Abstract
Background and aims
The potential impact of coronary atherosclerosis, as detected by coronary artery calcium, on clinical outcomes in COVID-19 patients remains unsettled. We aimed to evaluate the prognostic impact of clinical and subclinical coronary artery disease (CAD), as assessed by coronary artery calcium score (CAC), in a large, unselected population of hospitalized COVID-19 patients undergoing non-gated chest computed tomography (CT) for clinical practice.
Methods
SARS-CoV 2 positive patients from the multicenter (16 Italian hospitals), retrospective observational SCORE COVID-19 (calcium score for COVID-19 Risk Evaluation) registry were stratified in three groups: (a) “clinical CAD” (prior revascularization history), (b) “subclinical CAD” (CAC >0), (c) “No CAD” (CAC = 0). Primary endpoint was in-hospital mortality and the secondary endpoint was a composite of myocardial infarction and cerebrovascular accident (MI/CVA).
Results
Amongst 1625 patients (male 67.2%, median age 69 [interquartile range 58–77] years), 31%, 57.8% and 11.1% had no, subclinical and clinical CAD, respectively. Increasing rates of in-hospital mortality (11.3% vs. 27.3% vs. 39.8%, p < 0.001) and MI/CVA events (2.3% vs. 3.8% vs. 11.9%, p < 0.001) were observed for patients with no CAD vs. subclinical CAD vs clinical CAD, respectively.
The association with in-hospital mortality was independent of in-study outcome predictors (age, peripheral artery disease, active cancer, hemoglobin, C-reactive protein, LDH, aerated lung volume): subclinical CAD vs. No CAD: adjusted hazard ratio (adj-HR) 2.86 (95% confidence interval [CI] 1.14–7.17, p=0.025); clinical CAD vs. No CAD: adj-HR 3.74 (95% CI 1.21–11.60, p=0.022). Among patients with subclinical CAD, increasing CAC burden was associated with higher rates of in-hospital mortality (20.5% vs. 27.9% vs. 38.7% for patients with CAC score thresholds≤100, 101–400 and > 400, respectively, p < 0.001). The adj-HR per 50 points increase in CAC score 1.007 (95%CI 1.001–1.013, p=0.016). Cardiovascular risk factors were not independent predictors of in-hospital mortality when CAD presence and extent were taken into account.
Conclusions
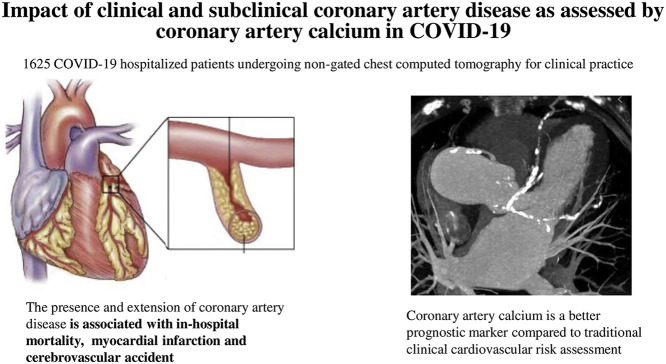
The presence and extent of CAD are associated with in-hospital mortality and MI/CVA among hospitalized patients with COVID-19 disease and they appear to be a better prognostic gauge as compared to a clinical cardiovascular risk assessment.
Keywords: COVID-19, Coronary artery disease, Atherosclerosis, Agatston score, Coronary artery calcifications, Calcium score, In-hospital mortality
Graphical abstract
1. Introduction
Since December 2019, SARS-CoV-2, the causative agent of the ongoing coronavirus disease 2019 (COVID-19) pandemic, has infected more than 118 million people worldwide. The number of affected individuals is continuing to escalate, which places a significant burden upon national healthcare systems. In this context, precise prognostication to optimize resource allocation and patient management is of utmost importance. Furthermore, understanding the pathophysiological reasons for adverse prognosis in COVID-19 disease may aid the development of patient specific management strategies.
Several predictors of COVID-19-related mortality have been described, including patient comorbidities, clinical, radiological and ultrasound features of lung disease severity and serum biomarkers of inflammation and organ damage [1]. The presence of cardiovascular risk factors and previous comorbidities in patients with COVID-19 disease is associated with adverse outcomes and higher in-hospital mortality [[2], [3], [4]].
However, the pathophysiological link between clinical cardiovascular risk factors and outcomes in COVID-19 remains largely unexplored. In this regard, atherosclerotic disease may be a substratum of more severe COVID-19 disease manifestations. As a disconnection exists between the cardiovascular clinical risk and the actual presence of atherosclerosis [5,6], coronary artery calcium (CAC), a highly specific marker of atherosclerotic burden [7], may be a better gauge of COVID-19 outcomes than clinical profile. This hypothesis seems to be supported by some recent small, single center exploratory studies [[8], [9], [10]].
We tested the hypothesis that combining clinical history details with CAC score on routine non gated chest computed tomography (CT) could better explore the spectrum of coronary artery disease (CAD) and stratify prognosis amongst patients admitted with COVID-19.
2. Patients and methods
2.1. Study design and population
SCORE COVID-19 (calcium score for COVID-19 Risk Evaluation) is a multicenter, retrospective, observational registry comprising 1691 SARS-CoV 2 positive consecutive patients admitted to 16 Italian hospitals between March 1, 2020 to April 24, 2020 with an available non-contrast chest CT scan performed for pneumonia severity assessment. Of 1691 patients, 66 were excluded (6 for missing CT images, 32 because of no computable CAC score, 27 for missing outcome data) leading to a final population of 1625 patients.
