Abstract
The health risks of nicotine are well known, but there is some evidence of its beneficial effects on cognitive function. The present review focused on the reported benefits of nicotine in the brain and summarizes the associated underlying mechanisms. Nicotine administration can improve cognitive impairment in Alzheimer's disease (AD), and dyskinesia and memory impairment in Parkinson's disease (PD). In terms of its mechanism of action, nicotine slows the progression of PD by inhibiting Sirtuin 6, a stress-responsive protein deacetylase, thereby decreasing neuronal apoptosis and improving neuronal survival. In AD, nicotine improves cognitive impairment by enhancing protein kinase B (also referred to as Akt) activity and stimulating phosphoinositide 3-kinase/Akt signaling, which regulates learning and memory processes. Nicotine may also activate thyroid receptor signaling pathways to improve memory impairment caused by hypothyroidism. In healthy individuals, nicotine improves memory impairment caused by sleep deprivation by enhancing the phosphorylation of calmodulin-dependent protein kinase II, an essential regulator of cell proliferation and synaptic plasticity. Furthermore, nicotine may improve memory function through its effect on chromatin modification via the inhibition of histone deacetylases, which causes transcriptional changes in memory-related genes. Finally, nicotine administration has been demonstrated to rescue long-term potentiation in individuals with sleep deprivation, AD, chronic stress and hypothyroidism, primarily by desensitizing α7 nicotinic acetylcholine receptors. To conclude, nicotine has several cognitive benefits in healthy individuals, as well as in those with cognitive dysfunction associated with various diseases. However, further research is required to shed light on the effect of acute and chronic nicotine treatment on memory function.
Keywords: nicotine, memory impairment, protein kinases, histone deacetylases, hypothyroidism
1. Introduction
Nicotine, or 3-(1-Methylpyrrolidin-2-yl) pyridine, is an alkaloid that is found in the tobacco plant (1,2). Nicotine use can lead to a number of health complications, including heart and lung diseases, and increases the risk of cancer occurrence (3) and the susceptibility to several infectious diseases, including tuberculosis, pneumonia and sexually transmitted diseases such as chlamydia (4). However, increasing evidence suggests that nicotine also has beneficial health effects, particularly in terms cognitive function.
Nicotine acts as an agonist of nicotinic cholinergic receptors (nAChRs), which are found in both the central nervous system (CNS) and the peripheral nervous system (2,5,6). Each nAChR comprises five α or β subunits (7). There are nine potential α subunits and three β subunits, and different nAChR receptor subtypes possess varying compositions of these subunits (8,9). The most abundant receptor subtypes present in the human brain are α4β2, α3β4 (heterogenic) and α7 (homomeric) (10). The α3β4 nAChR is known to mediate the cardiovascular effects of nicotine (11), while the homomeric α7 nAChR is speculated to be involved in synaptic transmission, as well as in learning and sensory gating (12,13). Stimulation of nAChRs in the CNS by nicotine or acetylcholine regulates the release of a variety of neurotransmitters, such as dopamine, glutamate, serotonin, norepinephrine and γ-aminobutyric acid (14,15). Therefore, alterations in the expression or function of nAChRs, as a result of a disease, may alter the release of other neurotransmitters and, thus, affect brain function.
It is commonly known that long-term exposure to nicotine causes nAChR desensitization (16), leading to memory impairment in otherwise healthy individuals (17). Such nicotine-induced cognitive dysfunction is associated with several mechanisms, including activation of the phosphodiesterase-5 (PDE-5) signaling pathway and inhibition of estrogen biosynthesis (18,19). In particular, nicotine stimulates the expression of PDE-5 (19,20), which plays a role in cleaving cyclic guanosine monophosphate and cyclic adenosine monophosphate that activate downstream signaling pathways contributing to memory impairment (21–23). Nicotine also blocks estrogen synthase (aromatase) in the brain, which is important for estrogen biosynthesis (18,24). Estrogen activates estrogen receptors in the brain, which function as transcriptional factors and enhance the expression of several neurotransmitters (including glutamate, acetylcholine, serotonin and noradrenaline), and thus stimulate the neuronal circuits required for memory encoding (25). Therefore, alterations in estrogen biosynthesis due to nicotine (20,26), as well as the nicotine-induced elevation of PDE-5 levels, can lead to cognitive impairment in healthy individuals.
