Abstract
Decades of comparative and experimental work suggest that testosterone (T) promotes mating effort at the expense of parental effort in many vertebrates. There is abundant evidence that T-mediated trade-offs span both evolutionary and seasonal timescales, as T is often higher in species or breeding stages with greater mating competition and lower in association with parental effort. However, it is less clear whether transient elevations in T within a male’s own reactive scope can affect parental effort in the same way, with effects that are visible to natural selection. Here, we injected free-living male tree swallows (Tachycineta bicolor) with gonadotropin-releasing hormone (GnRH), thus temporarily maximizing T production within an individual’s own limit. Passive loggers at each nest showed that GnRH-injected males provisioned more frequently than saline males for the subsequent day, and their offspring gained more mass during that time. The degree of offspring growth was positively correlated with the father’s degree of T elevation, but provisioning was not proportional to changes in T, and GnRH- and saline-injected males did not differ in corticosterone secretion. These results suggest that prior knowledge of T-mediated trade-offs garnered from seasonal, evolutionary, and experimental research cannot necessarily be generalized to the timescale of transient fluctuations in T secretion within an individual. Instead, we propose that GnRH-induced T fluctuations may not result in visible trade-offs if selection has already sculpted an individual male’s reactive scope based on his ability to handle the competing demands of mating and parental care.
Keywords: Hypothalamo-pituitary-gonadal axis, paternal care, nestling growth, Radio-frequency identification, feeding
INTRO
Evidence of life history trade-offs can be seen across multiple timescales: among species, between different seasons or breeding stages, and in moment-to-moment changes in phenotypes that respond to variable environmental conditions (reviewed in Roff, 1993; Stearns, 1992). A major goal of evolutionary biology has been to understand how these trade-offs arise. One hypothesis is that short-term plastic responses give rise to macro-evolutionary variation (West-Eberhard, 2005). This hypothesis relies on assumptions that trade-offs occurring during moment-to-moment changes in the environment operate in the same manner as trade-offs that have arisen over evolutionary time, and that any short-term adjustments affect fitness in such a way that selection can act. Hormonal mechanisms are a promising avenue for testing these assumptions because their effects on the phenotype traverse different timescales from early life to adulthood (Adkins-Regan, 2005), and hormone secretion has been linked with key life history variables among diverse animal taxa (Vitousek et al., 2019; Wingfield et al., 1990).
The steroid hormone testosterone (T) is known as a proximate mediator of life history and behavioral trade-offs in vertebrates. T has pleiotropic effects that coordinate expression of multiple traits promoting mating effort, oftentimes at the expense of parental effort (Ketterson et al., 2009). In species with greater mating competition, males tend to have higher circulating T levels, whereas males that provide more parental care tend to have lower T levels (e.g., blue-headed vs. red-eyed vireos, Van Roo et al., 2003); these interspecific patterns hold at the seasonal level as well, with higher T levels generally seen during territory establishment and initial competition for mates, and lower levels seen in breeding stages with parental care (Wingfield et al., 1990). Likewise, when males are given implants that increase T levels for days or weeks, they often exhibit an increase in mating effort (e.g., increased territorial aggression or advertisement) alongside a reduction in parental effort (reviewed in Lynn, 2016; Stiver and Alonzo, 2009). This large body of comparative and experimental work suggests that T mediates trade-offs at both interspecific and seasonal scales. However, T secretion also can vary over the course of minutes or hours, in response to social interactions or breeding opportunities (Goymann et al., 2007; Goymann et al., 2019; Wingfield et al., 1990). It is far less clear whether these sorts of short-lived intra-individual differences in T also generate trade-offs between mating effort and parental effort, in the way that they do over longer timescales. Understanding the behavioral effects of naturalistic T fluctuations on a moment-to-moment timescale is necessary to know how such seasonal and evolutionary patterns can arise, and how they may be shaped by current evolutionary forces.
One way to experimentally induce brief fluctuations in T is an injection of gonadotropin-releasing hormone (GnRH). By flooding an individual’s system with GnRH, this treatment essentially maximizes gonadotropin output from the pituitary and maximizes gonadal T secretion 30-60min later. These ‘GnRH challenges’ show how individuals respond hormonally to standardized stimulation of the HPG axis, and as such are commonly used to assay an individual’s natural T production capabilities, or reactive scope (e.g., Jawor et al., 2006; Rosvall et al., 2016). The use of GnRH challenges as a bioassay has led to important insights in comparative evolutionary endocrinology, beyond those gained from sampling basal T levels or measuring behavioral responses to exogenous T. For instance, GnRH challenges used to track the seasonal breeding cycle of woodchucks (Marmota monax) showed that males can facultatively increase luteinizing hormone (LH) and T about 1-2 months before they increase basal LH levels, thus revealing the true timing of gonadal recrudescence (Concannon et al., 1998). GnRH challenges also revealed divergence in the hormonal profiles of two subspecies of dark-eyed juncos (Junco hyemalis) that differ in a suite of T-mediated traits: though the more aggressive and ornamented South Dakota subspecies did not have higher baseline T levels than the Virginia subspecies, they did elevate T more rapidly and to a higher peak in response to GnRH (Rosvall et al., 2016). Within a population, individual differences in T responses to a GnRH challenge are often predictive of other aspects of the phenotype measured separately, including territorial aggression, parental care, body mass, and ornamentation (McGlothlin et al., 2008; McGlothlin et al., 2007). Critically, T responses to GnRH are repeatable (Jawor et al., 2006) and predictive of fitness measurements (Ambardar and Grindstaff, 2017; Cain and Pryke, 2016; McGlothlin et al., 2010), adding further biological relevance to this assay of an individual’s hormonal phenotype.
Less commonly, GnRH challenges have been used as experimental treatments to temporarily increase gonadal T production within an individual’s own reactive scope, which in turn can affect behavior. Although GnRH affects multiple downstream hormones beyond just T (Adkins-Regan, 2005), the use of GnRH injections as a method for manipulating T levels has one key advantage over direct treatment with T, at least in the context of evolutionary endocrinology. Specifically, treating all individuals with a standardized dose of T fails to account for evolutionarily significant inter-individual variation in T production capabilities. The GnRH challenge limits each individual’s T production to his own physiological maximum, and in doing so, better positions us to understand how naturalistic T elevation within a male’s own reactive scope feeds back to shape other components of the phenotype. Behavioral effects of GnRH injections have historically been attributed to known or assumed effects on T production. For instance, male European ground squirrels (Spermophilus citellus) increase plasma T levels 40 min after GnRH treatment, and they exhibit more aggression in the day post-GnRH treatment (Millesi et al., 2002). In the black redstart (Phoenicurus ochruros), however, GnRH injection does not alter male aggression (Goymann et al., 2015), but it does reduce paternal care in the 2 hours post-treatment; notably, T levels were not quantified in these same individuals (Goymann and Dávila, 2017; Goymann et al., 2015), leaving some uncertainty as to whether observed behavioral shifts could be attributed to changes in systemic T levels. Thus, it is still unclear whether transient fluctuations of T within an individual’s reactive scope lead to short-term deleterious effects on parental care, as is typically assumed in previous literature focused on longer timescales.
The process by which fluctuations in T affect other aspects of the phenotype should take time: one of the primary mechanisms of T action occurs via androgen receptors, which alter transcription and translation. Therefore, T-mediated changes in behavior may not become visible until several hours after changes in T levels in circulation (Adkins-Regan, 2005). More rapid effects via non-genomic mechanisms are also possible, e.g., via T that is converted into estradiol (Heimovics et al., 2018). In either case, if any T-mediated changes in parental effort can be shaped by selection, then these behavioral changes should result in costs or benefits to current reproductive success; however, this idea is also untested.