Demographic characteristics, cardiovascular risk factors, along with clinical, laboratory and chest CT imaging variables related to the index hospitalization were collected in a dedicated pre-specified dataset by each center and analyzed by the coordinating center (Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy).
The diagnosis of COVID-19 disease was established by a positive qualitative polymerase-chain-reaction assay for SARS-CoV-2. Chest CT scans were acquired with a standard non-gated chest CT protocol, on multidetector scanners with at least 16 detector rows. Imaging data were collected by each center and sent to the central core-lab for image analysis (Experimental Imaging center, IRCCS, Ospedale San Raffaele, Milano) by three expert cardio-thoracic radiologists of the core-lab blinded to patients clinical data. The presence and vessel location (left main, left anterior descending, left circumflex or right coronary artery) of calcification was visually assessed if CAC was detected, the Agatston CAC score was quantitatively computed with a validated commercial software (IntelliSpace v 8.0 software, Philips, The Netherlands).
For the purpose of this analysis, COVID-19 patients were stratified into three groups as follows: (a) “clinical CAD” as defined by a history of previous surgical or percutaneous coronary revascularization, (b) “subclinical CAD” as defined by a CAC >0, (c) “No CAD” as defined by a CAC = 0.
Patient with subclinical CAD were further stratified according to validated CAC score thresholds (≤100: mild; 100–400: moderate; >400: severe) [10]. Sensitivity analyses for age groups were also performed.
2.2. Study endpoints
The primary endpoint was in-hospital mortality.
The secondary endpoint was a composite of in-hospital myocardial infarction and cerebrovascular accident (MI/CVA).
Study outcomes were retrospectively ascertained by a physician at each participating center, who reviewed all the medical records. MI was defined according the Fourth Universal definition of Myocardial infarction [11], while the diagnosis of CVA was assessed according to clinical practice.
2.3. Statistical analysis
Categorical variables are expressed as number and percentages, continuous variables are expressed as mean ± standard deviation or median and interquartile range (IQR) as appropriate. Unpaired t-test or nonparametric Mann-Whitney U test was used for comparisons of continuous variables and chi-square test was used for categorical variables. Kaplan-Meier curves were performed to evaluate cumulative event rates at follow-up. Log-rank p-value was determined to test if Kaplan Meier event estimates differed over time. Outcomes were then adjusted with a multivariate Cox proportional hazards model. All baseline variables presented in Table 1, Table 2 were tested for univariate significance. Those found to be significantly associated with the primary outcome in univariable models (p < 0.05) were included in the final Cox regression model, provided that they had less than 30% missing data. The proportional hazards assumption of the Cox regression model was checked using time-dependent Cox models. Results are presented as hazard ratio (HR) with 95% confidence interval (CI). We assessed multicollinearity among the variables included in the final multivariate model by calculating the variance inflation factor (VIF). VIFs are reported in Supplementary Table 1 and were below 5 for all the independent variables suggesting no significant multicollinearity. Receiver Operating Characteristic (ROC) curves were elaborated for several predictive models and their associated areas under the curve (AUCs) were compared with DeLong et al. approach [12].
Table 1.
Clinical and laboratory characteristics of the overall population and stratified by CAD categories.
| Total (n) (N = 1625) |
No CAD (N = 504) | Subclinical CAD (N = 940) | Clinical CAD (N = 181) | p-value | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age [IQR] (n) | 69 [58, 77] (1625) | 57 [50, 66] (504) | 72 [63, 79] (940) | 75 [69, 81] (181) | <0.001 |
| Male gender % (n) | 67.2 (1092) | 55.2 (276) | 70.9 (666) | 81.1 (150) | <0.001 |
| BMI [IQR] (n) | 26 [24 29] (560) | 26 [24, 29] (208) | 27 [24, 29] (297) | 26 [23, 28] (55) | 0.191 |
| Hypertension % (n) | 54.8 (885) | 34.3 (171) | 60.4 (565) | 82.3 (149) | <0.001 |
| Diabetes % (n) | 19.1 (309) | 7.8 (39) | 21.3 (199) | 39.2 (71) | <0.001 |
| Smoke % (n) | 6.5 (79) | 3.7 (15) | 6.8 (46) | 13.4 (18) | <0.001 |
| CKD % (n) | 7.3 (87) | 2.3 (9) | 7.2 (49) | 23.4 (29) | <0.001 |
| Atrial fibrillation % (n) | 9 (140) | 3.5 (17) | 10.1 (90) | 18.3 (33) | <0.001 |
| Peripheral artery disease %(n) | 6.1 (98) | 1.4 (7) | 6 (56) | 19.3 (35) | <0.001 |
| Chronic lung disease % (n) | 9.9 (160) | 5 (25) | 11.3 (106) | 16 (29) | <0.001 |
| Active malignancy % (n) | 5.1 (83) | 3 (15) | 5.9 (55) | 7.2 (13) | 0.009 |
| Laboratory data at admission | |||||
| Hb g/dl [IQR] (n) | 14 [12,15] (1617) | 14 [12, 15] (501) | 14 [12, 15] (936) | 13 [12, 15] (180) | 0.580 |
| WBC*103/mm3 [IQR] (n) | 6.8 [5.0, 9.9] (1548) | 6.2 [4.7, 9.2] (485) | 7.1 [5.3, 10.1] (884) | 7.3 [5.2, 10.6] (179) | <0.001 |
| Creat. Mg/dL [IQR] (n) | 1.0 [0.9, 1.2] (324) | 1.0 [0.8, 1.1] (115) | 1.0 [0.9, 1.2] (177) | 1.1 [1.0, 1,6] (32) | <0.001 |
| HS-TnI ng/L baseline [IQR] (n) | 10.9 [5.4, 32.9] (275) | 6.0 [2.7, 15.0] (91) | 10.9 [6.0, 35.5] (140) | 32.8 [14.8, 93.0] (44) | <0.001 |
| HS-TnI ng/L peak [IQR] (n) | 10.9 [5.4, 32.9] (265) | 9.9 [4, 31] (87) | 19.5 [9, 59.1] (140) | 53.1 [22.5, 150.1] (38) | <0.001 |
| LDH mg/dl [IQR] (n) | 354 [254, 480] (1140) | 330 [240, 442] (357) | 365 [264, 495] (665) | 350 [252, 463] (118) | 0.010 |
| CRP mg/L [IQR] (n)< | 11.5 [5.3, 20] (1594) | 10.5 [4.2,20.3] (486) | 10.9 [5.6, 20.1] (929) | 11.7 [6.6, 18.4] (179) | 0.070 |
BMI = body mass index, Creat. = creatinine, CKD = chronic kidney disease, CRP=C-reactive protein, Hb = haemoglobin, IQR = interquartile range, LDH = lactate dehydrogenase, HS-TnI = high-sensitivity troponin I, WBC = white blood cells.
