comparison to sole cardiac progenitor cells. Besides, the authors have revealed that miR-98 overexpression exaggerated the reduction in proliferation and apoptosis of cardiac progenitor cells induced by H2O2. However, in vivo transplantation of cardiac progenitor cells using miR-98 silencing led to remarkable improvement in cardiac functioning in the infarcted hearts in comparison to sole cardiac progenitor cells. The authors have also shown that miR-98 mimics prevented phosphorylation of signal transducer and activator of the transcription-3 (STAT-3) and decreased the expression of its downstream signaling molecule Bcl-2 (anti-apoptotic protein) but administration of miR-98 inhibitor increased the Bcl-2 protein level. This indicates that melatonin exerts protective effect by down-regulating miR-98 but augmenting STAT3 protein to suppress H2O2-induced apoptosis (Ma et al., 2018b).
3.7.2. H19/miR-675/USP10 pathway
A previous study by Cai et al. reported that melatonin pretreatment tends to antagonize the senescence of cardiac progenitor cells in response to sub-lethal concentration of H2O2, through inhibition of expression of senescence associated β-galactosidase, senescence-associated heterochromatin loci, IL-6 level, p53 and p21. Also, melatonin reduced senescence-associated proliferation reduction in cardiac progenitor cells. However, these protective effects were abolished in the presence of luzindole. Moreover, it was reported that melatonin prevented cardiac progenitor cells through protecting H2O2 dependent down-regulation of long noncoding RNA H19 and miR-675. Thus, melatonin treatment prevented premature senescence of cardiac progenitor cells through H19/miR-675/USP10 pathway (Cai et al., 2016).
Eventually, Ma and co-workers have designed nano drug delivery carriers to release the drug in controlled fashion to protect the drug from degradation in insurmountable odds owing to the inability of melatonin to stay in unfavorable ischemic microenvironment for a longer duration. Both in-vitro and in-vivo studies revealed that melatonin encapsulated with Poly(lactide-co-glycolide)-monomethoxy-poly-(polyethylene glycol) (PLGA-mPEG) nanoparticles exerted enhanced protective effects on adipose-derived mesenchymal stem cells in comparison to free melatonin. The in-vitro study indicated that melatonin nanoparticles remarkably reduced the formation of the p53-cyclophilin D complex, and salvaged adipose-derived mesenchymal stem cells from hypoxia/reoxygenation injury. Besides, the delivery of melatonin nanoparticles improved the survival rates of adipose-derived mesenchymal stem cells in comparison to free melatonin in the infarcted areas. Thus, the combination of melatonin nanoparticles and stem cell transplantation may serve as a promising strategy for treating myocardial infarction (Ma et al., 2018a).
3.8. Chronic treatment
3.8.1. Chronic pain (spared nerve injury)
In the heart, Ca2+/calmodulin-dependent protein kinase (CaMKII) is predominantly expressed and plays a crucial role in maintaining the cardiac performance (Ding et al., 2010). It is well known that chronic pain sensitizes the heart to IRI (Torrance et al., 2010). Yang et al. have shown that mice subjected to spared nerve injury exhibited an exaggerated response to myocardial IRI in comparison to the control animals. Besides, spared nerve injury group animals showed significant increase in TNF- α. Furthermore, it was seen that receptor-interacting protein kinase 3 (RIP3; apoptosis inducing enzyme) dependent phosphorylation of mixed lineage kinase domain (MLK) like and CaMKII was significantly higher in the spared nerve injury mice. However, melatonin treatment led to significant decline in the levels of TNF-α, negatively regulated RIP3-dependent phosphorylation of MLK and CaMKII signaling to reduce subsequent ROS production. Thus, melatonin administration reduced cardiomyocyte apoptosis and improved cardiac function (Yang et al., 2018).
3.9. Possible relationship among these pathways
It is manifested that the binding of melatonin to the MTRs activates MAPK-ERK pathway which subsequently inactivates GSK-3β to reduce MPTP formation (Nduhirabandi et al., 2014). Also, the activation of MAPK-ERK pathway increases the generation of NO via endothelial NOS which subsequently activates guanylyl cyclase enzyme to increase the production of cGMP (cyclic guanosine monophosphate). The increase in cGMP level in turn activates PKG-1α such that it inhibits p-38 MAPK and subsequent release of apoptotic proteins. The activation of PKG-1α also facilitates nuclear translocation of Nrf-2 (Nuclear factor erythroid 2-related factor 2) into the nucleus and triggers the transcription of genes with ARE sequence including haem oxygenase (HO) to limit MPTP (mitochondrial permeability transition pore) formation (Yu et al., 2018).
