Abstract
The purpose of the present study was to evaluate whether tanshinone IIA (TIIA) could treat cardiac dysfunction and fibrosis in heart failure (HF) by inhibiting oxidative stress. An HF model was induced by ligation of the left anterior descending artery to cause ischemia myocardial infarction (MI) in Sprague-Dawley rats. Cardiac fibrosis was evaluated using Masson's staining, and the levels of collagen I, collagen III, TGF-β, α-smooth muscle actin (α-SMA), matrix metalloproteinase (MMP) 2 and MMP9 were determined using PCR or western blotting. TIIA treatment reversed the decreases of left ventricular (LV) ejection fraction, fractional shortening (FS), LV systolic pressure and the maximum of the first differentiation of LV pressure (LV ± dp/dtmax), the increases of LV volume in systole, LV volume in diastole, LV end-systolic diameter and LV end-diastolic diameter in MI rats. TIIA administration also reversed the increases of expression levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in the heart of MI rats and in angiotensin (Ang) II-treated cardiac fibroblasts (CFs). TIIA reversed the decreases of superoxide dismutase activity and malondialdehyde and the increases of superoxide anions and NADPH oxidase (Nox) activity in both MI rats and Ang II-treated CFs. Nox4 overexpression inhibited the effects of TIIA of improving cardiac dysfunction and fibrosis in MI rats and Ang II-treated CFs. These results demonstrated that TIIA improved cardiac dysfunction and fibrosis via inhibiting oxidative stress in HF rats. Nox4 could regulate the inhibitory effects of TIIA on HF and cardiac fibrosis.
Keywords: tanshinone IIA, heart failure, myocardial infarction, oxidative stress, fibrosis
Introduction
Heart failure (HF) remains a globally epidemic cardiac disease (1,2). HF is preceded by left ventricular (LV) remodeling, such as enlarged myocardial size or increased LV mass due to pressure-overload (3). Myocardial infarction (MI), the coronary artery disease, can increase the risk of HF and is even life-threatening (4).
LV remodeling is characterized by increased cardiac interstitial fibrosis resulting from the accumulation of collagen type I, collagen type III, matrix metalloproteinase (MMP) 2, MMP9, TGF-β and α-smooth muscle actin (α-SMA) (5–7). Cardiac fibrosis is common in many cardiovascular diseases, such as myocardial infarction (8), hypertension (9) and cardiomyopathy (10) and is critical to the evolution of structural LV remodeling and the development of HF. Currently, the mechanisms of cardiac fibrosis in HF remains to be elucidated.
Tanshinone IIA (TIIA) is a natural compound extracted from the roots of Salvia miltiorrhiza Bunge and has been used in traditional Chinese medicine to protect against organ injuries (11). Studies have demonstrated that TIIA exhibits antioxidative, anti-cancer and anti-inflammatory effects (12–14). TIIA is also been used to treat cardiovascular diseases (15) because it can increase coronary blood flow and ameliorates myocardial metabolic disorder induced by hypoxia (16). Furthermore, TIIA treatment can reduce the infarction and increase myocardial regeneration and contractility (16). However, the effects of TIIA on HF remain to be elucidated.
Oxidative stress serves important roles in pathological cardiac remodeling and cardiac failure (17,18). Coronary ligation can result in dilatation of left ventricle with compensatory hypertrophy of the remaining LV myocardium and the right ventricle (19). This remodeling is associated in LV non-infarcted myocardium with increased oxidative stress and collagen infiltration (19). TIIA can inhibit hydrogen peroxide-induced cardiomyocyte apoptosis (20). However, whether TIIA administration can reverse cardiac fibrosis in MI-induced HF rats via inhibiting oxidative stress is not known. In addition, it is not clear whether NADPH oxidase (Nox) 4 is involved in the oxidative stress in the fibrosis of cardiac fibroblasts (CFs) induced by Ang II. These questions were addressed by the present study.
Materials and methods
Animals
Experiments were carried out on male Sprague-Dawley (SD) rats (age, 5–6 weeks; weight, 180–200 g; Vital River Biological Co., Ltd, Beijing, China). A total of 80 rats were used in the present study. All procedures were approved by the Experimental Animal Care and Use Committee of Nanjing University of Chinese Medicine (approval no. 17064512), and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85–23, revised in 1996; pubmed.ncbi.nlm.nih.gov/25121211/). The rats were kept in a temperature (22±1°C) and humidity (30–60%)-controlled room on a 12-h light/dark cycle with free access to standard chow and tap water. The rats were sacrificed using an overdose of sodium pentobarbital (100 mg/kg intravenously) for sample collection. Death was confirmed by the absence of heartbeat, corneal reflexes and paw withdrawal response to a noxious pinch.
Myocardial infarction model
The myocardial infarction (MI) in rats was induced by coronary artery ligation with sterile techniques as previously reported (21). Briefly, the rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and randomly subjected to the ligation of the left anterior descending (LAD) coronary artery or sham-operation. The heart was exposed through a left intercostal thoracotomy and the left coronary artery was looped by a single nylon suture (7–0). The LAD was ligated under 1–1.5 mm from the left atrial appendage in rats. The heart was then quickly repositioned into the chest. The sham rats were treated in the same way as the coronary ligation rats except that their coronary arteries were not ligated. After 24 h, the rats were randomly assigned to four groups: i) Sham + saline group, ii) sham + TIIA group, iii) MI + saline group and iv) MI + TIIA group (n=8 for each group). TIIA (1.5 mg/kg/d/500 µl, Sigma-Aldrich; Merck KGaA) (22) was orally gavaged to the rats in sham + TIIA and MI + TIIA groups for 28 days, while the rats in the other two groups received the same volume of saline. The rats were euthanized using an overdose of sodium pentobarbital (100 mg/kg intravenously) if malaise or surgical site putrescence presented.
