Abstract
Background/purpose
Dental pulp stem cells (DPSCs) contribute to the regeneration of various tissues and have superior proliferation, immune privilege, and anti-inflammation properties to other mesenchymal stem cells. 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (THSG) not only enhances the aforementioned properties of DPSCs but also promotes self-renewal and reprogramming-like ability. However, whether THSG enhances the aforementioned properties and abilities through direct or indirect interaction mechanisms remains unclear. To address this knowledge gap, we examined the effects of THSG-stimulated DPSC-derived conditioned medium (THSG-CM) on the activity and anti-inflammation properties of cells.
Materials and methods
DPSCs were treated with various concentrations of THSG to produce THSG-CM, which was then collected, analyzed, and lyophilized. A cytokine profiling antibody assay was used to compare protein components between THSG-treated and nontreated CM. Human skin fibroblasts (HSFs) and human gingival fibroblasts (HGFs) were used to investigate the effect of THSG-CM on cell proliferation, anti-inflammation, and wound healing abilities; for this investigation, MTS assay, quantitative real-time PCR analysis, and 2-well silicone inserts wound model were conducted.
Results
We observed that THSG enhanced the secretion of growth- and immune-associated proteins in THSG-CM and increased the proliferation of HSFs and HGFs. Furthermore, THSG-CM significantly attenuated lipopolysaccharide-stimulated mRNA levels of cytokines in both cells and improved wound healing abilities.
Conclusion
We conclude that THSG-CM had more beneficial effects on cell activity and anti-inflammation in the HSFs and HGFs than DPSC-derived CM. DPSC-derived CM can be developed into a cell-free regenerative strategy in the future, and its therapeutic efficacy may be improved by THSG-CM.
Keywords: Dental pulp stem cell; 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside; Conditioned medium; Proliferation; Anti-inflammation; Wound healing
Introduction
Adult stem cells are undifferentiated cells that can be isolated from adult tissues. These cells have self-renewal and multipotent differentiation capacities and exhibit less teratogenic potential than do embryonic stem cells.1 Bone marrow currently constitutes the most accepted source of adult stem cells for clinical treatment; however, the associated harvesting and collecting procedures are painful and invasive.2 Investigations have thus shifted to adult stem cell sources that can be obtained through less invasive procedures without ethical concerns. The oral cavity contains numerous connective dental soft tissues that constitute an attractive option for tissue regeneration through minimally invasive procedures, thus eliminating ethical concerns. Adult stem cells can be isolated from various tissues in oral cavity, such as the pulp of exfoliated deciduous teeth3 or permanent teeth,4, 5, 6 periodontal ligament,7 apical papilla,8 dental follicle,9 human periapical cyst,10 and the gingiva.11 Dental pulp is a major source of adult stem cells; these cells can be obtained easily, can be isolated and amplified from deciduous or permanent teeth, and have immunomodulatory and anti-inflammatory capacities.12 Over the past two decades, our group and numerous other groups have isolated and established mesenchymal stem cells (MSCs) derived from human dental pulp, namely dental pulp stem cells (DPSCs), which are available in teeth extracted in orthodontic treatment. DPSCs can contribute to the regeneration of various tissues (bone, adipose, or neuronal tissue)13,14 and have higher potential for proliferation and mineralization induction than do bone marrow MSCs.15 Furthermore, DPSCs can be combined with various scaffolds to regenerate defective tissues and have immune privilege and anti-inflammatory properties. Therefore, DPSCs were considered prime candidates among MSCs for in vivo allogeneic transplantations.15 In addition, dental pulp tissue is an excellent noninvasive source for regenerative therapies.14,16
The therapeutic potential of adult stem cells depends considerably on the release of molecules and factors in the extracellular environment.17,18 Such molecules are secreted through paracrine activity and are called the secretome, which includes soluble factors (proteins, lipids, and nucleic acids) and extracellular vesicles.18,19 Conditioned medium (CM) from adult stem cells, particularly MSCs, contains growth factors, cytokines, and other active substances and has been revealed to mimic the regulatory effects of stem cells on immunocompetent cells.20,21 CM from adipose stem cells was reported to enhance the secretion of the anti-inflammatory cytokine interleukin (IL)-10 from T-helper cells in vitro.22 In addition, CM from bone marrow stromal cells was confirmed to induce new bone and cementum formation in intrabony defects with minimal inflammatory cell infiltration.23 Research also revealed that CM derived from MSCs exhibits angiogenesis-promoting, cell growth–supporting, and regeneration-enhancing properties in animal wound models.24
2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside (THSG) is a major bioactive components of Polygonum multiflorum Thunb. (also called “He Shou Wu” in Chinese), which exhibits anti-inflammatory activity and cell proliferation in human gingival fibroblasts (HGFs),25 in addition to enhancing self-renewal and reprogramming-like abilities in human DPSCs.6 However, whether these THSG-enhanced effects in DPSCs are engendered through direct or indirect interaction mechanisms remains unclear. In addition, increasing bodies of evidence indicate that the therapeutic efficacy of stem cell-based therapy is not reliant on the engraftment of stem cells at the site of injury or the differentiation capability of the transplanted stem cells; instead, such efficacy is based on the cells' paracrine signaling.26,27 In this study, we first collected, analyzed, and lyophilized THSG-stimulated DPSC-derived CM (THSG-CM). Subsequently, we treated HGFs and human skin fibroblasts (HSFs) with lyophilized THSG-CM to examine the augmentative effects of THSG-CM on cell activity and anti-inflammation.
Materials and methods
Reagents
THSG (purity: 95%, dissolved in dimethyl sulfoxide for cell treatment) was kindly provided by Dr. Ching-Chiung Wang, as previously described.6,25 Lipopolysaccharides (LPS, Cat. No.: L2630) (Sigma–Aldrich, Inc., St. Louis, Mo, USA) were dissolved in water for cell treatment. Charcoal stripped fetal bovine serum (stripped FBS; Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for THSG treatment at a medium concentration of 0.25%.
Cell cultures
DPSCs were obtained from Axol Bioscience Ltd. (Cambridge, UK). According to information provided by the supplier, the DPSCs were >90% positive for the markers CD29, CD44, CD90, CD105 and < 10% positive for the markers CD34 and CD45. HSFs (BCRC08C0011) were obtained from Bioresource Collection and Research Center (Hsinchu, Taiwan). HGFs (AG09319) were purchased from the Coriell Cell Repository (Camden, NJ, USA). In accordance with protocols of passaging procedures for cell line maintenance and expansion to obtain a sufficient number of cells, we passaged the cells used in our experiments up to five times. Among the cells, the DPSCs were maintained in low-glucose Dulbecco's modified Eagle's medium (DMEM), HSFs were maintained in high-glucose DMEM, and HGFs were maintained in DMEM/F-12 supplied with 10% FBS in an incubator with 5% CO2 at 37 °C. Before the experiments, the cells were placed in the medium contained 0.25% stripped FBS for 2 days.