Apart from this, melatonin triggers AMPK-PKG1α signaling, whereby Nrf2 is a downstream signaling mediator of the pathway (Yu et al., 2017b) to exert cytoprotective effects. This gives us an indication that MAPK-ERK and AMPK-PKG1α signaling act in alliance via enhancing the nuclear translocation of Nrf2 molecule to limit oxidative stress. Besides, the activation of AMPK signaling also prevents the activation of motile protein Drp 1 which results in reduced VDAC1 oligomerization, induces tight coupling of HK2 on the mitochondria to reduce mitochondrial fission. Also, SIRT1 and SIRT3 signaling tend to increase the level of anti-oxidant enzymes which boosts the level of Nrf factor to reduce MPTP formation (Han et al., 2016; Yu et al., 2017b). Melatonin dependent increase in Notch-Hes signaling decreases PTEN levels and subsequently activates PI3/Akt pathway to reduce MPTP formation in the myocardium (Yu et al., 2015). Apart from this, melatonin augments the expression of the myocardial SERCA2a, NCX1, CAMKII, KCa, KATP to enhance the ability of cells to handle calcium ions to reduce the stress injury and apoptosis of the cardiomyocytes (Yeung et al., 2015) (Figs. 1 and 2).
3.10. Melatonin and other endogenous substances
Melatonin interacts with a variety of endogenous molecules including angiotensin, adrenaline, corticosteroids. Stressful stimuli including ischemia can provoke excessive release of adrenaline (sympathetic nervous system and adrenal medulla) in the body which may increase ROS formation due to its pro-oxidant and auto-oxidant potential (Nishi et al., 2019). The inordinate ROS generation is deleterious for the cells which can be counteracted with endogenous/exogenous melatonin. Melatonin tends to abrogate sympathetic overactivity, scavenges the free radicals and augments the formation of anti-oxidant enzymes (Vazan et al., 2005; Pal et al., 2018; Nishi et al., 2019) to exert cardioprotective effects. Apart from this, the renin angiotensin system also interacts with melatonin both at central and peripheral level to modulate the cardiovascular functioning (Campos et al., 2013). It has been reported that melatonin and angiotensin work in an antagonistic manner in the cardiovascular system. Angiotensin tends to induce vasoconstriction in the blood vessels whereas melatonin exerts vasodilatory effect (Campos et al., 2013). Overall, the endogenous melatonin exhibits counter-regulatory effect against sustained sympathetic stimulation (Simko et al., 2019) due to its property to scavenge free radicals and limit the release of inflammatory cytokines (Campos et al., 2013).
An in-vitro study by Rogers et al. revealed that the combination of melatonin and corticosteroids significantly decreased proliferation in mitogen-stimulated human lymphocytes. This indicates that their combination may curb the in-vivo inflammatory response (Rogers et al., 1997). On the other hand, it has also been reported that in-vitro melatonin administration reduces the synthesis of corticosteroids in beef adrenal slices (Mehdi and Sandor, 1977). Perhaps, further studies are required to elucidate the impact of corticosteroid-melatonin interaction in eliciting cardioprotective effects.
4. Conclusion
Despite being a neurohormone, melatonin plays a crucial role in modulating IRI in the heart. Melatonin can elicit cardioprotective effects either via direct anti-oxidant action or by activating membrane or nuclear receptors widely expressed in the heart. Melatonin evokes the protective effects against cardiac IRI via modulating intricately interlinked signaling pathways that regulate various physiological processes ranging from opening of ion channels, oxidative stress, NO release, autophagy, generation of inflammatory cytokines, ROS production, mitochondrial biogenesis, glycosaminoglycans and collagen accumulation. Perhaps, there is an inverse relationship between serum melatonin level and occurrence of heart attack. Thus, this indicates that modulating the circulating melatonin levels can serve as potential target for alleviating myocardial IRI.
