Abstract
AIM
To compare the effect of myopia and astigmatism correction and postoperative change in higher-order aberration as results of receiving small-incision lenticule extraction (SMILE) and femtosecond laser-assisted in situ keratomileusis (FS-LASIK).
METHODS
A prospective and non-randomized controlled study was conducted. The subjects are divided into two groups according to different operations received: 229 eyes of 116 patients in the SMILE group and 168 eyes of 86 patients in the FS-LASIK group. All subjects were followed up for 3mo by monitoring their uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), spherical equivalent, higher-order aberrations, and the preoperative and postoperative complications.
RESULTS
At 1wk, 1, and 3mo post-surgery, 224 eyes (97.8%), 227 eyes (99.1%) and 229 eyes (100%) had UCVA≥20/20 in the SMILE group, while 165 eyes (98.2%), 167 eyes (99.4%) and 167 eyes (99.4%) had UCVA≥20/20 in the FS-LASIK group, respectively (χ2=0.146, 2.135, and 1.124; all P>0.05). BCVA reduction was not observed in both groups at 1 and 3mo of post-surgery (χ2=0.734 and 1.898, P>0.05). There was no statistically significant difference in the spherical equivalent between the two groups at 1 and 3mo post-surgery, though the percentage of the spherical equivalent within ±0.50 D at 3mo post-surgery was 98% in the SMILE group, which was higher than that of the FS-LASIK group (92%, χ2=1.872, P>0.05). The root mean square (RMS) values of total high-order aberration, coma, and spherical aberration of the two groups increased significantly in the early postoperative period and decreased after 3mo, but the values were still higher than the preoperative levels (P<0.05); there was no significant difference between the two groups in the RMS values of total higher-order aberrations and specific higher-order aberrations (P>0.05). The incidence of complications in the SMILE group was lower than that in the FS-LASIK group (χ2=14.52, P<0.05).
CONCLUSION
SMILE and FS-LASIK can effectively treat myopia, significantly improve visual acuity, and increase the total high-order aberration, spherical aberration, and coma. The incidence of complications after SMILE is relatively low.
Keywords: myopia, femtosecond laser in situ keratomileusis, small incision lenticule extraction, high order aberrations
INTRODUCTION
There are two areas of applications of femtosecond laser: femtosecond laser in situ keratomileusis (FS-LASIK) and small incision lenticule extraction (SMILE). FS-LASIK is capable of producing corneal flap and cutting the stroma of corneal with femtosecond laser, having the advantages of broad area of effectiveness and outstanding outcomes[1]–[2]. On the other hand, completing a SMILE only requires one piece of equipment while being minimally invasive. SMILE can maintain the wholesomeness of the front surface of the corneal, resulting in minimal effect on the corneal in terms of biomechanics. Therefore, SMILE can lead to a low chance of having postoperative dry eye symptom and a high quality of vision[3]–[4]. According to research[5]–[6], patients are usually easily affected by glares, halos, and reduction of vision in dark environment. Such symptoms are suggested to be related to postoperative higher-order aberrations. Through paired observations of preoperative and postoperative vision, diopter, higher-order aberrations, and complications of patients after receiving SMILE and FS-LASIK, our research compared the clinical effects of both operations so that helpful supports can be provided when patients are making choices.
SUBJECTS AND METHODS
Ethical Approval
The study was approved by the Ethics Committee of Beijing Tongren Hospital, and conducted in accordance with the principles of the Declaration of Helsinki. The informed consent was signed by the patients.
Subjects
Totally 397 eyes of 202 patients with myopia and astigmatism who were admitted to the Refractive Center of Beijing Tongren Hospital from June 2016 to June 2018. The subjects have a range of age from 17 to 51y with a mean of 31.26±3.44y. There were 94 males (182 eyes) and 108 females (215 eyes). The subjects are divided into two groups according to different operations received: 229 eyes of 116 patients in the SMILE group and 168 eyes of 86 patients in the FS-LASIK group. Inclusion criteria: central corneal thickness ≥450 µm; postoperative corneal stroma thickness >280 µm; no systemic diseases, other eye diseases, history of eye surgery and history of trauma; no history of wearing contact lenses or discontinued soft contact lens for more than 2wk and, rigid contact lens for more than 8wk, and orthokeratology lens for more than 3 to 6mo.
