Abstract
Objective:
Persons with human immunodeficiency virus (HIV) have double the risk of developing cardiovascular disease (CVD) compared to the general population. A persistent and heightened immune response to cytomegalovirus (CMV) co-infection may be one contributing factor, but the relationship between CMV replication, virus-specific immune cells and plaque burden is unclear.
Approach and Results:
We assessed the relationship between CD4+ T-cell subsets and carotid plaque burden in a cohort of 70 HIV-positive participants with sustained viral suppression on a single antiretroviral regimen and without known CVD. We evaluated relationships between immune parameters, carotid plaque burden, and brachial artery flow-mediated vasodilation (FMD) using multivariable linear and logistic regression models. We found that participants with carotid plaque had increased circulating CX3CR1+~GPR56+~CD57+ (i.e., C~G~C)+ CD4+ T cells (p=0.03), which is a marker combination associated with anti-viral and cytotoxic responses. In addition, a median of 14.4% [IQR 4.7, 32.7%] of the C~G~C+ CD4+ T-cells expressed antigen receptors that recognized a single CMV glycoprotein-B epitope. Notably, using immunofluorescence staining we found that CX3CR1+ CD4+ T-cells were present in coronary plaque from deceased HIV-positive persons. C-G-C+ CD4+ T cells were also present in cells isolated from the aorta of HIV-negative donors.
Conclusions:
HIV-positive persons with carotid atheroma have a higher proportion of circulating CD4+ T-cells expressing the C~G~C surface marker combination associated with antiviral and cytotoxic responses. These cells can be CMV-specific and are also present in the aorta.
Keywords: Cardiovascular Disease, Atherosclerosis, Cytomegalovirus, HIV, CD4+ T-cells, inflammation
Graphical Abstract
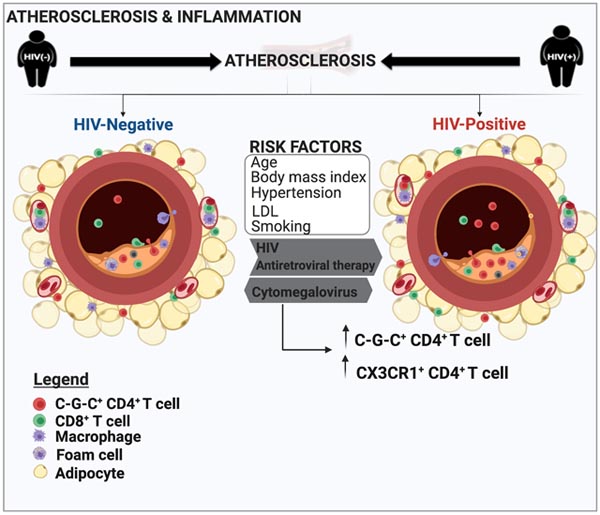
Introduction
Persons with human immunodeficiency virus (HIV) can survive decades on antiretroviral therapy (ART), but this success is offset by an approximately two-fold increased risk of cardiovascular disease (CVD) compared to HIV-negative persons through mechanisms that are not well-defined1–7. The effect of cytomegalovirus (CMV) co-infection on the immune system is amplified in HIV-positive persons, who often have chronic, low-level CMV replication, asymptomatic shedding, and higher levels of anti-CMV T cells8–11. Prior studies show infection with CMV is accompanied by accelerated atherosclerosis in cardiac transplant recipients, and an approximately 20% increased risk of cardiovascular events in the general population12,1, 13, 14. Infection with CMV is lifelong, and the persistent immune response required to suppress active viremia alters the memory T cell repertoire and generates inflated anti-CMV T cell responses and antibody titers9, 10, 15–17. The immune response to CMV, including both innate and adaptive components, may also contribute to CVD, as higher CMV antibody titers are linked to coronary atherosclerosis18 and impaired brachial artery flow-mediated dilation (FMD)19, 20.
The heightened adaptive immune response to CMV in HIV-positive persons is referred to as “memory inflation” and has been described against specific CMV-epitopes. Two examples of CD4+ epitopes that may drive an anti-CMV response are the HLA-DR7-restricted epitope, glycoprotein B217–227 DYSNTHSTRYV (DYS) in the long-term HIV non-progressors9, and the HLA-DQ6-restricted matrix phosphoprotein 6541–55 LLQTGIHVRVSQPSL21, 22, 23. For both epitopes, up to ~25–30% of circulating CD4+ cells have epitope-specific receptors in HIV-positive and HIV-negative persons in the absence of an acute infection9, 21. These cells have either a T effector memory (TEM) or CD45RA+ revertant (TEMRA) phenotype and are maintained over decades9. In HIV-positive persons, the inflated immune response to CMV results in a greater relative proportion of CMV-specific CD4+ T cells compared to HIV-negative persons.
Defining the innate and adaptive immune cells which promote atheroma formation and vascular disease has been challenging for many reasons, including the high degree of cell necrosis within atherosclerotic plaques. However, it is essential to delineate these immune mechanisms as a step towards targeted therapeutics, such as anti-viral agents, anti-inflammatory agents or combination drugs. Our group recently described a subset of CD4+ T cells co-expressing CX3CR1, GPR56 and CD57 (hereafter referred to as ‘C~G~C’ CD4+ T cells) enriched in the adipose tissue and peripheral blood of HIV-positive, CMV co-infected diabetic individuals24. We further described a higher proportion of GPR56 and CX3CR1 co-expression on CD4+ TEM and TEMRA cells in the context of metabolic disease. Prior studies have shown that virus-specific TEMRA cells are more frequently GPR56+25,21, and higher expression of GPR56 has been linked to greater CD4+ T cell proinflammatory cytokine expression26. Furthermore, GPR56+ CD4+ TEMRA cells frequently co-express the CX3CR1 receptor,25, 27, 28 which is found on many anti-CMV T cells and binds the fractalkine receptor expressed on endothelial cells9, 21, 28–30.
We hypothesized that C~G~C+ CD4+ T cells are largely CMV-specific and contribute to the development of atherosclerosis. To this end, we determined the circulating T cell subsets associated with greater carotid plaque burden in a well-characterized cohort of HIV-positive persons on a single ART regimen with long-term viral suppression and no history of cardiac events31–34. Thirty HIV-negative individuals were included as a comparison group. We additionally identified the phenotype of CMV-specific CD4+ T cells in a subset of the cohort participants using tetramer staining. We utilized immunofluorescent imaging to visualize CX3CR1+ CD4+ T cells within atherosclerotic plaque sections of HIV-positive and HIV-negative deceased donors. Lastly, we isolated cells from the aorta of two HIV-negative donors and use flow cytometry to demonstrate the presence of C~G~C+ CD4+ T cells.
Methods
The data and analytic methods for this study are available within the article and are available upon request.
Study cohort
We enrolled 70 adults with HIV from the Vanderbilt Comprehensive Care Clinic, an academic HIV treatment clinic, and 30 adults without HIV from the Vanderbilt general medicine outpatient clinic as previously described32, 33. All HIV-positive participants were on efavirenz, tenofovir disoproxil fumarate, and emtricitabine (i.e., the combination pill Atripla) for at least 6 months prior to enrollment and had been on ART with persistent HIV-1 RNA measurements <50 copies/ml for at least 2 years. Additional inclusion criteria include a CD4+ T cell count >350 cells/μl at the time of enrollment, no use of anti-diabetic medications or HMG CoA reductase inhibitors (i.e., statins) in the prior 6 months, and no known history of diabetes, CVD, or rheumatologic disease. The HIV-negative participants were all obese (median body mass index [BMI] ≥35.7 kg/m2) by study design and group matched on sex, race, and BMI with the obese HIV-positive participants (median BMI 35.9 kg/m2). The HIV-negative participants were similarly not receiving anti-diabetic or statin medications, and had no history of diabetes, CVD, or rheumatologic disease.
The study was approved by the Vanderbilt University Medical Center and CVPath Institutional Review Boards. Participants provided written informed consent. The investigators carried out studies in accordance with guidelines of the United States Department of Health and Human Services. This study is registered on clinicaltrials.gov (NCT04439448).
