This review highlights the emergent role of the cytokine IL-17 in orchestrating cellular and organismal metabolism. Metabolism is thereby integrated into the protective and pathogenic aspects of IL-17 responses in a temporally and spatially regulated manner.
Abstract
IL-17 was discovered nearly 30 yr ago, but it has only been recently appreciated that a key function of this cytokine is to orchestrate cellular and organismal metabolism. Indeed, metabolic regulation is integrated into both the physiological and the pathogenic aspects of IL-17 responses. Thus, understanding the interplay between IL-17 and downstream metabolic processes could ultimately inform therapeutic opportunities for diseases involving IL-17, including some not traditionally linked to this cytokine pathway. Here, we discuss the emerging pathophysiological roles of IL-17 related to cellular and organismal metabolism, including metabolic regulation of IL-17 signal transduction.
Introduction
IL-17A, referred to here as IL-17, is the founding cytokine of IL-17 family that includes five other members, IL-17B–F (Monin and Gaffen, 2018). IL-17 is best known as a host-defensive cytokine at barrier mucosal tissues, with essential roles in immunity to fungi and other extracellular pathogens (Conti and Gaffen, 2010; Conti et al., 2009; Conti et al., 2016; Verma et al., 2017). IL-17 also drives inflammation in a variety of autoimmune pathologies (McGeachy et al., 2019; Veldhoen, 2017). Consequently, we have witnessed an effective application of IL-17/Th17 cell basic biology research discoveries to treatment of psoriasis, psoriatic arthritis, ankylosing spondylitis, etc. Additional drugs targeting the IL-17/Th17 cell pathway are in preclinical development (Prinz et al., 2020; Vitiello and Miller, 2020; McGeachy et al., 2019). Nevertheless, surprising new aspects of IL-17 biology continue to be discovered, indicating that our overall understanding of this cytokine system remains incomplete.
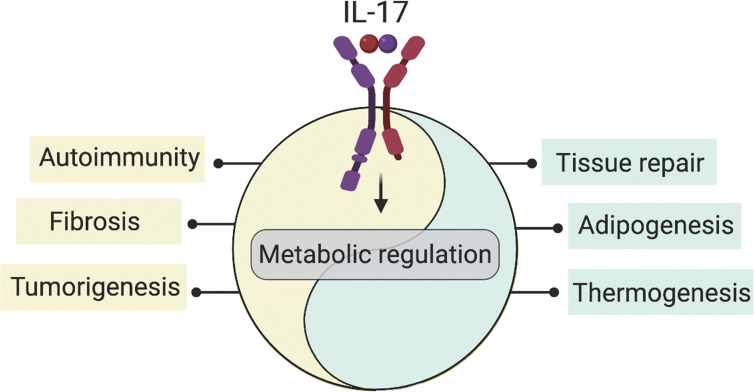
The metabolism-modulating activity of IL-17 is a relatively new feature of the events driven by this cytokine (Fig. 1). An intimate association between the immune and metabolic systems has emerged on many fronts, especially studies of metabolic syndrome where inflammatory cytokines from adipose tissue are linked to insulin resistance and related impairments (Makowski et al., 2020; Hotamisligil et al., 1993; Mathis and Shoelson, 2011). Paralleling immune regulation of the metabolic system, metabolism of immune cells themselves is highly regulated. “Immunometabolism” refers to two complementary facets of these interactions. The first relates to cellular metabolism, i.e., how metabolic regulation within immune cells leads to different cell fates and function (e.g., activation, proliferation, and differentiation; Gaber et al., 2017; O’Neill et al., 2016). The second focuses on the effect of inflammation and immune responses on the control of systemic metabolism (e.g., adipogenesis; Mathis, 2019; Troha and Ayres, 2020; Man et al., 2017; Lee et al., 2018; Hotamisligil, 2017a). Therefore, this fusion between immunity and metabolism has broadened our understanding of connections between autoimmune and metabolic diseases.
Figure 1.
Overview of IL-17–mediated metabolic regulation. IL-17–driven metabolic pathways deliver vital signals for tissue homeostasis. In parallel, metabolic regulation underlies many IL-17–driven pathologies. Cellular and organismal metabolic homeostasis cannot be sustained without appropriate temporally and spatially regulated IL-17 signaling, at the risk of damage to tissues with critical metabolic functions.
In mediating immunity to infections, IL-17 is expressed only transiently. However, in several chronic inflammatory and autoimmune diseases, IL-17 persists long term, and its capacity to influence metabolic syndrome may have severe consequences (McGeachy et al., 2019). In both settings, growing literature points to a role for IL-17 in cellular and organismal metabolism. At a cellular level, IL-17–mediated metabolic regulation promotes cell proliferation, a process that has been described in epithelial cells, keratinocytes, and stem cells to coordinate tissue repair and wound healing (Lee et al., 2015; Song et al., 2015; MacLeod et al., 2013; Chen et al., 2019). In addition, IL-17 promotes proliferation and survival of synovial fibroblasts (SFs; Kim et al., 2017) and fibroblastic reticular cells (FRCs; Majumder et al., 2019), which support IL-17–mediated inflammation in arthritis and models of multiple sclerosis, among other conditions (Yu et al., 2018; Majumder et al., 2019; Kim et al., 2017). Moreover, certain cellular metabolites (e.g., itaconate; O’Neill and Artyomov, 2019), influence IL-17 signal transduction. At the organismal level, IL-17 impacts adipogenesis, thermogenesis, and fibrosis (Ahmed and Gaffen, 2010; Kohlgruber et al., 2018; Hu et al., 2020; Okamoto et al., 2012; Madhur et al., 2010; Wilson et al., 2010). Elevated levels of IL-17 are detected in numerous metabolic disease settings, including obesity (Sumarac-Dumanovic et al., 2009; Winer et al., 2009), diabetes (Arababadi et al., 2010; Marwaha et al., 2010), and osteoporosis (Zhang et al., 2015). Moreover, some IL-17–driven conditions (e.g., psoriasis) constitute a major risk for metabolic syndrome (Davidovici et al., 2010; Azfar and Gelfand, 2008).
In this review, we outline the current view of metabolic pathways that underlie IL-17 function, including metabolic patterns of IL-17–target cells to metabolic homeostasis or diseases mediated by an IL-17 response. Changes in cellular metabolism that contribute to Th17 cell differentiation are also discussed.
The immunometabolic response in immune and structural cells
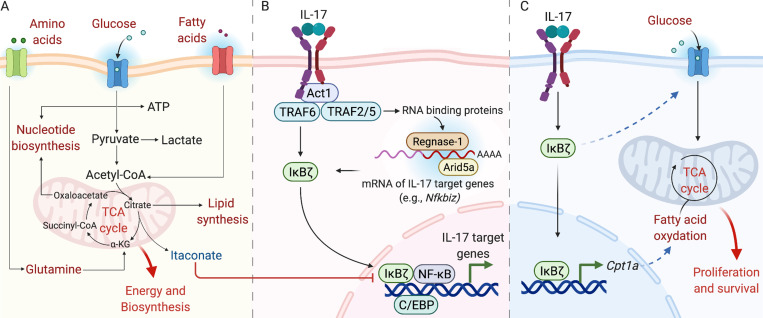
The immune system needs to be poised to respond rapidly to harmful triggers and continuously adapt to diverse environments and, hence, requires immediately available energy sources. At the same time, the potentially damaging effects of resulting metabolites must be avoided (Hotamisligil, 2017b; Wang et al., 2019; Buck et al., 2017). Hence, cells rely on a balance between catabolism and anabolism, which are dependent on various metabolic substrates, such as glucose, fatty acids, and amino acids (Wang et al., 2019). These substrates are involved in at least six major metabolic pathways: glycolysis, tricarboxylic acid (TCA) cycle, pentose phosphate pathway, fatty acid oxidation (FAO), fatty acid synthesis (FAS), and amino acid metabolism (Fig. 2 A; O’Neill et al., 2016). Although diverse in terms of intracellular metabolites that are generated, these pathways are interconnected and coregulated. For example, the FAS pathway, required for cellular proliferation, requires the availability of intermediate metabolites generated during glycolysis and the TCA cycle (Fig. 2 A; O’Neill et al., 2016). Proinflammatory stimuli are usually mitogenic, activating anabolic pathways (e.g., glycolysis and FAS) that lead to proliferation and differentiation (Wang et al., 2019). Alternatively, anti-inflammatory signals drive catabolic pathways (e.g., FAO) and promote cellular quiescence (Wang et al., 2019).
Figure 2.
IκBζ mediates IL-17–driven metabolic and immunological responses. (A) Cellular metabolism generates energy and regulates cell function in numerous ways. Glucose metabolism creates intermediates that supply different metabolic pathways. For example, pyruvate is converted to acetyl-CoA, which feeds into the TCA cycle to generate ATP. Oxaloacetate and citrate are used for nucleotide and lipid synthesis, respectively. Fatty acids are metabolized through β-oxidation to acetyl-CoA, whereas glutamine is metabolized via glutaminolysis to α-ketoglutarate. Some metabolites (e.g., itaconate) directly influence IL-17 responses. (B) IL-17 activates downstream signaling pathways through distinct TRAF proteins. TRAF6 induces IκBζ, NF-κB, C/EBPs, and MAPK pathways. Together, these pathways upregulate transcription of IL-17–dependent target genes. Additionally, IL-17 controls posttranscriptional events that increase target mRNA levels through various RBPs (e.g., Act1, HuR, Arid5a, Regnase-1). (C) IL-17 promotes stromal cell survival in lymph nodes (e.g., FRCs) through cell metabolic regulation involving an IκBζ-dependent increase in glycolysis and mitochondrial activity.
The fast-paced literature on immunometabolism in the past few years has revealed requirements for specific metabolic events in all immune cells: macrophages (O’Neill and Pearce, 2016; Covarrubias et al., 2015; Russell et al., 2019), neutrophils (Injarabian et al., 2019), dendritic cells (O’Neill and Pearce, 2016; Pearce and Everts, 2015), natural killer cells (O’Brien and Finlay, 2019), and T cells (Pearce et al., 2013; Bantug et al., 2018; Man and Kallies, 2015). The status of cellular metabolism governs the types of cytokines produced by immune cells and, consequently, the ensuing immune response. This concept was illustrated in macrophages, where M1 cells are highly glycolytic, whereas M2 macrophages rely largely on oxidative phosphorylation (O’Neill and Pearce, 2016; Russell et al., 2019; Vats et al., 2006). A recent study showed that subsets of γδ-T cells differ in their metabolic signatures and are influenced by the obesity status of the host (Lopes et al., 2021). Hence, metabolic requirements critically dictate cell fate and function.