Acknowledgement
The authors are thankful to National Heart, Lung and Blood Institute grant 1R01HL141045-01A1 for funding and supporting us.
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
References
- Aslan G, Gul HF, Tektemur A, Sahna E, 2020. Ischemic postconditioning reduced myocardial ischemia-reperfusion injury: the roles of melatonin and uncoupling protein 3. Anatol. J. Cardiol 23, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M, 2009. Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflügers Arch. 457, 979–988. [DOI] [PubMed] [Google Scholar]
- Cai B, Ma W, Bi C, Yang F, Zhang L, Han Z, Huang Q, Ding F, Li Y, Yan G, Pan Z, Yang B, Lu Y, 2016. Long noncoding RNA H19 mediates melatonin inhibition of premature senescence of c-kit(+) cardiac progenitor cells by promoting miR-675. J. Pineal Res 61, 82–95. [DOI] [PubMed] [Google Scholar]
- Campos La C-NJ, Amaral FG, Michelini LC, Bader M, Baltatu OC, 2013. The angiotensin-melatonin axis. Int J Hypertens 2013, 521783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Liu HB, Chen YD, Sha Y, Ma Q, Zhu PJ, Mu Y, 2018. Melatonin attenuates myocardial ischemia/reperfusion injury by inhibiting autophagy via an AMPK/mTOR signaling pathway. Cell. Physiol. Biochem 47, 2067–2076. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chua CC, Gao J, Chua KW, Ho YS, Hamdy RC, Chua BH, 2009. Prevention of ischemia/reperfusion-induced cardiac apoptosis and injury by melatonin is independent of glutathione peroxidase 1. J. Pineal Res 46, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosek J, Drobnik J, 2012. Function of the hypothalamo-neurohypophysial system in rats with myocardial infarction is modified by melatonin. Pharmacol. Rep 64, 1442–1454. [DOI] [PubMed] [Google Scholar]
- Diez ER, Prados LV, Carrion A, Ponce ZA, Miatello RM, 2009. A novel electrophysiologic effect of melatonin on ischemia/reperfusion-induced arrhythmias in isolated rat hearts. J. Pineal Res 46, 155–160. [DOI] [PubMed] [Google Scholar]
- Ding PHJ, Battiprolu PK, Hill JA, Kamm KE, Stull JT, 2010. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J. Biol. Chem 285, 40819–40829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobsak P, Siegelova J, Eicher JC, Jancik J, Svacinova H, Vasku J, Kuchtickova S, Horky M, Wolf JE, 2003. Melatonin protects against ischemia-reperfusion injury and inhibits apoptosis in isolated working rat heart. Pathophysiology 9, 179–187. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez AA-GP, de la Torre-Hernandez JM, Gonzalez-Gonzalez J, Garcia-Camarero T, Consuegra-Sanchez L, Garcia-Saiz MD, Aldea-Perona A, Virgos-Aller T, Azpeitia A, Reiter RJ, Maria Investigators, 2017. Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: results of the Melatonin Adjunct in the acute myocaRdial Infarction treated with Angioplasty trial. J. Pineal Res 62, 2017. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez M, Ferrer-Hita J, Vargas M, Reiter RJ, 2005. Elevated levels of oxidized low-density lipoprotein and impaired nocturnal synthesis of melatonin in patients with myocardial infarction. Atherosclerosis 180, 101–105. [DOI] [PubMed] [Google Scholar]
- Domínguez-Rodríguez AA-GP, García MJ, Sanchez J, Marrero F, de Armas-Trujillo D, 2002. Decreased nocturnal melatonin levels during acute myocardial infarction. J. Pineal Res 33, 248–252. [DOI] [PubMed] [Google Scholar]
- Drobnik J, Karbownik-Lewinska M, Szczepanowska A, Slotwinska D, Olczak S, Jakubowski L, Dabrowski R, 2008. Regulatory influence of melatonin on collagen accumulation in the infarcted heart scar. J. Pineal Res 45, 285–290. [DOI] [PubMed] [Google Scholar]
- Drobnik J, Olczak S, Owczarek K, Hrabec Z, Hrabec E, 2010. Melatonin augments expression of the procollagen alpha1 (I) and alpha1 (III) genes in the infarcted heart scar of pinealectomized rats. Connect. Tissue Res 51, 491–496. [DOI] [PubMed] [Google Scholar]