Here we set out to test the effects of GnRH injections on circulating T and parental behavior using male tree swallows (Tachycineta bicolor), a cavity-nesting songbird with biparental care. In a study repeated over three years, we captured males while they provisioned, injected each male with either GnRH or saline, measured changes in circulating T levels 30 min later, and then observed changes in provisioning behavior and offspring growth over the subsequent day. In this way, a GnRH injection served as both (i) an assay of a male’s T production capabilities, which reflect his investment in mating effort, and (ii) an experimental treatment to increase a male’s T levels within his own reactive scope. At the time of the post-injection bleed, we also measured circulating corticosterone (CORT) levels, since 30 min of restraint commonly induce acute stress responses (Romero and Reed, 2005), which in turn can affect parental behavior (Wingfield et al., 1998). We monitored parental visits to the nest using RFID technology, allowing us to collect data continuously for a full day after the treatment. To assess potential fitness-related consequences of a single activation of the HPG axis, we focused on the subset of nests with chicks at the peak of postnatal growth, and we measured treatment effects on chick growth for one day. We predicted that GnRH-treated males would increase T levels and reduce parental care, leading to slowed chick growth; such a finding would be consistent with the hypothesis that brief, naturalistic elevations in T negatively affect parental behavior. In fact, we did find significant effects of GnRH treatment on T levels, paternal behavior, and offspring development, but these effects were not consistent with what we have come to expect from previous work on T-mediated trade-offs.
METHODS
(a). Capture and GnRH treatments
This study used male tree swallows breeding in artificial nesting cavities (nest boxes) located around Bloomington, IN, USA (39°9 N, 86°31 W). The experiment occurred during three successive breeding seasons, from 2016 to 2018 (hereafter Y1 to Y3) and included 62 unique males. Six of these males were sampled in two years, leading to 68 total nests studied. Only two of these repeated males received the same treatment, and randomly excluding data from one year for these two males does not change our results. Sample sizes and exact dates for each year are summarized in supplemental table 1.
We used nest box traps (Stutchbury and Robertson, 1986) to capture males that were provisioning. Offspring ranged from 5-10 days post-hatch (mean=7 days old, where hatch day=1). All males were captured between 0700 and 1300 EDT. Upon capture, we immediately collected blood (40-70 μL) from the alar vein using heparinized microcapillary tubes and a 27G needle (latency from capture to initial bleed: 5.1±0.5min). We then used a 50 μL Hamilton syringe to inject either a solution of 1.25 μg chicken GnRH-I (Bachem H-3106, Torrence, CA, USA; n=40 males) in 50 μL phosphate-buffered saline (PBS) or a control solution of 50 μL PBS (hereafter ‘saline’; n=28) into the left pectoral muscle. This method has been used with female tree swallows (George and Rosvall, 2018) and other songbirds of similar mass to bring about a peak in T about 30 min later, which subsides within 2h (Rosvall et al., 2016). We gave each bird a unique numbered USGS band on one leg and a plastic color band on the other leg. In Y2 and 3 of this study, each plastic band contained a PIT tag (personal integrated transponder; 2.3mm with an EM4102 transponder from Eccel Technologies, UK) that transmits a unique hexadecimal number when activated by an RFID reader/antenna, thus allowing us to record individual time-stamped visits to nests (elaborated below). Males were held in an opaque paper bag until 30 min post-injection, when we collected another 40-70 μL blood. Using a spring-loaded scale, we measured each male’s body mass as a proxy of his condition or quality. After applying pressure and confirming that bleeding had ceased, we released each male.
(b). Quantifying parental visits to nest
In Y2 and 3 of this study, we equipped each nest box with a low-cost radio-frequency identification (RFID) reader (“Generation 2” of the design in Bridge and Bonter, 2011; Cellular Tracking Technology, PA, USA). In all we collected RFID data for n=22 GnRH- and 15 saline-injected males (see supplemental table 1 for sample size per year). Readers were attached to a copper coil antenna at the nest box entrance. Previous work in this species has shown that RFID-detected visits are highly correlated with observed visit counts (Lendvai et al., 2015), and >95% of parental visits to the nest are feeding events (McCarty, 2002); thus, RFID visit counts accurately reflect provisioning efforts. RFID readers were programmed to scan every 500 ms to detect any PIT tags at the box entrance and record date, time, and tag identity. To conserve battery life, readers were programmed to be inactive overnight (2100 to 0600 EDT). To account for individuals perching or hovering at the antenna before entering the box, we filtered out any repeated reads by the same individual within 3 sec of one another, and used the remaining reads per individual as estimated counts of entrances and exits combined. We then divided the remaining reads in half to estimate the number of unique visits, assuming one entrance and one exit per visit. In a separate study, our lab confirmed that RFID visits calculated in this exact way are highly correlated (r=0.90) with counts obtained via focal observation of provisioning over 30 min periods (Wolf et al., preprint). For all analyses of provisioning behavior, we calculated visitation rate as the average number of visits per hour for the 28-hour period post-release (excluding overnight or incomplete hours). This time period was chosen because it was the maximum elapsed time over which we measured chick growth (see below). Additionally, we used RFID data to find each male’s latency to return to his nest (time elapsed between a male’s release post-injection and his first post-injection box visit). We also obtained visitation rates from RFID-tagged female social partners at 34 of these 37 boxes, which allowed us to separately address whether male treatment altered female behavior.
(c). Quantifying effects on offspring
At the time of each male’s injection, we measured the mass of each chick in the nest, to the nearest 0.1 g (Ohaus HH120D digital scale, Parsippany, NJ, USA). We returned to each nest approximately one day later to mass chicks again, recording time elapsed between measurements (mean=25.0±0.2h, range= 21 to 28 h). We also measured chick mass on day 12 post-hatch, at which point we routinely band and measure chicks because they have typically reached their asymptotic mass (Teather, 1996).
We calculated average chick growth for each male as each brood’s change in mass normalized for brood size:. To maximize our ability to detect differences in chick mass change over a short period of time (one day), we limited any chick growth analyses to only nests whose chicks had the potential to gain the most mass in a single 24-hour period. We determined which nests to include by fitting a logistic growth curve to chick mass by age in our population (data collected for a separate study; Wolf et al., in press) and calculating the inflection point (i.e., the point on the curve with the steepest slope). This maximal growth rate occurred at day 6 post-hatch, consistent with prior work in this species (Quinney et al., 1986). Thus, growth analyses included only nests whose chicks were 6 ± 1 days post-hatch on the date the father was treated with GnRH (n=30) or saline (n=24). We did not apply this same restriction to the provisioning analyses described above because tree swallow parental behavior increases from days 1-5 post-hatching and then remains fairly constant until a few days before fledging (Leffelaar and Robertson, 1986; Lombardo, 1991). Given that all males in our study had chicks ranging in age from 5-10 days post-hatch (i.e., the period of maximal provisioning), we included all injected males with RFID data in analyses of provisioning. When testing for treatment effects on average day 12 mass, we limited our analysis to only those nests included in earlier growth analyses, excluding 3 additional nests that failed for unrelated reasons before day 12 (n=51 nests included).
