Abstract
Cervical cancer is a common public health issue with high morbidity worldwide. Paeonol (Pae) has been recognized as a traditional Chinese medicine used for the treatment of various cancer types. However, whether Pae could exert a protective effect on cervical cancer remains to be investigated. The aim of the present study was to explore the role of Pae in cervical cancer cells and identify the potential mechanism. Cell Counting Kit-8 and colony-formation assays were conducted to test the proliferation of HeLa cells. Additionally, wound healing and transwell assays were used to detect the migratory and invasive abilities of cells. The plasmid that overexpressed 5-lipoxygenase (5-LO) or control vector was constructed and transfected into the cells. Subsequently, flow cytometry was used to monitor the apoptotic rate of cells. The expression levels of apoptosis-associated proteins and 5-LO were detected using western blot analysis. Reverse transcription-quantitative PCR analysis detected the expression of 5-LO. Pae inhibited the proliferation, invasion and migration of HeLa cells, promoted cell apoptosis and downregulated the expression of 5-LO. Overexpression of 5-LO, however, attenuated these effects. Thus, Pae could inhibit the proliferation, migration and invasion, as well as promote apoptosis of HeLa cells by regulating the expression of 5-LO.
Keywords: paeonol, HeLa cells, 5-lipoxygenase, cervical cancer
Introduction
Cervical cancer has a considerably high mortality rate and is the fourth most common cancer in women worldwide (1). In total, >130,500 individuals are diagnosed with cervical cancer in China every year, accounting for 30% of the overall cancer-affected population worldwide (2). Cervical cancer is caused by the infection of the human papillomavirus (HPV) to the uterine epithelia. Although cervical cancer can affect other parts of the body, it progresses slowly and can be treated effectively if diagnosed at an early stage. However, current treatments, including chemotherapy and radiotherapy are not ideal due to the side effects caused. Therefore, it is important to discover novel methods to effectively treat cervical cancer.
Paeonol (Pae) is a natural product derived from the root of Cynanchum paniculatum (Bunge) K. Schum and the root of Paeonia suffruticosa Andr. (Ranunculaceae). It has received extensive attention due to its multiple biological activities, such as anti-oxidative, anti-inflammatory and anti-cancer effects (3–6). Pae relieved the induction of oxidative stress and inflammation in rats with testicular ischaemia-reperfusion injury (4). A previous study demonstrated that by downregulating the expression of Erb-B2 receptor tyrosine kinase 2 and inhibiting the nuclear factor-κB signaling pathway, Pae induced the apoptosis of gastric cancer cells (7). Another study demonstrated the significant role of Pae in inhibiting the growth of breast cancer cells, possibly by its ability to induce cell apoptosis (8). However, whether Pae can exert significant effects on the proliferation and apoptosis of cervical cancer cells has not been fully elucidated, thus, requiring an in-depth study to be conducted on its role. Through STITCH, it was found that paeonol could regulate prostaglandin-endoperoxide synthase (PTGS2), and the interaction between PTGS2 and 5-lipoxygenase (ALOX5; 5-LO) can be found on the String website.
5-LO is considered the major enzyme involved in the biosynthesis of a class of bioactive lipids signaling molecules known as eicosanoids (9). The roles of lipoxygenase in the pathogenesis of cancer have been recently identified and novel lipoxygenase inhibitors have been developed with promising anti-cancer activity (10). Monga et al (11) revealed that pharmacological and genetic targeting of 5-LO induced prostate cancer cell apoptosis. Increased expression of 5-LO was detected in clinical samples from patients with breast cancer (12). The inhibition of 5-LO impeded the invasion of breast cancer cells by regulating the production of interleukin-8 and matrix metlloprotease-9 (MMP-9) (13). However, the specific role of 5-LO in cervical cancer has not been fully investigated.
In the present study, the role of Pae on the proliferation, migration and invasion of cervical cancer cells was investigated and the potential mechanisms underlying these processes were explored in association with 5-LO expression.
Materials and methods
Cell culture and treatment
The HeLa cell line was obtained from the Shanghai Cell Bank of Chinese Academy of Sciences. The human immortalized cervical epithelial cell line H8 was obtained from Bnbio. Subsequently, the cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.), containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in an incubator with 5% CO2 at 37°C. Pae (purity >98%) was purchased from Dalian Meilun Biotechnology Co., Ltd. (cat. no. MB1762-S). Pae was dissolved in DMSO and preserved for further experiments. The cells were exposed to Pae for 24 h at the concentrations of 0.1, 0.2, 0.4 and 0.6 mg/ml. The culture medium was replaced every 2–3 days.