Table 2.
Clinical and laboratory characteristics of patients with subclinical CAD stratified by increasing CAC burden.
| CAC <100 (N = 465) | CAC 100–400 (N = 219) | CAC ≥ 400 (N = 256) | p-value | |
|---|---|---|---|---|
| Age [IQR] (n) | 67.5 [60, 75] (465) | 73 [66, 80] (219) | 77 [71, 83] (256) | <0.001 |
| Male gender % (n) | 66.8 (310) | 71.9 (156) | 78.1 (200) | 0.003 |
| BMI - median [IQR] (n) | 27 [25, 29] (151) | 27 [24, 29] (68) | 26.6 [25, 29] (78) | 0.678 |
| Arterial hypertension % (n) | 60.5 (565) | 59.9 (130) | 65.9 (168) | 0.040 |
| Diabetes % (n) | 21.3 (199) | 24.0 (52) | 27.1 (69) | <0.001 |
| Smoker % (n) | 6.8 (46) | 5.7 (9) | 8.5 (16) | 0.412 |
| Chronic kidney disease % (n) | 7.2 (49) | 8.8 (14) | 9.3 (17) | 0.002 |
| Atrial fibrillation % (n) | 10.1 (90) | 8.1 (17) | 14.2 (35) | 0.050 |
| Peripheral artery disease % (n) | 6.0 (56) | 6.0 (13) | 10.6 (27) | <0.001 |
| Chronic lung disease % (n) | 11.4 (106) | 11.1 (24) | 14.1 (36) | 0.103 |
| Active malignancy % (n) | 5.9 (55) | 6.0 (13) | 8.6 (22) | 0.070 |
| Laboratory data at admission | ||||
| Hemoglobin g/dl [IQR] (n) | 14 [12, 15] (463) | 13.9 [12, 15] (218) | 13.3 [12, 15] (255) | 0.320 |
| WBC*103/mm3 [IQR] (n) | 7.1 [5.3, 9.7] (430) | 6.9 [5.0, 9.9] (210) | 7.1 [5.4, 10.6] (244) | 0.280 |
| Creatinine mg/dL [IQR] (n) | 1.0 [0.8, 1.1] (97) | 1.0 [0.9, 1.3] (34) | 1.1 [0.9, 1.4] (46) | 0.250 |
| HS-TnI baseline ng/L [IQR] (n) | 10.0 [6.0, 29.1] (60) | 8.8 [5.3, 35.0] (34) | 15.5 [7.2, 37.4] (46) | 0.365 |
| LDH mg/dl [IQR] (n) | 392 [274, 520] (338) | 342 [252, 473] (158) | 360 [258, 462] (203) | 0.780 |
| CRP mg/L - [IQR] (n) | 23 [13.3, 55.8] (460) | 28.6 [15.5,61.2] (214) | 29.1 [16.3, 68.5] (255) | 0.390 |
| HS-TnI ng/L peak [IQR] (n) | 17.0 [8.5, 49.0] (74) | 21.0 [8.5, 48.9] (31) | 34.9 [13.6, 74.8] (35) | 0.110 |
BMI = body mass index, Creat. = creatinine, CKD = chronic kidney disease, CRP=C-reactive protein, Hb = haemoglobin, IQR = interquartile range, LDH = lactate dehydrogenase, HS-TnI = high-sensitivity troponin I, WBC = white blood cells.
A p < 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (version 24.0, SPSS Inc., Chicago, Illinois, US).
3. Results
3.1. Baseline characteristics
Amongst 1625 COVID-19 patients, 181 (11.1%) had a history of prior surgical or percutaneous revascularization (clinical CAD). Of the remaining 1444 patients, 940 (57.8%) were diagnosed with subclinical CAD and 504 (31%) had no evidence of CAC on chest CT.
Demographic, clinical and laboratory features stratified by CAD status are presented in Table 1. Overall, median age was 69 years [IQR 58, 77], 67.2% were males. Older age, higher burden of cardiovascular comorbidities including arterial hypertension, diabetes, smoke, atrial fibrillation and peripheral artery disease along with more frequent male sex and chronic kidney and lung diseases were observed across the spectrum from No CAD to clinical CAD. The same increasing trend across the groups was also observed for laboratory data at admission, including white blood cell count, creatinine level, high-sensitivity Troponin I (hs-TnI) at admission and at peak.
