Abstract
Background/purpose
The colonization of microorganisms onto denture bases is one common problem that can contribute to oral diseases. Herein, three food preservatives, including zinc oxide, potassium sorbate, and sodium metabisulfite were introduced as anti-microbial additives into a heat-polymerized poly(methyl methacrylate) (PMMA).
Materials and methods
Relative microbial reductions of the modified PMMA resins against Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans were evaluated. The in vitro cytotoxicity of the materials was measured against mouse fibroblast L929 cells. A three-point flexural test was performed to determine a flexural strength and modulus properties of the materials.
Results
The incorporation of all preservative agents into the material diminished the microbial growth of three microbial species. The PMMA resin combined with sodium metabisulfite exhibited the greatest anti-microbial activity that reduced almost all bacterial cells and about 40% of C. albicans. All modified resins showed no significant cytotoxicity against L929 cells. The addition of food preservatives did not significantly alter the flexural strength of the PMMA resin (∼84–92 MPa). However, the flexural modulus of the PMMA incorporated with food preservatives (∼2,024–2,144 MPa) was significantly lower than the unmodified PMMA.
Conclusion
Three food preservatives, especially sodium metabisulfite, could be applied as anti-microbial additives into the denture base resin. The PMMA incorporated with the additives did not show cytotoxicity. Although, the addition of the food preservatives altered the mechanical properties, the materials still provided acceptable flexural properties.
Keywords: Anti-bacterial property, Anti-fungal property, Denture base material, Food preservatives, Poly(methyl methacrylate)
Introduction
Poly (methyl methacrylate) (PMMA) has been commonly used as denture base materials for several decades. It has advantages of easy manipulation and processing, low cost, good appearance, and acceptable color stability.1,2 However, the denture base materials are prone to be colonized by microorganisms in the oral environment, including Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans.3, 4, 5 These can contribute diseases in the oral cavity, especially denture stomatitis that is one major problem among denture wearers.6 Moreover, microbial infections can also cause systemic effects, such as pneumonia and endocarditis.7,8 Thus, an improvement of denture base materials to include anti-microbial activity is essential.
One attempt to promote anti-microbial properties of denture base materials is an incorporation of drugs or anti-septics such as ketoconazole, chlorhexidine, and quaternary ammonium salts.9, 10, 11 Although, anti-microbial drugs are effective, the drug resistance and toxicity issue might occur. Herein, preservative agents, including zinc oxide microparticles (ZnO; ∼45 μm), potassium sorbate (PS; C6H7KO2), and sodium metabisulfite (SM; Na2S2O5) were introduced as alternative compounds. The preservatives have been used in foods and cosmetics to prevent microbial growth.12,13 Therefore, the anti-microbial effect of the denture base combined with these additives could be expected with favorable biocompatibility.
The purpose of this study was to incorporate different food preservatives (ZnO, PS, and SM) into a PMMA denture base resin and evaluate in vitro anti-microbial activity against various species and cytotoxicity to L929 fibroblast cells. An influence of the additives on mechanical properties of the materials was also investigated.
Materials and methods
PMMA denture base resin
The heat polymerized denture base acrylic resin was manufactured by the Siam Cement Public Company Limited. (SCG; Bangkok, Thailand) and used throughout the study. The product composition was mentioned in the patent.14 Briefly, the powder was predominantly PMMA particles with average molecular weight 1.2 × 106 g/mol and particle size around 80 μm. The liquid compartment mainly consisted of methyl methacrylate (MMA) monomer and also a small amount of initiator, plasticizer, and stabilizer. For anti-microbial denture base formulae, the food preservatives were mixed with the PMMA powder to certain concentrations (0.25 %w/w of ZnO, 1.0 %w/w of PS, and 0.5 %w/w of SM) according to the previous patent.14
To fabricate the denture base specimens in all experiments, the metal moulds were designed to achieve uniform sized specimens. The powder and liquid were mixed according to the manufacturer's recommendations (20 g: 11 mL). The materials were packed into the mould at dough stage under pressure of 2–4 bar for 20 min. The mould containing specimens was places in water tank for 45 min at 90 °C and then cooled for an hour before deflasking. The excess resin was trimmed off with high-speed burs and finished using silicon carbide papers. Prior to the testing, the specimens were wet-grinded under water cooling using metallographic grinding papers with a grain size of approximately 30 μm (P500). For biologic tests, the materials were cleaned by 70% ethanol and sterilized both sides by UV-C radiation for 15 min.