Cell transfection
The overexpression plasmids pcDNA 3.1–5-LO and control pcDNA 3.1 were generated by Shanghai GenePharma Co., Ltd. HeLa cells were respectively transfected with 2.5 µg pcDNA 3.1–5-LO or vector using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h according to the manufacturer's instructions. The transfection efficiency of the cells was determined by reverse transcription-quantitative PCR (RT-qPCR).
Cell Counting Kit-8 (CCK-8) assay
Following transfection, the cells were seeded into 96-well plates (1×105 cells per well) and re-suspended in RPMI-1640 containing 10% FBS. Then, 10 µl CCK-8 reagent (Thermo Fisher Scientific, Inc.) was added to cells treated for 24, 48 and 72 h at 37°C. Subsequently, the absorbance at 450 nm was measured with a microplate reader (Thermo Fisher Scientific, Inc.) at each time point according to the manufacturer's instructions.
Colony-formation assay
Treated cells were detached by 0.25% trypsin and re-suspended in medium. The cells were seeded into culture dishes at a density of 2,000 cells/well. Following 2 weeks of incubation, the colonies were visible to the naked eye. The cells were fixed with 4% paraformaldehyde for 20 min at room temperature and stained with 0.2% crystal violet for 10 min at room temperature. The number of colonies was counted using ImageJ software (v.1.52s; National Institutes of Health).
Wound healing assay
The cells were cultured in 6-well plates (5×105 cells/well) with RPMI-1640 containing 10% FBS. A scratch was created on the cell surface with a 200-µl pipette tip. Following washing to remove the detached cells, the medium was replaced with serum-free RPMI-1640 medium and cultured at 37°C for 24 h. The images were obtained after 24 h by a light microscope (Olympus Corporation; magnification ×100).
Transwell assay
Following transfection, the cells were plated in a serum-free medium at a density of 1×104 cells/ml in the upper chamber, which was coated with Matrigel (Corning Inc.). Medium containing 20% FBS was added into the lower chamber. Following 24-h incubation, 0.05% crystal violet was used to stain the cells for 30 min at room temperature in the lower chamber. The cells were counted under a light microscope at a magnification of ×100.
Flow cytometry
Cell apoptosis was measured using the Annexin-FITC Apoptosis Detection kit (Beyotime Institute of Biotechnology). Briefly, the cells were collected and washed with PBS twice, gently resuspended in Annexin V binding buffer and incubated with Annexin V-FITC/PI at room temperature in dark. The number of apoptotic cells was analyzed using a flow cytometer (Becton, Dickinson and Company).
Western blotting
The cells were collected and the total proteins were extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology). BCA assay was used to determine the protein concentration. Briefly, protein samples (20 µg) were loaded at the same concentration on each lane of the 12% SDS-polyacrylamide gel. The proteins were transferred to PVDF membranes (EMD Millipore). Then, 5% skimmed milk was used for blocking the membranes at room temperature for 1 h. Primary antibodies such as MMP-2 (cat. no. ab92536; dilution, 1:1,000; Abcam), MMP-9 (cat. no. ab76003; dilution, 1:1,000; Abcam), Bcl-2 (cat. no. ab182858; dilution, 1:2,000; Abcam), Bax (cat. no. ab32503; dilution, 1:1,000; Abcam), cleaved-caspase-3 (cat. no. ab32042; dilution, 1:500; Abcam), cleaved-caspase-9 (cat. no. 20750; dilution, 1:1,000; Cell Signaling Technology, Inc.), caspase-3 (cat. no. ab32351; dilution, 1:5,000; Abcam), caspase-9 (cat. no. ab32539; dilution, 1:500; Abcam), ALOX5 (cat. no. ab169755; dilution, 1:1,000; Abcam) and GAPDH (cat. no. ab8245; dilution, 1:1,000; Abcam) were incubated with the membranes overnight at 4°C. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (cat. no. 7074; dilution, 1:2,000; Cell Signaling Technology, Inc.) at room temperature for 2 h prior to ECL detection. Image J. v.1.52s (National Institutes of Health) was used to analyze the density of the immunoblots. GAPDH was used as an internal control.
RT-qPCR
Total RNA was extracted from HeLa cells transfected with pcDNA 3.1–5-LO and control vector using a Takara MiniBEST RNA Extraction kit (Takara Bio, Inc). Total RNA was reverse-transcribed into cDNA using SuperScript IV First-Strand Synthesis system (Thermo Fisher Scientific, Inc.) at the following thermocycling conditions: 42°C for 60 min, 70°C for 5 min, preserved at 4°C. RT-qPCR was detected using a TaqMan gene expression assay kit (Thermo Fisher Scientific, Inc.). PCR was performed as follows: Pretreatment at 95°C for 10 min, followed by 35 cycles at 94°C for 15 sec, 60°C for 1 min, 60°C for 1 min and preserved at 4°C. The 2−ΔΔCq method was used to analyze the relative gene expression (14) and GAPDH was used for normalization. The primer sequences were as follows: 5-LO forward: 5′-TGGAATGACTTCGCCGACTTTGAG-3′ and reverse: 5′-TAGCCAAACATCAGGTCTTCCTGC-3′; and GAPDH forward: 5′-ACCACAGTCCATGCCATCAC-3′ and reverse: 5′-TCCACCACCCTGTTGCTGTA-3′.