3.1.1. Patients with subclinical CAD stratified by CAC burden
Demographic and clinical characteristics of patients with subclinical CAD stratified by CAC burden are presented in Table 2. Increasing CAC burden was associated with male sex, older age and higher rates of hypertension, diabetes, peripheral artery disease and chronic kidney disease.
3.2. Study outcomes
3.2.1. Overall population
After a mean follow-up of 14 (10) days, in-hospital death occurred in 385 (23.7%) patients while in-hospital MI/CVA in 39 (2.4%) patients.
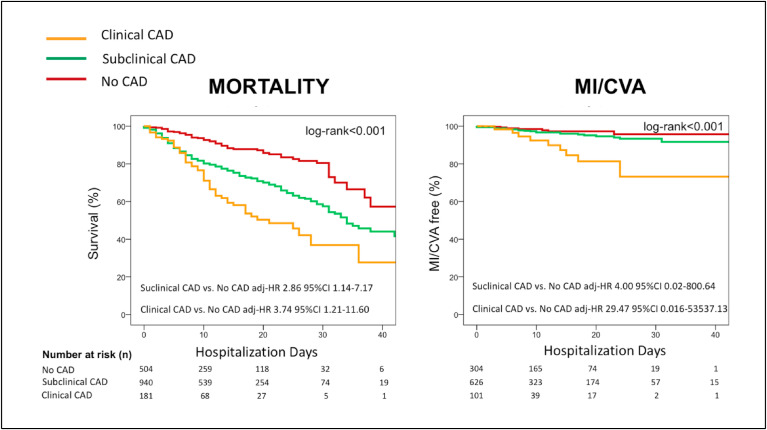
Increasing rates of in-hospital mortality (57 [11.3%, 0.8% person-day] vs. 256 [27.3%, 1.7% person-day] vs. 72 [39.8%, 2.9% person-day], p < 0.001) and MI/CVA (7 [2.3%, 0.1% person-day] vs. 20 [3.8%, 0.3% person-day] vs. 12 (11.9%, 0.9% person-day), p < 0.001) were observed for patients with No CAD vs. subclinical CAD vs . clinical CAD, respectively. Univariate predictors of the primary endpoint are presented in Supplementary Table 2.
In a Cox multivariate model with demographics and in-study outcome predictors (Table 3 ), “CAD stratification” was identified as an independent predictor of in-hospital mortality: subclinical CAD vs. No CAD: HR 2.86 95% CI 1.14–7.17, p=0.025; clinical CAD vs. No CAD: HR 3.74 95% CI 1.21–11.60, p=0.022 (Fig. 1 ), while no cardiovascular risk factor remained significantly associated with in-hospital mortality. A multivariate model with CAC as a continuous variable tested in patients with no CAD and subclinical CAD showed consistent results (Supplementary Table 4).
Table 3.
Independent predictors of in-hospital mortality at Cox regression analysis.
| Variables | Multivariate analysis |
|
|---|---|---|
| HR (95% CI) | p-value | |
| Age | 1.07 (1.04–1.1) | 0.000 |
| Sex | 0.71 (0.38–1.31) | 0.267 |
| Arterial hypertension | 0.66 (0.38–1.13) | 0.131 |
| Diabetes | 1.3 (0.76–2.21) | 0.343 |
| Smoke | 0.94 (0.38–2.3) | 0.890 |
| Chronic lung disease | 1.01 (0.47–2.14) | 0.986 |
| Peripheral artery disease | 2.03 (1.19–3.67) | 0.02 |
| Atrial fibrillation | 2.01 (0.96–4.24) | 0.065 |
| Active malignancy | 2.9 (1.33–6.34) | 0.007 |
| Chronic kidney disease | 1.56 (0.79–3.04) | 0.198 |
| Hemoglobin | 1.15 (1.01–1.30) | 0.038 |
| WBCa103/mm3 [IQR] | 1.0 (1.0–1.0) | 0.745 |
| LDH (U/L) | 1.0 (1.0–1.0) | 0.002 |
| CRP (m) | 1.01 (1.0–1.01) | 0.038 |
| SatO2 in AA | 1.01 (0.98–1.03) | 0.687 |
| Clinical CADa | 3.74 (1.21–11.60) | 0.022 |
| Subclinical CADa | 2.86 (1.14–7.17) | 0.025 |
| Well aerated lung volume [cc] | 0.999 (0.999–1.00) | 0.001 |
| Pericardial effusion | 1.27 (0.62–2.6) | 0.523 |
| Pleural effusion | 0.84 (0.48–1.47) | 0.532 |
| Pneumonia | 0.91 (0.62–1.0) | 0.244 |
HR = hazard ratio, CAD = coronary artery disease, CRP=C-reactive protein, LDH = lactate dehydrogenase, Sat O2 in AA = oxygen saturation in ambient air.
vs. No CAD. All the listed variables are univariate predictors of in-hospital mortality, the p-values refer to the final level of significance in the Cox multivariate model.
Fig. 1.
Kaplan Meyer estimates of in-hospital mortality and a composite of in-hospital myocardial infarction and cerebrovascular accident stratified by CAD status.
A multivariate model including “CAD stratification” was superior to a model based on clinical cardiovascular risk profile exclusively (AUCs: 0.71, 95%CI 0.66–0.76 vs. 0.62, 95%CI 0.56–0.68, p-value for AUCs comparison = 0.028) (Supplementary Table 4 and Supplementary Fig. 1). The final model, which included comorbidities and markers of COVID-19 disease severity, was superior to the model based on clinical cardiovascular risk profile and “CAD stratification” (final model AUC: 0.87, 95%CI 0.83–0.90, p-value for AUCs comparison <0.001). Despite being an independent predictor of the primary outcome, “CAD stratification” did not provide incremental prognostic value in terms of AUC to the full model of in-study outcome predictors including markers of COVID-19 disease severity (p-value for AUCs comparison = 0.933) (Supplementary Fig. 1).