Methods
Routine examination items
All selected patients undergo routine preoperative examinations and the following data were recorded: including uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), non-contact tonometry for the intraocular pressure, slit-lamp examination, binocular ophthalmoscope retinal examination, refractive error check before and after cycloplegia, curvature of the cornea, and corneal thickness measurement using an ultrasound and corneal tomography.
Wavefront aberration measuring devices and methods
The wavefront device used was the WaveScan WavePrint system (VISX, Santa Clara, Calif), which is based on the Shack-Hartmann principle. The wavefront aberration was measured by the same examiner alone, and the aberration measurement was carried out in a dark room after 10min of dark adaptation. Images were collected within 3s after blinking, and the measurement was repeated 3 times for each eye. The interpretation and selection of wavefront aberration measurement results were referred to the clinical standard of “wavefront-guided keratorefractive surgery”. The measured result of original images with optimal focus, least center shift, low-order aberrations closest to the manifest refraction results (spherical degree: ±0.75 D, cylinder degree: ±0.5 D, astigmatism axis: within ±15°), and the most reproducible high-order aberration graphs and root mean square (RMS) values were selected for the present study. A standardized pupil diameter of 6.0 mm was used for the analysis of aberration.
Surgical procedures
During the FS-LASIK procedure, a Femto LDV femtosecond laser machine (Ziemer Ophthalmic Systems AG, Port, Switzerland) or a VisuMax full femtosecond laser machine (Zeiss, Germany) was used to produce corneal flaps with an estimated thickness of 100-110 µm and a diameter of 8.5-9.0 mm. After flap was lifted, ablations were performed using the Visx S4 excimer laser (VISX Inc., Santa Clara, USA). The corneal flap and stroma surface were irrigated with balanced normal saline solution after ablation and the flap was repositioned. During the SMILE procedure, corneal cutting was performed based on the prepared process. The related parameters are as follows: the distance between the laser spot and the matrix lens was 4.5 µm, the edge of the lens was 2.0 µm, the corneal cap thickness was 110-120 µm, the diameter was 7.0-7.6 mm, the diameter of the lens was 6.0-6.6 mm, the thickness of the thinnest part of the lens was 10-15 µm, the incision position was 90° or 120°, and the width was 2.0-2.1 mm. Levofloxacin eye drops and artificial tears were given for 2wk after surgery. 0.1% fluorometholone eye drops were given 4 times/d, which was gradually reduced after 3d, and stopped 2wk later.
Observation Index
Observed the UCVA, BCVA, spherical equivalents, and RMS of higher-order aberrations of the subjects from both groups before, one week after, one month after, and three months after the operations. Recorded the conditions of complications during and after the operations.
Statistical Analysis
The data were analyzed by SPSS22.0. Count data were expressed as a rate (%), and the χ2 test was used for analysis. Measurement data were expressed as, t-test, or analysis of variance was used for the comparison between groups. P<0.05 was considered statistically significant.
RESULTS
Preoperative Comparisons Between the Two Groups Under Normal Conditions
In terms of age, uncorrected vision, and corrected vision, subjects from both groups had no statistical differences: the two groups are comparable (Table 1).
Table 1. Comparison of general data of two groups.
| Parameters | SMILE group | FS-LASIK group | t | P |
| Age (y) | 31.22±2.87 | 32.22±3.41 | 0.307 | 0.804 |
| Spherical equivalent (D) | -5.11±1.05 | -5.13±1.01 | 0.432 | 0.787 |
| Corneal thickness (µm) | 543.62±24.11 | 517.85±21.24 | -5.092 | 0.000 |
| UCVA | 0.16±0.19 | 0.14±0.12 | 0.727 | 0.402 |
| BCVA | 0.92±0.18 | 0.91±0.20 | 0.566 | 0.724 |
Comparison of Postoperative UCVA Between the Two Groups
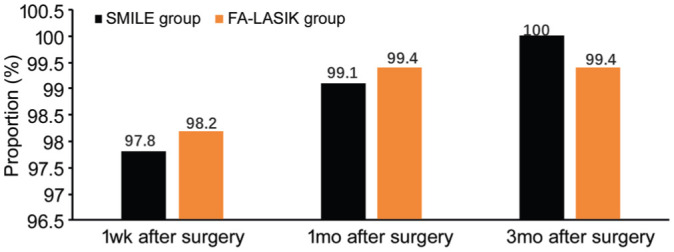
At 1wk, 1, and 3mo post-surgery, 224 eyes (97.8%), 227 eyes (99.1%), and 229 eyes (100%) had UCVA≥20/20 in the SMILE group, while 165 eyes (98.2%), 167 eyes (99.4%,) and 167 eyes (99.4%) had UCVA≥20/20 in the FS-LASIK group, and there were no significant differences between the two groups (χ2=0.146, 2.135, and 1.124; P=0.874, 0.313 and 0.524; Figure 1).