Echocardiography
Transthoracic echocardiography was performed using an ultrasound system (VisualSonics Inc.) with a 21-MHz probe under isoflurane anesthesia (2.5%) to measure the LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV volumes in diastole (LVVd) and LV volumes in systole (LVVs). The LV ejection fraction (EF) and fractional shortening (FS) were calculated as EF = (LVVd-LVVd)/LVVd ×100 and FS = (LVEDD-LVESD)/LVEDD ×100. The measurements over three consecutive cardiac cycles were averaged.
Hemodynamic monitoring
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). A conductance micromanometer catheter (1.4F, Millar Instruments, Inc.) was inserted into the LV chamber via the left carotid artery across the aortic valve. The left ventricular systolic pressure (LVSP) and LV end-diastolic pressure (LVEDP), maximum of the first differentiation of LV pressure (LV + dp/dt) and decline (LV - dp/dt) were obtained with a PowerLab data acquisition system (ADInstruments Pty Ltd.).
Masson trichrome staining
The rats were sacrificed with an overdose of sodium pentobarbital (100 mg/kg, i.p.) and the hearts were harvested. Serial dehydration was performed using an ethanol concentration gradient, followed by paraffin embedding. Sections of the heart (5 µm) were examined by Masson's trichrome staining according to the manufacturer's instructions (Wuhan Servicebio Technology Co., Ltd.) to determine the extent of fibrosis. Three to five random fields of view were selected in three sections from each animal for observation under a light microscope (Zeiss AG). Captured images were analyzed using Image-Pro Plus software 6.0 (Media Cybernetics, Inc.).
Culture of CFs
Rat CFs were isolated from 1–3 day-old SD rats (Beijing Vital River Laboratory Animal Technology Co., Ltd.). A total of 60 cubs were used in the present study. The hearts were collected following the pups anesthesia under isoflurane (3.5%). Briefly, CFs were separated from cardiomyocytes by gravity separation and grown to confluence on 10 cm cell culture dishes with growth media [DMEM (Gibco; Thermo Fisher Scientific, Inc.] including 10% FBS, 1% penicillin and 1% streptomycin) at 37°C in humid air with 5% CO2 and 95% O2. The CFs from the second passage were incubated with Ang II (10-6 M, Sigma-Aldrich; Merck KGaA) for 24 h to induce the fibrotic phenotype. The CFs were then assigned to four groups: i) PBS group, ii) TIIA (10 µM) group, iii) Ang II group and iv) Ang II + TIIA (10 µM) group.
Reverse transcription-quantitative PCR
The total RNA in LV or CFs (1×106 cells/ml) was extracted with TRIzol® (Thermo Fisher Scientific, Inc.). cDNA was extracted from RNA with reverse transcription using 10 µl random primers according to the instructions of the PrimeScript™ RT Master Mix (Takara Biotechnology Co., Ltd.) and stored at −70°C before use. Collagen I, collagen III, α-smooth muscle actin (SMA), TGF-β, matrix metalloproteinase (MMP) 2 and MMP9 mRNA levels were determined with Synergy Brands Green I fluorescence (Applied Biosystems) in accordance with the manufacturer's protocols. All samples were amplified in triplicates for 45 cycles in a 384-well plate (95°C for 15 sec, then 60°C for 1 min). The relative gene expression was determined by calculating the values of ΔCq as a relative quantity to the endogenous control (23). These experiments were replicated three times. The primers sequences are listed in Table I.
Table I.
List primers used for reverse transcription-quantitative PCR.
| Gene | Species | Forward primer, 5′-3′ | Reverse primer, 5′-3′ |
|---|---|---|---|
| Collagen I | Rat | TCAAGATGGTGGCCGTTAC | CTGCGGATGTTCTCAATCTG |
| Collagen III | Rat | CGAGATTAAAGCAAGAGGAA | GAGGCTTCTTTACATACCAC |
| TGF-β | Rat | CAGGGAGTAAGGGACACGA | ACAGCAGTTAGGAACCCAGAT |
| α-SMA | Rat | GTCCCAGACATCAGGGAGTAA | TCGGATACTTCAGCGTCAGGA |
| MMP2 | Rat | CCCCATGTGTCTTCCCCTTC | AGCTCCTGGATCCCCTTGAT |
| MMP9 | Rat | AGGGCCCCTTTCTTATTGCC | CGAGTAACGCTCTGGGGATC |
| GAPDH | Rat | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
α-SMA, α-smooth muscle actin; MMP, matrix metalloproteinase.
Western blotting
CF samples were lysed in modified RIPA buffer (BioChannel Biological Technology Co., Ltd.). The protein concentration was determined using a BCA assay (Beyotime Institute of Biotechnology). A total of 30–50 µg of protein was separated using SDS-PAGE on 8% gels, then transferred to a PVDF membrane. The membrane was blocked with 5% skimmed milk powder at room temperature for 1 h and probed overnight at 4°C with primary antibodies against collagen I (1:1,000; cat. no. ab254113; Abcam), collagen III (1:5,000; cat. no. ab7778; Abcam), TGF-β (1:1,000; cat. no. ab215715; Abcam), α-SMA(1:10,000; cat. no. ab124964; Abcam), MMP2 (1:2,000; cat. no. ab92536; Abcam) and MMP9 (1:1,000; cat. no. ab228402; Abcam) primary antibodies (Abcam), followed by incubation with a HRP-conjugated goat anti-rabbit secondary antibody (1:10,000, cat. no. ab7090; Abcam) at 37°C for 1 h. The bands were visualized using the enhanced chemiluminescence (ECL) substrate (BioChannel Biological Technology Co., Ltd.). The total AT1R protein level was normalized to the protein level of GAPDH (Bioworld Technology Inc.). Images were analyzed using Image-Pro Plus software (version 6.0; XRayScan; CAD/CAM Services, Inc.).
Nox4 overexpression
Recombinant adenoviral vectors harboring green fluorescent protein (Ad-GFP) and Nox4 (Ad-Nox4) were constructed and packaged by Shanghai GeneChem Co., Ltd. In the in vivo experiment, adenovirus (200 µl/rat, 1×1012 plaque-forming units/ml) was injected into the rats via the tail vein. In the in vitro experiment, 20 µl original solution was diluted in 2 ml Enhance Infection Solution (Shanghai GeneChem Co., Ltd.). The CFs were transfected with serum-free medium containing Ad-Nox4 or Ad-GFP at 37°C for 24 h.