Statistical analysis
All experimental data are expressed as mean ± standard deviation, and were statistically analyzed with SPSS 17.0 software (SPSS, Inc.). The Student' s t-test was used to analyze the comparison between the two groups and the one-way analysis of variance test followed by the Tukey's post hoc test was performed to analyze significant differences among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Pae inhibits the migration and invasion of HeLa cells
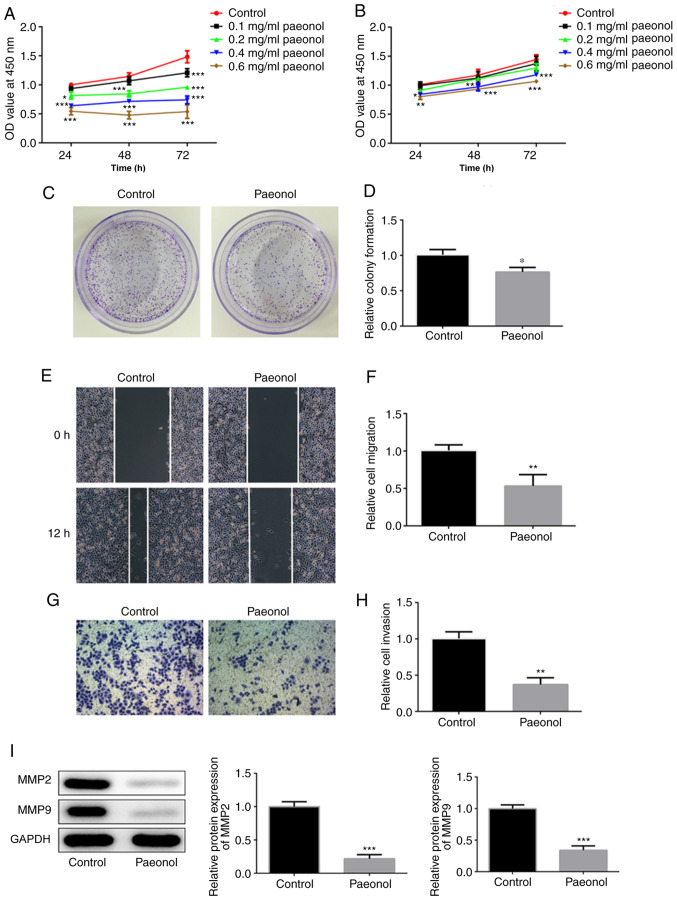
The viability of HeLa cells was decreased with the increasing doses of Pae (Fig. 1A). H8 cells treated with low concentrations of Pae demonstrated no significant changes on cell viability compared with control H8 cells. However, cell viability was decreased with time when the dose of Pae increased to 0.4 mg/ml (Fig. 1B). Therefore, 0.2 mg/ml Pae was selected for further experiments. Untreated HeLa cells were used as the control group, whereas cells treated with 0.2 mg/ml Pae were used as the Pae group. The colony-formation ability of HeLa cells treated with Pae was decreased compared with that of the control group (Fig. 1C and D). Wound healing and Transwell assays were conducted to assess the invasion and migration of HeLa cells. The data indicated that the invasive and migratory activities of HeLa cells were markedly inhibited following their exposure to Pae (Fig. 1E-H). Western blot analysis was conducted to detect changes in the expression levels of the invasion- and migration-associated proteins MMP-2 and MMP-9. The results indicated considerably lower expression levels in the Pae group compared with those noted in the control group (Fig. 1I). These results suggested that Pae inhibited the proliferation, migration and invasion of HeLa cells.
Figure 1.
Paeonol inhibits the migration and invasion of HeLa cells. (A) HeLa cells and (B) H8 cells were treated with different concentrations of Pae, and the cell viability at 24, 48 and 72 h was estimated via Cell Counting Kit-8 assay. (C and D) Colony-formation assay was performed to determine the colony-forming capacity of HeLa cells. (E and F) Wound healing assay was conducted to evaluate the migratory capacity of HeLa cells (magnification, ×100). (G and H) Transwell assay was performed to assess the invasive capacity of HeLa cells (magnification, ×100). (I) The protein levels of MMP-2, MMP-9 in HeLa cells were detected by western blot. *P<0.05, **P<0.01, ***P<0.001 vs. control group. MMP, matrix metalloprotease.