With regard to the IL-17 pathway, immunometabolism has been most extensively studied in Th17 cells. However, nonhematopoietic cells, the main targets of IL-17 signaling, are less well defined in terms of immunometabolic events (Monin and Gaffen, 2018; McGeachy et al., 2019). The sensitivity of IL-17 target cells is thought to be mainly due to restricted expression of the essential IL-17RC subunit. With the exception of in vitro–differentiated Th17 cells (Chong et al., 2020), IL-17RC is generally expressed at low or undetectable levels in hematopoietic cells (Kuestner et al., 2007; Ishigame et al., 2009). IL-17–responsive cells reside in highly diverse tissues and organs, and hence, it is not surprising that each cell type has distinct metabolism and nutrient usage. Recently, advances in single-cell transcriptomic technologies have uncovered the heterogeneity of mesenchymal and epithelial cells, even within single tissues (Koliaraki et al., 2020; Krausgruber et al., 2020). Therefore, the consequence of global single-gene deletion models in mice may often not mirror what is happening at the tissue level. For example, IL-17 deficiency predisposes mice to age-associated obesity and impaired glucose tolerance but improves glucose uptake and insulin responses of adipocytes in vitro (Zúñiga et al., 2010). In addition, the actions of IL-17 are context dependent and may vary in physiological and pathological outcomes, leading to somehow contradictory IL-17–mediated metabolic regulation. Several examples are provided in Table 1. Of note, IL-17 signaling outcomes are not only influenced by the specificity of its target cells but also by concomitant signals from other cytokines present in the same environment. IL-17 is notorious for its ability to mediate synergistic or cooperative signaling with other cytokines or inflammatory stimuli (Li et al., 2019), which may constitute another compelling reason behind the pleiotropic roles of IL-17 on both local and systemic metabolism.
Table 1. IL-17–driven metabolic pathways in health and disease.
| IL-17–mediated metabolic regulation during homeostasis | ||||
|---|---|---|---|---|
| IL-17 target cells | Metabolic regulation | Cellular outcome | Organismal outcome | References |
| Epithelial cells/Mesenchymal cells | Unknown | Proliferation | Tissue repair and wound healing | Lee et al. (2015), Song et al. (2015), MacLeod et al. (2013), Chen et al. (2019), Floudas et al. (2017) |
| Adipocytes | Decreased expression of proadipogenic TFs | Suppressed differentiation of adipocytes | Decreased adipose tissue mass accumulation | Ahmed and Gaffen (2013) |
| Decreased insulin-mediated glucose uptake in vitro | Unknown | Unknown | Zúñiga et al. (2010) | |
| Increased expression of thermogenic genes | Enhanced metabolic respiration via uncoupled oxidative phosphorylation | Thermogenesis | Kohlgruber et al. (2018), Papotto and Silva-Santos (2018) | |
| Stromal adipose cells | Unknown | IL-33 production | Insulin sensitivity | Kohlgruber et al. (2018), Papotto and Silva-Santos (2018) |
| IL-17–mediated metabolic regulation during disease | ||||
| IL-17 target cells | Metabolic regulation | Cellular outcome | Organismal outcome | References |
| Epithelial cells/Mesenchymal cells | Unknown | ECM production | Fibrosis | Okamoto et al. (2012), Madhur et al. (2010), Wilson et al. (2010), Wu et al. (2014), Mi et al. (2011) |
| Increased glycolysis | Proliferation | Tumorigenesis | Terzić et al. (2010), Straus (2013) | |
| Keratinocytes | Reprogramming of urea cycle | Proinflammatory gene expression | Psoriasis | Lou et al. (2020), Bandyopadhyay and Larregina (2020) |
| Increased cholesterol uptake | Proinflammatory gene expression | Psoriasis | Varshney et al. (2016) | |
| SFs | Decreased expression of oxidative phosphorylation genes | Cell survival | RA | Kim et al. (2017) |
| Increased expression of amino acid transporter 1 | Increased cell migratory capacity | RA | Yu et al. (2018) | |
| FRCs | Increased glycolysis/Increased FAO | Cell survival and proinflammatory genes expression | EAE/Colitis | Majumder et al. (2019) |
The metabolism-modulating activities of IL-17 are context dependent and may vary on the basis of its target cells.
Beyond their well-established role in providing structural support to organs, epithelial and mesenchymal cells are actively involved in immune responses, which has become increasingly appreciated (Tuong and Clatworthy, 2020; Koliaraki et al., 2020; Nowarski et al., 2017; Malhotra et al., 2013). Several prominent examples support the immunomodulatory role of structural cells (Koliaraki et al., 2020): mesenchymal cells in adipose tissue promote T reg cell differentiation (Spallanzani et al., 2019; Vasanthakumar et al., 2015; Kohlgruber et al., 2018), FRCs in lymphoid organs regulate T-cell function (Jalkanen and Salmi, 2020; Krishnamurty and Turley, 2020), and stromal cells in the synovium (SFs) are key determinants of the inflammatory response during arthritis (Nygaard and Firestein, 2020). Generally, IL-17–mediated metabolic regulation in target cells promotes proliferation (e.g., in SFs and FRCs; Kim et al., 2017; Majumder et al., 2019); migration (e.g., in SFs; Kim et al., 2017) as well as the expression of metabolic enzymes (e.g., Cpt1a in FRCs [Majumder et al., 2019]; arginase-1 in keratinocytes [Lou et al., 2020]); and inflammatory mediators, which are involved in immune regulation. Consequently, dysfunctional metabolic regulation in IL-17 target cells may contribute to IL-17–driven diseases.
Metabolic regulation in IL-17–driven physiological responses
IL-17–mediated metabolic regulation in barrier tissue
IL-17 is a potent driver of barrier tissue repair (Zhao et al., 2020a), a metabolically demanding process (Eming et al., 2017). The regenerative role for IL-17 in the gut was inferred from patients with inflammatory bowel disease, whose symptoms were unexpectedly aggravated after clinical trials of anti–IL-17A/IL-17RA biologics for Crohn’s disease (Hueber et al., 2012; Targan et al., 2016). These observations were consistent with prior studies in mice showing that Act1, the essential IL-17R adaptor, promotes tissue repair in response to gut injury (Qian et al., 2007). Consistent with this, IL-17 neutralization or Il17a deficiency in mice caused exacerbated dextran sodium sulfate–induced intestinal injury (Ogawa et al., 2004; O’Connor et al., 2009; Lee et al., 2015). Similarly, IL-17 promotes wound healing in the skin, and impaired IL-17 in murine models of skin injury resulted in delayed wound closure, decreased barrier integrity, and skin microbiome dysbiosis (MacLeod et al., 2013; Floudas et al., 2017). Collectively, in homeostasis and acute models of inflammation, IL-17 promotes skin and gut tissue repair rather than drives pathogenic inflammation, as it does in psoriasis.
IL-17–dependent wound healing properties are explained by several mechanisms. IL-17 promotes proliferation of keratinocytes and epithelial cells via mitogenic signals, such as the ERK pathway (Shen et al., 2009; Zepp et al., 2017; Wu et al., 2015). Moreover, IL-17 induces expression of antimicrobial peptides (e.g., β-defensins, RegIIIγ, and S100A8/9; Lee et al., 2015; Whibley and Gaffen, 2015; MacLeod et al., 2013; Ha et al., 2014) and metalloreductase Steap4 (Wu et al., 2015), which promote proliferation (Lee et al., 2015; Whibley and Gaffen, 2015; MacLeod et al., 2013; Ha et al., 2014). Although the expression of genes associated with epithelial tight junction integrity was not modified in Il17a-deficient mice, IL-17 regulates cellular localization of the tight junction protein occludin proposed to underlie gut epithelial barrier function (Lee et al., 2015). In addition, synergistic signals mediated by other cytokines present in the local inflammatory milieu appear to tune IL-17–mediated tissue repair. Specifically, IL-17 synergizes with fibroblast growth factor 2 to induce genes that repair damaged epithelium (Song et al., 2015). In principle, IL-17–induced repair responses could be regulated by nutrient availability. Nevertheless, our understanding of how the different bioenergetic pathways (e.g., glycolysis, FAO) impact IL-17–mediated tissue repair and regeneration is still limited.
Although IL-17 signaling drives cell proliferation and tissue repair, this process may lead to tumorigenesis when dysregulated (Wang et al., 2014; Ernst and Putoczki, 2014; Zhang et al., 2017). Indeed, chronic inflammation is known to promote colorectal tumorigenesis (Terzić et al., 2010). In this context, IL-17 synergizes with TNF-α to induce an NF-κB– and HIF1α-dependent glycolytic pathway that promotes the survival and proliferation of cultured human colorectal cancer cells (Straus, 2013). IL-17 signaling in skin stem cells represents another example of the link between inflammation and tumorigenesis (Chen et al., 2019). In this setting, IL-17 promotes recruitment of epidermal growth factor receptor (EGFR) to the IL-17R complex in skin stem cells. This allows the phosphorylation of EGFR and subsequent activation of the ERK5 pathway, resulting in cell proliferation and wound-induced tumorigenesis (Chen et al., 2019). Accordingly, the cross talk between IL-17 and EGFR signaling in skin stem cells represents a surprisingly direct integration of IL-17 and growth factor signaling in tissue repair and tumorigenesis.
IL-17–mediated metabolic regulation in nonbarrier tissue
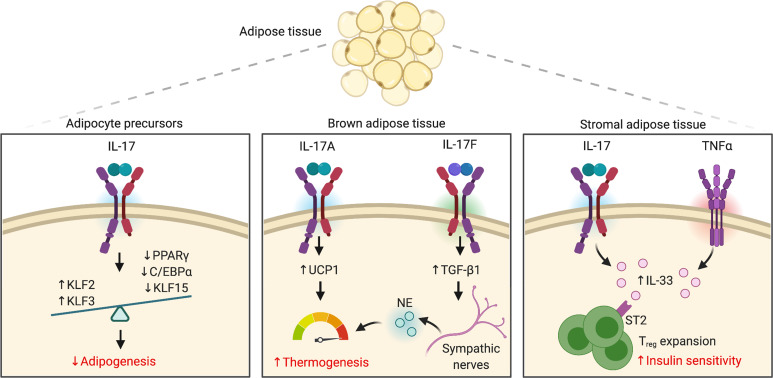
It has long been noted that mice lacking IL-17R or IL-17A are overweight, gaining fat mass with age (Zúñiga et al., 2010; Goswami et al., 2009; Ahmed and Gaffen, 2010). These observations were consistent with prior studies investigating the role of IL-17 in osteoporosis, where IL-17 was shown to suppress mesenchymal differentiation of adipocytes and promote differentiation of osteoblasts (Shin et al., 2009; Huang et al., 2009). These studies support a model in which lack of IL-17 establishes an environment where adipogenesis is favored. Mechanistically, IL-17 activation of a preadipocyte model cell line suppressed expression of proadipogenic transcription factors (TFs; e.g., CCAAT enhancer binding protein [C/EBP]-α, PPARγ, and Krüppel-like factor [KLF] 5) while enhancing antiadipogenic TFs (e.g., KLF2, KLF3; Ahmed and Gaffen, 2013). Thus, IL-17 restrains adipogenesis through the combined effects of TFs that regulate adipocyte differentiation (Fig. 3).