Vascular ultrasound evaluations
All antihypertensive and vasoactive drugs, alcohol and caffeine were held for at least 12 hours prior to vascular function measurements. Brachial-artery endothelium-dependent flow mediated dilation (FMD), carotid artery plaques and carotid intima-media thickness (CIMT) were measured by ultrasound as previously described33. Briefly, brachial artery diameter was measured using B-mode ultrasonography (Philips iE33) equipped with a high-resolution linear array transducer (L9–3, 7.5 mHz). A longitudinal image (parallel to the artery) was acquired just proximal to the antecubital fossa with the transducer positioned to optimize images of the near and far wall interfaces. A simultaneous electrocardiographic (ECG) signal was recorded and images were digitally acquired at end-diastole, synchronized to the R wave on the ECG at 30, 60, 90, and 120 seconds. To assess endothelium-dependent vasodilation, brachial artery diameter was measured under basal conditions and during reactive hyperemia. Reactive hyperemia was assessed after 5 minutes of an ischemic stimulus produced by inflating a blood pressure cuff on the upper arm to suprasystolic pressures. We have found that following cuff deflation, the maximal increase in brachial artery diameter occurs at approximately 1 minute of reactive hyperemia, a response mediated by endothelium-derived nitric oxide35, 36. Decreased FMD indicates reduced bioavailability of endothelium-derived nitric oxide. The video output and electrocardiographic signal of the ultrasound machine was connected to a computer equipped with a Data Translation frame grabber video card. The R wave on the electrocardiogram is used as a trigger to acquire (digitize) frames. Acquisition and analysis of the stored images was performed using software designed for this purpose by Medical Imaging Applications.
The data were analyzed by a single blinded operator. The vessel wall lumen interface was determined by derivative based edge detection following identification of the region of the anterior and posterior walls by the investigator. The maximum diameter of the vessel was then determined and the percent change in diameter (% vasodilation) calculated. We have found that this technique yields an inter-observer variability of 0.05±0.16% and intra-observer variability of 0±0.15%35–39. Differences of baseline brachial artery diameter could affect vasodilation, but cross-sectional areas did not differ significantly by sex (Table I, Data Supplement).
Carotid plaques (lesions ≥1.5 mm) were defined by B-mode ultrasound at the right and left common carotid arteries, carotid bulbs, and internal carotid arteries. We calculated the total number of carotid plaques per individual. CIMT was measured in plaque free arterial segments at the carotid bulb, and 1 cm proximally in the common carotid artery, as the distance between the inner echogenic line representing the blood-intima interface and the outer echogenic line representing the media-adventitia border. A similar procedure was used to measure the right and left carotid bulb IMT at 1 cm, which were averaged for analysis (Table II, Data Supplement).
Flow cytometry immunophenotyping
Peripheral blood mononuclear cells (PBMCs) were isolated and stored in liquid nitrogen immediately after collection. Flow cytometry analysis was used to define activated, senescent, and memory CD4+ and CD8+ T cells, and the specific population of C~G~C+ (CX3CR1+ GPR56+ CD57+) CD4+ T cell subsets that we previously identified in adipose tissue, as published24. The panel of antibodies included CD3-BV786, CD4-PcPCy5.5, CD8-A700, CD57-FITC, CX3CR1-PE, CD45RO-PECF594, CD14-V500, CD19-V500, LIVE/DEAD Fixable Aqua, CD69-APC, CCR7-V450, GPR56-PECy7, and HLA-DR APC Cy7. Cells are resuspended in 200 μl PBS prior to the addition of antibodies (Additional details of antibody clones are outlined in the Data Supplement). Quantitation of CMV-specific T cells was performed in 10 HIV-positive participants with human leukocyte antigen (HLA) DR7 using a well characterized DYS MHC II tetramer (HLA-DR7: DYSNTHSTRYV (DYS) APC) that binds T cell receptors recognizing the immunodominant DYSNTHSTRYV epitope (DYS) within CMV glycoprotein B (gB) presented by HLA-DRB1*07:01 MHC molecules9. Three HIV-positive participants who did not express HLA-DR7 were included as negative controls. Human CLIP 87–101 | PVSKMRMATPLLMQA and A2:NLVPMVATV (A2:NLV; CMV pp65495–503) tetramers were included as controls. All tetramers were made by the National Institutes of Health Tetramer Core Facility (contract HHSN272201300006C, NIAID).
Serum CMV IgG measurement
CMV IgG levels were measured using enzyme-linked immunosorbent assay (ELISA) (GenWay Biotech, Inc San Diego, CA) according to the manufacturer’s instructions. The intra-assay coefficient of variation was 0–26% and the inter-assay coefficient of variation was 9–13%.
Droplet digital PCR
Droplet digital PCR (ddPCR) was used to measure CMV in circulating immune cells from the participants with HLA-DR7 included in the DYS tetramer staining assay as previously published9. The ddPCR assay quantifies CMV transcripts within cells. The positive droplet threshold was manually set using the negative droplet control (media only), and a positive CMV (DNA) control was included.
Isolation of immune cells from aorta
Fresh plaque and vascular tissue from the aorta of two HIV-negative persons was obtained from the operating room and immediately placed in normal saline on cold ice. The tissue was minced into small pieces and added into digestion buffer with collagenase type I (0.1%) and DNASE (final concentration 500μg/ml) at 37°C for 30 minutes with constant agitation using a bench top rocker. At the end of this incubation, the digestion reaction was stopped by spiking in 10μl of 0.5M EDTA. The digested tissue was passed through a 70μm strainer and collected into a 50ml conical tube using 1–2ml Dulbecco’s Modified Eagle Medium (DMEM). This was centrifuged at 1200 rotations per minute for 5 minutes. The supernatant was removed, and cells resuspended in 1X RBC lysis buffer (2ml for 2 minutes). Cells were then washed with DMEM media and counted. They were stored in 10% DMSO/FBS at −80°C for 24 hours and then transferred to liquid nitrogen. The same flow panel above was used to stain these cell suspensions.
Tissue immunofluorescence and immunohistochemical staining
Human coronary artery specimens characterized as late and early atheroma from 11 HIV-positive and 12 HIV-negative adults deceased from sudden death (cardiac and non-cardiac) were obtained from the CVPath Institute Registry (Table III, Data Supplement). Briefly, the artery segments were fixed in formalin and 2 to 3-millimeter segments were embedded in paraffin. Cross-sections 5 microns thick were cut from 3–4 serial sections of the segments and mounted on slides. Slides were deparaffinized and treated for antigen retrieval on the Ventana Discovery system (protocol CC1–32, Roche Diagnostics). Coronary plaque morphology was defined as previously published40. For immunohistochemical (IHC) staining, slides were incubated with anti-CX3CR1 antibody (Abcam), or anti-CD4 (Roche), anti-CD3 (Roche), anti-CD8, OmniMap anti-Ms Horseradish Peroxidase (HRP) (DISCOVERY) or anti-Rb HRP and developed by the NovaRed kit (Vector Laboratories). The IHC images were captured by an Axio Scan.Z1 (Zeiss, Germany) using a 20X objective. IHC staining was quantified in segments with the most severe stenosis using the area quantification module on the HALO image analysis platform (Indica Labs, Corrales, NM) and reported as % positive cells/area mm2.
Immunofluorescent staining (IF) of CD4, CX3CR1 and DAPI (Invitrogen, Thermo Fisher Scientific, catalog D3571) was performed on serial FFPE sections from an HIV-positive and HIV-negative donor. The sections were incubated with Dako protein block for 10 minutes to reduce non-specific binding. They were then incubated with primary antibodies against anti-CD4 (DAKO) and anti-CX3CR1 (Abcam) overnight. Secondary antibody detection was done using anti-rabbit Alexa Fluor 555 and anti-mouse Alexa Fluor 488 antibodies (Thermo Fisher Scientific), both 1:150 dilution) for 1h. DAPI was used as a nuclear counterstain. Negative controls were stained with the secondary antibody alone to detect autofluorescence. Laser scanning confocal microscopy was performed using a Nikon A1 laser-scanning confocal microscope equipped with a 1.25 NA 25x silicone oil immersion lens and 1.4 NA 60x oil immersion lens. Images for comparison were prepared with equal treatment, acquired with identical parameters (e.g. pinhole diameter, detector gain), and processed in an identical manner. Images were contrast-enhanced and cropped using ImageJ software (National Institutes of Health).
