Abstract
Recently, it has been reported that neonicotinoid pesticides (NNs) are transferred from mother to child and are assumed to affect the next generation, but the behavioral effects of NN exposure at different developmental stages have not been investigated. We exposed mice to no-observed-adverse-effect level (NOAEL) doses of clothianidin (CLO) during the fetal and lactational period, and then evaluated the neurobehavioral effects in juvenile and adult mice. Significant increases in anxiety-like behavior and locomotor activity were observed in juveniles and adults, respectively, and neuronal activity and neurogenesis in the hippocampal dentate gyrus were affected in both stages. These results suggest that fetal and lactational exposure to CLO may inhibit neurogenesis and cause different behavioral abnormalities at different developmental stages.
Keywords: behavioral test, clothianidin, developmental stage, fetal and lactational exposure, neurogenesis
Neonicotinoid pesticides (NNs) are a new class of pesticides developed in the 1980s and are widely used due to their greater convenience compared to conventional pesticides. NNs have a higher affinity for insect nicotinic acetylcholine receptors (nAChRs) than mammal nAChRs [29] and were originally considered safe for mammals. However, in the last decade it has been reported that NNs have nicotine-like excitatory effects on mammalian nAChRs [13], especially clothianidin (CLO), a type of NN, has been reported to be toxic to mammalian emotional behavior even at less than the no-observed-adverse-effect level (NOAEL) [8, 9, 26, 27, 33]. Recent studies have also reported that NNs were detected in the urine and breast milk of pregnant women and newborns [2, 10, 15], and Ohno et al. [19] clearly demonstrated rapid passage of clothianidin (CLO) through the placental barrier, suggesting exposure of NN to the next generation. Fetal and lactating individuals are thought to be vulnerable to chemicals because the blood-brain barrier is not yet developed and they are much more sensitive to chemicals than adults [1]. Because neurogenesis is active and neural circuits are developing during the fetal and lactational period, chemical exposure can affect the formation of neural circuits and cause abnormal behavior [21]. Previous studies have reported that the offspring of mice exposed to NNs during the developmental period have delayed behavioral development [28], abnormalities in anxiety-like and social behaviors in adulthood [23] and effects related to neurodevelopmental toxicity, such as reduced neurogenesis [18], and thus there are concerns about the effects of NNs on the next generation of the nervous system. Recently, it has also been shown that the environment in the fetal and lactational period influences future health and can pose risks for certain diseases, which may be related to the development including psychiatric and developmental disorders [17, 32]. Indeed, there is growing evidence that developmental exposure to chemicals, including pesticides, induces adult hyperactivity and that one of the causes is impaired development of the dopaminergic nervous system [11, 24]. Therefore, the effects of fetal and lactational exposure to CLO may extend to adults as well, so risk assessment for the next generation is necessary not only in juveniles but also in adulthood. The present study focused on the emotional behavior and nervous system of juvenile and adult mice exposed to the NOAEL of CLO during the fetal and lactational period in order to examine the effects of NNs on the next generation.
C57BL/6NCrSlc pregnant mice were purchased from Japan SLC (Hamamatsu, Japan) and maintained as described elsewhere [9]. This study was approved by the Institutional Animal Care and Use Committee (Permission #26-05-07) and carried out according to the Kobe University Animal Experimental Regulations. The dams were divided into two groups: CLO-0 (0 mg/kg body weight/day, dams n=17, pups n=39) and CLO-65 (65 mg/kg body weight/day, dams n=15, pups n=28). The administration concentration was set with reference to the NOAEL (ICR female mice: 65.1 mg/kg/day) [5, 30]. To eliminate the risk of adverse effects associated with gavage, the dams were given MediGel (MediGel® Sucralose; ClearH2O, Portland, ME, USA) with CLO or vehicle (1% Dimethyl sulfoxide: DMSO) as a substitute for filtered water from embryonic day (E) 1.5 to postnatal day (PND) 21 of age of the offspring. Dams ingested the gel actively, and the CLO concentration of 65 mg/kg/day did not affect the amount of gel intake. Each litter was randomly selected to a maximum of six pups on PND 2 to standardize the amount of milk, and a litter whose size was three or less was removed from the experiment for the same reason. One or two pups per litter were used in order to avoid a litter bias. Exposure to CLO was estimated by measuring body weight and gel intake in pregnant mice. The amounts of isolated CLO added to the gel were calculated as follows: the CLO purity was 90%; the daily gel intake and average body weight were 6 g/day and 22 g for early pregnancy (–E10.5), 6.5 g/day and 30 g for late pregnancy (E10.5–19.5), 16 g/day and 29 g for the first week of lactation, and 20 g/day for the second and third weeks of lactation; and the total gel weight was 60 g, excluding the cup weight. The calculated amount of CLO was dissolved in 600 µl of DMSO (1% volume of gel), injected into the cups containing the gel, and shaken vigorously to mix the CLO evenly in the gel.