(d). Hormone assays
Testosterone
We measured pre- and post-injection T levels using competitive-binding commercial ELISA kits (Enzo ADI# 900-176) that have already been validated in tree swallows (George and Rosvall, 2018). In general, we extracted hormones using three rounds of liquid-liquid ether extraction and reconstituted in 250μL assay buffer. For >80% of samples, we began with 20 μL plasma. For 19 of 107 samples, we did not have 20μL and so we instead used 5-15 μL and checked whether their results still fell between 20-80% binding. 2 of those low-volume samples and 13 additional samples did fall >80% binding (concentrations too low), and so we conservatively estimated their T concentrations based on the assay sensitivity (7.81 ng/mL), corrected for plasma volume. Plates were balanced by treatment and date, with both samples from the same individual run on the same plate, but each year was run separately. T concentration was calculated by comparing a sample’s absorbance to the absorbance of the assay’s 9-point standard curve (Gen5 curve-fitting software, Biotek EPOCH plate reader, Winooski, VT, USA). We used a previously extracted hormone pool (leftover from another study) to measure intra-and inter-plate variation, with three pairs of duplicates uniformly dispersed across each plate. The same pool was used throughout the entire study, and was stored at −20°C between assays and years. Intra-plate variation of the pool was 5.5±3.5% (average ±se), and inter-plate variation was 14.9%. Sample duplicates showed a CV of 6.9±0.7%. All analyses of T focused on pre-injection T levels and ΔT (post- minus pre- injection levels).
Corticosterone
In Y1 and 2 of the study, we used additional plasma from post-injection bleeds (n=16 GnRH, 13 saline males) to measure corticosterone (CORT) with commercial ELISA kits (Caymann Chemical, # 501320) previously validated for tree swallows (Virgin and Rosvall, 2018). We did not assay pre-injection samples because latencies from capture to initial bleed were 5.6±0.5 min, but CORT levels are known to change within only 3 min (Romero and Reed, 2005). Extraction methods were the same as above, except that we began with 5-10 μL of plasma and reconstituted in 500 μL assay buffer. We again used a pool of plasma extract to quantify variability of the assay. Inter-plate variation was 26.8% (plate 1 was higher than plates 2-3), but intraplate variation was 12.3 ± 5.3 % and sample duplicates showed a CV of 9.1±1.3% (average ±se). Due to the high inter-plate variation, we used a plate correction factor; that is, we multiplied each measurement by the pool grand mean divided by each plate’s pool mean (as in Jawor et al., 2007; McGlothlin et al., 2008). Samples were balanced by treatment and year across three plates, meaning any effects of those predictor variables should be apparent despite inter-plate variation.
(e). Statistical analyses
Statistical analyses used R (v. 3.6.1, R Core Team, 2019). All reported means are followed by ± standard errors. In general, we used a series of linear models to address our questions. For each separate question, we developed a set of a priori model options that included the main predictor in question (e.g., treatment) as well as probable predictors and interactions based on the literature (e.g., date, year, male body mass, chick age, brood size). Some response variables (ΔT, pre-injection T, and latency to return) were log-transformed to better meet model assumptions of normality. The full set of candidate models are overviewed below and detailed in the supplementary materials. We used AICc scores to systematically compare candidate models with one another and the intercept-only model, and used the multcomp R package (Hothorn et al., 2016) to select the top model that best fit the data (Burnham et al., 2011). In cases with multiple models with ΔAICc<2, reported results are from the most parsimonious model (Tables 1–3). Exceptions occurred when the intercept-only model was the best fit; instead, we present the most parsimonious model that still includes the key predictor variable (e.g., treatment), in order to quantify null results. We visually inspected histograms and normal quantile plots of final model residuals as well as plots of predicted versus residual values to verify that assumptions of normality and homoscedasticity were met (Figures S1–12; Zuur et al., 2010). This process identified three situations in which the most parsimonious model insufficiently met assumptions of homoscedasticity: testing for an effect of treatment on ΔT, an effect of treatment on pre-injection T, and an effect of GnRH-induced ΔT on male provisioning. In all three cases, the next most parsimonious model, still within ΔAICc<2 alleviated this problem with the addition of an important explanatory variable, and therefore we present those results in Tables 1–3, with further details in the supplement.
Table 1:
Linear models testing effects of treatment and other covariates on male hormone levels. Estimates use saline as the reference for treatment and Y1 as reference for year. Pre-injection T and ΔT values were log-transformed. Inclusion and exclusion of covariates determined via systematic comparison of candidate models (supplemental tables 2–4). Signficant predictors are bolded (p<0.05). Letters denote separate models.
| Response variable | Predictor variable | Estimate (± SE) | F-statistic | p-value |
|---|---|---|---|---|
|
ΔTestosterone
(post minus pre-injection) | ||||
| (a) | Treatment | 1.37 ± 0.50 | F1,65= 20.9 | p<0.0001 |
| Date | 0.0020 ± 0.0026 | F1,65= 3.24 | p=0.077 | |
| Treatment*Date | −0.0077 ± 0.0034 | F1,65= 5.19 | p=0.026 | |
| Mass | 0.029 ± 0.016 | F165= 4.05 | p=0.049 | |
|
Pre-injection
Testosterone | ||||
| (b) | Treatment | −0.247 ± 0.20 | F1,65= 1.27 | p=0.26 |
| Date | −0.017 ± 0.011 | F1,65=5.62 | p=0.021 | |
| Year | 0.49 ± 0.24 (Y2) | F2,65=3.08 | p=0.053 | |
| −0.50 ± 0.26 (Y3) | ||||
| Post-injection
Corticosterone | ||||
| (c) | Treatment | 6.11 ± 9.62 | F1,33=0.40 | p=0.53 |
Table 3:
Linear models testing effects of treatment, hormone levels, and other covariates on chick growth . Estimates use saline as the reference for treatment and Y1 as the reference for year. Inclusion and exclusion of covariates was determined via systematic comparison of candidate models (supplemental tables 10–13). Signficant predictors are bolded (p<0.05). Letters denote separate models. Analyses a-c focus on effects occuring within one day of male treatment at the time of peak growth; analysis d measured delayed effects at d12 post-hatch
| Response variable | Predictor variable | Estimate (± 95% CI) | F-statistic | p-value |
|---|---|---|---|---|
| Avg chick growth (Δg) | ||||
| (a) | Treatment | 0.37 ± 0.16 | F1,53=5.41 | p=0.024 |
| Date | −0.020 ± 0.007 | F1,53=7.38 | p=0.009 | |
| Elapsed time | 0.27 ± 0.052 | F1,53 =23.24 | p<0.0001 | |
| (b) | Avg M visits/hr | 0.085 ± 0.034 | F1,30 =9.49 | p=0.0046 |
| Elapsed time | 0.22 ± 0.067 | F1,30 =11.01 | p=0.0025 | |
| (c) | GnRH ΔT | 0.59 ± 0.15 | F1,28 =14.87 | p=0.0007 |
| Elapsed time | 0.30 ± 0.05 | F1,28 =30.52 | p<0.0001 | |
|
Avg d12 mass
(g) | ||||
| (d) | Treatment | −0.16 ± 0.64 | F1,50=1.24 | p=0.27 |
| Brood size | −1.00 ± 0.39 | F1,50=9.36 | p=0.0037 | |
| Male mass | 1.19 ± 0.31 | F1,50=11.38 | p=0.0015 | |
| Year | 1.93 ± 0.71 (Y2) | F2,50=5.21 | p=0.0092 | |
| 2.28 ± 0.82 (Y3) | ||||
Briefly, we tested for treatment effects on male hormone levels and responses (baseline T; ΔT; post-injection CORT), male parental behavior (latency to return to nest; average provisioning rates), female parental behavior (average provisioning rates), chick growth over the one day period post-treatment, and chick mass at day 12 post-hatch.
To assess how treatment-induced changes in hormones affected male provisioning and chick growth, we also tested for relationships between hormone variables (GnRH-induced ΔT; post-injection CORT) and male behavior or chick growth.
