Abstract
In canine lymphoma, drug resistance is the major factor hindering treatment. In this study, we performed immunohistochemical examination of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which are considered as transporters related to multidrug resistance in three recurrent canine lymphomas. All cases were negative for both transporters before anticancer drug administration, but became positive after this administration. The expression was confirmed in capillary endothelial cells, such as in brain capillaries acting as the blood-brain barrier (BBB). It is suggested that both transporters expressed on capillary endothelial cells in lymphoma tissue may inhibit the spread of anticancer drugs into tumor tissues from blood, the same as the BBB. Therefore, capillary endothelial cells could act as a blood-tumor barrier, which might be involved in drug resistance in canine lymphoma.
Keywords: breast cancer resistance protein, dog, immunohistochemistry, lymphoma, P-glycoprotein
Canine lymphoma is the most common hematopoietic neoplasia in dogs [15]. Its incidence rate is estimated to be 20–107 cases per 100,000 dogs [2, 3, 9] and it shows similarities to human non-Hodgkin lymphoma in terms of the clinical features [6]. Canine lymphoma is histologically classified according to the World Health Organization classification; within this category, B-cell lymphomas are reported to be prevalent and large B-cell lymphomas are the most common [17, 18]. Canine lymphoma is routinely treated with a multi-agent chemotherapy protocol [20], such as cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP-protocol), which is currently the standard of care. Although the initial response rate for multi-agent chemotherapy is high, in most cases the tumor recurs. The main reason for the development of non-responsiveness to chemotherapy is thought to be drug resistance of lymphoma. The acquisition of drug resistance in canine lymphoma is a major issue clinically, but the mechanism behind it has not been clarified.
Drug resistance can be present at the start of chemotherapy (intrinsic drug resistance) or develop during or following chemotherapy (acquired drug resistance). Acquired resistance is the major factor impeding survival. Some molecules involved in the mechanism of drug resistance in anticancer therapies are considered as transporters related to multidrug resistance, such as P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which efflux the drugs from the CHOP-protocol from tumor tissue. Studies have investigated the expression of P-gp and BCRP in canine lymphoma, but their localization has not been comprehensively clarified [20]. In this study, we performed immunohistochemical examination of three recurrent cases of canine lymphoma and confirmed the expression of P-gp and BCRP at different sites from those in previous reports.
Tumor samples were obtained from three dogs diagnosed with lymphoma, which presented at Patho Labo Co., Ltd. (Ito, Japan) for the examination of lymphoma. Detailed information of the samples (breed, sex, treatment, age, timing of sample collection) is presented in Table 1.
Table 1. Clincal data for dogs.
| Case | Sample | Age | Breed | Sex | Sampling method | Treatment |
|---|---|---|---|---|---|---|
| 1 | 1 (1st test) | 7 y and 7 m | Miniature pinscher | Male | Biopsy or resection | Anticancer drug started after diagnosis. Achieved CR but soon relapsed |
| 1 (2nd test) | 8 y | Biopsy or resection | ||||
| 2 | 2 (1st test) | - | Welsh corgi | Female | Biopsy or resection | Chemotherapy after diagnosed |
| 2 (2nd test) | 9 y | Biopsy or resection | ||||
| 2 (3rd test) | 10 y | Biopsy or resection | ||||
| 3 | 3 (1st test) | 9 y | Beagle | Male | Biopsy or resection | Not achieved CR after L-asparaginase, steroids, chlorambucil then vincristine |
| 3 (2nd test) | 9 y and 10 m | Biopsy or resection | ||||
CR=complete response, m=month, y=year.
To examine the expression of P-gp and BCRP and T-cell and B-cell typing for lymphoma, an enzyme antibody method was performed. All samples were fixed with 10% buffered formalin for less than 7 days, embedded in paraffin by a conventional method, and the sections (3–5 µm) were prepared from the blocks. For T-cell and B-cell typing, polyclonal rabbit CD20 antibody (CD20 Polyclonal Antibody; Thermo Fisher Scientific Inc., Waltham, MA, USA) and monoclonal mouse CD3 antibody (Monoclonal Mouse Anti-Human CD3; DAKO Denmark A/S, Glostrup, Denmark) were used as primary antibodies. To examine the expression of P-gp and BCRP, monoclonal rabbit P-gp antibody (Anti-P-Glycoprotein antibody; EPR10364-57; Abcam Plc., Cambridge, UK) and monoclonal mouse BCRP antibody (Anti-BCRP/ABCG2 antibody; BXP-21; Abcam Plc.) were used as primary antibodies.