The male pups were divided into four groups: 3 wk CLO-0 (3-week-old CLO-0), 3 wk CLO-65 (3-week-old CLO-65), 10 wk CLO-0 (10-week-old CLO-0), 10 wk CLO-65 (10-week-old CLO-65) and subjected to the open field test (OF) and the elevated plus maze test (EPM). Both behavioral tests were performed under the conditions described elsewhere [16]. The OF is based on the property that mice prefer wall borders and decreasing in time spent in the center zone (30 × 30 cm) of the open field is defined as increasing in anxiety-like behavior. In addition, we recorded total distance and moving speed [total distance (cm)/ total movement duration (sec)] as an index of the locomotor activity in a novel environment. Next, the EPM was performed to evaluate behavior under the fear condition of no walls at high altitude. Similar to the OF, this test uses the property which mice prefer wall borders and decreasing percentage of open arm entries is defined as increasing in anxiety-like behavior. In addition, we recorded total distance as an index of the locomotor activity. The results of each behavioral test were analyzed with Image J software (National Institutes of Health, Bethesda, MD, USA).
After the behavioral test, all mice were deeply anesthetized with isoflurane using an inhalation anesthesia apparatus (BS-400T; Brain Science Idea, Osaka, Japan), after which, the animals were euthanized. The brains were excised, weighed, cryoprotected in ascending solutions of sucrose (10%, 20%, 30%) in 0.1 M phosphate buffer overnight and then frozen in liquid nitrogen in an embedding solution consisting of Tissue-Tek® O.C.T. compound (Sakura Finetek, Tokyo, Japan). Some excised brains above mentioned were immersed in the 4% paraformaldehyde solution for 20 hr at 4°C, dehydrated through a graded series of ethanol followed by xylene and embedded in paraffin. Coronal sections (−1.58 mm to −2.06 mm and 0.38 mm to −0.10 mm from the bregma) were cut at 10-µm-thickness, and mounted on a glass slide precoated with 0.2% 3-aminopropyltriethoxysilane (Shin-Etsu Chemical Co., Tokyo, Japan).
We used the following antibodies: rabbit monoclonal anti‐c-fos (1:10,000; #2353; Cell Signaling Technology, Beverly, MA, USA) and rabbit polyclonal anti‐doublecortin (DCX, 1:3,000; ab18723; Abcam, Cambridge, UK) in the dentate gyrus of the hippocampus (DG); rabbit monoclonal anti‐NeuN (1:3,000; ab177487; Abcam) in the DG and the cerebral cortex; and mouse monoclonal anti‐dopamine receptor D1 (DRD1, 1:1,000; MAB5290; Merck Millipore, Darmstadt, Germany) and rabbit polyclonal anti‐dopamine receptor D2 (DRD2, 1:1,200; AB5084P; Merck Millipore) in the striatum. Secondary antibodies were conjugated with EnVision + System- HRP Labeled Polymer (Dako, Glostrup, Denmark) and Histofine MAX-PO (M) (Histofine Simplestain System) (Nichirei Bioscience, Tokyo, Japan). Immunostaining of all sections was performed as previously described [9]. We counted the number of c-fos-positive cells in the DG, and DCX in the subgranular zone (SGZ), the inner layer of the DG, and the number of immunopositive cells per area by using ImageJ software.
Statistical analyses were performed with Excel Statistics 2012 (Version 1.00; SSRI, Tokyo, Japan). The behavioral and quantitative immunohistochemical data were analyzed by two-way ANOVA (CLO × age) followed by Tukey-Kramer post hoc test to determine the main effects and their interaction. Welch’s t-test or Mann-Whitney U-test was used to detect differences in the gel intake, litter size, or body and brain weight between the control and administration groups. The results were considered significant when the P-value was less than 0.05.