Preparation and lyophilization of THSG-CM
DPSCs were cultured in their growth media to reach 80% confluence. The cells were washed once with phosphate-buffered saline (PBS), and the medium was replaced with the medium contained 0.25% stripped FBS for 2 days of starvation. Subsequently, the cells were treated with THSG (0, 0.1, 1, 10, 25, 50, 75, and 100 μM) in 0.25% stripped FBS medium for 48 h. CM of THSG-stimulated DPSCs was collected after 48 h of culture and centrifuged at 1000×g for 10 min. The supernatants were re-centrifuged at 10,000×g for 10 min, followed by the collection of the second supernatants, namely THSG-CM. To prepare lyophilized THSG-CM, THSG-CM was filtered using a 0.22-μm syringe filter and then frozen and stored at −80 °C. The frozen THSG-CM was then freeze dried at −51 °C overnight by using the FDU-1200 Freeze Dryer (Eyela, Tokyo, Japan). The freeze-dried powders of lyophilized THSG-CM were weighted and reconstituted in sterilized ultrapure water (QH2O) for subsequent experiments.
Cytokine profiling antibody array assay
To investigate the effect of THSG, we analyzed 310 cytokines and factors by performing the cytokine profiling antibody array (Full Moon BioSystems, Sunnyvale, CA, USA) in CM derived from DPSCs alone (1.0 × 107 cells) and DPSCs (1.0 × 107 cells) treated with 10 μM THSG for 3 days. The CM obtained from each culture was filtered and assessed using the BCA protein assay kit (Pierce, Rockford, Ill, USA) using VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA). For the antibody array assay, we used the SCK100 antibody array assay kit (Full Moon BioSystems) and sent medium samples to the agent of the manufacturer (Rainbow Biotechnology co., LTD, Taipei, Taiwan) for analysis according to the manufacturer's protocol. After the analysis, data on protein information were annotated using UniProt DB (www.uniprot.org). The signal intensity was proportional to the expression level of an individual cytokine in each sample. The array data were then normalized on the basis of the negative control signal intensity of each array.
Cell proliferation assay (MTS assay)
To determine cell viability, the HSFs and HGFs were cultured in their growth media until they reached 80% confluence. The cells were seeded on 96 well plates at a density of 5 × 103 cells per in a total volume of 100 μl in each well. After seeding, the cells were incubated at 37 °C and in 5% CO2 for 24 h to allow for cell attachment. The cells were then washed once with PBS, and the medium was replaced with the medium contained 0.25% stripped FBS for 2 days of starvation. Media supplemented with 0.25% stripped FBS and THSG-CM at different concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 0.5:1000, 1:1000, 2.5:1000, 5:1000) was replaced and treated for 4 days. The THSG-CM–medium mixtures were refreshed every other day. After 4 days of treatment, media containing 20% MTS solution (CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit; Promega, Madison, WI, USA) were replaced with growth media and incubated for 2 h. The absorbance of each well at 490 nm was measured using the VersaMax ELISA microplate reader (Molecular Devices).
Wound healing assay and analysis
To examine the wound healing effects of the HSFs and HGFs after THSG-CM treatment, wound healing experiments were performed using silicone insert (culture-insert, 2-wells plate, ibiTreat; Ibidi, Martinsried, Germany), with the cells being placed in a 24-well plate. Specifically, the cells were seeded at a density 2.5 × 105 cells/cm2 in two compartments of a silicone insert. The cells were grown for 24 h, after which the medium was replaced with starvation medium containing 0.25% stripped FBS and cultured for another 24 h. The culture-inserts were removed using sterile tweezers, resulting in a 500-μm-wide gap. Media supplemented with 0.25% stripped FBS and THSG-CM at different concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 0.5:1000, 1:1000, 2.5:1000, 5:1000) were added and incubated for 24 h.
The subsequent healing process was recorded for one well using the Olympus IX50 inverted microscope (Olympus, Hamburg, Germany). In addition, images of the starting conditions (500-μm gaps) were captured for at least two to three wells; all wells of the 24-well plate were also assessed visually for any anomalies. After the wounding process was executed for 24 h, cell growth was stopped, and the cells were fixed in 75% ethanol. The fixed cells were stained with 0.2% crystal violet (dissolved in 2% ethanol), and micrographs of all wells were captured for wound size measurement. According to procedures described in a previous study,28 wound sizes were determined and analyzed using ImageJ software with the MRI wound-healing tool macro. Wound sizes were determined for four replicates for each treatment, and the mean value and standard deviation of healed wound areas were calculated.
Quantitative real-time polymerase chain reaction analysis
To examine the pro-inflammatory gene expression in the HGFs and HSFs treated with THSG-CM, we conducted quantitative real-time polymerase chain reaction (QPCR) analysis. The HSFs and HGFs were starved and then treated with 0, 0.1, 1, 10 μg/ml Escherichia coli (E. Coli) LPS for 6 h; alternatively, they were cotreated with 1 μg/mL E. Coli LPS and THSG-CM (THSG-CM concentration [μl]:fresh medium concentration [μl] = 5:1000) for 6 h. The cells were then collected and subjected to QPCR analysis. According to procedures described in previous research,25 total RNA was extracted using the GENEzol™ TriRNA Pure Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan), with eliminating genomic DNA. cDNA was prepared using 1 μg of DNase I-treated total RNA by employing the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). The prepared cDNA was used as the template for QPCR processes, which were conducted using the QuantiNovaTM SYBR® Green PCR Kit (QIAGEN, Hilden, Germany) on CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, U.S.A.). The QPCR processes involved an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 5 s and combined annealing/extension at 60 °C for 10 s, as detailed in the manufacturer's instructions. The Homo sapiens primer sequences are presented in Table 1. Calculations of relative gene expression (normalized to 18 S as reference gene) were performed according to the 2−ΔΔCT method. QPCR fidelity was determined through melting temperature analysis.
Table 1.
The primer sequences used for QPCR analysis.
| Gene name | Forward | Backward | Accession No. |
|---|---|---|---|
| IL-1β | GCAGCCATGGCAGAAGTACC | AGTCATCCTCATTGCCACTGTAAT | NM_000576.2 |
| TNF-α | TAGCCCATGTTGTAGCAAACCC | TTATCTCTCAGCTCCACGCCA | NM_000594.3 |
| IL-6 | ACCCCCAGGAGAAGATTCCA | GATGCCGTCGAGGATGTACC | M54894.1 |
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | NR_003286 |
IL-1β: interleukin 1 β; TNF-α: tumor necrosis factor α; IL-6: interleukin 6.
Statistical analysis
The collected data of cell viability (MTS) and genes expression (QPCR) were analyzed using IBM®SPSS® Statistics version 19.0 (SPSS Inc., Chicago, IL, USA). A two-tailed Student's t-test was conducted, and results were considered significant when p-values < 0.05 (∗, or #), 0.005 (∗∗ or ##) and 0.001 (∗∗∗ or ###).