Figure 3.
IL-17–mediated metabolic regulation in adipose tissue. The IL-17 pathway exerts an antiadipogenic role by suppressing adipocyte differentiation through decreased expression of proadipogenic TFs. IL-17A promotes thermogenesis in BAT by supporting a UCP1-dependent thermogenic response. Moreover, IL-17 synergizes with TNF-α to induce IL-33 by stromal adipose cells, leading to T reg cell expansion. IL-17F is reported to trigger sympathetic innervation and norepinephrine (NE) release.
IL-17 contributes to the homeostatic regulation of glucose metabolism as well (Zúñiga et al., 2010). In vitro, IL-17 inhibited insulin-induced glucose uptake by adipocytes (Zúñiga et al., 2010). The capacity of IL-17 to regulate glucose homeostasis was further confirmed in vivo. Indeed, young IL-17–deficient mice present with slightly increased fasting glucose levels, lower insulin levels, and improved glucose clearance upon glucose tolerance test (Zúñiga et al., 2010), an effect that was lost upon development of age-associated obesity (Zúñiga et al., 2010). Like IL-17, the effects of IL-6 on glucose metabolism are context dependent, leading to both positive (improved insulin sensitivity) and negative (impaired glucose tolerance) outcomes (Hotamisligil, 2017b). In contrast to IL-17 and IL-6, the metabolic effects of TNF-α are highly consistent among mouse models, where neutralization of TNF-α results in increased insulin sensitivity (for a complete list, see https://www.metaflammation.org; Borst et al., 2004; Araújo et al., 2007; Hotamisligil, 2017b).
Adaptive thermogenesis is the process of heat production, mainly in brown adipose tissue (BAT), and recent evidence shows that IL-17 contributes to this process. Thermogenesis is mediated by uncoupling protein 1 (UCP1), which uncouples mitochondrial FAO from ATP production, thereby releasing the energy of substrate oxidation as heat (Chouchani et al., 2019). In addition, BAT is richly innervated by sympathetic nerves, which release norepinephrine upon external stimuli (e.g., cold), and consequently activate lipolysis and thermogenic regulation (Chouchani et al., 2019). In this context, a homeostatic adipose population of IL-17–secreting γδ-T cells is required for the maintenance of body temperature (Kohlgruber et al., 2018; Papotto and Silva-Santos, 2018). IL-17 and TNF-α signaling in BAT trigger expression of UCP1, driving adaptive thermogenesis (Fig. 3). Consistent with this, IL-17–deficient mice are susceptible to hypothermia as demonstrated by decreased expression of thermogenic genes (e.g., Ucp1) and a failure to increase energy expenditure after cold challenge (Kohlgruber et al., 2018; Papotto and Silva-Santos, 2018). A related study found that IL-17F, rather than IL-17A, impacted thermogenesis. IL-17F was suggested to promote expression of TGF-β1 in adipocytes, which in turn supported sympathetic innervation of adipose tissue and hence activated lipolysis and thermogenesis via norepinephrine (Ciofani, 2020; Hu et al., 2020). Accordingly, these studies point to intriguing cross talk between IL-17 and adipose tissue in homeostatic body temperature control (Fig. 3).
In addition to its role in regulating adipogenesis and energy homeostasis, IL-17 mediates metabolic-immunological coordination in adipose tissue. In aging mice, the number of IL-17–producing γδ-T cells increase concomitantly with T reg cells in adipose tissue (Fig. 3; Kohlgruber et al., 2018; Papotto and Silva-Santos, 2018). Consequently, Il17a−/− mice show a decrease in T reg cell numbers in fat, suggesting that IL-17 is implicated in homeostatic expansion of T reg cells (Kohlgruber et al., 2018; Papotto and Silva-Santos, 2018). This effect is thought to be initiated by IL-17 and TNF-α signaling in adipose stromal cells leading to IL-33 production (Kohlgruber et al., 2018; Papotto and Silva-Santos, 2018), thereby maintaining the expansion of T reg cells expressing the IL-33 receptor (ST2). Although these studies did not report the consequences of IL-17–mediated T reg cell accumulation on insulin sensitivity (Mathis, 2019), T reg cells are known to promote this process (Feuerer et al., 2009; Kolodin et al., 2015). Hence, the ability of stromal adipose tissue to provide a niche that supports T reg cell differentiation reveals that mesenchymal-immune interactions are not restricted to lymphoid organs but can also happen in peripheral tissues.
A key question that emerges from these explorations of IL-17 in adipose tissue is the impact on human obesity and metabolic disorders, such as diabetes. Is there any advantage for IL-17–mediated metabolic-immune regulation in adipose tissue? One could speculate that the increase in IL-17 production observed in metabolic syndrome (Ahmed and Gaffen, 2010; Sumarac-Dumanovic et al., 2009) may be viewed as an attempt from the immune system to correct the metabolic disorders associated with the increase in fat storage and insulin resistance. Indeed, by inhibiting adipogenesis and promoting T reg cell expansion, IL-17 may play a beneficial role in maintaining insulin sensitivity and limiting inflammation in adipose tissue. However, obesity-linked metabolic diseases, such as type 2 diabetes and cardiovascular diseases, are associated with chronic low-grade inflammation (Mathis and Shoelson, 2011; Donath and Shoelson, 2011). Therefore, once obesity is established, the beneficial metabolic effects of IL-17 may be offset by other detrimental mechanisms (e.g., high levels of TNF-α), shifting the balance toward inflammation and leading to obesity-associated metabolic complications.
Metabolic regulation in IL-17–driven pathological responses
IL-17 and cellular immunometabolism during inflammation
Psoriasis is considered a metabolism-associated disease, but the impact of metabolic changes in the main IL-17 target cells in this disease—keratinocytes—are not fully understood (Pohla et al., 2020; Harden et al., 2016; Kamleh et al., 2015; Herbert et al., 2018; Zhang et al., 2018; Kang et al., 2017). It has been shown that arginase-1, a catalytic enzyme in the urea cycle, is upregulated in skin from psoriatic patients (Bruch-Gerharz et al., 2003). Interestingly, IL-17 downregulates protein phosphatase 6, a regulatory signaling intermediate, resulting in increased arginase-1 expression in psoriatic keratinocytes (Lou et al., 2020; Bandyopadhyay and Larregina, 2020). Accumulation of arginase-1 increases polyamine production from the urea cycle, which then binds to self-RNA–antigen complexes released by damaged keratinocytes. Consequently, these complexes seem to act as danger signals and activate dermal dendritic cells to produce proinflammatory cytokines. In particular, dendritic cell–derived IL-6 promotes IL-17 secretion. Therefore, arginase-1 activation maintains a positive feedback loop that sustains inflammation in psoriatic skin. Consistently, inhibition of arginase-1 ameliorated skin inflammation in imiquimod (IMQ)-induced experimental dermatitis, a common mouse model of psoriasis (Lou et al., 2020; Bandyopadhyay and Larregina, 2020). Another study showed that IL-17 induces cholesterol accumulation in keratinocytes, leading to secretion of proinflammatory cytokines (Varshney et al., 2016). However, in contrast to IL-17–mediated reprogramming of the urea cycle, our knowledge on how IL-17 induces its target genes through regulation of cholesterol metabolism is quite limited. Collectively, IL-17–mediated metabolic regulation in keratinocytes is a key component of a coordinated ensuing immune response.
During rheumatoid arthritis (RA), SFs expand, become invasive, and promote joint inflammation, thereby destroying cartilage and promoting development of the hyperplastic RA synovium as a tertiary lymphoid organ (Nygaard and Firestein, 2020). During this process, the metabolism of SFs is “reprogrammed” as a consequence of the inflamed microenvironment (Bustamante et al., 2017). This metabolic switch in the RA synovium is characterized by mitochondrial dysfunction (Bustamante et al., 2017; Harty et al., 2012; Biniecka et al., 2016) and disruption of glucose metabolism (Bustamante et al., 2018; de Oliveira et al., 2019). In SFs, IL-17 decreases expression of oxidative phosphorylation component genes and, consequently, induces autophagy and inhibition of cell death (Kim et al., 2017; Bustamante et al., 2017; Clarke and Simon, 2019). Thus, activated fibroblasts shift from oxidative phosphorylation to glycolytic ATP production, and therefore, inhibition of glycolysis decreases cell proliferation, migration, and invasion (de Oliveira et al., 2019; Bustamante et al., 2018). Moreover, IL-17 induces expression of the amino acid transporter LAT1 in SFs, resulting in enhanced leucine uptake and potentiated cell migratory capacity (Yu et al., 2018). Accordingly, IL-17–activated SFs upregulate the expression of nutrient transporters to perpetuate inflammation. However, the efficacy of IL-17 blockade in treating RA was disappointingly modest (Taams, 2020; Genovese et al., 2014; Mease et al., 2018; Pavelka et al., 2015). Although the reasons remain unclear, RA is a heterogenous disease, and not all patients present with high IL-17 levels; thus, IL-17 blockade alone may not be sufficient to disrupt all the requisite inflammatory triggers effectively (Taams, 2020). Moreover, effects of IL-17 may be temporal in early versus late disease, an issue difficult to address in standard RA clinical trials (Taams, 2020).
FRCs are specialized cells located in the T cell areas of lymphoid organs that produce collagen-rich reticular fibers to support immune cell organization (Jalkanen and Salmi, 2020; Krishnamurty and Turley, 2020; Koliaraki et al., 2020). However, FRCs not only provide structural support to immune cells but also actively participate in determining the magnitude of immune responses as well as immune cell trafficking and peripheral tolerance (Jalkanen and Salmi, 2020; Krishnamurty and Turley, 2020; Koliaraki et al., 2020). Interestingly, recent studies demonstrated an unexpected role for IL-17 in driving metabolic regulation in FRCs during experimental autoimmune settings, including experimental autoimmune encephalomyelitis (EAE) and colitis (Majumder et al., 2019). Like stromal cells in the RA synovium, IL-17 promotes proliferation and survival of FRCs by increasing metabolic fitness. Majumder et al. (2019) showed that IL-17 drives these effects at least in part through induction of the Cpt1a gene, a rate-limiting enzyme of mitochondrial FAO, which was driven by the IκBζ TF (Fig. 2 C). Consistently, FRCs fail to expand in mice lacking IL-17RA in FRCs during EAE. Moreover, these mice show compromised B-cell activation and autoantibody production (Majumder et al., 2019; Bordon, 2019; Mueller, 2019). Thus, IL-17 drives successful metabolic reprogramming of FRC, which allows activation of the adaptive immune response. In contrast to SFs where IL-17–mediated metabolic regulation is detrimental, IL-17–driven metabolic changes in FRCs may have more broad consequences in a context-dependent manner.