Figure 1. Comparison of the proportion of UCVA≥20/20 in the SMILE group and the FS-LASIK group at different periods post-surgery.
Comparison of Postoperative BCVA Between the Two Groups
BCVA decrease was not observed in both groups at 1 and 3mo post-surgery, and there was no statistically significant difference between the two groups (P>0.05). At 1 and 3mo post-surgery, the BCVA was unchanged in 140 eyes (61.1%) and 137 eyes (59.8%) in the SMILE group, while it was unchanged in 109 eyes (64.9%) and 107 eyes (63.7%) in the FS-LASIK group, and there were no significant differences between two groups (χ2=3.637 and 1.173, P=0.209 and 0.534). At 1 and 3mo post-surgery, the BCVA was increased by more than 1 line in 89 eyes (38.9%) and 92 eyes (40.2%) in the SMILE group, and in 59 eyes (35.1%) and 61 eyes (36.3%) in the FS-LASIK group, and there were no significant differences between two groups (χ2=3.012 and 2.113, P=0.254 and 0.339; Tables 2 and 3).
Table 2. Proportion of BCVA increase or decrease at different periods after surgery in the SMILE group.
| Time | More than 1 line of decrease | Unchanged | More than 1 line of increase | Total |
| 1mo | 0 | 140 (61.1) | 89 (38.9) | 229 (100.0) |
| 3mo | 0 | 137 (59.8) | 92 (40.2) | 229 (100.0) |
eyes (%)
Table 3. Proportion of BCVA increase or decrease at different periods after surgery in the FS-LASIK group.
| Time | More than 1 line of decrease | Unchanged | More than 1 line of increase | Total |
| 1mo | 0 | 109 (64.9) | 59 (35.1) | 168 (100.0) |
| 3mo | 0 | 107 (63.7) | 61 (36.3) | 168 (100.0) |
eyes (%)
Comparison of Postoperative Diopter Between the Two Groups
No statistically significant difference was noted in the spherical equivalent between the two groups at 1 and 3mo post-surgery. The percentage of the spherical equivalent within ±0.50 D at 3mo post-surgery was 98% in the SMILE group, which was higher than that of the FS-LASIK group (92%), but the difference was not statistically significant (χ2=1.872, P>0.05; Table 4).
Table 4. Comparison of spherical equivalent between the two groups at different periods after surgery.
| Time | SMILE group | FS-LASIK group | t | P |
| Preop. | -5.11±1.05 | -5.13±1.01 | 0.432 | 0.787 |
| 1mo after surgery | 0.35±0.21 | 0.54±2.41 | 0.594 | 0.671 |
| 3mo after surgery | 0.16±0.25 | 0.52±3.03 | 0.651 | 0.583 |
D
Comparison of High-Order Aberrations at a Pupil Diameter of 6.0 mm
Comparison of specific high-order aberrations before and after surgery
The RMS values of total high-order aberration, coma, and spherical aberration of the two groups in the early postoperative period increased significantly compared with the preoperative levels and decreased a little after 3mo, but the values were still higher than the preoperative levels, and the differences were statistically significant (P<0.05; Tables 5 and 6).
Table 5. Comparison of RMS values of specific high-order aberrations before and after surgery in the SMILE group.
| Parameters | Preop. | 1mo after surgery | 3mo after surgery |
| Total HOA | 0.38±0.12 | 0.56±0.17a | 0.52±0.15a |
| Coma | 0.21±0.14 | 0.30±0.19a | 0.27±0.11a |
| Trefoil | 0.19±0.1 | 0.18±0.13 | 0.19±0.13 |
| Spherical aberration | 0.11±0.11 | 0.18±0.19a | 0.16±0.15a |
| 3rd order | 0.30±0.16 | 0.33±0.12 | 0.33±0.10 |
| 4th order | 0.17±0.09 | 0.20±0.09 | 0.19±0.11 |
| 5th order | 0.11±0.07 | 0.13±0.08 | 0.13±0.07 |
aCompared with preoperative value, P<0.05.
