Abstract
Invasive pulmonary mucormycosis and aspergillosis are rare, life-threatening fungal infections. Most documented cases have been reported in patients with diabetes mellitus, neutropenia, or treatment with corticosteroids. Both infections have been recognized as secondary complications of COVID-19, especially among critically ill patients. We report the first case of combined probable pulmonary aspergillosis and possible mucormycosis in a male with COVID-19 in the ICU.
Keywords: Invasive, Pulmonary, Mucormycosis, Aspergillosis, COVID-19
1. Introduction
Mucormycosis and Aspergillosis are rare, life-threatening fungal infections with mortality rates over 50% despite surgical debridement and antifungal therapy [1,2]. Rhizopus arrhizus, which is responsible for 70% of all cases of mucormycosis, is associated with several clinical diseases (rhino-orbital-cerebral, cutaneous, gastric, and pulmonary) in adults [1]. This fungal infection is particularly recognized due to its highly pathogenic nature, which is characterized by rapid tissue destruction and invasion across tissue planes [3]. The major risk factors for mucormycosis include uncontrolled diabetes mellitus with ketoacidosis, hematological malignancy, stem cell and solid organ transplantations, iron chelation therapy with deferoxamine, and corticosteroid usage [3]. The diagnosis of mucormycosis is made by the identification of causative fungal organisms by histopathological analysis of tissue specimens from patients with suspected signs and symptoms [3]. Cultures are occasionally positive [3]. Initial treatment of mucormycosis typically requires early aggressive surgical debridement of infected tissues, combined with administration of amphotericin B deoxycholate (AmB) or liposomal amphotericin B (L-AmB) [3].
Aspergillus fumigatus is responsible for over 70% of all cases of invasive aspergillosis [4]. Invasive aspergillosis usually occurs in immunosuppressed patients such as those receiving chronic corticosteroid treatment or with prolonged neutropenia from malignancy [2,4]. The diagnosis of proven, probable, or possible invasive aspergillosis is made by the combination of host status and imaging and mycological findings [5]. The optimal treatment of aspergillosis is administration of voriconazole or isavuconazole [2,4].
Both infections have been recognized as secondary complications of coronavirus disease 2019 (COVID-19), especially among critically ill patients in the intensive care unit (ICU) [[6], [7], [8]]. Reports have shown that up to 35% of these patients have invasive pulmonary aspergillosis, which has been associated with prior corticosteroid usage and has led to higher mortality [[6], [7], [8], [9]]. In contrast, the incidence of invasive pulmonary mucormycosis following COVID-19 is rare, with only two cases currently reported [10,11]. To our knowledge, we report the first case of combined probable pulmonary aspergillosis and possible mucormycosis in a male with COVID-19 in the ICU.
2. Case
A 79-year old Latino male was found confused at home and was taken by ambulance to our emergency department (ED). The patient was minimally responsive, and history was obtained from the daughter. Over the past 10 days, he had fevers, rigors, dry cough, and worsening shortness of breath. The patient has a past medical history of diabetes mellitus and hypertension. Initial vital signs in the ED demonstrated a temperature of 38.4 °C, heart rate of 103 beats per minute, respiratory rate of 25 breaths per minute, oxygen saturation of 92% while breathing 60 L of oxygen per minute via high-flow nasal canula, and blood pressure of 154/78 mmHg. On examination, he was ill appearing and in respiratory distress. The patient had tachycardia, but no cardiac murmurs. Rales and rhonchi were heard at both lung bases.