The primary antibodies were diluted with phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA) and the secondary antibodies were diluted with polyclonal goat anti-immunoglobulins [Histofine Simple Stain MAX-PO (MULTI); Nichirei Biosciences Inc., Tokyo, Japan]. The slides were deparaffinized with xylene and rehydrated in a series of ethanol solutions with decreasing concentrations. For antigen activation, pretreatment was performed using an autoclave for CD3 and Immunosaver (Nisshin EM Co., Ltd., Tokyo, Japan) for P-gp and BCRP. Afterwards, the sections were saturated with 3% hydrogen peroxide for 20 min to inactivate endogenous peroxidase. For the inhibition of nonspecific reactions, the sections were incubated with block-ace at room temperature for 30 min. The sections were incubated overnight with each primary antibody (4°C). After washing with PBS (5 min, three times), the sections were incubated with secondary antibody for 1 hr. The sections were stained with 3,3′-diaminobenzidine tetrahydrochloride (DAB)/hydrogen peroxide and counterstained with hematoxylin, dehydrated with a series of ethanol solutions and xylene, and then covered using mounting solution.
For the pathological diagnosis of canine lymphoma, not only CD3/CD20 immunohistochemistry but also hematoxylin and eosin staining were performed.
The normal lymph nodes and normal cerebrum of dogs who died of pulmonary edema were also immunostained with P-gp and BCRP for comparison with lymphoma cases. Normal lymph node was collected from a 17 year old female mix dog, and normal cerebrum was collected from a 7 year and 11 month old male Welsh corgi by Laboratory of Veterinary Pathology, School of Veterinary Medicine, Azabu University.
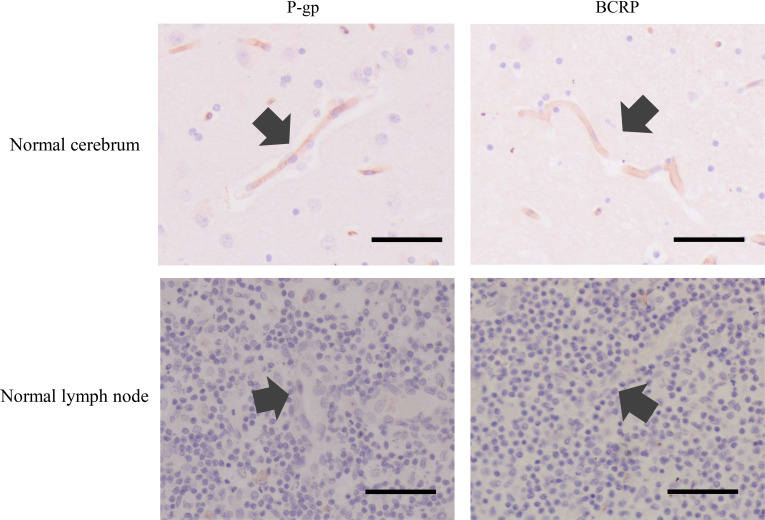
In the normal cerebrum, P-gp, and BCRP expression on the capillary endothelial cells was observed. In the normal lymph node, P-gp and BCRP expression was not observed (Fig. 1).
Fig. 1.
Results of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) immunohistochemistry in normal cerebrum and normal lymph node. In the normal cerebrum, both P-gp and BCRP were expressed on capillary endothelial cells (arrows). In the normal lymph node, no expression of P-gp and BCRP was detected (arrows show capillary). Scale bar=50 µm.
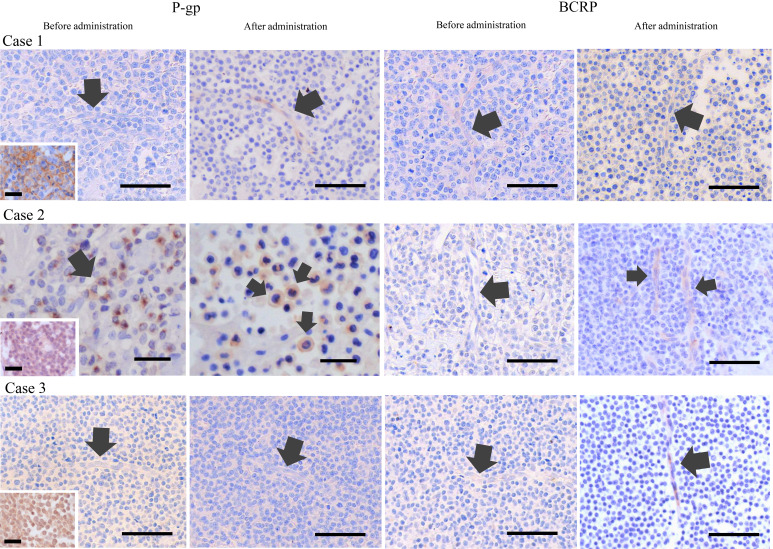
Case 1 was diagnosed as lymphoma by pathological examination due to multiple masses (first test). Thereafter, treatment with anticancer drugs was initiated, and the patient achieved complete response, but then relapsed and underwent pathological examination due to swelling of the inguinal lymph node (second test). In this case, the first test showed negativity for P-gp and BCRP, but the second test showed staining of capillary endothelial cells with P-gp. In the second test, BCRP was negative (Fig. 2 and Table 2). This case was CD3-negative and CD20-positive and diagnosed with diffuse large B-cell lymphoma.