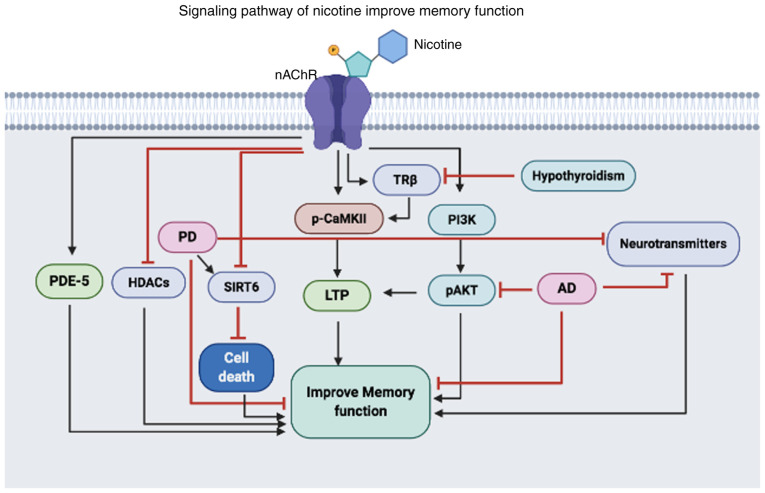
In contrast to these detrimental effects of nicotine on cognitive function, some studies report that nicotine also has beneficial effects on memory and learning processes. Thus, the present review summarizes the potential benefits of nicotine on cognition (Fig. 1).
Figure 1.
Illustration of the proposed mechanisms of nicotine in improving memory dysfunction. Nicotine activates nAChR, which can activate or inhibit the expression and functions of various proteins. Nicotine can activate PDE-5, TRβ and CaMKII, and activation of these proteins can lead to increased neuronal communication that ultimately improves memory function. In addition, nicotine activates the pro-survival PI3K/AKT pathway that increases LTP and improves memory dysfunction caused by AD. Also, nicotine can inhibit HDACs and SIRT6, which are increased in PD, thus reducing the activity of these proteins reduces neural apoptosis and improves memory dysfunction. PDE-5, phosphodiesterase-5; HDAC, histone deacetylases; PD, Parkinson's disease; SIRT6, Sirtuin 6; LTP, long-term potentiation; p-, phosphorylated; CAMKII, calmodulin-dependent protein kinase II; TRβ, thyroid receptor subunit β; PI3K, phosphoinositide 3-kinase; AD, Alzheimer's disease; nAChR, nicotinic cholinergic receptors.
2. Benefits of nicotine in Alzheimer's disease (AD)
AD is a neurodegenerative disease that primarily affects older adults and causes dementia (27). AD is characterized by the deposition of toxic amyloid-β (Aβ) and tau proteins in the brain (28,29). In particular, the accumulation of Aβ has been demonstrated to inhibit mitochondrial function, leading to increased reactive oxygen species formation and the stimulation of inflammatory processes (30). Indeed, several studies have revealed that Aβ deposition alters the physiological function of the brain and causes neuronal dysfunction (31,32). Unfortunately, there is still no cure for AD, and the disease is currently managed by slowing its progression with the administration of antioxidants and drugs such as cholinesterase inhibitors (33).
According to the cholinergic hypothesis, the cognitive decline in AD arises from deficiencies in central cholinergic neurotransmission due to the loss of acetylcholine (34). Therefore, cholinesterase inhibitors (such as donepezil and galantamine), which block the degradation of acetylcholine, remain the first-line approach to restore central cholinergic function in AD. Moreover, changes in the expression and density of α7 nAChRs in the hippocampus have been observed in AD and appear to have the most impact on cognitive function (35). Such α7 nAChRs have also been found to be co-localized with plaques in AD (36). Therefore, agonists of α7 nAChRs, including nicotine, may be useful for treating AD.