Anti-microbial property of the modified PMMA denture base
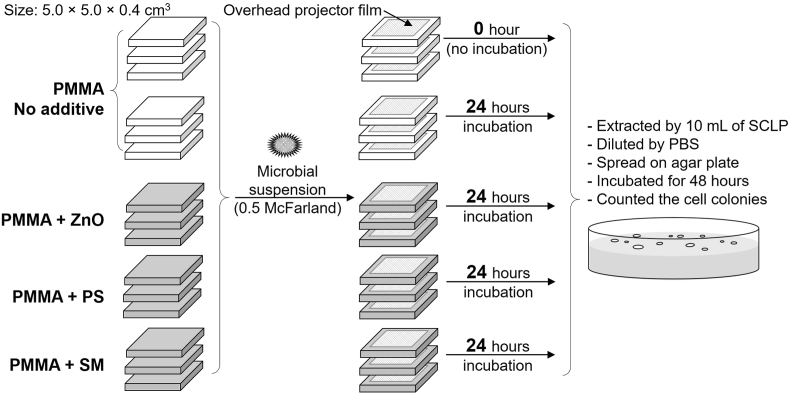
Three oral microbial species were selected, including S. aureus (ATCC 6538p), P. aeruginosa (ATCC 27853), and C. (ATCC 10231). The cell culture media and condition are described in Supplementary data. The evaluation of anti-microbial effect of the denture base resin incorporated with the preservative agents was adapted from the International Organization for Standardization (ISO) 22196:2011.15 In this method, the microbial suspension was adjusted to the turbidity of 0.5 McFarland standard (∼2.5 × 105 to 1.0 × 106 cells/mL). Then, 400 μl of the cell suspension was inoculated on the acrylic surface (5.0 × 5.0 × 0.4 cm3 in size) and covered by sterile overhead projector film (4.0 × 4.0 × 0.1 cm3 in size). The specimens were incubated at 35 ± 1 °C for 24 h. After incubation, the cells were extracted by Soybean Casein Lecithin Polysorbate (SCLP) broth. The extracted cells were diluted and swirled onto sterile agar plates with potato dextrose agar. The plates were incubated at 35 ± 1 °C for 48 h. The number of colonies was counted and then calculated into cells/cm2. The unmodified PMMA resin contacted to the cell suspension for 0 and 24 h were used as the controls. Each group was done by three parallel specimens (as shown in Fig. 1). Three independent experiments (n = 3) were performed. In this study, the anti-microbial effect of modified denture base materials was represented by the relative microbial reduction (%) compared with the unmodified PMMA after incubation for 24 h as Equation (1).
| Relative microbial reduction = [(N0 – N1) / N0] × 100 | (Equation 1) |
where: N0 was the number of viable cells (cells/cm2) on the unmodified PMMA after 24 h.
Figure 1.
Experimental design for the evaluation of the anti-microbial property of the denture base resins incorporated with food preservatives. PBS: Phosphate buffer saline; PMMA: Poly(methyl methacrylate); PS: Potassium sorbate; SCLP: Soybean Casein Lecithin Polysorbate; SM: Sodium metabisulfite; ZnO: Zinc oxide particles.
N1 was the number of viable cells (cells/cm2) on the modified PMMA after 24 h.
In vitro cytotoxic assay
The cytotoxicity of the PMMA resin incorporated with food preservatives were conducted by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the ISO 10993-5:2009.16 The mouse fibroblast L929 cells at 105 cells/cm3 were plated in a 96-well plate at 100 μL each well. The cells were incubated at 37 °C, 95% humidity, and 5% CO2 for 24 h.