µm
Table 6. Comparison of RMS values of specific high-order aberrations before and after surgery in the FS-LASIK group.
| Parameters | Preop. | 1mo after surgery | 3mo after surgery |
| Total HOA | 0.38±0.16 | 0.59±0.17a | 0.54±0.18a |
| Coma | 0.22±0.18 | 0.32±0.18a | 0.29±0.19a |
| Trefoil | 0.19±0.11 | 0.21±0.13 | 0.21±0.11 |
| Spherical aberration | 0.11±0.09 | 0.23±0.19a | 0.19±0.17a |
| 3rd order | 0.31±0.15 | 0.35±0.10 | 0.34±0.11 |
| 4th order | 0.19±0.08 | 0.22±0.10 | 0.21±0.12 |
| 5th order | 0.10±0.07 | 0.11±0.09 | 0.11±0.08 |
aCompared with preoperative value, P<0.05.
µm
Comparison of various high-order aberrations between the two groups
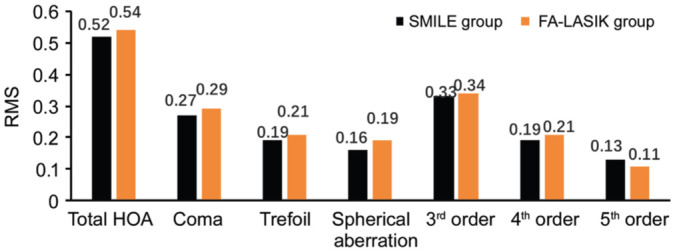
There were no statistically significant differences in the RMS values of the total high-order aberration and specific high-order aberrations between the two groups at different periods post-surgery (Figures 2 and 3).
Figure 2. Comparison of the RMS values of the total high-order aberration and specific high-order aberrations between the two groups at 1mo post-surgery.
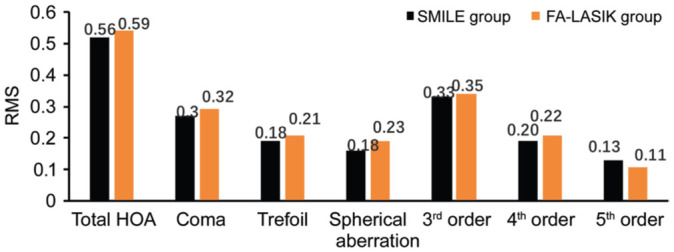
Figure 3. Comparison of the RMS values of the total high-order aberration and specific high-order aberrations between the two groups at 3mo post-surgery.
Comparison of Intraoperative and Postoperative Complications Between the Two Groups
Operations were conducted smoothly in both groups, no serious complications such as negative pressure loss and corneal infections happened. However, after the operation, sub conjunctional hemorrhage happened to one eye from the SMILE group (0.44%) and two eyes (1.19%) from the FS-LASIK group. There were 5 eyes (2.18%) from the SMILE group had the symptoms of glare, halo, and night vision loss while there were 9 eyes (5.36%) from the FS-LASIK group caught the same symptoms. Also, seven eyes (3.06%) from the SMILE group had the sensations of dryness, foreign body, burning, fatigue, and discomfort which were the typical symptoms of dry eye, and there were 11 eyes (6.55%) from the FS-LASIK group had the same symptoms. No complications such as diffuse interlamellar keratitis, corneal epithelium implantation, and refractive back happened to either of the groups. The complication rate of the SMILE group (13 eyes, 5.68%) was lower than that of the FS-LASIK group (22 eyes, 13.1%), and the difference was statistically significant (χ2=14.52, P<0.05; Table 7).