Superoxide dismutase (SOD) activity level
The rats were sacrificed with an overdose of pentobarbital (100 mg/kg, i.p.). The LV samples were collected and homogenized in lysis buffer (Thermo Fisher Scientific, Inc.). SOD was measured using a commercial kit (Nanjing Jiancheng Bioengineering Institute; cat. no. A001-3-2) according to the manufacturer's instructions using a microplate reader (BioTek Instruments, Inc.).
Malondialdehyde level in the heart
After homogenizing the LV samples, malondialdehyde (MDA) levels in the LV were determined using an ELISA kit (Wuhan USCN Business Co., Ltd.; cat. no. CEA597Ge) following the manufacturer's instructions.
Measurement of Nox activity
The Nox activity in the heart was measured by enhanced lucigenin chemiluminescence. Briefly, NADPH (100 µM) was added to the media as a substrate to react with Nox and generate superoxide anions. The light emission produced by the reaction of lucigenin (5 µM) with superoxide anions was measured with a microplate reader (BioTek Instruments, Inc.) once every minute for 10 min. The values representing Nox activity were expressed as the mean light units (MLU) per min per mg of protein.
Measurement of superoxide anions
The level of superoxide anions in the heart was determined by lucigenin-derived chemiluminescence. Briefly, the reaction with superoxide anions was started by adding dark-adapted lucigenin (5 µM) to each sample to cause photon emission, which was measured with a microplate reader (BioTek Instruments, Inc.) once every minute for 10 min. The values representing the superoxide anions level were expressed as the MLU per min per mg of protein.
Statistical analysis
Data are presented as the mean ± standard error of the mean and were analyzed using GraphPad Prism 7.0 (GraphPad software Inc.). Statistics were completed using one-way ANOVA, followed by Bonferroni test for post hoc analysis when multiple comparisons were made. P<0.05 was considered to indicate a statistically significant difference.
Results
TIIA improves cardiac function of rats with MI-induced HF
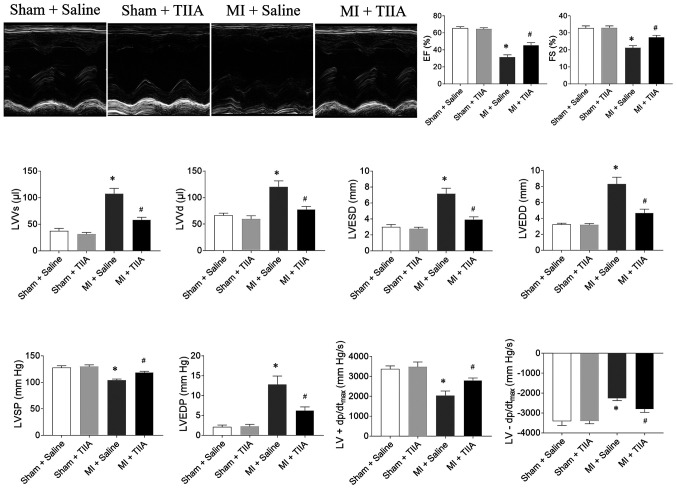
EF, FS, LVSP and LV ±dp/dtmax were reduced in rats with MI-induced HF and this reduction was reversed by TIIA treatment. LVVs, LVVd, LVESD, LVEDD and LVEDP were increased in MI-induced HF rats and this increase was attenuated by TIIA treatment (Fig. 1).
Figure 1.
TIIA improves cardiac dysfunction in rats with MI-induced heart failure. TIIA treatment improved the decreases of LV EF, FS, LVSP and the LV ± dp/dtmax and the increases of LVVS, LVVD, LVESD and LVEDD in MI rats. The results are expressed as mean ± standard error of the mean. n=8. *P<0.05 vs. the Sham + Saline group; #P<0.05 vs. the MI + Saline group. TIIA, tanshinone IIA; MI, myocardial infarction; LV, left ventricular; EF, ejection fraction; FS, fractional shortening; LVSP, LV systolic pressure; LV ± dp/dtmax, maximum of the first differentiation of LV pressure; LVVS, LV volume in systole; LVVD, LV volume in diastole; LVESD, LV end-systolic diameter; LVEDD, LV end-diastolic diameter.
TIIA attenuates LV fibrosis in rats with MI-induced HF
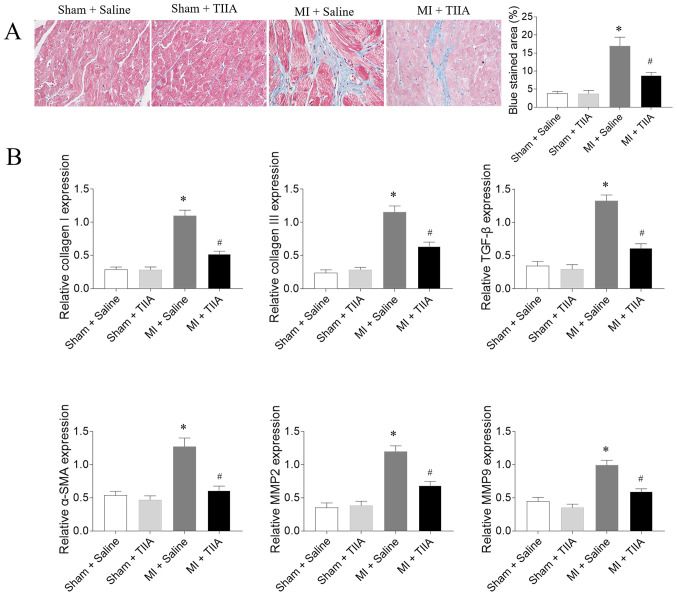
The mRNA expression levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in LV of MI rats were increased compared with sham rats. The increased levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in the LVs of MI rats were inhibited after TIIA administration (Fig. 2).