The stimulation of nAChRs by nicotine also likely affects downstream signaling molecules, including protein kinases, which are important regulators of synaptic plasticity and memory (37). In particular, protein kinase B (also referred to as Akt) is a central molecule of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, which plays a vital role in the regulatory functions of neurons in the CNS, including neuronal survival (38–42), and learning and memory encoding (38,43,44). Therefore, it is hypothesized that the stimulation of nAChRs by nicotine or its analogs activates the PI3K/Akt signaling pathway, which, in turn, regulates learning and memory processes (42,45). Indeed, acute and chronic administration of nicotine was reported to improve cognitive impairment in patients with AD (46–48). Moreover, acute nicotine administration during electroencephalography (EEG) performed in patients with AD who received cholinesterase inhibitors was found to shift the EEG readings towards normal levels (49). Thus, nicotine administration may have a beneficial effect on the cognitive decline observed in AD.
3. Benefits of nicotine in Parkinson's disease (PD)
PD is the second most common neurodegenerative disorder after AD that affects older individuals (50). Although the exact cause of PD is still not fully understood, its pathogenesis involves the loss or degeneration of the dopaminergic neurons (dopamine-producing neurons) in the substantia nigra of the midbrain (51). This loss of dopaminergic neurons causes impairment of motor control, tremors, rigidity and bradykinesia, and cognitive impairment (52,53). Studies in animal models of PD have revealed that nicotine can protect the brain cells from damage (54,55). Smoking cigarettes is also reported to reduce the risk of PD occurrence (53), and nicotine may help improve some symptoms of PD, such as dyskinesia and memory impairments (55). Indeed, the neuroprotective effects of nicotine in PD have been examined in vitro and in vivo, and are hypothesized to be primarily due to its pro-survival effects on dopaminergic neurons (56).
In addition to activating pro-survival signaling pathways in the brain, such as the aforementioned PI3K/Akt pathway, nicotine may also slow the progression of PD by inhibiting Sirtuin 6 (SIRT6), an NAD+-dependent class III deacetylase (57). This suppression of SIRT6 was found to reduce apoptosis and increase neuron survival (57). Consistently, several studies reported that the overexpression of SIRT6 impairs contextual fear memory formation (58,59). Despite this, another study found that loss of SIRT6 in the brain also causes memory impairment (60). Therefore, the downstream effects of nicotine on SIRT6 in PD require further investigation.
4. Benefits of nicotine on memory processes in patients with thyroid disease
Studies have revealed that thyroid hormones (61), including thyroxine (T4) and triiodothyronine (T3), regulate brain development, neurogenesis, synaptogenesis and myelination (62,63). T3 and T4 are synthesized in the thymus (64,65), released into the bloodstream, and eventually exert their effects by binding to a nuclear receptor termed the thyroid hormone receptor (TR), which is present in two different isoforms, α and β (66). The expression levels of these isoforms differ among tissues: The α1 receptor is primarily expressed in the heart and the skeletal muscle (67), whereas β1 is mainly expressed in the liver, kidney and brain (68).
TRs are also abundantly expressed in the hippocampus, which is the part of the brain that is responsible for memory formation (63). Therefore, in diseases such as hyperthyroidism, hypothyroidism and cretinism, in which abnormal thyroid hormone levels are present (69,70), hippocampal function may be affected, thus resulting in cognitive impairment (71). Indeed, neuroimaging studies have demonstrated that the structure and function of the hippocampus are altered in patients with hypothyroidism (72–74).
Of note, acute nicotine administration has been reported to activate TRs (particularly TRβ in the brain) and, thus, may enhance learning and memory processes in certain individuals (66). Furthermore, TRβ knockout in mice did not affect memory function following nicotine administration, confirming the role of TRβ in memory processes (75). In addition, memory impairment caused by hypothyroidism was revealed to be improved by nicotine via the modulation of calcineurin, which regulates the function of calmodulin-dependent protein kinase II (CaMKII) to improve synaptic plasticity (76). However, the precise underlying mechanisms of nicotine administration in improving cognitive impairments in patients with thyroid diseases require further investigation.