All models initially included date, year, and male body mass. Hormone models also considered latency to bleed. Models looking at effects of CORT levels on behavior also considered a CORT*treatment interaction. The final model testing for effects of treatment on ΔT contained a treatment *date interaction, so we used the emtrends() function from package emmeans (Lenth et al., 2018) to test whether the estimated simple slopes of ΔT by date within each treatment group significantly differed from zero. All models of behavior or chick growth considered effects of chick age and brood size, and growth models also considered the exact time elapsed between mass measures.
RESULTS
Treatment effects on hormone secretion
We found a significant main effect of treatment on ΔT, such that males injected with GnRH elevated T more (i.e., had higher ΔT) than saline males (GnRH ΔT=0.36±0.09 ng/mL plasma, saline ΔT=−0.13±0.07 ng/mL plasma; Table 1a, Figure 1a). We also found a significant main effect of male body mass on ΔT, indicating higher T elevation in heavier males. Finally, ΔT showed a marginal main effect with date, but a significant treatment by date interaction (Table 1a, Figure 1b): the slope of the relationship between ΔT and date significantly differed from zero in the GnRH group (t=2.63, p=0.011), but not the saline group (t=0.79, p=0.44; see Figure 1b). Pre-injection T levels significantly differed by year and date, with lower T levels at later dates; critically, however, pre-injection T levels did not differ between treatment groups (Table 1b). Therefore, treatment differences in ΔT are due to differences in T responses, rather than differences in initial values.
Figure 1:
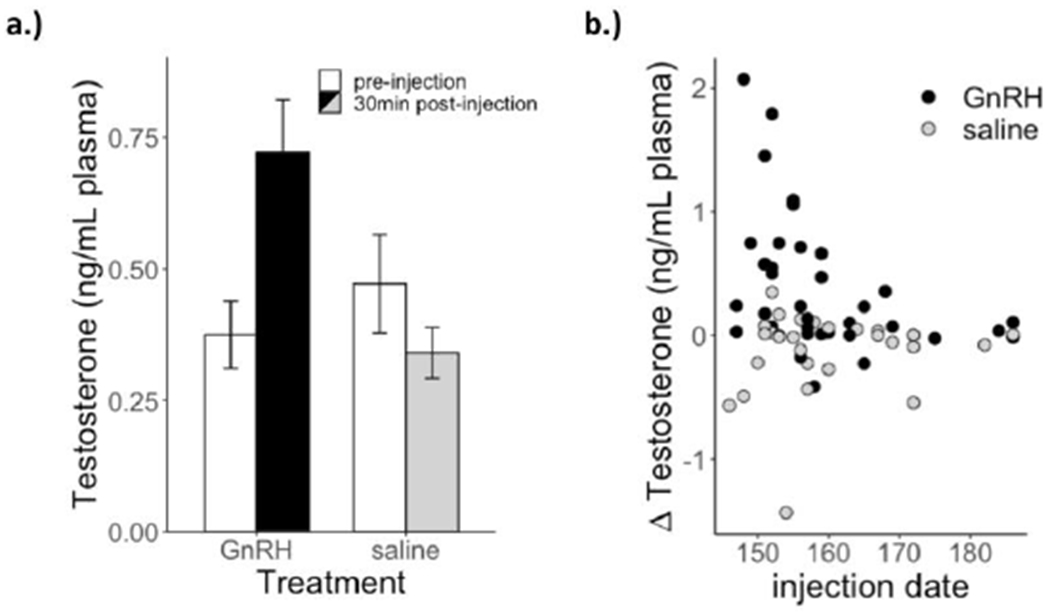
(a). Average T concentrations (untransformed) by pre/post-injection and treatment (with standard error bars) (b). T response (ΔT, untransformed) by day of year.
Males of the two treatment groups did not have significantly different CORT levels 30min post-injection (Table 1c; combined mean= 48.2±4.5 ng/mL plasma). Observed CORT levels resembled those seen in adult male songbirds sampled 30 min after a standardized stressor (similar to Bókony et al., 2009).
Effects on visitation rates
Contrary to our predictions, GnRH-injected males visited their nests more frequently over the 28 hours post-injection (Table 2a; Figure 2). ΔT in response to GnRH did not predict male visitation rates, nor did male body mass (Table 2b). In a separate model including only Y2 data, from which we had both RFID visit counts and CORT levels, we found no relationship between CORT levels and visitation rates, though there was still an effect of treatment, with GnRH males visiting more (Table 2c). There was no treatment effect on latency to return to the nest (Table 2d; GnRH=2h 47min ± 16min, saline=3h 38min ± 55min). Average female visitation rates during the same period did not differ between treatments (Table 2e), suggesting that female responses to the male treatment did not drive any downstream effects.
Table 2:
Linear models testing effects of treatment and other covariates on parental behavior in the 28 h following male injection. Latency to return values were log-transformed. Estimates use saline as the reference for treatment. Inclusion and exclusion of covariates was determined via systematic comparison of candidate models (supplemental tables 5-9). Signficant predictors are bolded (p<0.05). Letters denote separate models.
| Response variable | Predictor variable | Estimate (± SE) | F-statistic | p-value |
|---|---|---|---|---|
| Avg male visits per h | ||||
| (a) | Treatment | 2.07 ± 0.90 | F1,36=5.28 | p=0.028 |
| (b) | GnRH ΔT | −0.46 ± 1.57 | F1,21=0.004 | p=0.95 |
| mass | 0.90 ± 0.54 | F1,21=2.75 | p=0.11 | |
| (c) | Treatment | 2.81 ± 1.25 | F1,22=5.01 | p=0.04 |
| Post-inject CORT | −0.043 ± 0.02 | F1,22=2.78 | p=0.11 | |
|
Latency to return
(h) | ||||
| (d) | Treatment | −0.030 ± 0.12 | F1,36=0.037 | p=0.85 |
|
Avg female visits per
h | ||||
| (e) | Male treatment | 0.51 ± 0.98 | F1,33=0.27 | p=0.60 |
Figure 2:
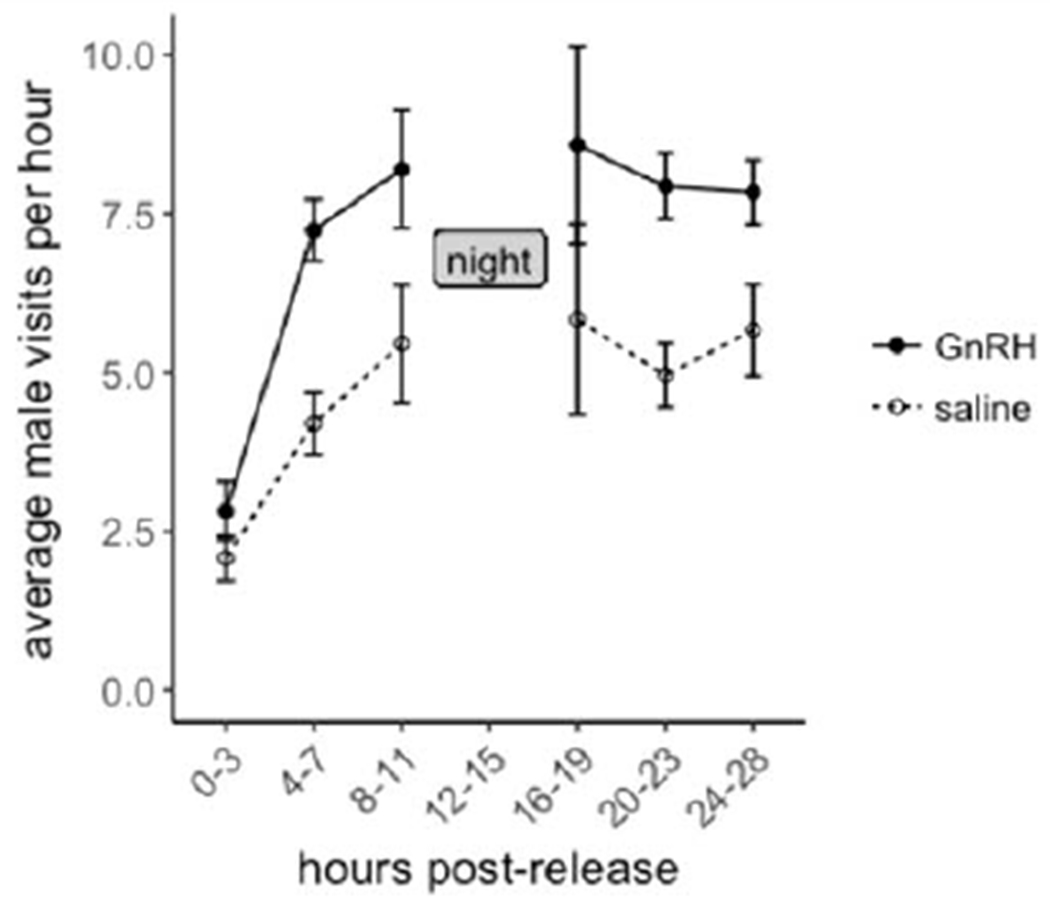
Average nest visitation rate by treatment. Although we analyzed these data averaged across the entire 28h period, relative to when each male was released post-injection, we visualized the data here in 3-hr intervals for heuristic purposes.