- Drobnik J, Owczarek K, Piera L, Tosik D, Olczak S, Ciosek J, Hrabec E, 2013a. Melatonin-induced augmentation of collagen deposition in cultures of fibroblasts and myofibroblasts is blocked by luzindole–a melatonin membrane receptors inhibitor. Pharmacol. Rep 65, 642–649. [DOI] [PubMed] [Google Scholar]
- Drobnik J, Slotwinska D, Olczak S, Tosik D, Pieniazek A, Matczak K, Koceva-Chyla A, Szczepanowska A, 2011. Pharmacological doses of melatonin reduce the glycosaminoglycan level within the infarcted heart scar. J. Physiol. Pharmacol 62, 29–35. [PubMed] [Google Scholar]
- Drobnik J, Tosik D, Piera L, Szczepanowska A, Olczak S, Zielinska A, Liberski PP, Ciosek J, 2013b. Melatonin-induced glycosaminoglycans augmentation in myocardium remote to infarction. J. Physiol. Pharmacol 64, 737–744. [PubMed] [Google Scholar]
- Dwaich KH, Al-Amran FG, Al-Sheibani BI, Al-Aubaidy HA, 2016. Melatonin effects on myocardial ischemia-reperfusion injury: impact on the outcome in patients undergoing coronary artery bypass grafting surgery. Int. J. Cardiol 221, 977–986. [DOI] [PubMed] [Google Scholar]
- Ekelof SV, Halladin NL, Jensen SE, Zaremba T, Aaroe J, Kjaergaard B, Simonsen CW, Rosenberg J, Gogenur I, 2016. Effects of intracoronary melatonin on ischemia-reperfusion injury in ST-elevation myocardial infarction. Heart Ves. 31, 88–95. [DOI] [PubMed] [Google Scholar]
- Fu ZJY, Wang J, Zhang Y, Shen M, Reiter RJ, Xi Q, Chen Y, 2020. Cardioprotective role of melatonin in acute myocardial infarction. Front. Physiol 11, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genade S, Genis A, Ytrehus K, Huisamen B, Lochner A, 2008. Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. J. Pineal Res 45, 449–458. [DOI] [PubMed] [Google Scholar]
- Gogenur I, Kucukakin B, Panduro Jensen L, Reiter RJ, Rosenberg J, 2014. Melatonin reduces cardiac morbidity and markers of myocardial ischemia after elective abdominal aortic aneurism repair: a randomized, placebo-controlled, clinical trial. J. Pineal Res 57, 10–15. [DOI] [PubMed] [Google Scholar]
- Green Ea BB, Biaggioni I, et al. , 2014. Melatonin reduces tachycardia in postural tachycardia syndrome: a randomized, crossover trial. Cardiovasc. Ther 32, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Pereira GC, Pasdois P, 2015. The role of hexokinase in cardioprotection - mechanism and potential for translation. Br. J. Pharmacol 172, 2085–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Huang W, Li X, Gao L, Su T, Li X, Ma S, Liu T, Li C, Chen J, Gao E, Cao F, 2016. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J. Pineal Res 60, 178–192. [DOI] [PubMed] [Google Scholar]
- Han D, Wang J, Ma S, Chen Y, Cao F, 2017. SIRT1 as a promising novel therapeutic target for myocardial ischemia reperfusion injury and cardiometabolic disease. Curr. Drug Targets 18, 1746–1753. [DOI] [PubMed] [Google Scholar]
- Han DWY, Chen J, Zhang J, Yu P, Zhang R, Li S, Tao B, Wang Y, Qiu Y, Xu M, Gao E, Cao F, 2019. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res 67, e12571. [DOI] [PubMed] [Google Scholar]
- He B, Zhao Y, Xu L, Gao L, Su Y, Lin N, Pu J, 2016. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res 60, 313–326. [DOI] [PubMed] [Google Scholar]