The acrylic resin specimens were prepared by metal mould and cut in small pieces weighted 0.2 g. The specimens were extracted by 1 mL of Minimum Essential Medium (MEM) at 37 °C for 24 h. Thermanox® Plastic coverslip and a polyurethane film containing 0.1% Zinc diethyldithiocarbamate (ZDEC) were employed as negative and positive controls, respectively. 100 μL of the liquid extracts was then transferred to the well containing L929 cell monolayer. For untreated wells, the cell monolayer was added by 100 μL of MEM. All groups were incubated at 37 °C under 95% humidity and 5% CO2 for 24 h. Six replicates for each group were performed. Thereafter, the well containing MEM without the cell line was used as a blank. The cell line was gently washed with phosphate buffer saline (PBS). The viable cells were stained with 50 μL of 0.1% MTT in Dulbecco's Modified Eagle's medium (DMEM) without supplement and phenol red. The cells were incubated in 95% humidity, 5% CO2 atmosphere for 2 h. Then, MTT was removed and washed with PBS. After adding 100 μL of isopropanol for each well, the 96-well plate were shaken for 30 min and measured the absorbance at 570 nm (OD570) using a microplate reader. The percentage of viable L929 cells was calculated by comparing the OD570 with the untreated group. Eight independent experiments (n = 8) were done.
Three-point flexural test of the anti-microbial denture base materials
Flexural strength and modulus of the denture base incorporated with anti-microbial additives were investigated by three-point flexural tests according to the ISO 20795-1:2013.17 The heat-cured acrylic were mixed with recommended P:L ratio at 20:11 as the manufacturer's instructions. The specimens were fabricated by flasking technique using the stainless steel mold mounted with dental stone internally to control the dimension (length 64 mm; width 10 mm; thickness 3.3 mm). The prepared acrylic was grinded with Silicon carbide (15 μm) waterproof abrasive papers under running water and then immersed in distilled water at 37 °C for 48 h. Seven specimens (n = 7) for each material were fabricated and tested by TG-5kN universal testing machine (Shimadzu, Japan). For the three-point flexural test, the test condition was set with a support span of 50 mm and a crosshead speed of 5 mm/min. Flexural strength and modulus of the materials were calculated by following formulae (Equations (Equation 2), (Equation 3), respectively):
| Flexural strength = 3PL/2bh2, | (Equation 2) |
where P is the maximum load in fracture (N), L is the support span (mm), b is the test piece width (mm) and h is the test piece thickness (mm).
| Flexural modulus = FL3/4dbh3, | (Equation 3) |
where F is the load at the proportionate point on the load–deformation curve (N) and d is the deformation at a load of F (mm).
Data analysis
All statistical computations were performed by SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc. The significant differences between cell viabilities and mechanical properties of the anti-microbial resins compared with the unmodified PMMA control were analyzed by one-way ANOVA followed with Dunnett's test at α = 0.05.
Results
Relative microbial reduction of the acrylic resin incorporated with preservatives
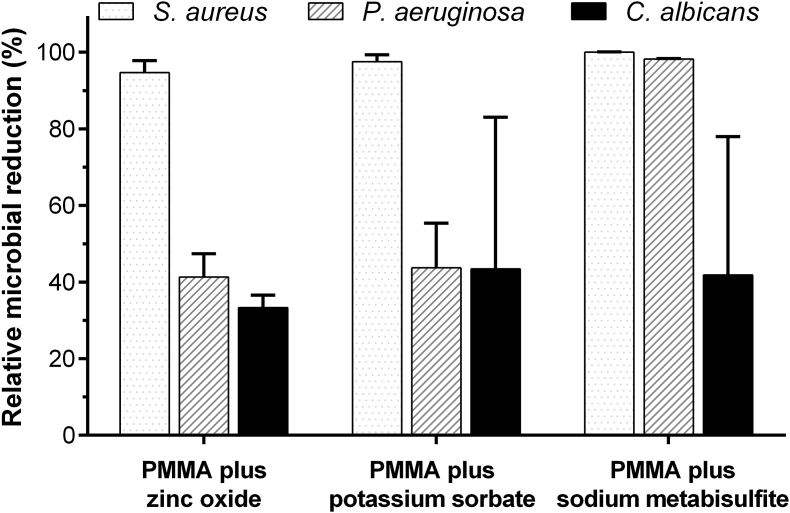
The numbers of microbial cells at contact periods for 0 and 24 h onto the unmodified PMMA resin are shown in Table 1. For all microbial species, the numbers of viable cells on the unmodified PMMA surface significantly increased after 24 h (p < 0.05). For the modified PMMA resins, their anti-microbial effect was represented by a decrease in the number of microbial cells compared with the control PMMA. Three additives provided an inhibition effect against all microbial species (Fig. 2). The gram-positive bacterium, S. aureus, was highly susceptible (almost 100% microbial reduction) to the anti-microbial acrylic resins. Whereas, the moderate microbial inhibitory effect (about 40%) was observed in the gram-negative bacterium, P. aeruginosa. Only the acrylic resin with SM showed strongly anti-microbial effect (∼98%). For opportunistic fungus, C. albicans exhibited less susceptibility (30–40% microbial reduction) among all materials.