Effects on chick growth
Focused on chicks at the peak of nestling growth (6 ± 1 day old), we found a significant main effect of treatment: chicks of GnRH males gained approximately 0.4 ±0.2 g more than those of saline males in the day post-treatment (Figure 3a, Table 3a), at a time when pre-treatment chick mass averaged 10.1± 0.4 g. Chick growth was also significantly related to the number of hours elapsed between the first and second mass measurement and date, such that chicks with more time between measurements and chicks hatched earlier in the spring gained more mass (Table 3a). Outside the period of rapid post-natal growth (age 5-7 days), these effects were more limited (see supplemental table 14). Across treatment groups, average male visitation rates predicted variation in chick growth (Figure 3b), as did elapsed time between measurements (Table 3b). Looking solely within the GnRH treatment group, males with higher T elevation (ΔT) had chicks that gained more mass compared to the chicks of low ΔT males, in addition to a significant effect of elapsed time (Table 3c).
Figure 3:
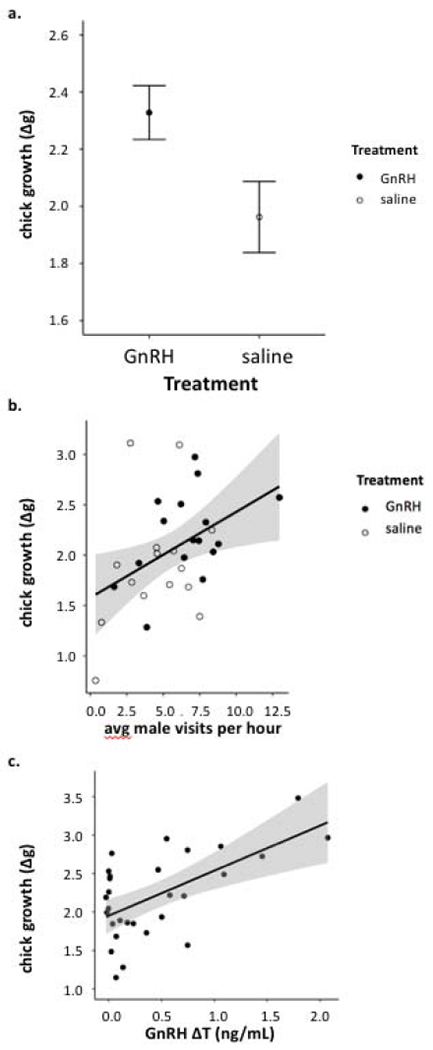
At the age of their peak growth, (a) chicks of GnRH males gained more mass in the day following treatment; (b) average growth per nest was positively correlated with the rate of paternal visits; and (c) within the GnRH treatment, the degree of T elevation was correlated with average chick growth in the day following paternal treatment. Error bars are ± SE and shaded areas are 95% confidence intervals. These conditional plots are generated using the package visreg (Breheny and Burchett, 2017), which represents the relationship between one predictor and response variable by holding other predictors constant (see Table 3).
By 12 days post-hatch there was no longer a significant treatment effect, though there were significant effects of year, brood size, and male mass, indicating that chicks in smaller broods with heavier fathers were larger by day 12 post-hatch (Table 3d).
DISCUSSION
Testosterone is considered a mechanistic driver in the evolution of life history strategies, but how this evolutionary process unfolds is unclear. One hypothesis is that relationships between hormones and behavior on longer timescales result from accumulation of shorter-term effects that are canalized or fixed over evolutionary time (sensu Goymann et al., 2007; Wingfield et al., 1990). To begin to test whether this hypothesis applies to short term changes in T and parental care, we injected parental male songbirds with either saline or gonadotropin-releasing hormone, which stimulated T secretion within each male’s current reactive scope. We calculated subsequent changes in T secretion (ΔT), which we used as a metric of each male’s own investment in putatively T-mediated mating effort (Ketterson et al., 2009; McGlothlin et al., 2007). We quantified downstream effects over the subsequent day and found treatment effects on both paternal care and offspring mass at the peak of growth, but not in the direction predicted by decades of prior work. Below we offer alternative hypotheses for our results and discuss their implications for broader questions about hormones and the evolution of life history trade-offs.
We were surprised to find that GnRH-injected males visited their nests more frequently in the day following treatment. At the same time, chicks of GnRH-injected males grew more (discussed in greater depth below) and male visits positively correlated with chick growth, suggesting that RFID-detected visits to the nest were in fact accompanied by food delivery to chicks. Therefore, in this instance we found that HPG axis activation actually promoted, rather than inhibited, paternal provisioning. One interpretation of this result is that T itself may have enhanced the expression of parental care in this context. Although experimentation with T or androgen receptor blockers is needed to directly assess this possibility, we believe it is unlikely because of the marked inter-male variation in T production among GnRH-injected males. This variation in T production, which was only partly explained by date, allowed us to tease apart effects of GnRH vs. T to some degree. We found no relationship between a male’s T elevation and provisioning: even GnRH-injected males who did not significantly elevate T still provisioned more than saline-injected controls. This suggests that some other mechanism downstream of GnRH release is responsible for the observed effects on paternal care.
There are certainly ways in which stimulation of the HPG axis could positively affect parenting, independent of T. For instance, the secretion of LH can occur in response to GnRH injection even without a detectable effect on T (e.g., Meddle et al., 2002; Wingfield and Lewis, 1993). Therefore, GnRH may promote parental care via one or more of its other downstream products, such as estradiol (E2), which can promote paternal care in mammals (Trainor and Marler, 2001). GnRH can also stimulate release of prolactin (Henderson et al., 2008; Weber et al., 1997), which is one of the main hormones affecting parental care in birds (Smiley, 2019). Other HPG axis-related hormones that have previously been implicated in paternal care in birds include progesterone and GnIH (reviewed in Lynn, 2016), but it is unclear whether these hormones directly promote provisioning behaviors, as observed in the present study.
