Abstract
There is uncertainty regarding carotenoid intake recommendations, because positive and negative health effects have been found or are correlated with carotenoid intake and tissue levels (including blood, adipose tissue, and the macula), depending on the type of study (epidemiological vs intervention), the dose (physiological vs supraphysiological) and the matrix (foods vs supplements, isolated or used in combination). All these factors, combined with interindividual response variations (eg, depending on age, sex, disease state, genetic makeup), make the relationship between carotenoid intake and their blood/tissue concentrations often unclear and highly variable. Although blood total carotenoid concentrations <1000 nmol/L have been related to increased chronic disease risk, no dietary reference intakes (DRIs) exist. Although high total plasma/serum carotenoid concentrations of up to 7500 nmol/L are achievable after supplementation, a plateauing effect for higher doses and prolonged intake is apparent. In this review and position paper, the current knowledge on carotenoids in serum/plasma and tissues and their relationship to dietary intake and health status is summarized with the aim of proposing suggestions for a “normal,” safe, and desirable range of concentrations that presumably are beneficial for health. Existing recommendations are likewise evaluated and practical dietary suggestions are included.
Keywords: adipose tissue, β-carotene, liver, lutein, lycopene, macula, recommended dietary intake
This review article is dedicated in memoriam to Catherine Caris-Veyrat (+29.2.2019), a great carotenoid researcher, our EUROCAROTENE / LYCOCARD partner, a phantastic person and a good friend.
INTRODUCTION
Carotenoids are a group of > 1100 tri-, tetra-, and pentaterpenoid lipophilic pigments (but mostly tetraterpenoid) produced by plants and many bacteria and fungi, but not by humans, who rely on dietary intake as the exclusive source.1 Green leafy vegetables are a major source of these compounds in the human diet, but they are also present in other foods, such as kiwis, maize, peppers, eggs, dairy products, oils, and some types of fish.2,3 Intake of carotenoids through diet, as well as their concentrations in various body compartments, especially in plasma/serum, has been correlated with the reduced incidence of several chronic diseases, including type 2 diabetes4; cardiovascular diseases,5 including stroke6; and several types of cancer, such as those of the upper intestinal tract.7,8 Moreover, meta-analyses of prospective cohort studies9,10 have found reduced all-cause mortality among participants with increasing circulating β-carotene concentrations.
The National Health and Nutrition Examination Survey III confirmed that carotenoid concentrations in serum correlate with mortality, though relationships differed by carotenoid type.11 Although the all-cause mortality was reduced until serum levels were ∼1000 nmol/L, little impact of higher concentrations was found. Lower concentrations of serum lycopene most strongly predicted all-cause mortality, followed by total carotenoid concentration.11 Donaldson,12 on the basis of 62 non-intervention studies, proposed a carotenoid health index for total plasma carotenoids, with <1000 nmol/L reflecting a high risk for developing chronic diseases, including cardiometabolic diseases and cancer, whereas participants with plasma concentrations ≥ 2500 nmol/L appeared to be generally “protected.” Of note, it is possible that the observed health benefits may be due to other bioactive compounds, or overall dietary pattern, because blood carotenoid levels are useful biomarkers of fruit and vegetable intake13 and carotenoid levels reflect, indirectly, a generally healthier lifestyle.
Carotenoid-related health benefits previously were attributed mainly to their antioxidant properties, such as radical quenching.14,15 More recently, the relevance of this effect has been questioned at typical endogenous (ie, non-supplemented), physiological, and nutritionally relevant concentrations.16 Alternative antioxidant and anti-inflammatory effects mediated via carotenoids and their metabolites, acting at the gene-expression level, have recently been proposed and summarized,17 though their physiological and nutritional relevance has not been conclusively proven and additional confirmation in humans is required. In addition, some carotenoids can also act as vitamin A precursors (eg, α- and β-carotene, β-cryptoxanthin) because they are cleaved in the intestinal mucosa, as well as after uptake in various other human tissues,18–20 by β-carotene oxygenase 1 and β-carotene oxygenase 2 into retinal and other apo-carotenals, respectively.21 Other carotenoids, especially lutein and zeaxanthin, appear to be important in protecting the macula of the retina, aiding in the prevention of age-related macular degeneration (AMD),22 the major cause of vision loss in the elderly.
These previously reported positive health effects have inspired intervention trials. Supplementation trials with smokers were launched because of the postulation that the effects of smoking are mediated via antioxidant effects of carotenoids. The aim of the trials was to ameliorate or inhibit proradical effects induced by smoking. Two large, randomized intervention trials investigated the effect of β-carotene supplementation on lung cancer: The Alpha Tocopherol, Beta Carotene Prevention trial (ATBC) included 29,000 participants who received 20 mg of β-carotene daily for 5–8 years,23 and the β-Carotene and Retinol Efficacy Trial (CARET),24 which included >18,000 participants who received 30 mg of β-carotene daily for 4 years. β-Carotene was given as water-soluble beadlets, resulting in much higher blood concentrations of β-carotene (up to ∼10 times higher) compared with typical dietary intake. The average β-carotene plasma concentrations after supplementation were ∼3800 and ∼5600 nmol/L for the CARET and ATBC trials, respectively, compared with, for example, the 95th percentile concentration of 90–900 nmol/L found in the US population.25 Both studies showed an increased lung cancer rate in the β-carotene groups (16% and 28% in the ATBC trial and the CARET study, respectively). Also, overall mortality was significantly increased in both intervention groups. The interaction of smoking and high β-carotene supplementation was later shown in animal models to increase CYP activation and nuclear hormone receptor–mediated signaling via β-carotene metabolites (ie, these interactions via retinoid-mediated effects were demonstrated in follow-up studies using ferret and mice models). These studies focused on the lung, where, after high β-carotene supplementation, reduced local retinoic acid concentrations and expression levels of retinoic acid receptor target genes were described, resulting in a more vulnerable local tissue status with respect to smoking.26,27 On the other hand, in the Physician’s Health Study28 and the Heart Protection Study,29 in which participants received 50 mg of β-carotene every other day for 13 years and 20 mg/day for 5 years, respectively, resulting in 2200 and 1220 nmol/L serum concentrations, respectively, of β-carotene, supplements did not increase lung cancer risk. Furthermore, the Chinese Linxian intervention trial30 (n = 30,000 participants, mostly nonsmokers) reported preventive effects of a combined supplement of 15 mg β-carotene, 30 mg α-tocopherol, and 50 µg selenium (resulting in 1000 nmol/L mean plasma carotenoid concentration) for stomach cancer and total mortality. Perhaps participants were originally marginally deficient in some of these micronutrients. Also, blood concentrations of β-carotene may serve as a better health marker than intake alone.
In a systematic review and meta-analysis,31 it was emphasized that supplementation with β-carotene (alone or in combination with other antioxidants) was associated with increased total mortality and other adverse effects in a mixed population. However, results were strongly biased by the ATBC and the CARET trials, whereas other trials did not suggest negative effects. Thus, the negative findings are possibly only relevant for high doses of carotenoids in smokers and perhaps asbestos workers, as included in CARET. Nevertheless, the results of these studies added to the controversy regarding carotenoid health and safety, emphasizing that dosing, form of preparation, combination with other nutrients, duration of intervention, and subject characteristics (nutritional status, physiological vs pathological condition) are important factors.32 Although it was emphasized that typical dietary carotenoid intakes (ie, via fruits and vegetables) appear to show beneficial health effects, supplemental intake of >20 mg/day may result in adverse effects, including increased cancer and total mortality risks, especially in smokers.
Because of this continuing controversy, no recommendations regarding carotenoid intake or desired blood or tissue concentrations have been issued by the majority of health authorities; no dietary reference intake recommendations (DRIs) exist, to our knowledge. However, agencies in the United Kingdom have proposed safe upper intake levels for supplemental carotenoid intake (7 mg/day for β-carotene33), whereas the German Nutrition Society, in response to the CARET and ABTC trials, recommended 2 mg/day total dietary carotenoid intake.34 Median intakes in European countries currently are ∼2.7 mg/day and 2.9 mg/day for men and women, respectively.35 Despite contradictory scientific results, supplemental “antioxidants” are widely used by the general population in many countries. Data from the United States indicate that 70% of surveyed adults (all age groups) used dietary supplements, mostly multivitamins and minerals, and thus not only antioxidants.36 For Europe, values range between 4% and 58%,37 with higher adoption in northern countries.
For individual carotenoids, there is also dispute. The importance of lutein and zeaxanthin for eye health has been acknowledged for people at risk for AMD,38 and intakes of 10 mg/day have been advocated,39 equivalent to ∼100 g spinach/day.40 Recommendations are impeded, however, because carotenoid bioavailability (ie, the fraction of an ingested carotenoid that can be absorbed and used for physiological function and/or stored) depends on several factors, adding to their interindividual variability after intake.21 These include dietary and host-related factors. For example, although a diet rich in lipids can aid in solubilizing carotenoid levels, dietary fiber may reduce their bioavailability.41,42 Furthermore, an individual’s genetic makeup, through single nucleotide polymorphisms in genes coding for digestion enzymes (eg, lipase), intestinal transporters (eg, SR-BI, CD36, NPC1L1) and cleavage enzymes (ie, β-carotene oxygenase 1) influence carotenoid bioavailability.43–46 In this review, we highlight the relationship between carotenoid intake and carotenoid blood plasma/serum and tissue levels and emphasize how the present knowledge could be used to develop dietary intake recommendations for carotenoids.
EXISTING DIETARY RECOMMENDATIONS FOR CAROTENOID INTAKE
Recommendations regarding provitamin A carotenoids
When investigating published recommendations for carotenoid intake, provitamin A carotenoids and non–provitamin A carotenoids must be discussed separately. Because vitamin A is an essential micronutrient with recommendations differing between countries, the provitamin A carotenoids, of which β-carotene is the most prominent, is discussed first. Two additional main dietary carotenoids, α-carotene and β-cryptoxanthin, can also be cleaved to vitamin A. In 1967, the Food and Drug Administration, in conjunction with the World Health Organization, defined that 6 µg of β-carotene would be of equivalent vitamin A activity as 1 µg of retinol. Other provitamin A carotenoids were defined as being half as active as β-carotene. These proposed conversion factors remained unchanged over decades.47,48 In 1988, a joint Food and Agriculture Organization and World Health Organization Expert Consultation, on the basis of controlled depletion–repletion studies in adult men, confirmed these conversion factors for mixed diets, considering that these were only best approximations that could under- or overestimate bioavailability, depending on various factors, such as items consumed cooked or raw, whole or pureed, with or without dietary fat.48,49
In 2001, the US Institute of Medicine, on the basis of various studies50–54 resulting in bioefficacy ratios between 1:2 and 1:28, revised the bioefficacy of β-carotene in a mixed diet from 1:6 to 1:12 and to 1:24 for other provitamin A carotenoids.55 In 2004, Food and Agriculture Organization and World Health Organization, in view of the most recently available data, proposed revised equivalency factors of 1:14 for β-carotene and 1:28 for other provitamin A carotenoids from usual vegetable diets.49 In contrast, other countries (eg, Germany, Italy, United Kingdom) continued using the factor 1:6.56–58 In 2012, in the Nordic Nutrition Recommendations, the bioconversion factors proposed by the Institute of Medicine in 200159 were adopted. In 2015, the European Food Safety Authority published its Scientific Opinion on Dietary Reference Values for Vitamin A and considered current evidence insufficient to support a change of the conversion factors proposed earlier47,48 for the European population, confirming 1 μg of retinol equivalent being equivalent to 1 μg of retinol, 6 μg of β-carotene, and 12 μg for other carotenoids with provitamin A activity.
Vitamin A recommendations are generally expressed as retinol equivalents49 or as retinol activity equivalents.55 According to these definitions, the contribution of each food to the vitamin A intake (mg/d) is expressed as retinol equivalent = retinol + (β-carotene / 6) + (α-carotene / 12) + (β-cryptoxanthin / 12) or as retinol activity equivalent = retinol + (β-carotene / 12) + (α-carotene / 24) + (β-cryptoxanthin / 24). In the latter form, the contribution of provitamin A carotenoids is half compared to that using retinol equivalents. For provitamin A carotenoids, it can be calculated that persons not consuming other sources of vitamin A (due to lack of availability, as in many developing countries, or for vegetarians, especially vegans), ∼10.8 mg/day β-carotene or 21.6 mg/day α-carotene (or other provitamin A carotenoids) would fulfil the dietary reference intake RDA for vitamin A (900 µg) for healthy adult men. However, no recommendations exist specifically for vegetarians or vegans, though it was recommended to increase β-carotene consumption to 7 mg/day in people with low preformed vitamin A intake.61 Having pregnant women take β-carotene supplements has also been recommended for the development of mammalian tissues because it is considered “safer” than preformed vitamin A.62 The mean dietary intake of β-carotene is in the range of 1.5–1.8 mg/day, and provitamin A intake is <3 mg/day in most European countries (Table 1).
Table 1.
Average daily intake of carotenoids and characteristics of studies examining carotenoid serum/plasma concentrations and intake in women and men
| Reference | Country | Women/men, age (no.) | Method | Intake (mg/d)a |
Serum or plasma (nmol/L)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACAR | BCAR | L/Z | BCRY | LYC | Total carosb | ACAR | BCAR | L/Z | BCRY | LYC | Total caros * | ||||||
| Costa Rica | |||||||||||||||||
| El-Sohemy et al (2002)63 |
|
FFQ | 0.73 | 4.67 | 2.89 | 0.55 | 5.77 | 14.61 | 180 | 821 | 328 | 275 | 585 | 2189 | |||
|
FFQ | 0.45 | 3.41 | 2.41 | 0.38 | 5.45 | 12.10 | 135 | 484 | 316 | 181 | 501 | 1617 | ||||
|
| |||||||||||||||||
| France | |||||||||||||||||
|
FFQ | 0.74 | 5.84 | 2.50 | 0.45 | 4.75 | 14.28 |
|
|
|
|
|
|
||||
|
| |||||||||||||||||
| Ireland | |||||||||||||||||
| Carroll et al (1999)66 |
|
FFQ |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
FFQ |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
| |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
| |||||||||||||||||
| Italy | |||||||||||||||||
| Lucarini et al (2006)67 | Men and women, total diet (1968) | Individual dietary survey, 7-d diaries | 0.15 | 2.60 | 2.21 (F), 2.27 (M) | 0.17 | 5.12 (F), 6.54 (M) | 10.25 (M) | |||||||||
|
| |||||||||||||||||
| Sette et al (2011)68 | Men and women, total diet with supplements (3323) | Individual dietary survey, 3-d consecutive food records | 0.18 (F), 0.15 (M) | 2.37 | |||||||||||||
|
| |||||||||||||||||
| Luxembourg | |||||||||||||||||
| Biehler et al (2012)69 | Luxembourg population (1432) | FFQ | 7.6c | 1.5 | 1.4 | 1.8 | 17d | ||||||||||
|
| |||||||||||||||||
| Netherlands | |||||||||||||||||
|
FFQ64 | 0.68 | 4.35 | 2.01 | 0.97 | 4.86 | 12.87 |
|
|
|
|
|
|
||||
|
| |||||||||||||||||
| Spain | |||||||||||||||||
|
FFQ64 | 0.29 | 2.96 | 3.25 | 1.36 | 1.64 | 9.50 |
|
|
|
|
|
|
||||
|
| |||||||||||||||||
|
|
3 x 24-h dietary recalls |
|
|
|||||||||||||
|
| |||||||||||||||||
|
Individual dietary intake survey (24-h dietary recall and 3-d diet diary | 0.27 | 1.49 | 1.24 | 0.32 | 3.06 | 6.38 | ||||||||||
|
| |||||||||||||||||
|
Individual dietary intake survey (24-h dietary recall and 3-d diet diary) | 0.25 | 1.26 | 0.78 | 0.02 | 2.64 | 4.95 | ||||||||||
|
| |||||||||||||||||
| National Survey of Dietary Intake in Spain (2009–2010) From fruits only. 18–64 y (3000) | Individual dietary intake survey (24-h dietary recall and 3-d diet diary) | 0.01 | 0.10 | 0.06 | 0.31 | 0.33 | 0.81 | ||||||||||
|
| |||||||||||||||||
| Sweden | |||||||||||||||||
| Wawrzyniak et al (2013)73 |
|
FFQ | 1.03 | 3.47 | 2.64 | 0.46 | 2.15 | 9.75 | 80 | 459 | 293 | 505 | 611 | 1948 | |||
|
| |||||||||||||||||
| United Kingdom | |||||||||||||||||
| Pezdirc et al (2016)74 |
|
FFQ | 1.46 | 5.24 | 1.70 | 0.32 | 4.63 | 13.35 | 559 | 527 | 181 | 931 | 2198 | ||||
|
| |||||||||||||||||
| United States | |||||||||||||||||
| Young et al (1994)75 |
|
|
|
|
|
|
|
|
110 | 340 | 460 | 170 | 580 | 1660 | |||
|
| |||||||||||||||||
| Tucker et al (1999)76 |
|
FFQ | 0.86 | 4.51 | 3.09 | 0.08 | 7.00 | 15.54 | 117 | 510 | 560 | 270 | 610 | 2067 | |||
|
FFQ | 0.66 | 3.79 | 2.68 | 0.06 | 7.64 | 14.38 | 82 | 330 | 520 | 200 | 640 | 1772 | ||||
|
| |||||||||||||||||
| Curran-Celentano et al (2001)77 |
|
FFQ | 2.94 | 1.10 | 8.37 | 12.41 | 280 | 371 | 601 | 1252 | |||||||
|
| |||||||||||||||||
| Tangney et al (2004)78 |
|
|
0.64 |
|
3.19 | 0.08 | 8.16 | 6.08 | 70 | 400 | 360 | 160 | 350 | 1340 | |||
|
|
0.63 |
|
70 | 520 | 350 | 180 | 510 | 1630 | ||||||||
|
| |||||||||||||||||
| Burke et al (2005)79 |
|
FFQ | 4.52 | 1.83 | 7.40 | 13.75 | 723 | 450 | 438 | 1611 | |||||||
|
FFQ | 2.85 | 1.47 | 8.14 | 12.46 | 463 | 401 | 435 | 1299 | ||||||||
|
| |||||||||||||||||
| Talegawkar et al (2008)80 |
|
|
|
|
|
|
|
|
70 | 640 | 320 | 180 | 1240 | 2450 | |||
|
|
|
|
|
|
|
|
70 | 510 | 320 | 170 | 1440 | 2510 | ||||
|
| |||||||||||||||||
| George et al (2012)81 |
|
|
|
|
|
|
|
|
130 | 460 | 274 | 199 | 762 | 1825 | |||
|
|
|
|
|
|
|
|
103 | 341 | 243 | 176 | 818 | 1681 | ||||
|
| |||||||||||||||||
| United States and Canada | |||||||||||||||||
| Fraser et al (2016)82 |
|
|
|
|
|
|
|
|
170 | 670 | 410 | 250 | 550 | 2050 | |||
|
| |||||||||||||||||
| Range | 0.01-2.43 | 0.1-8.8 | 0.06-4.84 | 0.02-1.4 | 0.28-10.70 | 0.81-22.63 | 70-180 | 280-870 | 200-560 | 117-505 | 91-1440 | 989-2510 | |||||
|
| |||||||||||||||||
| Mean | 0.7 | 4.1 | 2.2 | 0.3 | 4.6 | 11.8 | 104 | 495 | 331 | 229 | 594 | 1725 | |||||
|
| |||||||||||||||||
| SD | 0.5 | 1.7 | 0.8 | 0.4 | 2.4 | 4.2 | 36 | 138 | 101 | 94 | 271 | 377 | |||||
|
| |||||||||||||||||
| Relative contribution (%) | 5.9 | 14.4 | 18.6 | 2.5 | 39.0 | 100 | 6.0 | 28.7 | 19.2 | 13.3 | 34.4 | 100 | |||||
Blank cells represent nondetermined carotenoid concentrations or no data are available.