Results
THSG enhanced the secretion of growth-associated and immune-associated proteins in CM from DPSCs
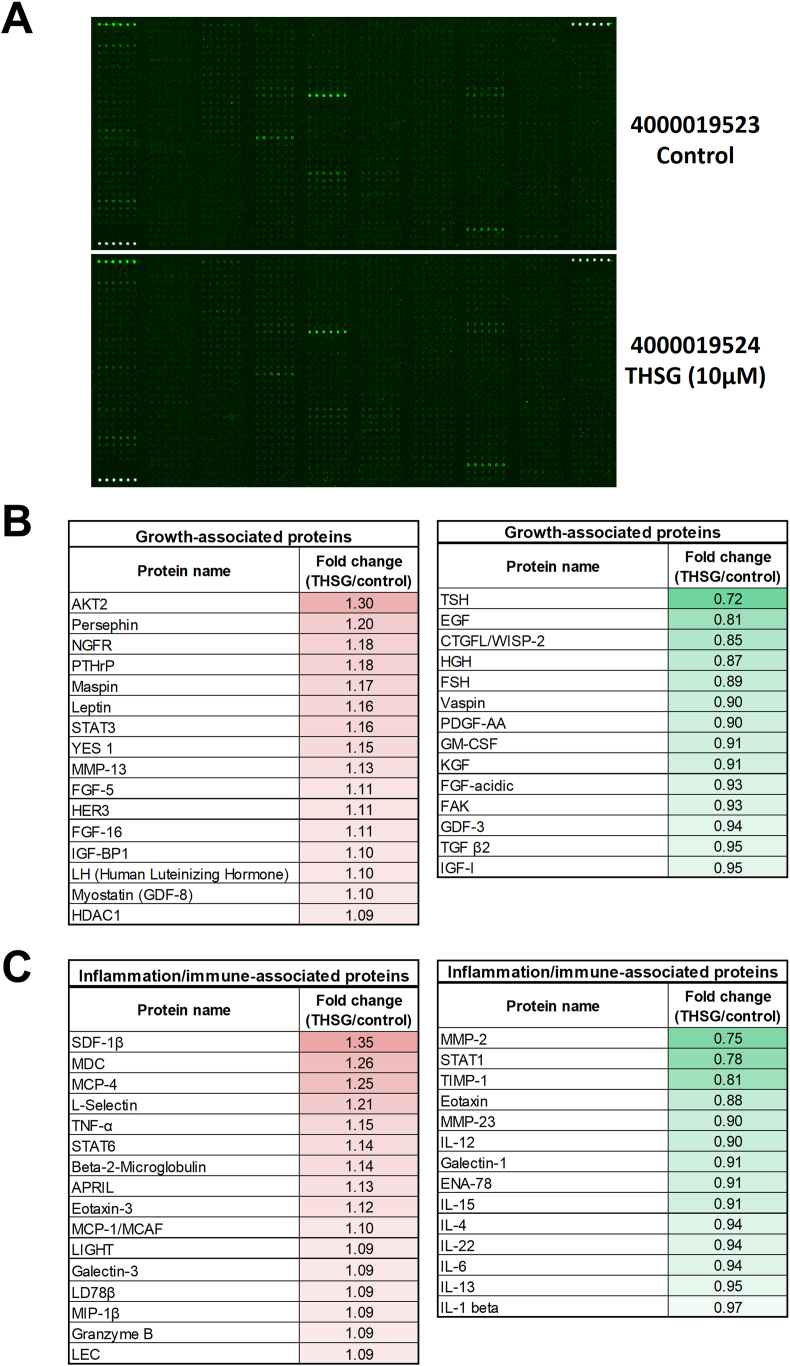
To explore the differences in protein content between CM derived from THSG-treated DPSCs and that derived from non-THSG-treated DPSCs, we applied a cytokine profiling antibody array to examine the protein composition and expression fold changes (Fig. 1A). Treatment of the DPSCs with 10 μM THSG for 48 h induced the secretion of growth-associated proteins in CM, including AKT2, persephin, nerve growth factor receptor (NGFR), and parathyroid hormone-related protein (PTHrP) (left panel of Fig. 1B). THSG treatment also reduced the secretion of several growth-associated proteins, including EGF, HGH, vaspin, and PDGF-AA, in CM (right panel of Fig. 1B). Furthermore, THSG treatment enhanced the secretion of inflammation/immune-associated proteins in CM, such as SDF-1β, MDC, and MCP-4 (left panel of Fig. 1C). THSG-treated DPSCs exhibited reduced secretion of inflammation/immune-associated proteins in CM, including MMP-2, TIMP-1, and eotaxins (right panel of Fig. 1C).
Figure 1.
Screening of DPSC-secreted proteins in THSG-treated CM. (A) Differences in protein content between THSG-treated CM and nontreated CM were assessed using a cytokine profiling antibody array. (B) Comparison list of THSG-stimulated DPSC-secreted growth-associated proteins in CM. (C) Comparison list of THSG-stimulated DPSC-secreted immune-associated proteins in CM.
THSG-CM enhanced cell proliferation
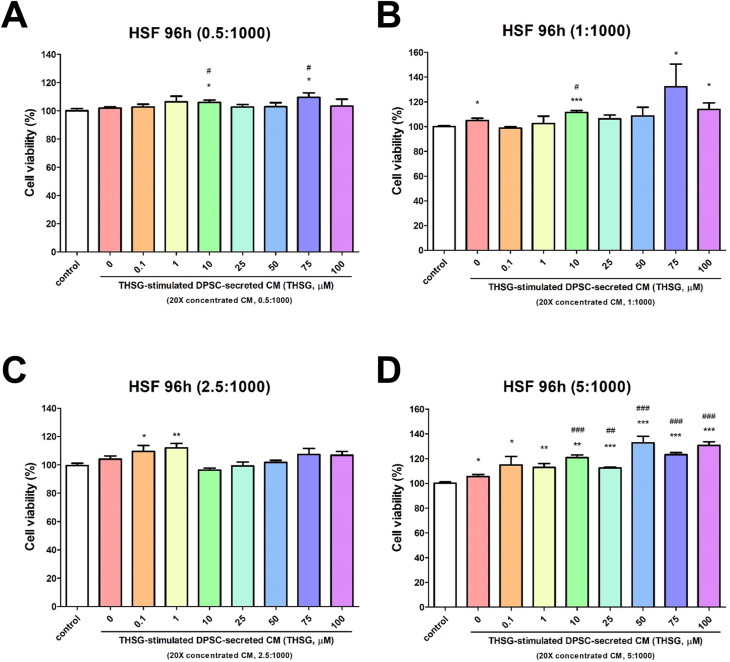
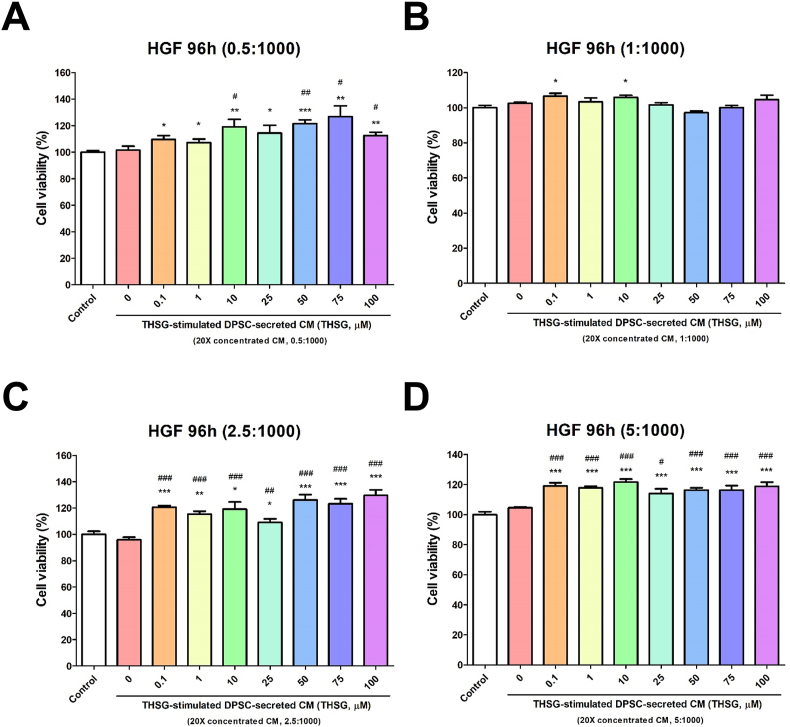
To examine the cell proliferative effect of THSG-CM, we performed the MTS assay. Media supplemented with 0.25% stripped FBS and THSG-CM at different concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 0.5:1000, 1:1000, 2.5:1000, 5:1000) were used to treat the HSFs and HGFs for 4 days. When the HSFs were treated with a low concentration of THSG-CM (0.5:1000), only 10 and 75 μM THSG-CM significantly enhanced cell proliferative activity relative to 0 μM THSG-CM (Fig. 2A). However, when the HSFs were treated with a high concentration of THSG-CM (5:1000), 10–100 μM THSG-CM significantly augmented cell proliferative activity relative to 0 μM THSG-CM (Fig. 2D). We observed similar results for the HGFs treated with various THSG-CM concentrations (Fig. 3). Nevertheless, when the HGFs were treated with relatively high concentrations of THSG-CM (2.5:1000 and 5:1000), 0.1–100 μM THSG-CM significantly augmented cell proliferative activity relative to 0 μM THSG-CM (Fig. 3C and D).