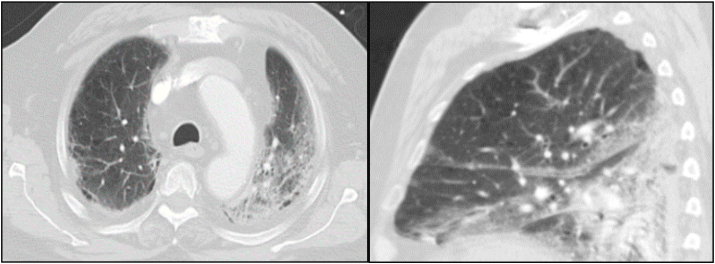
Pertinent abnormal laboratory studies on initial presentation revealed a white blood cell count of 3.5 × 103 cells/um3, absolute lymphocyte count of 0.2 × 103 cells/um3, random glucose of 147 mg/dL, HgA1c of 6.9%, lactate of 2.2 mg/dL, D-dimer of 709 ng/mL, C-reactive protein of 10.3 mg/dL, ferritin of 433 ng/mL, and lactate dehydrogenase of 475 U/L. Nasopharyngeal swab polymerase chain reaction (PCR) test was positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). A portable chest radiograph (CXR) showed patchy bibasilar infiltrates. Computed tomography (CT) of the chest revealed moderate bilateral ground-glass opacities and infiltrates (Fig. 1).
Fig. 1.
Transverse and sagittal images (slices) from CT of chest scan on day 0 of hospitalization demonstrating bilateral ground-glass opacities and infiltrates.
The patient was treated with intravenous (IV) fluid hydration and empiric antibiotic treatment with IV ceftriaxone 1 g daily and IV azithromycin 250 mg daily for community-acquired pneumonia. A 5-day course of IV remdesivir (200 mg X 1, then 100 mg daily) and 10-day course of IV dexamethasone 6 mg daily were initiated. He was admitted to the ICU for closer respiratory monitoring on day 0.
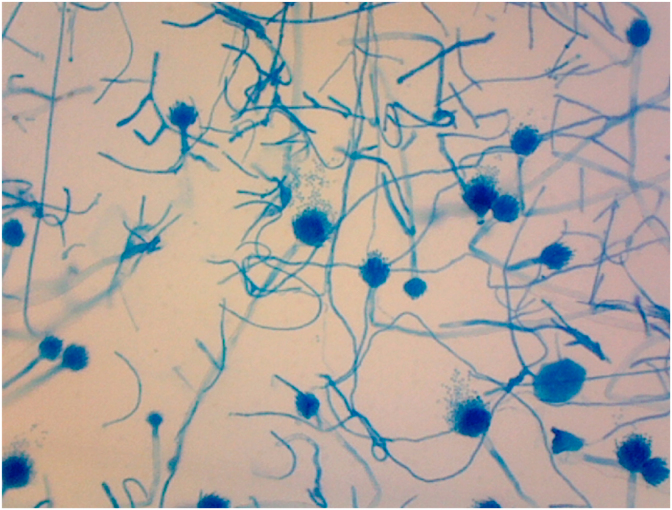
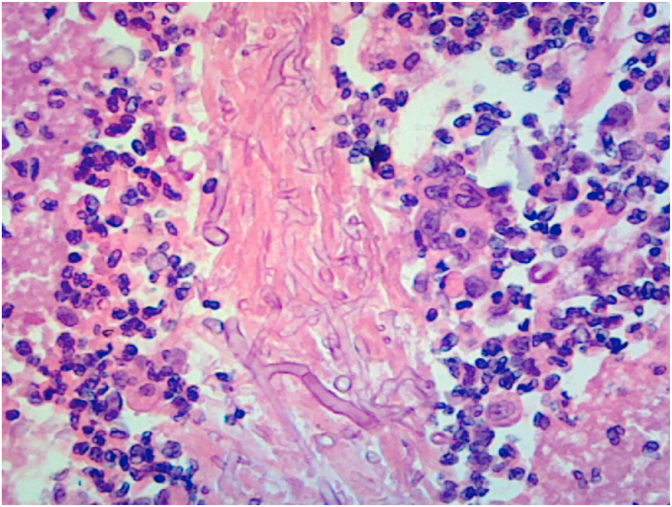
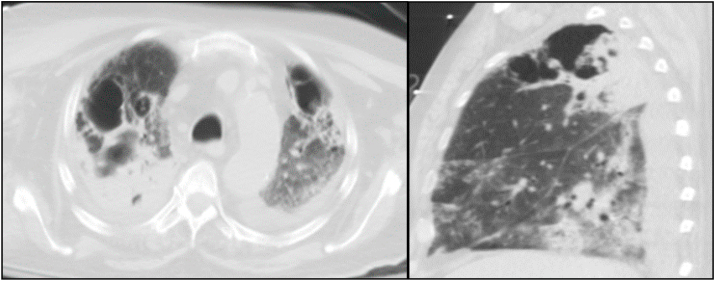
On day +5, the patient completed his treatment of ceftriaxone, azithromycin, and remdesivir. On day +6, his respiratory status deteriorated and was intubated due to hypoxic respiratory failure. On day +9, the patient was diagnosed with ventilator-associated pneumonia from methicillin-resistant Staphylococcus aureus and Klebsiella pneumoniae, in which he started a 7-day course of IV vancomycin 1250 mg every 8 hours and IV ceftriaxone 1 g daily. On day +11, the patient continued to have fevers and required vasopressor support with norepinephrine. On day +13, a bronchoalveolar lavage (BAL) was performed and thick frothy respiratory secretions were seen. On day +14, the BAL culture grew fungus which was suspicious for Aspergillus species. Hyaline septate hyphae and characteristic conidial heads (or fruiting bodies) were seen on microscopic image of the BAL specimen (Fig. 2). Empiric antifungal treatment with IV voriconazole 200 mg twice daily was initiated. Fungal culture was ordered, and a specimen was sent to an outside reference laboratory for identification. The Aspergillus galactomannan assays of serum (2.04, normal <0.49) and of the BAL fluid (7.86, normal <0.49) were positive. Serum 1,3 β-D glucan (94, normal <80) was positive. On day +19, a second fungus grew in the BAL culture. This fungus