Abstract
Histiocytic sarcoma was investigated histopathologically and immunohistochemically in 17 four-toed hedgehogs (Atelerix albiventris), along with a review of their clinical data. Cases were histopathologically classified into two types: round-polygonal cell type (6 cases) and spindle cell type (11 cases). Round-polygonal cell type was found in visceral organs such as the spleen, lymph nodes, and more, and most cases of this type were consistent with disseminated histiocytic sarcoma. On the other hand, spindle cell type occurred mainly in skin, and almost all cases were consistent with localized histiocytic sarcoma. The prognosis of patients with round-polygonal cell type appeared worse than that of spindle cell type. Immunohistochemically, neoplastic cells of spindle cell type showed stronger reactivity against human leukocyte antigen-DR than round-polygonal cell type. Neoplastic cells of all cases showed strong reactivity against ionized calcium-binding adapter molecule-1 (Iba-1) and various reactivities against cluster of differentiation (CD) 204. Regardless of morphological classification, most tumor cells were negative for CD163, suggesting that this marker is less effective for the diagnosis of histiocytic sarcoma. The results of this study suggest that Iba-1 is the most effective marker for histiocytic sarcoma.
Keywords: four-toed hedgehog, histiocytic sarcoma, immunohistochemistry
Four-toed hedgehogs (Atelerix albiventris) are among the most popular pets in Japan. It is known that the incidence of neoplasia is high in this species. In one study, 63 of 105 samples (60%) of four-toed hedgehogs submitted to histopathological examination were classified as neoplastic lesions [18]. Uterine tumors, oral squamous cell carcinomas, mammary gland tumors, lymphomas, and fibrosarcomas are common [18, 20].
Histiocytic sarcoma is a malignant neoplasm originating from interstitial dendritic cells or bone marrow macrophages (hemophagocytic histiocytic sarcoma). This neoplasm occurs in various organs, such as the spleen, lymph nodes, bone marrow, lung, central nervous system, skin, and more. Two forms of histiocytic sarcoma are known: 1) localized histiocytic sarcoma, which occurs in one organ; and 2) disseminated histiocytic sarcoma, which metastasizes from local lymph nodes or disseminates to systemic organs [14, 15]. Histiocytic sarcoma is more common in dogs, and fewer cases have been reported in many domestic animal species such as cats, rabbits, hamsters, and ferrets [2, 4, 6, 7, 13, 23, 25].
There are a few reports of histiocytic sarcoma in four-toed hedgehogs [10, 16, 17, 21]. However, most are single or case series-based reports [10, 16, 17]. A single comprehensive study including clinical, histopathological, and immunohistochemical features of this tumor has recently been reported [21]. In the present study, the clinical and histopathological features of histiocytic sarcoma were examined in 17 four-toed hedgehogs, and the immunohistochemical efficacy of various antibodies for histiocytes was evaluated.
MATERIALS AND METHODS
The samples included 10 biopsies and 7 necropsies submitted by seven veterinary clinics to the Laboratory of Veterinary Pathology at Nihon University. In all necropsy cases, the necropsy was performed by clinicians (so-called necropsy in a jar). These cases were collected from June 2017 to February 2020, and all cases were diagnosed as histiocytic sarcoma. All tissue samples were fixed in 10% neutral-buffered formalin solution. After trimming of tissues, the samples were embedded in paraffin wax. For histopathological examination, paraffin-embedded tissues were sectioned at a thickness of 5 µm and stained with hematoxylin and eosin (HE). Clinical information, including signalment, clinical signs, tumor location, survival time, metastasis, and recurrence (if applicable), was provided by the referring clinicians.
All cases were reviewed by all authors, and the tumors were classified into 2 histological types according to the previous literature in dogs: 1) round-polygonal cell type; and 2) spindle cell type [3, 22]. The criteria for round-polygonal cell type are more than 50% of the neoplastic cells are round to polygonal-shaped and have distinct cell borders, whereas spindle cell type is composed of spindle-shaped cells with indistinct cell borders in more than 50% of neoplastic tissues. Mitoses were randomly counted in 10 high-power fields (400×). Hemophagocytosis and multinucleated giant cells were also noted.
For immunohistochemistry, the primary antibodies used in the present study and the antigen retrieval of each antibody are summarized in Table 1. Primary antibody diluted appropriately at 4°C overnight was applied to tissue sections. Subsequently, tissue sections to which cluster of differentiation (CD) 208 was applied were dropped into Histofine simple cysteine Max-PO (Rat) (Nichireibioscience, Tokyo, Japan), and the other sections were dropped into Histofine simple cysteine Max-PO (MULTI) (Nichireibioscience) at room temperature for 30 min. In all tumor tissues, 10 high-power fields (400×) were examined randomly and divided into 4 categories as follows: negative (−)=0% of neoplastic cells were positive; weakly positive (+)=0–25% of neoplastic cells were positive; moderately positive (++)=25–50% of neoplastic cells were positive; and strongly positive (+++)=more than 50% of neoplastic cells were positive. Tissues of normal spleen, lymph nodes, bone marrow, lung, liver, and skin from four four-toed hedgehogs were used for positive and negative controls. Tissues of normal spleen and lymph nodes from two dogs were also used as controls.