Analysis of T cell receptor specificity
Single cell suspensions of aorta were enriched for CD3+ T cells by flow cytometry (BD FACS ARIA™ III) and processed for single-cell sequencing using the Chromium single cell kit (10x genomics) as published41. V(D)J feature barcodes allowed us to identify TCRαβ pairs. For the analysis, CellRanger (version 3.0.0) was used to demultiplex the raw sequencing data and extract filter and correct barcodes and unique molecular identifiers (UMIs), remove cDNA PCR duplicates and align reads to the human transcriptome (GRCh38) using STAR. TCR clonal analysis was performed using the Loupe VDJ browser.
Statistical Analyses
We calculated medians (with interquartile ranges) of continuous variables and percentages of categorical variables. Continuous variables with a skewed distribution were square root transformed. Clinical and demographic differences were compared using Mann-Whitney U test for continuous variables and chi-square tests for categorical variables. Univariable and multivariable linear regression analyses adjusted for age, sex, BMI, hypertension, smoking status and fasting low-density lipoprotein (LDL) were used to assess the relationship between CD4+ T cell subsets, CMV IgG and the presence and number of carotid plaques, CIMT, and brachial-artery FMD. Statistical analysis was performed using R version 3.6.1 (http://www.R-project.org), and GraphPad Prism version 7.0 and 8.0 (GraphPad Software, San Diego CA). Figure 6 and graphical abstract were created with BioRender.
Figure 6. Theoretical model illustrating the role of CMV-specific CX3CR1+ GPR56+ CD57+ (C~G~C) T cells in promoting adipose tissue inflammation and CVD.
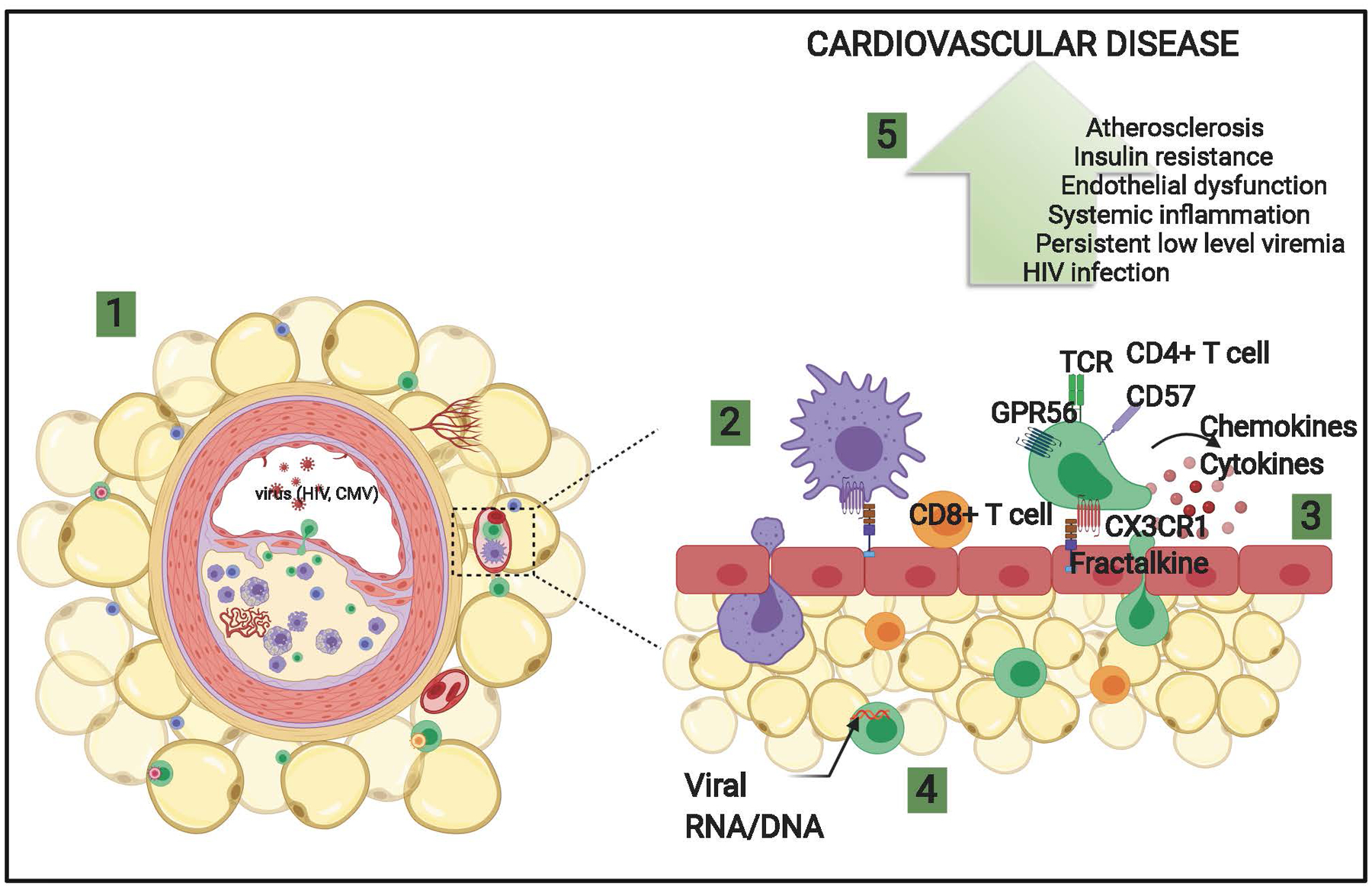
1) Schematic of coronary plaque and perivascular adipose tissue in HIV-positive persons. 2) Peripheral leukocytes expressing CX3CR1 bind to fractalkine/CX3CL1 on endothelial cells and migrate to the perivascular adipose tissue. 3) Inflammatory cytokines and chemokines are expressed by immune cells recruited coronary vessels and perivascular adipose tissue, including virus-specific T cells that disrupt endothelial function and may contribute to plaque deposition in large arteries. 4) Clonal CD4+ (green) and CD8+ memory T cells (orange) predominate in perivascular adipose tissue of HIV-positive persons and are proinflammatory. 5) Increased inflammation, vascular dysfunction, and atherosclerosis contributes to cardiovascular disease.
Results
Cohort clinical demographics
Of the 70 HIV-positive persons recruited to the study, 30 were female (Table IV, Data Supplement). The median age of female participants was higher than males (46 vs. 44 years, p=0.02), and female participants were more likely to be non-white (73% vs. 40%, p=0.006). The median duration of ART therapy was shorter among males compared to females (5.1 vs. 8.4 years, p=0.001). Fasting laboratory values were similar by sex except for greater high sensitivity C-reactive protein (hsCRP) (5.5 vs. 1.7 mg/dL, p=0.002) and high-density lipoprotein (HDL) (49.5 vs. 40.5 mg/dL, p<0.001) in females. A sub-analysis revealed that the higher hsCRP in females was primarily driven by the high BMI participants (Figure IA, Data Supplement). There was no sex difference observed for hsCRP in the HIV-negative participants (Figure IB, Data Supplement). The median CMV IgG levels and proportion with hepatitis C virus seropositivity did not differ between females and males. Similarly, there were no sex differences in the FMD, carotid plaque and CIMT measurements (Table I, Data Supplement).
The 30 obese, HIV-negative participants did not differ based on sex or BMI from the obese HIV-positive participants but were significantly younger (median 37 vs. 46 yrs., p=0.005) and had lower CMV IgG levels (0.8 vs. 1.4 ab index, p<0.001) (Table V, Data Supplement). Due to the smaller sample size of the HIV-negative cohort, we compared differences in immune subsets by HIV status, including a subset of participants matched by age, but the HIV-negative study arm was underpowered for the adjusted regression models performed in the HIV-positive group.