The gel intake, litter size, brain weight and body weight of 3- and 10-week-old pups were not significantly different between the administration and control groups (Tables 1, 2).
Table 1. Gel intake and litter size of the mothers.
| Groups |
||
|---|---|---|
| CLO-0 | CLO-65 | |
| Gel intake (g/day) | ||
| Early pregnancy | 5.67 ± 0.97 | 5.57 ± 1.28 |
| Late pregnancy | 6.55 ± 0.95 | 6.43 ± 1.02 |
| Litter size | 8.00 ± 1.50 | 8.07 ± 1.24 |
CLO, clothianidin. Mean ± SD, n=15–17.
Table 2. Body weight and brain weight of the pups.
| Groups |
||||
|---|---|---|---|---|
| 3 wk CLO-0 | 3 wk CLO-65 | 10 wk CLO-0 | 10 wk CLO-65 | |
| Body weight (g) | 9.29 ± 0.82 | 9.04 ± 0.83 | 26.66 ± 1.56 | 27.11 ± 2.10 |
| Brain weight (g) | 0.45 ± 0.02 | 0.45 ± 0.01 | 0.487 ± 0.02 | 0.499 ± 0.02 |
CLO, clothianidin. Mean ± SD, n=18–37.
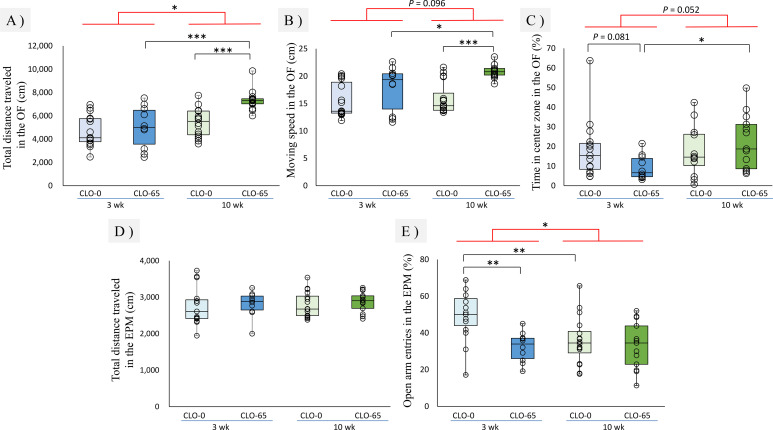
In regard to locomotor activity, there was a significant main effect of CLO and age with a significant interaction on the total distance traveled in the OF [F(1, 53)=9.287, P<0.01; F(1, 53)=16.76, P<0.001; F(1, 53)=4.829, P<0.05]. In regard to the moving speed in the OF, there was a significant main effect for CLO, and a interaction was close to significant [F(1, 53)=19.51, P<0.001; F(1, 53)=4.189, P<0.05; F(1, 53)=2.872, P=0.096]. Both parameters were significantly increased in the 10 wk CLO-65 group, although no significant effects were found between the juvenile group (Fig. 1A, 1B). On the other hand, the total distance traveled in the EPM had no significant effect in either the juvenile or adult group (Fig. 1D). As for anxiety-like behavior, the main effect of age and a interaction was close to significant on the time spent in the center zone in the OF, [F(1, 53)=0.399, P=0.083; F(1, 53)=3.976, P=0.052] and this parameter tended to decrease in the 3 wk CLO-65 group compared to the 3 wk CLO-0 group (Fig. 1C). On the open arm entries in the EPM, there was a significant main effect of CLO and a close to significant main effect of age and a significant interaction [F(1, 53)=8.496, P<0.05; F(1, 53)=3.616, P=0.063; F(1, 53)=4.560, P<0.05] and significant decrease in the 3 wk CLO-65 group compared to the CLO-0 group, which meant an increase in anxiety-like behaviors (Fig. 1E). There were no significant changes in these parameters in the adult group. The results of the behavioral tests revealed that exposure to the NOAEL dose of CLO during the fetal and lactational period increased anxiety-like behaviors in juvenile mice, and then increased locomotor activity in adulthood despite the absence of CLO intake. That is, exposure to CLO in the fetal and lactational period was found to cause different behavioral abnormalities between juveniles and adults.
Fig. 1.