Table 1. Primary antibodies used for immunohistochemical staining.
| Antibody* | Type (Clone) | Dilution | Antigen retrieval | Target | Source |
|---|---|---|---|---|---|
| Iba-1 | Rabbit polyclonal antibody | 1:1,000 | Autoclaving with citrate buffer (pH 6.0) at 121°C for 10 min | Dendritic cell, macrophage, microglia | Wako, Osaka, Japan |
| HLA-DR | Mouse monoclonal antibody (TAL.1B5) | 1:20 | Autoclaving with citrate buffer (pH 6.0) at 121°C for 10 min | Antigen-presenting cell | Dako, Tokyo, Japan |
| CD204 | Mouse monoclonal antibody (SRA-E5) | 1:100 | Autoclaving with citrate buffer (pH 6.0) at 121°C for 10 min | Macrophage | TransGenic, Kobe, Japan |
| CD163 | Mouse monoclonal antibody (AM-3K) | 1:80 | Autoclaving with citrate buffer (pH 6.0) at 121°C for 10 min | Macrophage | TransGenic, Kobe, Japan |
| CD208 | Rat monoclonal antibody (1010E1.01) | 1:50 | Autoclaving with citrate buffer (pH 6.0) at 121°C for 10 min | Dendritic cell | Dendritics, Lyon, France |
*Iba-1: ionized calcium-binding adapter molecule-1, HLA-DR: human leukocyte antigen-DR., CD: cluster of differentiation
RESULTS
Clinical findings and histological examination
The clinical and histopathological findings are summarized in Table 2. Morphologically, 6 cases (35.3%) were categorized as round-polygonal cell type, and 11 cases (64.7%) were categorized as spindle cell type. Of the cases of round-polygonal cell type, 4 (66.7%) were male and 2 (33.3%) were female. The median age was 2 years and 2 months, and the median survival time was 27.5 days in the 4 cases with information available. The most common clinical signs were anorexia, hypoactivity, and diarrhea. In this cell type, the neoplasm was widely disseminated to multiple visceral organs in 5 (83.3%) of 6 cases. In these cases, the primary sites were spleen (n=2), mesenteric lymph nodes (n=1), intra-abdominal mass (n=1), and undetermined (n=1), and the neoplasm was disseminated to heart, lung, lymph nodes, salivary gland, spleen, pancreas, liver, gastrointestinal tract, adrenal gland, kidney, female genital system, and male accessory gland in all 5 cases. The organs that were the most severely affected or formed the largest apparent lesion on gross examination and/or ultrasonography were considered the possible primary sites of the tumors. Grossly, in the primary sites, a focal, tan to light-gray mass, with multifocal variably sizes masses in some cases, was observed. The spleen in most cases was diffusely enlarged with rounded edges. In metastatic lesions, multifocal, tan to light gray masses were randomly distributed throughout the organs. The other case primarily involved the small intestine, which metastasized to the mesenteric lymph nodes. In all cases, the neoplastic tissues were highly cellular, invasive, and effaced normal tissue architectures. The neoplasm consisted of round or polygonal cells arranged in a loose to dense sheet pattern. The neoplastic cells had distinct cell borders with moderate amounts of eosinophilic cytoplasm. Nuclei were variably sized, round, with coarsely stippled chromatin and distinct nucleoli. Anisocytosis and anisokaryosis were moderate, and one to 17 mitoses were seen per 10 high-power fields (400×). Hemophagocytosis was often observed in the spleen, liver, and lung of 3 cases (50.0%) (Fig. 1). Multinucleated giant cells were also confirmed, mainly in the spleen.
Table 2. Summary of clinical and histopathological findings.
| Cell type | Case No. | Age a) | Sex b) | Clinical signs | Survival time | Tumor location c) | Mitoses d) | Hemophagocytosis e) | Multinucleated giant cells f) | Metastasis/ recurrence | Sample |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Round-polygonal cell | |||||||||||
| 1 | 1y 9m | M | Diarrhea, mesenteric lymph node enlargement | Unknown | Small intestine*, mesenteric lymph node | 4 | - | - | Metastasis | Biopsy | |
| 2 | 2y | M | Mass formation | 36 days | Spleen*, vascular, lung, liver, kidney | 2 | Spleen, liver | Spleen | Dissemination | Necropsy | |
| 3 | 1y 11m | F | Anorexia | 19 days | Heart, lung, spleen, liver, omentum majus, kidney, ovary, uterus, vagina *unknown | 8 | Lung, spleen, liver | Spleen, omentum majus | Dissemination | Necropsy | |
| 4 | 2y 3m | F | Anorexia | 178 days | Spleen*, liver, adrenal gland, kidney | 1 | Spleen | - | Dissemination | Necropsy | |
| 5 | 3y 5m | M | Anorexia, hypoactivity, reduction of evacuation | 5 days | Mesenteric lymph node*, heart, vascular, lung, spleen, anterior mediastinal lymph node, salivary gland, liver, pancreas, stomach, intestine, kidney | 17 | - | Spleen | Dissemination | Necropsy | |
| 6 | 4y | M | Anorexia, hypoactivity, diarrhea | Unknown | Abdominal mass*, intestine, mesenterium, kidney, accessory gland | 6 | - | - | Dissemination | Necropsy | |
| Spindle cell | |||||||||||
| 7 | 2y 10m | F | Pubis swelling | Unknown | Caudal aspect of urethra | 0 | - | - | ‐ | Biopsy | |
| 8 | 1y | F | Mass formation | Unknown | Skin (left armpit)*, axillary lymph node | 15 | - | - | Metastasis | Biopsy | |
| 9 | 2y 4m | M | Mass formation | 359 days | Skin (left back) | 0 | - | - | Recurrence | Biopsy | |
| 10 | 3y | F | Mass formation | Unknown | Skin (left gena)*, skin (anterior thorax) | 28 | - | - | Recurrence | Biopsy | |
| 11 | Unknown | F | Mass formation | Unknown | Skin (central abdomen)*, right inguinal lymph node | 6 | - | Skin | Metastasis | Biopsy | |
| 12 | 3y 11m | F | Mass formation | 129 days | Skin (left head) | 2 | - | Skin | Recurrence | Biopsy | |
| 13 | 2y | F | Mass formation | Unknown | Skin (left abdomen) | 30 | - | - | - | Biopsy | |
| 14 | 3y 6m | M | Penis prolapse | 96 days | Skin (penis) | 6 | - | - | - | Biopsy | |
| 15 | 4y | M | Mass formation | More than 25 days | Skin (left cubital joint) | 34 | - | - | - | Biopsy | |
| 16 | 2y 6m | M | Anorexia, hypoactivity, diarrhea, abdominal lymph node enlargement | Unknown | Abdominal lymph node*, spleen, liver | 2 | Spleen | Spleen, abdominal lymph node | Dissemination | Necropsy | |
| 17 | 5y 11m | M | Progressive paralysis of hind limb, progressive lumbar osteolysis | 91 days | Lumbar vertebral body | 9 | - | Lumbar vertebral body | - | Necropsy | |
a) y=year(s); m=month(s), b) M=male; F=female, c) *=primary location of tumor, d) number of mitotic figures (10 high-power fields (400×)), e) location of hemophagocytosis, f) location of multinucleated giant cells.