HIV-positive persons have significantly higher proportions of circulating C~G~C+ CD4+ T cells compared to HIV-negative persons
Given the frequent co-occurrence of cardiovascular and metabolic abnormalities in HIV-positive persons, and our recent description of greater circulating C~G~C+ CD4+ T cells in HIV-positive diabetic individuals, we hypothesized that C~G~C+ CD4+ T cells might contribute to the development of vascular disease. Sample flow cytometry plots and gating strategies from PBMCs stained with the 12-antibody panel are shown in Figure 1A. To visualize the multidimensional data, we used dimensional reductional algorithms. We again observed the C~G~C markers cluster in a distinct population of CD4+ T cells using algorithms on visualization of t-distributed stochastic neighbor embedding (viSNE) plots (Figure II, Data Supplement). Furthermore, there were significantly more C~G~C+ CD4+ T cells in the HIV-positive compared to the HIV-negative participants (median 3.4 vs. 1.3%, p<0.01) (Figure 1B–D). This remained statistically significant (p < 0.01) even after removal of the highest percentage outlier from each group in figure 1D (data not shown). The difference in C~G~C+ CD4+ T cells did not appear to be due to differences in BMI (Figure 1E). We also compared C~G~C+ CD4+ T cell proportions between obese individuals by HIV status, and these cells remained higher in the HIV-positive group (Figure 1F). In all the samples analyzed regardless of HIV-status, CD57+ CD4+ and C~G~C+ CD4+ T cells were positively correlated with age, while CD4+ naïve cells were negatively correlated with age (Figure 1G).
Figure 1. HIV-positive persons have a greater proportion of circulating CX3CR1+ GPR56+ CD57+ (C~G~C)+ CD4+ T cells.
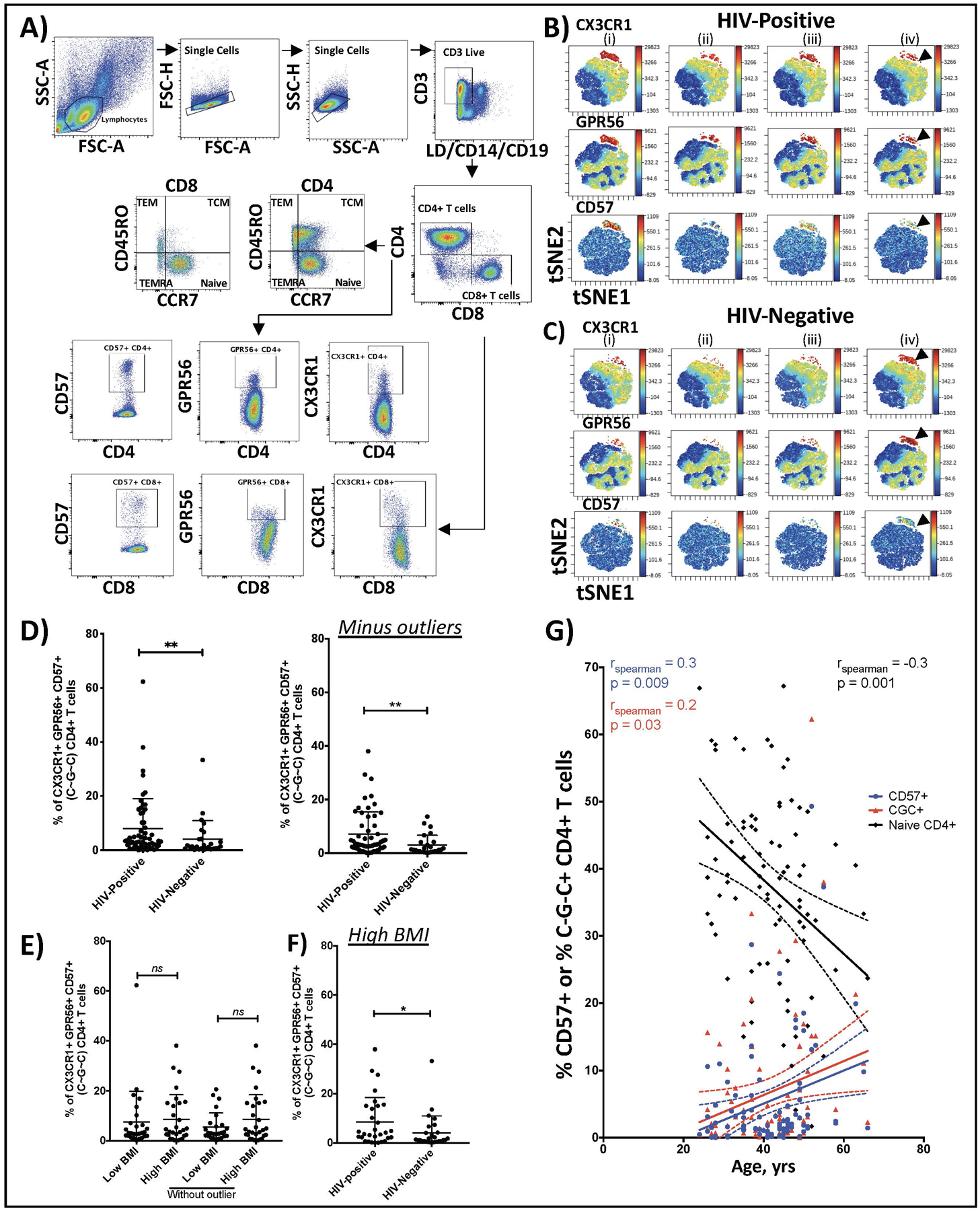
Representative flow cytometry plots of PBMCs showing gating of live CD3+, CD4+ and CD8+ T cells. CCR7 and CD45RO expression on CD4+ T cells was used to delineate CD4 naïve (CCR7hi CD45ROlo), TCM (CCR7hi CD45ROhi), TEM (CCR7lo CD45ROhi) and TEMRA (CCR7lo CD45ROlo) cells (A). Representative viSNE plots of PBMCs with arrows showing CX3CR1+ GPR56+ CD57+ (C~G~C)+ CD4+ T cells in 4 HIV-positive (B) and 4 HIV-negative persons (C). Plot shows the distribution of C~G~C+ CD4+ T cells as a proportion of total CD4+ T cells in HIV-negative (n=27) and HIV-positive persons (n=57) (D). Percentage of C~G~C+ CD4+ T cells in HIV-positive participants by BMI (low BMI < 30kg/m2 and high BMI >30kg/m2) (E). Comparison of percentage of C~G~C+ CD4+ T cells in obese HIV-positive and negative participants (F). Correlation plot of age (years) and percentage CD57+ CD4+, C~G~C+ CD4+ and naïve CD4+ T cells (G). Mann-Whitney test comparison, * p<0.05, ** p<0.01; Spearman rank test, p<0.05 significant.
Given the difference in age between HIV-negative and positive participants, we performed a sensitivity analysis with the HIV-positive participants in the lower half of the age distribution. The median age of this subgroup was 42.5 years (IQR 34.3, 48.3) in HIV-positive and 37.0 years (IQR 29.5, 44.0) in HIV-negative (p=0.11). The median C~G~C+ CD4+ T cell cells were 5.2%(IQR 1.7, 15.2) in HIV-positive and 1.3%(IQR 0.7, 4.2) in HIV-negative (p=0.04), indicating the difference observed with the full HIV-positive cohort was not confounded by age. We also dichotomized the HIV-positive participants based on the percentage of C~G~C+ CD4+ T cells (we categorized using the median as >3.4% [high] versus <3.4% [low]). The HIV-positive participants with high C~G~C+ CD4+ T cells had more carotid plaque burden (47.6% vs. 14.3%, p = 0.046), shorter duration of ART (5.2 vs. 7.3 years, p=0.04), lower CD4-to-CD8 ratio (0.9 vs. 1.1, p=0.03) and higher CMV IgG levels (1.7 vs. 1.4, p=0.007). Notably, there was no significant difference in age, sex, BMI, or smoking status between these groups.