Figure 2.
TIIA attenuates LV fibrosis in rats with MI-induced heart failure. (A) TIIA treatment attenuated LV fibrosis in MI rats, the blue area represents the fibrosis. Magnification, ×400. (B) TIIA treatment inhibited the increases of collagen I, collagen III, SMA, TGF-β, MMP2 and MMP9 in MI rats. The results are expressed as mean ± standard error of the mean. n=8. *P<0.05 vs. the Sham + Saline group; #P<0.05 vs. the MI + Saline group. TIIA, tanshinone IIA; LV, left ventricular; MI, myocardial infarction; SMA, α-smooth muscle actin; MMP, matrix metalloproteinase.
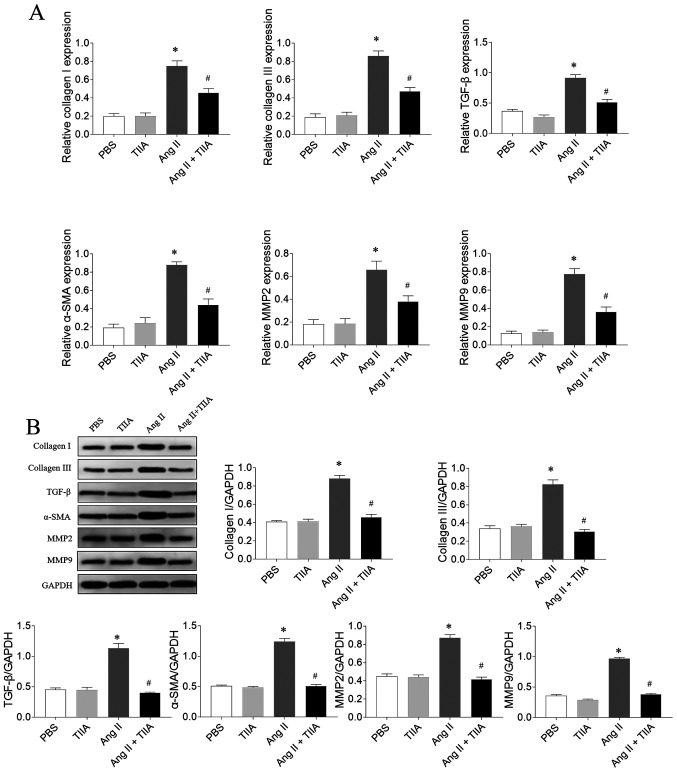
TIIA attenuates Ang II-induced fibrosis of CFs
The mRNA expression levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 increased in Ang II-treated CFs and this increase was reversed after TIIA treatment (Fig. 3A). The Ang II-induced increases in protein expression levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in CFs were inhibited after TIIA treatment (Fig. 3B).
Figure 3.
TIIA attenuates CFs fibrosis induced by Ang II. (A) TIIA treatment inhibited the increased mRNA expression levels of collagen I, collagen III, SMA, TGF-β, MMP2 and MMP9 in Ang II-treated CFs. (B) TIIA treatment inhibited the increased protein expression of collagen I, collagen III, α-SMA, TGF-β, MMP2 and MMP9 in Ang II-treated CFs. The results are expressed as mean ± SE. *P<0.05 vs. the PBS group; #P<0.05 vs. the Ang II group. TIIA, tanshinone IIA; CFs, cardiac fibroblasts; Ang II, angiotensin II; SMA, α-smooth muscle actin; MMP, matrix metalloproteinase.
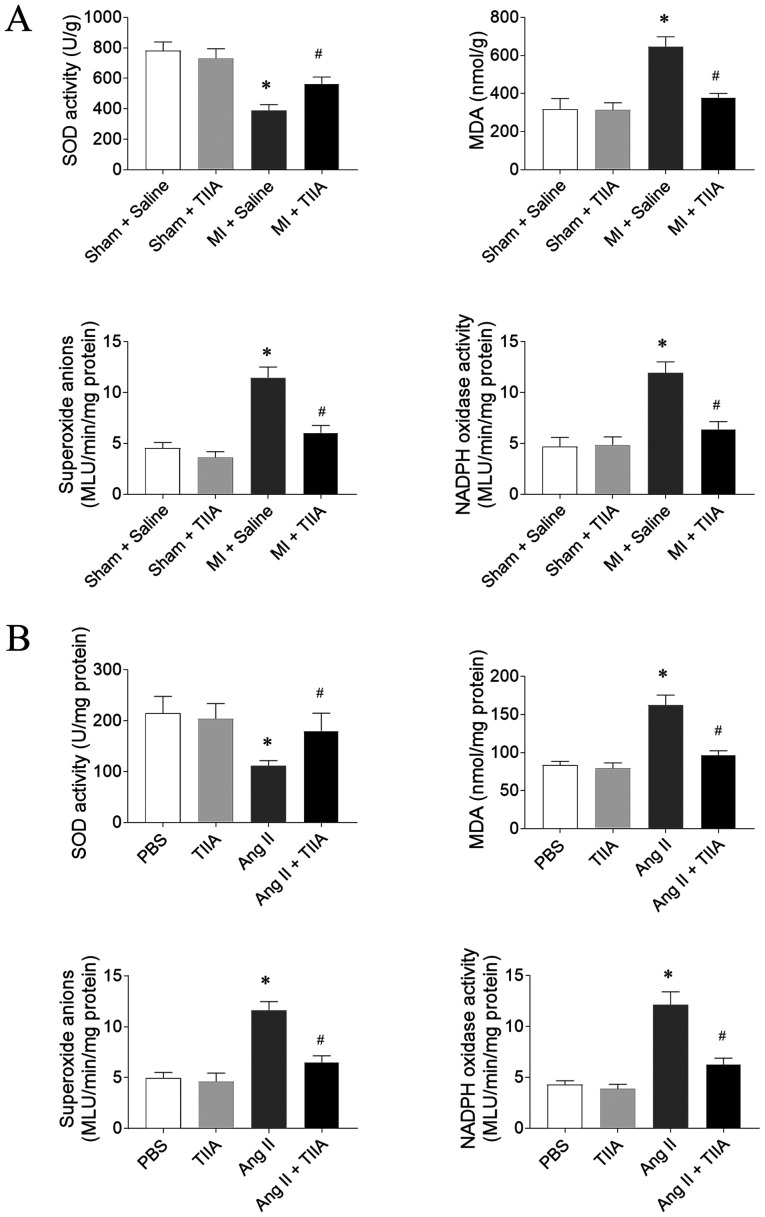
TIIA attenuates oxidative stress in the LVs of HF rats and Ang II-treated CFs
SOD activity level was reduced in the LVs of MI-induced HF rats and this reduction was reversed by TIIA. MDA, superoxide anions and Nox activity levels were significantly increased in LV of MI-induced HF rats and this increase was inhibited after TIIA treatment (Fig. 4A). The decrease of SOD activity and the increases of MDA, superoxide anions and Nox activity levels in Ang II-treated CFs were reversed by TIIA administration (Fig. 4B).