Fig. 2.
Results of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) immunohistochemistry in Case 1 to 3. In Case 1, P-gp was not expressed before the administration of anticancer drugs (arrow shows capillary) but expressed on capillary endothelial cells (arrow) after the administration of anticancer drugs (2nd test). BCRP was not expressed before and after the administration of anticancer drugs (arrows show capillary). Inset shows CD20 immunohistochemistry result of Case 1. In Case 2, cytoplasmic staining (arrow) with P-gp was observed before the administration of anticancer drugs (1st test) but was considered negative. P-gp was expressed on the cell membrane (arrow) after the administration of anticancer drugs (3rd test). BCRP was not expressed before the administration of anticancer drugs (arrow shows capillary) but expressed on capillary endothelial cells (arrows) after the administration of anticancer drugs (2nd test). Inset shows CD3 immunohistochemistry result of Case 2. In Case 3, P-gp was not expressed before and after the administration of anticancer drugs (arrows show capillary). BCRP was not expressed before the administration of anticancer drugs (arrow shows capillary) but expressed on capillary endothelial cells (arrow) after the administration of anticancer drugs (2nd test). Inset shows CD3 immunohistochemistry result of Case 3. Scale bar=50 µm (except for Case 2 P-gp and Inset). Scale bar=25 µm for Case 2 P-gp. Scale bar=20 µm for inset.
Table 2. Immunohistochemical Examination.
| P-gp test |
BCRP test |
|||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | |
| (Before administration) | (After administration) | (After administration) | (Before administration) | (After administration) | (After administration) | |
| Case 1 | - | + | ND | - | - | ND |
| (Capillary endothelial cells) | ||||||
| Case 2 | - | - | + | - | + | - |
| (Cell membrane) | (Capillary endothelial cells) | |||||
| Case 3 | - | - | ND | - | + | ND |
| (Capillary endothelial cells) | ||||||
Before/after administration represents before/after administration of anti-cancer drugs. P-gp=P-glycoprotein, BCRP=breast cancer resistance protein, ND=not determined.
In Case 2, pathological examination was performed because of enlargement of the lymph nodes and swelling of the lips (first test). This case was CD3-positive and CD20-negative and diagnosed with extranodal/peripheral T-cell lymphoma. After being diagnosed with lymphoma by pathological examination, chemotherapy was started. Lymph node enlargement was observed about half a year later, for which a pathological examination was performed (second test). About 1 year later, lymph node enlargement was observed and a test was again performed (third test). At the first test, cytoplasmic staining for P-gp was observed. In the second test, similarly, cytoplasmic staining for P-gp was observed. In the third test, not only cytoplasm but also cell membrane staining for P-gp was observed. We considered that the cytoplasmic staining of P-gp was a false positive result because P-gp must be present in the cell membrane to function as a drug transporter. In the third test, P-gp was expressed on the cell membrane of lymphoma cells, so the staining on the cell membrane was considered P-gp rather than nonspecific staining. For BCRP immunohistochemistry, BCRP was expressed on capillary endothelial cells only at the second test (Fig. 2 and Table 2). The BCRP expression site of capillary endothelial cells was judged to be BCRP positive as same as P-gp in Case 1.
In Case 3, swelling of the mandibular lymph node was noted. After the administration of steroids and antibiotics for 2 months, a biopsy of the swollen lymph node was performed, and the patient was diagnosed with T-cell lymphoma (first test). Then, the administration of L-asparaginase, steroid, and chlorambucil was started. This case maintained stable disease for a while, but lymph node enlargement was observed, after which vincristine was initiated. The size of the lymph nodes remained unchanged after the start of vincristine, but the left lower lymph node was removed for diagnosis (second test). BCRP was negative at the first test, but a second test showed BCRP expression on the capillary endothelial cells. In this case, P-gp was negative in both tests (Fig. 1 and Table 2). This case was CD3-positive and CD20-negative and diagnosed with T-zone lymphoma.