Figure 2.
Proliferative effect of THSG-CM on HSFs. HSFs were starved for 2 days and then transferred to media supplemented with 0.25% stripped FBS and THSG-CM with different concentrations and incubated for 4 days. Cell proliferation was evaluated using the MTS assay. (A) THSG-CM concentration (μl):fresh medium concentration (μl) = 0.5:1000; (B) THSG-CM concentration (μl):fresh medium concentration (μl) = 1:1000; (C) THSG-CM concentration (μl):fresh medium concentration (μl) = 2.5:1000; (D) THSG-CM concentration (μl):fresh medium concentration (μl) = 5:1000. (N = 8, data are expressed as mean ± standard deviation [SD]; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared with control group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with 0 μM group).
Figure 3.
Proliferative effect of THSG-CM on HGFs. HGFs were starved for 2 days and then transferred to media supplemented with 0.25% stripped FBS and THSG-CM with different concentrations and incubated for 4 days. Cell proliferation was evaluated using the MTS assay. (A) THSG-CM concentration (μl):fresh medium concentration (μl) = 0.5:1000; (B) THSG-CM concentration (μl):fresh medium concentration (μl) = 1:1000; (C) THSG-CM concentration (μl):fresh medium concentration (μl) = 2.5:1000; (D) THSG-CM concentration (μl):fresh medium concentration (μl) = 5:1000. (N = 8, data are expressed as mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared with control group; #p < 0.05, ##p < 0.01, ###p < 0.001, compared with 0 μM group).
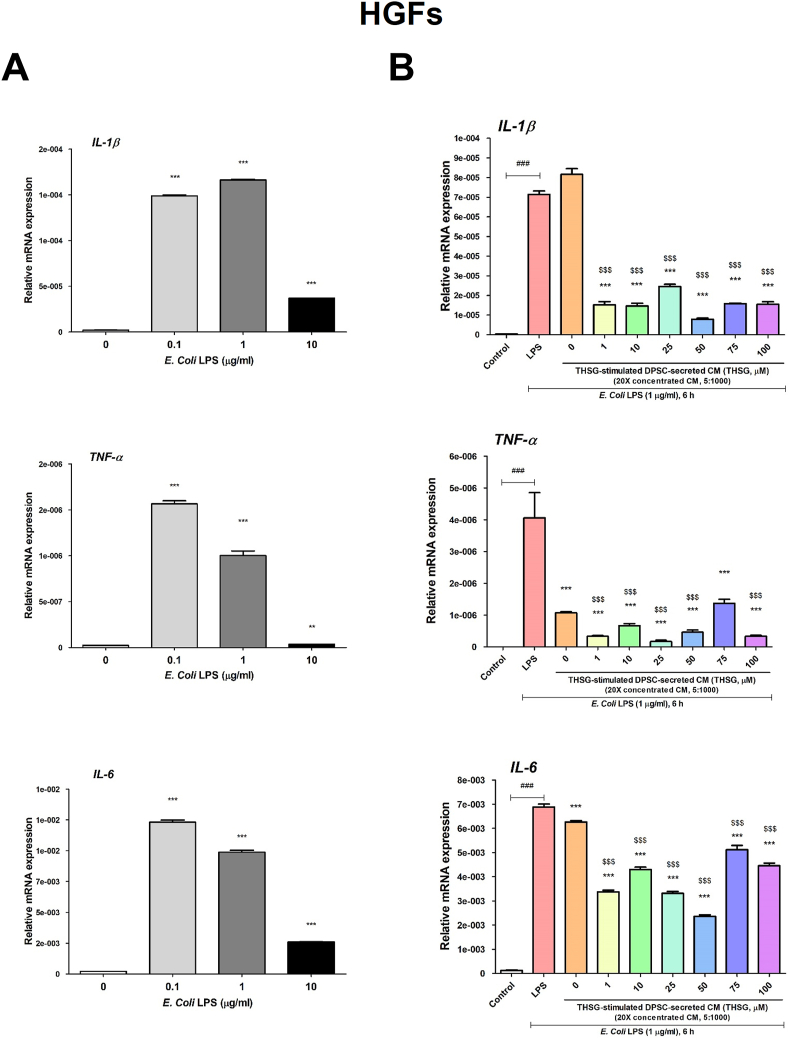
THSG-CM ameliorated LPS-induced inflammation
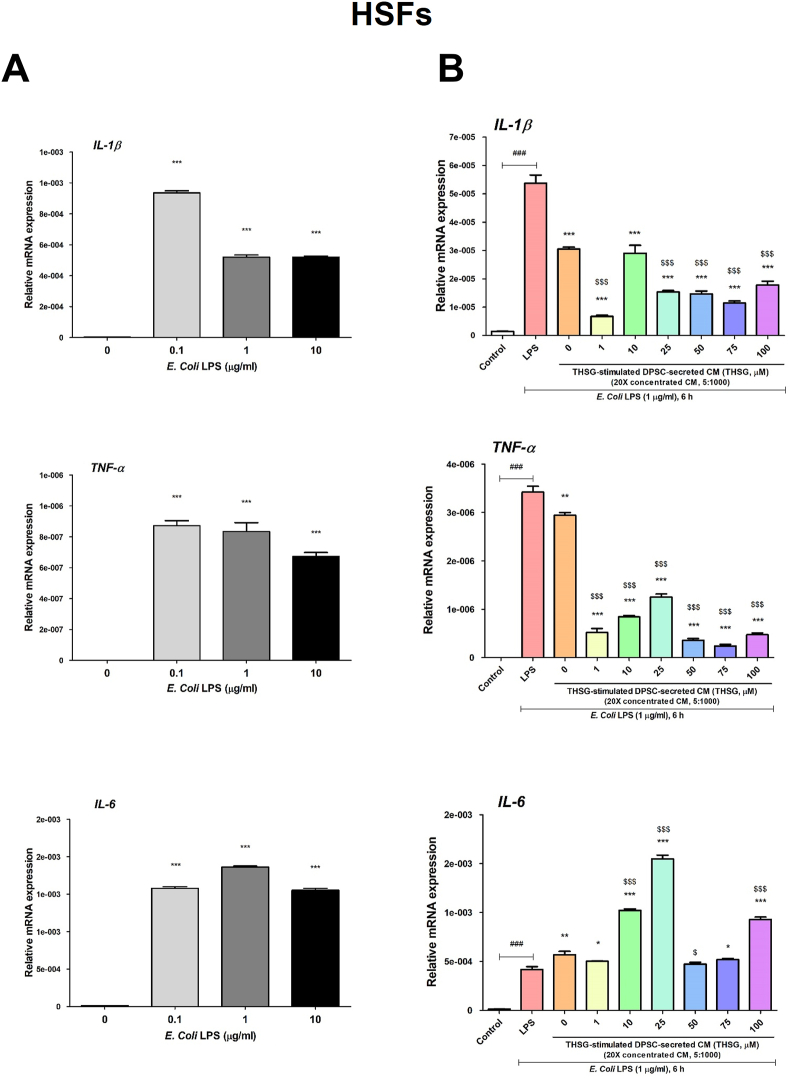
To evaluate the anti-inflammatory potential of THSG-CM, we applied E. coli LPS to stimulate inflammation in HSFs and HGFs. The HSFs were starved and treated with 0, 0.1, 1, 10 μg/ml E. Coli LPS for 6 h. We observed that LPS treatment induced significant gene expression levels of the pro-inflammatory cytokines IL-1β, tumor necrosis factor (TNF)-α, and IL-6 (Fig. 4A). Therefore, 1 μg/ml LPS was selected as the ideal concentration for subsequent experiments on the anti-inflammatory effects of THSG-CM. CM derived from the untreated DPSCs significantly reduced IL-1β and TNF-α, but not IL-6, expression in the HSFs (Fig. 4B). THSG-CM significantly ameliorated IL-1β and TNF-α expression in the HSFs (Fig. 4B). However, 10, 25, 50, and 100 μM THSG-CM significantly enhanced IL-6 expression in the HSFs relative to 0 μM THSG-CM (Fig. 4B).