If not measured, estimates were based on individual carotenoids.
Combined α- and β-carotene. In the Olmedilla et al65 report, mean values for serum and median values were used for the intake.
Including other carotenoids (eg, violaxanthin, neoxanthin, phytoene, phytofluene).
Abbreviations: 24 H, 24 h dietary recalls; ACAR, α-carotene; BCAR, β-carotene; BCRY, β-cryptoxanthin; BP, black participant; caros, carotenoids; DHQ, diet history questionnaire; FFQ, food frequency questionnaire; LUT, lutein; LYC, lycopene; L/Z, lutein/zeaxanthin; NBP, non-black participant; SD, standard deviation; ZEA, zeaxanthin.
Besides the carotenoid content in foods, their bioavailability is of utmost importance to establish recommendations, because there are many diet- and host-related factors that may affect vitamin A equivalency of β-carotene.83 These include the food matrix, food-processing techniques, β-carotene dose, and amounts of dietary fat, fiber, vitamin A, and other carotenoids in the diet, as well as vitamin A status, nutrient deficiencies, gut integrity, and genetic polymorphisms associated with β-carotene metabolism.21,43,84–88 We recently discussed these aspects influencing individual carotenoid responses in a separate review,21 emphasizing that approximate calculations, as given in previous paragraphs, would be accurate only on average.
There is also controversy about the bioavailability between various carotenoids. For example, it is assumed that the bioavailabilities of α-carotene and β-cryptoxanthin are equal, with each of the 2 compounds having half the bioconversion factor of β-carotene, as shown in humans for α-carotene.89 However, β-cryptoxanthin, mainly supplied by red and orange fruits, seems to be more efficiently absorbed and converted into vitamin A than is α-carotene.90,91 This is supported by a study from Estevez-Santiago et al,92 in which the authors indicated the bioaccessibility (ie, the fraction of a compound that is released from the matrix and available for additional uptake) of β-cryptoxanthin was greater than that of β-carotene in nearly one-half of the fruits analyzed. This has also been corroborated by human studies, as reviewed recently.93
Recommendations regarding non-provitamin A carotenoids
There are no generally accepted dietary recommendations for non-provitamin A carotenoid intake, because their absence from the diet does not cause specific deficiency symptoms.94 Thus, suggestions for intake are mainly based on epidemiologic and intervention studies and beneficial health effects. Among the non-provitamin A carotenoids, the 2 xanthophylls (oxygen-carrying carotenoids) lutein and zeaxanthin have received much interest in the past decades. These are, besides the in vivo–formed meso-zeaxantin,95 the UV- and blue light–protecting compounds in the macula lutea (the yellow spot of the human eye), discussed as protective agents against AMD. The large Age-Related Eye Disease Study 2 (AREDS2), an intervention study, showed that lutein and zeaxanthin should be used rather than β-carotene for eye health, because of safety concerns regarding β-carotene.23,24 In general, main sources of lutein and zeaxanthin are vegetables; the contribution from oils, fats,96 and eggs and egg products appears to be rather small.72 Lutein and zeaxanthin in foods or in supplements increased the macular pigment optical density (MPOD) in several human intervention studies. Stringham and Stringham97 showed that an intake of 7.4 mg/day of macular carotenoids (6.2 mg lutein;0.7 mg zeaxanthin;0.5 mg meso-zeaxanthin) was the most efficient dose regarding serum response, whereas MPOD most efficiently increased after intakes of 13.1 mg/day (10.9 mg lutein;1.3 mg zeaxanthin;0.9 mg meso-zeaxanthin). This intake, thus, seems to be a good basis for discussion about developing recommendations, at least for dietary lutein and zeaxanthin. However, in a study with healthy volunteers (n = 108), MPOD was lower in older (45–65 years) vs younger (20–35 years) participants, despite a higher dietary intake and higher serum concentrations of lutein and zeaxanthin.70 It was concluded that age ranges should be considered when establishing normal or reference ranges for lutein and zeaxanthin in serum and that the levels of these xanthophylls should be expressed in relation to blood lipid concentration as a better predictor of MPOD, at least in persons >45 years old.70 Thus, a recommendation for a minimum serum concentration or daily intake of lutein and zeaxanthin appear to be needed, though they may be difficult to attain98 via dietary intake alone.
Recently, lutein by itself has been proposed for intake recommendations because of its relation to chronic disease prevention and health promotion99; based on the following criteria: (1) accepted definition of the compound; (2) a reliable analysis method; (3) inclusion in a food database; (4) conducted cohort studies; (5) conducted clinical trials regarding metabolic processes; (6) clinical trials regarding dose response and efficacy; (7) availability of safety data; (8) systematic reviews and/or meta-analyses; and (9) a plausible biological function. A lutein intake of 6 mg/day has been associated with a decreased risk of several chronic diseases.98 These levels are much higher than its intake in many populations (Table 1).72 For instance, an intake of 15 mg 3 times a week during 2 years increased serum lutein concentrations to 600–1050 nmol/L.100 Such concentrations were associated with lower risk of AMD, cataract disease, and atherosclerosis,100,101 but they are typically greater than the 95th percentile regarding lutein plasma concentration in persons in Westernized countries.98 Available evidence suggests that such concentrations (600 and 1050 nmol/L) produce no obvious adverse effects, are achievable through diet, and constitute a desirable target.98
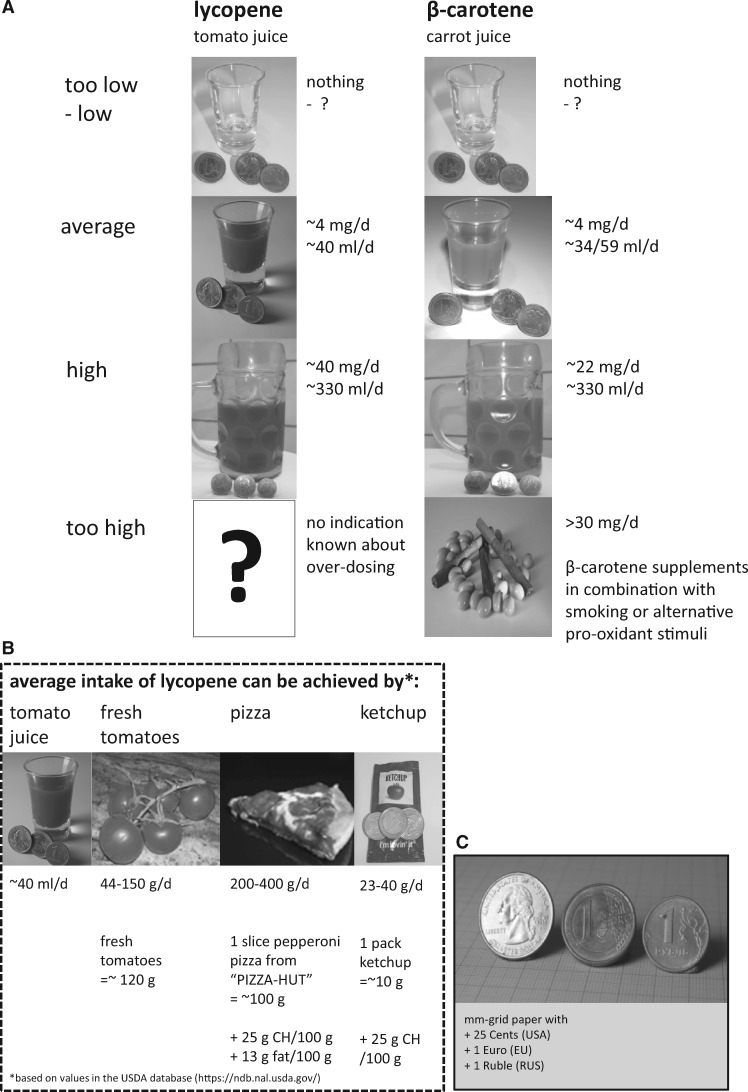
Potential adverse effects cannot be excluded, however, so developing carotenoid intake recommendations requires a risk assessment for the individual compounds. Such an assessment was published for lutein and lycopene102 (Table 2) on the basis of results from human intervention studies investigating effects of different doses. None of these studies found any adverse effects. The only documented adverse effect for supplementing high concentrations of carotenoids is carotenodermia, resulting in a yellow to red skin, which is reversible.111 In the Spanish cohort of a European multicenter intervention trial with β-carotene, lycopene, or lutein (15 mg/day for 4 months), carotenodermia was reported by 95% of participants supplemented with α- and β-carotene, 40% of those in the lutein group and 25% of those taking lycopene capsules.65 Thus, there appears to be no basis for identifying lowest observed or no-observed adverse effect levels. As a consequence, observed safe levels for humans were defined (Table 2) that are difficult to achieve by dietary means without fortified foods or supplements. Finally, the European Food Safety Authority has published acceptable daily intake recommendations for several carotenoids (Table 2), typically made on the basis of milligrams per kilogram of body weight, with doses that, likewise, appear relatively high. For example, for lycopene, this estimate is 0.5 mg/kg body weight, which is normally not reached from dietary sources, despite its variable intake from natural sources (Table 1), especially from tomatoes and tomato-derived products. High consumption of such items may result in intakes of 20 mg of lycopene per day. An average intake between 0.5 and 5 mg/day has been reported (Table 1). In southern European countries such as Italy, the main lycopene sources are raw tomatoes, cooked tomatoes, and pizza, whereas main contributors for other European regions (eg, United Kingdom, Ireland, France, the Netherlands) are canned tomatoes and soups, and, in the United States, pasta sauces.112
Table 2.
Recommendations for dietary and supplemental intakes of carotenoids
| National RDIs | National RUL or similar |
|||
|---|---|---|---|---|
| Carotenoid | (mg/d) | (mg/kg body weight/d) | (mg/d) | Reference |
| Astaxanthin | 0.043a | EFSA (2014)103 | ||
| β-Apo-8′-carotenal | 0.05a | EFSA (2012)104 | ||
| β-Carotene | 15b,c | EFSA (2012)105 | ||
| β-Carotene | EFSA (2012)106 EFSA (2012)106 | |||
| 7f | Expert Group on Vitamins and Minerals (2003)33 | |||
| 2g | Müller (1996)34 | |||
| 8g,h | Müller (1996)34 | |||
| Canthaxanthin | 0.03a | EFSA (2010)107 | ||
| Lutein | 20i | Shao (2006)102 | ||
| 1.0a | EFSA (2010)108 | |||
| 10j | Huang (2015)39 | |||
| Lycopene | 75b | Shao (2006)102 | ||
| 0.5a | EFSA (2010)109 | |||
| Zeaxanthin | 53b | EFSA (2012)110 | ||
EFSA acceptable daily intake.
EFSA safe intake.
For smokers.
From food additives and supplements alone.
From all sources.
United Kingdom, safe upper intake level.
German Nutrition Society’s recommended daily intake.
Vegetarians.
Council for Responsible Nutrition, observed safe level.
Dietary intake for eye health.
Abbreviations: EFSA, European Food Safety Authority; RDI, recommended daily intake; RUL, recommended upper limit.
INTERRELATION OF DIETARY CAROTENOID INTAKE AND PLASMA/SERUM CONCENTRATION
Regarding the carotenoid type, the majority of the dietary intakes across a variety of studies, as well as serum/plasma measurements included only 6 carotenoids, namely, α-carotene, β-carotene, lutein, zeaxanthin, β-cryptoxanthin, and lycopene (Table 1), and significant correlations between intake and serum concentrations have been reported. In Europe, blood carotenoid concentrations have been shown to be influenced by body mass index (BMI), sex, and smoking status, though country-specific carotenoid intake patterns also were discussed.13 At the individual level, the intake of fruits; root vegetables, including carrots; and tomato products are good predictors of β-cryptoxanthin, α-carotene, and lycopene plasma concentrations, respectively. Importantly, carotenoid serum concentrations can help discriminate population-level consumption of fruits and vegetables113 (Table 1).
Several studies have suggested that women, on average, consume 19% to 63% higher amounts of α-carotene, β-carotene, and β-cryptoxanthin, and have higher blood concentrations of 33% to 69% than men do,63,76 resulting, in part, in higher correlations between intake and plasma concentrations for women than for men. Similarly, Burke et al79 reported elevated carotenoid concentrations in blood of women vs men for β-carotene, lutein, zeaxanthin, and lycopene, but only when the intake of β-carotene was significantly higher. Also, Olmedilla et al114–116 found higher blood concentrations in women than in men, whereas Tangney et al78 observed higher lycopene concentrations in men (46% higher), together with a 31% higher intake, perhaps due to higher consumption of pizza and pasta-related tomato products. Of note, because women have lower body weight and plasma volume than men, it can be assumed that a similar intake results in somewhat higher plasma concentrations in women. This is in line with a study by George et al,81 who found no significant difference for total carotenoid intake, though slightly higher serum levels for women were encountered. In women, their menstrual cycle also could influence plasma carotenoid concentrations. In studies with premenopausal women, total and individual carotenoid plasma concentrations (lycopene, β-carotene, lutein, and zeaxanthin) were lowest in the early follicular phase and significantly higher thereafter, which may affect estimating plasma carotenoid-disease relationships.117 Taken together, the results imply that intake is the main predictor of blood carotenoid concentrations, and the overall absorption and clearance of carotenoids do not seem to significantly differ between sexes, though similar intake may result in slightly higher plasma concentrations in women.
Dosing, especially at prolonged high and non-nutritionally relevant concentrations, can result in a plateau of plasma carotenoid levels. For example, in a dose-escalating study with lycopene administered at 15, 30, 45, 60, 90, or 120 mg/day for 12 months in elderly men (mean age, 74 years), lycopene concentration plateaued after 3 months, and plasma levels for 15- and 90-mg doses were similar, resulting in 500–700 nmol/L lycopene (120 mg resulting in 1300 nmol/L).118 Likewise, the intake of 60 g of tomato puree (containing 17 mg of lycopene) for 3 weeks increased plasma lycopene concentration by 500 nmol/L,119 whereas 25 g of the same product for 2 weeks120 produced an increase of 400 nmol/L, emphasizing that plasma lycopene concentrations do not respond in a linear dose-dependent manner and that low amounts of a bioavailable source suffice to improve and maintain plasma levels.
Similarly, in a dose-escalating study with lutein supplements,121 with either 2.5, 5, or 10 mg lutein/day for 6 months, final serum concentrations were 620, 1040, 1180 nmol/L, respectively. Contrarily, no plasma plateau was reached for participants ingesting various amounts of fruits or vegetables per day during a 1-year intervention study, with <250 g/day vs 250–500 g/day, 500–750 g/day, and >750 g/day, resulting in β-carotene concentrations of 250, 420, 450, and 530 nmol/L, respectively,122 perhaps due to a more even distribution of carotenoid intake over time and rather physiological doses.
Regarding time, volunteers receiving carotenoid supplements showed a plateau in serum concentrations of 1500, 2000, and 1200 nmol/L for lutein, β-carotene, and lycopene, respectively, after ∼15 mg/day of each carotenoid, starting from 8 weeks and maintained until week 20.65 Similarly, administration of 180 mg/day β-carotene resulted in a plateau of ∼9500–18,500 nmol/L between 1.5 and 4 weeks.123 Ranges and average concentrations of plasma β-carotene after supplementation trials have been published25 and highlight that only easily bioavailable doses (eg, dissolved in oil) of 20 mg/day or more (such as in the CARET or ATBC study) resulted in β-carotene plasma concentrations >1900 nmol/L—doses that were related with adverse effects and clearly higher-than-typical average concentrations in populations (>500 nmol/L) (Table 1). Interestingly, when effects of dose, supplementation duration, formulation, sex, smoking status, and study design were evaluated among 57 human studies, dosing was the most prominent factor affecting β-carotene plasma response. Multiple-unit dosing was more effective than single-unit dosing and outweighed the effect of dose within the range of 2–30 mg/day.124 It is possible that the amount of carotenoids that can be absorbed at 1 time, or those that can be additionally secreted via chylomicrons and transported in lipoproteins, is limited.
Age has also been proposed to be associated with carotenoid blood concentrations, but effects are inconsistent and may be heavily confounded by dietary intake and habits. In women >65 years old compared with women 56–65 years old, significantly lower β-carotene, lutein, zeaxanthin, β-cryptoxanthin, and lycopene concentrations were found,73 likely due, at least in part, to lower dietary intake. Contrarily, Wu et al125 did not confirm a trend of lycopene plasma concentrations with age and, likewise, Palli et al126 found no such age effects for, α-, β-, and γ-carotene. Similarly, Grolier et al127 reported no effect of age on lutein, β-cryptoxanthin, and β- and α-carotene concentrations in blood but noted significantly lower lycopene levels (50% lower) in elderly compared with young persons (<35 years vs >60 years), in agreement with Hodge et al.128 Lower lycopene concentrations in the elderly were emphasized in several studies (reviewed by Bohn et al21), though it is unclear whether lower intake or less efficient absorption at older age plays the main role. Interestingly, Olmedilla-Alonso et al70 found even higher serum concentrations in older vs younger study participants, though this was due to higher lutein and zeaxanthin dietary intake (both crude and energy adjusted). Likewise, Anlasik et al129 found a statistically significant effect of carotenoid intake (for lower [0–100 g/day] and higher [>350 g/day] consumption of fruits and vegetables), on plasma concentrations, independent of age and sex in elderly participants (65–102 years old). Thus, the observed relationship between age and circulating carotenoid concentrations is most likely be dominated and explained by differences in dietary intake.130
In some studies, a positive association between education and plasma/serum concentrations with predominant carotenoids was noted.73 The EPIC study indicated higher fruit and vegetable intakes in the United Kingdom compared to EPIC centers in Spain and Italy, similar to the report by Buijsse et al.9 It is likely that people with a higher education are more health conscious than people with a lower education and also can afford a healthier diet (ie, rich in fresh fruits and vegetables).131
Studies assessing the effect of smoking on carotenoid concentrations in serum/plasma demonstrated up to 44% reduced concentrations in smokers.128,132–135 Furthermore, Palli et al126 confirmed that the highest β-carotene levels were found in women who had quit smoking, whereas the lowest concentrations were found in present smokers. Inverse associations of serum carotenoids with the metabolic syndrome136 and diabetes137 were more evident in current smokers than in nonsmokers.138 Although not all studies adjusted for dietary carotenoid intake, some did. Walmsley et al139 suggested that the reduced dietary intake in observed smokers could only partly explain lower circulating plasma carotenoid concentrations. Similarly, in their small-scale study, Rust et al132 did not find different dietary patterns, but they did report lower plasma carotenoid concentrations. Finally, in the large-scale National Health and Nutrition Examination Survey III study, which included almost 8000 apparently healthy participants, smoking, even after dietary adjustments, was associated with significantly reduced β-carotene concentrations.133 Thus, though not all studies found lower carotenoid concentrations in smokers after adjustment for dietary intake,140 most did, suggesting, indeed, either lower bioavailability or enhanced turnover.