Pae promotes the induction of HeLa cell apoptosis
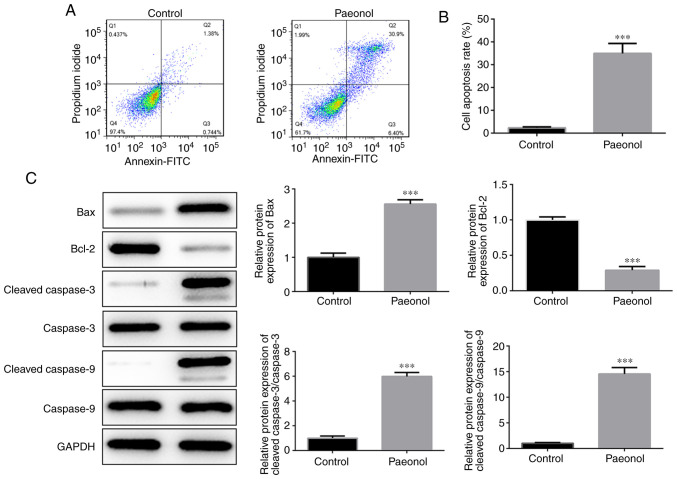
Flow cytometry was utilized to detect the induction of apoptosis in HeLa cells. The results indicated that the percentage of apoptotic cells in the Pae group was significantly increased compared with that of the control cells (Fig. 2A and B). Western blot analysis indicated that the expression levels of Bcl-2 were markedly decreased, while those of the pro-apoptotic proteins Bax, cleaved caspase-3 and cleaved caspase-9 were significantly increased (Fig. 2C). Therefore, these results indicated that Pae promoted the induction of apoptosis in HeLa cells.
Figure 2.
Pae promotes the apoptosis of HeLa cells. (A and B) Apoptotic HeLa cells were detected by flow cytometry. (C) The protein levels of Bcl-2, Bax, cleaved caspase-3, cleaved caspase-9, caspase-3 and caspase-9 in HeLa cells were detected by western blotting. GAPDH served as an internal control. ***P<0.001 vs. control group.
Pae inhibits the expression of 5-LO in HeLa cells
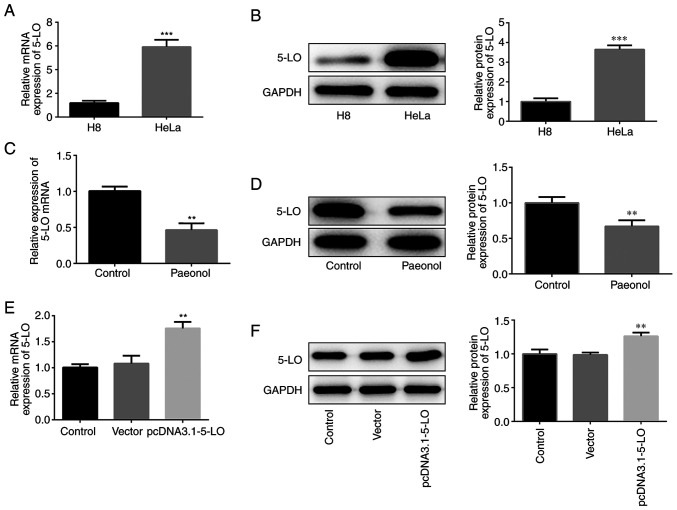
In order to detect the expression of 5-LO in HeLa cells, western blotting and RT-qPCR analysis were performed. 5-LO mRNA and protein levels were elevated in HeLa cells (Fig. 3A and B). Pae-treated HeLa cells exhibited downregulated expression of 5-LO compared with that of the control cells (Fig. 3C and D). The transcription and protein levels of 5-LO were increased following construction and transfection of the overexpression plasmid of 5-LO into HeLa cells (Fig. 3E and F), indicating that the overexpression plasmid was effective. Collectively, these results confirmed that 5-LO was highly expressed in HeLa cells and that Pae inhibited the expression of 5-LO.
Figure 3.
Pae inhibits the expression of 5-LO in HeLa cells. (A and B) The mRNA and protein levels of 5-LO in H8 or HeLa cells. ***P<0.001 vs. H8 group. (C and D) The mRNA and protein levels of 5-LO in HeLa cells treated with or without Pae. **P<0.01 vs. control group. (E and F) The transfection efficiency of HeLa cells transfected with pcDNA 3.1–5-LO was validated by western blotting and reverse transcription-quantitative PCR. **P<0.01 vs. control group. 5-LO, 5-lipoxygease.