IL-17 and organismal immunometabolism during inflammation
Autoimmune diseases such as psoriasis and RA are often accompanied by impacts on the cardiovascular system, and emerging data illustrate how far reaching these consequences can be (Davidovici et al., 2010; Gisondi et al., 2018; Ferguson et al., 2019; Frostegård, 2005; Katz et al., 2019; Liu and Kaplan, 2018). However, the mechanisms underlying autoimmunity-associated cardiovascular consequences are largely unknown. Even so, recent studies provide interesting mechanistic insights. In IMQ-induced experimental psoriasis, IL-17 was found to promote collagen deposition in psoriatic lesions and distal skin regions and arteries, thereby altering lipoprotein traffic. Consequently, lipoproteins accumulate within the vessel wall, promoting atherosclerosis. IL-17 neutralization rescued lipoprotein transit, suggesting a therapeutic avenue for treating this complication (Huang et al., 2019). These observations are consistent with other studies in mouse models of psoriasis where IL-17 was linked to thrombosis and where IL-17 neutralization reduced skin and cardiovascular manifestations (Li et al., 2018). Cumulatively, these studies suggest that treating the noncardiovascular symptoms of autoimmunity may be effective in reducing cardiovascular comorbidity. Nevertheless, a recent case-time-control study suggested that the administration of ustekinumab, which blocks the IL-12/IL-23 p40 subunit, may trigger severe cardiovascular events among patients at high cardiovascular risk (Poizeau et al., 2020). Consistently, low serum levels of IL-17 were associated with a higher risk of major cardiovascular events in patients with myocardial infraction (Simon et al., 2013). In a murine model of hypercholesterolemic atherosclerosis, administration of IL-17 reduced endothelial VCAM-1 expression, vascular T cell infiltration, and atherosclerotic lesion development (Taleb et al., 2009). Even so, the contexts in which IL-17 exerts proatherogenic effects versus protective signals is unclear (Liuzzo et al., 2013), and close monitoring may be needed when using anti–IL-17 therapy in patients with cardiovascular risk factors.
Different fibroblasts and mesenchymal cell types across many organs are responsible for regulating extracellular matrix (ECM) homeostasis (Zhao et al., 2019; Rabinowitz and Mutlu, 2019; Zhao et al., 2020b). Hence, it is not surprising that IL-17, like other cytokines (e.g., TNF-α, TGF-β), mediates fibrosis as a result of the activation, proliferation, and persistence of its target cells after a dysregulated healing response (Eming et al., 2017). IL-17 is linked to collagen production in angiotensin-induced hypertension (Wu et al., 2014; Madhur et al., 2010), bleomycin-induced lung fibrosis (Wilson et al., 2010; Mi et al., 2011), and skin fibrosis (Okamoto et al., 2012). Moreover, blockade of IL-17 attenuates tissue injury, inflammation, and fibrosis. However, in a model of kidney injury, IL-17 plays a surprising antifibrotic role by promoting production of bradykinin through the kallikrein-kinin system in renal epithelial cells, resulting in production of matrix metalloproteinases that constrain fibrosis (Ramani et al., 2018). Therefore, IL-17 function in tissue remodeling is influenced by the responding cell type and the local inflammatory environment. Metabolic perturbation is increasingly appreciated as an important pathogenic process that underlies fibrosis, with upregulation of glycolysis favoring ECM deposition over degradation (Zhao et al., 2019; Rabinowitz and Mutlu, 2019; Zhao et al., 2020b). TGF-β is a well-known activator of glycolysis in mesenchymal cells, leading to collagen synthesis (Zhao et al., 2020b; Jiang et al., 2015). Considerable data show that the IL-17A–TGF-β axis is important to the development of fibrosis (Wilson et al., 2010; Fabre et al., 2014). Hence, understanding the metabolic milieu that sustains IL-17–mediated fibrosis might offer new therapeutic avenues for treating fibrosis.
Metabolic pathways as potential therapeutic targets in IL-17–driven pathology
Metabolic regulation of IL-17 signaling
Cellular metabolism is not just limited to the delivery of energy and substrates for biosynthesis but can regulate downstream signaling by cytokines. IL-17 signaling events are initiated by the multifunctional adaptor protein Act1, which is recruited to the IL-17R after ligand engagement. Act1 in turn recruits diverse TNF receptor–associated factor (TRAF) proteins, which initiate transcriptional and posttranscriptional pathways (Fig. 2 B; Gaffen, 2009; Amatya et al., 2017; Li et al., 2019; Qian et al., 2007). IL-17 signaling is well known to activate the classical NF-κB pathway. Additional downstream IL-17 TFs include C/EBP and AP-1 components (Shen et al., 2006; Ruddy et al., 2004; Patel et al., 2007; Karlsen et al., 2010; Slowikowski et al., 2020; Qian et al., 2007; Chang et al., 2006; Shen et al., 2009). A major transcriptional target of NF-κB is IκBζ (Nfkbiz), a noncanonical member of the NF-κB family (Karlsen et al., 2010; Yamazaki et al., 2005). IκBζ is now recognized to be a vital mediator of many IL-17 metabolic activities (Fig. 2 C). As noted, IL-17 induction of IκBζ in FRCs drives expression of Cpt1a and is required for elevated glucose uptake needed to support cell survival (Mueller, 2019; Majumder et al., 2019). IκBζ is implicated in psoriasis both genetically and functionally (Tsoi et al., 2015; Johansen et al., 2015), and Nfkbiz−/− mice, similar to IL-17–deficient mice, are resistant to pathology in IMQ dermatitis (Johansen et al., 2015). Notably, in keratinocytes, IL-17–mediated induction of IκBζ and its downstream target genes is blocked by a derivative of itaconate (Bambouskova et al., 2018; Bordon, 2018), a metabolite from the TCA cycle (Fig. 2 A; O’Neill and Artyomov, 2019). Hence, itaconate-mediated regulation of IκBζ represents a striking example of how a standard metabolic pathway can exert immunoregulatory potential (Bambouskova et al., 2018; Bordon, 2018).
From the perspective of downstream IL-17 signaling, IκBζ is at a nexus of not only transcriptional events but also posttranscriptional events. It has become increasingly apparent that a major mechanism by which IL-17 mediates gene expression is through stabilization/destabilization of inflammatory mRNAs and control of translation (Herjan et al., 2013; Amatya et al., 2018; Monin et al., 2017; Garg et al., 2015; Tanaka et al., 2019; Herjan et al., 2018). Strikingly, the Nfkbiz mRNA encoding IκBζ is intrinsically unstable. Nfkbiz is subject to posttranscriptional regulation by Arid5a and Regnase-1, two IL-17–induced RNA-binding proteins (RBPs) that play opposing roles in determining mRNA longevity (Fig. 2 B). Whereas Regnase-1 is an endoribonuclease that degrades Nfkbiz mRNA (Garg et al., 2015), Arid5a binds to a similar site and promotes Nfkbiz stability and, hence, total protein levels (Amatya et al., 2018). By these means, posttranscriptional regulation pathways activated by IL-17 exhibit cross talk with transcriptional signaling. Hence, regardless of the mechanism by which IL-17 target genes are upregulated transcriptionally, RBPs can regulate their expression by controlling the stability/translation of TFs such as IκBζ. Nevertheless, it is still not clear how IL-17–mediated posttranscriptional events are integrated with its broader signaling to generate metabolic responses or, conversely, how metabolic intermediates influence RBP function.
Metabolic regulation of Th17 cells
Cellular metabolism may affect IL-17 activity by acting upstream via Th17 cell differentiation and proliferation (O’Neill et al., 2016; Shi et al., 2011). For instance, FAS in Th17 cells generates RORγt ligands, which favor IL-17 production over IL-10, as demonstrated in in vitro T cell differentiation experiments as well as during EAE (Wang et al., 2015; Gaublomme et al., 2015; Davis et al., 2015). In addition, the balance between Th17 and T reg cells is determined by FAO. T reg cells exhibit increased FAO gene expression (e.g., Cpt1a) compared with other T cells (Michalek et al., 2011; Gerriets et al., 2015). Numerous studies demonstrated that the glycolytic pathway affects Th17 cell differentiation (Shi et al., 2011; Woodman, 2011; Revu et al., 2018; DiToro et al., 2020). Specifically, under Th17 cell–polarizing conditions, mammalian target of rapamycin upregulates HIF1α, which is not observed in Th1 or Th2 cells. HIF1α promotes increased glycolysis and enhances IL-17 production, whereas deficiency in HIF1α diverts cells toward a T reg cell profile (Shi et al., 2011; Woodman, 2011). Moreover, Th17 cell differentiation is maintained by the conversion of glutamate-derived α-ketoglutarate (Fig. 2 A) into 2-hydroxyglutarate, promoting Foxp3 methylation and silencing (Xu et al., 2017; Pearce and Pearce, 2018). Thus, metabolism dictates T cell fate via epigenetic regulation. Cumulatively, modulation of glycolytic pathway, lipid metabolism, or glutamate-dependent metabolic pathway could affect Th17 cell differentiation, possibly offering therapeutic avenues to treat certain autoimmune conditions. Therefore, it may be useful to consider the effects of metabolic alterations on both Th17 cells and IL-17 signaling targets when designing therapeutic strategies. Indeed, small molecules, such as dimethylfumarate, used to treat psoriasis and multiple sclerosis, downregulate aerobic glycolysis in Th17 cells (Kornberg et al., 2018, Angiari and O’Neill, 2018) as well as dampen IL-17–induced IκBζ expression in keratinocytes (Ohgakiuchi et al., 2020). As we learn more about the implication of cellular metabolic processes in inflammation, novel therapeutic opportunities are likely to be revealed for the treatment of IL-17–related immune disorders.