5. Effects of nicotine on cognitive function in healthy individuals
There is mounting evidence that nicotine administration may improve memory in otherwise healthy individuals. For example, research revealed that sleep deprivation causes memory impairment by downregulating the phosphorylation of CaMKII, which is an essential regulator of cell proliferation and synaptic plasticity (77–79). CaMKII was previously found to regulate the expression of glutamate receptor subunit-1 and its trafficking to the synaptic surface, which is necessary for normal brain function and memory formation (80). Consistently, acute nicotine administration was found to improve memory impairments caused by sleep deprivation by enhancing the phosphorylation of CaMKII (81). Therefore, nicotine may improve memory impairments caused by a lack of sleep in otherwise healthy individuals.
6. Nicotine-induced chromatin modifications may improve memory and learning
Some studies have indicated that nicotine affects chromatin in the cell nucleus (82–84). Chromatin is composed of four subunits, called histones, which can be modified via acetylation, methylation or phosphorylation (85), thereby regulating gene transcription (86,87). In particular, histone acetyltransferases and histone deacetylases (HDACs) play essential roles in the chromatin modifications involved in various cellular functions, including memory and synaptic plasticity (88,89). For example, inhibition of HDACs can increase the expression of key genes involved in memory processes, which are regulated by the cAMP response element-binding protein (CREB)-CREB-binding protein transcriptional complex (89). In particular, HDAC4 has been demonstrated to be crucial for learning and memory processes (89,90). As cigarette smoking has been reported to modulate the regulation of chromatin by altering the functionality of HDACs, such as HDAC6, in the lungs (83), it may also have a similar effect in the CNS. Indeed, it has been revealed that nicotine can inhibit HDACs in the brain, and, thus, improve memory function (84). However, further study is required to investigate the effect of nicotine on cognitive function through chromatin modulation.
7. Electrophysiological effects of nicotine: Strengthening synapses
The neurons in the brain interconnect to form networks, which are organized according to function (91). Therefore, understanding these connections allows certain areas to be stimulated and recorded, to monitor neurotransmitter release and receptor response in particular regions of the brain. Long-term potentiation (LTP) is used to measure synaptic plasticity, and can provide a cellular model of learning and memory encoding. For example, an increase in the level of glutamate released from the presynaptic to the postsynaptic neurons was found to enhance excitatory postsynaptic potential in the hippocampus during spatial learning tasks (92). Previously, studies have reported that acute nicotine exposure rescues LTP in individuals with sleep deprivation (81). In addition, chronic administration of nicotine has been revealed to improve LTP in AD, chronic stress models and hypothyroidism models (74,93,94). There is also mounting evidence that the restoration of LTP due to nicotine exposure is related to the normalization of the phosphorylation of essential kinases, such as CREB and CaMKIV (48,78,95). Therefore, nicotine administration may strengthen synapses between two neurons, leading to improved memory in both healthy individuals and those with diseases such as AD or hypothyroidism.
8. Conclusions
The findings reported in the studies included in the present review article indicate that nicotine can stimulate memory function. Therefore, although nicotine is similar to other psychoactive substances, in that it can induce dependence or abuse, it also has certain beneficial effects, including enhancing cognitive function in healthy individuals and restoring memory function in patients with diseases, such as AD, PD and hypothyroidism.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Author's contributions