5-LO inhibits Pae-mediated anti-migratory and anti-invasive effects
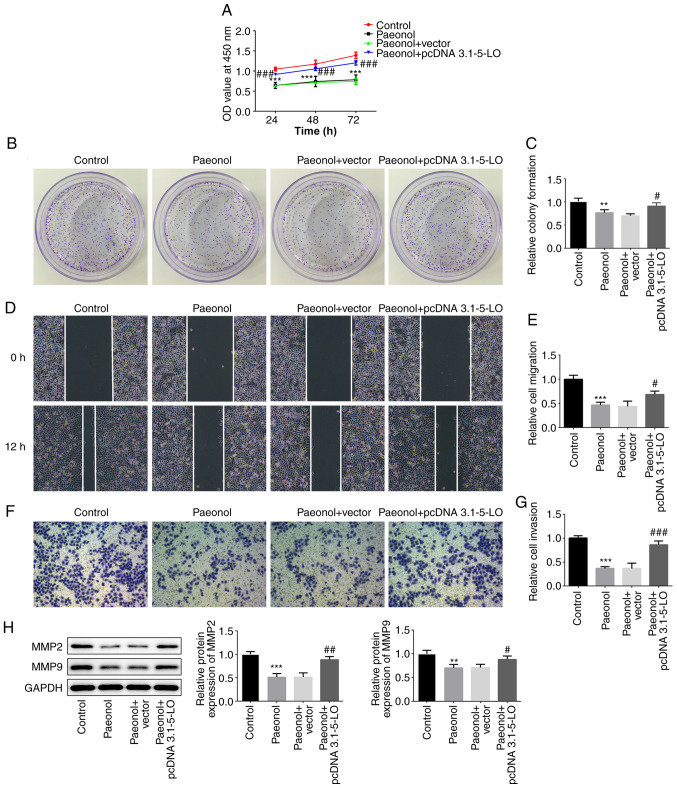
The cells were classified into four groups, namely control, Pae, Pae+vector and Pae+pcDNA 3.1–5-LO groups. Cell viability and clone-formation ability of the Pae or the Pae+vector groups were decreased to a relatively low level compared with those of the control group, while the addition of the 5-LO overexpression plasmid into HeLa cells disrupted this effect (Fig. 4A-C). As shown in Fig. 4D-G, the Pae or Pae+vector groups demonstrated decreased invasive and migratory activities compared with those of the control group, while these effects were recovered partially in the Pae+pcDNA 3.1–5-LO group as determined by the expression levels of the invasion- and migration-associated proteins MMP-2 and MMP-9 (Fig. 4H). The latter were significantly decreased following treatment of HeLa with Pae. However, when HeLa cells were transfected with pcDNA 3.1–5-LO the effects of Pae were weakened and the levels of MMP-2 and MMP-9 were increased. Taken together, the results demonstrated that the anti-migratory and anti-invasive effects of Pae were 5-LO-dependent.
Figure 4.
5-LO inhibits Pae from exerting the anti-migratory and anti-invasive effects. (A) The cell viability at 24, 48 and 72 h was estimated via Cell Counting Kit-8 assay in HeLa cells divided into control, Pae, Pae+vector and Pae+pcDNA 3.1–5-LO groups. (B and C) Colony-formation assay was performed to determine the colony-forming capacity of HeLa cells in different groups. (D and E) Wound-healing assay was conducted to evaluate the migratory capacity of HeLa cells (magnification, ×100). (F and G) Transwell assay was performed to assess the invasive capacity of HeLa cells (magnification, ×100). (H) The protein levels of MMP-2, MMP-9 in HeLa cells were detected by western blotting. **P<0.01, ***P<0.01 vs. control group. #P<0.05, ##P<0.01, ###P<0.001 vs. Pae+vector group. 5-LO, 5-lipoxygease; MMP, matrix metalloprotease.