In previous reports, P-gp and BCRP antibodies in this study were used in western blotting or immunocytochemistry of canine cell lines and show specificity for P-gp or BCRP [11, 19]. Therefore, the signal of immunohistochemical staining observed in this study is considered to be P-gp or BCRP except for cytoplasmic staining.
In all cases, the staining pattern of P-gp and BCRP in capillary endothelial cells was partial and not all capillaries were stained in sections.
To date, a few studies have immunohistochemically evaluated the expression of P-gp and BCRP in canine lymphoma; the sites of P-gp and BCRP expression as confirmed by immunohistochemical examination are the cytoplasm or cell membrane [16]. In this study, we evaluated the expression of P-gp and BCRP in tumor tissue in lymph nodes before and after anticancer drug administration. The results showed that, in all cases, the expression of P-gp and BCRP was negative before anticancer drug administration, but became positive thereafter. In addition, we found P-gp and BCRP expression on capillary endothelial cells, which contrasts with previous reports.
In case 3, BCRP was expressed after treatment with a steroid drug, which is one of the substrates of BCRP [14]. Some studies have reported that glucocorticoid before the initiation of chemotherapy has an adverse effect on treatment in canine lymphoma [12, 13]. It can be said that substrates of BCRP such as steroid drugs may be involved in the expression of BCRP.
Experiments using cultured cell lines have shown that P-gp gene expression was increased after exposure to P-gp substrates such as doxorubicin [5]. Therefore, it is possible that the expression of P-gp and BCRP became positive in the cases in this study because the transcriptional activities of these drug resistance transporters may be increased by anticancer drugs including P-gp/BCRP substrates.
In this study, P-gp was expressed in B-cell lymphoma after anticancer drug treatment and BCRP was expressed in T-cell lymphoma after anticancer drug treatment, which corresponds to the report of a study evaluating the mRNA expression of ABC transporters in canine lymphoma [20].
As mentioned above, in this study immunohistochemistry revealed the expression of P-gp and BCRP in capillary endothelial cells of lymph nodes. This expression was similar to the expression in the normal cerebrum (Fig. 1). Capillary endothelial cells in the brain are known to be associated with the blood-brain barrier (BBB) and P-gp and BCRP act in biological defense mechanisms against chemicals [4, 8, 10]. P-gp and BCRP expressed in capillary endothelial cells may enable the efflux of anticancer drugs from tumor tissues, the same as their roles at the BBB. Therefore, the expression of capillary transporters in tumor sites may constitute a blood-tumor barrier, which might be involved in drug resistance in lymphoma. However, there is usually a gap between capillary endothelial cells [1]. Otherwise, capillary endothelial cells at the BBB form a closed structure due to tight junctions, physically blocking the passage of small molecules [7]. If tight junctions are not seen in the capillary endothelial cells and the intercellular space is wide, low-molecular-weight substances pass through the intercellular space, regardless of the presence of P-gp and BCRP substrates, and may reach tumor cells. Therefore, this study suggested that the expression of P-gp and BCRP might be related to drug resistance to anticancer drugs but to prove the association between P-gp and BCRP expression and drug resistance, it will be necessary to evaluate tight junctions of intertumoral capillary endothelial cells and accumulate more data in the future.
In conclusion, P-gp and BCRP were observed after the administration of anticancer drugs. P-gp and BCRP were identified in the capillary endothelial cells in canine lymphoma, which can be suggested to be associated with formation of the blood-tumor barrier as a mechanism of drug resistance. To prove that the blood-tumor barrier has the same function of excreting drugs from tumor tissues as the BBB, further studies such as demonstrating tight junctions at the blood-tumor barrier are required.
CONFLICT OF INTEREST
There are no conflicts of interest on this article.
Acknowledgments
We gratefully thanks to Patho Labo Co., Ltd. who provided specimens.