The effect of alcohol consumption on plasma concentrations of several carotenoids was studied in healthy men consuming low or moderate amounts of alcohol, patients with alcohol addiction but without severe liver disease, and in a control population. Plasma concentrations of all carotenoid fractions were significantly lower in the alcohol-addiction group than in the low-drinking group. After withdrawal, plasma concentrations of all carotenoids increased.141 On the other hand, lycopene concentrations in serum were 8% higher among drinkers compared with nondrinkers,125,142 and higher alcohol consumption (up to 20 g or ∼ 25 mL/day or 1.5 drinks) was associated with increased serum/plasma carotenoid levels in men but not in women. However, alcohol consumption exceeding 40 g/day (∼ 50 mL or 3 drinks) was associated with decreased β-carotene levels in serum/plasma, and this decline was more pronounced in men. Lyle et al143 also demonstrated lower β-carotene serum concentrations in drinkers with intakes of ≥ 91 g of alcohol per week, suggesting that increased alcohol intake is also co-associated with an altered dietary pattern high in certain processed food items. For example, poor diet quality, and thereby increased convenient food intake, such as pizza and ketchup, which are high in lycopene,144 was associated in middle-aged men from France with increased alcohol intake.145
In conclusion, carotenoid intake and plasma/serum concentrations appear reasonably well correlated. Women tend to have higher serum/plasma carotenoid concentrations than men, and age is associated with a decline of lycopene levels,130 though different dietary patterns of the elderly with lower consumption of processed tomato products could play a role.
INTERRELATION OF DIETARY CAROTENOID INTAKE AND TISSUE CONCENTRATION
Interestingly, a human postmortem study showed that the distribution pattern of carotenoids in serum/plasma was similar to that in the organs of the individuals, although there were significant quantitative differences in the levels of various carotenoids between organs.146 This was corroborated by additional studies investigating tissue carotenoid concentrations147–149 (Table 3). Thus, there is a correlation between intake and blood and tissue levels. Care should also be taken that many body compartments are assessed for carotenoid concentrations by different techniques, noting that absence of data does not necessarily mean absence of carotenoids. Furthermore, the association between intake and plasma/tissue concentrations is influenced on the individual level by genetic factors, which have been recently reviewed by our group and others, and we refer the reader to these comprehensive overviews.21,164
Table 3.
Concentrations of carotenoids in various tissues, all data in nmol/L (nmol/kg or L)
| Tissue | BCAR | ACARa | BCRY | LYC | LUT | ZEA | PHYE | PHYF | Total carosb | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum/plasma | 360 ± 10 | 120 ± 10 | 230 ± 10 | 740 ± 10 | 380 ± 10 | 90 ± 10 | 40 ± 20 | 170 ± 70 | 1940 ± 20 | Al-Delaimy (2005)150 |
| Serum/plasma | ATBC:
|
ATLYC:
|
Schierle et al (1997) 151, Fröhlich (2007)152 | |||||||
| Abdominal adipose tissue | 1472 ± 286 | 280 ± 74 | 417 ± 462 | 3329 ± 448 | 456 ± 62c | – | – | – | – | Chung et al (2009)153 |
| Liver | 5900 ± 6300 | – | – | 8400 ± 11,500 | 2200 ± 1600c | – | – | – | 16,500 | Bohn et al (2017) 21 |
| Skind | 430 ± 45 | 95 ± 20 | 225 ± 35 | 695 ± 45 | 180 ± 35 | 175 ± 35 | 320 ± 90 | 46 ± 20 | 0-7001730 | |
| Lung | 350 ± 440 | 230 ± 270 | 420 ± 750 | 570 ± 1110 | 480 ± 660 | – | – | – | 1905 ± 2820 | Schmitz et al (1991)147 |
| Kidney | 550 ± 730 | 300 ± 400 | 450 ± 1040 | 620 ± 620 | 1210 ± 2830 | – | – | – | 3050 ± 4210 | Schmitz et al (1991)147 |
| Braine | 10–30 | – | <10 | – | 20–80 | 10–30 | – | – | – | Vishwanathan et al (2014)157 |
| Adrenals |
|
|
|
|
– | – | – | – | 9400 ± 7800g | Stahl (1992)148 |
| Breast tissue | 38,000–50,000 | 158 | ||||||||
| Testes |
|
|
|
|
– | – | – | – | 7550 | Stahl (1992)148 |
| Bone | 745 ± 95 | 95 ± 35 | 125 ± 35 | 280 ± 35 | 175 ± 35 | 140 ± 60 | 825 ± 185 | 275 ± 45 | – | Ermakov et al (2013)155 |
| Colon tissue | 60 ± 30 | – | – | – | – | – | – | – | – | Pappalardo et al (1997)159 |
| Breast milk | 60–200 | 20–40 | 2–10 | 5–25 | 10–25 | – | – | – | – | Gossage et al (2002)160 |
| Uterus | 503h | – | 870 | – | – | – | – | – | 12,500 ± 6000 | Czeczuga-Semeniuk et al (2008)161 |
| Prostate | 600 | 300 | 100 | 700 | 300 | 200 | – | – | 2700 | Clintonet al (1996)162 |
| Eye (retina) | – | – | – | – | ∼3125–12,496i | – | – | – | Rapp et al (2000)163 | |
All values represent mean ± SD, with data reported as nmol/L (nmol/kg or L). Values in brackets reflect ranges.
Blank cells represent nondetermined carotenoids or no data were available.
Sum of listed carotenoids, unless otherwise stated.
Sum of lutein and zeaxanthin.
Dermis and epidermis of back, forehead, inner forearm, and hand.
Infants, prefrontal cortex, frontal cortex, hippocampus, auditory cortex, and occipital cortex.
Including upper and lower level of this range.
Standard error of the mean.
Values given in literature as “carotenes.”
This concentration is based on a total amount of 0.25 nmol in a retina, which is calculated on a predicted retina weight of 10–80 mg.
Abbreviations: 5CLYC, 5-cis-lycopene; 9CBC, 9-cis-β-carotene; 9CLYC, 9-cis-lycopene; 13CBC, 13-cis-β-carotene; 13/15CLYC, 13/15-cis-lycopene; ACAR, α-carotene; ATBC, Alpha Tocopherol, Beta Carotene Prevention Trial; ATLYC, all-trans lycopene; BCAR, β-carotene; BCRY, β-cryptoxanthin; caros, carotenoids; LUT, lutein; LYC, lycopene; PHYE, phytoene; PHYF, phytofluene; ZEA, zeaxanthin.
Intestine and liver
The intestine is among the first organs exposed to dietary carotenoids. Carotenoid concentrations in the small intestine are poorly documented. In the colon, β-carotene tissue levels were 60 ± 30 nmol/kg and were significantly increased (2.6 times) by supplementation (30 mg/day for 43 days).159 Patients with colon cancer displayed a lower total carotenoid content compared with healthy participants.159 This is in line with a previous study in which researchers showed that β-carotene concentrations were lower in colon and rectum cancer samples, as well as in other cancer tissues (cervix, endometrium, ovary, breast, lung, and liver), compared with control tissues.165 Whether this reflects altered cellular uptake, distribution, or a faster degradation is currently unknown.
The liver is acknowledged to accumulate carotenoids, especially β-carotene 148 and lycopene.147 The liver may constitute a rather fast-exchanging carotenoid pool, compared with, for example, adipose tissue.166 In adults, the total carotenoid concentration in liver varied from 2500 to 77,000 nmol/kg,147,149 and significant correlations were observed between serum and liver α- and β-carotene levels.167 Conversely, there were no correlations between liver vitamin A and individual or total carotenoids in normal livers,149 perhaps due to the limited contribution of total carotenoids to vitamin A, emphasizing the importance of preformed vitamin A, at least for participants regularly consuming animal products. It has also been suggested that liver diseases could interfere with the uptake, excretion, or metabolism of carotenoids.167
Adipose tissue
It has been established that carotenoids are stored to a notable extent in adipose tissue,153,168–171 with lycopene and β-carotene predominating.170,172 Chung et al153 identified lycopene as the most prevalent carotenoid in this tissue (3329 ± 448 nmol/kg; >50% of total carotenoids), followed by β-carotene (1472 ± 286 nmol/kg), lutein and zeaxanthin (456 ± 62 nmol/kg), β-cryptoxanthin (418 ± 462 nmol/kg) and α-carotene (280 ± 74 nmol/kg).153
Adipose tissue concentrations of carotenoids appear to be similar in men and women. The total carotenoid concentration appears to be site specific, with abdominal concentrations being higher than in the buttocks or thigh.153 Interestingly, circulating concentrations of most carotenoids are inversely correlated to fat mass, and to both general and central adiposity in mostly normal-weight and overweight persons.153,173 This may suggest that in people with higher BMI, carotenoids are sequestered within the adipose tissue, though lower intake or increased turnover rates may also play a role. Indeed, most studies revealed a strong inverse correlation between BMI and all measured carotenoids in plasma in normal, overweight, and obese participants,9,73,79,125,126,128,130 except for few studies finding otherwise, such as for lycopene,174 suggesting that the adipose tissue acts as a sink for circulating carotenoids.
Researchers have also measured carotenoids in adipose tissues. Studies63,175 have revealed statistically significant positive correlations between individual carotenoid concentrations (P < 0.01 for lutein and zeaxanthin, lycopene, β-cryptoxanthin, and β-carotene) in blood and adipose tissue. Similarly, β-carotene content in adipose tissue correlated weakly (r = 0.2) with plasma.176,177 In another study, total carotenoid content, except for lycopene and lutein/zeaxanthin, in adipose tissue was strongly associated with serum levels.153 Similarly, breast adipose tissue carotenoid content was correlated with levels in plasma, except for β-cryptoxanthin.178 It is noteworthy that, at least for β-carotene, even though its serum concentration was lower in obese people, the total body pool of β-carotene was similar in obese and nonobese people, when taking into account the total fat mass.179 Thus, higher fat mass appears to be related with lower plasma concentrations, which, in turn, appear to correlate with lower adipose tissue concentration of carotenoids. In other words, higher fat mass seems related to lower concentration of carotenoids in adipose tissue, also.
Adipose tissue carotenoid content is correlated not only with plasma levels but also with other tissue concentrations. For example, lutein adipose tissue content was positively correlated with macular pigment density in men (though not in women).180 Conversely, weight loss was associated with increased lutein and zeaxanthin serum concentrations.181 Though hypercarotenemia could be expected to develop at the onset of the pronounced postoperative weight loss after bariatric surgery, a consistent and continuous drop of all serum carotenoids to levels at or below the fifth percentile of the reference ranges was observed in patients followed up for 18 months.182
Factors influencing carotenoid distribution in adipose tissue uptake and turnover are poorly understood. On the basis of single-dose studies such as those by Diwadkar-Navsariwalaet al183 and Moran et al,166 it was suggested that adipose tissue is a major component of a slow exchanging pool, as proposed for lycopene and phytoene. The uptake of carotenoids by adipose tissue was not linked to carotenoid physicochemical properties,184 suggesting the involvement of transporters. In accordance, the involvement of CD36 in lycopene and lutein uptake by adipose tissue and adipocytes has been demonstrated.185 Thus, adipose tissue carotenoid content may be considered a reasonable mid- to long-term indicator of dietary carotenoid intake,176 though it has been shown to increase after supplementation. β-Carotene concentrations in adipose tissue increased from 1470 nmol/kg to 2090 nmol/kg after 5 days of a high, single, oral dose (120 mg).171 Lutein and zeaxanthin levels (230,000 ± 70,000 nmol/kg dry tissue) in adipose tissue significantly increased after spinach and corn consumption (10.8 mg/day lutein; 0.3 mg/day zeaxanthin) in healthy participants, with a maximum measured at 8 weeks of intervention (470,000 ± 80,000 nmol/kg dry tissue).186 Finally, tomato-oleoresin supplementation (15 mg lycopene/day) significantly increased lycopene concentration in adipose tissue (from 230 ± 160 nmol/kg to 340 ± 230 nmol/kg).187
Dietary carotenoid intake also correlated strongly with abdominal adipose tissue concentration, but less so with buttock or thigh adipose tissue, for α- and β-carotene, β-cryptoxanthin, cis-lycopene isomers and total carotenoids (NB, we use cis/trans terminology in this article, rather than E/Z).153 However, correlations varied largely and were strongly influenced by sex. El-Sohemy et al63 reported mostly significant correlations in women between intake and concentrations in adipose tissues of α- and β-carotene, β-cryptoxanthin, and lutein/zeaxanthin of 0.25, 0.29, 0.44, and 0.17, respectively, but not in men (r < 0.23 for all). The origin of this discrepancy is unknown, but carotenoid adipose tissue concentrations may be affected by factors other than intake, such as circulating hormones.
Breast milk
Few studies have been dedicated to exploring carotenoid concentrations in human milk, which appears to be an important source of both provitamin A and non-provitamin A carotenoids during the first months of life.160 In an American cohort, carotenoid concentrations in milk at day 4 ranged from 50 to 380 nmol/L, depending on the carotenoid (α-carotene < β-cryptoxanthin < lutein ≈ lycopene ≈ β-carotene), with a high interindividual variability. Similar concentrations were found by others. Khachik et al188 found carotenoid concentrations between 2 and 49 nmol/L, whereas Johnson et al189 reported higher concentrations (ie, ∼800 nmol/L for β-carotene, and 165–185 nmol/L were reported by Alien et al190 for lycopene, though all were rather small-scale studies with < 10 participants. As observed for fat-soluble vitamins, milk carotenoid concentrations decreased during the first month to reach mature milk concentrations of ∼10–130 nmol/L. These concentrations were equivalent to 5% to 10% of plasma concentrations, except for lutein, which was present at concentrations equivalent to 30%, constituting 50% of total milk carotenoids. This suggests a specific flow of lutein into milk.160 Some lutein in milk may be present in the form of esters, and re-esterification of lutein has been proposed.191 Supplementation with β-carotene (30 mg/day) during the first month of lactation affected neither milk β-carotene nor other carotenoid concentrations,160 contrary to previous work.192 In this latter study, by Canfield et al,192 breast milk retinol was not significantly different among the groups over the treatment period, but breast milk β-carotene concentration was greater after palm oil supplementation (90 mg over 10 days), compared with an equivalent supplementation with pure β-carotene and vs a placebo. The difference observed between the 2 trials may be due to differing efficacy of milk enrichment in β-carotene, which seems to be directly linked to milk fat content.160,192 The accumulation of lutein in mothers’ milk and the association of carotenoids in breast milk with plasma concentrations has been confirmed in recent studies.193–196 This highlights the possible importance of neonatal exposure to carotenoids during development and may help establish dietary recommendations and in the design of human milk mimetics. It has been suggested that lutein can accumulate in various brain tissues157 and may constitute an important microconstituent for optimal brain health during the early phases of life.
Lung, kidney, brain, and bone
Different studies have shown that total carotenoid contents of kidney and lung ranged between 200 and 12,700 (mean, 3100) nmol/kg and 100–8400 (mean, 1900) nmol/kg in tissues, respectively.147 Interestingly, and similar to liver, lung and kidney β-carotene concentrations were positively correlated with α-carotene, lycopene, and total carotenoids.147
Major carotenoids identified in the brain were lutein, zeaxanthin, anhydrolutein, α-cryptoxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene, similar to blood, with concentrations of 10–80 nmol/kg (Table 4). Xanthophylls accounted for 66% to 77% of total carotenoids in all brain regions examined. This differs from plasma, perhaps suggesting discriminative steps in brain-tissue uptake. As for the neural retina, the ratio of zeaxanthin to lutein was high, and both xanthophylls were significantly correlated.199 Interestingly, the frontal lobes, but not the occipital lobes, exhibited an age-related decline in total xanthophylls and total carotenoids.199
Table 4.
Correlation coefficients between blood plasma and/or serum concentrations and dietary intake of carotenoids in humans
| Blood serum or plasma (nmol/L)a,b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Location; women and/ or men, age (no.) | Method | ACAR | BCAR | LUT | ZEA | L/Z | BCRY | LYC | |
| Fraser et al (2016)82 |
|
|
|
|
|
|
|
|||
|
| ||||||||||
| Olmedilla-Alonso et al (2014)70 |
|
3 24-h diet recalls |
|
|
|
|||||
|
| ||||||||||
| Wawrzyniak et al (2013)73 |
|
FFQ | 0.25 | 0.37 | 0.29 | 0.30 | 0.24 | |||
|
| ||||||||||
| George et al (2012)81c |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|||
|
| ||||||||||
| Talegawkar et al (2008)197 |
|
|
|
|
|
|
|
|||
|
| ||||||||||
| Tangney et al (2004)78 |
|
|
0.47 | 0.03d | 0.44 | −0.02d | ||||
|
|
0.54 | 0.13d | 0.46 | 0.09d | |||||
|
| ||||||||||
| El-Sohemy et al (2002)63 |
|
FFQ | 0.26 | 0.13d | 0.22 | 0.55 | 0.19 | |||
|
FFQ | 0.24 | 0.22 | 0.20 | 0.44 | 0.35 | ||||
|
| ||||||||||
| Curran-Celentano et al (2001)77 | American women and men, 18–50 y (280) | FFQ | 0.16 | 0.19 | 0.03d | |||||
|
| ||||||||||
| Carroll et al (1999)66 |
|
FFQ |
|
|||||||
|
FFQ |
|
|
|
||||||
|
| ||||||||||
| Tucker et al (1999)76 | American women 67–93 y (346) | FFQ | 0.33 | 0.36 | 0.27 | 0.44 | 0.35 | |||
| American men 68–91 y (201) | FFQ | 0.18 | 0.25 | 0.10d | 0.32 | 0.21 | ||||
|
| ||||||||||
| Yong et al (1994)198 |
|
|
|
|
|
|
|
|||
| Range | 0.14–0.70 | 0.09–0.52 | 0.14–0.34 | 0.03–0.37 | 0.02–0.52 | 0.25–0.68 | 0.02–0.50 | |||
|
| ||||||||||
| Mean | 0.39 | 0.29 | 0.27 | 0.17 | 0.24 | 0.45 | 0.29 | |||
|
| ||||||||||
| Standard deviation | 0.16 | 0.14 | 0.07 | 0.11 | 0.14 | 0.11 | 0.11 | |||
Pearson correlation coefficient is reported unless otherwise indicated.
Either serum or plasma.
Blank cells represent nondetermined correlations due to no data, data not determined, or missing values of measured carotenoids.
Spearman coefficient reported.
Not statistically significant.
Abbreviations: 24 H, 24 h dietary recalls; ACAR: α-carotene; BCAR: β-carotene; BCRY, β-cryptoxanthin; DHQ, diet history questionnaire; FFQ, food frequency questionnaire; LUT, lutein; LYC, lycopene; L/Z, lutein/zeaxanthin; ZEA, zeaxanthin.