Table 7. Comparison of complication rates in the two groups.
| Complications | SMILE group |
FS-LASIK group |
||
| No. (eyes) | Incidence (%) | No. (eyes) | Incidence (%) | |
| Subconjunctival hemorrhage | 1 | 0.44 | 2 | 1.19 |
| Diffuse lamellar keratitis | 0 | 0 | 0 | 0 |
| Postoperative glare | 5 | 2.18 | 9 | 5.36 |
| Regression | 0 | 0 | 0 | 0 |
| Dry eye | 7 | 3.06 | 11 | 6.55 |
| Infection | 0 | 0 | 0 | 0 |
| Epithelial ingrowth | 0 | 0 | 0 | 0 |
| Total | 13 | 5.68 | 22 | 13.1 |
DISCUSSION
The application of femtosecond laser has led to the rapid development of keratorefractive surgery. Compared with the microkeratome, the femtosecond laser makes flap creation safer during LASIK[7]–[8], and the appearance of SMILE is an important innovation in the practice of refractive surgery, which set up the era of minimally invasive keratorefractive surgery[9]–[10]. Compared with FS-LASIK, SMILE does not require the production of corneal flaps to ensure that it will not cause intraoperative and postoperative complications caused by corneal flap production, and its impact on nerve tissue becomes smaller[11]. However, it is not still understood which surgical method is more suitable for the patient. The present study observed and compared the 3-month follow-up examination results of these two surgical procedures to provide a reference for the selection of clinical surgical procedures.
The safety, effectiveness, predictability, and stability are the main indicators used for evaluating refractive surgery[12]. The results of this study showed that although the visual acuity of patients in the FS-LASIK group was better in the early stage after surgery, the SMILE group showed a steady improvement in the later stage. At 1wk, 1 and 3mo post-surgery, 224 eyes (97.8%), 227 eyes (99.1%), and 229 eyes (100%) had UCVA≥20/20 in the SMLIE group, while 165 eyes (98.2%), 167 eyes (99.4%), and 167 eyes (99.4%) had UCVA≥20/20 in the FS-LASIK group, and there were no significant differences between the two groups, which suggests that both surgical procedures are highly effective, and this result is consistent with the result of Yang et al[13] who also compared the above two surgical procedures. The reason for the slow recovery of visual acuity after SMILE may be that the surgical procedure (lenticule creation and extraction) causes a certain degree of corneal stromal edema, which in turn affects early visual acuity[14]. In the present study, comparison of the diopter showed that there was no statistically significant difference in the spherical equivalent at each time point post-surgery. At 3mo post-surgery, the percentage of the spherical equivalent within ±0.50 D was 98% in the SMILE group, which was higher than that of the FS-LASIK group (92%), but the difference was not statistically significant (P>0.05). This result shows that both surgical procedures have high predictability. BCVA decrease was not observed in both groups at 1 and 3mo post-surgery, and there were no postoperative serious complications such as infection and epithelial ingrowth, which shows that the two surgical procedures are highly safe in the correction of myopia.
Although the two surgical procedures have relatively high safety, effectiveness, predictability, and stability in correcting myopia, how to better improve the visual quality of patients after surgery is still the focus of attention for refractive surgeons. Visual quality can not only reflect the clinical outcome after surgery to a certain extent but also reflect the subjective feelings of patients, such as the comfort of vision, etc[15]. Keratorefractive surgery can cause changes in the morphology and structure of the cornea, and corneal cutting may also result in corresponding changes in the postoperative optical quality. Studies[16]–[17] showed that the high-order aberrations, especially spherical aberration and coma increased after SMILE and FS-LASIK. In the present study, the RMS values of total high-order aberration, coma, and spherical aberration of the two groups in the postoperative period increased significantly compared with the preoperative levels and decreased after 3mo, but the values were still higher than the preoperative levels, and the differences were statistically significant. Corneal flap healing after FS-LASIK can lead to an increase in postoperative aberrations, especially coma. SMILE does not need to make a corneal flap, which can avoid the increase in flap-derived aberrations. However, after lenticule extraction, corneal morphology changes and remodeling of the corneal structure caused by the postoperative wound healing can cause an increase in the postoperative high-order aberrations.