Effects of clothianidin (CLO) exposure during the fetal and lactational period at 3 and 10 weeks of age on total distance traveled (A), moving speed (B), and the time spent in the center zone (C) in the open field test (OF) and on total distance traveled (D) and open arm entries (E) in the elevated plus maze test (EPM). Data are reported in the form of a box plot, and each result is plotted. The numbers of mice in each group were as follows: 3 wk CLO-0 (n=15); 3 wk CLO-65 (n=10); 10 wk CLO-0 (n=16); 10 wk CLO-65; (n=13). A, B: In regard to the total distance traveled, there was a significant interaction between CLO and age. In the 10 wk CLO-65 mice, the total distance traveled and moving speed in the OF were significantly greater and faster than those in the 3 wk CLO-65 and 10 wk CLO-0 group. C: In the 3 wk CLO-65 mice, the time spent in the center zone in the OF has a tendency or significant low level compared to that in the 3 wk CLO-0 and 10 wk CLO-65 group, respectively. D: There was no significant difference in the total distance in the EPM among the groups. E: There was a significant interaction between CLO and age. In the 3 wk CLO-65 mice, the number of open arm entries in the EPM was significantly lower than that in the 3 wk CLO-0 group. *P<0.05, **P<0.01, ***P<0.001 vs. other groups (two-way ANOVA followed by Tukey-Kramer post hoc test).
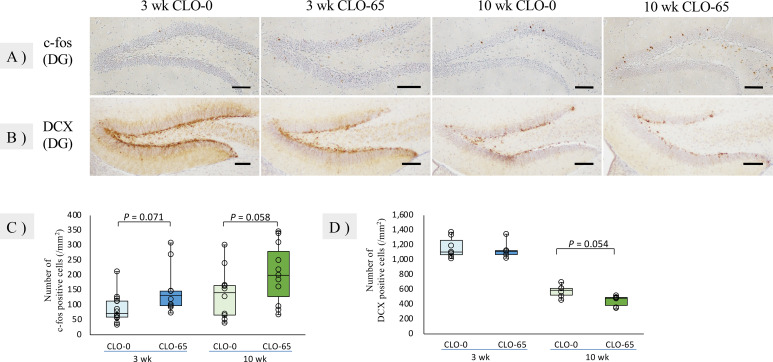
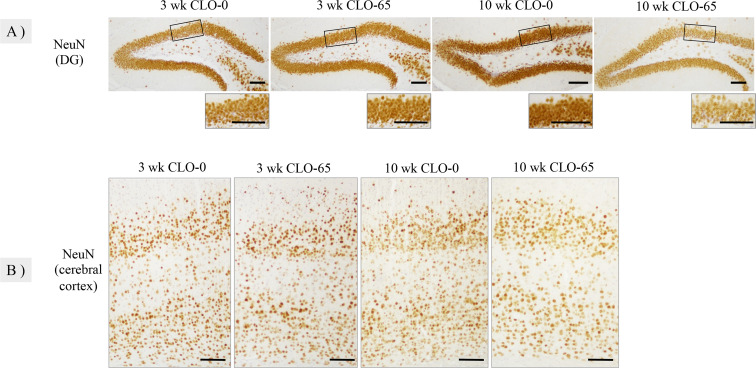
The number of c-fos-positive cells per unit area in the DG after behavioral tests had a significant main effect on CLO and age [F(1, 43)=7.276, P<0.05; F(1, 43)=4.740, P<0.05], and tended to be increased in the administration group compared to the control group (Fig. 2A, 2C). The expression of c-fos is an indirect marker of neuronal activity, because c-fos is often expressed when neurons fire action potentials [4, 31]. The present results indicate that neuronal activity in the DG was disrupted by fetal and lactational exposure to CLO. We visualized DCX and NeuN in DGs by immunohistochemistry to examine their effects on neurogenesis. DCX is a microtubule-associated protein expressed by neuronal precursor cells and immature neurons. Immunohistochemistry of DCX showed positive reactions in all groups, mainly in the cell bodies and dendrites of SGZ (Fig. 2B). The number of DCX-positive cells per unit area in the SGZ showed a main effect of age [F(1, 23)=198.6, P<0.05]. There was no significant difference in the number of positive cells in the juvenile group, but the density and branching of dendrites decreased, suggesting that the maturation of immature neurons may have been inhibited in the administration group. The number of DCX-positive cells decreased in the 10 wk CLO-65 group compared to the control group (Fig. 2D). NeuN was expressed in the nucleus of post-mitotic neurons and immunohistochemistry for NeuN showed positive reactions to the nuclei of DG and cerebral cortex neurons in all groups (Fig. 3). There was no significant difference in the number or sequence of NeuN-positive cells in the cerebral cortex in either group or in the DG in the juvenile group. Positive cells were densely distributed in the 10 wk CLO-0 group DG, whereas the number of positive cells in the 10 wk CLO-65 group was reduced, and the distribution was relatively sparse. NeuN-positive cells were not altered in the cerebral cortex on the same section, proving that the changes in DG were not