Figure 4.
Anti-inflammatory effect of THSG-CM on HSFs. (A) HSFs were starved and then treated with 0, 0.1, 1, 10 μg/ml E. Coli LPS for 6 h. The gene expression of pro-inflammatory cytokines, IL-1β, TNF-α and IL-6 was evaluated. (N = 6, data are expressed as mean ± SD; ∗∗∗p < 0.001, compared with control group) (B) Cells were starved and then co-treated with 1 μg/ml E. Coli LPS and THSG-CM at different concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 5:1000) for 6 h. The gene expression of IL-1β, TNF-α and IL-6 was also evaluated. (N = 6, data are expressed as mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared with LPS group; ###p < 0.001, compared with control group; $p < 0.05, $$$p < 0.001, compared with 0 μM group).
We observed similar results regarding the effects of treatment on IL-1β and TNF-α expression in the HGFs (Fig. 5). Nevertheless, the effects on IL-6 expression differed in the HGFs (Fig. 5B). Specifically, in the HGFs, IL-6 expression was significantly reduced in CM derived from the untreated DPSCs; THSG-CM also significantly ameliorated IL-6 expression in the HGFs (Fig. 5B).
Figure 5.
Anti-inflammatory effect of THSG-CM on HGFs. (A) HGFs were starved and then treated with 0, 0.1, 1, 10 μg/ml E. Coli LPS for 6 h. The gene expression of pro-inflammatory cytokines, IL-1β, TNF-α and IL-6 were evaluated. (N = 6, data are expressed as mean ± SD; ∗∗p < 0.01, ∗∗∗p < 0.001, compared with control group) (B) Cells were starved and then co-treated 1 μg/ml E. Coli LPS and THSG-CM at different concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 5:1000) for 6 h. The gene expression of IL-1β, TNF-α, and IL-6 was also evaluated. (N = 6, data are expressed as mean ± SD; ∗∗∗p < 0.001, compared with LPS group; ###p < 0.001, compared with control group; $$$p < 0.001, compared with 0 μM group).