3.2.2. Patients with subclinical CAD stratified by CAC burden
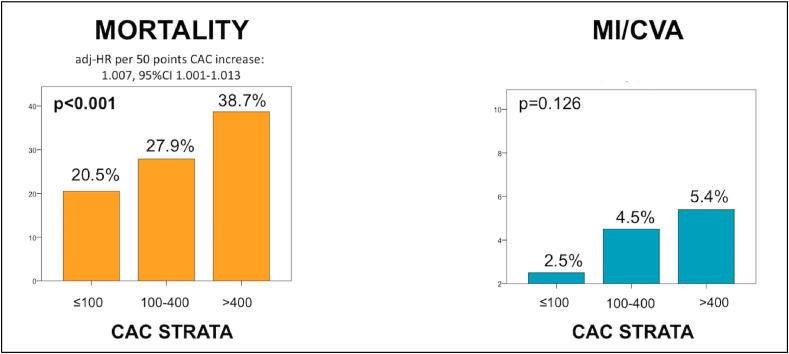
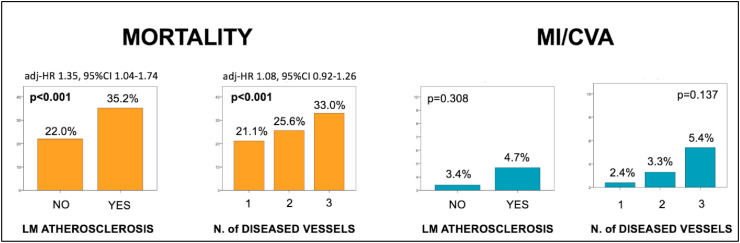
Among patients with subclinical CAD, increasing CAC burden was associated with higher rates of in-hospital mortality (20.5% vs. 27.9% vs. 38.7% for patients with mild, moderate and severe CAC, respectively, p < 0.001) also after multivariate adjustment (adj-HR per 50 points CAC increase: 1.007, 95%CI 1.001–1.013, p=0.016). A trend for higher rates of MI/CVA was also noted (2.5% vs. 4.5% vs. 5.4%, p=0.106) (Fig. 2 ). Similar results were observed when patients with subclinical CAD were stratified by the presence vs. absence of left main coronary artery calcification (in-hospital mortality 22.0% vs. 35.2%, p < 0.001, MI/CVA 3.4% vs. 4.7% p < 0.001, adj-HR 1.35, 95%CI 1.04–1.74, p=0.023) and by the number of calcified vessels (in-hospital mortality one: 21.1% vs. two: 25.6% vs. three: 33%, p < 0.001, MI/CVA one: 2.4% vs. two: 3.3% vs. three: 5.4%, p=0.137), though non-significant after multivariate adjustment (adj-HR 1.08, 95%CI 0.92–1.26, p=0.355) (Fig. 3 ).
Fig. 2.
In-hospital mortality and a composite of in-hospital myocardial infarction and cerebrovascular accident as stratified by increasing CAC burden.
Adjusted hazard ratio for demographics and in-study outcome predictors is provided for the primary endpoint. CAC = coronary artery calcium; HR = hazard ratio, MI/CVA = myocardial infarction/cerebrovascular accident.
Fig. 3.
In-hospital mortality and a composite of in-hospital myocardial infarction and cerebrovascular accident according to left main coronary artery involvement and number of diseased vessels.
Adjusted hazard ratio for demographics and in-study outcome predictors is provided for the primary endpoint. LM = left main; HR = hazard ratio, MI/CVA = myocardial infarction/cerebrovascular accident.
3.2.3. Sensitivity analysis by age
When stratifying the population according to the median age, increasing rates of in-hospital mortality were observed for patients with no CAD vs. subclinical CAD vs. clinical CAD in both age subgroups (<70 years: 6.3% vs. 14.8% vs. 22.9%, p < 0.001; ≥70 years: 35.2% vs. 36.3% vs. 50%). Among patients <70 years, as compared to no CAD, subclinical CAD was associated with an increased risk of in-hospital mortality (adj-HR 2.11 95%CI 1.11–4.01, p=0.023), while numerical trends were observed for clinical CAD (adj-HR 2.83 95%CI 0.92–8.74, p=0.071). In contrast, in patients ≥70 years, subclinical CAD compared to no CAD was not associated with increased risk of in-hospital mortality despite numerical trends (adj-HR 1.44 95%CI 0.86–2.43, p=0.167), while increased risk was observed for patients with clinical CAD (adj-HR 2.19 95%CI 1.16–4.13, p=0.016).
4. Discussion
This study evaluated the impact of clinical and subclinical CAD, as assessed by coronary artery calcium, on in-hospital mortality in a large, unselected population of hospitalized COVID-19 patients undergoing non gated chest CT for pneumonia severity assessment. The main findings were:
-
1.
Clinical and subclinical CAD assessed by CAC score on a routine non-gated chest CT are associated with in-hospital mortality and MI/CVA.
-
2.
This association is independent of comorbidities, clinical, laboratory and lung imaging predictors of COVID-19-related mortality.
-
3.
The extension and location of subclinical coronary artery disease are associated with COVID-19 in-hospital mortality.
-
4.
Clinical cardiovascular risk factors are not independently associated with COVID-19 in-hospital mortality when the extent and presence of coronary atherosclerosis is considered.