due to staining differences caused by the procedure or antibody concentration gradient. These results show that fetal and lactational exposure to CLO does not affect the proliferation of juvenile neural progenitor cells but inhibits the maturation of immature neurons, and in adults such prior exposure inhibits the proliferation or viability of neural progenitor cells and reduces the number of neural cells. Previous studies have shown that the α7 and β2 nAChR subunits with CLO binding affinity are expressed in DG neural progenitor cells [12], and that stimulation of α7 nAChR in cultured hippocampal cells activates ERK 1/2, which promotes the proliferation of neural progenitor cells [3], acetamiprid and imidacloprid exposure from the postnatal period (PND12-26) by oral gavage has also been shown to decrease the number of DCX- and NeuN-positive cells, resulting in decreased neurogenesis [18], and administration of donepezil, which suppresses the degradation of acetylcholine, promotes hippocampal neurogenesis in the adult brain [14]. Thus, many reports suggest that nAChRs play an important role in hippocampal neurogenesis. Also, it has been reported that neurogenesis is up-regulated by the administration of anxiolytics [20] and that neurogenesis inhibitors administered during the developmental period can induce hyperactivity and anxiety in adulthood [6]. In addition, adult neurogenesis is thought to play an important role in the basic functions of the hippocampus, such as spatial cognition, learning, memory, and emotion [22, 25], and is closely related the behavior. These findings and the results of immunohistochemistry of c-fos, DCX, and NeuN suggested that exposure to CLO in the fetal and lactational period interfered with neurogenesis and the normal formation of neurocircuitry, disrupted signaling in the hippocampal DG, and induced abnormal behavior in both the juvenile period and adulthood.
Fig. 2.
Representative histology and immunohistochemistry for c-fos and doublecortin (DCX) in the dentate gyrus of the hippocampus (DG) at 3 and 10 weeks of age (A, B) and the numbers of c-fos-positive cells in the DG and DCX-positive cells in the subgranular zone (SGZ) (C, D). Bar=100 µm. Data are reported in the form of a box plot, and each result is plotted. A, C: The number of c-fos-positive cells in the DG showed a substantial but not significant increase in both the 3 and 10 wk clothianidin (CLO)-65 group compared to the control groups. The numbers of mice were as follows: 3 wk CLO-0 (n=14); 3 wk CLO-65 (n=9); 10 wk CLO-0 (n=10); 10 wk CLO-65 (n=11). B, D: The density and branching of dendrites decreased in the 3 wk CLO-65 group compared to the 3 wk CLO-0 group and the number of DCX-positive cells in the SGZ showed a substantial but not significant decrease in the 10 wk CLO-65 group compared to the 10 wk CLO-0 group. The numbers of mice were as follows: 3 wk CLO-0 (n=7); 3 wk CLO-65 (n=5); 10 wk CLO-0 (n=6); 10 wk CLO-65 (n=6). All data were analyzed by two-way ANOVA followed by Tukey-Kramer post hoc test.
Fig. 3.
Representative histology and immunohistochemistry for NeuN in the dentate gyrus of the hippocampus (DG) and the cerebral cortex at 3 and 10 weeks of age. Bar=100 µm. A: The number of NeuN-positive cells in the DG was reduced in the 10 wk clothianidin (CLO)-65 group. B: No significant effect on the number or sequence of NeuN-positive cells in the cerebral cortex was observed in any group.