REFERENCES
- 1.Dejana E., Corada M., Lampugnani M. G.1995. Endothelial cell-to-cell junctions. FASEB J. 9: 910–918. doi: 10.1096/fasebj.9.10.7615160 [DOI] [PubMed] [Google Scholar]
- 2.Dobson J. M., Samuel S., Milstein H., Rogers K., Wood J. L.2002. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 43: 240–246. doi: 10.1111/j.1748-5827.2002.tb00066.x [DOI] [PubMed] [Google Scholar]
- 3.Edwards D. S., Henley W. E., Harding E. F., Dobson J. M., Wood J. L.2003. Breed incidence of lymphoma in a UK population of insured dogs. Vet. Comp. Oncol. 1: 200–206. doi: 10.1111/j.1476-5810.2003.00025.x [DOI] [PubMed] [Google Scholar]
- 4.Fromm M. F.2004. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol. Sci. 25: 423–429. doi: 10.1016/j.tips.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Hu X. F., Slater A., Wall D. M., Kantharidis P., Parkin J. D., Cowman A., Zalcberg J. R.1995. Rapid up-regulation of mdr1 expression by anthracyclines in a classical multidrug-resistant cell line. Br. J. Cancer 71: 931–936. doi: 10.1038/bjc.1995.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito D., Frantz A. M., Modiano J. F.2014. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: recent progress and applications. Vet. Immunol. Immunopathol. 159: 192–201. doi: 10.1016/j.vetimm.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W. Y., Wang Z. B., Zhang L. C., Wei X., Li L.2012. Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci. Ther. 18: 609–615. doi: 10.1111/j.1755-5949.2012.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao Q., Unadkat J. D.2015. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—an update. AAPS J. 17: 65–82. doi: 10.1208/s12248-014-9668-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merlo D. F., Rossi L., Pellegrino C., Ceppi M., Cardellino U., Capurro C., Ratto A., Sambucco P. L., Sestito V., Tanara G., Bocchini V.2008. Cancer incidence in pet dogs: findings of the animal tumor registry of Genoa, Italy. J. Vet. Intern. Med. 22: 976–984. doi: 10.1111/j.1939-1676.2008.0133.x [DOI] [PubMed] [Google Scholar]
- 10.Miller D. S., Bauer B., Hartz A. M.2008. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 60: 196–209. doi: 10.1124/pr.107.07109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita A., Aoshima K., Gulay K. C. M., Onishi S., Shibata Y., Yasui H., Kobayashi A., Kimura T.2019. High drug efflux pump capacity and low DNA damage response induce doxorubicin resistance in canine hemangiosarcoma cell lines. Res. Vet. Sci. 127: 1–10. doi: 10.1016/j.rvsc.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 12.Piek C. J., Rutteman G. R., Teske E.1999. Evaluation of the results of a L-asparaginase-based continuous chemotherapy protocol versus a short doxorubicin-based induction chemotherapy protocol in dogs with malignant lymphoma. Vet. Q. 21: 44–49. doi: 10.1080/01652176.1999.9694990 [DOI] [PubMed] [Google Scholar]
- 13.Price G. S., Page R. L., Fischer B. M., Levine J. F., Gerig T. M.1991. Efficacy and toxicity of doxorubicin/cyclophosphamide maintenance therapy in dogs with multicentric lymphosarcoma. J. Vet. Intern. Med. 5: 259–262. doi: 10.1111/j.1939-1676.1991.tb03131.x [DOI] [PubMed] [Google Scholar]
- 14.Sissung T. M., Baum C. E., Kirkland C. T., Gao R., Gardner E. R., Figg W. D.2010. Pharmacogenetics of membrane transporters: an update on current approaches. Mol. Biotechnol. 44: 152–167. doi: 10.1007/s12033-009-9220-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teske E.1994. Canine malignant lymphoma: a review and comparison with human non-Hodgkin’s lymphoma. Vet. Q. 16: 209–219. doi: 10.1080/01652176.1994.9694451 [DOI] [PubMed] [Google Scholar]
- 16.Vajdovich P., Koltai Z., Dékay V., Kungl K., Harnos A.2018. Evaluation of Pgp (MDR1) immunohistochemistry in canine lymphoma - prognostic and clinical aspects. Acta Vet. Hung. 66: 309–328. doi: 10.1556/004.2018.028 [DOI] [PubMed] [Google Scholar]
- 17.Valli V. E.2002. Histological classification of hematopoietic tumors of domestic animals. WHO International Histological Classification of Tumors of Domestic Animals. [Google Scholar]
- 18.Vezzali E., Parodi A. L., Marcato P. S., Bettini G.2010. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet. Comp. Oncol. 8: 38–49. doi: 10.1111/j.1476-5829.2009.00201.x [DOI] [PubMed] [Google Scholar]
- 19.Zandvliet M., Teske E., Schrickx J. A.2014. Multi-drug resistance in a canine lymphoid cell line due to increased P-glycoprotein expression, a potential model for drug-resistant canine lymphoma. Toxicol. In Vitro 28: 1498–1506. doi: 10.1016/j.tiv.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Zandvliet M., Teske E., Schrickx J. A., Mol J. A.2015. A longitudinal study of ABC transporter expression in canine multicentric lymphoma. Vet. J. 205: 263–271. doi: 10.1016/j.tvjl.2014.11.002 [DOI] [PubMed] [Google Scholar]