- Hosseini L, Vafaee MS, Badalzadeh R, 2020. Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J. Cardiovasc. Pharmacol. Therapeut 25, 240–250. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhu P, Zhou H, Zhang Y, Chen Y, 2018. Melatonin-induced protective effects on cardiomyocytes against reperfusion injury partly through modulation of IP3R and SERCA2a via activation of ERK1. Arq. Bras. Cardiol 110, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung MW, Yeung HM, Lau CF, Poon AMS, Tipoe GL, Fung ML, 2017. Melatonin attenuates pulmonary hypertension in chronically hypoxic rats. Int. J. Mol. Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani N, Skeffington KL, Beck C, Niu Y, Giussani DA, 2016. Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J. Pineal Res 60, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiki Z, Lecour S, Nduhirabandi F, 2018. Cardiovascular benefits of dietary melatonin: a myth or a reality? Front. Physiol 9, 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung FJ, Yang L, Harter L, Inci I, Schneiter D, Lardinois D, Keel M, Weder W, Korom S, 2004. Melatonin in vivo prolongs cardiac allograft survival in rats. J. Pineal Res 37, 36–41. [DOI] [PubMed] [Google Scholar]
- Kashimoto S, Kume M, Ikeya K, Ishiyama T, Kumazawa T, 1999. Effects of melatonin and superoxide dismutase on free radical formation in the postischemic reperfused heart. J. Anesth 13, 23–28. [DOI] [PubMed] [Google Scholar]
- Knowlton AA, Liu TT, 2015. Mitochondrial dynamics and heart failure. Comp. Physiol 6, 507–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Wei M, Sun N, Zhu J, Su X, 2017. Role of melatonin in calcium overload-induced heart injury, 42, 611–616. Zhong Nan Da Xue Xue Bao Yi Xue Ban. [DOI] [PubMed] [Google Scholar]
- Lamont KT, Somers S, Lacerda L, Opie LH, Lecour S, 2011. Is red wine a SAFE sip away from cardioprotection? Mechanisms involved in resveratrol- and melatonin-induced cardioprotection. J. Pineal Res 50, 374–380. [DOI] [PubMed] [Google Scholar]
- Lan H, Su Y, Liu Y, Deng C, Wang J, Chen T, Jules KED, Masau JF, Li H, Wei X, 2019. Melatonin protects circulatory death heart from ischemia/reperfusion injury via the JAK2/STAT3 signalling pathway. Life Sci. 228, 35–46. [DOI] [PubMed] [Google Scholar]
- Liu LF, Qian ZH, Qin Q, Shi M, Zhang H, Tao XM, Zhu WP, 2015. Effect of melatonin on oncosis of myocardial cells in the myocardial ischemia/reperfusion injury rat and the role of the mitochondrial permeability transition pore. Genet. Mol. Res 14, 7481–7489. [DOI] [PubMed] [Google Scholar]
- Liu LF, Qin Q, Qian ZH, Shi M, Deng QC, Zhu WP, Zhang H, Tao XM, Liu Y, 2014. Protective effects of melatonin on ischemia-reperfusion induced myocardial damage and hemodynamic recovery in rats. Eur. Rev. Med. Pharmacol. Sci 18, 3681–3686. [PubMed] [Google Scholar]
- Lochner A, Genade S, Davids A, Ytrehus K, Moolman JA, 2006. Short- and long-term effects of melatonin on myocardial post-ischemic recovery. J. Pineal Res 40, 56–63. [DOI] [PubMed] [Google Scholar]
- Lochner A, Marais E, Huisamen B, 2018. Melatonin and cardioprotection against ischaemia/reperfusion injury: what’s new? A review. J. Pineal Res 65, e12490. [DOI] [PubMed] [Google Scholar]
- Ma QYJ, Huang X, Guo W, Li S, Zhou H, Li J, Cao F, Chen Y, 2018a. Poly (Lactide-Co-Glycolide)-Monomethoxy-Poly-(Polyethylene glycol) nanoparticles loaded with melatonin protect adipose-derived stem cells transplanted in infarcted heart tissue. Stem Cell. 36, 540–550. [DOI] [PubMed] [Google Scholar]
- Ma S, Dong Z, 2019. Melatonin attenuates cardiac reperfusion stress by improving OPA1-related mitochondrial fusion in a yap-hippo pathway-dependent manner. J. Cardiovasc. Pharmacol 73, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, He F, Ding F, Zhang L, Huang Q, Bi C, Wang X, Hua B, Yang F, Yuan Y, Han Z, Jin M, Liu T, Yu Y, Cai B, Lu Y, Du Z, 2018b. Pre-treatment with melatonin enhances therapeutic efficacy of cardiac progenitor cells for myocardial infarction. Cell. Physiol. Biochem 47, 1287–1298. [DOI] [PubMed] [Google Scholar]
- Mc Namara K AH, Jackson JK, 2019. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integrated Pharm. Res. Pract 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullan CJ, Rimm EB, Schernhammer ES, Forman JP, 2017. A nested case-control study of the association between melatonin secretion and incident myocardial infarction. Heart 103, 694–701. [DOI] [PubMed] [Google Scholar]