Table 1.
Number of viable microbial cells treated on the unmodified heat-polymerized PMMA resin for 0 and 24 h.
| Microbial species | Number of viable microbial cells (×104 cells/cm2) |
|
|---|---|---|
| PMMA contact perioda 0 hour |
PMMA contact perioda 24 hours |
|
| S. aureus | 1.19 ± 0.62 | 68.27 ± 16.35∗ |
| P. aeruginosa | 2.21 ± 0.25 | 94.2 ± 15.37∗ |
| C. albicans | 0.84 ± 0.06 | 12.33 ± 2.32∗ |
Values were represented in mean ± standard deviation (n = 3). Asterisk indicated significant different from the unmodified PMMA with 0 h-contact period (p < 0.05).
PMMA: Poly(methyl methacrylate).
Contact period can be defined as an exposure time of the microbial cells onto the PMMA surface.
Figure 2.
Relative microbial reductions (mean ± standard deviation) against three microbial species at 24 h of the heat-cured PMMA resins with different food preservatives. The data were represented as percentages compared to the control PMMA without additive at 24 h. PMMA: Poly(methyl methacrylate).
In comparison between the PMMA combined with different preservative agents, the denture base with SM as the additive appeared the most effective composition to inhibit microorganisms, especially against both positive and negative gram bacteria (more than 90% microbial reduction). While, the formulae with ZnO and PS provided strong microbial inhibition for only S. aureus and showed moderate effect less than 50% reduction for both P. aeruginosa and C. albicans.
Cytotoxicity of the anti-microbial PMMA materials
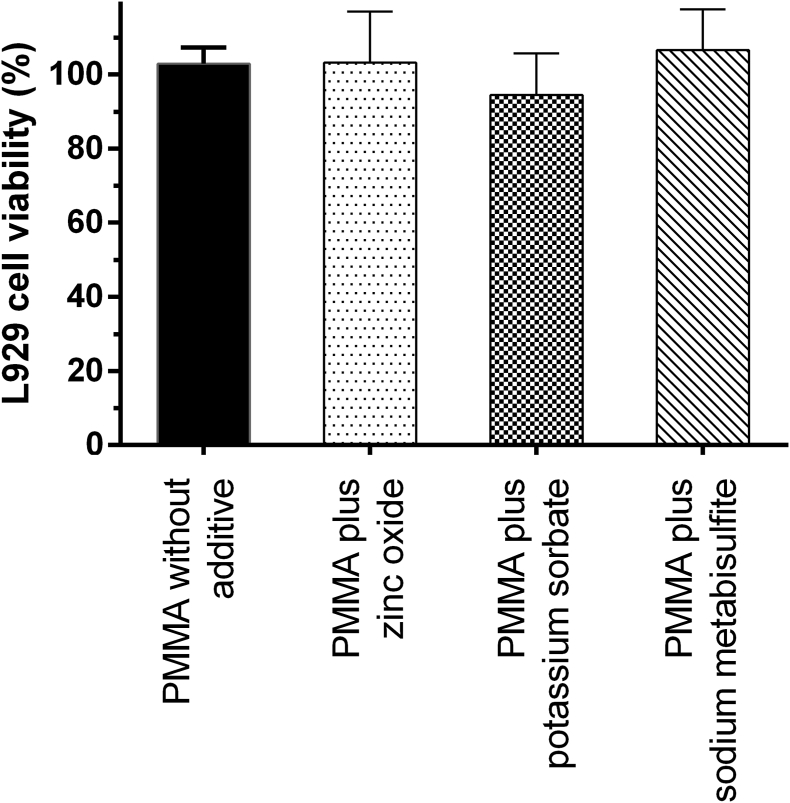
The short-term cytotoxicity against L929 fibroblast cells of the heat-cured acrylic resin incorporated with preservative agents is shown in Fig. 3. The PMMA denture base without additive had no cytotoxic effect to L929 cells. The incorporation of all preservatives did not significantly cause the cytotoxicity when compared with the PMMA control (p < 0.05).