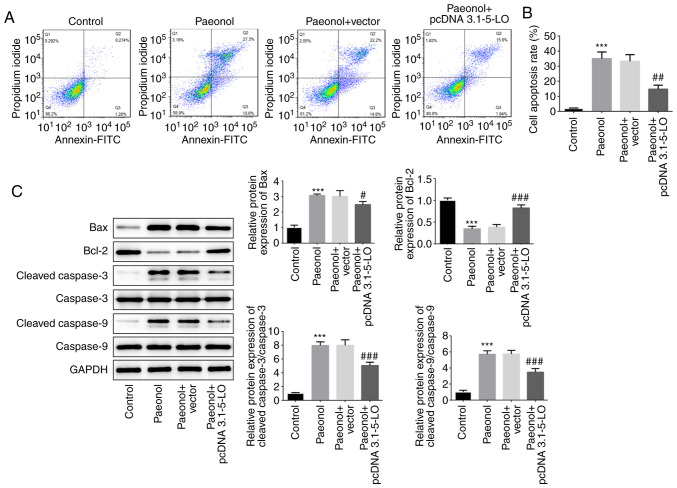
5-LO is required for the pro-apoptotic effect of Pae
The pro-apoptotic effects of Pae on HeLa cells were investigated following transfection of the cells with pcDNA 3.1–5-LO. The Pae group indicated enhanced apoptotic rate compared with that of the control group, whereas the apoptotic rate of the Pae+pcDNA 3.1–5-LO group was alleviated compared with that of the Pae+vector group, indicating that overexpression of 5-LO drastically decreased the pro-apoptotic effects of Pae (Fig. 5A and B). Subsequently, the protein levels of Bcl-2, Bax, cleaved caspase-3 and cleaved caspase-9 were estimated. The results indicated that the levels of Bax, cleaved caspase-3 and cleaved caspase-9 were increased following treatment of the cells with Pae, while overexpression of 5-LO reversed the effects of Pae (Fig. 5C). These results suggested that Pae promoted the induction of cell apoptosis by regulating 5-LO.
Figure 5.
5-LO is required for the pro-apoptotic effect of Pae. (A and B) Apoptotic HeLa cells in different groups were detected by flow cytometry. (C) The protein levels of Bcl-2, Bax, cleaved caspase-3, cleaved caspase-9, caspase-3 and caspase-9 in HeLa cells were detected by western blot. GAPDH served as an internal control. ***P<0.001 vs. control group. #P<0.05, ##P<0.01, ###P<0.001 vs. Pae+vector group. 5-LO, 5-lipoxygease.
Discussion
As a common malignant tumor of the female reproductive system (15), cervical cancer is the third most diagnosed cancer type with a considerably high frequency worldwide (16). Due to the advanced healthcare systems, the incidence of cervical cancer has decreased over the past decade in developed countries (17). However, it is still high in developing countries (17). Despite the unclear pathogenesis of cervical cancer, it has been widely accepted that HPV infection, due to sexual intercourse, is closely associated with this disease. Recently a high mortality has been noted among women under the age of 30 due to cervical cancer (18). Therefore, the identification of the mechanism underlying the occurrence and development of cervical cancer is of considerable importance.
Pae is a phenolic compound isolated from Paeonia suffruticosa that exerts numerous pharmacological effects (19), including anti-oxidation and anti-inflammation (20), which may benefit the recovery from diseases such as gastric ulcer (21), myocardial infarction (22) and cancer (23). A previous study demonstrated that Pae plays a protective role against acute lung injury in an endotoxic rat model by downregulating the expression levels of the pro-inflammatory cytokine HMGB1 (24), a highly conserved non-histone DNA-binding protein in the nucleus (25). However, studies that have investigated the anti-inflammatory effects of Pae and its interaction with a certain disease are limited. Moreover, a limited number of studies exist on the effects of traditional Chinese medicine Pae on the progression of cervical cancer. Therefore, a series of experiments were conducted to dissect the mechanism underlying the anticancer effects of Pae with regard to the inhibition of cell proliferation, migration and invasion of HeLa cells.
It has been previously shown that Pae regulates the expression levels of proliferation-associated proteins in order to exert its anti-metastasis activities (26). The present study demonstrated that Pae exerted potent anticancer effects by regulating the proliferation, migration and invasion of HeLa cells. Previous studies have reported that 5-LO is implicated in the pathogenesis of certain cancer types. Bai et al (27) reported that 5-LO expression was associated with poor prognosis in esophageal squamous cell carcinoma (ESCC), whereas its inhibition decreased the viability and migration of ESCC cells. Moreover, 5-LO promoted the invasion of papillary thyroid carcinoma cells by inducing the expression of MMP-9 (28). It was found that 5-LO was highly expressed in HeLa cells and that Pae could significantly decrease its levels. Given the important role of 5-LO in cell viability, migration and invasion, it was hypothesized that Pae could hinder the proliferation and migration of cervical cancer cells and activate the apoptotic cascade by downregulating the expression levels of 5-LO. In the present study, the elevated expression levels of 5-LO in HeLa cells were decreased following treatment of the cells with Pae. Additional investigations indicated that overexpression of 5-LO recovered the effects noted on proliferation, invasion and migration of Pae-treated HeLa cells to a certain extent.
In summary, the data demonstrated that Pae played an inhibitory role on the proliferation, invasion and migration of HeLa cells, while inducing apoptosis by regulating the expression of 5-LO. The current study may offer new insight and provide novel targets for the treatment of cervical cancer.
Acknowledgements
Not applicable.