AA designed the review paper, performed the literature search and wrote the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broide RS, Winzer-Serhan UH, Chen Y, Leslie FM. Distribution of alpha7 nicotinic acetylcholine receptor subunit mRNA in the developing mouse. Front Neuroanat. 2019;13:76. doi: 10.3389/fnana.2019.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015;36:24–31. doi: 10.4103/0971-5851.151771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unwin N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Q Rev Biophys. 2013;46:283–322. doi: 10.1017/S0033583513000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skok VI. Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci. 2002;97:1–11. doi: 10.1016/S1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]
- 7.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol. 2015;124:3–19. doi: 10.1016/bs.irn.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hone AJ, McIntosh JM. Nicotinic acetylcholine receptors in neuropathic and inflammatory pain. FEBS Lett. 2018;592:1045–1062. doi: 10.1002/1873-3468.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. Novel α3β4 nicotinic acetylcholine receptor-selective ligands. Discovery, structure-activity studies, and pharmacological evaluation. J Med Chem. 2010;53:8187–8191. doi: 10.1021/jm1006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aberger K, Chitravanshi VC, Sapru HN. Cardiovascular responses to microinjections of nicotine into the caudal ventrolateral medulla of the rat. Brain Res. 2001;892:138–146. doi: 10.1016/S0006-8993(00)03250-9. [DOI] [PubMed] [Google Scholar]
- 12.Levin ED, Bettegowda C, Blosser J, Gordon J. AR-R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hajos M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, Groppi VE. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)- 1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther. 2005;312:1213–1222. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- 14.Benowitz NL. Pharmacology of nicotine: Addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: Implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6:4–16. [PMC free article] [PubMed] [Google Scholar]
- 16.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not ‘either/or’: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, Smyth K, Garcia K, Mattson E, Li L, Xiao Z. Nicotine inhibits memory CTL programming. PLoS One. 2013;8:e68183. doi: 10.1371/journal.pone.0068183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echeverria Moran V. Brain effects of nicotine and derived compounds. Front Pharmacol. 2013;4:60. doi: 10.3389/fphar.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotston MR, Jeremy JY, Bloor J, Koupparis A, Persad R, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-alpha in cavernosal vascular smooth muscle cells: Mediation by superoxide. BJU Int. 2007;99:612–618. doi: 10.1111/j.1464-410X.2006.06618.x. [DOI] [PubMed] [Google Scholar]
- 20.Henderson VW. Cognitive changes after menopause: Influence of estrogen. Clin Obstet Gynecol. 2008;51:618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domek-Łopacińska K, Strosznajder JB. Cyclic GMP metabolism and its role in brain physiology. J Physiol Pharmacol. 2005;56(Suppl 2):S15–S34. [PubMed] [Google Scholar]
- 22.Cui Q, So KF. Involvement of cAMP in neuronal survival and axonal regeneration. Anat Sci Int. 2004;79:209–212. doi: 10.1111/j.1447-073x.2004.00089.x. [DOI] [PubMed] [Google Scholar]
- 23.Peixoto CA, Nunes AK, Garcia-Osta A. Phosphodiesterase-5 inhibitors: Action on the signaling pathways of neuroinflammation, neurodegeneration, and cognition. Mediators Inflamm. 2015;2015:940207. doi: 10.1155/2015/940207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biegon A, Kim SW, Logan J, Hooker JM, Muench L, Fowler JS. Nicotine blocks brain estrogen synthase (aromatase): In vivo positron emission tomography studies in female baboons. Biol Psychiatry. 2010;67:774–777. doi: 10.1016/j.biopsych.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20:534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luine VN. Estradiol and cognitive function: Past, present and future. Horm Behav. 2014;66:602–618. doi: 10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neugroschl J, Wang S. Alzheimer's disease: Diagnosis and treatment across the spectrum of disease severity. Mt Sinai J Med. 2011;78:596–612. doi: 10.1002/msj.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MP, LeVine H., III Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deshpande A, Mina E, Glabe C, Busciglio J. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schilling T, Eder C. Amyloid-β-induced reactive oxygen species production and priming are differentially regulated by ion channels in microglia. J Cell Physiol. 2011;226:3295–3302. doi: 10.1002/jcp.22675. [DOI] [PubMed] [Google Scholar]