Figure 3.
In vitro cytotoxicity (mean ± standard deviation) against L929 cells of the PMMA acrylic resin with zinc oxide particles, potassium sorbate, and sodium metabisulfite. PMMA: Poly(methyl methacrylate).
Flexural modulus and flexural strength of the anti-microbial acrylic resins
From three-point flexural test according to the ISO 20795-1, two mechanical properties, including flexural strength and modulus, of the materials are shown in Table 2. The acrylic denture base produced by SCG company showed flexural strength and modulus about 83 and 2,400 MPa, respectively. The addition of preservative agents did not provide a significant alteration in the flexural strength of the materials. Nevertheless, all additives significantly decreased the flexural modulus of the materials to about 2,000 MPa (p < 0.05).
Table 2.
Flexural strength and modulus of the heat-polymerized PMMA resin with different food preservatives.
| Acrylic resin | Flexural strength (MPa) | Flexural modulus (MPa) |
|---|---|---|
| PMMA (control) | 82.65 ± 4.59 | 2,376.71 ± 172.76 |
| PMMA plus ZnO | 83.75 ± 7.77 | 2,023.57 ± 119.13∗ |
| PMMA plus PS | 85.01 ± 4.27 | 2,143.75 ± 53.89∗ |
| PMMA plus SM | 91.84 ± 10.05 | 2,030.43 ± 58.75∗ |
Values were represented in mean ± standard deviation (n = 7). Asterisk indicated significant different from the PMMA control (p < 0.05).
PMMA: Poly(methyl methacrylate); PS: Potassium sorbate; SM: Sodium metabisulfite; ZnO: Zinc oxide particles.
Discussion
As described previously, denture base materials prone to be reservoir for microbial colonization from several pathogens.3,4,18 In this study, the incorporation of food preservatives into the acrylic denture base was introduced to promote anti-microbial effect. Various food preservatives including zinc oxide (ZnO), potassium sorbate (PS), and sodium metabisulfite (SM) were preliminarily studied their optimal compositions which were mentioned in the previous patent.14
Three problematic oropharyngeal microorganisms were selected including S. aureus, P. aeruginosa, and C. albicans as representatives for gram-positive bacteria, gram-negative bacteria, and fungi, respectively. The anti-microbial mechanism of these additives have been purposed.12,19, 20, 21, 22, 23 ZnO generated reactive oxygen species (ROS) that caused cellular damage and apoptosis.20 While plausible mechanism for two weak acid preservatives (PS and SM) could be an interruption of cellular metabolism by an accumulation of protons inside the microbial cells.12,21 Both PS and SM also induced lipid peroxidation that deteriorated the cell membrane.22 Additionally, SM that is the sulfur-containing compound was reported about the releasing of sulfur dioxide molecules. These compounds can cleave disulfide bonds of in protein structures causing enzyme destruction and cellular damage.23
From the anti-microbial testing, the unmodified PMMA resin itself did not provide the inhibitory effect against the microbial species (Table 1). The incorporation of all food preservatives into the PMMA resins could reduce the microbial growth as illustrated in Fig. 2. The anti-microbial effect of the materials against S. aureus was stronger than that against P. aeruginosa, and C. albicans. One possible reasons could be the difference in their cellular protective structures. Because the gram positive S. aureus has only one cytoplasmic membrane, it would be more susceptible to anti-microbial agents than other species that possess cell membrane bilayer.24 While the cell wall composition of C. albicans, consisting glucan and mannan structures, mostly differs from that of both nonsporulating bacteria.25 This might lead to the lowest microbial inhibition of the materials against C. albicans. Furthermore, there was a difference in the anti-bacterial activity against P. aeruginosa among three compositions. The PMMA with SM showed greater inhibitory effect to P. aeruginosa than that combined with PS. This could be explained by the minimum inhibition concentration (MIC) of SM itself against P. aeruginosa of was lower than that of PS in many studies (Table 3).12,26,27 Whereas, ZnO particles into the PMMA exhibited less microbial reduction against P. aeruginosa in spite of its low MIC in previous articles.28,29 This could be explained by size-dependent anti-bacterial activity of ZnO particles.30 In this study, the particle size of ZnO was 45 μm, approximately. Therefore, the surface-to-volume ratio was lower than that of nano-size ZnO in previous studies.