An additional possibility is that GnRH treatment may have interacted with stress-related impacts on parenting. Given that our protocol (measuring T 30min post-injection) mirrors a standardized restraint stress assay (reviewed in Small et al., 2017), it is unsurprising that post-injection blood samples indicated high levels of CORT secretion in both treatment groups. There is a wide body of literature suggesting that stress-induced CORT elevation may temporarily reduce parental effort (Lynn, 2016; Wingfield et al., 1998). We found no correlation between post-injection CORT levels and visitation rates in the subset of birds for which we had both types of data; however, relationships between hormones and behavior need not be linear for them to exist (Adkins-Regan, 2003). Without unhandled controls, we cannot say for certain whether saline-males provisioned less than ‘usual’ in this system. But if so, our results may suggest that simultaneous activation of both HPA and HPG axes in GnRH males acted as a buffer against the negative effects of CORT on provisioning. With roughly equal CORT levels in the two treatment groups, such mitigative effects would have to occur downstream of CORT secretion. In fact, GnRH can alleviate anxiety and depressive behaviors in rodents (Massol et al., 1989; Umathe et al., 2008), and there is some evidence that sex steroids can modulate glucocorticoid receptor signaling (reviewed in Bourke et al., 2012). However, these effects are not as well understood as the more well characterized effects of HPA-axis activation on the HPG-axis (as reviewed in Acevedo-Rodriguez et al., 2018). Regardless of the exact mechanism, we show that a temporary increase in GnRH promotes parental effort immediately following an acute stressor, a pattern ripe for future investigation.
GnRH treatment of fathers also had real effects on offspring growth at the age of their most rapid daily mass gain. Across both treatment groups, male visitation rates positively correlated with chick growth, and male treatment did not affect female parental behavior, suggesting that the observed treatment effect on chick growth was the direct result of the treatment effect on male parental behavior. In a single day, chicks of GnRH-injected males gained roughly 0.4g more than chicks in saline nests. This difference amounts to a 14% difference in growth rate, during a period of time when chicks grow by 4x in a matter of days (Wolf et al., in press). While this treatment effect on chick mass was subtle and gone by 12 days post-hatch (5-7 days after male injection), we can extrapolate how this difference in growth would affect development if applied to the entire pre-fledging period. Floaters can intrude at nestboxes, even during the chick period (Stutchbury and Robertson, 1987) and extra-pair paternity is likewise high (76% of broods), with most extra-pair matings occurring among neighbors (Kempenaers et al., 2001). Therefore, it is reasonable to expect males could have social interactions that activate the HPG axis, even during chick rearing, in a way comparable to that of our one-time treatment. If such activation occurred repeatedly, longer-term changes in paternal behavior could influence the overall pace of nestling growth, as well as asymptotic mass or feather length achieved prior to fledging (Gebhardt-Henrich and Richner, 1998), particuarly in light of the strong relationship we observed between male visitation rates and chick growth. Each of these factors have clear effects on the probability of survival to fledging (Martin et al., 2011) or after fledging (Martin et al., 2018). Thus, changes in offspring development that stem from HPG axis activation in a parent could have real consequences that are visible to natural selection.
Regardless of how HPG axis activation may have promoted parental care and chick growth, a key question remains: how can we reconcile our finding that brief GnRH-induced T elevations did not negatively affect paternal care, despite decades of correlative and experimental work showing a negative relationship between T and paternal care in birds?
Resolution of this issue may rest on the idea that a male’s hormonal reactive scope — and thus his ability to reallocate resources towards T-mediated mating effort — is condition-dependent. It is well established that quality, or condition, can vary greatly among individuals (Rowe and Houle, 1996). Such individual differences in resource acquisition can generate positive correlations of traits or behaviors among individuals, even when negative correlations (i.e., trade-offs) are anticipated or observed within individuals (Laskowski et al., 2021; Van Noordwijk and de Jong, 1986). In our experiment, we found that ΔT of GnRH males positively correlated with body mass and chick growth, suggesting that a male’s T reactive scope reflects his condition or quality, as previously seen in other species (McGlothlin et al., 2008; Millesi et al., 2002). Higher quality individuals are likely to have greater overall resources to allocate between mating, parental, and somatic effort (Magrath and Komdeur, 2003); therefore, they may be able to reallocate resources toward mating effort without measurable consequences to current offspring. This is not to say that T elevation is universally cost-free for high quality individuals, but rather that they have the somatic resources to provide parental care in spite of elevated T.
Extending this reasoning, lower quality males should have fewer resources available to reallocate toward pursuing additional mating opportunities. For them, an increase in mating effort could lead to a fall in parental or somatic effort in such a way that survival and/or current reproductive success is affected. But critically, in our study, these low quality males hardly increased T at all, which may in turn have limited their ability to reallocate resources toward T-mediated mating phenotypes during the parental breeding stage. In single-brooded and highly synchronous breeders such as the tree swallow, new mating opportunities during the chick-rearing stage are not abundant, as most females have already laid eggs. Furthermore, extra-pair paternity in this species is often biased toward males in better condition (i.e., heavier and fewer ectoparasites; Kempenaers et al., 2001) and with brighter dorsal plumage coloration (Whittingham and Dunn, 2016). Therefore, low quality males may have little to gain by investing in putative T-mediated mating phenotypes while simultaneously rearing chicks. Our observations suggest that selection has already acted to ‘turn off’ the HPG axes of some males during this breeding stage. In contrast, males of multi-brooded or asynchronous breeding species may face greater selective pressure to maintain reproductive capabilities throughout the breeding season. In those cases, lower quality males could still be induced to elevate T and reallocate resources toward mating, even at a cost to parental effort. This could explain the negative effect of GnRH injection on parental behavior seen in the multi-brooded black redstart (Goymann and Dávila, 2017), which stands in contrast to our own findings.
Moving forward, one way to test for condition-dependent effects of T on parental care would be to measure the effects of standardized doses of T (via injections) on males with different reactive scopes. We predict that if lower quality males’ T levels were elevated beyond their reactive scope, this may in fact negatively affect their parental care, as we initially expected to see in this study. Indeed, how individual variation in condition or other components of the phenotype interacts with hormonal responses or manipulations is a poorly understood but critical aspect of life history and evolutionary trajectories (e.g., Bonier and Cox, 2020).
In summary, we found that, contrary to predictions based on decades of prior research on T-mediated trade-offs in birds, a single activation of the HPG axis did not reduce parental care. Instead, GnRH-injected males provisioned their chicks more than saline-injected males, regardless of how much they increased T in response to the treatment. These effects have the potential to influence reproductive fitness, as rapidly-growing chicks of GnRH-injected males experienced faster growth during the next day. Among GnRH-injected males, we found that chick growth and male body mass were positively correlated with the magnitude of a male’s T response, supporting the idea that GnRH ΔT positively reflects male quality. We propose that only the highest-quality individuals maintain the ability to elevate T during parental stages, effectively preventing any visible costs to parental care from naturally-occurring T elevations. Thus, seasonal and evolutionary patterns of T-mediated trade-offs cannot necessarily be generalized to the timescale of transient changes in T experienced by an individual, if selection has already limited the potential scope of T production in a condition-dependent manner. Moving forward, we urge further research on how even the potential for T-mediated trade-offs can vary among individuals, species, and ecological settings, and how trade-off parameters can become fixed over evolutionary time.
Supplementary Material
Highlights.
We injected parental male tree swallows with either GnRH or saline.
GnRH-males elevated testosterone (T) and provisioned more than saline males.
Compared to saline, GnRH-males’ chicks gained more mass during the following day.
GnRH males’ ΔT positively correlated with chick growth but not parental behavior.
T-mediated trade-offs may not be visible within a male’s own reactive scope.
ACKNOWLEDGEMENTS
Many thanks to Indiana University’s Research and Teaching Preserve, Indiana Department of Natural Resources, and Bloomington Parks and Recreation for access to field sites. We are also grateful to SD Myers, KR Content, EK Dossey, AM Bishop, and the 2016-2018 TRES crew for help in the field; and to SE Wolf, AB Bentz, SE Lipshutz, CL Fitzpatrick, GE Demas, and CR Lattin, and two anonymous reviewers for valuable feedback.