In previous study, Luo et al[18] found the values of total higher-order aberration and coma in the SMILE group were lower than those in the FS-LASIK group at 1 and 3mo after surgery, and the difference was statistically significant. In the study of Tan and Ma[19], the values of total high-order aberration, spherical aberration, coma, and trefoil aberration in the FS-LASIK group and the SMILE group increased at 3mo post-surgery compared to the preoperative values, and the values of spherical aberration and trefoil aberration in the SMILE group were less than those of the FS-LASIK group at 3mo post-surgery; the incidence and degree of the halo were significantly reduced in the SMILE group at 6mo post-surgery compared with those measured 1mo post-surgery, however, there were no significant differences in the values of high-order aberrations between the two groups. The results of this study showed that except for the fifth-order aberration, the RMS values of the total high-order aberration and specific high-order aberrations in the SMILE group at 1 and 3mo post-surgery were lower than those of the FS-LASIK group, but the difference was not statistically significant. The main reason is that SMILE does not need to make corneal flaps, which can avoid the corneal biomechanical changes caused by FS-LASIK making corneal flaps, and also avoid the increase of high-order aberrations that may be caused by the abnormal position of the corneal flaps. Therefore, the flap-derived aberrations rarely increase after SMILE. However, the corneal asphericity changes after refractive surgery, which results in increased aberrations, especially spherical aberration. Therefore, the effects of the two surgical procedures on the postoperative high-order aberrations are also the same.
This study also compared the postoperative complication rates of the two surgical procedures. The results showed that the complication rate of the SMILE group (5.68%) was lower than that of the FS-LASIK group (13.1%), and the difference was statistically significant. Especially, the incidence of postoperative complications such as dry eye was significantly lower in the SMILE group than that in the FS-LASIK group, which is consistent with the study of Tian and Wang[20]. The cornea is innervated by the ophthalmic branch of the trigeminal nerve, which enters the anterior 1/3 layer of the corneal stroma radially from the nasal and temporal corneal limbi, and enters the epithelial layer from the anterior elastic layer[21]. During the FS-LASIK surgery, in the process of making the corneal flap and laser cutting, the corneal nerve is partially cut off. The larger the corneal flap, the more serious the nerve damage, which results in decreased corneal perception, damage to the function of the lacrimal reflex, and reduced tear secretion[12],[22]. However, during the SMILE surgery, only a small incision of 2 to 4 mm is made at the edge of the corneal cap, and the scan is closer to the middle stromal layer, so the damage to the corneal nerve is small, the corneal perception recovers quickly, and the occurrence of postoperative dry eye is reduced.
In conclusion, SMILE and FS-LASIK are both safe and effective in the treatment of myopia and astigmatism. Patients have good visual quality after surgery, and the incidence of complications after SMILE is relatively low. These two surgical procedures should be widely used in clinical practice.
Acknowledgments
Conflicts of Interest: Zhao PF, None; Hu YB, None; Wang Y, None; Fu CY, None; Zhang J, None; Zhai CB, None.
REFERENCES
- 1.Bao FJ, Huang W, Zhu R, Lu NJ, Wang Y, Li HC, Wu S, Lin HN, Wang JJ, Zheng XB, Huang JH, Li YY, Wang QM, Elsheikh A. Effectiveness of the goldmann applanation tonometer, the dynamic contour tonometer, the ocular response analyzer and the corvis ST in measuring intraocular pressure following FS-LASIK. Curr Eye Res. 2020;45(2):144–152. doi: 10.1080/02713683.2019.1660794. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, He FL, Liu Y, Fan XQ. Comparison of choroidal thickness in high myopic eyes after FS-LASIK versus implantable collamer lens implantation with swept-source optical coherence tomography. Int J Ophthalmol. 2020;13(5):773–781. doi: 10.18240/ijo.2020.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao KW, Liu LN, Yu T, Chen F, Bai J, Liu T. Changes in corneal biomechanics during small-incision lenticule extraction (SMILE) and femtosecond-assisted laser in situ keratomileusis (FS-LASIK) Lasers Med Sci. 2020;35(3):599–609. doi: 10.1007/s10103-019-02854-w. [DOI] [PubMed] [Google Scholar]