Conclusion and perspectives
This review highlights a facet of IL-17 function that has been underappreciated until quite recently. IL-17 is best known as a critical host-defensive cytokine against extracellular fungal and bacterial species in conjunction with its widely accepted proinflammatory role. However, IL-17 is not simply a proinflammatory cytokine, and the above-described studies pointed to an emerging role of IL-17 signaling in metabolism at the cellular, tissue, and organismal level. In addition, cellular metabolism can influence IL-17 signal transduction. During inflammation, a feed-forward loop appears to be set in motion wherein effector molecules released from IL-17 target cells modulate systemic metabolism, in turn regulating the metabolism and function of IL-17 target cells.
Metabolism is integrated into the protective and pathogenic aspects of IL-17 responses (Fig. 1), and it is likely that some transcriptional regulators (e.g., IκBζ) that are central to IL-17 signaling activities also control specific metabolic programs that support this cytokine’s functions. In addition, cellular metabolism may impact gene expression through epigenetic modifications, and chromatin-modifying enzymes require different metabolites as cofactors or substrates (Montellier and Gaucher, 2019; Lio and Huang, 2020). Hence, a future challenge will be to define the relationships between IL-17–mediated metabolic regulation and epigenetics or chromatin remodeling. Such epigenetic changes may in turn be the basis of chronic, sustained transcriptional responses that are seen in autoimmune settings, which thereby modulate the activity of IL-17 and related proinflammatory cytokine programs.
Acknowledgments
Figures were made using BioRender.
S.L. Gaffen was supported by National Institutes of Health grant AI147383 and the Rheumatology Research Foundation. M.J. McGeachy was supported by National Institutes of Health grant AI148356.
Author contributions:Conceptualization:R. Bechara and S.L. Gaffen; Writing – original draft: R. Bechara; Writing – review and editing: S.L. Gaffen and M.J. McGeachy; Funding acquisition: S.L. Gaffen; Supervision: S.L. Gaffen.
References
- Ahmed, M., and Gaffen S.L.. 2010. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 21:449–453. 10.1016/j.cytogfr.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, M., and Gaffen S.L.. 2013. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Krüppel-like factors. Cytokine. 61:898–905. 10.1016/j.cyto.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatya, N., Garg A.V., and Gaffen S.L.. 2017. IL-17 signaling: The yin and the yang. Trends Immunol. 38:310–322. 10.1016/j.it.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatya, N., Childs E.E., Cruz J.A., Aggor F.E.Y., Garg A.V., Berman A.J., Gudjonsson J.E., Atasoy U., and Gaffen S.L.. 2018. IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA binding protein Arid5a. Sci. Signal. 11:eaat4617. 10.1126/scisignal.aat4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiari, S., and O’Neill L.A.. 2018. Dimethyl fumarate: targeting glycolysis to treat MS. Cell Res. 28:613–615. 10.1038/s41422-018-0045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arababadi, M.K., Nosratabadi R., Hassanshahi G., Yaghini N., Pooladvand V., Shamsizadeh A., Hakimi H., and Derakhshan R.. 2010. Nephropathic complication of type-2 diabetes is following pattern of autoimmune diseases? Diabetes Res. Clin. Pract. 87:33–37. 10.1016/j.diabres.2009.09.027 [DOI] [PubMed] [Google Scholar]
- Araújo, E.P., De Souza C.T., Ueno M., Cintra D.E., Bertolo M.B., Carvalheira J.B., Saad M.J., and Velloso L.A.. 2007. Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology. 148:5991–5997. 10.1210/en.2007-0132 [DOI] [PubMed] [Google Scholar]
- Azfar, R.S., and Gelfand J.M.. 2008. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr. Opin. Rheumatol. 20:416–422. 10.1097/BOR.0b013e3283031c99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambouskova, M., Gorvel L., Lampropoulou V., Sergushichev A., Loginicheva E., Johnson K., Korenfeld D., Mathyer M.E., Kim H., Huang L.H., et al. 2018. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 556:501–504. 10.1038/s41586-018-0052-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay, M., and Larregina A.T.. 2020. Keratinocyte-polyamines and dendritic cells: a bad duet for psoriasis. Immunity. 53:16–18. 10.1016/j.immuni.2020.06.015 [DOI] [PubMed] [Google Scholar]
- Bantug, G.R., Galluzzi L., Kroemer G., and Hess C.. 2018. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18:19–34. 10.1038/nri.2017.99 [DOI] [PubMed] [Google Scholar]
- Biniecka, M., Canavan M., McGarry T., Gao W., McCormick J., Cregan S., Gallagher L., Smith T., Phelan J.J., Ryan J., et al. 2016. Dysregulated bioenergetics: a key regulator of joint inflammation. Ann. Rheum. Dis. 75:2192–2200. 10.1136/annrheumdis-2015-208476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordon, Y. 2018. Itaconate charges down inflammation. Nat. Rev. Immunol. 18:360–361. 10.1038/s41577-018-0016-4 [DOI] [PubMed] [Google Scholar]
- Bordon, Y. 2019. Stromal support from IL-17. Nat. Rev. Immunol. 19:270–271. [DOI] [PubMed] [Google Scholar]
- Borst, S.E., Lee Y., Conover C.F., Shek E.W., and Bagby G.J.. 2004. Neutralization of tumor necrosis factor-α reverses insulin resistance in skeletal muscle but not adipose tissue. Am. J. Physiol. Endocrinol. Metab. 287:E934–E938. 10.1152/ajpendo.00054.2004 [DOI] [PubMed] [Google Scholar]
- Bruch-Gerharz, D., Schnorr O., Suschek C., Beck K.F., Pfeilschifter J., Ruzicka T., and Kolb-Bachofen V.. 2003. Arginase 1 overexpression in psoriasis: limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hyperproliferation. Am. J. Pathol. 162:203–211. 10.1016/S0002-9440(10)63811-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, M.D., Sowell R.T., Kaech S.M., and Pearce E.L.. 2017. Metabolic instruction of immunity. Cell. 169:570–586. 10.1016/j.cell.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante, M.F., Garcia-Carbonell R., Whisenant K.D., and Guma M.. 2017. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 19:110. 10.1186/s13075-017-1303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante, M.F., Oliveira P.G., Garcia-Carbonell R., Croft A.P., Smith J.M., Serrano R.L., Sanchez-Lopez E., Liu X., Kisseleva T., Hay N., et al. 2018. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann. Rheum. Dis. 77:1636–1643. 10.1136/annrheumdis-2018-213103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S.H., Park H., and Dong C.. 2006. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 281:35603–35607. 10.1074/jbc.C600256200 [DOI] [PubMed] [Google Scholar]
- Chen, X., Cai G., Liu C., Zhao J., Gu C., Wu L., Hamilton T.A., Zhang C.J., Ko J., Zhu L., et al. 2019. IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1+ stem cells. J. Exp. Med. 216:195–214. 10.1084/jem.20171849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, W.P., Mattapallil M.J., Raychaudhuri K., Bing S.J., Wu S., Zhong Y., Wang W., Chen Z., Silver P.B., Jittayasothorn Y., et al. 2020. The cytokine IL-17A limits Th17 pathogenicity via a negative feedback loop driven by autocrine induction of IL-24. Immunity. 53:384–397.e5. 10.1016/j.immuni.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani, E.T., Kazak L., and Spiegelman B.M.. 2019. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29:27–37. 10.1016/j.cmet.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Ciofani, M. 2020. Tγδ17 cells build up the nerve. Nat. Immunol. 21:367–368. 10.1038/s41590-020-0642-4 [DOI] [PubMed] [Google Scholar]
- Clarke, A.J., and Simon A.K.. 2019. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19:170–183. 10.1038/s41577-018-0095-2 [DOI] [PubMed] [Google Scholar]
- Conti, H.R., and Gaffen S.L.. 2010. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 12:518–527. 10.1016/j.micinf.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Jung J.W., et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206:299–311. 10.1084/jem.20081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, H.R., Bruno V.M., Childs E.E., Daugherty S., Hunter J.P., Mengesha B.G., Saevig D.L., Hendricks M.R., Coleman B.M., Brane L., et al. 2016. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 20:606–617. 10.1016/j.chom.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias, A.J., Aksoylar H.I., and Horng T.. 2015. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin. Immunol. 27:286–296. 10.1016/j.smim.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovici, B.B., Sattar N., Prinz J., Puig L., Emery P., Barker J.N., van de Kerkhof P., Ståhle M., Nestle F.O., Girolomoni G., and Krueger J.G.. 2010. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J. Invest. Dermatol. 130:1785–1796. 10.1038/jid.2010.103 [DOI] [PubMed] [Google Scholar]
- Davis, F.P., Kanno Y., and O’Shea J.J.. 2015. A metabolic switch for Th17 pathogenicity. Cell. 163:1308–1310. 10.1016/j.cell.2015.11.033 [DOI] [PubMed] [Google Scholar]
- de Oliveira, P.G., Farinon M., Sanchez-Lopez E., Miyamoto S., and Guma M.. 2019. Fibroblast-like synoviocytes glucose metabolism as a therapeutic target in rheumatoid arthritis. Front. Immunol. 10:1743. 10.3389/fimmu.2019.01743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiToro, D., Harbour S.N., Bando J.K., Benavides G., Witte S., Laufer V.A., Moseley C., Singer J.R., Frey B., Turner H., et al. 2020. Insulin-like growth factors are key regulators of T helper 17 regulatory t cell balance in autoimmunity. Immunity. 52:650–667.e10. 10.1016/j.immuni.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath, M.Y., and Shoelson S.E.. 2011. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11:98–107. 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- Eming, S.A., Wynn T.A., and Martin P.. 2017. Inflammation and metabolism in tissue repair and regeneration. Science. 356:1026–1030. 10.1126/science.aam7928 [DOI] [PubMed] [Google Scholar]
- Ernst, M., and Putoczki T.. 2014. IL-17 cuts to the chase in colon cancer. Immunity. 41:880–882. 10.1016/j.immuni.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Fabre, T., Kared H., Friedman S.L., and Shoukry N.H.. 2014. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 193:3925–3933. 10.4049/jimmunol.1400861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, L.D., Siebert S., McInnes I.B., and Sattar N.. 2019. Cardiometabolic comorbidities in RA and PsA: lessons learned and future directions. Nat. Rev. Rheumatol. 15:461–474. 10.1038/s41584-019-0256-0 [DOI] [PubMed] [Google Scholar]