- Mehdi AZ, Sandor T, 1977. The effect of melatonin on the biosynthesis of corticosteroids in beef adrenal preparations in vitro. J. Steroid Biochem 8, 821–823. [DOI] [PubMed] [Google Scholar]
- Misaka T, Yoshihisa A, Yokokawa T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Takeishi Y, 2019. Plasma levels of melatonin in dilated cardiomyopathy. J. Pineal Res 66, e12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Ghosh AK, Bandyopadhyay A, Basu A, Datta S, Pattari SK, Reiter RJ, Bandyopadhyay D, 2012. Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 53, 166–179. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D, 2015. Mechanisms of isoproterenol-induced cardiac mitochondrial damage: protective actions of melatonin. J. Pineal Res 58, 275–290. [DOI] [PubMed] [Google Scholar]
- Mukherjee DRS, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D, 2010. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res 48, 251–262. [DOI] [PubMed] [Google Scholar]
- Nduhirabandi F, Du Toit EF, Blackhurst D, Marais D, Lochner A, 2011. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res 50, 171–182. [DOI] [PubMed] [Google Scholar]
- Nduhirabandi F, Huisamen B, Strijdom H, Blackhurst D, Lochner A, 2014. Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J. Pineal Res 57, 317–332. [DOI] [PubMed] [Google Scholar]
- Nduhirabandi F, Lamont K, Albertyn Z, Opie LH, Lecour S, 2016. Role of toll-like receptor 4 in melatonin-induced cardioprotection. J. Pineal Res. 60, 39–47. [DOI] [PubMed] [Google Scholar]
- Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, Cerretani D, 2015. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr. Vasc. Pharmacol 13, 26–36. [DOI] [PubMed] [Google Scholar]
- Nishi EE, Almeida VR, Amaral FG, Simon KA, Futuro-Neto HA, Pontes RB, Cespedes JG, Campos RR, Bergamaschi CT, 2019. Melatonin attenuates renal sympathetic overactivity and reactive oxygen species in the brain in neurogenic hypertension. Hypertens. Res. 42, 1683–1691. [DOI] [PubMed] [Google Scholar]
- Pal PBB, Ghosh A, Chattopadhyay A, Bandyopadhyay D, 2018. Adrenaline induced disruption of endogenous melatoninergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1, 109–131. [Google Scholar]
- Patel V, Upaganlawar A, Zalawadia R, Balaraman R, 2010. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol 644, 160–168. [DOI] [PubMed] [Google Scholar]
- Pei HF, Hou JN, Wei FP, Xue Q, Zhang F, Peng CF, Yang Y, Tian Y, Feng J, Du J, He L, Li XC, Gao EH, Li Yang YJ, 2017. Melatonin attenuates postmyocardial infarction injury via increasing Tom70 expression. J. Pineal Res 62. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, Fiore T, Paradies G, 2009. Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am. J. Physiol. Heart Circ. Physiol 297, H1487–H1493. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G, 2010. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J. Pineal Res 48, 340–346. [DOI] [PubMed] [Google Scholar]
- Rogers N, van den Heuvel C, Dawson D, 1997. Effect of melatonin and corticosteroid on in vitro cellular immune function in humans. J. Pineal Res 22, 75–80. 10.1111/j.1600-079x.1997.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Rouleau JL, Warnica WJ, Baillot R, Block PJ, Chocron S, Johnstone D, Myers MG, Calciu CD, Dalle-Ave S, Martineau P, Mormont C, van Gilst WH, Investigators I, 2008. Effects of angiotensin-converting enzyme inhibition in low-risk patients early after coronary artery bypass surgery. Circulation 117, 24–31. [DOI] [PubMed] [Google Scholar]
- Sahna E AA, Ozer MK, Olmez E, 2002. Myocardial ischemia-reperfusion in rats: reduction of infarct size by either supplemental physiological or pharmacological doses of melatonin. J. Pineal Res 33, 234–238. [DOI] [PubMed] [Google Scholar]