Carotenoids also exist in human bone and surrounding fatty tissue, both in significant and individually variable concentrations, up to almost 1000 nmol/kg for individual carotenoids (eg, phytoene). Measurements of biopsied tissue samples, determined by Raman spectrometry, revealed that all carotenoids known to exist in human skin (ie, β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin) are also present in human bone, but additional studies are needed to establish correlations with plasma or skin levels.155
Buccal mucosal cells and skin
Strong correlations (R) between plasma and buccal mucosal cell concentrations of lutein (up to 0.873), β-cryptoxanthin (up to 0.815), α-carotene (up to 0.796), and β-carotene (up to 0.775) were observed.200 In supplementation studies, responses in β-carotene concentrations varied considerably between participants. Data suggested the existence of weak and strong responders,201 a concept well documented in postprandial studies.202 Conversely, although lycopene cellular content increased after supplementation (70 mg/day lycopene via oleoresin, tomato juice, or beadlets), correlations between lycopene concentrations in plasma and in buccal mucosal cells were weak and not significant for any treatment.200 This contradicted another study, showing that both β-carotene and lycopene were incorporated into mucosal tissues within 7 days, but it was not clear whether the change in carotenoid plasma concentrations was reflected in existing buccal mucosal cells or in those produced during the elevated plasma concentrations.203 Interestingly, buccal mucosal cell concentrations of β-carotene were correlated with (1) skin type (the darker the skin, the more β-carotene),204 perhaps suggesting a fundamental role of epithelial cells and protection from light; and (2) smoking, with smokers having lower carotenoid concentrations in these cells vs nonsmokers,204,205 due to lower consumption or increased turnover.
Predominant carotenoids detected in the skin are lycopene, β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, phytoene, and phytofluene, with highest amounts for lycopene.156 Skin carotenoids contribute to skin color and photoprotection.154,206–208 The skin carotenoid score (SCS) can be assessed by Raman spectroscopy and may well reflect carotenoid intake. Skin carotenoid concentrations of up to 650 nmol/kg for individual carotenoids have been reported (Table 3). Indeed, a trial showed that SCS increased with daily consumption of a carotenoid-rich juice (∼15 mg/day), but returned to initial levels 3 days after the last intake.209 This is in line with a previous study that showed SCS predicted plasma concentrations (r = 0.72; P < 0.001), indicating that changes in SCS closely follow changes in plasma across a broad range of intakes (from carotenoid-depleted diets to 59–65 mg mixed carotenoids per day). Moreover, at the individual level, skin carotenoids predicted plasma levels (r = 0.70; P < 0.001), confirming that SCS can be a noninvasive, objective biomarker of vegetable and fruit intake.210 Among children aged 5–17 years, consuming 30–120 mL (2.8–11 mg carotenoids) per day of a carotenoid-rich juice significantly increased skin carotenoid status over 8-week periods.211 However, Walfisch et al187 showed that skin lycopene only slightly increased (1.6-fold) after 1–7 weeks of supplementation with tomato oleoresin (30 mg lycopene/day)1. As stated by Stahl and Sies,212 optimal skin protection against UV-light–induced erythema may take 8–10 weeks following increased carotenoid intake, perhaps explaining limited changes found in some previous studies, though increased concentrations of carotenoids were detected after 4 weeks of intake.213
Breast and reproductive organs: ovary, uterus, testes, and prostate
Thirteen carotenoids were found in female breast adipose tissue around neoplastic tissue in fairly high concentrations (mean, ≈ 38,000–50,000 nmol/kg). Lutein-epoxide and violaxanthin were predominant in breast adipose tissue, in malignant and benign areas. Mutatoxanthin, lutein epoxide, zeaxanthin, canthaxanthin, lutein, and neoxanthin were predominant in neoplastic material. β-Carotene and lutein epoxide were found in all samples, whereas α-carotene was found only in 50% of the samples. The total carotenoid tissue content was slightly lower for cancerous tissue and the surrounding adipose compared with benign tissues, and was significantly higher in the adipose tissue surrounding the tumors, irrespective of their histological structure.158
Similarly, up to 14 carotenoids, including β-carotene, β-cryptoxanthin, lutein, lutein epoxide, violaxanthin, and mutatoxanthin, were identified in uterine161 and ovarian tissue.214 In normal uteri, the mean carotenoid concentration was highest in the follicular phase endometrium (18,000 nmol/kg), whereas the highest percentage of provitamin A carotenoids (β-carotene and β-cryptoxanthin) was found in the luteal phase (18.2%).161 In all ovarian pathological lesions, total carotenoid concentration was relatively low (mean ∼3000 nmol/kg), whereas it was higher in the ovarian endometriosis group (4000 nmol/kg).214 High levels were also found in uterus endometrioid adenocarcinoma (20,000 nmol/kg), suggesting that certain enzymatic defects in carotenoid metabolism occur during lesion evolution.161 Another trial showed that α-carotene and β-carotene cervical tissue concentrations were significantly correlated.215 Thus, though a high diversity of carotenoids in the physiologic, benign, and malignant tissues of both breast and reproductive tract in women has been highlighted, differences in carotenoid patterns do not allow drawing conclusions on its relation to the pathophysiological state.216
Testes also accumulated significant amounts of carotenoids, with lycopene being predominant (4300 nmol/kg) in a small German cohort.148 A study of elderly men in the United States showed that concentrations of specific carotenoids in the benign and malignant prostate tissue from the same participants were highly correlated. Lycopene and all-trans-β-carotene were the predominant carotenoids, with concentrations ranging from 0 to 2580 nmol/kg and 90 to 1700 nmol/kg, respectively. Also detected were 9-cis-β-carotene, α-carotene, lutein, zeaxanthin, and β-cryptoxanthin. Although no significant correlations between the concentration of lycopene and any other carotenoid were observed, strong correlations between prostate β-carotene and α-carotene concentrations and between several other carotenoid pairs were highlighted, possibly reflecting similar dietary origins.162
Macular carotenoids and macular pigment optical density
An important target tissue to consider for developing dietary recommendations for carotenoids is the macula, with MPOD constituting the most accessible marker for xanthophyll concentration in the macula. The macula (or macula lutea) is a small, specialized tissue at the center of the retina (being 4% of the retina area), mediating sharp, central, and color vision. The fovea, the center of the macula, is a small, central pit composed of closely packed cones and is responsible for almost all useful day vision.217 Only primates accumulate the macular carotenoids at the fovea. Several carotenoids, due to their conjugated double bonds, have short-wavelength (blue) light-filtering properties.218–220 The xanthophylls, lutein, zeaxanthin, and meso-zeaxanthin (MZ) accumulate in the central retina (Figure 1). Previous studies220,221 have demonstrated that macula optical density peaks at the center of the fovea, with a concentration 3 orders of magnitude above that found in normal serum. Bone et al221 quantified foveal total carotenoid concentrations as ranging from 0.05 ng/mm2 in the peripheral retina to 13 ng/mm2 at the fovea. The lutein-to-zeaxanthin ratio changes drastically from the serum of humans (4:1 to 2:1)222,223 to the macula, where the ratio varies across the fovea. Zeaxanthin is concentrated in the central fovea (ratio of lutein to zeaxanthin, ∼1:2.4), whereas lutein is concentrated in the periphery (ratio of lutein to zeaxanthin, ∼2:1).221
Figure 1.
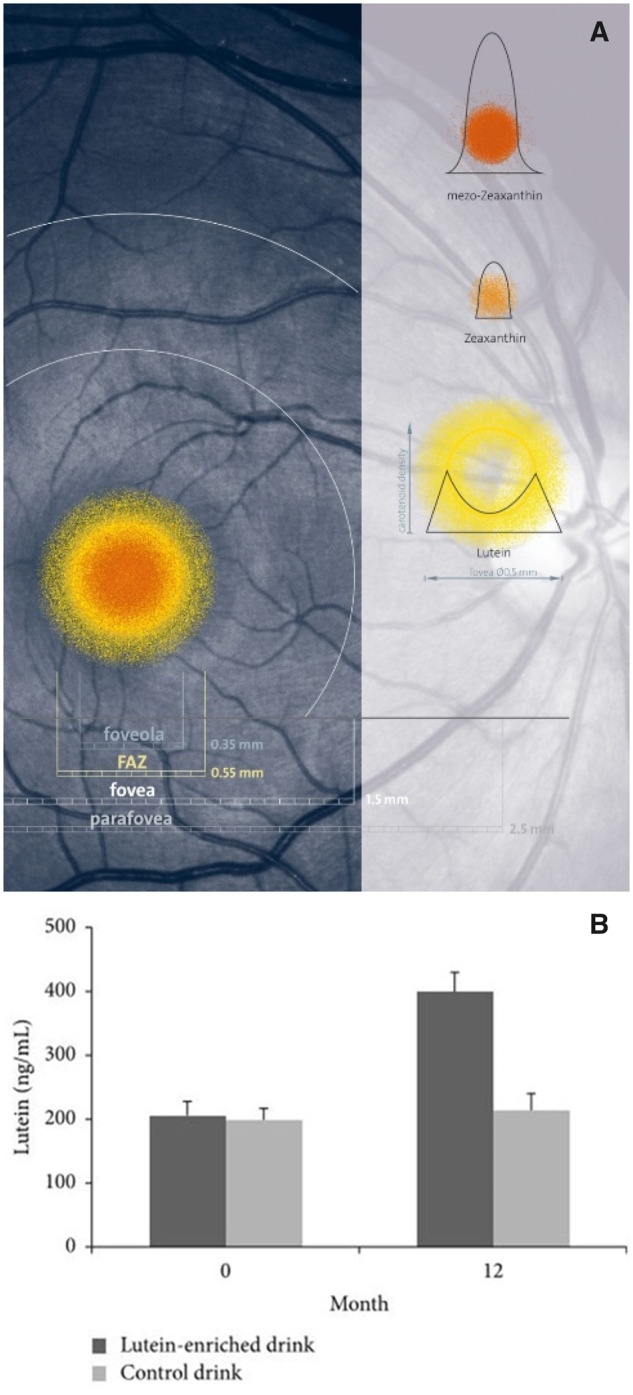
(A) Distribution of macular pigments lutein, meso-zeaxanthin, and zeaxanthin, presented in scale in a photograph of a healthy human retina. Image from Robert Kochling, Berlin, Germany; and John Nolan, Waterford, Ireland, with permission. (B) Mean ± standard error of plasma lutein concentration at 0 and 12 months for the lutein (dark grey) and the placebo (light grey) groups.207Abbreviation: FAZ, foveal avascular zone.
Recent work highlighted that enrichment of macular pigment (MP) in patients with early AMD enhances visual function by improving contrast sensitivity,224 consistent with findings that supplementing with lutein, zeaxanthin, and MZ enhances visual performance in persons with and without retinal disease.225–227 A recent meta-analysis in humans also emphasized that the increase in MPOD was significantly greater when MZ was included in the supplement.228 Thus, supplementing with all 3 carotenoids for 12 months, at ∼20 mg/day total, resulted in the best outcomes in terms of MPOD and visual function.228 In formulations lacking MZ, up to 25% of study participants exhibited a nonresponse regarding MP levels.229,230
As for plasma carotenoid concentrations, the source and type of foods consumed have a strong impact also on MPOD. For humans, vegetables provide >7 times the concentration of macular carotenoids compared with eggs and nearly 20 times that of fruits.231 However, carotenoids from plant sources are of limited bioavailability. In contrast, carotenoids in egg yolk are highly bioavailable because they are present in a digestible lipid matrix. Recently, a human intervention with a buttermilk drink with egg yolk proved to be a highly bioavailable source of lutein and zeaxanthin232 (Figure 1B). MPOD also increased significantly, from 0.45 to 0.52 optical density units in the active treatment group (P < 0.001) but was unchanged in the placebo group (same drink without egg yolk). The Egg Xanthophyll Intervention Trial showed that carotenoid-enriched eggs (2/day for 8 weeks) significantly increased serum concentrations of lutein, zeaxanthin, and MZ compared with standard eggs. However, neither of these interventions significantly increased MPOD,233 perhaps because study participants were healthy and middle aged at study onset.
Lutein and zeaxanthin are evenly distributed between low-density lipoprotein and high-density lipoprotein,234 the lipoprotein profile is likely to affect MP levels.234 Apolipoproteins can act as cofactors of enzymes involved in lipoprotein metabolism, such as lipoprotein lipase.235 One study found that apolipoprotein E (ApoE) levels were higher in patients with AMD than in controls.235 The ApoE lipoprotein APOE-ε4 allele is likely associated with a reduced risk of AMD,236,237 possibly due to increased MP at the retina. It was postulated that ApoE could influence the transport, capture, and stabilization of lutein and zeaxanthin at the macula.238 There is a specific deposition of MP within the eye (Figure 1), which suggests a biological process governing the capture, deposition, and stabilization of carotenoids at the macula. This regulation is believed to be elicited by binding proteins. Bernstein et al239 identified tubulin as a possible locus for concentrating MP in the fovea; Li et al240 identified pi isoform glutathione S-transferase as a binding protein for zeaxanthin and MZ, and steroidogenic acute regulatory domain was identified as a lutein binding protein.241 However, mechanisms controlling the deposition and stabilization of MP are not fully understood.242 An atypical spatial profile of MP exists in the fovea of some people.229 This central dip in MP has been proposed to be due to a lack of MZ in these individuals, potentially due to an inability to convert lutein to MZ.243,244 The clinical trial reported by Nolan et al229 demonstrated that supplements containing MZ could rebuild central dips in MP.
In some trials, the MPOD of participants supplemented with lutein and zeaxanthin did not increase245–247 despite increased serum concentrations. Micozzi et al246 observed that supplementing β-carotene reduced serum concentrations of lutein in men, possibly due to competition for absorption. Also, in AREDS2, lutein and zeaxanthin serum concentrations were lower in the group receiving additional β-carotene supplements.248 The Lutein Nutrition Effects Measured by Autofluorescence (LUNA) study suggested that malabsorption or impaired serum transport of the macular carotenoids was not responsible for the failure of supplements to improve MPOD in retinal nonresponders (because serum concentrations of these carotenoids increased with supplementation) but that impaired capture and/or stabilization of lutein and zeaxanthin within the retina contributed to poor macular response.230 One explanation was that the supplement was lacking MZ, because when MZ was present in the formulation, 100% of participants exhibited an increase in MP.226
MP determinants can be either modifiable (eg, cigarette smoking) or nonmodifiable (eg, age). A family history of AMD was associated with significantly lower MPOD despite normal serum MP concentrations.249 A significant but modest age-related MPOD decline in study participants >50 years old was observed,250 a finding supported by other studies.70,249,251–253 Moran et al254 reported that even when controlling for supplemental intake, MPOD showed age-specific correlations in older but not younger study participants.254 Oxidative stress, being higher in older individuals, may be a contributing factor.255,256 Therefore, any carotenoid intake recommendation targeting MPOD may need adjustment for age. Sex is also related to MPOD, with men having significantly higher MPOD than women, even after adjusting for serum lutein and zeaxanthin levels.249,257 This difference was due to the lack of women in the highest MP range,258 as corroborated previously.259 Removal of the top 5% of men with the highest MP values resulted in minimal differences for MP. BMI is also inversely related to serum and MPOD levels.249,254,257,260 An association between oxidative stress and BMI, as well as competition between adipose tissue and the retina for uptake of MP, are possible causes.181,254,261–263 Hammond et al observed an inverse relationship between MPOD and BMI (n = 680, r = −0.12), stating that the relationship was driven by participants with higher BMI (>29), as these had 21% less MP compared to participants with a BMI < 29.262 Also, carotenoid intake of participants with higher BMI was lower than of those with normal BMI. Similar as for plasma, Hammond et al258 found that cigarette smokers had, on average, 25% less MP compared with nonsmokers, consistent with studies by Nolan et al258,264 in which an inverse relationship between MPOD and smoking frequency was demonstrated.249,265 Finally, an association between education and MPOD was found.265,266 Education was a positive predictor of MPOD even after adjustment for confounders (eg, age, sex, diet). Heritability of MP was estimated in 1 study at 84%, indicating that genetic factors play a key role in the distribution profile of MP.267 Furthermore, it was shown that genetic factors explain 27% of the variation in MPOD in response to supplemental lutein and zeaxanthin.268 These factors have been reviewed elsewhere.21,87
In summary, it appears that carotenoid accumulation in human organs is highly variable, both between and within individuals, and tissue carotenoid concentration can be modulated by (1) host factors (eg, low vs high responders, age, smoking status); (2) dietary intake, food matrix, and processing; (3) types of carotenoid; and (4) the pathophysiological state of the organs,42,269 though factors governing distribution between tissues in an individual are not well understood.
Although analytically challenging, compartmental approaches based on isotope administration are interesting and allow the study of fluxes between body compartments, as done for β-carotene.270,271 Moran et al272 developed a model for 13C-labelled lycopene that was based on an earlier model.183 These studies indicated that the major body pools are a slow turnover pool, likely representing body tissues such as adipose tissues, and a fast turnover pool, possibly including the liver. Transport rates across the pools were mentioned, with irreversible losses from the slow turnover pool, given with 2500 nmol/day (1.3 mg/day) lycopene, which eventually would need to be replenished. However, it thus is difficult to predict the amount needed to ingest to maintain certain tissue levels in individuals, though on population levels, estimates appear possible.
INSIGHTS FROM LESS FREQUENTLY CONSUMED CAROTENOIDS
In addition to β-carotene, α-carotene, lycopene, lutein, zeaxanthin, and β-cryptoxanthin, other less frequently and locally consumed, or investigated, carotenoids and apo-carotenoids are also present in the human diet. These have also been detected in human plasma. Though their nutritional relevance remains largely unclear, because they mostly cannot be converted into vitamin A active metabolites, some of these compounds are bioavailable and bioactive. For example, phytoene and phytofluene are present at high concentrations in a variety of fruits and vegetables, including carrots, tomatoes, apricots, and oranges.69,273 Despite occurring together with lycopene, many bioavailability studies, focusing on tomato-based foods, regularly overlook phytoene and phytofluene. Yet, they are among the predominant carotenoids found in plasma.274–276 The bioaccessibility273,277 and even bioavailability166 of these colorless carotenoids appear to be even superior to that of lycopene.
Astaxanthin is a marine carotenoid bioavailable in humans278–280 and found in aquatic animals. Synthetic asthaxanthin is used in high amounts in “blue farming” (ie, in the open ocean) and also as a food colorant (E161f). This yields the consumer-preferred orange to pink color of fish such as trout and salmon.281 Main dietary sources of astaxanthin include salmon, trout, red seabream, shrimp, and lobster.282 However, the role of astaxanthin as a novel food ingredient has also received increasing attention on the European market, as published in a Scientific Opinion by a European Food Safety Authority Panel.103 Guerin et al283 have reviewed the potential of astaxanthin in human health and nutrition and highlighted its role as an antioxidant and photoprotective agent. In fact, astaxanthin has been reported to be a more potent antioxidant than lutein or β-carotene.218 In a double-blind supplementation trial, participants were randomly allocated to either a supplement (4 mg of astaxanthin twice per day) or to a placebo for 3 months. The authors reported a significant reduction of plasma levels of 12- and 15-hydroxy fatty acids.278 These results highlight the potential role of astaxanthin against lipid peroxidation in vivo.