Table 3.
Minimum inhibition concentrations against P. aeruginosa of three anti-microbial agents from some published articles.
| Compound | MIC (%) | Method | Refs. |
|---|---|---|---|
| ZnO | 0.05 | Microtiter plate based assay | 28 |
| ∼0.02 | Agar plate diffusion assay | 29 | |
| PS | 0.15 | Agar plate diffusion assay | 12 |
| 1 | Broth microdilution technique | 26 | |
| >1 | Microtiter plate based assay | 27 | |
| SM | 0.05 | Agar plate diffusion assay | 12 |
| 0.125 | Broth microdilution technique | 26 | |
| 1 | Microtiter plate based assay | 27 |
MIC: Minimum inhibition concentration; PS: Potassium sorbate; SM: Sodium metabisulfite; ZnO: Zinc oxide particles.
The preliminary cytotoxic testing of the modified denture base resins was done according to the ISO standard about biological testing of medical devices.16 The PMMA acrylic resin from SCG company did show cytotoxic effect to L929 fibroblast cells (Fig. 3). Thus, the denture base itself appeared to be biocompatible. The anti-microbial additives in this study have been widely used as preservatives in food and cosmetic products. The concentrations of all preservative agents into the PMMA resin were within safety range.31, 32, 33, 34 Therefore, the denture base incorporated with these anti-microbial agents did not cause cytotoxic issue. However, the products should also be evaluated in animal models before applying in a clinical trial.
Besides biocompatibilty assessment, the mechanical properties of the materials were also evaluated. The additives could influence or deteriorate the material properties.35 In this study, the PMMA resin manufactured by SCG company showed flexural properties about 83 MPa and 2,400 MPa for flexural strength and modulus, respectively. The incorporation of the food preservatives raised the flexural strength but reduced the flexural modulus of the PMMA resin. This should be further investigated. Although the anti-microbial PMMA resins showed the alteration in the flexural properties, these materials still provided acceptable flexural strength and modulus according to the ISO 20795-1:2013 (65 and 2,000 MPa, respectively).17
Within the limitation in this study, it can be concluded that the incorporation of three preservative agents into the denture base resin promoted anti-microbial activity (Table 4). The PMMA combined with 0.5 %w/w of sodium metabisulfite appeared to be an effective composition providing the great inhibition effect against both gram-positive and gram-negative bacteria. All additives did not cause cytotoxicity. Regarding to the mechanical properties, the modified resins still provided acceptable flexural properties according to the ISO 20795-1:2013.
Table 4.
Summarized properties including anti-microbial activity against various microorganisms, L929 cell viability, and flexural properties of the heat-cured PMMA resin incorporated with food preservatives.
| Acrylic resin |
aAnti-microbial activity |
In vitro cytotoxicity |
bMechanical properties |
||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | C. albicans | |||
| PMMA + ZnO | √√ | √ | √ | Biocompatible | Acceptable |
| PMMA + PS | √√ | √ | √ | Biocompatible | Acceptable |
| PMMA + SM | √√ | √√ | √ | Biocompatible | Acceptable |
PMMA: Poly(methyl methacrylate); PS: Potassium sorbate; SM: Sodium metabisulfite; ZnO: Zinc oxide particles.