- Feuerer, M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A.B., Benoist C., Shoelson S., and Mathis D.. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15:930–939. 10.1038/nm.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floudas, A., Saunders S.P., Moran T., Schwartz C., Hams E., Fitzgerald D.C., Johnston J.A., Ogg G.S., McKenzie A.N., Walsh P.T., and Fallon P.G.. 2017. IL-17 receptor A maintains and protects the skin barrier to prevent allergic skin inflammation. J. Immunol. 199:707–717. 10.4049/jimmunol.1602185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård, J. 2005. SLE, atherosclerosis and cardiovascular disease. J. Intern. Med. 257:485–495. 10.1111/j.1365-2796.2005.01502.x [DOI] [PubMed] [Google Scholar]
- Gaber, T., Strehl C., and Buttgereit F.. 2017. Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 13:267–279. 10.1038/nrrheum.2017.37 [DOI] [PubMed] [Google Scholar]
- Gaffen, S.L. 2009. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9:556–567. 10.1038/nri2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, A.V., Amatya N., Chen K., Cruz J.A., Grover P., Whibley N., Conti H.R., Hernandez Mir G., Sirakova T., Childs E.C., et al. 2015. MCPIP1 endoribonuclease activity negatively regulates interleukin-17-mediated signaling and inflammation. Immunity. 43:475–487. 10.1016/j.immuni.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaublomme, J.T., Yosef N., Lee Y., Gertner R.S., Yang L.V., Wu C., Pandolfi P.P., Mak T., Satija R., Shalek A.K., et al. 2015. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 163:1400–1412. 10.1016/j.cell.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese, M.C., Greenwald M., Cho C.S., Berman A., Jin L., Cameron G.S., Benichou O., Xie L., Braun D., Berclaz P.Y., and Banerjee S.. 2014. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol. 66:1693–1704. 10.1002/art.38617 [DOI] [PubMed] [Google Scholar]
- Gerriets, V.A., Kishton R.J., Nichols A.G., Macintyre A.N., Inoue M., Ilkayeva O., Winter P.S., Liu X., Priyadharshini B., Slawinska M.E., et al. 2015. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125:194–207. 10.1172/JCI76012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisondi, P., Fostini A.C., Fossà I., Girolomoni G., and Targher G.. 2018. Psoriasis and the metabolic syndrome. Clin. Dermatol. 36:21–28. 10.1016/j.clindermatol.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Goswami, J., Hernández-Santos N., Zuniga L.A., and Gaffen S.L.. 2009. A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur. J. Immunol. 39:2831–2839. 10.1002/eji.200939670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, H.L., Wang H., Pisitkun P., Kim J.C., Tassi I., Tang W., Morasso M.I., Udey M.C., and Siebenlist U.. 2014. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl. Acad. Sci. USA. 111:E3422–E3431. 10.1073/pnas.1400513111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden, J.L., Lewis S.M., Lish S.R., Suárez-Fariñas M., Gareau D., Lentini T., Johnson-Huang L.M., Krueger J.G., and Lowes M.A.. 2016. The tryptophan metabolism enzyme L-kynureninase is a novel inflammatory factor in psoriasis and other inflammatory diseases. J. Allergy Clin. Immunol. 137:1830–1840. 10.1016/j.jaci.2015.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty, L.C., Biniecka M., O’Sullivan J., Fox E., Mulhall K., Veale D.J., and Fearon U.. 2012. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 71:582–588. 10.1136/annrheumdis-2011-200245 [DOI] [PubMed] [Google Scholar]
- Herbert, D., Franz S., Popkova Y., Anderegg U., Schiller J., Schwede K., Lorz A., Simon J.C., and Saalbach A.. 2018. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. J. Invest. Dermatol. 138:1999–2009. 10.1016/j.jid.2018.03.1522 [DOI] [PubMed] [Google Scholar]
- Herjan, T., Yao P., Qian W., Li X., Liu C., Bulek K., Sun D., Yang W.P., Zhu J., He A., et al. 2013. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J. Immunol. 191:640–649. 10.4049/jimmunol.1203315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herjan, T., Hong L., Bubenik J., Bulek K., Qian W., Liu C., Li X., Chen X., Yang H., Ouyang S., et al. 2018. IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nat. Immunol. 19:354–365. 10.1038/s41590-018-0071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil, G.S. 2017a. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 47:406–420. 10.1016/j.immuni.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil, G.S. 2017b. Inflammation, metaflammation and immunometabolic disorders. Nature. 542:177–185. 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- Hotamisligil, G.S., Shargill N.S., and Spiegelman B.M.. 1993. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 259:87–91. 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- Hu, B., Jin C., Zeng X., Resch J.M., Jedrychowski M.P., Yang Z., Desai B.N., Banks A.S., Lowell B.B., Mathis D., and Spiegelman B.M.. 2020. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 578:610–614. 10.1038/s41586-020-2028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H., Kim H.J., Chang E.J., Lee Z.H., Hwang S.J., Kim H.M., Lee Y., and Kim H.H.. 2009. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 16:1332–1343. 10.1038/cdd.2009.74 [DOI] [PubMed] [Google Scholar]
- Huang, L.H., Zinselmeyer B.H., Chang C.H., Saunders B.T., Elvington A., Baba O., Broekelmann T.J., Qi L., Rueve J.S., Swartz M.A., et al. 2019. Interleukin-17 drives interstitial entrapment of tissue lipoproteins in experimental psoriasis. Cell Metab. 29:475–487.e7. 10.1016/j.cmet.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber, W., Sands B.E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P.D., Wehkamp J., Feagan B.G., Yao M.D., Karczewski M., et al. Secukinumab in Crohn’s Disease Study Group . 2012. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 61:1693–1700. 10.1136/gutjnl-2011-301668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Injarabian, L., Devin A., Ransac S., and Marteyn B.S.. 2019. Neutrophil metabolic shift during their lifecycle: impact on their survival and activation. Int. J. Mol. Sci. 21:287. 10.3390/ijms21010287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame, H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 30:108–119. 10.1016/j.immuni.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Jalkanen, S., and Salmi M.. 2020. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 20:566–578. 10.1038/s41577-020-0281-x [DOI] [PubMed] [Google Scholar]
- Jiang, L., Xiao L., Sugiura H., Huang X., Ali A., Kuro-o M., Deberardinis R.J., and Boothman D.A.. 2015. Metabolic reprogramming during TGFβ1-induced epithelial-to-mesenchymal transition. Oncogene. 34:3908–3916. 10.1038/onc.2014.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, C., Mose M., Ommen P., Bertelsen T., Vinter H., Hailfinger S., Lorscheid S., Schulze-Osthoff K., and Iversen L.. 2015. IκBζ is a key driver in the development of psoriasis. Proc. Natl. Acad. Sci. USA. 112:E5825–E5833. 10.1073/pnas.1509971112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamleh, M.A., Snowden S.G., Grapov D., Blackburn G.J., Watson D.G., Xu N., Ståhle M., and Wheelock C.E.. 2015. LC-MS metabolomics of psoriasis patients reveals disease severity-dependent increases in circulating amino acids that are ameliorated by anti-TNFα treatment. J. Proteome Res. 14:557–566. 10.1021/pr500782g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H., Li X., Zhou Q., Quan C., Xue F., Zheng J., and Yu Y.. 2017. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br. J. Dermatol. 176:713–722. 10.1111/bjd.15008 [DOI] [PubMed] [Google Scholar]
- Karlsen, J.R., Borregaard N., and Cowland J.B.. 2010. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-β nor C/EBP-δ. J. Biol. Chem. 285:14088–14100. 10.1074/jbc.M109.017129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz, G., Smilowitz N.R., Blazer A., Clancy R., Buyon J.P., and Berger J.S.. 2019. Systemic lupus erythematosus and increased prevalence of atherosclerotic cardiovascular disease in hospitalized patients. Mayo Clin. Proc. 94:1436–1443. 10.1016/j.mayocp.2019.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E.K., Kwon J.E., Lee S.Y., Lee E.J., Kim D.S., Moon S.J., Lee J., Kwok S.K., Park S.H., and Cho M.L.. 2017. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 8:e2565. 10.1038/cddis.2016.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlgruber, A.C., Gal-Oz S.T., LaMarche N.M., Shimazaki M., Duquette D., Koay H.F., Nguyen H.N., Mina A.I., Paras T., Tavakkoli A., et al. 2018. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat. Immunol. 19:464–474. 10.1038/s41590-018-0094-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliaraki, V., Prados A., Armaka M., and Kollias G.. 2020. The mesenchymal context in inflammation, immunity and cancer. Nat. Immunol. 21:974–982. 10.1038/s41590-020-0741-2 [DOI] [PubMed] [Google Scholar]
- Kolodin, D., van Panhuys N., Li C., Magnuson A.M., Cipolletta D., Miller C.M., Wagers A., Germain R.N., Benoist C., and Mathis D.. 2015. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21:543–557. 10.1016/j.cmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, M.D., Bhargava P., Kim P.M., Putluri V., Snowman A.M., Putluri N., Calabresi P.A., and Snyder S.H.. 2018. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 360:449–453. 10.1126/science.aan4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausgruber, T., Fortelny N., Fife-Gernedl V., Senekowitsch M., Schuster L.C., Lercher A., Nemc A., Schmidl C., Rendeiro A.F., Bergthaler A., and Bock C.. 2020. Structural cells are key regulators of organ-specific immune responses. Nature. 583:296–302. 10.1038/s41586-020-2424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurty, A.T., and Turley S.J.. 2020. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21:369–380. 10.1038/s41590-020-0635-3 [DOI] [PubMed] [Google Scholar]
- Kuestner, R.E., Taft D.W., Haran A., Brandt C.S., Brender T., Lum K., Harder B., Okada S., Ostrander C.D., Kreindler J.L., et al. 2007. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 179:5462–5473. 10.4049/jimmunol.179.8.5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.S., Tato C.M., Joyce-Shaikh B., Gulen M.F., Cayatte C., Chen Y., Blumenschein W.M., Judo M., Ayanoglu G., McClanahan T.K., et al. 2015. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 43:727–738. 10.1016/j.immuni.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.S., Wollam J., and Olefsky J.M.. 2018. An integrated view of immunometabolism. Cell. 172:22–40. 10.1016/j.cell.2017.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Golden J.B., Camhi M.I., Zhang X., Fritz Y., Diaconu D., Ivanco T.L., Simon D.I., Kikly K., McCormick T.S., et al. 2018. Protection from psoriasis-related thrombosis after inhibition of IL-23 or IL-17A. J. Invest. Dermatol. 138:310–315. 10.1016/j.jid.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Bechara R., Zhao J., McGeachy M.J., and Gaffen S.L.. 2019. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 20:1594–1602. 10.1038/s41590-019-0514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio, C.J., and Huang S.C.. 2020. Circles of Life: linking metabolic and epigenetic cycles to immunity. Immunology. 161:165–174. 10.1111/imm.13207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., and Kaplan M.J.. 2018. Cardiovascular disease in systemic lupus erythematosus: an update. Curr. Opin. Rheumatol. 30:441–448. 10.1097/BOR.0000000000000528 [DOI] [PubMed] [Google Scholar]