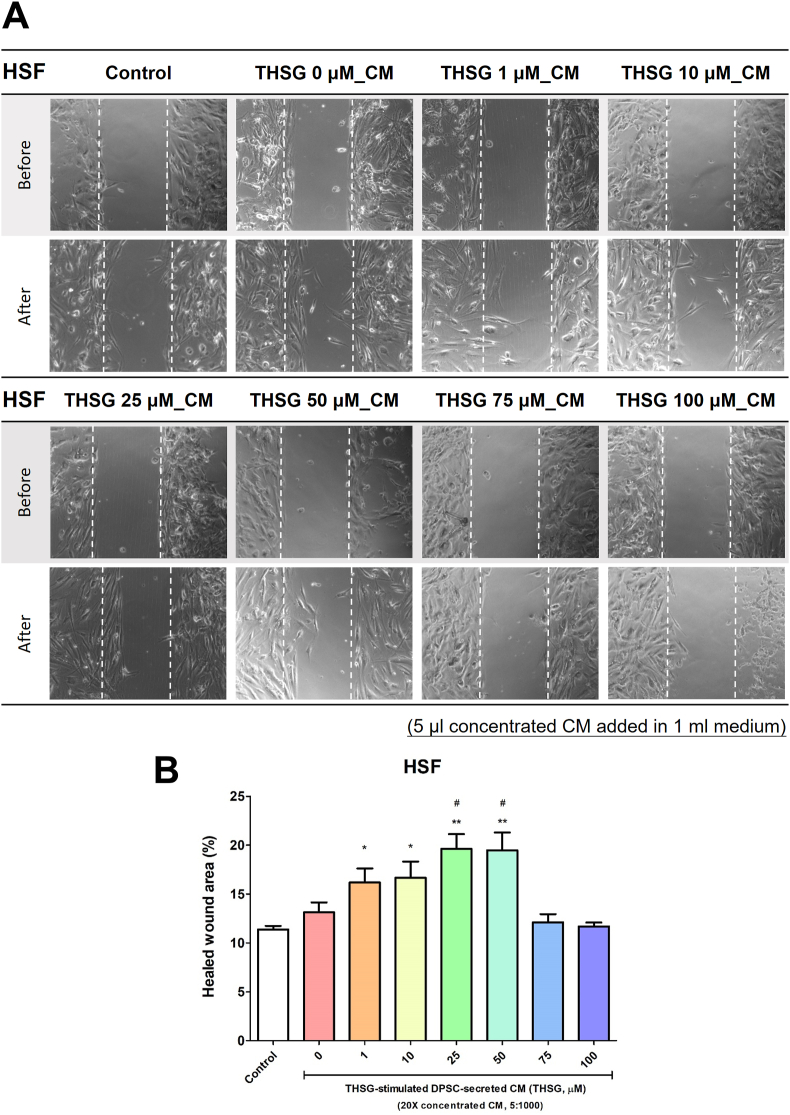
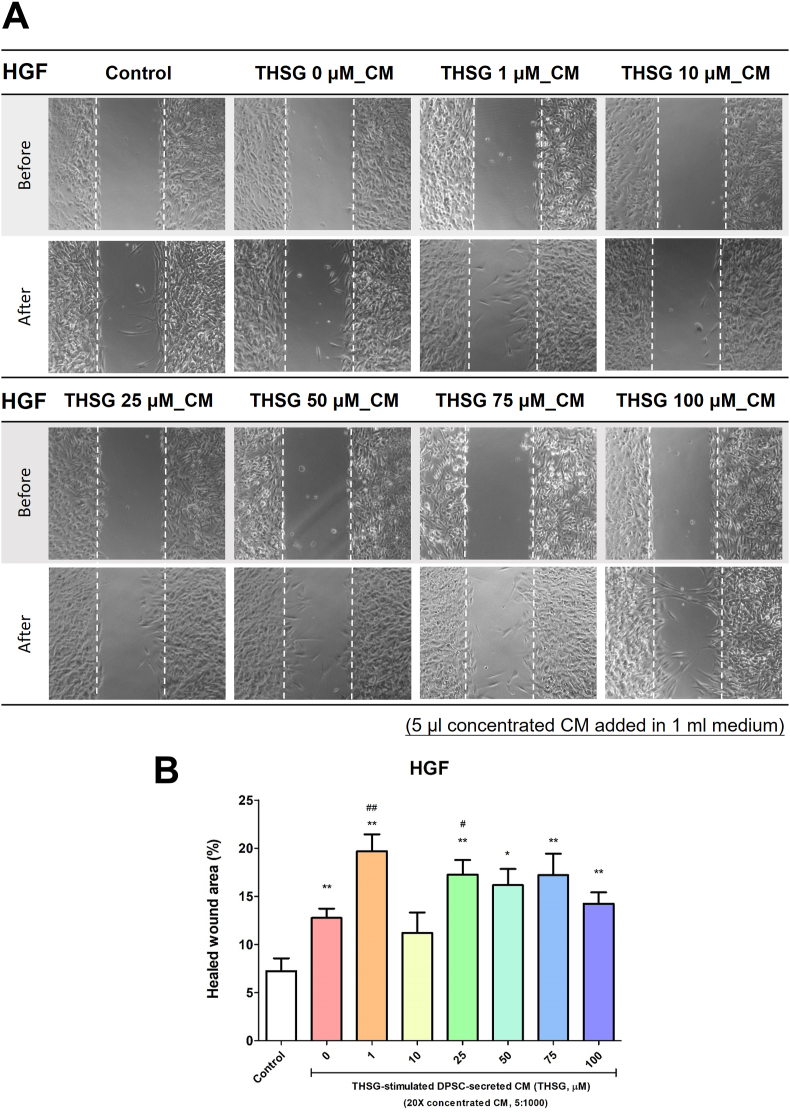
THSG-CM accelerated wound healing
The HSFs and HGFs were seeded in silicone inserts and starved for 2 days. After removing the inserts, we transferred the cells to media supplemented with 0.25% stripped FBS and THSG-CM at different concentrations and incubated them for 24 h. The wound healing effects observed for the HSFs and HGFs are illustrated in Fig. 6A and Fig. 7A. CM derived from the untreated DPSCs did not improve wound healing in the HSFs. However, only 25 and 50 μM THSG-CM significantly enhanced wound healing in the HSFs relative to 0 μM THSG-CM (Fig. 6B). We noted similar results for the HGFs (Fig. 7). Nevertheless, CM derived from the untreated DPSCs significantly improved wound healing in the HGFs. Moreover, only 1 and 25 μM THSG-CM significantly enhanced wound healing relative to 0 μM THSG-CM (Fig. 7B).
Figure 6.
Wound healing effect of THSG-CM on HSFs. HSFs were seeded and starved for 2 days in silicone inserts. After the removal of inserts, the cells were transferred to media supplemented with 0.25% stripped FBS and THSG-CM at different concentrations and incubated for 24 h. (A) Wound healing effect of the treatment. (B) Wound closure area (%) determined in the wound healing assays. (N = 4, data are expressed as mean ± SD; ∗p < 0.05, ∗∗p < 0.01, compared with control group; #p < 0.05, compared with 0 μM group).
Figure 7.
Wound healing effect of THSG-CM on HGFs. HGFs were seeded and starved for 2 days in silicone inserts. After the removal of the inserts, the cells were transferred to media supplemented with 0.25% stripped FBS and THSG-CM at different concentrations and incubated for 24 h. (A) Wound healing effect of the treatment. (B) Wound closure area (%) determined in the wound healing assays. (N = 4, data are expressed as mean ± SD; ∗p < 0.05, ∗∗p < 0.01, compared with control group; #p < 0.05, ##p < 0.01, compared with 0 μM group).
Discussion
This study revealed that THSG-CM enhanced cell proliferation and wound healing in HSFs and HGFs and that it ameliorated the expression of LPS-induced pro-inflammatory cytokines (IL-1β and TNF-α). In our previous studies, we have confirmed that THSG could stimulate cell proliferation in HGFs and DPSCs and that it could ameliorate the expression of pro-inflammatory genes (IL-1β, TNF-α, and IL-6) in HGFs.6,25 A review article reported that CM can be obtained through various methods: Specifically, CM can be acquired from 24 to 72 h or every 2 or 4 days after cell conditioning at different cell confluences (from 50% to 80%).16 Cells can also be starved by maintaining them in culture medium supplemented with serum or by changing the medium to a serum-free or low-serum-concentration medium (approximately 0.1%–0.5%). Subsequently, the culture medium can be collected, centrifuged, or concentrated, and the supernatant constituting the CM can be obtained and stored or frozen at −30 or −80 °C.16 In the present study, the CM isolation and production protocol entailed starving the DPSCs (at 80% confluence) for 2 days in 0.25% stripped FBS medium from which nonpolar material (such as certain growth factors, hormones, and cytokines) was removed. After the DPSCs in 0.25% stripped FBS medium were treated with THSG for 48 h, we collected, isolated, centrifuged, and lyophilized THSG-CM. Thus, we concentrated and preserved a sufficient amount of active ingredients.