Funding Statement
The study was supported by Medical Health Science and Technology Project of Zhejiang Province (grant no. 2019KY477) and Key project of Traditional Medical Science and Technology of Zhejiang Province (grant no. 2018ZZ013).
Funding
The study was supported by Medical Health Science and Technology Project of Zhejiang Province (grant no. 2019KY477) and Key project of Traditional Medical Science and Technology of Zhejiang Province (grant no. 2018ZZ013).
Availability of data and materials
All data generated or analyzed during this study are included in the present manuscript.
Authors' contributions
SQS and LYY acquired the data and confirmed the authenticity of all the raw data. XWZ and HYP contributed to analysis and interpretation of data. SQS, FYH and JLL contributed to the design of the study and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wei J, Wang Y, Shi K, Wang Y. Identification of core prognosis-related candidate genes in cervical cancer via integrated bioinformatical analysis. Biomed Res Int. 2020;2020:8959210. doi: 10.1155/2020/8959210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei H, Wang XW, Chen KM, Ling SR, Yi CJ. Analysis of gene mutation associated with tyrosine kinase inhibitor sensitivity of epidermal growth factor receptor in cervical cancer patients. Eur Rev Med Pharmacol Sci. 2018;22:6280–6287. doi: 10.26355/eurrev_201810_16036. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CS, Chen JX, Tang J, Geng YW, Zheng L, Lv LL, Chen LY, Chen Z. Paeonol inhibits pancreatic cancer cell migration and invasion through the inhibition of TGF-β1/smad signaling and epithelial-mesenchymal-transition. Cancer Manag Res. 2020;12:641–651. doi: 10.2147/CMAR.S224416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohamed MZ, Morsy MA, Mohamed HH, Hafez HM. Paeonol protects against testicular ischaemia-reperfusion injury in rats through inhibition of oxidative stress and inflammation. Andrologia. 2020;52:e13599. doi: 10.1111/and.13599. [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Wang Z, Lu D, Huang J, Liu J, Hong L. Paeonol induces cytoprotective autophagy via blocking the Akt/mTOR pathway in ovarian cancer cells. Cell Death Dis. 2019;10:609. doi: 10.1038/s41419-019-1849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Liu Q, Qian R, Liu S, Hu W, Liu Z. Paeonol antagonizes oncogenesis of osteosarcoma by inhibiting the function of TLR4/MAPK/NF-κB pathway. Acta histochemica. 2020;122:151455. doi: 10.1016/j.acthis.2019.151455. [DOI] [PubMed] [Google Scholar]
- 7.Fu J, Yu L, Luo J, Huo R, Zhu B. Paeonol induces the apoptosis of the SGC7901 gastric cancer cell line by downregulating ERBB2 and inhibiting the NF-κB signaling pathway. Int J Mol Med. 2018;42:1473–1483. doi: 10.3892/ijmm.2018.3704. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Saahene RO, Wang J, Wang ML, Agbo E, Pang D. The antitumor mechanism of paeonol on CXCL4/CXCR3-B signals in breast cancer through induction of tumor cell apoptosis. Cancer Biother Radiopharm. 2018;33:233–240. doi: 10.1089/cbr.2018.2450. [DOI] [PubMed] [Google Scholar]
- 9.Moore GY, Pidgeon GP. Cross-talk between cancer cells and the tumour microenvironment: The role of the 5-Lipoxygenase pathway. Int J Mol Sci. 2017;18:236. doi: 10.3390/ijms18020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahboubi-Rabbani M, Zarghi A. Lipoxygenase inhibitors as cancer chemopreventives: Discovery, recent developments, and future perspectives. Curr Med Chem. 2019 doi: 10.2174/0929867326666191210104820. [DOI] [PubMed] [Google Scholar]
- 11.Monga J, Subramani D, Bharathan A, Ghosh J. Pharmacological and genetic targeting of 5-lipoxygenase interrupts c-Myc oncogenic signaling and kills enzalutamide-resistant prostate cancer cells via apoptosis. Sci Rep. 2020;10:6649. doi: 10.1038/s41598-020-62845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa H, Touma J, Davoudi B, Benard M, Sauer T, Geisler J, Vetvik K, Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection is correlated with enhanced cyclooxygenase-2 and 5-lipoxygenase protein expression in breast cancer. J Cancer Res Clin Oncol. 