Fig. 1.
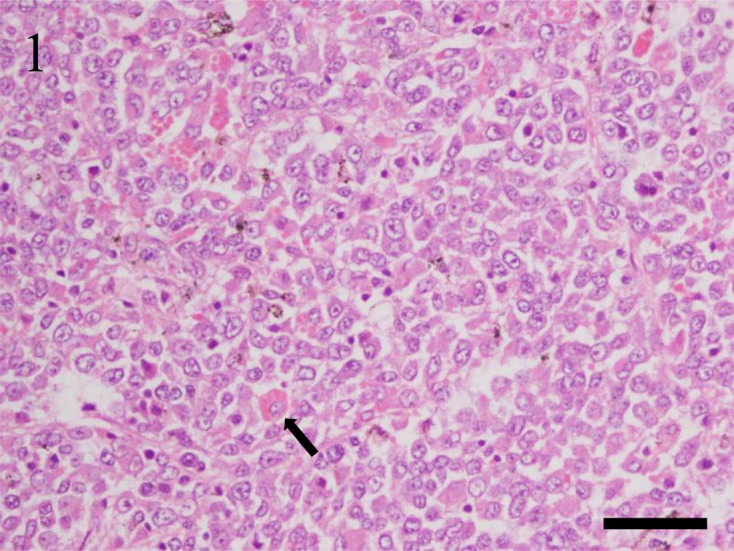
Case No. 2, spleen. Histiocytic sarcoma of round-polygonal cell type. The neoplasm consists of round to polygonal cells arranged in a dense sheet pattern. Neoplastic cells have distinct cell borders and moderate amounts of eosinophilic cytoplasm. Nuclei are various sized and round. Hemophagocytosis (arrow) is often observed. Hematoxylin and eosin stain. Bar=50 µm.
In cases of spindle cell type, 5 cases (45.5%) were male, and 6 cases (54.5%) were female. The median age was 2 years and 11 months, and the median survival time was 112.5 days in the 5 cases with survival information available. The common clinical sign was mass formation. The neoplasm of 8 (72.7%) of 11 cases occurred primarily in the skin of the trunk (n=4), head (n=2), joint (n=1), and penis (n=1). In these cases, local recurrence in 3 cases and lymph node metastasis in 2 cases were confirmed. In addition, the neoplasm occurred in the caudal aspect of the urethra (n=1), a lumbar vertebral body (n=1), and an abdominal lymph node (n=1). In the case that primarily occurred in an abdominal lymph node, the neoplasm disseminated to the spleen and liver. Grossly, the dermis to subcutaneous tissue was expanded by a tan to light-gray mass in most cutaneous lesions. In the neoplasm of the skin, neoplastic tissue invaded from the dermis to the subcutis. The neoplasm consisted of spindle-shaped to angular cells arranged in interlacing bundles. The neoplastic cells had indistinct cell borders with small amounts of eosinophilic cytoplasm. Nuclei were variably sized, oval to elongated, with coarsely stippled chromatin and distinct nucleoli. Anisocytosis and anisokaryosis were moderate, and 0 to 34 mitoses were seen per 10 high-power fields (400×). Hemophagocytosis was confirmed in the spleen of only 1 case. In addition, multinucleated giant cells and mild to moderate infiltration of inflammatory cells, such as neutrophils, eosinophils, and lymphocytes, were often seen (Fig. 2).
Fig. 2.