CMV-specific CD4+ T cells predominantly express the C~G~C surface marker combination in HIV-positive persons
We used the DYS tetramer to identify CD4+ T cells with antigen receptors against the CMV DYSNTHSTRYV epitope in the ten participants with HLA-DR7 (Table VI, Data Supplement). Three HLA-mismatched participants (Non-HLA-DR7) were included as controls to show the specificity of the DYS tetramers. We verified DYS-tetramer sensitivity and specificity by staining with DR7:CLIP as a negative control in HLA-DR7-positive and -negative participants (Figure III, Data Supplement). A median of 14.4% (interquartile range (IQR), 4.7–32.7%) of C~G~C+ CD4+ T cells from HIV-positive participants in this study expressed antigen receptors that recognized this single epitope from the glycoprotein B of CMV. The DYS+ CD4+ T cells were mainly TEM and TEMRA cells (Figure 2A–B) and dimensional reduction analysis showed that the DYS tetramer-positive cells were predominantly C~G~C+ CD4+ cells (Figure 2C). It is noteworthy that CX3CR1 and GPR56 formed a tight group whereas CD57 was split, with high and low expression. Two-dimensional flow cytometry analysis showed that a median of 60.5% (IQR 21.9, 96.3%) of DYS-positive cells were CD4+ GPR56+ CX3CR1+, whereas only 2.6% (IQR 0.8, 6.9%) of cells in the DYS-negative gate were GPR56+ CX3CR1+ (Figure IV, Data Supplement). Two dimensional plots of CD3+ T cells confirmed that DYS tetramer stained CD4+ T cells with minimal background on CD8+ T cells (Figure 2D). Similarly, viSNE plots generated including CD4+ and CD8+ T cells showed that DYS tetramer specifically bound C~G~C+ CD4+ T cells (Figure 2E).
Figure 2. CMV-specific CD4+ T cells predominantly express the C~G~C surface marker combination.
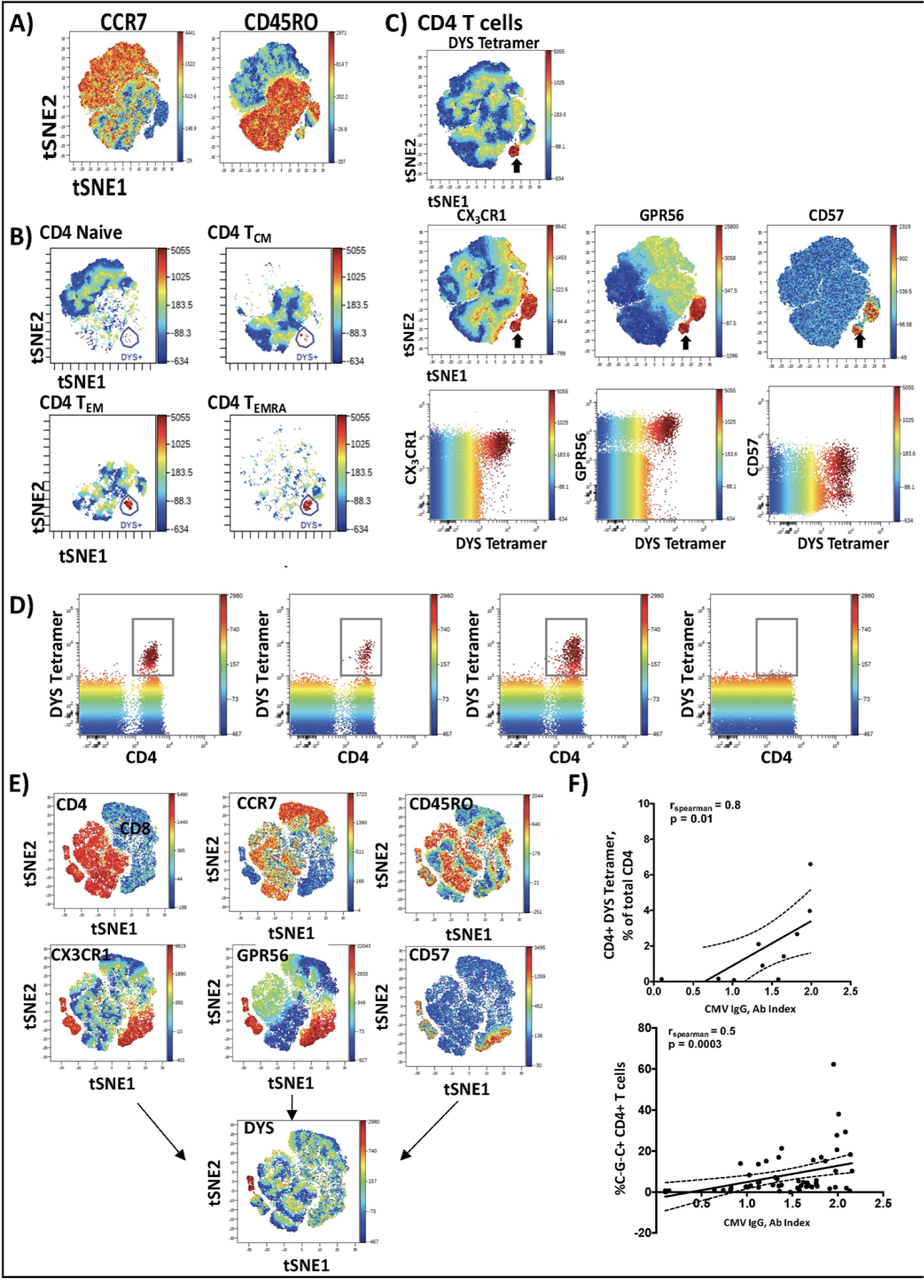
PBMCs from 10 individuals with HLA-DR7 were stained with fluorescently tagged antibodies against surface marker proteins and the DYS tetramer. viSNE plots (n=10) were generated in Cytobank software and concatenated using flowjo software (A). DYS tetramer-positive CD4+ T cells were mainly TEM and TEMRA cells (B). DYS tetramer-positive cells (C, top panel) express CX3CR1, GPR56 and CD57 (i.e., ‘C~G~C cells’; C, middle panel). The Blue-Green-Yellow-Red gradient is the mean fluorescence intensity of DYS tetramer, with the surface markers on the right (C, middle panel). Two dimensional Flowjo plots showing CX3CR1and GPR56 expression on tetramer-positive and tetramer-negative CD4+ T cells (C, lower panel). DYS tetramer gating on total CD3+ T cells shows that it specifically binds to CD4+ T cells with no binding of CD8 T cells (D). Representative viSNE plot (n=1) of CD3+ T cells confirms that bright DYS tetramer+ cells bind CD4+ T cells and not CD8+ T cells (E). Correlation between CD4+ DYS tetramer-positive (n=10) and CD4+ C~G~C+ T cells with CMV IgG titers (n=57) (F). Correlations assessed by Spearman’s test, p<0.05 significant.
CMV-specific CD4+ T cells and C~G~C+ CD4+ T cells are correlated with CMV IgG levels in persons with HIV
Serum CMV IgG was positively correlated with both C~G~C+ CD4+ cells (rho=0.5, p=0.0003) and the DYS tetramer-positive subset (rho=0.8, p=0.01) (Figure 2F). None of the ten HLA-DR7+ individuals had an active infection or subclinical CMV viremia, based on negative CMV quantitation by ddPCR of PBMCs (Figure V, Data Supplement). CMV IgG titer remained significantly associated with C~G~C+ CD4+ T cells after adjustment in a multivariable model (β=2.3, p=0.001) (Table VII, Data Supplement).