Fucoxanthin, a carotenoid commonly found in the marine environment, is present in brown seaweeds such as Undaria pinnatifida (wakame), Hijikia fusiformis (hijiki), Laminaria japonica (ma-kombu), and Sargassum fulvellum. All are popular foodstuffs in East Asia.284 This carotenoid has an unusual allenic bond (C = C = C) and a 5,6-monoepoxide in its molecule that is responsible for its radical scavenging and singlet oxygen-quenching activity. Its antioxidant potential has been reported to be higher than that of β-carotene, lycopene, or astaxanthin.285 Furthermore, fucoxanthin has also been shown to have anti-obesity and anti-diabetic properties in vitro in a mouse-derived white adipose cell culture.286,287 Metabolism of fucoxanthin can result in fucoxanthinol accumulating in plasma and liver of mice,288 as well as in human plasma,289 but their physiological and nutritional relevance is unknown.290
Violaxanthin and neoxanthin are frequently consumed epoxycarotenoids, especially from green leafy vegetables such as spinach and kale.69 In a previous study, it was estimated that their intake constitutes ∼10% of total carotenoid intake.69 However, it is also known that the bioavailability of these compounds and their gastrointestinal degradation products such as neochrome, a metabolite of neoxanthin, is low291,292 because they can undergo epoxy-furanoid transition in the acid milieu of the stomach. Nevertheless, cellular trials with Caco-2 cells have shown that they can be taken up by enterocytes.293,294 Their fate still remains to be elucidated. It is possible they are shuffled out of the enterocyte back to the lumen or are transformed into yet-unknown compounds. However, recent studies have suggested their presence in humans, such as in breast cell tissues.158
Of note, other apo-carotenoids are also bioavailable and bioactive, though they are possibly taken up by the diet rather than produced in humans. However, the intake of these compounds is likely inferior to the native carotenoids This is the case, for example, of crocetin from safflower, norbixin and bixin from annatto seeds, and abscisic acid, a plant hormone present in many leafy vegetables. For example, plasma crocetin concentrations of up to 760 nmol/L were measured in humans after the consumption of a single 22.5-mg dose.295 Likewise, abscisic acid was absorbed in humans, with cytokine-like activity in human granulocytes,296 ameliorating experimental inflammatory bowel disease in rats.297 Abscisic acid also improved glucose tolerance in rats and humans,298 interacting on apoptosis via the retinoic acid receptor.299 This may allow conclusions to be drawn also for apo-carotenoid metabolites produced in vivo. However, compared with the main native carotenoids, these apo-carotenoids remain marginally examined.
DISCUSSION AND PERSPECTIVES
Several carotenoids are implicated in health-related outcomes, from AMD (lutein and zeaxanthin) to possible effects regarding cardiometabolic diseases (predominantly β-carotene and lutein) and cancer (predominantly lycopene). However, because of the large variability of bioavailability according to carotenoid source and host factors,164 in conjunction with possible negative effects of some carotenoids at higher doses (at least for specific target populations), it is difficult to establish dietary intake recommendations. The only possible recommendation is to consume a diet rich in a variety of fruits and vegetables and their products to provide a sufficient combination of health-maintaining and promoting bioactive compounds. In opposition to these suggestions, it has to be considered that in modern Western societies, a strong desire for convenience food exists, which is generally satisfied by large food companies. These food items are processed and “ready to eat” and are based on economically more favorable basic ingredients rich in saturated fats, sugar, and added ingredients for preservation and coloring purposes, rather than being dense in micronutrients and secondary plant compounds.300,301 This is in contrast to the dietary suggestion of a diet rich in fruits and vegetables promoted mainly by the scientific community. Unfortunately, these latter recommendations are mainly reaching people of higher socioeconomic status who tend to be more sensitive and aware of a healthier lifestyle, including diet,302 whereas the problem rests rather with the less educated, often following a more unhealthy lifestyle including poor dietary choices,303 smoking,304 and alcohol abuse.305 Thus, these suggestions are engrossed and partly opposite from common consumer demands and availability or affordability, and also lack strong advertising and lobbying efforts by the media and political forces.
Intake of artificial carotenoids occurs at multiple levels, either as direct vitamin or carotenoid supplements or as added food colorants. These range from the E160 (carotenoids)/E161 (xanthophylls) cluster (β-carotene [E160a], capsanthin [E160c], lycopene [E160d], apo-8´-carotenal [E160e]/-esters [E160f], lutein [E161b], violaxanthin [E161e], canthaxanthin [E161g], and zeaxanthin [E161h]) to astaxanthin [E161j].306 In addition, indirect supplementation with often synthetically produced carotenoids, vitamins, and food-colorant mixtures occurs via livestock, farm animals, and blue farming.307 Thus, a considerable or even the major fraction of dietary carotenoids and metabolites (retinol) may originate from direct or indirect supplementation of artificially synthesized carotenoids or retinoids,308,309 which are regulated by governmental bodies. Dietary recommendations regarding single carotenoids are quickly adapted by the food industry, with fortification to the food chain, even adding higher amounts to ensure sufficient levels in food products lasting throughout their shelf life, to also meet governmental requirements for the quantity of listed food ingredients.
Governmental bodies, therefore, should make intake recommendations based on scientific expertise beyond the common “more fruits and vegetables” suggestion. This should encompass direct and indirect supplementation with single or multiple carotenoids, considering age, sex, and possibly genetic background and disease status for prevention strategies. By contrast, a recommended balanced diet rich in fruits and vegetables does not appear to require upper tolerable levels of intake, due to lack of evidence for harmful effects.
Dietary intake of carotenoids has also changed over time. Although lycopene intake was uncommon in the preindustrialized human diet, especially considering the primarily European-focused worldview, it strongly increased in Western society due to a high consumption of tomatoes and tomato products.310 Lycopene-associated anticancer effects may be expected to contribute to lower cancer incidence in Western societies,112 though such results have not been observed, possibly due to other confounding factors also influencing cancer prevalence.311
Major studies using lutein and zeaxanthin supplementation are often sponsored by the industry and have been focusing on eye-related effects, promoting higher dietary intake. These studies, however, neglected the possibility that higher intake of lutein or zeaxanthin and resulting higher serum or plasma and tissue concentrations may interfere with beneficial signaling mediated by other carotenoids, as indicated by endogenously relevant β-carotene–mediated retinoid signaling.20,312 Proposing recommendations based on single carotenoids without evaluating the entire picture of carotenoid-mediated signaling within the whole organism is preliminary and risky.
Dietary recommendations of single and multiple carotenoids should focus on end points clearly related to beneficial health effects and conclusive mechanisms of action. However, this connection between mechanistic effects and observed health outcomes has not been clearly established in humans. Carotenoids are generally considered precursor lipids (mainly for bioactive vitamin A or retinoids) in the diet, whereas their complex and multistep metabolic pathways and the relation to health beneficial effects are still poorly understood.
As a suggestion, a first aim should be to estimate (1) the “normal” concentration ranges of serum and plasma carotenoids, based on a basal healthy diet, and (2) plasma and serum concentrations associated with specific deficiencies like those related to optimal protection against AMD. On the basis of these results, supplementation strategies and recommendations should aim to identify (1) the dose and type of carotenoids needed and which carotenoid mixtures can be recommended, at least for well-defined objectives; and (2) for foods and supplements enriched in carotenoids, concentration ranges should be calculated on the basis of carotenoid intake suggestions and their amount already consumed as part of a healthy diet, to avoid unneeded and/or potential adverse oversupplementation.
CONCLUSIONS AND RECOMMENDATIONS
On the basis of current knowledge, the following statements can be made:
A high intake of a variety of fruits and vegetables is advised.
Supplementation with individual carotenoids may be beneficial for specific purposes, such as for lutein- and zeaxanthin-associated eye diseases.
Additional associations between carotenoids and health are based on observational studies and are not yet proven as causal. Because carotenoid intake via fruits and vegetables has never been associated with negative effects, dietary intake recommendations should especially focus on carotenoid intake from fortified foods or supplements, including food ingredients and additives such as colorants, to fully evaluate a risk and benefit ratio considering the bioactivity of carotenoids and their metabolites.
The adverse effects observed after individually administered carotenoid supplementation in smokers, resulting in high circulating concentrations, as found in the CARET or the ATBC study, are not generalizable to the whole population, especially non-smokers. Under such circumstances, carotenoids are not working endogenously as nutritionally relevant antioxidants by neutralizing free radicals and cannot be recommended as anti-smoking dietary supplements.313 Pro-oxidative events, free-radical formation, and enhanced CYP activity are just some of the potential negative effects of tobacco smoking, which is clearly the major risk factor for increased lung cancer incidence found in the CARET and the ATBC study.23,24
As a cornerstone, benchmark concentrations for carotenoids should be suggested, including both normal and deficiency threshold ranges. These ranges should correlate with well-defined and established disease markers, including early markers of diseases and impairments of physiological important functions, also including novel “omics” markers related to diseases.
The basal benchmark concentration indicating a higher risk for chronic diseases appears to constitute a total carotenoid plasma and serum concentration <1.000 nmol/L. The second benchmark concentration reflecting normal carotenoid intake are average plasma and serum concentrations of individual and total carotenoids indicating, and here defined as, a healthy, varied diet. These may be estimated from the data in Table 1, with ∼1725 nmol/L total carotenoids: 100 nmol/L α-carotene, 500 nmol/L β-carotene, 600 nmol/L lycopene, 230 nmol/L β-cryptoxanthin, and 330 nmol/L lutein and zeaxanthin. On the basis of these 2 benchmark concentrations and the correlating observed carotenoid intake (Table 1), one can estimate normal daily carotenoid intakes (ie, total carotenoids [11.8 mg] and individual carotenoids (0.7 mg α-carotene; 4.1 mg β-carotene; 2.2 mg lutein/zeaxanthin; 0.3 mg β-cryptoxanthin, 4.6 mg lycopene) (Table 1). Such levels can then be translated into the intake of relevant food items rich in carotenoids (Table 5), on the basis of correlations between reported average intakes for lutein, β-carotene, and lycopene with serum concentrations (Table 1) and considering intervention with carotenoid-rich foods (Table 5). It can be calculated that a combination of ∼50 mL of carrot juice, 65 mL tomato juice, and 20 g of cooked spinach per day are sufficient to achieve such average plasma and serum concentrations of the major carotenoids (summarized in Figure 2). This estimation is intended to serve as an example and this calculation can be transferred to alternative food sources and combinations.
Table 5.
Translation of food intake to recommendations: carotenoid concentrations in serum/plasma following dietary intervention trials
| Food source and conditions | Reference | BCAR (nmol/L) | LUT (nmol/L) | LYC (nmol/L) |
|---|---|---|---|---|
| Deficiency | ||||
| Carotenoid free diet for 14 d, average intake before supplementation | Watzl et al (1999)315 | 600 ± 360 | 350 ± 120 | 160 ± 80 |
| Average dietary intake | ||||
| Unknown intake of tomato juice, carrot juice, and dried spinach | Watzl et al (1999)315 | 740 ± 440 | 370 ± 140 | 160 ± 70 |
| β-Carotene supplements or β-carotene–rich food | ||||
| 330 mL carrot juice/d for 14 d (15.7 mg ACAR/21.6 mg BCAR/0.5 mg LUT) | Watzl et al (1999)315 | 2050 ± 720* | 360 ± 110 | 150 ± 50 |
|
3800*/5600* | |||
| Lycopene supplements or lycopene-rich food | ||||
| 330 mL tomato juice/d for 8 wk (22 mg LYC; 1.0 mg BCAR) | Bohn et al (2013)316 | 390 ± 160* | 1240 ± 30* | |
| Tomato extract (45 mg LYC/d) for 7 d | Wood et al (2008)317 | 540* | 250 | 127* |
| 30 mg/d LYC supplement (tomato extract) for 8 wk | Vrieling et al (2007)318 | 610 ± 220* | ||
| 15 mg/d LYC supplement (extract, tomato oleorosin) for 6 mo | Schwarz et al (2008)319 | 1240 ± 310* | ||
| 330 mL tomato juice/d for 14 d (40 mg LYC / 1.5 mg BCAR) | Bub et al (2000)320 | 650 ± 250 | 330 ± 120 | 380 ± 130* |
| Lutein/zeaxanthin supplements or lutein-rich food | ||||
| 6-mo L/Z supplementation (10 mg lutein; 2 mg zeaxanthin) | Korobelnik et al (2017)321 | 590 ± 390* | ||
|
Bub et al (2000)320 | 1210 ± 510* |
|
140 ± 60 |
Values are reported as mean ± standard deviation.
Statistical significance vs control group or beginning of trial (baseline), P < 0.05.
Abbreviations: ACAR, α-carotene; ATBC, Alpha-Tocopherol, Beta Carotene Prevention Trial; BCAR, β-carotene; CARET, β-Carotene and Retinol Efficacy Trial; LUT, lutein; LYC, lycopene; L/Z, lutein and zeaxanthin.
Figure 2.
(A, B) Summary of foods and amounts as examples of a balanced and recommended diet or, alternatively, a common Western diet rich in calories and fats, to fulfil dietary recommended intake of the 2 reference carotenoids: lycopene and β-carotene, as exemplified by the intake of frequently consumed standardized food items available virtually worldwide. (C) For the tomato juice and carrot juice pictures, 3 coins of international currencies were used: 1 Euro, 25 US cents, and 1 Russian ruble, shown exemplified on millimeter-grid paper as an extra figure. A standard liquor glass (40 ml) and a half litre beer tankard (500 ml) were used as a standard measure for the liquid display. Abbreviations: CH, carbohydrates; USDA, US Department of Agriculture.
Because carotenoids usually exhibit a large interindividual variability in their bioavailability,164 depending on several host-related factors,21 establishing recommended daily allowances for carotenoids on the basis of the relationship between carotenoid intake and their blood or tissue concentrations is sensible at the population level. An important aspect will be to refine our knowledge about the individual determinants of carotenoid bioavailability, further metabolism and carotenoid-metabolite and retinoid-mediated signaling and resulting omics-based disease-marker expression in target tissues to provide possibly more personalized dietary recommendations based on individual characteristics (eg, sex, age, disease state, genetics, eating behavior).
Carotenoid intake recommendations may have to consider individual carotenoids or at least groups of carotenoids with similar effects (ie, relevant chronic health problems related to the respective carotenoid, and possibly the food matrix and bioavailability aspects) in addition to lifestyle factors such as gastrointestinal diseases, age, sex, or smoking status.21
However, more data, especially including accepted health and disease markers and including novel omics approaches, including lipidomics, transcriptomics, and proteomics, regarding any potential adverse effects, are needed to establish a better correlation and additional metabolic links between carotenoid intake, carotenoid status, and chronic disease prevention. Instead of dozens of individual studies focusing on selected aspects of the carotenoid-signaling pathway, a highly concerted action is advised involving a critical mass of competent groups in single- or multi-centered human supplementation trial focusing on low, average, and high carotenoid supplementation and targeting multiple relevant end points.
As a final simple and general take-home message regarding carotenoid intake, the following is suggested: Consumption of a large variety of food items is encouraged* especially combining several green-yellowish, yellow, orange, or pink-red food items, indicating a variety of carotenoids in food, with not too little** or too much# of each single food component&$.
*“food items” include naturally generated carotenoids and those present in foods, including indirect food fortification via previously supplemented animals, direct fortified human food as well as dietary supplements including fruit/vegetable extract, natural carotenoid extracts or supplements with synthetic carotenoids; **“not to less” is based on sufficient dietary carotenoids calculated to achieve a proposed 1000 nM (1 µM) total plasma carotenoid concentration and #to avoid excess intakes of single carotenoids >30 mg/d or more; &for smokers carotenoid supplements should not be recommended; $chronic inflammatory diseases are associated with higher carotenoid targeted or untargeted utilization or carotenoid excretion, these are not included in this general recommendation.
Acknowledgments
This article is based on work from the European Cooperation in Science and Technology (COST) Action CA15136, European Network to Advance Carotenoid Research and Applications in Agro-Food and Health (www.eurocaroten.eu), supported by COST. Opinions contained herein are those of the authors and do not necessarily represent the views of any institutions.
Author contributions. T.B. and R.R. proposed and drafted the concept of the publication and contributed to writing the manuscript. V.B., G.L., B.O.A., D.P., and E.R. contributed to writing the manuscript. D.B., P.B., J.C.R., A.R.L., C.D., J.D.L., J.F.L., I.M., J.N., M.P., P.R., J.M.R., E.V., A.W., and B.M.W.R. contributed to writing the manuscript and worked on tables. All authors read and agreed on the final version.
Funding. This research received support from the EU Cooperation in Science and Technology Action CA15136 EUROCAROTEN. Support was also received from the Instituto de Salud Carlos III (grant PI16/01991), co-funded by ERDF/European Social Fund.
Declaration of interest. The authors declare no conflict of interest.
References
- 1.Britton G, Liaaen-JEnsen S, Pfander H. Carotenoids handbook. Compiled by: Mercadante AZ; Egeland ES. Basel: Birkhäuser; 2004. [Google Scholar]
- 2.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids. Volume 5: Nutrition and Health. Basel: Birkhäuser; 2009. [Google Scholar]
- 3. Sommerburg O, Keunen JE, Bird AC, et al. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Brit J Ophthalmol. 1998;82:907–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamer M, Chida Y.. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens. 2007;25:2361–2369. [DOI] [PubMed] [Google Scholar]
- 5. Leermakers ET, Darweesh SK, Baena CP, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103:481–494. [DOI] [PubMed] [Google Scholar]
- 6. Li X, Xu J.. Dietary and circulating lycopene and stroke risk: a meta-analysis of prospective studies. Sci Rep. 2015;4:5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ge XX, Xing MY, Yu LF, Shen P.. Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:1911–1918. [DOI] [PubMed] [Google Scholar]
- 8. Zhou Y, Wang T, Meng Q, et al. Association of carotenoids with risk of gastric cancer: a meta-analysis. Clin Nutr. 2016;35:109–116. [DOI] [PubMed] [Google Scholar]
- 9. Buijsse B, Feskens EJ, Schlettwein-Gsell D, et al. Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: The Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am J Clin Nutr. 2005;82:879–886. [DOI] [PubMed] [Google Scholar]
- 10. Zhao LG, Zhang QL, Zheng JL, et al. Dietary, circulating beta-carotene and risk of all-cause mortality: a meta-analysis from prospective studies. Sci Rep. 2016;6:26983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shardell MD, Alley DE, Hicks GE, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res. 2011;31:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donaldson MS. A carotenoid health index based on plasma carotenoids and health outcomes. Nutrients 2011;3:1003–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Delaimy WK, Ferrari P, Slimani N, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. 2005;59:1387–1396. [DOI] [PubMed] [Google Scholar]
- 14. Krinsky NI, Yeum KJ.. Carotenoid-radical interactions. Biochem Biophys Res Commun. 2003;305:754–760. [DOI] [PubMed] [Google Scholar]
- 15. Bohn T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: implications for chronic diseases. Antioxidants (Basel, Switzerland). 2019;8: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erdman JW Jr, Ford NA, Lindshield BL.. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys. 2009;483:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaulmann A, Bohn T.. Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34:907–929. [DOI] [PubMed] [Google Scholar]
- 18. Olson JA. Provitamin A function of carotenoids: the conversion of beta-carotene into vitamin A. J Nutr. 1989;119:105–108. [DOI] [PubMed] [Google Scholar]
- 19. Wang XD, Krinsky NI.. Identification and quantification of retinoic acid and other metabolites from beta-carotene excentric cleavage in human intestine in vitro and ferret intestine in vivo. Methods Enzymol. 1997;282:117–130. [DOI] [PubMed] [Google Scholar]
- 20. van Vliet T, van Schaik F, Schreurs WH, et al. In vitro measurement of beta-carotene cleavage activity: methodological considerations and the effect of other carotenoids on beta-carotene cleavage. Int J Vitam Nutr Res. 1996;66:77–85. [PubMed] [Google Scholar]
- 21. Bohn T, Desmarchelier C, Dragsted LO, et al. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res. 2017;61; doi:10.1002/mnfr.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. [DOI] [PubMed] [Google Scholar]
- 24. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: Institute of Medicine; 2000. [Google Scholar]
- 26. Wang XD, Liu C, Bronson RT, et al. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. [DOI] [PubMed] [Google Scholar]
- 27. Goralczyk R. Beta-carotene and lung cancer in smokers: review of hypotheses and status of research. Nutr Cancer. 2009;61:767–774. [DOI] [PubMed] [Google Scholar]
- 28. Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. [DOI] [PubMed] [Google Scholar]
- 29.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23–33.12114037 [Google Scholar]
- 30. Blot WJ, Li JY, Taylor PR, et al. The Linxian trials: mortality rates by vitamin-mineral intervention group. Am J Clin Nutr. 1995;62:1424s–1426s. [DOI] [PubMed] [Google Scholar]
- 31. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;Cd007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am J Clin Nutr. 2010;91:1468S–1473S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Expert Group on Vitamins and Minerals. Safe Upper Levels for Vitamins and Minerals. London: Food Standards Agency; 2003.