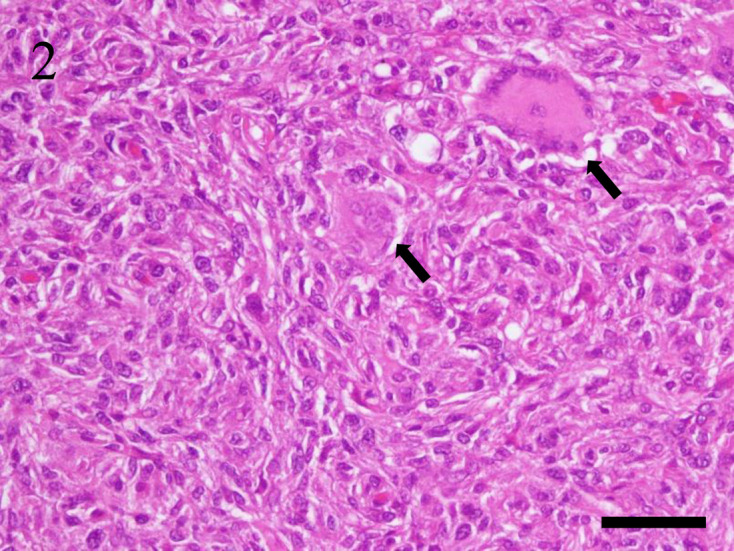
Case No. 12, skin. Histiocytic sarcoma of spindle cell type. The neoplasm consists of spindle-shaped to angular cells arranged in interlacing bundles. Neoplastic cells have indistinct cell borders and small amounts of eosinophilic cytoplasm. Nuclei are variable in size and oval to elongated in shape. Multinucleated giant cells (arrow) are often observed. Hematoxylin and eosin stain. Bar=50 µm.
Immunohistochemical evaluation
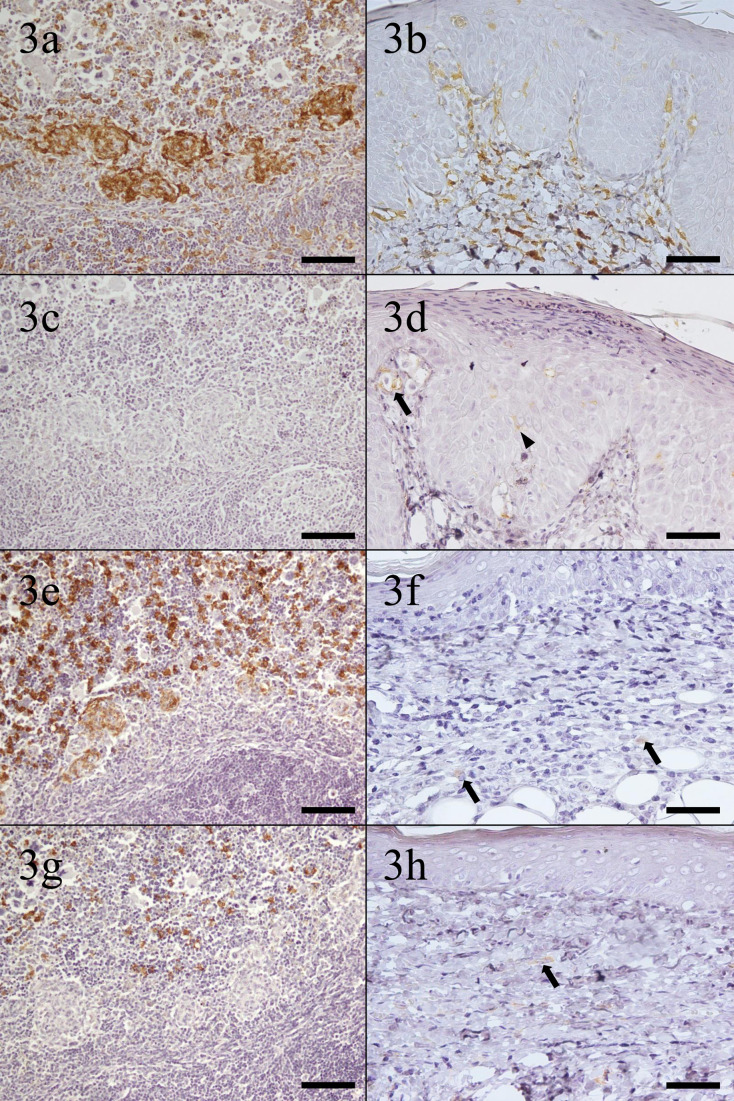
The immunohistochemical results of the normal tissues of four-toed hedgehogs are summarized in Table 3. Dendritic cells and macrophages in all tissues examined were immunopositive for ionized calcium-binding adapter molecule-1 (Iba-1) (Fig. 3a and 3b). Only bone marrow macrophages, epidermal Langerhans cells, and dermal dendritic cells in the skin showed positive immunostaining for anti-human leukocyte antigen-DR (HLA-DR) antibody (Fig. 3c and 3d). Macrophages in most tissues examined were immunopositive for CD204 (Fig. 3e and 3f). Only macrophages in red pulp and dermal macrophages were immunopositive for CD163 (Fig. 3g and 3h). With CD208, only type II pneumocytes were immunopositive, but dendritic cells and macrophages in each tissue were immunonegative. Since sufficient results could not be obtained, further research using CD208 was excluded from the present study.
Table 3. Immunohistochemical results of normal tissues in four-toed hedgehogsa).
| Tissue | Cell | Iba-1 | HLA-DR | CD204 | CD163 | CD208 b) |
|---|---|---|---|---|---|---|
| Spleen | Interdigitating dendritic cells | + | – | – | – | – |
| Macrophages in red pulp | + | – | + | + | – | |
| Macrophages in marginal zone | + | – | + | – | – | |
| Macrophages in white pulp (germinal centre/mantle zone) | + | – | + | – | – | |
| Lymph node | Interdigitating dendritic cells | + | – | – | – | – |
| Macrophages in sinus | + | – | + | – | – | |
| Bone marrow | Macrophages | + | + | + | – | – |
| Lung | Alveolar macrophages | + | – | + | – | – |
| Liver | Kupffer cells | + | – | + | – | – |
| Skin | Epidermal langerhans cells | + | + | – | – | – |
| Dermal dendritic cells | + | + | – | – | – | |
| Dermal macrophages | + | – | + | + | – | |
a) − =Negative, + =positive, Iba-1: ionized calcium-binding adapter molecule-1, HLA-DR: human leukocyte antigen-DR, CD: cluster of differentiation. b) Only type II pneumocytes in lung were positive for CD208.