Figure 4.
TIIA attenuates the increase of oxidative stress in the heart of myocardial infarction MI rats and the Ang II-treated CFs. (A) TIIA treatment increased the decreased SOD activity and inhibited the increases of MDA, superoxide anions and NADPH oxidase activity in the LV of MI rats. (B) TIIA treatment increased the decrease of SOD activity and inhibited the increases of MDA, superoxide anions and NADPH oxidase activity in Ang II-treated CFs. The results are expressed as mean ± standard error of the mean. n=8. *P<0.05 vs. the (A) Sham + Saline or (B) PBS group; #P<0.05 vs. the (A) MI + Saline or (B) Ang II group. TIIA, tanshinone IIA; MI, myocardial infarction; Ang II, angiotensin II; CFs, cardiac fibroblasts; SOD, superoxide dismutase; MDA, malondialdehyde; LV, left ventricular.
Nox4 overexpression reverses the improving effects of TIIA on cardiac function in HF rats
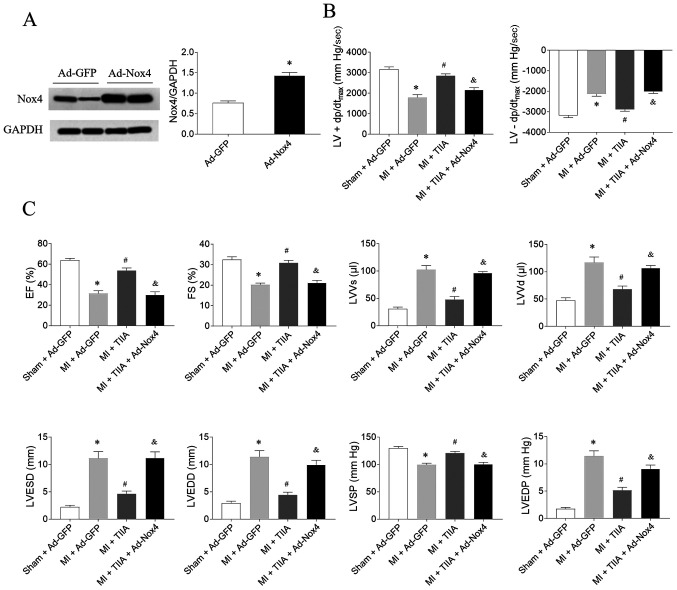
The expression level of Nox was increased in the heart rats treated with Ad-Nox4 (Fig. 5A). The effects of TIIA in improving LV ± dp/dtmax, EF, FS and LVSP in MI-induced HF rats were reversed by Nox4 overexpression. Moreover, Nox4 overexpression also reversed the effects of TIIA in decreasing LVVs, LVVd, LVESD, LVEDD and LVEDP in MI-induced HF rats (Fig. 5B and C.).
Figure 5.
Nox4 overexpression reverses the effects of TIIA in improving cardiac dysfunction on rats with MI-induced heart failure. (A) The expression level of Nox4 was increased in the heart rats treated with adenoviral (Ad)-Nox4. (B) Nox4 overexpression reversed the improving effects of TIIA on the decreases of LV ± dp/dtmax in MI rats. (C) Nox4 overexpression reversed the improving effects of TIIA on the decreases of LV EF, FS and LVSP and the increases of LVVS, LVVD, LVESD and LVEDD in MI rats. The results are expressed as mean ± standard error of the mean. n=8. *P<0.05 vs. the (A) Ad-GFP or (B and C) Sham + Ad-GFP group; #P<0.05 vs. the MI + Ad-GFP group; &P<0.05 vs. the MI + TIIA group. Nox, NADPH oxidase; TIIA, tanshinone IIA; MI, myocardial infarction; LV ± dp/dtmax, maximum of the first differentiation of LV pressure; LV, left ventricular; EF, ejection fraction; FS, fractional shortening; LVSP, LV systolic pressure; LVVS, LV volume in systole; LVVD, LV volume in diastole; LVESD, LV end-systolic diameter; LVEDD, LV end-diastolic diameter.
Nox4 overexpression reverses the inhibitory effects of TIIA on LV fibrosis in HF rats
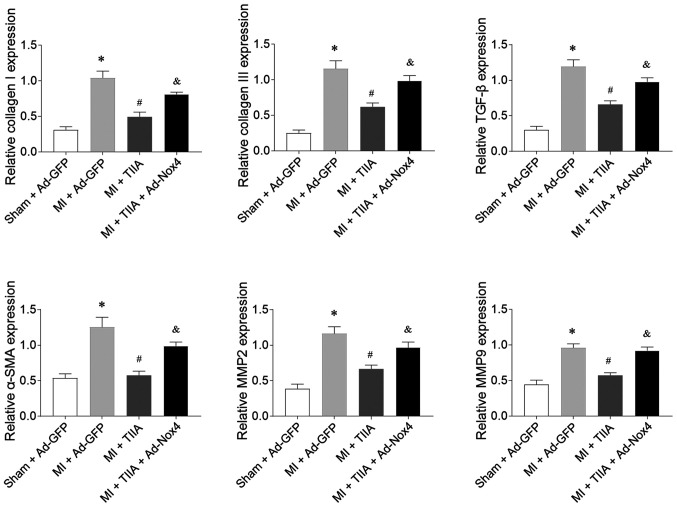
Nox4 overexpression reversed the inhibiting effects of TIIA on the increases in the mRNA expression levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in the LVs of rats with MI-induced HF (Fig. 6).