- Liuzzo, G., Trotta F., and Pedicino D.. 2013. Interleukin-17 in atherosclerosis and cardiovascular disease: the good, the bad, and the unknown. Eur. Heart J. 34:556–559. 10.1093/eurheartj/ehs399 [DOI] [PubMed] [Google Scholar]
- Lopes, N., McIntyre C., Martin S., Raverdeau M., Sumaria N., Kohlgruber A.C., Fiala G.J., Agudelo L.Z., Dyck L., Kane H., et al. 2021. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 22:179–192. 10.1038/s41590-020-00848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, F., Sun Y., Xu Z., Niu L., Wang Z., Deng S., Liu Z., Zhou H., Bai J., Yin Q., et al. 2020. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity. 53:204–216.e10. 10.1016/j.immuni.2020.06.004 [DOI] [PubMed] [Google Scholar]
- MacLeod, A.S., Hemmers S., Garijo O., Chabod M., Mowen K., Witherden D.A., and Havran W.L.. 2013. Dendritic epidermal T cells regulate skin antimicrobial barrier function. J. Clin. Invest. 123:4364–4374. 10.1172/JCI70064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhur, M.S., Lob H.E., McCann L.A., Iwakura Y., Blinder Y., Guzik T.J., and Harrison D.G.. 2010. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 55:500–507. 10.1161/HYPERTENSIONAHA.109.145094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder, S., Amatya N., Revu S., Jawale C.V., Wu D., Rittenhouse N., Menk A., Kupul S., Du F., Raphael I., et al. 2019. IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat. Immunol. 20:534–545. 10.1038/s41590-019-0367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski, L., Chaib M., and Rathmell J.C.. 2020. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 295:5–14. 10.1111/imr.12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, D., Fletcher A.L., and Turley S.J.. 2013. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol. Rev. 251:160–176. 10.1111/imr.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man, K., and Kallies A.. 2015. Synchronizing transcriptional control of T cell metabolism and function. Nat. Rev. Immunol. 15:574–584. 10.1038/nri3874 [DOI] [PubMed] [Google Scholar]
- Man, K., Kutyavin V.I., and Chawla A.. 2017. Tissue immunometabolism: development, physiology, and pathobiology. Cell Metab. 25:11–26. 10.1016/j.cmet.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha, A.K., Crome S.Q., Panagiotopoulos C., Berg K.B., Qin H., Ouyang Q., Xu L., Priatel J.J., Levings M.K., and Tan R.. 2010. Cutting edge: Increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J. Immunol. 185:3814–3818. 10.4049/jimmunol.1001860 [DOI] [PubMed] [Google Scholar]
- Mathis, D. 2019. Organismal immunometabolism: advances in both directions. Nat. Rev. Immunol. 19:83–84. 10.1038/s41577-018-0118-z [DOI] [PubMed] [Google Scholar]
- Mathis, D., and Shoelson S.E.. 2011. Immunometabolism: an emerging frontier. Nat. Rev. Immunol. 11:81–83. 10.1038/nri2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy, M.J., Cua D.J., and Gaffen S.L.. 2019. The IL-17 family of cytokines in health and disease. Immunity. 50:892–906. 10.1016/j.immuni.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease, P.J., Jeka S., Jaller J.J., Kitumnuaypong T., Louthrenoo W., Mann H., Matsievskaia G., Soriano E.R., Jia B., Wang C., et al. 2018. CNTO6785, a fully human antiinterleukin 17 monoclonal antibody, in patients with rheumatoid arthritis with inadequate response to methotrexate: a randomized, placebo-controlled, phase II, dose-ranging study. J. Rheumatol. 45:22–31. 10.3899/jrheum.161238 [DOI] [PubMed] [Google Scholar]
- Mi, S., Li Z., Yang H.Z., Liu H., Wang J.P., Ma Y.G., Wang X.X., Liu H.Z., Sun W., and Hu Z.W.. 2011. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J. Immunol. 187:3003–3014. 10.4049/jimmunol.1004081 [DOI] [PubMed] [Google Scholar]
- Michalek, R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., MacIver N.J., Mason E.F., Sullivan S.A., Nichols A.G., and Rathmell J.C.. 2011. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186:3299–3303. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin, L., and Gaffen S.L.. 2018. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 10:a028522. 10.1101/cshperspect.a028522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin, L., Gudjonsson J.E., Childs E.E., Amatya N., Xing X., Verma A.H., Coleman B.M., Garg A.V., Killeen M., Mathers A., et al. 2017. MCPIP1/Regnase-1 restricts IL-17A- and IL-17C-dependent skin inflammation. J. Immunol. 198:767–775. 10.4049/jimmunol.1601551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montellier, E., and Gaucher J.. 2019. Targeting the interplay between metabolism and epigenetics in cancer. Curr. Opin. Oncol. 31:92–99. 10.1097/CCO.0000000000000501 [DOI] [PubMed] [Google Scholar]
- Mueller, S.N. 2019. IL-17 instructs lymphoid stromal cells. Nat. Immunol. 20:524–526. 10.1038/s41590-019-0375-4 [DOI] [PubMed] [Google Scholar]
- Nowarski, R., Jackson R., and Flavell R.A.. 2017. The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell. 168:362–375. 10.1016/j.cell.2016.11.040 [DOI] [PubMed] [Google Scholar]
- Nygaard, G., and Firestein G.S.. 2020. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16:316–333. 10.1038/s41584-020-0413-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien, K.L., and Finlay D.K.. 2019. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 19:282–290. 10.1038/s41577-019-0139-2 [DOI] [PubMed] [Google Scholar]
- O’Connor, W. Jr., Kamanaka M., Booth C.J., Town T., Nakae S., Iwakura Y., Kolls J.K., and Flavell R.A.. 2009. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10:603–609. 10.1038/ni.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill, L.A.J., and Artyomov M.N.. 2019. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 19:273–281. 10.1038/s41577-019-0128-5 [DOI] [PubMed] [Google Scholar]
- O’Neill, L.A., and Pearce E.J.. 2016. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213:15–23. 10.1084/jem.20151570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill, L.A., Kishton R.J., and Rathmell J.. 2016. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16:553–565. 10.1038/nri.2016.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, A., Andoh A., Araki Y., Bamba T., and Fujiyama Y.. 2004. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110:55–62. 10.1016/j.clim.2003.09.013 [DOI] [PubMed] [Google Scholar]
- Ohgakiuchi, Y., Saino Y., Muromoto R., Komori Y., Sato A., Hirashima K., Kitai Y., Kashiwakura J.I., Oritani K., and Matsuda T.. 2020. Dimethyl fumarate dampens IL-17-ACT1-TBK1 axis-mediated phosphorylation of Regnase-1 and suppresses IL-17-induced IκB-ζ expression. Biochem. Biophys. Res. Commun. 521:957–963. 10.1016/j.bbrc.2019.11.036 [DOI] [PubMed] [Google Scholar]
- Okamoto, Y., Hasegawa M., Matsushita T., Hamaguchi Y., Huu D.L., Iwakura Y., Fujimoto M., and Takehara K.. 2012. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum. 64:3726–3735. 10.1002/art.34643 [DOI] [PubMed] [Google Scholar]
- Papotto, P.H., and Silva-Santos B.. 2018. Got my γδ17 T cells to keep me warm. Nat. Immunol. 19:427–429. 10.1038/s41590-018-0090-6 [DOI] [PubMed] [Google Scholar]
- Patel, D.N., King C.A., Bailey S.R., Holt J.W., Venkatachalam K., Agrawal A., Valente A.J., and Chandrasekar B.. 2007. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-kappaB and C/EBPbeta activation. J. Biol. Chem. 282:27229–27238. 10.1074/jbc.M703250200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelka, K., Chon Y., Newmark R., Lin S.L., Baumgartner S., and Erondu N.. 2015. A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J. Rheumatol. 42:912–919. 10.3899/jrheum.141271 [DOI] [PubMed] [Google Scholar]
- Pearce, E.J., and Everts B.. 2015. Dendritic cell metabolism. Nat. Rev. Immunol. 15:18–29. 10.1038/nri3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, E.J., and Pearce E.L.. 2018. Immunometabolism in 2017: Driving immunity: all roads lead to metabolism. Nat. Rev. Immunol. 18:81–82. 10.1038/nri.2017.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, E.L., Poffenberger M.C., Chang C.H., and Jones R.G.. 2013. Fueling immunity: insights into metabolism and lymphocyte function. Science. 342:1242454. 10.1126/science.1242454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohla, L., Ottas A., Kaldvee B., Abram K., Soomets U., Zilmer M., Reemann P., Jaks V., and Kingo K.. 2020. Hyperproliferation is the main driver of metabolomic changes in psoriasis lesional skin. Sci. Rep. 10:3081. 10.1038/s41598-020-59996-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poizeau, F., Nowak E., Kerbrat S., Le Nautout B., Droitcourt C., Drici M.D., Sbidian E., Guillot B., Bachelez H., Ait-Oufella H., et al. 2020. Association between early severe cardiovascular events and the initiation of treatment with the anti-interleukin 12/23p40 antibody ustekinumab. JAMA Dermatol. 156:1208. 10.1001/jamadermatol.2020.2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz, I., Sandrock I., and Mrowietz U.. 2020. Interleukin-17 cytokines: Effectors and targets in psoriasis-A breakthrough in understanding and treatment. J. Exp. Med. 217:e20191397. 10.1084/jem.20191397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y., Liu C., Hartupee J., Altuntas C.Z., Gulen M.F., Jane-Wit D., Xiao J., Lu Y., Giltiay N., Liu J., et al. 2007. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 8:247–256. 10.1038/ni1439 [DOI] [PubMed] [Google Scholar]
- Rabinowitz, J.D., and Mutlu G.M.. 2019. A metabolic strategy to reverse fibrosis? Nat. Metab. 1:12–13. 10.1038/s42255-018-0013-8 [DOI] [PubMed] [Google Scholar]
- Ramani, K., Tan R.J., Zhou D., Coleman B.M., Jawale C.V., Liu Y., and Biswas P.S.. 2018. IL-17 receptor signaling negatively regulates the development of tubulointerstitial fibrosis in the kidney. Mediators Inflamm. 2018:5103672. 10.1155/2018/5103672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revu, S., Wu J., Henkel M., Rittenhouse N., Menk A., Delgoffe G.M., Poholek A.C., and McGeachy M.J.. 2018. IL-23 and IL-1β drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep. 22:2642–2653. 10.1016/j.celrep.2018.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy, M.J., Wong G.C., Liu X.K., Yamamoto H., Kasayama S., Kirkwood K.L., and Gaffen S.L.. 2004. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279:2559–2567. 10.1074/jbc.M308809200 [DOI] [PubMed] [Google Scholar]