To reveal the different components of CM derived from the THSG-stimulated DPSCs and nonstimulated DPSCs, we performed a cytokine profiling antibody array assay to analyze 310 cytokines and factors. THSG (10 μM) induced the DPSCs to secrete AKT2, persephin, NGFR, and PTHrP in CM (Fig. 1B). AKT2 is a serine/threonine protein kinase (AKT kinase) that regulates numerous processes, including metabolism, proliferation, cell survival, growth, and angiogenesis. Persephin is a neurotrophic factor in the glial cell line–derived neurotrophic factor family and can influence the survival and function of peripheral and central neurons.29 NGFR binds to nerve growth factor and other neurotrophins that stimulate nerve growth and regulate sympathetic neuron differentiation.30 PTHrP is a protein member of the parathyroid hormone family secreted by MSCs; this protein has normal functions in bone, tooth, vascular, and other tissues.31 We also observed that THSG reduced growth-associated proteins in CM (Fig. 1B), such as thyroid-stimulating hormone, a pituitary hormone that stimulates the thyroid gland to produce thyroid hormones that stimulate the metabolism of almost every tissue in the body.32 Moreover, THSG treatment enhanced the cell secretion of inflammation/immune-associated proteins in CM (Fig. 1C), such as stromal cell-derived factor 1β (SDF-1β or CXCL12), which typically exhibits reduced chemotactic or inflammatory activity.33 THSG also reduced inflammation/immune-associated proteins in CM (Fig. 1C), such as matrix metalloproteinase-2 (MMP-2), which cleaves and activates CCL7 and CXCL12. Therefore, MMP-2 may contribute to the creation of a chemotactic gradient and subsequent immune cell recruitment to sites of vascular injury.34 The limitation of fold changes was decided according to the manufacturer's protocol and suggestion. As a result, we chose the fold change ≥1.09 as induced expression and that ≤0.97 as reduced expression. Although the fold changes were rather minimal, the samples were not concentrated and lyophilized before performing this assay.
Our group and other research groups have reported that THSG induces proliferation of rat cardiac stem cells,35 HGFs25 and DPSCs.6 In addition, numerous studies have confirmed that CM derived from DPSCs can enhance cell proliferation and migration activity,36 alter endothelial cell behavior,37 direct osteo/odontogenic differentiation,38 promote vascular-like structures formation and angiogenic gene expression,12 impair cell death and induce tissue formation inside the root canal.12 In the present study, we prepared and concentrated confluent DPSC–derived CM in culture with THSG to examine the effect of THSG-CM on HSF and HGF proliferation. The DPSC-derived CM (without THSG stimulation; 0 μM THSG-CM) enhanced the proliferation of the HSFs when THSG-CM was applied at several concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 1:1000 and 5:1000) (Fig. 2B and D) but did not affect the proliferation of the HGFs. When THSG-CM was applied at low concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 0.5:1000), compared with 0 μM THSG-CM, only 10 and 75 μM THSG significantly enhanced the proliferation of both HSFs and HGFs (Figure 2, Figure 3A). However, when THSG-CM was applied at high concentrations (THSG-CM concentration [μl]:fresh medium concentration [μl] = 5:1000), most of the THSG-CM treatments (concentrations) significantly enhanced the proliferation of both HSFs and HGFs relative to 0 μM THSG-CM (Figure 2, Figure 3D). Furthermore, at some concentrations of THSG-CM, not all THSG-CM treatments increased cell proliferation, especially in the HSFs (Figure 2, Figure 3). This may be because cell metabolic waste increased in CM, thereby affecting its capability. However, THSG-CM at a high concentration may contain sufficient factors to overcome the inhibitory effect of cell metabolic waste.
A report revealed that mesenchymal stromal cell–derived CM reduced inflammation or enhanced epithelialization in the wounds of rats.39 We applied the in vitro inflammatory cell model (E. coli LPS treatment) and wound healing model of cells to evaluate the anti-inflammatory and wound healing activity of THSG-CM. Both HSFs and HGFs were treated with E. coli LPS for 6 h; the treatment significantly induced the expression of IL-1β, TNF-α, and IL-6 (Figure 4, Figure 5A). However, a high dose of LPS (10 μg/ml) inhibited the mRNA expression of these pro-inflammatory cytokines/mediators in the HGFs (Fig. 5A). This finding is similar to that of a previous study.40 The DPSC-derived CM (with or without THSG stimulation) attenuated the LPS-induced expression of IL-1β and TNF-α in the HSFs but enhanced the IL-6 expression (Fig. 4B). A possible reason for this finding is that IL-6 is involved in the regulation of wound healing as inflammation progresses in skin;41 the factors in DPSC-derived CM may enhance the LPS-induced expression of IL-6, which is further enhanced by THSG-CM. In the HGFs, all LPS-induced mRNA expression levels of the pro-inflammatory cytokines/mediators were attenuated by THSG-CM (Fig. 5B). We also applied two-well silicone inserts to create defined cell-free gaps to evaluate the wound healing activity of THSG-CM in the HSFs and HGFs. After 24 h of treatment, only some of the THSG-CM treatments (25 and 50 μM THSG-CM treatments in the HSFs; 1 and 25 μM THSG-CM treatments in the HGFs) improved cell wound healing relative to 0 μM THSG-CM (Figure 6, Figure 7). This suggests that the HSFs and HGFs exhibited different levels of sensitivity levels THSG-CM.
We demonstrated that THSG-CM had more beneficial effects on cell activities (cell proliferation and wound healing capability) and anti-inflammation in HSFs and HGFs than did DPSC-derived CM. However, future studies should be done to identify and isolate the stimulatory components of THSG-CM, especially extracellular vesicles (EVs). EVs are secreted membranous structures of various subtypes, including exosomes (40–140 nm), microvesicles (50–1000 nm), and apoptotic bodies (1–5 μm).42 Recent studies have confirmed that MSC-derived EVs have beneficial effects on skin wound healing, kidney injury, graft versus host disease, stroke, and sepsis.42 Further investigation is warranted to elucidate the beneficial effects of EVs (especially exosomes) contained in THSG-CM that has been filtered through a 0.22-μm filter to sterilize and remove EVs of other sizes. The isolation, purification, characterization, and therapeutic evaluation of THSG-stimulated DPSC-derived exosomes should be conducted in the future.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This work was supported by a grant from Wan-Fang Hospital, Taipei Medical University, Taipei, Taiwan (Dr. Sheng-Yang Lee, 109-wf-eva-14). It was also supported in part by general a grant of Ministry of Science and Technology, Taiwan (Dr. Sheng-Yang Lee, MOST108-2314-B-038-033-MY2). This manuscript was edited by Wallace Academic Editing.