Fig. 3.
Immunohistochemical staining of normal tissues. (a) Spleen. Interdigitating dendritic cells in white pulp and macrophages in red pulp and the marginal zone are positive for ionized calcium-binding adapter molecule-1 (Iba-1). Bar=100 µm. (b) Skin. Epidermal Langerhans cells, dermal dendritic cells, and dermal macrophages are positive for Iba-1. Bar=50 µm. (c) Spleen. No cells positive for human leukocyte antigen-DR (HLA-DR). Bar=100 µm. (d) Skin. Epidermal Langerhans cells and dermal dendritic cells are positive for HLA-DR. Arrowhead: epidermal Langerhans cell; arrow: dermal dendritic cell. Bar=50 µm. (e) Spleen. Macrophages in red pulp and the marginal zone are positive for cluster of differentiation (CD) 204. Bar=100 µm. (f) Skin. Dermal macrophages are positive for CD204. Arrows: dermal macrophages. Bar=50 µm. (g) Spleen. Macrophages in red pulp are positive for CD163. Bar=100 µm. (h) Skin. Dermal macrophages are positive for CD163. Arrow: dermal macrophage. Bar=50 µm.
The reactivity to each antibody in histiocytic sarcoma of four-toed hedgehogs is summarized in Table 4. In round-polygonal cell type, the neoplastic cells of all cases (100.0%) were moderately to strongly positive for Iba-1. The neoplastic cells of 3 of 6 cases (50.0%) were weakly positive for HLA-DR. The neoplastic cells in 1 case (16.7%) were moderately positive, and the others of 5 cases (83.3%) were weakly positive for CD204. The neoplastic cells of 1 case (16.7%) were weakly positive for CD163 (Fig. 4).
Table 4. Summary of immunohistochemical resultsa).
| Cell type | Case No. (tissue) | Iba-1 | HLA-DR | CD204 | CD163 |
|---|---|---|---|---|---|
| Round-polygonal cell | 1 (small intestine) | +++ | – | + | – |
| 2 (spleen) | ++ | + | + | – | |
| 3 (spleen) | +++ | – | ++ | + | |
| 4 (spleen) | ++ | + | + | – | |
| 5 (lymph node) | +++ | – | + | – | |
| 6 (intra-abdominal mass) | +++ | + | + | – | |
| Spindle cell | 7 (caudal aspect of urethra) | +++ | + | ++ | – |
| 8 (skin) | ++ | + | + | – | |
| 9 (skin) | +++ | +++ | + | – | |
| 10 (skin) | +++ | +++ | + | – | |
| 11 (skin) | +++ | + | ++ | – | |
| 12 (skin) | +++ | ++ | ++ | – | |
| 13 (skin) | +++ | ++ | + | + | |
| 14 (skin) | +++ | + | ++ | – | |
| 15 (skin) | +++ | + | + | + | |
| 16 (abdominal lymph node) | +++ | – | +++ | + | |
| 17 (lumbar vertebral body) | ++ | ++ | ++ | + | |
a) Negative (−)=0% of tumor cells were positive; weakly positive (+)=0–25% of tumor cells were positive; moderately positive (+)=25–50% of tumor cells were positive; strongly positive (+++)=more than 50% of tumor cells were positive. Iba-1: ionized calcium-binding adapter molecule-1, HLA-DR: human leukocyte antigen-DR, CD: cluster of differentiation.
Fig. 4.
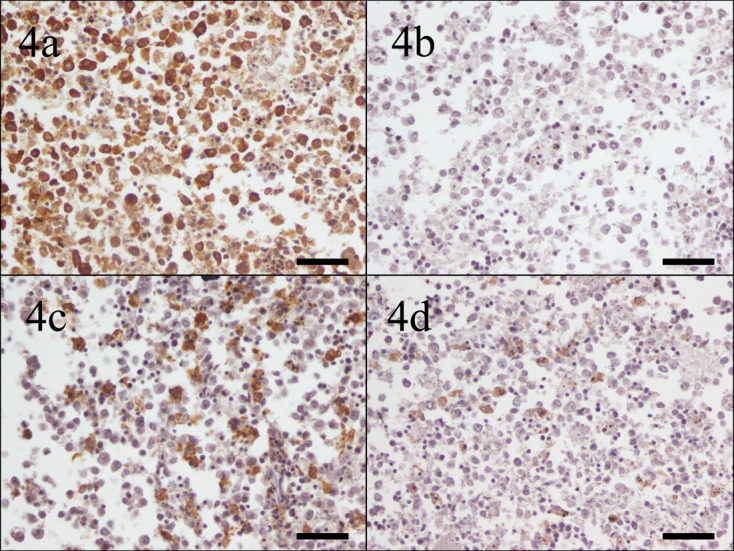
Case No. 3, spleen. Neoplastic cells are (a) strongly positive for ionized calcium-binding adapter molecule-1, (b) negative for human leukocyte antigen-DR, (c) moderately positive for cluster of differentiation (CD), and (d) weakly positive for CD163. Bar=50 µm.
In spindle cell type, the neoplastic cells of all 11 cases (100.0%) were moderately to strongly positive for Iba-1. The neoplastic cells in 2 cases (18.2%) were strongly positive, those of 3 cases (27.3%) were moderately positive, those of 5 cases (45.5%) were weakly positive, and those of 1 case (9.1%) were negative for HLA-DR. Positive reactivity for HLA-DR varied by case. The neoplastic cells of 1 case (9.1%) were strongly positive, those of 5 cases (45.5%) were moderately positive, and those of 5 cases (45.5%) were weakly positive for CD204. The neoplastic cells in only 4 cases (36.4%) were weakly positive for CD163 (Fig. 5).