Funding:
This project was funded by National Science Foundation grants to KAR (IOS-1656109), EMG (NSF graduate research fellowship) and IU Center for the Integrative Study of Animal Behavior REU site program (DBI-1460949) supported DN. KAR and EMG are also supported by NIH T32HD049336.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Acevedo-Rodriguez A, Kauffman AS, Cherrington BD, Borges CS, Roepke TA, Laconi M, 2018. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol, 30(10), e12590. doi: 10.1111/jne.12590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins-Regan E, 2005. Hormones and animal social behavior. Princeton University Press. [Google Scholar]
- Ambardar M, Grindstaff JL, 2017. Pre-GnRH and GnRH-induced testosterone levels do not vary across behavioral contexts: A role for individual variation. Gen Comp Endocrinol, 246, 51–62. doi: 10.1016/j.ygcen.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Bókony V, Lendvai Ádám Z., Liker A, Angelier F, Wingfield John C., Chastel O, 2009. Stress Response and the Value of Reproduction: Are Birds Prudent Parents? Am Nat, 173(5), 589–598. doi: 10.1086/597610 [DOI] [PubMed] [Google Scholar]
- Bonier F, Cox RM, 2020. Do hormone manipulations reduce fitness? A meta-analytic test of the Optimal Endocrine Phenotype Hypothesis. Mol Cell Endocrinol, 500, 110640. doi: 10.1016/j.mce.2019.110640 [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN, 2012. Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Horm Behav, 62(3), 210–218. doi: 10.1016/j.yhbeh.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breheny P, Burchett W, 2017. Visualization of regression models using visreg. R J, 9(2), 56. [Google Scholar]
- Bridge ES, Bonter DN 2011. A low-cost radio frequency identification device for ornithological research. J Field Ornithol, 82(1), 52–59. doi: 10.1111/j.1557-9263.2010.00307.x [DOI] [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP, 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol, 65(1), 23–35. doi: 10.1007/s00265-010-1029-6 [DOI] [Google Scholar]
- Cain KE, Pryke SR, 2016. Testosterone production ability predicts breeding success and tracks breeding stage in male finches. J Evol Biol, 430–436. doi: 10.1111/jeb.13005 [DOI] [PubMed] [Google Scholar]
- Concannon PW, Roberts P, Graham L, Tennant BC, 1998. Annual cycle in LH and testosterone release in response to GnRH challenge in male woodchucks (Marmota monax). Reproduction, 114(2), 299–305. doi: 10.1530/jrf.0.1140299 [DOI] [PubMed] [Google Scholar]
- Gebhardt-Henrich S, Richner H, 1998. Causes of growth variation and its consequences for fitness. Oxford Ornithology Series, 8, 324–339. [Google Scholar]
- George EM, Rosvall KA, 2018. Testosterone production and social environment vary with breeding stage in a competitive female songbird. Horm Behav, 103, 28–35. doi: 10.1016/j.yhbeh.2018.05.015 [DOI] [PubMed] [Google Scholar]
- Goymann W, Dávila PF, 2017. Acute peaks of testosterone suppress paternal care: evidence from individual hormonal reaction norms. Proc Biol Sci, 284(1857). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Landys MM, Wingfield JC, 2007. Distinguishing seasonal androgen responses from male–male androgen responsiveness—Revisiting the Challenge Hypothesis. Horm Behav, 51(4), 463–476. doi: 10.1016/j.yhbeh.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Goymann W, Moore IT, Oliveira RF, 2019. Challenge Hypothesis 2.0: A Fresh Look at an Established Idea. BioScience, 69(6), 432–442. doi: 10.1093/biosci/biz041 [DOI] [Google Scholar]
- Goymann W, Villavicencio CP, Apfelbeck B, 2015. Does a short-term increase in testosterone affect the intensity or persistence of territorial aggression? — An approach using an individual’s hormonal reactive scope to study hormonal effects on behavior. Physiol Behav, 149, 310–316. doi: 10.1016/j.physbeh.2015.06.029 [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Merritt JR, Jalabert C, Ma C, Maney DL, Soma KK, 2018. Rapid effects of 17β-estradiol on aggressive behavior in songbirds: Environmental and genetic influences. Horm Behav, 104, 41–51. doi: 10.1016/j.yhbeh.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HL, Hodson DJ, Gregory SJ, Townsend J, Tortonese DJ, 2008. Gonadotropin-Releasing Hormone Stimulates Prolactin Release from Lactotrophs in Photoperiodic Species Through a Gonadotropin-Independent Mechanism. Biol. Reprod, 78(2), 370–377. doi: 10.1095/biolreprod.107.064063 [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S, 2016. multcomp: simultaneous inference in general parametric models. R package version 1.4-6. [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED, 2006. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen Comp Endocrinol, 149(2), 182–189. doi:http://dx.doi.Org/10.1016/j.ygcen.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED, 2007. Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct Ecol, 21(4), 767–775. doi: 10.1111/j.1365-2435.2007.01280.x [DOI] [Google Scholar]
- Kempenaers B, Everding S, Bishop C, Boag P, Robertson RJ, 2001. Extra-pair paternity and the reproductive role of male floaters in the tree swallow (Tachycineta bicolor). Behav Ecol Sociobiol, 49(4), 251–259. doi: 10.1007/s002650000305 [DOI] [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW, 2009. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr Comp Biol, 49(4), 365–379. doi: 10.1093/icb/icp057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski KL, Moiron M, Niemelä PT, 2021. Integrating Behavior in Life-History Theory: Allocation versus Acquisition? Trends Ecol Evol, 36(2), 132–138. doi: 10.101/j.tree.2020.10.017 [DOI] [PubMed] [Google Scholar]
- Leffelaar D, Robertson RJ, 1986. Equality of feeding roles and the maintenance of monogamy in tree swallows. Behav Ecol Sociobiol, 18(3), 199–206. doi: 10.1007/bf00290823 [DOI] [Google Scholar]
- Lendvai ÁZ, Akçay Ç, Ouyang JQ, Dakin R, Domalik AD, St John PS, Stanback M, Moore IT, Bonier F, 2015. Analysis of the Optimal Duration of Behavioral Observations Based on an Automated Continuous Monitoring System in Tree Swallows (Tachycineta bicolor): Is One Hour Good Enough? PLoS ONE, 10(11), e0141194. doi: 10.1371/journal.pone.0141194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, Singmann H, Love J, Buerkner P, Herve M, 2018. Emmeans: Estimated marginal means, aka least-squares means. R package version, 1(1), 3. [Google Scholar]
- Lombardo MP, 1991. Sexual differences in parental effort during the nestling period in Tree Swallows (Tachycineta bicolor). Auk, 108(2), 393–404. [Google Scholar]
- Lynn SE, 2016. Endocrine and neuroendocrine regulation of fathering behavior in birds. Horm Behav, 77, 237–248. doi: 10.1016/j.yhbeh.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Magrath MJL. Komdeur J, 2003. Is male care compromised by additional mating opportunity? Trends Ecol Evol, 18(8), 424–430. doi: 10.1016/S0169-5347(03)00124-1 [DOI] [Google Scholar]
- Martin TE, Lloyd P, Bosque C, Barton DC, Biancucci AL, Cheng Y-R, Ton R, 2011. Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution, 65(6), 1607–1622. doi: 10.1111/j.1558-5646.2011.01227.x [DOI] [PubMed] [Google Scholar]