References
- 1.Preston S.L., Alison M.R., Forbes S.J., Direkze N.C., Poulsom R., Wright N.A. The new stem cell biology: something for everyone. Mol Pathol. 2003;56:86–96. doi: 10.1136/mp.56.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vezzani B., Pierantozzi E., Sorrentino V. Mesenchymal stem cells: from the perivascular environment to clinical applications. Histol Histopathol. 2018;33:1235–1246. doi: 10.14670/HH-11-998. [DOI] [PubMed] [Google Scholar]
- 3.Miura M., Gronthos S., Zhao M. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C.Y., Kuo P.J., Chin Y.T. Dental pulp stem cell transplantation with 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside accelerates alveolar bone regeneration in rats. J Endod. 2019;45:435–441. doi: 10.1016/j.joen.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Lin C.Y., Chin Y.T., Kuo P.J. 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-glucoside potentiates self-renewal of human dental pulp stem cells via the AMPK/ERK/SIRT1 axis. Int Endod J. 2018;51:1159–1170. doi: 10.1111/iej.12935. [DOI] [PubMed] [Google Scholar]
- 7.Seo B.M., Sonoyama W., Yamaza T. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14:428–434. doi: 10.1111/j.1601-0825.2007.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonoyama W., Liu Y., Yamaza T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morsczeck C., Gotz W., Schierholz J. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Tabatabaei F.S., Moezizadeh M., Javand F. Effects of extracts of Salvadora persica on proliferation and viability of human dental pulp stem cells. J Conserv Dent. 2015;18:315–320. doi: 10.4103/0972-0707.159740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S.M., Chiu H.C., Chin Y.T. Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res Ther. 2014;5:52. doi: 10.1186/scrt441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Cara S., Origassa C.S.T., de Sa Silva F. Angiogenic properties of dental pulp stem cells conditioned medium on endothelial cells in vitro and in rodent orthotopic dental pulp regeneration. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristaldi M., Mauceri R., Tomasello L. Dental pulp stem cells for bone tissue engineering: a review of the current literature and a look to the future. Regen Med. 2018;13:207–218. doi: 10.2217/rme-2017-0112. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.Y., Chiang P.C., Tsai Y.H. Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells. J Endod. 2010;36:1336–1340. doi: 10.1016/j.joen.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Alge D.L., Zhou D., Adams L.L. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73–81. doi: 10.1002/term.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kichenbrand C., Velot E., Menu P., Moby V. Dental pulp stem cell-derived conditioned medium: an attractive alternative for regenerative therapy. Tissue Eng B Rev. 2019;25:78–88. doi: 10.1089/ten.TEB.2018.0168. [DOI] [PubMed] [Google Scholar]
- 17.La Noce M., Paino F., Spina A. Dental pulp stem cells: state of the art and suggestions for a true translation of research into therapy. J Dent. 2014;42:761–768. doi: 10.1016/j.jdent.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Stanko P., Altanerova U., Jakubechova J., Repiska V., Altaner C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cell Int. 2018;2018:8973613. doi: 10.1155/2018/8973613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Moshy S., Radwan I.A., Rady D. Dental stem cell-derived secretome/conditioned medium: the future for regenerative therapeutic applications. Stem Cell Int. 2020;2020:7593402. doi: 10.1155/2020/7593402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantinieaux D., Quertainmont R., Blacher S. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PloS One. 2013;8 doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vizoso F.J., Eiro N., Cid S., Schneider J., Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova-Todorova E., Bochev I., Dimitrov R. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. J Biomed Biotechnol. 2012;2012:295167. doi: 10.1155/2012/295167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inukai T., Katagiri W., Yoshimi R. Novel application of stem cell-derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430:763–768. doi: 10.1016/j.bbrc.2012.11.074. [DOI] [PubMed] [Google Scholar]
- 24.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chin Y.T., Hsieh M.T., Lin C.Y. 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-glucoside isolated from Polygoni multiflori ameliorates the development of periodontitis. Mediat Inflamm. 2016;2016:6953459. doi: 10.1155/2016/6953459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toma C., Wagner W.R., Bowry S., Schwartz A., Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iso Y., Spees J.L., Serrano C. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamm A., Strauss S., Vogt P., Scheper T., Pepelanova I. Positive in vitro wound healing effects of functional inclusion bodies of a lipoxygenase from the Mexican axolotl. Microb Cell Factories. 2018;17:57. doi: 10.1186/s12934-018-0904-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu S., Li Y., Bennett S. The role of glial cell line-derived neurotrophic factor family member artemin in neurological disorders and cancers. Cell Prolif. 2020;53 doi: 10.1111/cpr.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker P.A., Mantyh P., Arendt-Nielsen L., Viktrup L., Tive L. Nerve growth factor signaling and its contribution to pain. J Pain Res. 2020;13:1223–1241. doi: 10.2147/JPR.S247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita Y., Ono W., Ono N. Growth plate skeletal stem cells and their transition from cartilage to bone. Bone. 2020;136:115359. doi: 10.1016/j.bone.2020.115359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paus R., Langan E.A., Vidali S., Ramot Y., Andersen B. Neuroendocrinology of the hair follicle: principles and clinical perspectives. Trends Mol Med. 2014;20:559–570. doi: 10.1016/j.molmed.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: a critical mediator of metastasis. Life Sci. 2020;249:117534. doi: 10.1016/j.lfs.2020.117534. [DOI] [PubMed] [Google Scholar]
- 34.de Jager S.C.A., Hoefer I.E. Beyond the matrix: MMP2 as critical regulator of inflammation-mediated vascular dysfunction. Cardiovasc Res. 2017;113:1705–1707. doi: 10.1093/cvr/cvx202. [DOI] [PubMed] [Google Scholar]
- 35.Song F., Zhao J., Hua F. Proliferation of rat cardiac stem cells is induced by 2, 3, 5, 4'-tetrahydroxystilbene-2-O-beta-D-glucoside in vitro. Life Sci. 2015;132:68–76. doi: 10.1016/j.lfs.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Ueda M., Nishino Y. Cell-based cytokine therapy for skin rejuvenation. J Craniofac Surg. 2010;21:1861–1866. doi: 10.1097/SCS.0b013e3181f43f0a. [DOI] [PubMed] [Google Scholar]
- 37.Gharaei M.A., Xue Y., Mustafa K., Lie S.A., Fristad I. Human dental pulp stromal cell conditioned medium alters endothelial cell behavior. Stem Cell Res Ther. 2018;9:69. doi: 10.1186/s13287-018-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Sharabi N., Xue Y., Fujio M. Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng. 2014;20:3063–3072. doi: 10.1089/ten.TEA.2013.0718. [DOI] [PubMed] [Google Scholar]
- 39.Payushina O.V., Butorina N.N., Sheveleva O.N., Domaratskaya E.I. Effect of mesenchymal stromal cells and conditioned media on healing of skin wound. Bull Exp Biol Med. 2018;165:572–575. doi: 10.1007/s10517-018-4215-6. [DOI] [PubMed] [Google Scholar]
- 40.Kang W., Hu Z., Ge S. Healthy and inflamed gingival fibroblasts differ in their inflammatory response to Porphyromonas gingivalis lipopolysaccharide. Inflammation. 2016;39:1842–1852. doi: 10.1007/s10753-016-0421-4. [DOI] [PubMed] [Google Scholar]
- 41.Johnson B.Z., Stevenson A.W., Prele C.M., Fear M.W., Wood F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines. 2020;8 doi: 10.3390/biomedicines8050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varderidou-Minasian S., Lorenowicz M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979–5997. doi: 10.7150/thno.40122. [DOI] [PMC free article] [PubMed] [Google Scholar]