- Sallinen P, Manttari S, Leskinen H, Ilves M, Vakkuri O, Ruskoaho H, Saarela S, 2007. The effect of myocardial infarction on the synthesis, concentration and receptor expression of endogenous melatonin. J. Pineal Res 42, 254–260. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Gebhard MM, Gross W, 2019. The effect of melatonin on hearts in ischemia/reperfusion experiments without and with HTK cardioplegia. Bioelectrochemistry 129, 170–178. [DOI] [PubMed] [Google Scholar]
- Shafiei EBM, Raj P, et al. , 2018. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: a randomized, open-labeled, placebo-controlled trial. Medicine (Baltim.) 97, e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko FBT, Krajcirovicova K, et al. , 2018. Effect of melatonin on the renin-angiotensin-aldosterone system in l-NAME-induced hypertension. Molecules 23, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko FRR, Paulis L, 2019. Melatonin as a rational alternative in the conservative treatment of resistant hypertension. Hypertens. Res 42, 1828–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroethoff M, Behmenburg F, Spittler K, Raupach A, Heinen A, Hollmann MW, Huhn R, Mathes A, 2018a. Activation of melatonin receptors by ramelteon induces cardioprotection by postconditioning in the rat heart. Anesth. Analg 126, 2112–2115. [DOI] [PubMed] [Google Scholar]
- Stroethoff M, Christoph I, Behmenburg F, Raupach A, Bunte S, Senpolat S, Heinen A, Hollmann MW, Mathes A, Huhn R, 2018b. Melatonin receptor agonist ramelteon reduces ischemia-reperfusion injury through activation of mitochondrial potassium channels. J. Cardiovasc. Pharmacol 72, 106–111. [DOI] [PubMed] [Google Scholar]
- Sun H, Gusdon AM, Qu S, 2016. Effects of melatonin on cardiovascular diseases: progress in the past year. Curr. Opin. Lipidol 27, 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarszoi O, Asemu G, Vanecek J, Ost’adal B, Kolar F, 2001. Effects of melatonin on ischemia and reperfusion injury of the rat heart. Cardiovasc. Drugs Ther 15, 251–257. [DOI] [PubMed] [Google Scholar]
- Tare M, Parkington HC, Wallace EM, Sutherland AE, Lim R, Yawno T, Coleman HA, Jenkin G, Miller SL, 2014. Maternal melatonin administration mitigates coronary stiffness and endothelial dysfunction, and improves heart resilience to insult in growth restricted lambs. J. Physiol 592, 2695–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topkara VK, Evans S, Zhang W, Epelman S, Staloch L, Barger PM, Mann DL, 2011. Therapeutic targeting of innate immunity in the failing heart. J. Mol. Cell. Cardiol 51, 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance N, Elliott AM, Lee AJ, Smith BH, 2010. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur. J. Pain 14, 380–386. [DOI] [PubMed] [Google Scholar]
- Vazan RPD, Béder I, Styk J, 2005. Ischemia-reperfusion injury–antiarrhythmic effect of melatonin associated with reduced recovering of contractility. Gen. Physiol. Biophys 24, 355–359. [PubMed] [Google Scholar]
- Wang K, Liu F, Liu CY, An T, Zhang J, Zhou LY, Wang M, Dong YH, Li N, Gao JN, Zhao YF, Li PF, 2016. The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ. 23, 1394–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhou S, Wu P, Li M, Ding X, Sun L, Xu X, Zhou X, Zhou L, Cao C, Fei G, 2018. Identifying involvement of H19-miR-675–3p-IGF1R and H19-miR-200a-PDCD4 in treating pulmonary hypertension with melatonin. Mol. Ther. Nucleic Acids 13, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Wang X, Kuukasjarvi P, Laurikka J, Rinne T, Honkonen EL, Tarkka M, 2004. Bradykinin preconditioning in coronary artery bypass grafting. Ann. Thorac. Surg 78, 492–497. [DOI] [PubMed] [Google Scholar]
- Wiese C, Heisig J, Gessler M, 2010. Hey bHLH factors in cardiovascular development. Pediatr. Cardiol 31, 363–370. [DOI] [PubMed] [Google Scholar]