had pauciseptate hyphae (Fig. 3), and an additional specimen was sent for identification. On day +19, repeat CT of the chest revealed extensive bilateral pneumonia and new development of bilateral upper lobe cavitations (Fig. 4). Antifungal treatment was changed to IV L-AmB 400 mg daily for suspected pulmonary mucormycosis. No other infections or treatment complications occurred. On day +29, Rhizopus arrhizus and Aspergillus fumigatus were identified by microscopic examination with potassium hydroxide (KOH) preparation, culture, and isolation from the BAL culture at our outside reference laboratory, indicating the presence of probable pulmonary aspergillosis and possible mucormycosis. No susceptibility tests were performed.
Fig. 2.
Septate hyphae and conidia heads of Aspergillus fumigatus from BAL specimen on day +14 (Lactophenol cotton blue, 200X). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Broad hyphae with right angle branching and rare septations of fungus from BAL cytology on day +19 (Hematoxylin and eosin, 200X).
Fig. 4.
Transverse and sagittal images (slices) from CT of chest scan on day + 19 of hospitalization demonstrating bilateral pneumonia and upper lobe cavitations.
Throughout his ICU stay, his random blood sugars ranged from 119 mg/dL to 228 mg/dL, and were managed with subcutaneous insulin glargine 50 units daily and insulin lispro sliding scale. The patient underwent tracheostomy on day +23 and percutaneous endoscopic gastrostomy on day +25 due to his chronic hypoxic respiratory failure and encephalopathy. Weekly serum Aspergillus galactomannan and 1,3 β-D glucan assays were recommended but not tested. Weekly sputum cultures were positive for both organisms from days +29 and + 36. On day +36, the patient remained on ventilator support, tolerated IV L-AmB treatment, and was discharged to a long-term acute care facility.
3. Discussion
The data indicate that our patient had both probable pulmonary aspergillosis and possible mucormycosis. The diagnosis of mucormycosis was made by the finding of broad non-septate hyphae on cytology and isolation of Rhizopus arrhizus on culture from the BAL specimen. Our patient also met the diagnostic criteria for probable COVID-19-associated pulmonary aspergillosis (CAPA) based on the presence of a second fungus with septate hyphae and conidia on microscopy, the isolation of Aspergillus fumigatus on culture from the BAL specimen, cavitating infiltrates, and refractory fever [12]. However, because no bronchoscopic biopsy was performed, we cannot classify this case as proven aspergillosis and mucormycosis [5,12]. Our patient most likely acquired both organisms from the natural environment.