- 34. Müller H. Daily intake of carotenoids (carotenes and xanthophylls) from total diet and the carotenoid content of selected vegetables and fruit. Z Ernahrungswiss. 1996;35:45–50. [DOI] [PubMed] [Google Scholar]
- 35. Jenab M, Salvini S, van Gils CH, et al. Dietary intakes of retinol, [beta]-carotene, vitamin D and vitamin E in the European Prospective Investigation into Cancer and Nutrition cohort. Eur J Clin Nutr. 2009;63:S150–S178. [DOI] [PubMed] [Google Scholar]
- 36. Buhr G, Bales CW.. Nutritional supplements for older adults: review and recommendations-part I. J Nutr Elder. 2009;28:5–29. [DOI] [PubMed] [Google Scholar]
- 37. Skeie G, Braaten T, Hjartaker A, et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur J Clin Nutr. 2009;63 suppl 4:S226–238. [DOI] [PubMed] [Google Scholar]
- 38. Mares J. Lutein and zeaxanthin isomers in eye health and disease. Annu Rev Nutr. 2016;36:571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang YM, Dou HL, Huang FF, et al. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed Res Int. 2015;2015:564738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference Release 28. Washington DC: US Department of Agriculture. http://ndb.nal.usda.gov/ndb/foods/show. Accessed November 05, 20152015.
- 41. Bohn T. Metabolic fate of bioaccessible and non-bioaccessible carotenoids. In: Preedy V, ed. Vitamin A and Carotenoids: Chemistry, Analysis, Function and Effects (Food and Nutritional Components in Focus). London: RSC Publishing; 2012. [Google Scholar]
- 42. Borel P. Factors affecting intestinal absorption of highly lipophilic food microconstituents (fat-soluble vitamins, carotenoids and phytosterols). Clin Chem Lab Med. 2003;41:979–994. [DOI] [PubMed] [Google Scholar]
- 43. Borel P, Desmarchelier C.. Bioavailability of fat-soluble vitamins and phytochemicals in humans: effects of genetic variation. Annu Rev Nutr. 2018;38:69–96. [DOI] [PubMed] [Google Scholar]
- 44. Borel P, Desmarchelier C, Nowicki M, et al. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic Biol Med. 2015;83:238–244. [DOI] [PubMed] [Google Scholar]
- 45. Borel P, Desmarchelier C, Nowicki M, et al. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary beta-carotene bioavailability in healthy men. J Nutr. 2015;145:1740–1747. [DOI] [PubMed] [Google Scholar]
- 46. Borel P, Desmarchelier C, Nowicki M, et al. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr. 2014;100:168–175. [DOI] [PubMed] [Google Scholar]
- 47.Scientific Committee for Food. Nutrient and Energy Intakes for the European Community. Reports of the Scientific Committee for Food (31st Series). Luxembourg: European Commission, 1993.
- 48.Food and Agriculture Organization of the United Nations. Requirements of Vitamin A, Iron, Folate and Vitamin B12. Report of a Joint FAO/WHO Expert Consultation FAO Food and Nutrition Series, No. 23. Rome: Food and Agriculture Organization of the United Nations, 1988.
- 49.World Health Organization, Food and Agriculture Organization of the United Nations). Vitamin and Mineral Requirements in Human Nutrition: report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21-30 September 1998. Rome: Food and Agriculture Organization of the United Nations, 2004.
- 50. Parker RS, Swanson JE, You CS, et al. Bioavailability of carotenoids in human subjects. Proc Nutr Soc. 1999;58:155–162. [DOI] [PubMed] [Google Scholar]
- 51. van het Hof KH, Brouwer IA, West CE, et al. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. Am J Clin Nutr. 1999;70:261–268. [DOI] [PubMed] [Google Scholar]
- 52. Bauernfeind JC. Carotenoid vitamin A precursors and analogs in foods and feeds. J Agric Food Chem. 1972;20:456–473. [DOI] [PubMed] [Google Scholar]
- 53. Deuel HJ Jr, Greenberg SM, Straub E, et al. Stereochemical configuration and provitamin A activity. 7. Neocryptoxanthin U. Arch Biochem. 1949;23:239–241. [PubMed] [Google Scholar]
- 54. Sauberlich HE, Hodges RE, Wallace DL, et al. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm. 1974;32:251–275. [DOI] [PubMed] [Google Scholar]
- 55.Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: Institute of Medicine; 2001.
- 56. West CE, Eilander A, van LM.. Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. J Nutr. 2002;132:2920S–2926S. [DOI] [PubMed] [Google Scholar]
- 57.German Nutrition Society (DGE), Swiss Society for Nutrition (SGE), Austrian Nutrition Society (ÖGE). D-A-CH Referenzwerte für die Nährstoffzufuhr. Frankfurt: Deutsche Gesellschaft Für Ernährung; 2013. [Google Scholar]
- 58.Società Italiana di Nutrizione Umana (SINU). LARN - Livelli di Assunzione di Riferimento di Nutrienti ed energia per la Popolazione Italiana. Milan: Società Italiana di Nutrizione Umana, 2012.
- 59.Nordic Council of Ministers. Nordic Nutrition Recommendations 2012, Norden, Copenhagen, 2014.
- 60.National Food Institute, Research Group for Risk Benefit. EFSA Panel on Dietetic Products N, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for vitamin A. No. 4028. EFSA J. 2015;15.
- 61. Grune T, Lietz G, Palou A, et al. Beta-carotene is an important vitamin A source for humans. J Nutr. 2010;140:2268s–2285s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim YK, Quadro L.. Who needs β-carotene? A focus on embryonic development. J Nutr Food Sci. 2012;2:1000e113. [Google Scholar]
- 63. El-Sohemy A, Baylin A, Kabagambe E, et al. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr. 2002;76:172–179. [DOI] [PubMed] [Google Scholar]
- 64. O’Neill ME, Carroll Y, Corridan B, et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br J Nutr. 2001;85:499–507. [DOI] [PubMed] [Google Scholar]
- 65. Olmedilla B, Granado F, Southon S, et al. A European multicentre, placebo-controlled supplementation study with alpha-tocopherol, carotene-rich palm oil, lutein or lycopene: analysis of serum responses. Clin Sci (Lond). 2002;102:447–456. [PubMed] [Google Scholar]
- 66. Carrol YL, Corridan BM, Morrissey PA.. Carotenoids in young and elderly healthy humans: dietary intakes, biochemical status and diet-plasma relationships. Eur J Clin Nutr. 1999;53:644–653. [DOI] [PubMed] [Google Scholar]
- 67. Lucarini M, Lanzi S, D’Evoli L, et al. Intake of vitamin A and carotenoids from the Italian population–results of an Italian total diet study. Int J Vitam Nutr Res. 2006;76:103–109. [DOI] [PubMed] [Google Scholar]
- 68. Sette S, Le Donne C, Piccinelli R, et al. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06–Part 1: nutrient intakes in Italy. Nutr Metab Cardiovasc Dis. 2011;21:922–932. [DOI] [PubMed] [Google Scholar]
- 69. Biehler E, Alkerwi A, Hoffmann L, et al. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: assessment in Luxembourg. J Food Comp Anal. 2012;25:56–65. [Google Scholar]
- 70. Olmedilla-Alonso B, Beltran-de-Miguel B, Estevez-Santiago R, et al. Markers of lutein and zeaxanthin status in two age groups of men and women: dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr J. 2014;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beltran-de-Miguel B, Estevez-Santiago R, Olmedilla-Alonso B.. Assessment of dietary vitamin A intake (retinol, alpha-carotene, beta-carotene, beta-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009-2010). Int J Food Sci Nutr. 2015;66:706–712. [DOI] [PubMed] [Google Scholar]
- 72. Estevez-Santiago R, Beltran-de-Miguel B, Olmedilla-Alonso B.. Assessment of dietary lutein, zeaxanthin and lycopene intakes and sources in the Spanish survey of dietary intake (2009-2010). Int J Food Sci Nutr. 2016;67:305–313. [DOI] [PubMed] [Google Scholar]
- 73. Wawrzyniak A, Hamułka J, Friberg E, et al. Dietary, anthropometric, and lifestyle correlates of serum carotenoids in postmenopausal women. Eur J Nutr. 2013;52:1919–1926. [DOI] [PubMed] [Google Scholar]
- 74. Pezdirc K, Hutchesson MJ, Williams RL, et al. Consuming high-carotenoid fruit and vegetables influences skin yellowness and plasma carotenoids in young women: a single-blind randomized crossover trial. J Acad Nutr Diet.2016;116:1257–1265. [DOI] [PubMed] [Google Scholar]
- 75. Yong LC, Forman MR, Beecher GR, et al. Relationship between dietary intake and plasma concentrations of carotenoids in premenopausal women: application of the USDA-NCI carotenoid food-composition database. Am J Clin Nutr. 1994;60:223–230. [DOI] [PubMed] [Google Scholar]
- 76. Tucker KL, Chen H, Vogel S, et al. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J Nutr. 1999;129:438–445. [DOI] [PubMed] [Google Scholar]
- 77. Curran-Celentano J, Hammond BR Jr, Ciulla TA, et al. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001;74:796–802., [DOI] [PubMed] [Google Scholar]
- 78. Tangney CC, Bienias JL, Evans DA, et al. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004;134:927–934. [DOI] [PubMed] [Google Scholar]
- 79. Burke JD, Curran-Celentano J, Wenzel AJ.. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. J Nutr. 2005;135:1208–1214. [DOI] [PubMed] [Google Scholar]
- 80. Talegawkar SA, Johnson EJ, Carithers TC, et al. Carotenoid intakes, assessed by food-frequency questionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: validation of the Jackson Heart Study Delta NIRI Adult FFQs. Public Health Nutr. 2008;11:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. George SM, Thompson FE, Midthune D, et al. Strength of the relationships between three self-reported dietary intake instruments and serum carotenoids: The Observing Energy and Protein Nutrition (OPEN) Study. Public Health Nutr. 2012;15:1000–1007. [DOI] [PubMed] [Google Scholar]
- 82. Fraser GE, Jaceldo-Siegl K, Henning SM, et al. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr. 2016;146:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: beta-carotene bioavailability and conversion–evidence in humans. Am J Clin Nutr. 2012;96:1193s–1203s. [DOI] [PubMed] [Google Scholar]
- 84. Van Loo-Bouwman CA, Naber TH, Schaafsma G.. A review of vitamin A equivalency of beta-carotene in various food matrices for human consumption. Br J Nutr. 2014;111:2153–2166. [DOI] [PubMed] [Google Scholar]
- 85. Lietz G, Oxley A, Boesch-Saadatmandi C, et al. Importance of beta, beta-carotene 15,15’-monooxygenase 1 (BCMO1) and beta, beta-carotene 9’,10’-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012;56:241–250. [DOI] [PubMed] [Google Scholar]
- 86. von Lintig J. Provitamin A metabolism and functions in mammalian biology. Am J Clin Nutr. 2012;96:1234s–1244s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res. 2012;56:228–240. [DOI] [PubMed] [Google Scholar]
- 88. de Pee S, West CE.. Dietary carotenoids and their role in combating vitamin A deficiency: a review of the literature. Eur J Clin Nutr. 1996;50(suppl 3):S38–S53. [PubMed] [Google Scholar]
- 89. Cooperstone JL, Goetz HJ, Riedl KM, et al. Relative contribution of alpha-carotene to postprandial vitamin A concentrations in healthy humans after carrot consumption. Am J Clin Nutr. 2017;106:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Burri BJ. Beta-cryptoxanthin as a source of vitamin A. J Sci Food Agric. 2015;95:1786–1794. [DOI] [PubMed] [Google Scholar]
- 91. de Pee S, West CE, Permaesih D, et al. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 1998;68:1058–1067. [DOI] [PubMed] [Google Scholar]
- 92. Estevez-Santiago R, Olmedilla-Alonso B, Fernandez-Jalao I.. Bioaccessibility of provitamin A carotenoids from fruits: application of a standardised static in vitro digestion method. Food Funct. 2016;7:1354–1366. [DOI] [PubMed] [Google Scholar]
- 93. Burri BJ, La Frano MR, Zhu C.. Absorption, metabolism, and functions of beta-cryptoxanthin. Nutr Rev. 2016;74:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Carpenter K, Harper A. Evolution of knowledge of essential nutrients. In: Shils M, Olson J, Shike M, Ross A, eds. Modern Nutrition in Health and Disease. Baltimore: Williams and Wilkins; 2006:3–9. [Google Scholar]
- 95. Johnson EJ, Neuringer M, Russell RM, et al. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. [DOI] [PubMed] [Google Scholar]
- 96. Franke S, Fröhlich K, Werner S, et al. Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. Eur J Lipid Sci Technol. 2010;112:1122–1129. [Google Scholar]
- 97. Stringham JM, Stringham NT.. Serum and retinal responses to three different doses of macular carotenoids over 12 weeks of supplementation. Exp Eye Res. 2016;151:1–8. [DOI] [PubMed] [Google Scholar]
- 98. Granado F, Olmedilla B, Blanco I.. Nutritional and clinical relevance of lutein in human health. Br J Nutr. 2003;90:487–502. [DOI] [PubMed] [Google Scholar]
- 99. Ranard KM, Jeon S, Mohn ES, et al. Dietary guidance for lutein: consideration for intake recommendations is scientifically supported. Eur J Nutr. 2017;56:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Olmedilla B, Granado F, Blanco I, et al. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19:21–24. [DOI] [PubMed] [Google Scholar]
- 101. Dwyer JH, Navab M, Dwyer KM, et al. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles Atherosclerosis Study. Circulation. 2001;103:2922–2927. [DOI] [PubMed] [Google Scholar]
- 102. Shao A, Hathcock JN.. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol. 2006;45:289–298. [DOI] [PubMed] [Google Scholar]
- 103.European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the safety of astaxanthin-rich ingredients (AstaREAL A1010 and AstaREAL L10) as novel food ingredients. EFSA J. 2014;12:3757. [Google Scholar]
- 104.European Food Safety Authority. Re-evaluation of β-apo-8′-carotenal (E 160e) as a food additive. EFSA J. 2012;10:2499–2545. [Google Scholar]
- 105.European Food Safety Authority. Statement on the safety of β-carotene use in heavy smokers. EFSA J. 2012;10:2953. [Google Scholar]
- 106.European Food Safety Authority. Scientific opinion on the re-evaluation of mixed carotenes (E 160a (i)) and beta-carotene (E 160a (ii)) as a food additive. EFSA J. 2012;10:2593. [Google Scholar]
- 107.European Food Safety Authority. Scientific opinion on the re-evaluation of canthaxanthin (E 161 g) as a food additive. EFSA J. 2010;8:1852. [Google Scholar]
- 108.European Food Safety Authority. Scientific opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010;8:1678. [Google Scholar]
- 109.European Food Safety Authority. Revised exposure assessment for lycopene as a food colour. EFSA J. 2010;8:1444. [Google Scholar]
- 110.European Food Safety Authority. Scientific opinion: statement on the safety of synthetic zeaxanthin as an ingredient in food supplements. EFSA J. 2012;10:2891. [Google Scholar]
- 111. Maharshak N, Shapiro J, Trau H.. Carotenoderma–a review of the current literature. Int J Dermatol. 2003;42:178–181. [DOI] [PubMed] [Google Scholar]
- 112. Porrini M, Riso P.. What are typical lycopene intakes? J Nutr. 2005;135:2042s–2045s. [DOI] [PubMed] [Google Scholar]
- 113. Ferrari P, Al-Delaimy WK, Slimani N, et al. An approach to estimate between- and within-group correlation coefficients in multicenter studies: plasma carotenoids as biomarkers of intake of fruits and vegetables. Am J Epidemiol. 2005;162:591–598. [DOI] [PubMed] [Google Scholar]
- 114. Olmedilla B, Granado F, Blanco I, et al. Determination of nine carotenoids, retinols, retinyl palmitate and alpha-tocopherol in control human serum using two internal standards. Food Chem. 1992;45:205–213. [Google Scholar]
- 115. Olmedilla B, Granado F, Blanco I, et al. Seasonal and sex-related variations in six serum carotenoids, retinol, and alpha-tocopherol. Am J Clin Nutr. 1994;60:106–110. [DOI] [PubMed] [Google Scholar]
- 116. Olmedilla B, Granado F, Gil-Martinez E, et al. Reference values for retinol, tocopherol, and main carotenoids in serum of control and insulin-dependent diabetic Spanish subjects. Clin Chem. 1997;43:1066–1071. [PubMed] [Google Scholar]
- 117. Forman MR, Johnson EJ, Lanza E, et al. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: a controlled dietary study. Am J Clin Nutr. 1998;67:81–87. [DOI] [PubMed] [Google Scholar]
- 118. Clark PE, Hall MC, Borden LS Jr, et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–1261., [DOI] [PubMed] [Google Scholar]
- 119. Riso P, Pinder A, Santangelo A, et al. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? Am J Clin Nutr. 1999;69:712–718. [DOI] [PubMed] [Google Scholar]
- 120. Porrini M, Riso P.. Lymphocyte lycopene concentration and DNA protection from oxidative damage is increased in women after a short period of tomato consumption. J Nutr. 2000;130:189–192. [DOI] [PubMed] [Google Scholar]
- 121. Rosenthal JM, Kim J, de Monasterio F, et al. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Invest Ophthalmol Vis Sci. 2006;47:5227–5233. [DOI] [PubMed] [Google Scholar]
- 122. Macdonald HM, Hardcastle AC, Duthie GG, et al. Changes in vitamin biomarkers during a 2-year intervention trial involving increased fruit and vegetable consumption by free-living volunteers. Br J Nutr. 2009;102:1477–1486. [DOI] [PubMed] [Google Scholar]
- 123. Mathews-Roth MM. Plasma concentrations of carotenoids after large doses of beta-carotene. Am J Clin Nutr. 1990;52:500–501. [DOI] [PubMed] [Google Scholar]
- 124. Kraemer K, Krennrich G, Obermüller-Jevic U, et al. Blood response to beta-carotene supplementation in humans: An evaluation oacross published studies. In: Lester Packer KK, Ute Obermueller-Jevic Helmut Sies, eds. Carotenoids and Retinoids; Molecular Aspects and Health Issues. Champaign, IL: AOCS Press; 2005. [Google Scholar]
- 125. Wu K, Schwartz SJ, Platz EA, et al. Variations in plasma lycopene and specific isomers over time in a cohort of U.S. men. J Nutr. 2003;133:1930–1936. [DOI] [PubMed] [Google Scholar]
- 126. Palli D, Decarli A, Russo A, et al. Plasma levels of antioxidant vitamins and cholesterol in a large population sample in central-northern Italy. Eur J Nutr. 1999;38:90–98. [DOI] [PubMed] [Google Scholar]
- 127. Grolier P, Boirie Y, Levadoux E, et al. Age-related changes in plasma lycopene concentrations, but not in vitamin E, are associated with fat mass. Br J Nutr. 2000;84:711–716. [PubMed] [Google Scholar]
- 128. Hodge A, Cunningham J, Maple-Brown L, et al. Plasma carotenoids are associated with socioeconomic status in an urban Indigenous population: an observational study. BMC Public Health. 2011;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Anlasik T, Sies H, Griffiths HR, et al. Dietary habits are major determinants of the plasma antioxidant status in healthy elderly subjects. Br J Nutr. 2005;94:639–642. [DOI] [PubMed] [Google Scholar]