2019;145:2083–2095. doi: 10.1007/s00432-019-02946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go JH, Wei JD, Park JI, Ahn KS, Kim JH. Wogonin suppresses the LPSenhanced invasiveness of MDAMB231 breast cancer cells by inhibiting the 5LO/BLT2 cascade. Int J Mol Med. 42:1899–1908. doi: 10.3892/ijmm.2018.3776. 20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Lee YY, Choi CH, Sung CO, Do IG, Huh S, Song T, Kim MK, Kim HJ, Kim TJ, Lee JW, et al. Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecol Oncol. 2012;124:92–97. doi: 10.1016/j.ygyno.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 16.Aishanjiang A, Rouzi N, Jiao Z, Wang L, Wusainahong K, Wumanjiang N, Musha M, Niyazi M. MicroRNA-9 enhances invasion and migration of cervical carcinomas by directly targeting FOXO1. Eur Rev Med Pharmacol Sci. 2018;22:2253–2260. doi: 10.26355/eurrev_201804_14812. [DOI] [PubMed] [Google Scholar]
- 17.Banno K, Iida M, Yanokura M, Kisu I, Iwata T, Tominaga E, Tanaka K, Aoki D. MicroRNA in cervical cancer: OncomiRs and tumor suppressor miRs in diagnosis and treatment. ScientificWorldJournal. 2014;2014:178075. doi: 10.1155/2014/178075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X, Chu Y, Pan Y, Han L, Meng Z, Wang X. Preoperative neoadjuvant chemotherapy combined with radical surgery in cervical cancer. J BUON. 2020;25:125–131. [PubMed] [Google Scholar]
- 19.Al-Taher AY, Morsy MA, Rifaai RA, Zenhom NM, Abdel-Gaber SA. Paeonol attenuates methotrexate-induced cardiac toxicity in rats by inhibiting oxidative stress and suppressing TLR4-Induced NF-κB inflammatory pathway. Mediators Inflamm. 2020;2020:8641026. doi: 10.1155/2020/8641026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin X, Wang J, Xia ZM, Shang CH, Chao QL, Liu YR, Fan HY, Chen DQ, Qiu F, Zhao F. Anti-inflammatory and anti-oxidative activities of paeonol and its metabolites through blocking MAPK/ERK/p38 signaling pathway. Inflammation. 2016;39:434–446. doi: 10.1007/s10753-015-0265-3. [DOI] [PubMed] [Google Scholar]
- 21.Hafez HM, Morsy MA, Mohamed MZ, Zenhom NM. Mechanisms underlying gastroprotective effect of paeonol against indomethacin-induced ulcer in rats. Hum Exp Toxicol. 2019;38:510–518. doi: 10.1177/0960327118818254. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Song F, Duan LR, Sheng JJ, Xie YH, Yang Q, Chen Y, Dong QQ, Zhang BL, Wang SW. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep. 2016;6:23693. doi: 10.1038/srep23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng CS, Chen JX, Tang J, Geng YW, Zheng L, Lv LL, Chen LY, Chen Z. Paeonol inhibits pancreatic cancer cell migration and invasion through the inhibition of TGF-beta1/smad signaling and epithelial-mesenchymal-transition. Cancer Manag Res. 2020;12:641–651. doi: 10.2147/CMAR.S224416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Xu Q, Mei L, Lei H, Wen Q, Miao J, Huang H, Chen D, Du S, Zhang S, et al. Paeonol attenuates acute lung injury by inhibiting HMGB1 in lipopolysaccharide-induced shock rats. Int Immunopharmacol. 2018;61:169–177. doi: 10.1016/j.intimp.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Mei L, He M, Zhang C, Miao J, Wen Q, Liu X, Xu Q, Ye S, Ye P, Huang H, et al. Paeonol attenuates inflammation by targeting HMGB1 through upregulating miR-339-5p. Sci Rep. 2019;9:19370. doi: 10.1038/s41598-019-55980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyu ZK, Li CL, Jin Y, Liu YZ, Zhang X, Zhang F, Ning LN, Liang ES, Ma M, Gao W, et al. Paeonol exerts potential activities to inhibit the growth, migration and invasion of human gastric cancer BGC823 cells via downregulating MMP2 and MMP9. Mol Med Rep. 2017;16:7513–7519. doi: 10.3892/mmr.2017.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai CY, Zhang JY, Shi TW, Bai YQ, Wu BL, Du ZP, Wu ZY, Xu XE, Wang SH, Wu JY, et al. Association between 5-lipoxygenase expression, and malignant behaviors and poor prognosis in esophageal squamous cell carcinoma. Oncol Lett. 2018;15:9353–9360. doi: 10.3892/ol.2018.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kummer NT, Nowicki TS, Azzi JP, Reyes I, Iacob C, Xie S, Swati I, Darzynkiewicz Z, Gotlinger KH, Suslina N, et al. Arachidonate 5 lipoxygenase expression in papillary thyroid carcinoma promotes invasion via MMP-9 induction. J Cell Biochem. 2012;113:1998–2008. doi: 10.1002/jcb.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the present manuscript.