- 31.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: From synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jagust W. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain. 2016;139:23–30. doi: 10.1093/brain/awv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendiola-Precoma J, Berumen LC, Padilla K, Garcia-Alcocer G. Therapies for prevention and treatment of Alzheimer's disease. Biomed Res Int. 2016;2016:2589276. doi: 10.1155/2016/2589276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossberg GT. Cholinesterase inhibitors for the treatment of Alzheimer's disease: Getting on and staying on. Curr Ther Res Clin Exp. 2003;64:216–235. doi: 10.1016/S0011-393X(03)00059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Q, Yakel JL. The effect of α7 nicotinic receptor activation on glutamatergic transmission in the hippocampus. Biochem Pharmacol. 2015;97:439–444. doi: 10.1016/j.bcp.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: Roles in Alzheimer's disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giese KP, Mizuno K. The roles of protein kinases in learning and memory. Learn Mem. 2013;20:540–552. doi: 10.1101/lm.028449.112. [DOI] [PubMed] [Google Scholar]
- 38.Diez H, Garrido JJ, Wandosell F. Specific roles of Akt iso forms in apoptosis and axon growth regulation in neurons. PLoS One. 2012;7:e32715. doi: 10.1371/journal.pone.0032715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Puerto A, Wandosell F, Garrido JJ. Neuronal and glial purinergic receptors functions in neuron development and brain disease. Front Cell Neurosci. 2013;7:197. doi: 10.3389/fncel.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/S0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 42.Shu Y, Zhang H, Kang T, Zhang JJ, Yang Y, Liu H, Zhang L. PI3K/Akt signal pathway involved in the cognitive impairment caused by chronic cerebral hypoperfusion in rats. PLoS One. 2013;8:e81901. doi: 10.1371/journal.pone.0081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- 44.Chiang HC, Wang L, Xie ZL, Yau A, Zhong Y. PI3 kinase signaling is involved in A beta-induced memory loss in Drosophila. Proc Natl Acad Sci USA. 2010;107:7060–7065. doi: 10.1073/pnas.0909314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi JH, Baek SJ, Heo S, Park HJ, Kwon H, Lee S, Jung J, Park SJ, Kim BC, Lee YC, et al. Direct pharmacological Akt activation rescues Alzheimer's disease like memory impairments and aberrant synaptic plasticity. Neuropharmacology. 2018;128:282–292. doi: 10.1016/j.neuropharm.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, Pfaff A, Wilkins H, Howard D, Levin ED. Nicotine treatment of mild cognitive impairment: A 6-month double-blind pilot clinical trial. Neurology. 2012;78:91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majdi A, Kamari F, Sadigh-Eteghad S, Gjedde A. Molecular insights into memory-enhancing metabolites of nicotine in brain: A systematic review. Front Neurosci. 2018;12:1002. doi: 10.3389/fnins.2018.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivareerat M, Tran TT, Salim S, Aleisa AM, Alkadhi KA. Chronic nicotine restores normal Aβ levels and prevents short-term memory and E-LTP impairment in Aβ rat model of Alzheimer's disease. Neurobiol Aging. 2011;32:834–844. doi: 10.1016/j.neurobiolaging.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Knott V, Engeland C, Mohr E, Mahoney C, Ilivitsky V. Acute nicotine administration in Alzheimer's disease: An exploratory EEG study. Neuropsychobiology. 2000;41:210–220. doi: 10.1159/000026662. [DOI] [PubMed] [Google Scholar]
- 50.Sherer TB, Chowdhury S, Peabody K, Brooks DW. Overcoming obstacles in Parkinson's disease. Mov Disord. 2012;27:1606–1611. doi: 10.1002/mds.25260. [DOI] [PubMed] [Google Scholar]
- 51.Barber M, Stewart D, Grosset D, MacPhee G. Patient and carer perception of the management of Parkinson's disease after surgery. Age Ageing. 2001;30:171–172. doi: 10.1093/ageing/30.2.171-a. [DOI] [PubMed] [Google Scholar]