- 130. Cardinault N, Tyssandier V, Grolier P, et al. Comparison of the postprandial chylomicron carotenoid responses in young and older subjects. Eur J Nutr. 2003;42:315–323. [DOI] [PubMed] [Google Scholar]
- 131. Mayen AL, Marques-Vidal P, Paccaud F, et al. Socioeconomic determinants of dietary patterns in low- and middle-income countries: a systematic review. Am J Clin Nutr. 2014;100:1520–1531. [DOI] [PubMed] [Google Scholar]
- 132. Rust P, Lehner P, Elmadfa I.. Relationship between dietary intake, antioxidant status and smoking habits in female Austrian smokers. Eur J Nutr. 2001;40:78–83. [DOI] [PubMed] [Google Scholar]
- 133. Wei W, Kim Y, Boudreau N.. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am J Public Health. 2001;91:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Trobs M, Renner T, Scherer G, et al. Nutrition, antioxidants, and risk factor profile of nonsmokers, passive smokers and smokers of the Prevention Education Program (PEP) in Nuremberg, Germany. Prev Med. 2002;34:600–607. [DOI] [PubMed] [Google Scholar]
- 135. Albanes D, Virtamo J, Taylor PR, et al. Effects of supplemental beta-carotene, cigarette smoking, and alcohol consumption on serum carotenoids in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1997;66:366–372. [DOI] [PubMed] [Google Scholar]
- 136. Sugiura M, Nakamura M, Ogawa K, et al. Associations of serum carotenoid concentrations with the metabolic syndrome: interaction with smoking. Br J Nutr. 2008;100:1297–1306. [DOI] [PubMed] [Google Scholar]
- 137. Hozawa A, Jacobs DR Jr, Steffes MW, et al. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2006;163:929–937. [DOI] [PubMed] [Google Scholar]
- 138. McClure JB, Divine G, Alexander G, et al. A comparison of smokers’ and nonsmokers’ fruit and vegetable intake and relevant psychosocial factors. Behav Med. 2009;35:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Walmsley CM, Bates CJ, Prentice A, et al. Relationship between cigarette smoking and nutrient intakes and blood status indices of older people living in the UK: further analysis of data from the National Diet and Nutrition Survey of people aged 65 years and over, 1994/95. Public Health Nutr. 1999;2:199–208. [DOI] [PubMed] [Google Scholar]
- 140. Brady WE, Mares-Perlman JA, Bowen P, et al. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–137. [DOI] [PubMed] [Google Scholar]
- 141. Lecomte E, Grolier P, Herbeth B, et al. The relation of alcohol consumption to serum carotenoid and retinol levels. Effects of withdrawal. Int J Vitam Nutr Res. 1994;64:170–175. [PubMed] [Google Scholar]
- 142. Mayne ST, Cartmel B, Silva F, et al. Plasma lycopene concentrations in humans are determined by lycopene intake, plasma cholesterol concentrations and selected demographic factors. J Nutr. 1999;129:849–854. [DOI] [PubMed] [Google Scholar]
- 143. Lyle BJ, Mares-Perlman JA, Klein BE, et al. Serum carotenoids and tocopherols and incidence of age-related nuclear cataract. Am J Clin Nutr. 1999;69:272–277. [DOI] [PubMed] [Google Scholar]
- 144. Zhou YE, Buchowski MS, Liu J, et al. Plasma lycopene is associated with pizza and pasta consumption in middle-aged and older African American and white adults in the southeastern USA in a cross-sectional study. PLoS One. 2016;11:e0161918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Herbeth B, Samara A, Stathopoulou M, et al. Alcohol consumption, beverage preference, and diet in middle-aged men from the STANISLAS study. J Nutr Metab. 2012;2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kaplan LA, Lau JM, Stein EA.. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem. 1990;8:1–10. [PubMed] [Google Scholar]
- 147. Schmitz HH, Poor CL, Wellman RB, et al. Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr. 1991;121:1613–1621. [DOI] [PubMed] [Google Scholar]
- 148. Stahl W, Schwarz W, Sundquist AR, et al. cis-trans Isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem Biophys. 1992;294:173–177. [DOI] [PubMed] [Google Scholar]
- 149. Tanumihardjo SA, Furr HC, Amedee-Manesme O, et al. Retinyl ester (vitamin A ester) and carotenoid composition in human liver. Int J Vitam Nutr Res. 1990;60:307–313. [PubMed] [Google Scholar]
- 150. Al-Delaimy WK, Slimani N, Ferrari P, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: ecological-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. 2005;59:1397–1408. [DOI] [PubMed] [Google Scholar]
- 151. Schierle J, Bretzel W, Bühler I, et al. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997;59:459–465. [Google Scholar]
- 152. Fröhlich K. Lycopin-Isomere in Lebensmitteln und Humanplasma - Strukturaufklärung, antioxidative Aktivität, Gehalte und relative (E)-(Z)-VerhältnisseFriedrich-Schiller University [PhD Thesis]. Germany: Universität Jena; 2007.
- 153. Chung HY, Ferreira AL, Epstein S, et al. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. Am J Clin Nutr. 2009;90:533–539. [DOI] [PubMed] [Google Scholar]
- 154. Alaluf S, Heinrich U, Stahl W, et al. Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr. 2002;132:399–403. [DOI] [PubMed] [Google Scholar]
- 155. Ermakov IV, Ermakova MR, Rosenberg TD, et al. Optical detection of carotenoid antioxidants in human bone and surrounding tissue. J Biomed Opt. 2013;18:117006. [DOI] [PubMed] [Google Scholar]
- 156. Ermakov IV, Sharifzadeh M, Ermakova M, et al. Resonance Raman detection of carotenoid antioxidants in living human tissue. J Biomed Opt. 2005;10:064028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Vishwanathan R, Kuchan MJ, Sen S, et al. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr. 2014;59:659–665. [DOI] [PubMed] [Google Scholar]
- 158. Czeczuga-Semeniuk E, Wołczyński S, Markiewicz W.. Preliminary identification of carotenoids in malignant and benign neoplasms of the breast and surrounding fatty tissue. Neoplasma. 2003;50:280–286. [PubMed] [Google Scholar]
- 159. Pappalardo G, Maiani G, Mobarhan S, et al. Plasma (carotenoids, retinol, alpha-tocopherol) and tissue (carotenoids) levels after supplementation with beta-carotene in subjects with precancerous and cancerous lesions of sigmoid colon. Eur J Clin Nutr. 1997;51:661–666. [DOI] [PubMed] [Google Scholar]
- 160. Gossage CP, Deyhim M, Yamini S, et al. Carotenoid composition of human milk during the first month postpartum and the response to beta-carotene supplementation. Am J Clin Nutr. 2002;76:193–197. [DOI] [PubMed] [Google Scholar]
- 161. Czeczuga-Semeniuk E, Wolczynski S.. Dietary carotenoids in normal and pathological tissues of corpus uteri. Folia Histochem Cytobiol. 2008;46:283–290. [DOI] [PubMed] [Google Scholar]
- 162. Clinton SK, Emenhiser C, Schwartz SJ, et al. cis-trans Lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- 163. Rapp LM, Maple SS, Choi JH.. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41:1200–1209. [PubMed] [Google Scholar]
- 164. Desmarchelier C, Borel P.. Overview of carotenoid bioavailability determinants: from dietary factors to host genetic variations. Trends Food Sci Technol. 2017; 69:270–280. [Google Scholar]
- 165. Palan PR, Mikhail M, Romney SL.. Decreased beta-carotene tissue levels in uterine leiomyomas and cancers of reproductive and nonreproductive organs. Am J Obstet Gynecol. 1989;161:1649–1652. [DOI] [PubMed] [Google Scholar]
- 166. Moran NE, Novotny JA, Cichon MJ, et al. Absorption and distribution kinetics of the 13C-labeled tomato carotenoid phytoene in healthy adults. J Nutr. 2016;146:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Leo MA, Rosman AS, Lieber CS.. Differential depletion of carotenoids and tocopherol in liver disease. Hepatology. 1993;17:977–986. [PubMed] [Google Scholar]
- 168. Peirce AW. Carotene and vitamin A in human fat. Med J Aust. 1954;41:589. [DOI] [PubMed] [Google Scholar]
- 169. Virtanen SM, van't Veer P, Kok F, et al. Predictors of adipose tissue carotenoid and retinol levels in nine countries. The EURAMIC study. Am J Epidemiol. 1996;144:968–979. [DOI] [PubMed] [Google Scholar]
- 170. Parker RS. Carotenoids in human blood and tissues. J Nutr. 1989;119:101–104. [DOI] [PubMed] [Google Scholar]
- 171. Johnson EJ, Suter PM, Sahyoun N, et al. Relation between beta-carotene intake and plasma and adipose tissue concentrations of carotenoids and retinoids. Am J Clin Nutr. 1995;62:598–603. [DOI] [PubMed] [Google Scholar]
- 172. Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr. 1988;47:33–36. [DOI] [PubMed] [Google Scholar]
- 173. Wallstrom P, Wirfalt E, Lahmann PH, et al. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr. 2001;73:777–785. [DOI] [PubMed] [Google Scholar]
- 174. Andersen LF, Jacobs DR Jr, Gross MD, et al. Longitudinal associations between body mass index and serum carotenoids: The CARDIA study. Br J Nutr. 2006;95:358–365. [DOI] [PubMed] [Google Scholar]
- 175. Kabagambe EK, Baylin A, Allan DA, et al. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol. 2001;154:1126–1135. [DOI] [PubMed] [Google Scholar]
- 176. Kardinaal AF, van’t Veer P, Brants HA, et al. Relations between antioxidant vitamins in adipose tissue, plasma, and diet. Am J Epidemiol. 1995;141:440–450. [DOI] [PubMed] [Google Scholar]
- 177. Su LC, Bui M, Kardinaal A, et al. Differences between plasma and adipose tissue biomarkers of carotenoids and tocopherols. Cancer Epidemiol Biomarkers Prev. 1998;7:1043–1048. [PubMed] [Google Scholar]
- 178. Yeum KJ, Booth SL, Roubenoff R, et al. Plasma carotenoid concentrations are inversely correlated with fat mass in older women. J Nutr Health Aging. 1998;2:79–83. [PubMed] [Google Scholar]
- 179. Osth M, Ost A, Kjolhede P, et al. The concentration of beta-carotene in human adipocytes, but not the whole-body adipocyte stores, is reduced in obesity. PLoS One.2014;9:e85610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Broekmans WM, Berendschot TT, Klopping-Ketelaars IA, et al. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr. 2002;76:595–603. [DOI] [PubMed] [Google Scholar]
- 181. Kirby ML, Beatty S, Stack J, et al. Changes in macular pigment optical density and serum concentrations of lutein and zeaxanthin in response to weight loss. Br J Nutr. 2011;105:1036–1046. [DOI] [PubMed] [Google Scholar]
- 182. Granado-Lorencio F, Herrero-Barbudo C, Olmedilla-Alonso B, et al. Hypocarotenemia after bariatric surgery: a preliminary study. Obes Surg. 2009;19:879–882. [DOI] [PubMed] [Google Scholar]
- 183. Diwadkar-Navsariwala V, Novotny JA, Gustin DM, et al. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res. 2003;44:1927–1939. [DOI] [PubMed] [Google Scholar]
- 184. Sy C, Gleize B, Dangles O, et al. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood and tissue concentrations. Mol Nutr Food Res. 2012;56:1385–1397. [DOI] [PubMed] [Google Scholar]
- 185. Moussa M, Gouranton E, Gleize B, et al. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol Nutr Food Res. 2011;55:578–584. [DOI] [PubMed] [Google Scholar]
- 186. Johnson EJ, Hammond BR, Yeum KJ, et al. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr. 2000;71:1555–1562. [DOI] [PubMed] [Google Scholar]
- 187. Walfisch Y, Walfisch S, Agbaria R, et al. Lycopene in serum, skin and adipose tissues after tomato-oleoresin supplementation in patients undergoing haemorrhoidectomy or peri-anal fistulotomy. Br J Nutr. 2003;90:759–766. [DOI] [PubMed] [Google Scholar]
- 188. Khachik F, Spangler CJ, Smith JC Jr, et al. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69:1873–1881., [DOI] [PubMed] [Google Scholar]
- 189. Johnson EJ, Qin J, Krinsky NI, et al. Beta-carotene isomers in human serum, breast milk and buccal mucosa cells after continuous oral doses of all-trans and 9-cis beta-carotene. J Nutr. 1997;127:1993–1999. [DOI] [PubMed] [Google Scholar]
- 190. Alien CM, Smith AM, Clinton SK, et al. Tomato consumption increases lycopene isomer concentrations in breast milk and plasma of lactating women. J Am Diet Assoc. 2002;102:1257–1262. [DOI] [PubMed] [Google Scholar]
- 191. Rios JJ, Xavier AAO, Diaz-Salido E, et al. Xanthophyll esters are found in human colostrum. Mol Nutr Food Res. 2017;61.doi: 10.1002/mnfr.201700296. [DOI] [PubMed] [Google Scholar]
- 192. Canfield LM, Kaminsky RG, Taren DL, et al. Red palm oil in the maternal diet increases provitamin A carotenoids in breastmilk and serum of the mother-infant dyad. Eur J Nutr. 2001;40:30–38. [DOI] [PubMed] [Google Scholar]
- 193. de Azeredo VB, Trugo NM.. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition. 2008;24:133–139. [DOI] [PubMed] [Google Scholar]
- 194. Song BJ, Jouni ZE, Ferruzzi MG.. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition. 2013;29:195–202. [DOI] [PubMed] [Google Scholar]
- 195. Lipkie TE, Morrow AL, Jouni ZE, et al. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS One. 2015;10: e0127729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lietz G, Mulokozi G, Henry JC, et al. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr. 2006;136:1821–1827. [DOI] [PubMed] [Google Scholar]
- 197. Talegawkar SA, Johnson EJ, Carithers TC, et al. Serum carotenoid and tocopherol concentrations vary by dietary pattern among African Americans. J Am Diet Assoc. 2008;108:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yang M, Wang Y, Davis CG, et al. Validation of an FFQ to assess short-term antioxidant intake against 30 d food records and plasma biomarkers. Public Health Nutr. 2014;17:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Craft NE, Haitema TB, Garnett KM, et al. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8:156–162. [PubMed] [Google Scholar]
- 200. Paetau I, Rao D, Wiley ER, et al. Carotenoids in human buccal mucosa cells after 4 wk of supplementation with tomato juice or lycopene supplements. Am J Clin Nutr. 1999;70:490–494. [DOI] [PubMed] [Google Scholar]
- 201. Gilbert AM, Stich HF, Rosin MP, et al. Variations in the uptake of beta-carotene in the oral mucosa of individuals after 3 days of supplementation. Int J Cancer. 1990;45:855–859. [DOI] [PubMed] [Google Scholar]
- 202. Reboul E, Borel P.. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res. 2011;50:388–402. [DOI] [PubMed] [Google Scholar]
- 203. Reifen R, Haftel L, Faulks R, et al. Plasma and buccal mucosal cell response to short-term supplementation with all trans-beta-carotene and lycopene in human volunteers. Int J Mol Med. 2003;12:989–993. [PubMed] [Google Scholar]
- 204. Gollnick HP, Siebenwirth C.. Beta-carotene plasma levels and content in oral mucosal epithelium is skin type associated. Skin Pharmacol Physiol. 2002;15:360–366. [DOI] [PubMed] [Google Scholar]
- 205. Gabriel HE, Liu Z, Crott JW, et al. A comparison of carotenoids, retinoids, and tocopherols in the serum and buccal mucosa of chronic cigarette smokers versus nonsmokers. Cancer Epidemiol Biomarkers Prev. 2006;15:993–999. [DOI] [PubMed] [Google Scholar]
- 206. Biesalski HK, Obermueller-Jevic UC.. UV light, beta-carotene and human skin - beneficial and potentially harmful effects. Arch Biochem Biophys. 2001;389:1–6. [DOI] [PubMed] [Google Scholar]
- 207. Bohm F, Edge R, Lange L, et al. Enhanced protection of human cells against ultraviolet light by antioxidant combinations involving dietary carotenoids. J Photochem Photobiol B. 1998;44:211–215. [DOI] [PubMed] [Google Scholar]
- 208. Lee J, Jiang SG, Levine N, et al. Carotenoid supplementation reduces erythema in human skin after simulated solar radiation exposure. Proc Soc Exp Biol Med. 2000;223:170–174. [DOI] [PubMed] [Google Scholar]
- 209. Massenti R, Perrone A, Livrea MA, et al. Regular consumption of fresh orange juice increases human skin carotenoid content. Int J Food Sci Nutr. 2015;66:718–721. [DOI] [PubMed] [Google Scholar]
- 210. Jahns L, Johnson LK, Mayne ST, et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. Am J Clin Nutr. 2014;100:930–937. [DOI] [PubMed] [Google Scholar]
- 211. Aguilar SS, Wengreen HJ, Dew J.. Skin carotenoid response to a high-carotenoid juice in children: a randomized clinical trial. J Acad Nutr Diet. 2015;115:1771–1778. [DOI] [PubMed] [Google Scholar]
- 212. Stahl W, Sies H.. Carotenoids and protection against solar UV radiation. Skin Pharmacol Physiol. 2002;15:291–296. [DOI] [PubMed] [Google Scholar]
- 213. Aust O, Stahl W, Sies H, et al. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int J Vitam Nutr Res. 2005;75:54–60. [DOI] [PubMed] [Google Scholar]
- 214. Czeczuga-Semeniuk E, Wolczynski S.. Identification of carotenoids in ovarian tissue in women. Oncol Rep. 2005;14:1385–1392. [PubMed] [Google Scholar]
- 215. Gamboa-Pinto AJ, Rock CL, Ferruzzi MG, et al. Cervical tissue and plasma concentrations of alpha-carotene and beta-carotene in women are correlated. J Nutr. 1998;128:1933–1936. [DOI] [PubMed] [Google Scholar]
- 216. Czeczuga-Semeniuk E, Wolczynski S.. Does variability in carotenoid composition and concentration in tissues of the breast and reproductive tract in women depend on type of lesion? Adv Med Sci. 2008;53:270–277. [DOI] [PubMed] [Google Scholar]
- 217. Loughman J, Davison PA, Nolan JM, et al. Macular pigment and its contribution to visual performance and experience. J Optom. 2010;3:74–90. [Google Scholar]
- 218. Naguib YMA. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. 2000;48:1150–1154. [DOI] [PubMed] [Google Scholar]
- 219. Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448s–1461s. [DOI] [PubMed] [Google Scholar]
- 220. Snodderly DM, Auran JD, Delori FC.. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–685. [PubMed] [Google Scholar]
- 221. Bone RA, Landrum JT, Fernandez L, et al. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 222.Craft NE. Carotenoid reversed-phase high-performance liquid chromatography methods: reference compendium. Methods Enzymol. 1992;213:185–205. [DOI] [PubMed] [Google Scholar]
- 223. Handelman GJ, Shen B, Krinsky NI.. High resolution analysis of carotenoids in human plasma by high-performance liquid chromatography. Methods Enzymol. 1992;213:336–346. [DOI] [PubMed] [Google Scholar]