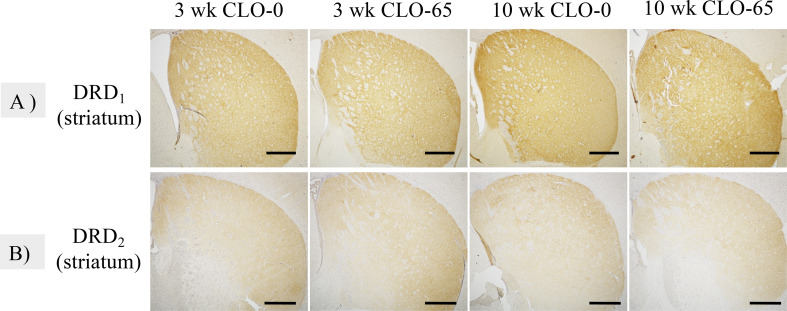
Dopamine (DA) is a neurotransmitter in the brain, and the major DA nerves in the midbrain belong to the nigrostriatal pathway. It is known that the amount of DA in the striatum is closely related to the increase in locomotion activity [7]. But we found no effect of CLO on DRD1 or DRD2 immunoreactivity in the striatum (Fig. 4).
Fig. 4.
Representative immunohistochemistry for dopamine receptor D1 (DRD1) and D2 (DRD2) in the striatum at 3 and 10 weeks of age. Bar=500 µm. A, B: No significant effect on the intensity of DRD1 or DRD2 positivity in the striatum was observed.
In the present study, the anxiety-like behaviors seen in juvenile mice disappeared and locomotor activity increased in adulthood. In regard to the possible mechanisms underlying these changes, the anxiety-like behaviors were erased in the process of growth or caused by juvenile-specific responses, but the difference in reactivity to CLO between the juvenile period and adulthood requires further investigation. On the other hand, the effects of fetal and lactational exposure may have manifested continuously from the juvenile period to adulthood, leading to an increase in hyperactivity in adulthood compared to the juvenile period. In fact, previous studies have reported that lactational exposure to bisphenol A induces apoptosis of substantia nigra neurons and increases locomotion activity in the adult rat brain [11].
Also, a single exposure of NOAEL doses of CLO to adult mice increased anxiety-like behavior in previous studies [9]. It has been shown that a sudden and transient increase in CLO levels causes abnormalities in emotional behavior, whereas the present study showed that chronic exposure to CLO in the fetal and lactational period caused abnormalities in emotional behavior associated with neurodevelopment disorders, indicating differences in behavioral effects depending on the timing and duration of CLO administration.
This study is the first to analyze the neurobehavioral effects of fetal and lactational exposure to a NOAEL dose of CLO on mice at different developmental stages. The results show that CLO inhibits neurogenesis, disrupts signaling, and increases anxiety-like behavior in the juvenile period and locomotor activity in adulthood. However, the detailed mechanisms by which CLO inhibits neurogenesis remain to be clarified, so additional studies are needed. The ongoing results of such studies will help to clarify the relationship between pesticides and psychiatric and developmental disorders.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was partly supported by Grants-in-Aid for Scientific Research A (#JP18H04132 to YI), B (#JP19H04277 to NH), and Grant-in-Aid for Early-Career Scientists (#JP19K19406 to TH) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by a grant from the Japan Environ and Organic-Farming Foundation (#2019004 to NH); and by an “Act Beyond Trust” (GIA) civil grant 2020 (to NH, YI). We also acknowledge financial support from the Nakajima Foundation, the Sumitomo Foundation, the Nihon Seimei Foundation and Triodos Foundation (to YI).
REFERENCES
- 1.Charnley G., Putzrath R. M.2001. Children’s health, susceptibility, and regulatory approaches to reducing risks from chemical carcinogens. Environ. Health Perspect. 109: 187–192. doi: 10.1289/ehp.01109187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen D., Liu Z., Barrett H., Han J., Lv B., Li Y., Li J., Zhao Y., Wu Y.2020. Nationwide biomonitoring of neonicotinoid insecticides in breast milk and health risk assessment to nursing infants in the Chinese population. J. Agric. Food Chem. 68: 13906–13915. doi: 10.1021/acs.jafc.0c05769 [DOI] [PubMed] [Google Scholar]
- 3.Dajas-Bailador F. A., Soliakov L., Wonnacott S.2002. Nicotine activates the extracellular signal-regulated kinase 1/2 via the α7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 80: 520–530. doi: 10.1046/j.0022-3042.2001.00725.x [DOI] [PubMed] [Google Scholar]
- 4.Dragunow M., Faull R.1989. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 29: 261–265. doi: 10.1016/0165-0270(89)90150-7 [DOI] [PubMed] [Google Scholar]
- 5.Food and Agriculture Organization of the United Nations. 2016. FAO Specifications and Evaluations for Agricultural Pesticide Clothianidin. http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/Clothianidin2011.pdf [accessed on January 10, 2020].