Anti-microbial activity was represented by relative microbial reductions (√ = less than 50% reduction; √√ = over than 90% reduction).
Acceptable mechanical properties according to the ISO 20795–1:2013 were more than 65 and 2,000 MPa for flexural strength and modulus, respectively.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
The authors would like to acknowledge the Siam Cement Public Company Limited, Thailand for providing the heat-polymerized denture base resin.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2020.09.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Naji S.A., Behroozibakhsh M., Hajizamani H., Habibzadeh S. Recent advances and future perspectives for reinforcement of poly (methyl methacrylate) denture base materials: a literature review. J Dent Biomater. 2018;5:490–502. [Google Scholar]
- 2.Gad M.M., Fouda S.M., Al-Harbi F.A., Näpänkangas R., Raustia A. PMMA denture base material enhancement: a review of fiber, filler, and nanofiller addition. Int J Nanomed. 2017;12:3801–3812. doi: 10.2147/IJN.S130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniluk T., Tokajuk G., Stokowska W. Occurrence rate of oral Candida albicans in denture wearer patients. Adv Med Sci. 2006;51:77–80. [PubMed] [Google Scholar]
- 4.Olms C., Yahiaoui-Doktor M., Remmerbach T., Stingu C. Bacterial colonization and tissue compatibility of denture base resins. Dent J (Basel) 2018;6:20. doi: 10.3390/dj6020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi Y., Nakajo K., Sato T., Koyama S., Sasaki K., Takahashi N. Quantification and identification of bacteria in acrylic resin dentures and dento-maxillary obturator-prostheses. Am J Dent. 2012;25:171–175. [PubMed] [Google Scholar]
- 6.Gendreau L., Loewy Z.G. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 7.Taylor G.W., Loesche W.J., Terpenning M.S. Impact of oral diseases on systemic health in the elderly: diabetes mellitus and aspiration pneumonia. J Publ Health Dent. 2000;60:313–320. doi: 10.1111/j.1752-7325.2000.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 8.Coulthwaite L., Verran J. Potential pathogenic aspects of denture plaque. Br J Biomed Sci. 2007;64:180–189. doi: 10.1080/09674845.2007.11732784. [DOI] [PubMed] [Google Scholar]
- 9.Quinn D.M. The effectiveness, in vitro, of miconazole and keteconazole combined with tissue conditioners in inhibiting the growth of Candida albicans. J Oral Rehabil. 1985;12:177–182. doi: 10.1111/j.1365-2842.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 10.Bertolini M.M., Portela M.B., Curvelo J.A.R., Soares R.M., Lourenço E.J., Telles D.M. Resins-based denture soft lining materials modified by chlorhexidine salt incorporation: an in vitro analysis of antifungal activity, drug release and hardness. Dent Mater. 2014;30:793–798. doi: 10.1016/j.dental.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Pesci-Bardon C., Fosse T., Serre D., Madinier I. In vitro antiseptic properties of an ammonium compound combined with denture base acrylic resin. Gerodontology. 2006;23:111–116. doi: 10.1111/j.1741-2358.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 12.Oladapo A., Abiodun O. The inhibitory effect of different chemical food preservatives on the growth of selected food borne pathogenic bacteria. Afr J Microbiol Res. 2014;8:1510–1515. [Google Scholar]
- 13.Brul S., Coote P. Preservative agents in foods: mode of action and microbial resistance mechanisms. Int J Food Microbiol. 1999;50:1–17. doi: 10.1016/s0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 14.Thamapipol S., Sae-Khow O., Kanchanavasit W., Suputtamongkol K., Wonglamsam A. 27 September 2018. The composition of antimicrobial denture and its preparation process. Thailand Patent Application no. TH 1801005936. [Google Scholar]
- 15.ISO 22196:2011 . vol. 2. 2011. p. 15. (Measurement of antibacterial activity on plastics and other non-porous surfaces. Switzerland). [Google Scholar]