- Wu D, Zhang K, Hu P, 2019. The role of autophagy in acute myocardial infarction. Front. Pharmacol 10, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jb KY, Zhang C, Yu XJ, Chen WS, 2019. Infusion of melatonin into the paraventricular nucleus ameliorates myocardial ischemia-reperfusion injury by regulating oxidative stress and inflammatory cytokines. J. Cardiovasc. Pharmacol 74, 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W, Yi D, Yu S, 2013. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res 55, 275–286. [DOI] [PubMed] [Google Scholar]
- Yang Z, Li C, Wang Y, Yang J, Yin Y, Liu M, Shi Z, Mu N, Yu L, Ma H, 2018. Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J. Mol. Cell. Cardiol 125, 185–194. [DOI] [PubMed] [Google Scholar]
- Yeung HM, Hung MW, Fung ML, 2008. Melatonin ameliorates calcium homeostasis in myocardial and ischemia-reperfusion injury in chronically hypoxic rats. J. Pineal Res 45, 373–382. [DOI] [PubMed] [Google Scholar]
- Yeung HM, Hung MW, Lau CF, Fung ML, 2015. Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res 58, 12–25. [DOI] [PubMed] [Google Scholar]
- Yu J, Wei J, Ji L, Hong X, 2014a. Exploration on mechanism of a new type of melatonin receptor agonist Neu-p11 in hypoxia-reoxygenation injury of myocardial cells. Cell Biochem. Biophys. 70, 999–1003. [DOI] [PubMed] [Google Scholar]
- Yu LFC, Li Z, Zhang J, Xue X, Xu Y, Zhao G, Yang Y, Wang H, 2017a. Melatonin rescues cardiac thioredoxin system during ischemia-reperfusion injury in acute hyperglycemic state by restoring Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner. J. Pineal Res 62. [DOI] [PubMed] [Google Scholar]
- Yu LGB, Duan W, Fan C, Zhang J, Li Z, Xue X, Xu Y, Meng D, Li B, Zhang M, Zhang Bin, Jin Z, Yu S, Yang Y, Wang H, 2017b. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci. Rep 7, 41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Li B, Zhang M, Jin Z, Duan W, Zhao G, Yang Y, Liu Z, Chen W, Wang S, Yang J, Yi D, Liu J, Yu S, 2016. Melatonin reduces PERK-eIF2alpha-ATF4-mediated endoplasmic reticulum stress during myocardial ischemia-reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis 21, 809–824. [DOI] [PubMed] [Google Scholar]
- Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang Y, Yang J, Yi D, Chen W, Wang X, Duan W, Jin Z, Yu S, 2015. Membrane receptor-dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia-reperfusion injury: in vivo and in vitro studies. J. Pineal Res 59, 420–433. [DOI] [PubMed] [Google Scholar]
- Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, Pei H, Wang X, Zhang H, Meng Q, Zhang Y, Yu S, Duan W, 2014b. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J. Pineal Res 57, 228–238. [DOI] [PubMed] [Google Scholar]
- Yu LM, Di WC, Dong X, Li Z, Zhang Y, Xue XD, Xu YL, Zhang J, Xiao X, Han JS, Liu Y, Yang Y, Wang HS, 2018. Melatonin protects diabetic heart against ischemia-reperfusion injury, role of membrane receptor-dependent cGMP-PKG activation. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis 1864, 563–578. [DOI] [PubMed] [Google Scholar]
- Zaslavskaia Rm SE, Lilitsa GV, Logvinenko SI, 2010. Melatonin in the combined treatment of cardiovascular diseases. Klin. Med. (Mosc.) 88, 26–30. [PubMed] [Google Scholar]
- Zhang Y, Qiao B, Gao F, Wang H, Miao S, Zhao H, 2018. Melatonin protects H9c2 cells against ischemia/reperfusioninduced apoptosis and oxidative stress via activation of the Nrf2 signaling pathway. Mol. Med. Rep 18, 3497–3505. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Xu J, Tian F, Hu S, Chen Y, Fu Z, 2019. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/ mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res 66, e12542. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y, 2017. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Prather ER, Garrison DE, Zuo L, 2018. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]