- 224. Akuffo KO, Beatty S, Peto T, et al. The impact of supplemental antioxidants on visual function in non-advanced age-related macular degeneration: a head-to-head randomized clinical trial. Invest Opthalmol Vis Sci 2017;58:5347–5360. [DOI] [PubMed] [Google Scholar]
- 225. Crosby-Nwaobi R, Hykin P, Peto T, et al. An exploratory study evaluating the effects of macular carotenoid supplementation in various retinal diseases. Clin Ophthalmol. 2016;10:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Nolan JM, Power R, Stringham J, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: central retinal enrichment supplementation trials - report 1. Invest Ophthalmol Vis Sci. 2016;57:3429–3439. [DOI] [PubMed] [Google Scholar]
- 227. Akuffo KO, Nolan JM, Howard AN, et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye. 2015;29:902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ma L, Liu R, Du JH, et al. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients. 2016;8:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Nolan JM, Akkali MC, Loughman J, et al. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res. 2012;101:9–15. [DOI] [PubMed] [Google Scholar]
- 230. Trieschmann M, Beatty S, Nolan JM, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: The LUNA Study. Exp Eye Res. 2007;84:718–728. [DOI] [PubMed] [Google Scholar]
- 231. Estévez-Santiago R, Olmedilla-Alonso B, Beltrán-de-Miguel B, et al. Lutein and zeaxanthin supplied by red/orange foods and fruits are more closely associated with macular pigment optical density than those from green vegetables in Spanish subjects. Nutr Res. 2016;36:1210–1221. [DOI] [PubMed] [Google Scholar]
- 232. van der Made SM, Kelly ER, Kijlstra A, et al. Increased macular pigment optical density and visual acuity following consumption of a buttermilk drink containing lutein-enriched egg yolks: a randomized, double-blind, placebo-controlled trial. J Ophthalmol. 2016;2016:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Kelly D, Nolan JM, Howard AN, et al. Serum and macular response to carotenoid-enriched egg supplementation in human subjects: The Egg Xanthophyll Intervention clinical Trial (EXIT). Br J Nutr. 2017;117:108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Goulinet S, Chapman MJ.. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. relevance to oxidative resistance and atherogenesis. Arterioscler Thromb Vasc Biol. 1997;17:786–796. [DOI] [PubMed] [Google Scholar]
- 235. Boerwinkle E, Utermann G.. Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am. J. Hum. Genet. 1988;42:104–112. [PMC free article] [PubMed] [Google Scholar]
- 236. Adams MKM, Simpson JA, Richardson AJ, et al. Apolipoprotein E gene associations in age-related macular degeneration: The Melbourne Collaborative Cohort Study. Am J Epidemiol. 2012;175:511–518. [DOI] [PubMed] [Google Scholar]
- 237. Zareparsi S, Reddick AC, Branham KE, et al. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest Ophthalmol Vis Sci. 2004;45:1306–1310. [DOI] [PubMed] [Google Scholar]
- 238. Loane E, Nolan JM, O'Donovan O, et al. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol. 2008;53:68–81. [DOI] [PubMed] [Google Scholar]
- 239. Bernstein PS, Balashov NA, Tsong ED, et al. Retinal tubulin binds macular carotenoids. Invest Ophthalmol Vis Sci. 1997;38:167–175. [PubMed] [Google Scholar]
- 240. Li B, Vachali P, Bernstein PS.. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010;9:1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Horvath MP, George EW, Tran QT, et al. Structure of the lutein-binding domain of human StARD3 at 1.74 A resolution and model of a complex with lutein. Acta Crystallogr F Struct Biol Commun. 2016;72:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Nolan JM, Meagher K, Kashani S, et al. What is meso-zeaxanthin, and where does it come from? Eye (Lond). 2013;27:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Kirby ML, Beatty S, Loane E, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci. 2010;51:6722–6728. [DOI] [PubMed] [Google Scholar]
- 244. Neuringer M, Sandstrom MM, Johnson EJ, et al. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3234–3243. [DOI] [PubMed] [Google Scholar]
- 245. Stahl W, Schwarz W, Sies H.. Human serum concentrations of all-trans beta- and alpha-carotene but not 9-cis beta-carotene increase upon ingestion of a natural isomer mixture obtained from Dunaliella salina (Betatene). J Nutr. 1993;123:847–851. [DOI] [PubMed] [Google Scholar]
- 246. Micozzi MS, Brown ED, Edwards BK, et al. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr. 1992;55:1120–1125. [DOI] [PubMed] [Google Scholar]
- 247. Kostic D, White WS, Olson JA.. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995;62:604–610. [DOI] [PubMed] [Google Scholar]
- 248. Chew EY, Clemons TE, Sangiovanni JP, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Nolan JM, Stack J, O' Donovan O, et al. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007;84:61–74. [DOI] [PubMed] [Google Scholar]
- 250. Nolan JM, Kenny R, O’Regan C, et al. Macular pigment optical density in an ageing Irish population: The Irish Longitudinal Study on ageing. Ophthalmic Res. 2010;44:131–139. [DOI] [PubMed] [Google Scholar]
- 251. Lima VC, Rosen RB, Prata TS, et al. Association of age and macular pigment optical density using dual-wavelength autofluorescence imaging. Clin Ophthalmol. 2013;7:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Berendschot TT, van Norren D.. On the age dependency of the macular pigment optical density. Exp Eye Res. 2005;81:602–609. [DOI] [PubMed] [Google Scholar]
- 253. Pipis A, Touliou E, Augustin AJ.. Macular pigment optical density in a Central European population. Ophthalmic Surg Lasers Imaging Retina. 2013;44:260–267. [DOI] [PubMed] [Google Scholar]
- 254. Moran R, Nolan JM, Stack J, et al. Non-dietary correlates and determinants of plasma lutein and zeaxanthin concentrations in the Irish population. J Nutr Health Aging. 2017;21:254–261. [DOI] [PubMed] [Google Scholar]
- 255. Junqueira VBC, Barros SBM, Chan SS, et al. Aging and oxidative stress. Mol Asp Med. 2004;25:5–16. [DOI] [PubMed] [Google Scholar]
- 256. Kregel KC, Zhang HJ.. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–R36. [DOI] [PubMed] [Google Scholar]
- 257. Nolan JM, Stack J, O’Connell E, et al. The relationships between macular pigment optical density and its constituent carotenoids in diet and serum. Inv Ophthalmol Vis Sci. 2007;48: 571–582. [DOI] [PubMed] [Google Scholar]
- 258. Hammond BR, Caruso-Avery M.. Macular pigment optical density in a Southwestern sample. Invest Ophthalmol Vis Sci. 2000;41:1492–1497. [PubMed] [Google Scholar]
- 259. Hammond BR, Curran-Celentano J, Judd S, et al. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vis Res. 1996;36: 2001–2012. [DOI] [PubMed] [Google Scholar]
- 260. Dietzel M, Zeimer M, Heimes B, et al. Determinants of macular pigment optical density and its relation to age-related maculopathy: results from the Muenster Aging and Retina Study (MARS). Invest Ophthalmol Vis Sci. 2011;52:3452–3457. [DOI] [PubMed] [Google Scholar]
- 261. Lin B-H, Morrison RM.. Higher fruit consumption linked with lower body mass index. Food Rev 2002;25:28–32. [Google Scholar]
- 262. Hammond BR Jr, Ciulla TA, Snodderly DM.. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci. 2002;43:47–50. [PubMed] [Google Scholar]
- 263. Keaney JF, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23:434–439. [DOI] [PubMed] [Google Scholar]
- 264. Hammond BR Jr, Wooten BR, Snodderly DM.. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res. 1996;36:3003–3009. [DOI] [PubMed] [Google Scholar]
- 265. Nolan JM, Feeney J, Kenny RA, et al. Education is positively associated with macular pigment: The Irish Longitudinal Study on Ageing (TILDA) education and The TILDA Study. Invest Ophthalmol Vis Sci. 2012;53:7855–7861. [DOI] [PubMed] [Google Scholar]
- 266. Nolan JM, Loskutova E, Howard AN, et al. Macular pigment, visual function, and macular disease among subjects with Alzheimer’s disease: an exploratory study. J Alzheimers Dis. 2014;42:1191–1202. [DOI] [PubMed] [Google Scholar]
- 267. Tariq A, Mahroo OA, Williams KM, et al. The heritability of the ring-like distribution of macular pigment assessed in a twin study. Invest Ophthalmol Vis Sci. 2014;55:2214–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Hammond CJ, Liew SH, Van Kuijk FJ, et al. The heritability of macular response to supplemental lutein and zeaxanthin: a classic twin study. Invest Ophthalmol Vis Sci. 2012;53:4963–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Bohn T. Bioavailabilty of non-provitamin A carotenoids. Cnf. 2008;4:240–258. [Google Scholar]
- 270. Green MH, Ford JL, Oxley A, et al. Plasma retinol kinetics and beta-carotene bioefficacy are quantified by model-based compartmental analysis in healthy young adults with low vitamin A stores. J Nutr. 2016;146:2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Novotny JA, Dueker SR, Zech LA, et al. Compartmental analysis of the dynamics of beta-carotene metabolism in an adult volunteer. J Lipid Res. 1995;36:1825–1838. [PubMed] [Google Scholar]
- 272. Moran NE, Cichon MJ, Riedl KM, et al. Compartmental and noncompartmental modeling of (1)(3)C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am J Clin Nutr. 2015;102:1436–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Mapelli-Brahm P, Corte-Real J, Melendez-Martinez AJ, et al. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017;229:304–311. [DOI] [PubMed] [Google Scholar]
- 274. Corte-Real J, Guignard C, Gantenbein M, et al. No influence of supplemental dietary calcium intake on the bioavailability of spinach carotenoids in humans. Br J Nutr. 2017;117:1560–1569. [DOI] [PubMed] [Google Scholar]
- 275. Cooperstone JL, Ralston RA, Riedl KM, et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol Nutr Food Res. 2015;59:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Melendez-Martinez AJ, Mapelli-Brahm P, Benitez-Gonzalez A, et al. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch Biochem Biophys. 2015;572:188–200. [DOI] [PubMed] [Google Scholar]
- 277. Corte-Real J, Bertucci M, Soukoulis C, et al. Negative effects of divalent mineral cations on the bioaccessibility of carotenoids from plant food matrices and related physical properties of gastro-intestinal fluids. Food Funct. 2017;8:1008–1019. [DOI] [PubMed] [Google Scholar]
- 278. Karppi J, Rissanen TH, Nyyssonen K, et al. Effects of astaxanthin supplementation on lipid peroxidation. Int J Vitam Nutr Res 2007;77:3–11. [DOI] [PubMed] [Google Scholar]
- 279. Mercke OJ, Lignell A, Pettersson A, et al. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur J Pharm Sci. 2003;19:299–304. [DOI] [PubMed] [Google Scholar]
- 280. Coral-Hinostroza GN, Ytrestoyl T, Ruyter B, et al. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’R/S isomers of astaxanthin fatty acyl diesters. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:99–110. [DOI] [PubMed] [Google Scholar]
- 281. Latscha T. The role of astaxanthin in shrimp pigmentation. Actes de Colloques Ifremer 1989;9:319–325. [Google Scholar]
- 282. Maoka T. Carotenoids in marine animals. Mar Drugs. 2011;9:278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Guerin M, Huntley ME, Olaizola M.. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. [DOI] [PubMed] [Google Scholar]
- 284. Hitoe S, Shimoda H.. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects. Funct Food Health Dis. 2017;7:246–262. [Google Scholar]
- 285. Kotake-Nara E, Asai A, Nagao A.. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005;220:75–84. [DOI] [PubMed] [Google Scholar]
- 286. Maeda H, Kanno S, Kodate M, et al. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar Drugs. 2015;13:4799–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Miyashita K, Nishikawa S, Beppu F, et al. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric. 2011;91:1166–1174. [DOI] [PubMed] [Google Scholar]
- 288. Yonekura L, Kobayashi M, Terasaki M, et al. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J Nutr. 2010;140:1824–1831. [DOI] [PubMed] [Google Scholar]
- 289. Hashimoto T, Ozaki Y, Mizuno M, et al. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br J Nutr. 2012;107:1566–1569. [DOI] [PubMed] [Google Scholar]
- 290. Sachindra NM, Sato E, Maeda H, et al. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem. 2007;55:8516–8522. [DOI] [PubMed] [Google Scholar]
- 291. Asai A, Terasaki M, Nagao A.. An epoxide-furanoid rearrangement of spinach neoxanthin occurs in the gastrointestinal tract of mice and in vitro: formation and cytostatic activity of neochrome stereoisomers. J Nutr. 2004;134:2237–2243. [DOI] [PubMed] [Google Scholar]
- 292. Asai A, Yonekura L, Nagao A.. Low bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr. 2008;100:273–277. [DOI] [PubMed] [Google Scholar]
- 293. Biehler E, Hoffmann L, Krause E, et al. Divalent minerals decrease micellarization and uptake of carotenoids and digestion products into Caco-2 cells. J Nutr. 2011;141:1769–1776. [DOI] [PubMed] [Google Scholar]
- 294. Sugawara T, Kushiro M, Zhang H, et al. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr. 2001;131:2921–2927. [DOI] [PubMed] [Google Scholar]
- 295. Umigai N, Murakami K, Ulit MV, et al. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine. 2011;18:575–578. [DOI] [PubMed] [Google Scholar]
- 296. Bruzzone S, Battaglia F, Mannino E, et al. Abscisic acid ameliorates the systemic sclerosis fibroblast phenotype in vitro. Biochem Biophys Res Commun. 2012;422:70–74. [DOI] [PubMed] [Google Scholar]
- 297. Guri AJ, Hontecillas R, Bassaganya-Riera J.. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin Nutr. 2010;29:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Magnone M, Ameri P, Salis A, et al. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015;29:4783–4793. [DOI] [PubMed] [Google Scholar]
- 299. Zhou N, Yao Y, Ye H, et al. Abscisic-acid-induced cellular apoptosis and differentiation in glioma via the retinoid acid signaling pathway. Int J Cancer. 2016;138:1947–1958. [DOI] [PubMed] [Google Scholar]
- 300. Jaworowska A, Blackham T, Davies IG, et al. Nutritional challenges and health implications of takeaway and fast food. Nutr Rev. 2013;71:310–318. [DOI] [PubMed] [Google Scholar]
- 301. Prentice AM, Jebb SA.. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4:187–194. [DOI] [PubMed] [Google Scholar]
- 302. de Ridder D, Kroese F, Evers C, et al. Healthy diet: health impact, prevalence, correlates, and interventions. Psychol. Health. 2017;32:907–941. [DOI] [PubMed] [Google Scholar]
- 303. Grosso G, Micek A, Godos J, et al. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: a comprehensive meta-analysis. PLoS One. 2017;12:e0183787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Hiscock R, Bauld L, Amos A, et al. Socioeconomic status and smoking: a review. Ann NY Acad Sci. 2012;1248:107–123. [DOI] [PubMed] [Google Scholar]
- 305. Collins SE. Associations between socioeconomic factors and alcohol outcomes. Alcohol Res. 2016;38:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Mortensen A. Carotenoids and other pigments as natural colorants. Pure Appl Chem.2006;78:1477–1491. [Google Scholar]
- 307.MarketsandMarkets. Feed Pigment Market: Forecasts until 2020. Northbrook, IL: MarketsandMarkets; 2016.
- 308. Flynn A, Hirvonen T, Mensink GB, et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res. 2009;53: 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Willers J, Heinemann M, Bitterlich N, et al. Vitamin intake from food supplements in a German cohort - is there a risk of excessive intake?. Int J Vitam Nutr Res. 2014;84:152–162. [DOI] [PubMed] [Google Scholar]
- 310. Jenab M, Ferrari P, Mazuir M, et al. Variations in lycopene blood levels and tomato consumption across European countries based on the European Prospective Investigation into Cancer and Nutrition (EPIC) study. J Nutr. 2005;135:2032s–2036s. [DOI] [PubMed] [Google Scholar]
- 311. Arab L, Steck-Scott S, Bowen P.. Participation of lycopene and beta-carotene in carcinogenesis: defenders, aggressors, or passive bystanders? Epidemiol Rev. 2001;23:211–230. [DOI] [PubMed] [Google Scholar]
- 312. Grolier P, Duszka C, Borel P, et al. In vitro and in vivo inhibition of beta-carotene dioxygenase activity by canthaxanthin in rat intestine. Arch Biochem Biophys. 1997;348:233–238. [DOI] [PubMed] [Google Scholar]
- 313. Satia JA, Littman A, Slatore CG, et al. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the VITamins And Lifestyle (VITAL) study. Am J Epidemiol. 2009;169:815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Rühl R, Bub A, Watzl B.. Modulation of plasma all-trans retinoic acid concentrations by the consumption of carotenoid-rich vegetables. Nutrition. 2008;24:1224–1226. [DOI] [PubMed] [Google Scholar]
- 315. Watzl B, Bub A, Brandstetter BR, et al. Modulation of human T-lymphocyte functions by the consumption of carotenoid-rich vegetables. Br J Nutr. 1999;82:383–389. [DOI] [PubMed] [Google Scholar]
- 316. Bohn T, Blackwood M, Francis D, et al. Bioavailability of phytochemical constituents from a novel soy fortified lycopene rich tomato juice developed for targeted cancer prevention trials. Nutr Cancer. 2013;65:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Wood LG, Garg ML, Powell H, et al. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. 2008;42:94–102. [DOI] [PubMed] [Google Scholar]
- 318. Vrieling A, Voskuil DW, Bonfrer JM, et al. Lycopene supplementation elevates circulating insulin-like growth factor binding protein-1 and -2 concentrations in persons at greater risk of colorectal cancer. Am J Clin Nutr. 2007;86:1456–1462. [DOI] [PubMed] [Google Scholar]
- 319. Schwarz S, Obermüller-Jevic UC, Hellmis E, et al. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008;138:49–53. [DOI] [PubMed] [Google Scholar]
- 320. Bub A, Watzl B, Abrahamse L, et al. Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr. 2000;130:2200–2206. [DOI] [PubMed] [Google Scholar]
- 321. Korobelnik JF, Rougier MB, Delyfer MN, et al. Effect of dietary supplementation with lutein, zeaxanthin, and omega-3 on macular pigment: a randomized clinical trial. JAMA Ophthalmol. 2017;135:1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]