- 6.Guo N., Yoshizaki K., Kimura R., Suto F., Yanagawa Y., Osumi N.2013. A sensitive period for GABAergic interneurons in the dentate gyrus in modulating sensorimotor gating. J. Neurosci. 33: 6691–6704. doi: 10.1523/JNEUROSCI.0032-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagino Y., Kasai S., Fujita M., Setogawa S., Yamaura H., Yanagihara D., Hashimoto M., Kobayashi K., Meltzer H. Y., Ikeda K.2015. Involvement of cholinergic system in hyperactivity in dopamine-deficient mice. Neuropsychopharmacology 40: 1141–1150. doi: 10.1038/npp.2014.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirano T., Yanai S., Omotehara T., Hashimoto R., Umemura Y., Kubota N., Minami K., Nagahara D., Matsuo E., Aihara Y., Shinohara R., Furuyashiki T., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2015. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 77: 1207–1215. doi: 10.1292/jvms.15-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano T., Yanai S., Takada T., Yoneda N., Omotehara T., Kubota N., Minami K., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Hoshi N.2018. NOAEL-dose of a neonicotinoid pesticide, clothianidin, acutely induce anxiety-related behavior with human-audible vocalizations in male mice in a novel environment. Toxicol. Lett. 282: 57–63. doi: 10.1016/j.toxlet.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa G., Kuribayashi R., Ikenaka Y., Ichise T., Nakayama S. M. M., Ishizuka M., Taira K., Fujioka K., Sairenchi T., Kobashi G., Bonmatin J. M., Yoshihara S.2019. LC-ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth. PLoS One 14: e0219208. doi: 10.1371/journal.pone.0219208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishido M., Yonemoto J., Morita M.2007. Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol. Lett. 173: 66–72. doi: 10.1016/j.toxlet.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 12.Kaneko N., Okano H., Sawamoto K.2006. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 11: 1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x [DOI] [PubMed] [Google Scholar]
- 13.Kimura-Kuroda J., Komuta Y., Kuroda Y., Hayashi M., Kawano H.2012. Nicotine-like effects of the neonicotinoid insecticides acetamiprid and imidacloprid on cerebellar neurons from neonatal rats. PLoS One 7: e32432. doi: 10.1371/journal.pone.0032432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotani S., Yamauchi T., Teramoto T., Ogura H.2006. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience 142: 505–514. doi: 10.1016/j.neuroscience.2006.06.035 [DOI] [PubMed] [Google Scholar]
- 15.López-García M., Romero-González R., Lacasaña M., Garrido Frenich A.2017. Semiautomated determination of neonicotinoids and characteristic metabolite in urine samples using TurboFlow™ coupled to ultra high performance liquid chromatography coupled to Orbitrap analyzer. J. Pharm. Biomed. Anal. 146: 378–386. doi: 10.1016/j.jpba.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 16.Maeda M., Yokoyama T., Kitauchi S., Hirano T., Mantani Y., Tabuchi Y., Hoshi N.2021. Influence of acute exposure to a low dose of systemic insecticide fipronil on locomotor activity and emotional behavior in adult male mice. J. Vet. Med. Sci. 83: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masarwa R., Levine H., Gorelik E., Reif S., Perlman A., Matok I.2018. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: a systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am. J. Epidemiol. 187: 1817–1827. doi: 10.1093/aje/kwy086 [DOI] [PubMed] [Google Scholar]
- 18.Nakayama A., Yoshida M., Kagawa N., Nagao T.2019. The neonicotinoids acetamiprid and imidacloprid impair neurogenesis and alter the microglial profile in the hippocampal dentate gyrus of mouse neonates. J. Appl. Toxicol. 39: 877–887. doi: 10.1002/jat.3776 [DOI] [PubMed] [Google Scholar]