- 52.Kinoshita KI, Tada Y, Muroi Y, Unno T, Ishii T. Selective loss of dopaminergic neurons in the substantia nigra pars compacta after systemic administration of MPTP facilitates extinction learning. Life Sci. 2015;137:28–36. doi: 10.1016/j.lfs.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Ma C, Liu Y, Neumann S, Gao X. Nicotine from cigarette smoking and diet and Parkinson disease: A review. Transl Neurodegener. 2017;6:18. doi: 10.1186/s40035-017-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu JYD, Su P, Barber JEM, Nash JE, Le AD, Liu F, Wong AHC. The neuroprotective effect of nicotine in Parkinson's disease models is associated with inhibiting PARP-1 and caspase-3 cleavage. PeerJ. 2017;5:e3933. doi: 10.7717/peerj.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quik M, O'Leary K, Tanner CM. Nicotine and Parkinson's disease: Implications for therapy. Mov Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barreto GE, Iarkov A, Moran VE. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson's disease. Front Aging Neurosci. 2015;6:340–340. doi: 10.3389/fnagi.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicholatos JW, Francisco AB, Bender CA, Yeh T, Lugay FJ, Salazar JE, Glorioso C, Libert S. Nicotine promotes neuron survival and partially protects from Parkinson's disease by suppressing SIRT6. Acta Neuropathol Commun. 2018;6:120. doi: 10.1186/s40478-018-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Kim HS, Kaang BK. Elevated contextual fear memory by SIRT6 depletion in excitatory neurons of mouse forebrain. Mol Brain. 2018;11:49. doi: 10.1186/s13041-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin X, Gao Y, Shi HS, Song L, Wang JC, Shao J, Geng XH, Xue G, Li JL, Hou YN. Overexpression of SIRT6 in the hippocampal CA1 impairs the formation of long-term contextual fear memory. Sci Rep. 2016;6:18982. doi: 10.1038/srep18982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaluski S, Portillo M, Besnard A, Stein D, Einav M, Zhong L, Ueberham U, Arendt T, Mostoslavsky R, Sahay A, Toiber D. Neuroprotective functions for the histone deacetylase SIRT6. Cell Rep. 2017;18:3052–3062. doi: 10.1016/j.celrep.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rousset B, Dupuy C, Miot F, Dumont J. Chapter 2 Thyroid Hormone Synthesis and Secretion. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA: 2000. [Sep 2;2015 ]. [Google Scholar]
- 62.Diez D, Grijota-Martinez C, Agretti P, De Marco G, Tonacchera M, Pinchera A, de Escobar GM, Bernal J, Morte B. Thyroid hormone action in the adult brain: Gene expression profiling of the effects of single and multiple doses of triiodo-L-thyronine in the rat striatum. Endocrinology. 2008;149:3989–4000. doi: 10.1210/en.2008-0350. [DOI] [PubMed] [Google Scholar]
- 63.Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mariotti S, Beck-Peccoz P. Physiology of the Hypothalamic-Pituitary Thyroidal System. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA: 2000. [Aug 14;2016 ]. [Google Scholar]
- 66.Cheng SY. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord. 2000;1:9–18. doi: 10.1023/A:1010052101214. [DOI] [PubMed] [Google Scholar]
- 67.Bradley DJ, Towle HC, Young WS., III Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams GR. Cloning and characterization of two novel thyroid hormone receptor beta isoforms. Mol Cell Biol. 2000;20:8329–8342. doi: 10.1128/MCB.20.22.8329-8342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–3043. doi: 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 71.Ge JF, Peng L, Hu CM, Wu TN. Impaired learning and memory performance in a subclinical hypothyroidism rat model induced by hemi-thyroid electrocauterisation. J Neuroendocrinol. 2012;24:953–961. doi: 10.1111/j.1365-2826.2012.02297.x. [DOI] [PubMed] [Google Scholar]
- 72.Cooke GE, Mullally S, Correia N, O'Mara SM, Gibney J. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid. 2014;24:433–440. doi: 10.1089/thy.2013.0058. [DOI] [PubMed] [Google Scholar]
- 73.Singh S, Rana P, Kumar P, Shankar LR, Khushu S. Hippocampal neurometabolite changes in hypothyroidism: An in vivo (1) H magnetic resonance spectroscopy study before and after thyroxine treatment. J Neuroendocrinol. 2016:28. doi: 10.1111/jne.12399. doi: 10.1111/jne.12399. [DOI] [PubMed] [Google Scholar]