Figure 6.
Nox4 overexpression reverses the effects of TIIA in inhibiting LV fibrosis in rats with MI-induced heart failure. Nox4 overexpression reversed the effects of TIIA in attenuating the increases of collagen I, collagen III, SMA, TGF-β, MMP2 and MMP9 in the LV of MI rats. The results are expressed as mean ± standard error of the mean. n=8. *P<0.05 vs. the Sham + Ad-GFP group; #P<0.05 vs. the MI + Ad-GFP group; &P<0.05 vs. the MI + TIIA group. Nox, NADPH oxidase; TIIA, tanshinone IIA; LV, left ventricular; MI, myocardial infarction; SMA, α-smooth muscle actin; MMP, matrix metalloproteinase.
Nox4 overexpression reverses the inhibitory effects of TIIA on Ang II-induced CF fibrosis
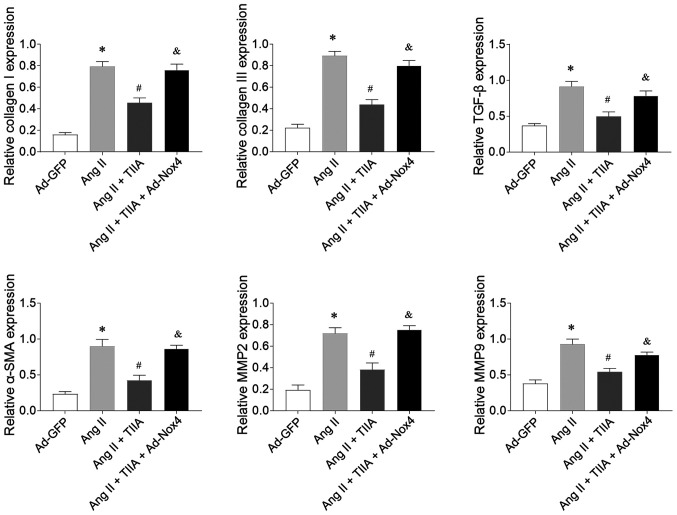
Nox4 overexpression reversed the inhibitory effects of TIIA on Ang II-induced increases in the mRNA expression levels of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in CFs (Fig. 7).
Figure 7.
Nox4 overexpression reversed the inhibiting effects of TIIA on Ang II-induced fibrosis of CFs. Nox4 overexpression reversed the effects of TIIA in attenuating the increases of collagen I, collagen III, SMA, TGF-β, MMP2 and MMP9 in the Ang II-treated CFs. The results are expressed as mean ± standard error of the mean. *P<0.05 vs. the Ad-GFP group; #P<0.05 vs. the Ang II group; &P<0.05 vs. the Ang II + TIIA group. Nox, NADPH oxidase; TIIA, tanshinone IIA; Ang II, angiotensin II; CFs, cardiac fibroblasts; SMA, α-smooth muscle actin; MMP, matrix metalloproteinase.
Discussion
The present study found that TIIA improved cardiac dysfunction in the rats with MI-induced HF. The fibrosis of LV in HF rats and Ang II-treated CFs was ameliorated following TIIA administration. Furthermore, oxidative stress was enhanced in LV of HF rats and in Ang II-treated CFs, as indicated by the decrease of SOD activity and the increases of MDA, superoxide anions and Nox activity levels, all reversed by TIIA treatment. Nox4 overexpression inhibited the effects of TIIA in improving cardiac dysfunction in HF rats and the fibrosis of LV in HF rats and Ang II-treated CFs.
The results of the present study demonstrated that TIIA treatment ameliorated the decreases of EF, FS, LVSP and LV ± dp/dtmax and the increases of LVVs, LVVd, LVESD, LVEDD and LVEDP in MI-induced HF rats, indicating that TIIA improved cardiac dysfunction in HF. This finding is consistent with a previous finding that TIIA could significantly improve heart function in left anterior descending (LAD) ligation-induced HF (22).
As a complex pathological process involving myocardial fibrosis, cardiac hypertrophy and cardiomyocyte apoptosis, LV remodeling is often caused by cardiovascular diseases (CVDs) such as HF, hypertension, or myocardial infarction (24,25). Cardiac fibrosis is a significant global health problem caused by pathological stimuli to the heart. Previous studies demonstrated that TIIA could inhibit the proliferation of mouse cardiac fibroblasts in cultures by MTT assay (26) and attenuate high glucose-mediated collagen synthesis through inhibiting the TGF-β1/Smad signaling pathway in cardiac fibroblasts (27). The present study found that the increases of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in LV of MI-induced HF rats were abolished following TIIA administration. In addition, TIIA treatment inhibited the increases of collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 in Ang II-treated CFs. These results suggested that TIIA attenuated the fibrosis of LV in HF.
As important molecules in the living organisms, reactive oxygen species (ROS) are involved in many signaling pathways (28). However, the overproduction of ROS serves a significant role in the development of CVDs (29,30). Oxidative stress, a key contributor to organ damage, is associated with various diseases (31), including cardiac fibrosis (32). Sodium TIIA sulfonate inhibits the increased production of ROS and expression of TGF-β1 stimulated by Ang II in human atrial fibroblasts (33). TIIA significantly inhibits H2O2-induced collagen synthesis via attenuating Nox activity and ROS generation (34). Due to its antioxidative property, TIIA protects two-kidney, two-clip hypertensive rats from cardiac dysfunction and fibrosis, partially via reducing Nox activity, but without changing blood pressure (35). In the present study, the results demonstrated that SOD activity was significantly reduced and that the MDA, superoxide anions and Nox activity levels were increased in LV of HF rats, then decreased by TIIA treatment. Furthermore, the decrease of SOD activity and the increases of MDA, superoxide anions and Nox activity in Ang II-treated CFs were reversed by TIIA administration. These results indicated that in the heart of MI-induced HF rats, the oxidants and antioxidants were imbalanced, which could be reversed by TIIA; TIIA could curb cardiac fibrosis in HF via inhibiting oxidative stress.