Our observations highlight the clinical relevance of the emerging link between SARS-Cov2 infection and the cardiovascular system. Our results suggest that the atherosclerotic spectrum identifies a vulnerability substratum for COVID-19 disease. The chronic inflammatory state and immune system dysregulation that characterize atherosclerosis may offer an ideal pro-inflammatory milieu for SARS-Cov2 infection. Endothelial cells are one of the targets of the virus and diffuse endothelitis has been described as a mechanism for multiorgan damage in COVID-19 disease. Atherosclerosis is associated with endothelial dysfunction, and together this may confer a higher susceptibility to more aggressive viral replication, inflammatory response and clinical manifestations [13,14]. Of note, both overall in-hospital mortality and MI/CVA events were significantly increased among COVID-19 patients with subclinical and clinical CAD as compared to those without, suggesting a specific interplay of SARS-Cov2 infection with the cardiovascular system. This consideration is supported by the particular thrombotic and clinical features of ST-elevation myocardial infarction among COVID-19 disease, reinforcing the importance of further investigations into developing specific management strategies amongst COVID-19 patients with (subclinical and clinical) CAD disease [15].
On the other hand, given that not all the incremental risk is explained by episode of acute coronary syndrome, one could speculate that CAC is a marker of reduced vascular reserve that penalizes the patients infected with SARS-Cov2.
The fact that clinical and subclinical CAD is associated with an increased risk of long-term mortality and cardiovascular events in the general population is well-known [16]. Our findings extend this concept to the acute setting and short-term prognosis of COVID-19 disease suggesting that a variety of complex mechanisms underlie the link between coronary atherosclerosis and adverse events, beyond acute and chronic coronary syndromes. This link was recently suggested by two small (53 and 209 patients) retrospective single-center experiences which evaluated the impact of subclinical CAD by CAC scoring on outcomes in COVID-19 patients. However, the studies used composite outcomes (death or intensive care unit admission [8], and death or occurrence of mechanical ventilation or extracorporeal membrane oxygenation [9]), with either no [8] or only partial [9] adjustment for important covariates. Our study provides a much broader multi-center experience, testing the hard endpoint of in-hospital mortality, which is less subject to heterogeneity in clinical practice. Furthermore, we provide more granular information on the extension and location of subclinical CAD and adjusted for a broad range of multiparametric covariates taking into account the clinical risk profile and markers of COVID-19 disease severity.
From a clinical standpoint, our results suggest that CAC score may be an objective, readily available tool to complement current COVID-19 disease risk stratification tools [15]. This may be particularly relevant in view of the third wave of the COVID-19 pandemic, which is creating significant demands upon the allocation of human and economic resources in healthcare systems worldwide. Specifically, we observed that the presence and extent of CAC disease is a much better gauge for COVID-19 prognosis as compared to a clinical cardiovascular risk assessment, which is frequently adopted in available COVID-19 prognostication tools [17]. This finding was clinically meaningful as demonstrated by the AUC improvement with “CAD stratification” over the clinical cardiovascular risk profile alone. Interestingly, the finding of additive independent value between the presence and extent of CAD and peripheral artery disease suggests the potential implication of a broader atherosclerotic burden assessment encompassing the whole arterial system. Moreover, CAD severity and extent demonstrated their independent prognostic value against age, peripheral artery disease, active malignancy, elevated lactic dehydrogenase (LDH) level and extent of pneumonia at chest CT which, besides being the independent prognostic predictors in our analysis, represent consistent and robust risk markers across most of the COVID-19 literature. As no significant AUC improvement with “CAD stratification” was observed when the markers of COVID-19 severity were taken into account, this association is rather of pathophysiological relevance and does not seem to justify a more aggressive use of chest CT in COVID-19 with the purpose to quantify CAC, but rather that CAC quantification may provide complementary information when chest CT is performed or available for other clinical reasons, especially in the setting of COVID-19 prevention and pre-emptive risk assessment.
In our cohort more than a third (39.8%) of patients with clinically manifest CAD died during the hospitalization and 12% developed a myocardial infarction or a cerebrovascular accident. Among patients with subclinical CAD, those with a CAC ≥400 showed a similar mortality rate (38.7%) to patients with clinical CAD, representing a subset of patients who could have been potentially overlooked by clinical assessment alone. The lower strata of CAC also maintained an additive prognostic value. This suggests that calcium deposition in the coronary artery is a mirror to a more general alteration in the vascular system, independently from overt CAD. Importantly, the subset of patients with zero CAC had a fairly low rate of adverse events (in-hospital mortality 11.3%, MI/CVA 2.3%) pointing at the protective role of a healthy vasculature in the course of COVID-19 disease natural history.
When a qualitative, rather than quantitative approach was applied, by stratifying COVID-19 patients according to the presence of absence of left main coronary artery calcification or number of calcified vessels, a significant association with in-hospital mortality was still observed. This suggests that coronary calcium evaluation either qualitatively or quantitatively on chest CT could potentially be a useful tool to guide physicians performing risk assessments in a busy clinical setting.
4.1. Limitations
This study must be interpreted in the context of some limitations. First, this was a retrospective observational study and outcomes were retrospectively adjudicated at each center without central adjudication. Second, some data regarding important cardiovascular risk factors such as dyslipidemia and CAD family history, along with important metrics of overall frailty status were not collected in the dataset and were thus not considered in the multivariate adjustment, which may have affected the study results [18,19]. Similarly, myocardial injury as defined by peak troponin value, was excluded from the multivariate adjustment because this data was only available for a minority of patients. Third, we only enrolled only COVID-19 patients who were hospitalized and underwent chest CT for lung assessment. Therefore, a selection bias might have occurred as patients with milder disease would not have been represented in the present analysis, although this bias could not be quantified from the data collected in the registry. Moreover, for this reason, most of the exams were not EKG-gated representing suboptimal imaging for quantitative CAC assessment. However, it has been previously demonstrated that CAC assessment based on non-EKG-gated CT is reliable [20] and, more importantly, the results of this analysis suggest its value in the setting of COVID-19 disease prognosis. Fourth, we used the absence of CAC evidence (CAC = 0) as a proxy for no coronary artery disease. Whilst this approach may not fully capture the extent of atherosclerosis from a pathophysiological standpoint, its negative predictive values for clinically and subclinically meaningful CAD approaches 100% and is thus of practical value [21]. To conclude, long-term outcomes of COVID-19 disease were beyond the scope of this investigation.