Fig. 5.
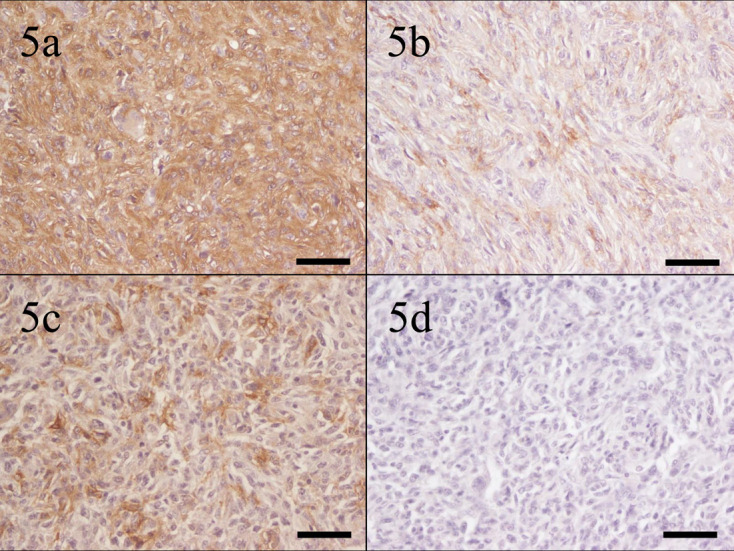
Case No. 12, skin. Neoplastic cells are (a) strongly positive for ionized calcium-binding adapter molecule-1, (b) moderately positive for human leukocyte antigen-DR, (c) moderately positive for cluster of differentiation (CD) 204, and (d) negative for CD163. Bar=50 µm.
DISCUSSION
No differences in sex distribution and age were confirmed when comparing round-polygonal cell type and spindle cell type. However, the median survival time of round-polygonal cell type appeared shorter than that of spindle cell type. In addition, the typical clinical signs of round-polygonal cell type were systemic symptoms such as anorexia, hypoactivity, and diarrhea, whereas those of spindle cell type were only local, such as mass formation. Thus, the clinical behavior and prognosis of round-polygonal cell type could be worse than that of spindle cell type.
Round-polygonal cell type occurred in abdominal organs such as spleen, lymph nodes, and intestine of all cases. On the other hand, spindle cell type occurred in skin in most cases. In addition, hemophagocytosis was more frequently confirmed in round-polygonal cell type than in spindle cell type. Interestingly, these morphological tendencies resemble previous reports on canine histiocytic sarcoma. Neoplasms of round-polygonal cell type occurred mainly in the spleen, whereas those of spindle cell type occurred mainly in the skin in dogs [3]. Furthermore, hemophagocytosis was more frequently observed in round-polygonal cell type [3, 22]. It has been suggested that these morphological features are related to the different microenvironment between visceral organs such as the spleen and skin. The dermis has abundant collagen and fibroblasts, which could be related to induction of spindle cell type histiocytic sarcoma [3]. It has also been suggested that the biological behavior of round-polygonal cell type is worse than that of spindle cell type [22].
Most neoplasms of round-polygonal cell type invaded widely into many internal organs. This feature is consistent with disseminated histiocytic sarcoma (DHS) [1, 5, 14, 15]. On the other hand, in spindle cell type, case 16 coincided with DHS. The other 10 cases were consistent with localized histiocytic sarcoma (LHS) because these neoplasms occurred in a single site [1, 5, 14, 15]. In these cases, recurrence and lymph node metastasis were observed in three cases and two cases, respectively. This feature of tumor distribution resembles canine histiocytic sarcoma. In dogs, DHS is mainly distributed in internal organs, and LHS occurs mainly in skin [1, 5]. However, in dogs, histiocytic sarcoma tends to occur in the skin of appendicular joints, whereas in four-toed hedgehogs, in most cases, histiocytic sarcoma occurred in the skin of the head and trunk, and in only one case, the neoplasm occurred in the skin of an appendicular joint [1, 5]. Thus, the location of LHS differed between dogs and four-toed hedgehogs.
Iba-1 is the antibody that reacts to dendritic cells, macrophages, and microglia [19]. It is useful to diagnose canine and feline histiocytic diseases and has cross-reactivity to the monocyte/macrophage lineage in many animal species, such as horses, sheep, cattle, mice, hamsters, and kangaroos [19]. In four-toed hedgehogs, dendritic cells and macrophages in internal organs and skin showed reactivity to Iba-1. Regardless of cell type, neoplastic cells of histiocytic sarcoma were moderately or strongly positive for Iba-1 in all cases. In this species, it is a useful marker for histiocytic sarcoma, as in other species.
HLA-DR is an anti-major histocompatibility complex class II antibody reacting to antigen-presenting cells. Reactivity is broad, but it reacts mainly to Langerhans cells and interstitial dendritic cells [15]. In dogs and ferrets, histiocytic sarcoma reacts strongly to HLA-DR [22, 23]. In four-toed hedgehogs, HLA-DR has higher reactivity to epidermal Langerhans cells and dermal dendritic cells in the skin than to dendritic cells and macrophages in visceral organs. Therefore, in this species, histiocytic sarcoma in the skin may originate from epidermal Langerhans cells or dermal dendritic cells, as suggested by one study [21].