A higher percentage of C~G~C+ CD4+ T cells is associated with subclinical atherosclerosis in HIV-positive persons
Higher proportions of CD4+ T cells co-expressing the C~G~C marker combination, and the individual component markers CX3CR1, GPR56, and CD57, were associated with greater carotid plaque burden (p<0.05 for all) after adjusting for age, sex, BMI, fasting LDL, hypertension and smoking status (Figure 3A). A similar association was observed for CD4+ TEM cells, a memory phenotype that expands with age and can include C~G~C+ CD4+ T cells, and therefore a second analysis model excluded age as a potentially collinear covariate. Removal of age from the model strengthened the association of CD4+ T cells expressing the C~G~C marker combination, and the individual markers, with carotid plaque burden (p<0.01 for all) (Figure 3B). Notably, a comparison of C~G~C+ CD4+ T cells in HIV-positive persons with and without plaque (as a categorical variable) confirmed that those with carotid plaque had significantly higher C~G~C+ CD4+ T cells (mean 14.4% vs 5.2%, p=0.003) (Figure 3C). This finding remained significant after adjusting for sex, age, BMI, fasting LDL, hypertension, and smoking status in a logistic regression (p=0.03), which again strengthened after age was removed from the model (p<0.01; data not shown). Note that this remained significant after removal of the highest percentage outlier (C~G~C+ CD4+ T cells > 60, p=0.01, data not shown). Results were similar when race was added to the model. We did not observe an association between C~G~C+ CD4+ T cells and CIMT.
Figure 3. Carotid plaques are associated with C~G~C+ CD4+ T cells in HIV-positive persons.
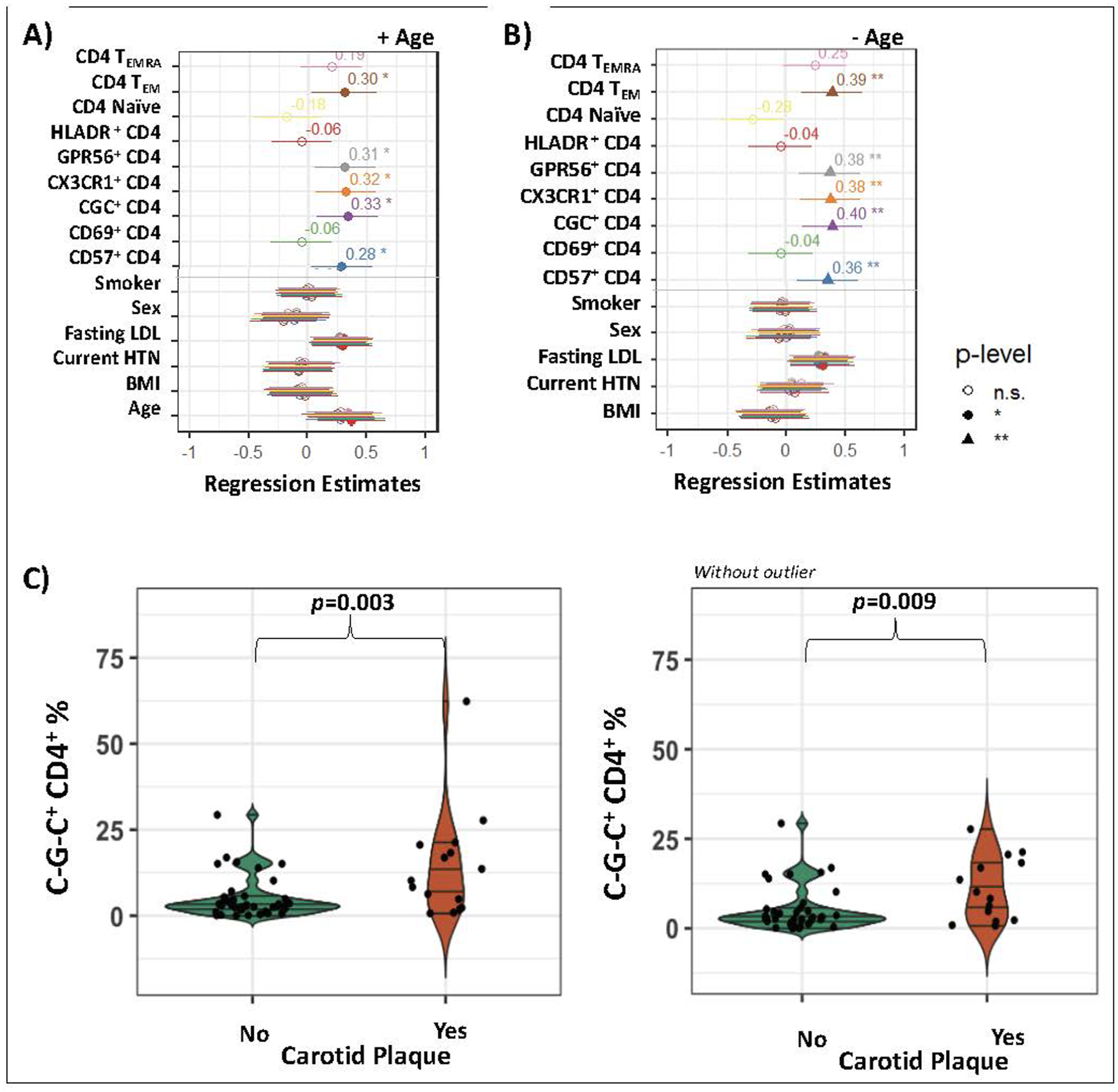
Relationships between CD4+ subsets (CD4 TEMRA, CD4 TEM, CD4 naïve, and HLADR+ CD4+, CD69+ CD4+, CD57+ CD4+, CX3CR1+ CD4+, GPR56+ CD4+ and C~G~C+ CD4+ T cells) and carotid plaque number after adjustment for sex, age, body mass (BMI) index, fasting low density lipoprotein (LDL), hypertension (HTN) and smoking status (A), and after removal of age from the model (B). The proportion of C~G~C+ CD4+ T cells was significantly higher in those with plaque (C). Linear regression, * p<0.05 ** p<0.01. T-test, p<0.05 considered significant.
Brachial artery flow mediated dilation is not associated with circulating C~G~C+ CD4+ T cells in HIV-positive persons
The median baseline brachial artery diameter for HIV-positive males was 0.39 cm (IQR: 0.35, 0.43) and for females was 0.31 cm (IQR: 0.29, 0.33; p<0.0001) (Table I, Data Supplement). There was no association between C~G~C+ CD4+ T cells and artery baseline diameter (data not shown) or FMD at 60 seconds after adjusting for sex, age, BMI, hypertension, LDL and smoking status (β=0.3, p=0.2) (Table VII, Data Supplement). A similar model also adjusted for race was not significant (data not shown).
CX3CR1+ CD4+ T cells are present within atheroma of coronary plaques from HIV-positive and HIV-negative persons
We previously showed that the peripheral blood and subcutaneous adipose tissue from HIV-positive diabetic individuals had higher percentages of C~G~C+ CD4+ T cells, suggesting that these cells can infiltrate tissue24. We hypothesized C~G~C+ CD4+ T cells may also infiltrate vascular structures and atherosclerotic plaques. We assessed the presence of CX3CR1+ CD4+ T cells (as a surrogate for C~G~C+ CD4+ T cells) in atheroma and vascular structures of postmortem coronary plaque samples from HIV-positive and HIV-negative adults who had sudden death (Table II, Data Supplement). We selected vessels with atherosclerotic plaques defined as early and late fibroatheromas. The coronary plaques were classified using a modified scheme based on the American Heart Association criteria as published40, 42 Serial sections were stained with hematoxylin/eosin and Movat stains. Immune cell markers including anti-CD3, CD4, and CX3CR1 were used to stain coronary plaque sections (Figure 4A–B). We quantified cells in early atheroma of HIV-negative (n=8) versus HIV-positive (n=4) donors: CD3 (median 0.31 vs. 0.53 staining/mm2, p=0.28), CD4 (0.04 vs. 0.35 staining/mm2, p=0.03) and CX3CR1 (0.49 vs. 1.18 staining/mm2, p=0.07) (Figure 4C). Similar quantification in late atheroma of HIV-negative (n=4) versus HIV-positive (n=7) showed: CD3 (median 0.39 vs. 0.34 staining/mm2, p=0.79), CD4 (0.38 vs. 0.05 staining/mm2, p=0.04) and CX3CR1 (0.93 vs. 0.62 staining/mm2, p=0.26). Histology of coronary plaques with immunofluorescent stains showed that CX3CR1+ CD4+ cells are present within the coronary plaques of both HIV-positive and negative persons (Figure 5A–D). Isotype controls were included as controls (Figure VI, Data Supplement). Due to our inability to match the cause of death categories between the HIV-positive and negative donors in this experiment, we did not compare the frequency of these cells by HIV-status. Notably our study supports a previous study in HIV-positive persons which found CX3CR1+ CD4+ T cells in the adventitia adjacent to coronary arteries of early but not late atheromas43.