TIIA might be a potent agent against the fibrosis in the hearts of lipopolysaccharide-treated mice partially via inhibiting Nox2 (36). The present study found that Nox4 overexpression reversed the effects of TIIA in ameliorating cardiac dysfunction in HF rats. The effects of TIIA in reducing collagen I, collagen III, TGF-β, α-SMA, MMP2 and MMP9 on LV of HF rats were inhibited following Nox4 overexpression. These results demonstrated that Nox4 could regulate the inhibitory effects of TIIA on HF and cardiac fibrosis.
In addition to the oxidative stress explored in the present study, TIIA is involved in various other signal pathways related to cardiac diseases. TIIA protects cardiomyocytes and improves cardiac function by activating the AMP-activated protein kinase-mammalian target of rapamycin signaling pathway to inhibit apoptosis and induce autophagy (22). TIIA prevents LV remodeling of MI rats, mainly through repressing the Toll-like receptor 4/Myeloid differentiation primary response 88/NF-κB signaling pathway (37). A previous study demonstrated that TIIA can activate the phosphatidylinositol 3-kinase/protein kinase B/mTOR signaling pathway to relieve myocardial ischemia reperfusion injury in rats (12).
In the present study, it was not clear how TIIA improved cardiac fibrosis of MI rats via inhibiting oxidative stress. Since only MI model-induced cardiac fibrosis was investigated in the present study, whether cardiac fibrosis in other models could also be attenuated by TIIA remains unknown.
In conclusion, oxidative stress was enhanced in the hearts of HF rats, then attenuated by TIIA. TIIA restored the imbalance between oxidant and antioxidant levels. TIIA, functioning as an antioxidant, could improve cardiac dysfunction and attenuate fibrosis in HF rats and Ang II-induced fibrosis in CFs. Nox4 regulated the inhibitory effects of TIIA in HF and cardiac fibrosis. It is hoped these results could provide evidence for the clinical application of TIIA in treating HF-related cardiac fibrosis.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the Key Discipline Construction Project of Jiangsu Province (grant no. js1303).
Funding
The present study was supported by the Key Discipline Construction Project of Jiangsu Province (grant no. js1303).
Availability of data and materials
The datasets used or analyzed during the present study are available from the corresponding author upon request.
Authors' contributions
RC and WC made substantial contributions to conception and design of the study, and were involved in drafting the manuscript or revising it critically for important intellectual content. XH acquired analysed and interpreted the data. QR made substantial contributions to conception and design of the study, and was involved in drafting and revising the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Experimental Animal Care and Use Committee of Nanjing University of Chinese Medicine (approval no. 2018031811).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raso A, Dirkx E, Philippen LE, Fernandez-Celis A, De Majo F, Sampaio-Pinto V, Sansonetti M, Juni R, El Azzouzi H, Calore M, et al. Therapeutic Delivery of miR-148a Suppresses Ventricular Dilation in Heart Failure. Mol Ther. 2019;27:584–599. doi: 10.1016/j.ymthe.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, et al. ESC Scientific Document Group European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J. 2018;39:508–579. doi: 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Yang CX, Chen XR, Liu BX, Li Y, Wang XZ, Sun W, Li P, Kong XQ. Alamandine attenuates hypertension and cardiac hypertrophy in hypertensive rats. Amino Acids. 2018;50:1071–1081. doi: 10.1007/s00726-018-2583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Liu C, Chen X, Li P. Alamandine attenuates long term hypertension induced cardiac fibrosis independent of blood pressure. Mol Med Rep. 2019;19:4553–4560. doi: 10.3892/mmr.2019.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract. 2017;133:124–130. doi: 10.1016/j.diabres.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X, Pan J, Wen L, Gong B, Li J, Gao H, Tan W, Liang S, Zhang H, Wang X. miR-144-3p Enhances Cardiac Fibrosis After Myocardial Infarction by Targeting PTEN. Front Cell Dev Biol. 2019;7:249. doi: 10.3389/fcell.2019.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai R, Sun T, Ramirez V, Lux E, Eren M, Vaughan DE, Ghosh AK. Acetyltransferase p300 inhibitor reverses hypertension-induced cardiac fibrosis. J Cell Mol Med. 2019;23:3026–3031. doi: 10.1111/jcmm.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan CY, Wong JX, Chan PS, Tan H, Liao D, Chen W, Tan LW, Ackers-Johnson M, Wakimoto H, Seidman JG, et al. Yin Yang 1 Suppresses Dilated Cardiomyopathy and Cardiac Fibrosis Through Regulation of Bmp7 and Ctgf. Circ Res. 2019;125:834–846. doi: 10.1161/CIRCRESAHA.119.314794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu QQ, Xu YJ, Yang C, Tang Y, Li L, Cai HB, Hou BN, Chen HF, Wang Q, Shi XG, et al. Sodium Tanshinone IIA Sulfonate Attenuates Scopolamine-Induced Cognitive Dysfunctions via Improving Cholinergic System. BioMed Res Int. 2016;2016:9852536. doi: 10.1155/2016/9852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Shen L, Wang Z, Jiang HP, Liu LX. Tanshinone IIA protects against myocardial ischemia reperfusion injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2016;84:106–114. doi: 10.1016/j.biopha.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wei Y, Yuan S, Liu G, Lu Y, Zhang J, Wang W. Potential anticancer activity of tanshinone IIA against human breast cancer. Int J Cancer. 2005;116:799–807. doi: 10.1002/ijc.20880. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Han X, Zhang H, Wu J, Li B. The interplay between autophagy and apoptosis induced by tanshinone IIA in prostate cancer cells. Tumour Biol. 2016;37:7667–7674. doi: 10.1007/s13277-015-4602-9. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Chen FY, Guo R, Zhang BK. Advances in cardiovascular effects of tanshinone II(A) Zhongguo Zhong Yao Za Zhi. 2015;40:1649–1653. (In Chinese) [PubMed] [Google Scholar]