- Martin TE, Tobalske B, Riordan MM, Case SB, Dial KP, 2018. Age and performance at fledging are a cause and consequence of juvenile mortality between life stages. Sci Adv, 4(6), eaar1988. doi: 10.1126/sciadv.aar1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JP, 2002. The number of visits to the nest by parents is an accurate measure of food delivered to nestlings in Tree Swallows. J Field Ornithol, 73(1), 9–14. doi: 10.1648/0273-8570-73.1.9 [DOI] [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED, 2008. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J Evol Biol, 21(1), 39–48. doi: 10.1111/j.1420-9101.2007.01471.x [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED, 2007. Natural Variation in a Testosterone-Mediated Trade-Off between Mating Effort and Parental Effort. Am Nat, 170(6), 864–875. doi: 10.1086/522838 [DOI] [PubMed] [Google Scholar]
- McGlothlin Joel W., Whittaker Danielle J., Schrock Sara E., Gerlach Nicole M., Jawor Jodie M., Snajdr Eric A., Ketterson Ellen D., 2010. Natural Selection on Testosterone Production in a Wild Songbird Population. Am Nat, 175(6), 687–701. doi: 10.1086/652469 [DOI] [PubMed] [Google Scholar]
- Meddle SL, Romero LM, Astheimer LB, Buttemer WA, Moore IT, Wingfield JC, 2002. Steroid Hormone Interrelationships with Territorial Aggression in an Arctic-Breeding Songbird, Gambel’s White-Crowned Sparrow, Zonotrichia leucophrys gambelii. Horm Behav, 42(2), 212–221. doi: 10.1006/hbeh.2002.1813 [DOI] [PubMed] [Google Scholar]
- Millesi E, Hoffmann IE, Steurer S, Metwaly M, Dittami JP, 2002. Vernal Changes in the Behavioral and Endocrine Responses to GnRH Application in Male European Ground Squirrels. Horm Behav, 41(1), 51–58. doi: 10.1006/hbeh.2001.1735 [DOI] [PubMed] [Google Scholar]
- Quinney TE, Hussell DJ, Ankney CD, 1986. Sources of variation in growth of Tree Swallows. Auk, 103(2), 389–400. [Google Scholar]
- Roff D, 1993. Evolution of life histories: theory and analysis. Springer Science & Business Media. [Google Scholar]
- Romero LM, Reed JM, 2005. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A: Mol Integr Physiol, 140(1), 73–79. doi: 10.1016/j.cbpb.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Jayaratna SP, Ketterson ED, 2016. Divergence along the gonadal steroidogenic pathway: Implications for hormone-mediated phenotypic evolution. Horm Behav, 84, 1–8. doi: 10.1016/j.yhbeh.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Houle D, 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc Biol Sci, 263(1375), 1415–1421. doi:doi: 10.1098/rspb.1996.0207 [DOI] [Google Scholar]
- Small TW, Bebus SE, Bridge ES, Elderbrock EK, Ferguson SM, Jones BC, Schoech SJ, 2017. Stress-responsiveness influences baseline glucocorticoid levels: Revisiting the under 3min sampling rule. Gen Comp Endocrinol, 247, 152–165. doi: 10.1016/j.ygcen.2017.01.028 [DOI] [PubMed] [Google Scholar]
- Smiley KO, 2019. Prolactin and avian parental care: New insights and unanswered questions. Horm Behav. doi: 10.1016/j.yhbeh.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Stearns SC, 1992. The Evolution of Life Histories. OUP Oxford. [Google Scholar]
- Stiver KA, Alonzo SH, 2009. Parental and Mating Effort: Is There Necessarily a Trade-Off? Ethology, 115(12), 1101–1126. doi: 10.1111/j.1439-0310.2009.01707.x [DOI] [Google Scholar]
- Stutchbury BJ, Robertson RJ, 1986. A Simple Trap for Catching Birds in Nest Boxes. J Field Ornithol, 57(1), 64–65. [Google Scholar]
- Stutchbury BJ, Robertson RJ, 1987. Behavioral tactics of subadult female floaters in the tree swallow. Behav Ecol Sociobiol, 20(6), 413–419. doi: 10.1007/BF00302984 [DOI] [Google Scholar]
- Teather K, 1996. Patterns of Growth and Asymmetry in Nestling Tree Swallows. J Avian Biol, 27(4), 302–310. doi: 10.2307/3677261 [DOI] [Google Scholar]
- Trainor BC, Marler CA, 2001. Testosterone, Paternal Behavior, and Aggression in the Monogamous California Mouse (Peromyscus californicus). Horm Behav, 40(1), 32–42. doi: 10.1006/hbeh.2001.1652 [DOI] [PubMed] [Google Scholar]
- Van Noordwijk AJ, de Jong G, 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat, 128(1), 137–142. [Google Scholar]
- Van Roo BL, Ketterson ED, Sharp PJ, 2003. Testosterone and prolactin in two songbirds that differ in paternal care: the blue-headed vireo and the red-eyed vireo. Horm Behav, 44(5), 435–441. doi: 10.1016/j.yhbeh.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Virgin EE, Rosvall KA, 2018. Endocrine-immune signaling as a predictor of survival: A prospective study in developing songbird chicks. Gen Comp Endocrinol, 267, 193–201. doi: 10.1016/j.ygcen.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Vitousek MN, Johnson MA, Downs CJ, Miller ET, Martin LB, Francis CD, Donald JW, Fuxjager MJ, Goymann W, Hau M, Husak JF, Kircher BK, Knapp R, Schoenle LA, Williams TD, 2019. Macroevolutionary Patterning in Glucocorticoids Suggests Different Selective Pressures Shape Baseline and Stress-Induced Levels. Am Nat, 193(6), 866–880. doi: 10.1086/703112 [DOI] [PubMed] [Google Scholar]
- Weber GM, Powell JF, Park M, Fischer WH, Craig AG, Rivier JE, Nanakorn U, Parhar IS, Ngamvongchon S, Grau EG, Sherwood NM, 1997. Evidence that gonadotropin-releasing hormone (GnRH) functions as a prolactin-releasing factor in a teleost fish (Oreochromis mossambicus) and primary structures for three native GnRH molecules. J Endocrinol, 155(1), 121–132. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ, 2005. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J Exp Zool B Mol Dev Evol, 304B(6), 610–618. doi: 10.1002/jez.b.21071 [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Dunn PO, 2016. Experimental evidence that brighter males sire more extra-pair young in tree swallows. Mol Ecol, 25(15), 3706–3715. doi: 10.1111/mec.13665 [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM Jr., Ball GF, 1990. The “Challenge Hypothesis”: Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. Am Nat, 136(6), 829–846. doi: 10.2307/2462170 [DOI] [Google Scholar]
- Wingfield JC, Lewis DM, 1993. Hormonal and behavioural responses to simulated territorial intrusion in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. Anim Behav, 45(1), 1–11. doi: 10.1006/anbe.1993.1001 [DOI] [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD, 1998. Ecological Bases of Hormone—Behavior Interactions: The “Emergency Life History Stage”1. Am Zool, 38(1), 191–206. doi: 10.1093/icb/38.1.191 [DOI] [Google Scholar]
- Wolf S, Stansberry K, Content K, Rosvall K, in press. Telomerase activation and tissue-specific consequences: implications for telomeres as drivers of fitness outcome in wild birds. J Avian Biol. [Google Scholar]
- Wolf SE, Sanders TL, Beltran SE, Rosvall KA, preprint. The telomere regulatory gene POT1 responds to stress and predicts performance in nature: implications for telomeres and life history evolution. bioRxiv. doi: 10.1101/2021.01.06.425609 [DOI] [PubMed] [Google Scholar]
- Zuur AF, leno EN, Elphick CS, 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol, 1(1), 3–14. doi: 10.1111/j.2041-210X.2009.00001.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