Figure 4. Immunohistochemical stain showing CX3CR1+ and CD4+ T cells within the coronary plaques of HIV-positive and HIV-negative persons.
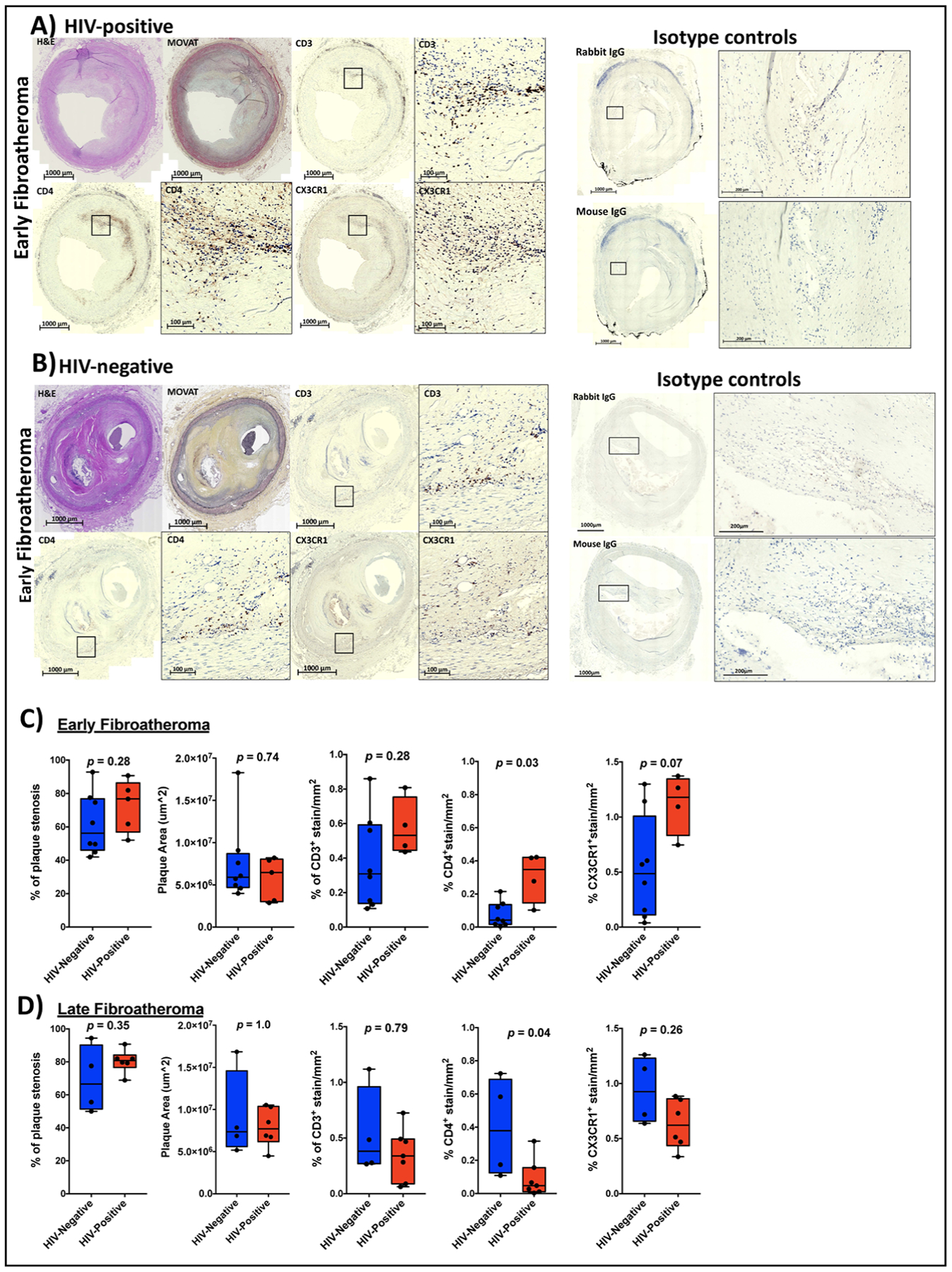
Representative early atheroma images from one HIV-positive (A) and HIV-negative coronary plaque (B) stained with H&E stain, Movat stain, CD3, CD4 and CX3CR1. Box plots showing % stenosis, plaque area, % of CD3+, CD4+ and CX3CR1+ staining per mm2 coronary plaque area by HIV status in early atheroma (n=8 in HIV negative and n=5 HIV-positive, CD3+ stains on fewer samples; n=4 in HIV negative and n=6 HIV-positive) (C) and late atheroma (n=8 in HIV negative and n=5 HIV-positive) (D). Statistical analysis, Mann-Whitney test; p<0.05 was considered significant.
Figure 5. CX3CR1+ GPR56+ CD57+ CD4+ T cells are present in coronary arteries and aorta.
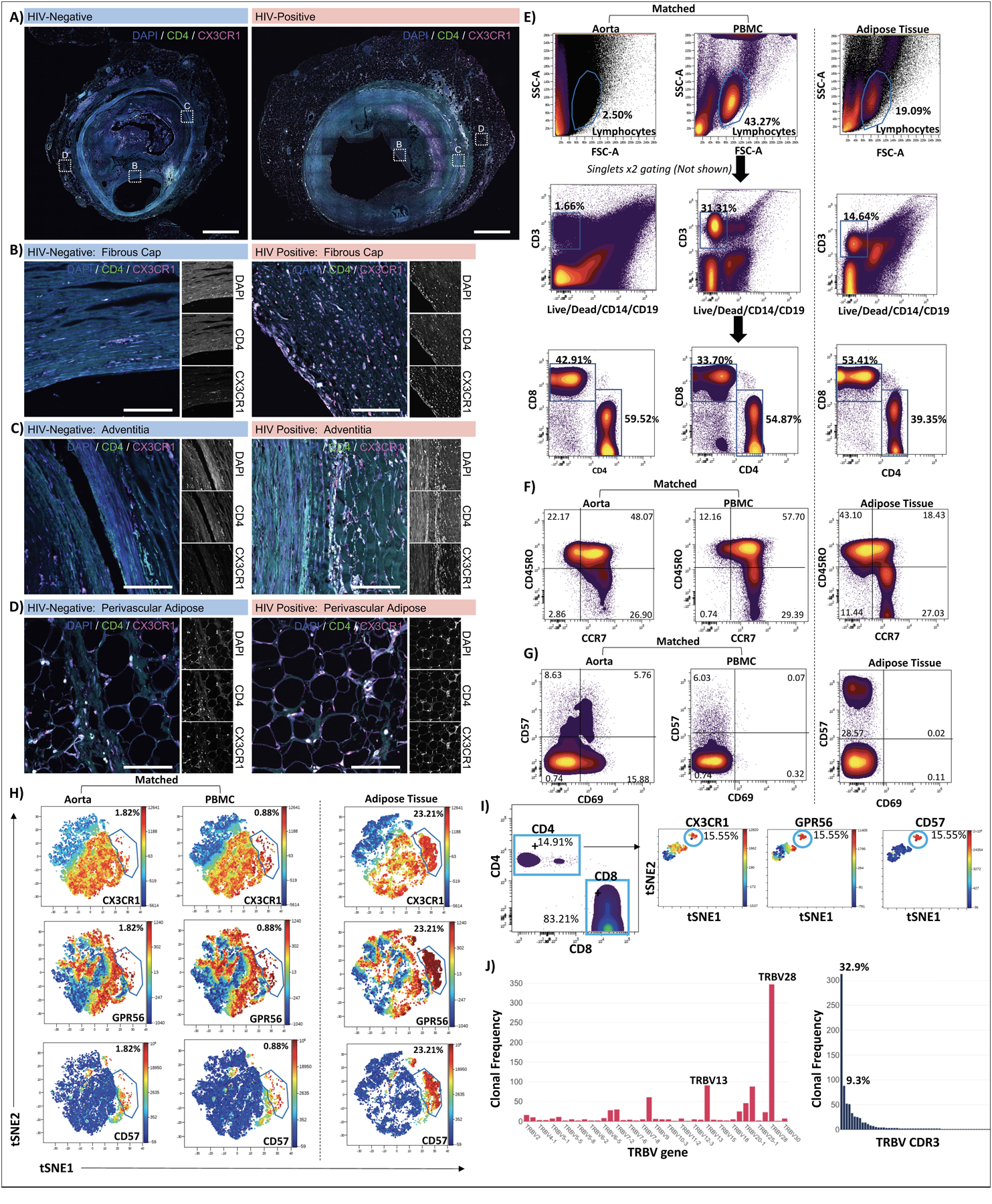
Low magnification confocal images of whole coronary plaques from HIV-negative (left) and HIV-positive (right) persons labeled with DAPI (blue), anti-CD4 (green), and anti-CX3CR1 (magenta), Scale bars 1000 μm (A). Enlarged images of the intima/ fibrous cap (B), adventitia (C) and perivascular adipose (D), scale bars 500 μm. Single cells isolated from the aorta of an HIV-negative individual were stained with the 12-panel antibodies including CX3CR1, GPR56 and CD57. PBMCs from the same person and subcutaneous adipose tissue from an HIV-positive participant as a positive control for tissue C-G-C+ CD4+ T cells were stained simultaneously. Lymphocytes, single cells, CD3+ live, CD4+ and CD8+ T cells were gated as shown (E). T cell memory populations were gated based on CCR7 and CD45RO co-expression (F). Two dimensional plots show expression of CD57 and CD69 (G). viSNE plots show C-G-C+ CD4+ T cells from aorta and PBMCs of HIV-negative donor and HIV-positive subcutaneous adipose tissue (H). C-G-C+ CD4+ T cells identified in a second HIV-negative aorta (I). Out of 949 CD3+ T cells sequenced from cells isolated from the aorta of donor 2; 240 clones were identified. The TCR β V gene and CDR3 clonal frequencies are shown (J).
