Correction to: Trials 22, 188 (2021)
https://doi.org/10.1186/s13063-021-05113-y
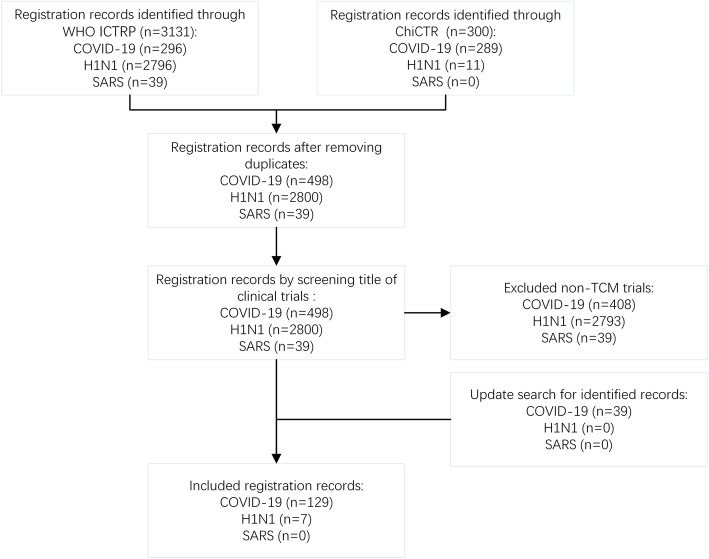
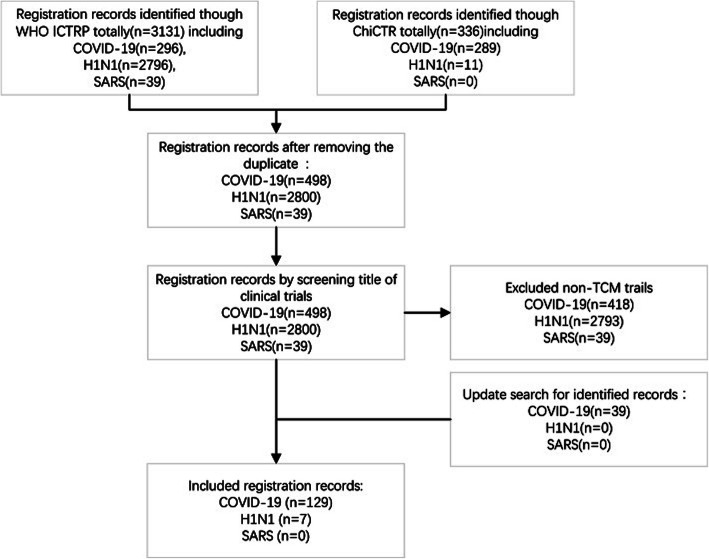
Following publication of the original article [1], we have been notified of a correction to Fig. 1 (Excluded non-TCM trials COVID-19).
Fig. 1.
Flow diagram of record screening. ICTRP, International Clinical Trials Registry Platform; ChiCTR, Chinese Clinical Trial Registry; TCM, traditional Chinese medicine; COVID-19, coronavirus disease 2019; H1N1, H1N1 influenza; SARS, severe acute respiratory syndrome
• Originally published Fig. 1:
• Corrected Fig. 1:
The original article has been corrected.
Contributor Information
Yaolong Chen, Email: chenyaolong@vip.163.com.
Xiaojia Ni, Email: grace1984325@126.com.
Reference
- 1.Kuang Z, et al. Calling for improved quality in the registration of traditional Chinese medicine during the public health emergency: a survey of trial registries for COVID-19, H1N1, and SARS. Trials. 2021;22:188. doi: 10.1186/s13063-021-05113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]