4.2. Conclusions
The presence and extension of CAD, as assessed by CAC obtained from non-gated chest CT, was associated with higher rates of in-hospital mortality, myocardial infarction and cerebrovascular accident among hospitalized patients with COVID-19 disease. Furthermore, CAC is a better prognostic marker compared to traditional clinical cardiovascular risk assessment amongst hospitalized COVID-19 patients.
Author contributions
Conception and design of study: Alessandra Scoccia, Guglielmo Gallone, Antonio Esposito, Francesco Giannini, Marco Toselli. Acquisition of data: Alessandra Scoccia, Guglielmo Gallone, Alberto Cereda, Anna Palmisano, Davide Vignale, Riccardo Leone, Valeria Nicoletti, Chiara Gnasso, Alberto Monello, Arif Khokhar, Alessandro Sticchi, Andrea Biagi, Carlo Tacchetti, Prof. Gianluca Campo, Claudio Rapezzi, Francesco Ponticelli, Gian Battista Danzi, Marco Loffi, Gianluca Pontone, Daniele Andreini, Gianni Casella, Gianmarco Iannopollo, Davide Ippolito, Giacomo Bellani, Gianluigi Patelli, Francesca Besana, Claudia Costa, Luigi Vignali, Giorgio Benatti, Mario Iannaccone, Paolo Giacomo Vaudano, Alberto Pacielli, Caterina Chiara De Carlini, Stefano Maggiolini, Pietro Andrea Bonaffini, Michele Senni, Elisa Scarnecchia, Fabio Anastasio, Antonio Colombo, Prof. Roberto Ferrari, Antonio Esposito, Francesco Giannini, Marco Toselli. Analysis and/or interpretation of data: Alessandra Scoccia1, Guglielmo Gallone, Antonio Esposito, Francesco Giannini, Anna Palmisano, Davide Vignale, Marco Toselli. Drafting of the manuscript: Alessandra Scoccia, Guglielmo Gallone. Revising the manuscript critically for important intellectual content: Gianluca Campo, Claudio Rapezzi, Arif Khokhar, Antonio Colombo, Roberto Ferrari, Antonio Esposito, Francesco Giannini, Marco Toselli. Approval of the version of the manuscript to be published: Alessandra Scoccia, Guglielmo Gallone, Alberto Cereda, Anna Palmisano, Davide Vignale, Riccardo Leone, Valeria Nicoletti, Chiara Gnasso, Alberto Monello, Arif Khokhar, Alessandro Sticchi, Andrea Biagi, Carlo Tacchetti, Gianluca Campo, Claudio Rapezzi, Francesco Ponticelli, Gian Battista Danzi, Marco Loffi, Gianluca Pontone, Daniele Andreini, Gianni Casella, Gianmarco Iannopollo, Davide Ippolito, Giacomo Bellani, Gianluigi Patelli, Francesca Besana, Claudia Costa, Luigi Vignali, Giorgio Benatti, Mario Iannaccone, Paolo Giacomo Vaudano, Alberto Pacielli, Caterina Chiara De Carlini, Stefano Maggiolini, Pietro Andrea Bonaffini, Michele Senni, Elisa Scarnecchia, Fabio Anastasio, Antonio Colombo, Roberto Ferrari, Antonio Esposito, Francesco Giannini, Marco Toselli.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.03.041.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., Italia L., Zaccone G., Tedino C., Fabbricatore D., Curnis A., Faggiano P., Gorga E., Lombardi C.M., Milesi G., Vizzardi E., Volpini M., Nodari S., Specchia C., Maroldi R., Bezzi M., Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020 May 14;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. Erratum in: Eur Heart J. 2020 Dec 21;41(48):4591. PMID: 32383763; PMCID: PMC7239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson E., Lo K.B., DeJoy R., Salacup G., Pelayo J., Bhargav R., Gul F., Albano J., Azmaiparashvili Z., Amanullah A., Patarroyo-Aponte G. The relationship between coronary artery disease and clinical outcomes in COVID-19: a single-center retrospective analysis. Coron. Artery Dis. 2020 Jul 23 doi: 10.1097/MCA.0000000000000934. Epub ahead of print. PMID: 32732512. [DOI] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotti G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M.V., Scandroglio A.M., Storti E., Cecconi M., Pesenti A., COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020 Apr 28;323(16):1574–1581. doi: 10.1001/jama.2020.5394. PMID: 32250385; PMCID: PMC7136855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rosendael A.R., Bax A.M., Smit J.M., van den Hoogen I.J., Ma X., Al'Aref S., Achenbach S., Al-Mallah M.H., Andreini D., Berman D.S., Budoff M.J., Cademartiri F., Callister T.Q., Chang H.J., Chinnaiyan K., Chow B.J.W., Cury R.C., DeLago A., Feuchtner G., Hadamitzky M., Hausleiter J., Kaufmann P.A., Kim Y.J., Leipsic J.A., Maffei E., Marques H., de Araújo Gonçalves P., Pontone G., Raff G.L., Rubinshtein R., Villines T.C., Gransar H., Lu Y., Peña J.M., Lin F.Y., Shaw L.J., Min J.K., Bax J.J. Clinical risk factors and atherosclerotic plaque extent to define risk for major events in patients without obstructive coronary artery disease: the long-term coronary computed tomography angiography CONFIRM registry. Eur Heart J Cardiovasc Imag. 2020 May 1;21(5):479–488. doi: 10.1093/ehjci/jez322. PMID: 32065624; PMCID: PMC7821703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadi A., Argulian E., Leipsic J., Newby D.E., Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019 Sep 24;74(12):1608–1617. doi: 10.1016/j.jacc.2019.08.012. PMID: 31537271. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J. Am. Coll. Cardiol. 2018 Jul 24;72(4):434–447. doi: 10.1016/j.jacc.2018.05.027. PMID: 30025580; PMCID: PMC6056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nai Fovino L., Cademartiri F., Tarantini G. Subclinical coronary artery disease in COVID-19 patients. Eur Heart J Cardiovasc Imag. 2020 Sep 1;21(9):1055–1056. doi: 10.1093/ehjci/jeaa202. PMID: 32671381; PMCID: PMC7454480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillinger J.G., Benmessaoud F.A., Pezel T., Voicu S., Sideris G., Chergui N., Hamzi L., Chauvin A., Leroy P., Gautier J.F., Sène D., Henry P., COVID Research Group of Lariboisiere Hospital Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imag. 2020 Nov;13(11):2468–2470. doi: 10.1016/j.jcmg.2020.07.004. PMID: 33153535; PMCID: PMC7605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosyns B., Motoc A., Luchian M.L., Lochy S., Belsack D. Coronary calcium score in COVID-19 hospitalized patients. JACC Cardiovasc Imag. 2020 Dec;13(12):2698. doi: 10.1016/j.jcmg.2020.09.038. Epub 2020 Oct 27. PMID: 33303104; PMCID: PMC7590636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S., Chairman B.R., Bax J.J., Morrow D.A., White H.D. Executive group on behalf of the joint European society of cardiology (ESC)/American college of cardiology (ACC)/American heart association (AHA)/World heart federation (WHF) task force for the universal definition of myocardial infarction. Fourth universal definition of myocardial infarction (2018) J. Am. Coll. Cardiol. 2018 Oct 30;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. Epub 2018 Aug 25. PMID: 30153967. [DOI] [PubMed] [Google Scholar]
- 12.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–845. PMID: 3203132. [PubMed] [Google Scholar]
- 13.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020 Sep;17(9):543–558. doi: 10.1038/s41569-020-0413-9. Epub 2020 Jul 20. PMID: 32690910; PMCID: PMC7370876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., Neil D., Hoefer I.E., Fragiadaki M., Waltenberger J., Weber C., Bochaton-Piallat M.L., Bäck M. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc. Res. 2020 Dec 1;116(14):2177–2184. doi: 10.1093/cvr/cvaa230. PMID: 32750108; PMCID: PMC7454368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudry F.A., Hamshere S.M., Rathod K.S., Akhtar M.M., Archbold R.A., Guttmann O.P., Woldman S., Jain A.K., Knight C.J., Baumbach A., Mathur A., Jones D.A. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2020 Sep 8;76(10):1168–1176. doi: 10.1016/j.jacc.2020.07.022. Epub 2020 Jul 14. PMID: 32679155; PMCID: PMC7833185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budoff M.J., Young R., Burke G., Jeffrey Carr J., Detrano R.C., Folsom A.R., Kronmal R., Lima J.A.C., Liu K.J., McClelland R.L., Michos E., Post W.S., Shea S., Watson K.E., Wong N.D. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA) Eur. Heart J. 2018 Jul 1;39(25):2401–2408. doi: 10.1093/eurheartj/ehy217. PMID: 29688297; PMCID: PMC6030975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., Li Y., Guan W., Sang L., Lu J., Xu Y., Chen G., Guo H., Guo J., Chen Z., Zhao Y., Li S., Zhang N., Zhong N., He J. China medical treatment expert group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020 Aug 1;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. PMID: 32396163; PMCID: PMC7218676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., Huang I., Lukito A.A., Suastika K., Kuswardhani R.A.T., Setiati S. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2021 Mar-Apr;93:104324. doi: 10.1016/j.archger.2020.104324. Epub 2020 Dec 15. PMID: 33352430; PMCID: PMC7832565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosco T.D., Best J., Davis D., Bryden D., Arkill S., van Oppen J., Riadi I., Wagner K.R., Conroy S. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing. 2021 Jan 14 doi: 10.1093/ageing/afab008. afab008. Epub ahead of print. PMID: 33448278; PMCID: PMC7929406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budoff M.J., Mayrhofer T., Ferencik M., Bittner D., Lee K.L., Lu M.T., Coles A., Jang J., Krishnam M., Douglas P.S., Hoffmann U. PROMISE investigators. Prognostic value of coronary artery calcium in the PROMISE study (prospective multicenter imaging study for evaluation of chest pain) Circulation. 2017 Nov 21;136(21):1993–2005. doi: 10.1161/CIRCULATIONAHA.117.030578. Epub 2017 Aug 28. PMID: 28847895; PMCID: PMC5698136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie X., Zhao Y., de Bock G.H., de Jong P.A., Mali W.P., Oudkerk M., Vliegenthart R. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imag. 2013 Jul;6(4):514–521. doi: 10.1161/CIRCIMAGING.113.000092. Epub 2013 Jun 11. PMID: 23756678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.