- Russell, D.G., Huang L., and VanderVen B.C.. 2019. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 19:291–304. 10.1038/s41577-019-0124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, F., Hu Z., Goswami J., and Gaffen S.L.. 2006. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 281:24138–24148. 10.1074/jbc.M604597200 [DOI] [PubMed] [Google Scholar]
- Shen, F., Li N., Gade P., Kalvakolanu D.V., Weibley T., Doble B., Woodgett J.R., Wood T.D., and Gaffen S.L.. 2009. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci. Signal. 2:ra8. 10.1126/scisignal.2000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L.Z., Wang R., Huang G., Vogel P., Neale G., Green D.R., and Chi H.. 2011. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208:1367–1376. 10.1084/jem.20110278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, J.H., Shin D.W., and Noh M.. 2009. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem. Pharmacol. 77:1835–1844. 10.1016/j.bcp.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Simon, T., Taleb S., Danchin N., Laurans L., Rousseau B., Cattan S., Montely J.M., Dubourg O., Tedgui A., Kotti S., and Mallat Z.. 2013. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 34:570–577. 10.1093/eurheartj/ehs263 [DOI] [PubMed] [Google Scholar]
- Slowikowski, K., Nguyen H.N., Noss E.H., Simmons D.P., Mizoguchi F., Watts G.F.M., Gurish M.F., Brenner M.B., and Raychaudhuri S.. 2020. CUX1 and IκBζ (NFKBIZ) mediate the synergistic inflammatory response to TNF and IL-17A in stromal fibroblasts. Proc. Natl. Acad. Sci. USA. 117:5532–5541. 10.1073/pnas.1912702117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X., Dai D., He X., Zhu S., Yao Y., Gao H., Wang J., Qu F., Qiu J., Wang H., et al. 2015. Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity. 43:488–501. 10.1016/j.immuni.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Spallanzani, R.G., Zemmour D., Xiao T., Jayewickreme T., Li C., Bryce P.J., Benoist C., and Mathis D.. 2019. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 4:eaaw3658. 10.1126/sciimmunol.aaw3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus, D.S. 2013. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Mol. Cancer. 12:78. 10.1186/1476-4598-12-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumarac-Dumanovic, M., Stevanovic D., Ljubic A., Jorga J., Simic M., Stamenkovic-Pejkovic D., Starcevic V., Trajkovic V., and Micic D.. 2009. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. 33:151–156. 10.1038/ijo.2008.216 [DOI] [PubMed] [Google Scholar]
- Taams, L.S. 2020. Interleukin-17 in rheumatoid arthritis: Trials and tribulations. J. Exp. Med. 217:e20192048. 10.1084/jem.20192048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb, S., Romain M., Ramkhelawon B., Uyttenhove C., Pasterkamp G., Herbin O., Esposito B., Perez N., Yasukawa H., Van Snick J., et al. 2009. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206:2067–2077. 10.1084/jem.20090545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, H., Arima Y., Kamimura D., Tanaka Y., Takahashi N., Uehata T., Maeda K., Satoh T., Murakami M., and Akira S.. 2019. Phosphorylation-dependent Regnase-1 release from endoplasmic reticulum is critical in IL-17 response. J. Exp. Med. 216:1431–1449. 10.1084/jem.20181078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan, S.R., Feagan B., Vermeire S., Panaccione R., Melmed G.Y., Landers C., Li D., Russell C., Newmark R., Zhang N., et al. 2016. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am. J. Gastroenterol. 111:1599–1607. 10.1038/ajg.2016.298 [DOI] [PubMed] [Google Scholar]
- Terzić, J., Grivennikov S., Karin E., and Karin M.. 2010. Inflammation and colon cancer. Gastroenterology. 138:2101–2114.e5. 10.1053/j.gastro.2010.01.058 [DOI] [PubMed] [Google Scholar]
- Troha, K., and Ayres J.S.. 2020. Metabolic adaptations to infections at the organismal level. Trends Immunol. 41:113–125. 10.1016/j.it.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi, L.C., Spain S.L., Ellinghaus E., Stuart P.E., Capon F., Knight J., Tejasvi T., Kang H.M., Allen M.H., Lambert S., et al. 2015. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat. Commun. 6:7001. 10.1038/ncomms8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuong, Z.K., and Clatworthy M.R.. 2020. Organ immune responses - don’t forget the structural cells. Nat. Rev. Nephrol. 16:570–571. 10.1038/s41581-020-00334-x [DOI] [PubMed] [Google Scholar]
- Varshney, P., Narasimhan A., Mittal S., Malik G., Sardana K., and Saini N.. 2016. Transcriptome profiling unveils the role of cholesterol in IL-17A signaling in psoriasis. Sci. Rep. 6:19295. 10.1038/srep19295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar, A., Moro K., Xin A., Liao Y., Gloury R., Kawamoto S., Fagarasan S., Mielke L.A., Afshar-Sterle S., Masters S.L., et al. 2015. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16:276–285. 10.1038/ni.3085 [DOI] [PubMed] [Google Scholar]
- Vats, D., Mukundan L., Odegaard J.I., Zhang L., Smith K.L., Morel C.R., Wagner R.A., Greaves D.R., Murray P.J., and Chawla A.. 2006. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 4:13–24. 10.1016/j.cmet.2006.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen, M. 2017. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 18:612–621. 10.1038/ni.3742 [DOI] [PubMed] [Google Scholar]
- Verma, A.H., Richardson J.P., Zhou C., Coleman B.M., Moyes D.L., Ho J., Huppler A.R., Ramani K., McGeachy M.J., Mufazalov I.A., et al. 2017. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2:eaam8834. 10.1126/sciimmunol.aam8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello, G.A., and Miller G.. 2020. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 217:e20190456. 10.1084/jem.20190456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K., Kim M.K., Di Caro G., Wong J., Shalapour S., Wan J., Zhang W., Zhong Z., Sanchez-Lopez E., Wu L.W., et al. 2014. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 41:1052–1063. 10.1016/j.immuni.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Yosef N., Gaublomme J., Wu C., Lee Y., Clish C.B., Kaminski J., Xiao S., Meyer Zu Horste G., Pawlak M., et al. 2015. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell. 163:1413–1427. 10.1016/j.cell.2015.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A., Luan H.H., and Medzhitov R.. 2019. An evolutionary perspective on immunometabolism. Science. 363:eaar3932. 10.1126/science.aar3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whibley, N., and Gaffen S.L.. 2015. Gut-busters: IL-17 ain’t afraid of no IL-23. Immunity. 43:620–622. 10.1016/j.immuni.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M.S., Madala S.K., Ramalingam T.R., Gochuico B.R., Rosas I.O., Cheever A.W., and Wynn T.A.. 2010. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 207:535–552. 10.1084/jem.20092121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer, S., Paltser G., Chan Y., Tsui H., Engleman E., Winer D., and Dosch H.M.. 2009. Obesity predisposes to Th17 bias. Eur. J. Immunol. 39:2629–2635. 10.1002/eji.200838893 [DOI] [PubMed] [Google Scholar]
- Woodman, I. 2011. T cells: a metabolic sHIFt to turn 17. Nat. Rev. Immunol. 11:503. 10.1038/nri3037 [DOI] [PubMed] [Google Scholar]
- Wu, J., Thabet S.R., Kirabo A., Trott D.W., Saleh M.A., Xiao L., Madhur M.S., Chen W., and Harrison D.G.. 2014. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 114:616–625. 10.1161/CIRCRESAHA.114.302157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., Chen X., Zhao J., Martin B., Zepp J.A., Ko J.S., Gu C., Cai G., Ouyang W., Sen G., et al. 2015. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 212:1571–1587. 10.1084/jem.20150204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, T., Stewart K.M., Wang X., Liu K., Xie M., Ryu J.K., Li K., Ma T., Wang H., Ni L., et al. 2017. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature. 548:228–233. 10.1038/nature23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, S., Muta T., Matsuo S., and Takeshige K.. 2005. Stimulus-specific induction of a novel nuclear factor-kappaB regulator, IkappaB-zeta, via Toll/Interleukin-1 receptor is mediated by mRNA stabilization. J. Biol. Chem. 280:1678–1687. 10.1074/jbc.M409983200 [DOI] [PubMed] [Google Scholar]
- Yu, Z., Lin W., Rui Z., and Jihong P.. 2018. Fibroblast-like synoviocyte migration is enhanced by IL-17-mediated overexpression of L-type amino acid transporter 1 (LAT1) via the mTOR/4E-BP1 pathway. Amino Acids. 50:331–340. 10.1007/s00726-017-2520-4 [DOI] [PubMed] [Google Scholar]
- Zepp, J.A., Zhao J., Liu C., Bulek K., Wu L., Chen X., Hao Y., Wang Z., Wang X., Ouyang W., et al. 2017. IL-17A-induced PLET1 expression contributes to tissue repair and colon tumorigenesis. J. Immunol. 199:3849–3857. 10.4049/jimmunol.1601540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Fu Q., Ren Z., Wang Y., Wang C., Shen T., Wang G., and Wu L.. 2015. Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis. Gynecol. Endocrinol. 31:183–190. 10.3109/09513590.2014.975683 [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Liu S., Parajuli K.R., Zhang W., Zhang K., Mo Z., Liu J., Chen Z., Yang S., Wang A.R., et al. 2017. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene. 36:687–699. 10.1038/onc.2016.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Zi Z., Lee E.E., Zhao J., Contreras D.C., South A.P., Abel E.D., Chong B.F., Vandergriff T., Hosler G.A., et al. 2018. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat. Med. 24:617–627. 10.1038/s41591-018-0003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Psarianos P., Ghoraie L.S., Yip K., Goldstein D., Gilbert R., Witterick I., Pang H., Hussain A., Lee J.H., et al. 2019. Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nat. Metab. 1:147–157. 10.1038/s42255-018-0008-5 [DOI] [PubMed] [Google Scholar]
- Zhao, J., Chen X., Herjan T., and Li X.. 2020a. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 217:e20190297. 10.1084/jem.20190297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Kwan J.Y.Y., Yip K., Liu P.P., and Liu F.F.. 2020b. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 19:57–75. 10.1038/s41573-019-0040-5 [DOI] [PubMed] [Google Scholar]
- Zúñiga, L.A., Shen W.J., Joyce-Shaikh B., Pyatnova E.A., Richards A.G., Thom C., Andrade S.M., Cua D.J., Kraemer F.B., and Butcher E.C.. 2010. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 185:6947–6959. 10.4049/jimmunol.1001269 [DOI] [PMC free article] [PubMed] [Google Scholar]