- 16.ISO 10993-5:2009 . vol. 3. 2009. p. 34. (Biological evaluation of medical devices - part 5 tests for in vitro cytotoxicity. Switzerland). [Google Scholar]
- 17.ISO 20795-1:2013 . vol. 2. 2013. p. 35. (Dentistry - base polymers - part 1: denture base polymers. Switzerland). [Google Scholar]
- 18.Lewis N., Parmar N., Hussain Z. Colonisation of dentures by Staphylococcus aureus and MRSA in out-patient and in-patient populations. Eur J Clin Microbiol Infect Dis. 2015;34:1823–1826. doi: 10.1007/s10096-015-2418-6. [DOI] [PubMed] [Google Scholar]
- 19.Sirelkhatim A., Mahmud S., Seeni A. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqi K.S., ur Rahman A., Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res lett. 2018;13:141. doi: 10.1186/s11671-018-2532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sofos J., Busta F. Antimicrobial activity of sorbate. J Food Protect. 1981;44:614–622. doi: 10.4315/0362-028X-44.8.614. [DOI] [PubMed] [Google Scholar]
- 22.Avis T.J., Michaud M., Tweddell R.J. Role of lipid composition and lipid peroxidation in the sensitivity of fungal plant pathogens to aluminum chloride and sodium metabisulfite. Appl Environ Microbiol. 2007;73:2820–2824. doi: 10.1128/AEM.02849-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arslan U. Evaluation of antifungal activity of sulfurcontaining salts against phytopathogenic fungi. Fresenius Environ Bull. 2015;24:1879–1886. [Google Scholar]
- 24.Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas G., Das S., Nipa M.N., Patwary R.H., Rahman A.M., Parveen S. A comparative study for the determination of efficacy of commonly used antimicrobials against specific bacterial strains in tomato (Solanum lycopersicum L.) juice. J Glob Biosci. 2015;4:3094–3103. [Google Scholar]
- 27.Barreteau H., Mandoukou L., Adt I., Gaillard I., Courtois B., Courtois J. A rapid method for determining the antimicrobial activity of novel natural molecules. J Food Protect. 2004;67:1961–1964. doi: 10.4315/0362-028x-67.9.1961. [DOI] [PubMed] [Google Scholar]
- 28.Premanathan M., Karthikeyan K., Jeyasubramanian K., Manivannan G. Selective toxicity of ZnO nanoparticles toward gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Elkady M.F., Shokry Hassan H., Hafez E.E., Fouad A. Construction of zinc oxide into different morphological structures to be utilized as antimicrobial agent against multidrug resistant bacteria. Bioinorgan Chem Appl. 2015;2015:1–20. doi: 10.1155/2015/536854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azam A., Ahmed A.S., Oves M., Khan M.S., Habib S.S., Memic A. Antimicrobial activity of metal oxide nanoparticles against gram-positive and gram-negative bacteria: a comparative study. Int J Nanomed. 2012;7:6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiwata H., Nishijima M., Fukasawa Y. Estimation of preservative concentrations in foods and their daily intake based on official inspection results in Japan in fiscal year 1998. J Food Hyg Soc Jpn. 2001;42:404–412. doi: 10.3358/shokueishi.42.404. [DOI] [PubMed] [Google Scholar]
- 32.Elder R. Final report on the safety assessment of sorbic acid and potassium sorbate. J Am Coll Toxicol. 1988;7:837–880. [Google Scholar]
- 33.Nair B., Elmore A. Final report on the safety assessment of sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, ammonium bisulfite, sodium metabisulfite and potassium metabisulfite. Int J Toxicol. 2003;22:63–88. doi: 10.1080/10915810305077x. [DOI] [PubMed] [Google Scholar]
- 34.Espitia P.J., Otoni C.G., Soares N.F. Zinc oxide nanoparticles for food packaging applications. In: Barros-Velazquez J., editor. Antimicrob food packag. 1st ed. Elsevier; London: 2016. pp. 425–431. [Google Scholar]
- 35.Addy M., Handley R. The effects of the incorporation of chlorhexidine acetate on some physical properties of polymerized and plasticized acrylics. J Oral Rehabil. 1981;8:155–163. doi: 10.1111/j.1365-2842.1981.tb00488.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.