- 19.Ohno S., Ikenaka Y., Onaru K., Kubo S., Sakata N., Hirano T., Mantani Y., Yokoyama T., Takahashi K., Kato K., Arizono K., Ichise T., Nakayama S. M. M., Ishizuka M., Hoshi N.2020. Quantitative elucidation of maternal-to-fetal transfer of neonicotinoid pesticide clothianidin and its metabolites in mice. Toxicol. Lett. 322: 32–38. doi: 10.1016/j.toxlet.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 20.Peng Z., Zhang R., Wang H., Chen Y., Xue F., Wang L., Yang F., Chen Y., Liu L., Kuang F., Tan Q.2013. Ziprasidone ameliorates anxiety-like behaviors in a rat model of PTSD and up-regulates neurogenesis in the hippocampus and hippocampus-derived neural stem cells. Behav. Brain Res. 244: 1–8. doi: 10.1016/j.bbr.2013.01.032 [DOI] [PubMed] [Google Scholar]
- 21.Rice D., Barone S., Jr. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 108 Suppl 3: 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahay A., Hen R.2007. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 10: 1110–1115. doi: 10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- 23.Sano K., Isobe T., Yang J., Win-Shwe T. T., Yoshikane M., Nakayama S. F., Kawashima T., Suzuki G., Hashimoto S., Nohara K., Tohyama C., Maekawa F.2016. In utero and lactational exposure to acetamiprid induces abnormalities in socio-sexual and anxiety-related behaviors of male mice. Front. Neurosci. 10: 228–240. doi: 10.3389/fnins.2016.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith T. F., Schmidt-Kastner R., McGeary J. E., Kaczorowski J. A., Knopik V. S.2016. Pre- and perinatal ischemia-hypoxia, the ischemia-hypoxia response pathway, and ADHD risk. Behav. Genet. 46: 467–477. doi: 10.1007/s10519-016-9784-4 [DOI] [PubMed] [Google Scholar]
- 25.Snyder J. S., Hong N. S., McDonald R. J., Wojtowicz J. M.2005. A role for adult neurogenesis in spatial long-term memory. Neuroscience 130: 843–852. doi: 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 26.Takada T., Yoneda N., Hirano T., Onaru K., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Nimako C., Ishizuka M., Ikenaka Y., Hoshi N.2020. Combined exposure to dinotefuran and chronic mild stress counteracts the change of the emotional and monoaminergic neuronal activity induced by either exposure singly despite corticosterone elevation in mice. J. Vet. Med. Sci. 82: 350–359. doi: 10.1292/jvms.19-0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takada T., Yoneda N., Hirano T., Yanai S., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Hoshi N.2018. Verification of the causal relationship between subchronic exposures to dinotefuran and depression-related phenotype in juvenile mice. J. Vet. Med. Sci. 80: 720–724. doi: 10.1292/jvms.18-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T.2012. Effects of maternal clothianidin exposure on behavioral development in F1 generation mice. Toxicol. Ind. Health 28: 697–707. doi: 10.1177/0748233711422726 [DOI] [PubMed] [Google Scholar]
- 29.Tomizawa M., Casida J. E.2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45: 247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930 [DOI] [PubMed] [Google Scholar]
- 30.Uneme H., Konobe M., Akayama A., Yokota T., Mizuta K.2006. Discovery and development of a novel insecticide ‘clothianidin’, Sumitomo Kagaku 2. pp. 1–14. https://www.sumitomo-chem.co.jp/english/rd/report/theses/docs/20060202_h6t.pdf [accessed on January 10, 2020].
- 31.VanElzakker M., Fevurly R. D., Breindel T., Spencer R. L.2008. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn. Mem. 15: 899–908. doi: 10.1101/lm.1196508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Lieshout R. J., Boylan K.2010. Increased depressive symptoms in female but not male adolescents born at low birth weight in the offspring of a national cohort. Can. J. Psychiatry 55: 422–430. doi: 10.1177/070674371005500705 [DOI] [PubMed] [Google Scholar]
- 33.Yoneda N., Takada T., Hirano T., Yanai S., Yamamoto A., Mantani Y., Yokoyama T., Kitagawa H., Tabuchi Y., Hoshi N.2018. Peripubertal exposure to the neonicotinoid pesticide dinotefuran affects dopaminergic neurons and causes hyperactivity in male mice. J. Vet. Med. Sci. 80: 634–637. doi: 10.1292/jvms.18-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]