- 4.Hamed AM, Heikal MA, Soliman TT, Daifalla A, Said-Ahmed KE. SMILE intraoperative complications: incidence and management. Int J Ophthalmol. 2019;12(2):280–283. doi: 10.18240/ijo.2019.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohac M, Koncarevic M, Dukic A, Biscevic A, Cerovic V, Merlak M, Gabric N, Patel S. Unwanted astigmatism and high-order aberrations one year after excimer and femtosecond corneal surgery. Optom Vis Sci. 2018;95(11):1064–1076. doi: 10.1097/OPX.0000000000001298. [DOI] [PubMed] [Google Scholar]
- 6.Zhao PF, Li SM, Lu J, Song HM, Zhang J, Zhou YH, Wang NL. Effects of higher-order aberrations on contrast sensitivity in normal eyes of a large myopic population. Int J Ophthalmol. 2017;10(9):1407–1411. doi: 10.18240/ijo.2017.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L. Changes of corneal curvature and astigmatism and anterior chamber depth after FS-LASIK. Guoji Yanke Zazhi(Int Eye Sci) 2020;20(2):378–381. [Google Scholar]
- 8.Zhou JH, Gao Y, Li SW, Gu W, Wu LJ, Guo XH. Predictors of myopic regression for laser-assisted subepithelial keratomileusis and laser-assisted in situ keratomileusis flap creation with mechanical microkeratome and femtosecond laser in low and moderate myopia. Ophthalmic Epidemiol. 2020;27(3):177–185. doi: 10.1080/09286586.2019.1704793. [DOI] [PubMed] [Google Scholar]
- 9.Elmassry A, Ibrahim O, Osman I, Said A, Sabry M, Seifelnasr M, Gaballah K, Abdalla M. Long-term refractive outcome of small incision lenticule extraction in very high myopia. Cornea. 2020;39(6):669–673. doi: 10.1097/ICO.0000000000002288. [DOI] [PubMed] [Google Scholar]
- 10.Han T, Shang JM, Zhou XY, Xu Y, Ang M, Zhou XT. Refractive outcomes comparing small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for high myopia. J Cataract Refract Surg. 2020;46(3):419–427. doi: 10.1097/j.jcrs.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 11.Jing CR. Clinical observation of visual acuity within 24h after SMILE and femtosecond LASIK. Guoji Yanke Zazhi(Int Eye Sci) 2019;19(1):172–174. [Google Scholar]
- 12.Jing CR. Clinical effect of SMILE and FS-LASIK and the effect on the stability of cornea and tear film. Guoji Yanke Zazhi(Int Eye Sci) 2018;18(10):1866–1869. [Google Scholar]
- 13.Yang WM, Liu ST, Li MY, Shen Y, Zhou XT. Visual outcomes after small incision lenticule extraction and femtosecond laser-assisted LASIK for high myopia. Ophthalmic Res. 2020;63(4):427–433. doi: 10.1159/000504304. [DOI] [PubMed] [Google Scholar]
- 14.Zou GC, Ye JJ. A comparative study of three excimer laser refractive surgical procedures for myopia and astigmatism. Journal of Clinical Research. 2019;36(11):2172–2175. 2178. [Google Scholar]
- 15.Luo WQ, Liu HT, Xiao X, et al. Research progress of visual quality after corneal refractive surgery. Chinese And Foreign Medical Research. 2020;18(5):181–183. [Google Scholar]
- 16.Zheng Y, Zhou YH, Zhang J, Zhang L, Zhai CB, Hu YB, Liu J, Wang Y. Comparison of the visual quality at 1 year following femtosecond laser-assisted LASIK, wavefront-guided femtosecond LASIK and small incision lenticule extraction for myopia and astigmatism. Zhonghua Yan Ke Za Zhi. 2020;56(2):118–125. doi: 10.3760/cma.j.issn.0412-4081.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Sun XY, Wang XR, et al. Comparison of two optical zones in visual quality after femtosecond laser-assisted LASIK. Guoji Yanke Zazhi(Int Eye Sci) 2019;19(8):1373–1376. [Google Scholar]
- 18.Luo FX, Yang C, Lu Q, et al. Effect of early postoperative visual quality femtosecond laser incision lens matrix removed. Guoji Yanke Zazhi(Int Eye Sci) 2019;19(3):414–417. [Google Scholar]
- 19.Tan Q, Ma DJ. Comparison of visual quality after SMILE and FS-LASIK. Chinese Journal of Optometry & Ophthalmology and Visual Science. 2017;19(8):468–475. [Google Scholar]
- 20.Tian Y, Wang ST. Observation on the effect of SMILE and FS-LASIK in treating myopia. Practical Journal of Medicine & Pharmacy. 2020;37(6):501–504. [Google Scholar]
- 21.Wang QM. Refractive surgery. Beijing: People's Medical Publishing House; 2011. pp. 8–9. [Google Scholar]
- 22.Wu XJ, Wu J, Huang HY, et al. Corneal densitometry after LASIK, FS-LASIK, and small-incision lenticule extraction. Journal of Clinical Ophthalmology. 2019;14(5):20–21. [Google Scholar]