The combined risk factors of diabetes mellitus, COVID-19, and recent corticosteroid treatment contributed to both infections in our patient. Airway epithelial damage and immune dysfunction are known complications of COVID-19, which may provide an opportunity for Aspergillus and Rhizopus species to invade into lung tissues [6]. Decline in cell-mediated immunity has been observed in symptomatic patients, including leukopenia, lymphopenia, and T-cell dysregulation [6]. In addition, the usage of corticosteroids in the treatment of COVID-19 may further predispose to these fungal infections [[6], [7], [8], [9]]. Failure to recognize and treat these infectious complications of COVID-19 will likely lead to higher mortality, which has been described [[7], [8], [9], [10]].
This is the first report of a case of combined probable pulmonary aspergillosis and possible mucormycosis in a patient with COVID-19. Our case differed from only two prior published cases of pulmonary mucormycosis following COVID-19 in that there was no diabetes mellitus [10,11]. However, our cases were similar given the development of extensive cavitary lung lesions, which may indicate a greater association with mucormycosis [10]. Interestingly, cavitary pneumonia was not observed in any of the published case series and reports of COVID-19 associated pulmonary aspergillosis [[13], [14], [15], [16]].
In conclusion, this case report highlights the need for healthcare professionals to be aware that combined pulmonary aspergillosis and mucormycosis can present as secondary complications of COVID-19 in critically ill patients.
Conflict of interest
There are none.
Funding
This study received no funding, and there are no potential conflicts of interests with respect to the research, authorship, and/or publication of this article.
Acknowledgements
None.
References
- 1.Ibrahim A., Spellberg B., Walsh T., Kontoyiannis D. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012;54:16–22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falcone M., Massetti A., Russo A., Vullo V., Venditti M. Invasive aspergillosis in patients with liver disease. Med. Mycol. 2011;49:406–413. doi: 10.3109/13693786.2010.535030. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim A., Kontoyiannis D. Update on mucormycosis pathogenesis. Curr. Opin. Infect. Dis. 2013;26:508–515. doi: 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicek N., Yildiz N., Kadayifci E., Gokce I., Alpay H. Invasive aspergillosis in a patient with end stage renal disease. Med Myco Case Rep. 2017;18:12–14. doi: 10.1016/j.mmcr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascioglu S., Rex J., De Pauw B., Bennett J., Bille J., Crokaert F. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 6.Thompson G., Cornely O., Pappas P., Patterson T., Hoenigl M., Jenks J. Invasive aspergillosis as an under-recognized superinfection in COVID-19. Open Forum Infect Dis. 2020;7:1–3. doi: 10.1093/ofid/ofaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado M., Valerio M., Alvarez-Uria A., Olmedo M., Veintimilla C., Padilla B. Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses. 2021;64:132–143. doi: 10.1111/myc.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai C., Yu W. COVID-19 associated with pulmonary aspergillosis: a literature review. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delliere S., Dudoignon E., Fodil S., Voicu S., Collet M., Oillic P. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasero D., Sanna S., Liperi C., Piredda D., Branca G., Casadio L. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection. 2020 doi: 10.1007/2Fs15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zurl C., Hoenigl M., Schulz E., Hatzl S., Gorkiewicz G., Krause R. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically ill COVID-19 patient with underlying hematological malignancy. J. Fungi. 2021 doi: 10.3390/jof7020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koehler P., Bassetti M., Chakrabarti A., Chen S., Colombo A., Hoenigl M. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beneditti M., Alava K., Sagardia J., Cadena R., Laplume D., Capece P. COVID-19 associated pulmonary aspergillosis in ICU patients: report of five cases from Argentina. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez N., Caceres D., Beer K., Irrazabal C., Delgado G., Farias L. Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID-19) from Argentina. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A., Hofmeyr A., Bansal A., Thakkar D., Lam L., Harrington Z. COVID-19 associated pulmonary aspergillosis (CAPA): an Australian case report. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prattes J., Valentin T., Hoenigl M., Talakic E., Reisinger A., Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU- A case report. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.200.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]