Both CD204 and CD163 are antibodies that bind to class A scavenger receptors on macrophages [8, 11, 12, 15, 24]. They are specific to monocytes/macrophages and have cross-reactivity to macrophages in various species, such as humans, dogs, cats, horses, cattle, pigs, rabbits, whales, and dolphins [8, 9, 11, 12, 24, 26]. It has been suggested that CD204 is a useful marker for histiocytic sarcoma in dogs, but expression is variable, and not all neoplastic cells necessarily show expression of CD204 [8, 15]. In four-toed hedgehogs, macrophages in each organ were reactive to CD204, consistent with previous reports [8, 24]. The present study suggested that CD204 had lower reactivity to histiocytic sarcoma than Iba-1, and not all neoplastic cells necessarily expressed CD204 in four-toed hedgehogs, as in dogs. On the other hand, only a few macrophages showed reactivity to CD163 in four-toed hedgehogs, and some neoplastic cells were weakly positive. CD163 has cross-reactivity to macrophages in various species [9, 26]. Furthermore, some neoplastic cells of histiocytic sarcoma have reactivity to CD163 in humans, dogs, and ferrets [12, 22, 23]. However, in four-toed hedgehogs, neoplastic cells of histiocytic sarcoma have almost no reactivity to CD163. The present study reports for the first time the expression of CD163 in tissues of four-toed hedgehogs. Based on these results, histiocytic sarcoma of four-toed hedgehogs has features of macrophages, as a previous study indicated [21].
In conclusion, this study focused on two cell types of histiocytic sarcoma in four-toed hedgehogs: 1) round-polygonal cell type; and 2) spindle cell type. Tumor location and biological behavior were different between the two types, and round-polygonal cell type often showed a disseminated form and worse prognosis than spindle cell type. A localized form occurred mainly in the skin, and spindle cell type was confirmed more frequently in skin than in visceral organs, consistent with the previous study [21]. The primary sites of a disseminated form were mainly observed in the spleen or lymph nodes, which was a different tendency from the previous study [21]. The utility of several histiocytic markers, including a marker that has not been reported previously, in many normal hedgehog tissues and neoplastic tissues has been reported. Iba-1 expression of neoplastic cells was strong in all cases, and Iba-1 was the most useful marker for histiocytic sarcoma in four-toed hedgehogs, which was consistent with an earlier study [21]. HLA-DR was more useful for neoplastic cells in skin than for those in visceral organs, but expression was variable. The present study showed the expression of CD163 for the first time in tissues of four-toed hedgehogs, but CD163 was less useful for the diagnosis of histiocytic sarcoma. It has been suggested that the origin of histiocytic sarcoma in this species is variable, such as dendritic cells and macrophages. Expressions of markers used in the present study were different from those in other species. Additional immunohistochemical studies of the histiocyte lineage (dendritic cells and macrophages) in four-toed hedgehogs are warranted to clarify histiocytic diseases in this species.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank the veterinary clinicians of seven animal clinics for providing clinical information.
REFERENCES
- 1.Affolter V. K., Moore P. F.2002. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet. Pathol. 39: 74–83. doi: 10.1354/vp.39-1-74 [DOI] [PubMed] [Google Scholar]
- 2.Coble D. J., Shoemaker M., Harrington B., Dardenne A. D., Bolon B.2015. Histiocytic sarcoma and bilateral facial vein thrombosis in a siberian hamster (Phodopus sungorus). Comp. Med. 65: 127–132. [PMC free article] [PubMed] [Google Scholar]
- 3.Constantino-Casas F., Mayhew D., Hoather T. M., Dobson J. M.2011. The clinical presentation and histopathologic-immunohistochemical classification of histiocytic sarcomas in the Flat Coated Retriever. Vet. Pathol. 48: 764–771. doi: 10.1177/0300985810385153 [DOI] [PubMed] [Google Scholar]
- 4.Friedrichs K. R., Young K. M.2008. Histiocytic sarcoma of macrophage origin in a cat: case report with a literature review of feline histiocytic malignancies and comparison with canine hemophagocytic histiocytic sarcoma. Vet. Clin. Pathol. 37: 121–128. doi: 10.1111/j.1939-165X.2008.00005.x [DOI] [PubMed] [Google Scholar]
- 5.Fulmer A. K., Mauldin G. E.2007. Canine histiocytic neoplasia: an overview. Can. Vet. J. 48: 1041–1043, 1046–1050. [PMC free article] [PubMed] [Google Scholar]