- 74.Alzoubi KH, Aleisa AM, Gerges NZ, Alkadhi KA. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: Behavioral and electrophysiological studies. J Neurosci Res. 2006;84:944–953. doi: 10.1002/jnr.21014. [DOI] [PubMed] [Google Scholar]
- 75.Leach PT, Kenney JW, Connor DA, Gould TJ. Thyroid receptor β involvement in the effects of acute nicotine on hippocampus-dependent memory. Neuropharmacology. 2015;93:155–163. doi: 10.1016/j.neuropharm.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alzoubi KH, Aleisa AM, Alkadhi KA. Molecular studies on the protective effect of nicotine in adult-onset hypothyroidism-induced impairment of long-term potentiation. Hippocampus. 2006;16:861–874. doi: 10.1002/hipo.20217. [DOI] [PubMed] [Google Scholar]
- 77.Pi HJ, Otmakhov N, El Gaamouch F, Lemelin D, De Koninck P, Lisman J. CaMKII control of spine size and synaptic strength: Role of phosphorylation states and nonenzymatic action. Proc Natl Acad Sci USA. 2010;107:14437–14442. doi: 10.1073/pnas.1009268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Chronic psychosocial stress-induced impairment of hippocampal LTP: Possible role of BDNF. Neurobiol Dis. 2006;22:453–462. doi: 10.1016/j.nbd.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Misrani A, Tabassum S, Wang M, Chen J, Yang L, Long C. Citalopram prevents sleep-deprivation-induced reduction in CaMKII-CREB-BDNF signaling in mouse prefrontal cortex. Brain Res Bull. 2020;155:11–18. doi: 10.1016/j.brainresbull.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Mao LM, Jin DZ, Xue B, Chu XP, Wang JQ. Phosphorylation and regulation of glutamate receptors by CaMKII. Sheng Li Xue Bao. 2014;66:365–372. [PMC free article] [PubMed] [Google Scholar]
- 81.Aleisa AM, Helal G, Alhaider IA, Alzoubi KH, Srivareerat M, Tran TT, Al-Rejaie SS, Alkadhi KA. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21:899–909. doi: 10.1002/hipo.20806. [DOI] [PubMed] [Google Scholar]
- 82.Shilatifard A. Chromatin modifications by methylation and ubiquitination: Implications in the regulation of gene expression. Annual Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 83.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 84.Volkow ND. Epigenetics of nicotine: Another nail in the coughing. Sci Transl Med. 2011;3:107ps143. doi: 10.1126/scitranslmed.3003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Brehove M, Wang T, North J, Luo Y, Dreher SJ, Shimko JC, Ottesen JJ, Luger K, Poirier MG. Histone core phosphorylation regulates DNA accessibility. J Biol Chem. 2015;290:22612–22621. doi: 10.1074/jbc.M115.661363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Griffin K, Mondal N, Parvin JD. Phosphorylation of histone H2A inhibits transcription on chromatin templates. J Biol Chem. 2004;279:21866–21872. doi: 10.1074/jbc.M400099200. [DOI] [PubMed] [Google Scholar]
- 88.Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003;4:944–947. doi: 10.1038/sj.embor.embor941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET, Olson EN, Monteggia LM. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci. 2012;32:10879–10886. doi: 10.1523/JNEUROSCI.2089-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pulvermuller F, Garagnani M, Wennekers T. Thinking in circuits: Toward neurobiological explanation in cognitive neuroscience. Biol Cybern. 2014;108:573–593. doi: 10.1007/s00422-014-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richter-Levin G, Canevari L, Bliss TV. Long-term potentiation and glutamate release in the dentate gyrus: Links to spatial learning. Behav Brain Res. 1995;66:37–40. doi: 10.1016/0166-4328(94)00121-U. [DOI] [PubMed] [Google Scholar]
- 93.Aleisa AM, Alzoubi KH, Alkadhi KA. Nicotine prevents stress-induced enhancement of long-term depression in hippocampal area CA1: Electrophysiological and molecular studies. J Neurosci Res. 2006;83:309–317. doi: 10.1002/jnr.20716. [DOI] [PubMed] [Google Scholar]
- 94.Alkadhi KA. Chronic stress and Alzheimer's disease-like pathogenesis in a rat model: Prevention by nicotine. Curr Neuropharmacol. 2011;9:587–597. doi: 10.2174/157015911798376307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alzoubi KH, Alkadhi KA. Chronic nicotine treatment reverses hypothyroidism-induced impairment of L-LTP induction phase: Critical role of CREB. Mol Neurobiol. 2014;49:1245–1255. doi: 10.1007/s12035-013-8594-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.