- 17.Sag CM, Santos CX, Shah AM. Redox regulation of cardiac hypertrophy. J Mol Cell Cardiol. 2014;73:103–111. doi: 10.1016/j.yjmcc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Madamanchi NR, Runge MS. Redox signaling in cardiovascular health and disease. Free Radic Biol Med. 2013;61:473–501. doi: 10.1016/j.freeradbiomed.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnefont-Rousselot D, Mahmoudi A, Mougenot N, Varoquaux O, Le Nahour G, Fouret P, Lechat P. Catecholamine effects on cardiac remodelling, oxidative stress and fibrosis in experimental heart failure. Redox Rep. 2002;7:145–151. doi: 10.1179/135100002125000389. [DOI] [PubMed] [Google Scholar]
- 20.Yan SH, Zhao NW, Geng ZR, Shen JY, Liu FM, Yan D, Zhou J, Nie C, Huang CC, Fang ZY. Modulations of Keap1-Nrf2 signaling axis by TIIA ameliorated the oxidative stress-induced myocardial apoptosis. Free Radic Biol Med. 2018;115:191–201. doi: 10.1016/j.freeradbiomed.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Gan XB, Duan YC, Xiong XQ, Li P, Cui BP, Gao XY, Zhu GQ. Inhibition of cardiac sympathetic afferent reflex and sympathetic activity by baroreceptor and vagal afferent inputs in chronic heart failure. PLoS One. 2011;6:e25784. doi: 10.1371/journal.pone.0025784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Wang Q, Wang X, Chen X, Shao M, Zhang Q, Guo D, Wu Y, Li C, Wang W, et al. Tanshinone IIA protects against heart failure post-myocardial infarction via AMPKs/mTOR-dependent autophagy pathway. Biomed Pharmacother. 2019;112:108599. doi: 10.1016/j.biopha.2019.108599. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Koshman YE, Patel N, Chu M, Iyengar R, Kim T, Ersahin C, Lewis W, Heroux A, Samarel AM. Regulation of connective tissue growth factor gene expression and fibrosis in human heart failure. J Card Fail. 2013;19:283–294. doi: 10.1016/j.cardfail.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou L, Guo J, Xu F, Weng X, Yue W, Ge J. Cardiomyocyte dimethylarginine dimethylaminohydrolase1 attenuates left-ventricular remodeling after acute myocardial infarction: Involvement in oxidative stress and apoptosis. Basic Res Cardiol. 2018;113:28. doi: 10.1007/s00395-018-0685-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhang S, Chen X. Tanshinone IIA protects against cardiac fibrosis through inhibition of β-tubulin expression. J Biol Regul Homeost Agents. 2018;32:1451–1455. [PubMed] [Google Scholar]
- 27.Tsai YT, Loh SH, Lee CY, Lee SP, Chen YL, Cheng TH, Tsai CS. Tanshinone IIA Inhibits High Glucose-Induced Collagen Synthesis via Nuclear Factor Erythroid 2-Related Factor 2 in Cardiac Fibroblasts. Cell Physiol Biochem. 2018;51:2250–2261. doi: 10.1159/000495870. [DOI] [PubMed] [Google Scholar]
- 28.Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int J Mol Sci. 2020;21:358. doi: 10.3390/ijms21010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Yun J, Kwon SM. Therapeutic Strategies for Oxidative Stress-Related Cardiovascular Diseases: Removal of Excess Reactive Oxygen Species in Adult Stem Cells. Oxid Med Cell Longev. 2016;2016:2483163. doi: 10.1155/2016/2483163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid Med Cell Longev. 2019;2019:6175804. doi: 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda T, Hirakawa Y, Nangaku M. The role of oxidative stress and hypoxia in renal disease. Kidney Res Clin Pract. 2019;38:414–426. doi: 10.23876/j.krcp.19.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kura B, Szeiffova Bacova B, Kalocayova B, Sykora M, Slezak J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int J Mol Sci. 2020;21:358. doi: 10.3390/ijms21010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Li M, Fan X, Cheng J, Wang L. Sodium Tanshinone IIA Sulfonate Prevents Angiotensin II–Induced Differentiation of Human Atrial Fibroblasts into Myofibroblasts. Oxid Med Cell Longev. 2018;2018:6712585. doi: 10.1155/2018/6712585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Zhou S, Xu L, Lu Y, Yuan X, Zhang H, Li R, Fang J, Liu P. Hydrogen peroxide-mediated oxidative stress and collagen synthesis in cardiac fibroblasts: Blockade by tanshinone IIA. J Ethnopharmacol. 2013;145:152–161. doi: 10.1016/j.jep.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Wu X, Bao Y, Fang J, Zhou S, Gao J, Pi R, Mou YG, Liu P. Tanshinone IIA prevents cardiac remodeling through attenuating NAD (P)H oxidase-derived reactive oxygen species production in hypertensive rats. Pharmazie. 2011;66:517–524. [PubMed] [Google Scholar]
- 36.Huang L, Zhu J, Zheng M, Zou R, Zhou Y, Zhu M. Tanshinone IIA protects against subclinical lipopolysaccharide induced cardiac fibrosis in mice through inhibition of NADPH oxidase. Int Immunopharmacol. 2018;60:59–63. doi: 10.1016/j.intimp.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Wu DM, Wang YJ, Han XR, Wen X, Li L, Xu L, Lu J, Zheng YL. Tanshinone IIA prevents left ventricular remodelling via the TLR4/MyD88/NF-κB signalling pathway in rats with myocardial infarction. J Cell Mol Med. 2018;22:3058–3072. doi: 10.1111/jcmm.13557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the present study are available from the corresponding author upon request.