- 6.Ishimori M., Michishita M., Yoshimura H., Azakami D., Ochiai K., Ishiwata T., Takahashi K.2017. Disseminated histiocytic sarcoma with hemophagocytosis in a rabbit. J. Vet. Med. Sci. 79: 1503–1506. doi: 10.1292/jvms.17-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karim M. R., Izawa T., Pervin M., Sasai H., Kuwamura M., Yamate J.2017. Cutaneous histiocytic sarcoma with regional lymph node metastasis in a Netherland dwarf rabbit (Oryctolagus cuniculus). J. Comp. Pathol. 156: 169–172. doi: 10.1016/j.jcpa.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Kato Y., Murakami M., Hoshino Y., Mori T., Maruo K., Hirata A., Nakagawa T. L. D. R., Yanai T., Sakai H.2013. The class A macrophage scavenger receptor CD204 is a useful immunohistochemical marker of canine histiocytic sarcoma. J. Comp. Pathol. 148: 188–196. doi: 10.1016/j.jcpa.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 9.Kawashima M., Nakanishi M., Kuwamura M., Takeya M., Yamate J.2004. Immunohistochemical detection of macrophages in the short-finned pilot whale (Globicephala macrorhynchus) and Risso’s dolphin (Grampus griseus). J. Comp. Pathol. 130: 32–40. doi: 10.1016/S0021-9975(03)00066-5 [DOI] [PubMed] [Google Scholar]
- 10.Koizumi I., Kondo H.2019. Clinical management and outcome of four-toed hedgehogs (Atelerix albiventris) with histiocytic sarcoma. J. Vet. Med. Sci. 81: 545–550. doi: 10.1292/jvms.18-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komohara Y., Hirahara J., Horikawa T., Kawamura K., Kiyota E., Sakashita N., Araki N., Takeya M.2006. AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J. Histochem. Cytochem. 54: 763–771. doi: 10.1369/jhc.5A6871.2006 [DOI] [PubMed] [Google Scholar]
- 12.Lau S. K., Chu P. G., Weiss L. M.2004. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am. J. Clin. Pathol. 122: 794–801. doi: 10.1309/QHD6YFN81KQXUUH6 [DOI] [PubMed] [Google Scholar]
- 13.Leissinger M., Brandão J., Wakamatsu N., Le Roux A., Rich G., Gaunt S.2013. Pulmonary histiocytic sarcoma in a rabbit. Vet. Clin. Pathol. 42: 364–367. doi: 10.1111/vcp.12058 [DOI] [PubMed] [Google Scholar]
- 14.Moore P. F.2014. A review of histiocytic diseases of dogs and cats. Vet. Pathol. 51: 167–184. doi: 10.1177/0300985813510413 [DOI] [PubMed] [Google Scholar]
- 15.Moore P. F.2015. Canine and feline histiocytic diseases. pp. 322–336. In: Tumors in Domestic Animals, 5th ed. (Meuten, D. J. eds.), Wiley Blackwell, Hoboken. [Google Scholar]
- 16.Ogihara K., Suzuki K., Madarame H.2017. Primary histiocytic sarcoma of the brain in an African hedgehog (Atelerix albiventris). J. Comp. Pathol. 157: 241–245. doi: 10.1016/j.jcpa.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 17.Ogihara K., Itoh T., Mizuno Y., Tamukai K., Madarame H.2016. Disseminated histiocytic sarcoma in an African hedgehog (Atelerix albiventris). J. Comp. Pathol. 155: 361–364. doi: 10.1016/j.jcpa.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 18.Okada K., Kondo H., Sumi A., Kagawa Y.2018. A retrospective study of disease incidence in African pygmy hedgehogs (Atelerix albiventris). J. Vet. Med. Sci. 80: 1504–1510. doi: 10.1292/jvms.18-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierezan F., Mansell J., Ambrus A., Rodrigues Hoffmann A.2014. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J. Comp. Pathol. 151: 347–351. doi: 10.1016/j.jcpa.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 20.Raymond J. T., Garner M. M.2001. Spontaneous tumours in captive African hedgehogs (Atelerix albiventris): a retrospective study. J. Comp. Pathol. 124: 128–133. doi: 10.1053/jcpa.2000.0441 [DOI] [PubMed] [Google Scholar]
- 21.Son N. V., Chambers J. K., Dung L. T., Kishimoto T. E., Nishimura M., Kita C., Takada Y., Miwa Y., Nakayama H., Uchida K.2020. Histological and immunohistochemical features of normal histiocytes and Langerhans cells, and histiocytic sarcomas in four-toed hedgehogs (Atelerix albiventris). J. Comp. Pathol. 178: 32–40. doi: 10.1016/j.jcpa.2020.06.009 [DOI] [PubMed] [Google Scholar]
- 22.Thongtharb A., Uchida K., Chambers J. K., Kagawa Y., Nakayama H.2016. Histological and immunohistochemical studies on primary intracranial canine histiocytic sarcomas. J. Vet. Med. Sci. 78: 593–599. doi: 10.1292/jvms.15-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thongtharb A., Uchida K., Chambers J. K., Miwa Y., Murata Y., Nakayama H.2016. Histological and immunohistochemical features of histiocytic sarcoma in four domestic ferrets (Mustela putorius furo). J. Vet. Diagn. Invest. 28: 165–170. doi: 10.1177/1040638715626485 [DOI] [PubMed] [Google Scholar]
- 24.Tomokiyo R., Jinnouchi K., Honda M., Wada Y., Hanada N., Hiraoka T., Suzuki H., Kodama T., Takahashi K., Takeya M.2002. Production, characterization, and interspecies reactivities of monoclonal antibodies against human class A macrophage scavenger receptors. Atherosclerosis 161: 123–132. doi: 10.1016/S0021-9150(01)00624-4 [DOI] [PubMed] [Google Scholar]
- 25.Warschau M., Hoffmann M., Dziallas P., Hansmann F., Baumgärtner W., Mischke R., Cichowski S., Fehr M.2017. Invasive histiocytic sarcoma of the lumbar spine in a ferret (Mustela putorius furo). J. Small Anim. Pract. 58: 115–118. doi: 10.1111/jsap.12632 [DOI] [PubMed] [Google Scholar]
- 26.Yamate J., Yoshida H., Tsukamoto Y., Ide M., Kuwamura M., Ohashi F., Miyamoto T., Kotani T., Sakuma S., Takeya M.2000. Distribution of cells immunopositive for AM-3K, a novel monoclonal antibody recognizing human macrophages, in normal and diseased tissues of dogs, cats, horses, cattle, pigs, and rabbits. Vet. Pathol. 37: 168–176. doi: 10.1354/vp.37-2-168 [DOI] [PubMed] [Google Scholar]