C-G-C+ CD4+ T cells in aorta samples
Due to the limitation of pre-fixed samples, we obtained aorta from two HIV-negative donors with coronary artery disease undergoing surgery (71 year-old-female and 62 year-old-male, unknown CMV status). Matched PBMCs were available from one donor. We isolated cells from the aorta and stained them with the 12-antibody panel as before. Subcutaneous adipose tissue from an HIV-positive donor was included as a positive control for the C-G-C+ CD4+ cell population (Figure 5E). We found that CD4+ T cells isolated from the aorta had higher proportions of TEM (CCR7lo CD45ROhi) and TEMRA (CCR7lo CD45ROlo) as compared to the matched PBMC sample (n=1, Figure 5F). Two dimensional plots show expression of CD57 and CD69 on these cells (Figure 5G). ViSNE plots confirm the presence of C~G~C cells in these samples (Figure 5H–I). TCR sequencing revealed the presence of clonal T cell populations with up to 313 cells out of 949 (32.9%) from a single clone (Figure 5J).
Discussion
HIV-positive adults have an approximately 2-fold higher risk of developing CVD compared to HIV-negative individuals, which is not explained by traditional cardiovascular risk factors and may be related to persistent, heightened innate and adaptive immune activation. In HIV-positive persons, CMV co-infection is highly prevalent and, unlike other herpes viruses, drives a large expansion of anti-CMV T cells and markedly increases chronic inflammation. As such, the inflation of CMV-specific memory T cells in HIV infection provides an ideal ‘experiment of nature’ to assess relationships between CMV-specific CD4+ T cells and CVD, which further studies may show are also present in the general population. Our group recently demonstrated that C~G~C+ CD4+ T cells comprise a distinct population increased in the blood and adipose tissue of HIV-positive persons with metabolic disease24. Here, we demonstrate that C~G~C+ CD4+ T cells can be CMV specific, are increased in the blood of HIV-positive persons with carotid plaque and are increased within plaques from HIV-positive compared to HIV-negative persons. This is noteworthy because CMV seropositivity in aging adults is related to increased deaths due to vascular disease44, and higher CMV antibody titers have been associated with carotid intima thickening in HIV-negative persons45, 46.
In HIV-positive persons, CMV-specific CD8+ T cells and CX3CR1+ CD4+ T cells have been associated with subclinical atherosclerosis43, 47. Our results build upon these findings by showing these CX3CR1+ cells also express GPR56, a marker that has been linked to a higher degree of cytotoxic function25. CX3CR1 is a chemokine receptor that binds fractalkine (CX3CL1), which is expressed on endothelial cells and facilitates extravasation of immune cells into tissues43. Our theoretical model, developed from our current findings and prior studies24, posits that C~G~C+ CD4+ T cells infiltrate coronary plaques, vascular structures, and peri-vascular adipose tissue, and promote recruitment of macrophages and additional immune cells, local inflammation, and tissue remodeling (Figure 6).
The previously reported role of adipose tissue as an important reservoir of HIV48 and CMV49 supports the possibility of active anti-viral immune responses within tissue compartments. Here, we show that C~G~C+ CD4+ T cells are frequently CMV-specific using a tetramer against a single, well-characterized CMV epitope, DYSNTHSTRYV from glycoprotein B. Defining these cells as CMV-specific using tetramer is more informative than ex vivo assays which stimulate cells and measure cytokine expression, as the surface phenotype of the target cells can be simultaneously characterized. While demonstrating the presence of frequently CMV-specific T cells at sites of vascular plaque formation implicates these cells in tissue inflammation and damage, future studies are needed to determine whether these cells are responding to the presence of viable CMV in situ versus migrating from the blood in a stochastic manner. If the former is correct, this finding would establish a physiologic basis for studies to determine if anti-CMV treatments could reduce the number or cytotoxic activity of anti-viral T cells in tissue and potentially reduce inflammation and structural changes.
Our study has several strengths. Our cohort of HIV-positive participants was receiving a single ART regimen and had maintained long-term plasma viral suppression. Furthermore, participants did not have advanced immunosuppression, and no diabetes nor prior cardiovascular events. The use of tetramer allowed the definitive identification of CMV-specific T cell receptors on C~G~C+ CD4+ T cells. Another strength was the use of immunohistochemical and immunofluorescence stains to visualize CX3CR1+ CD4+ T cells within coronary plaques and perivascular adipose tissue of HIV-positive and HIV-negative donors, and the isolation of immune cells from aorta samples. Limitations of this study include the cross-sectional design, which precludes assessments of causality, and a moderate cohort size of 70 HIV-positive persons. Within the HIV-positive group, females and males were not well matched and therefore sex-based comparisons were not performed. The HIV-negative control group was small, which allowed for the comparison of circulating CD4+ T cell subsets with the HIV-positive participants but precluded the use of adjusted regression models to determine the association of CGC+ CD4+ T cells and carotid plaque burden in the HIV-negative participants. Notably, the HIV-negative participants were also younger than the HIV-positive participants, therefore the effect of age on differences in immune cells by HIV-status could not be directly interpreted. For the flow cytometry data, a lack of CD56 in the antibody panel did not allow us to discriminate between T cells, NK cells and NK T cells. The coronary plaque samples from deceased donors allowed for characterization of immune markers within plaques of HIV-negative and HIV-positive persons. For the immunofluorescence stains, we did not include CD3 antibody stain and therefore cannot distinguish T CD4+ T cells from NK cells. However, due to differences in the cause of sudden death we did not directly compare by HIV status. Aorta samples allowed us to evaluate immune cells from this tissue, however there is a possibility of blood contamination in the aorta sample.
Conclusions
Therapeutic attempts to mitigate the progression of subclinical CVD have employed immune modulators in clinical trials50, 51. However, we still do not have a therapeutic approach that mitigates the progression of atherosclerotic CVD without increasing infection, which highlights the need for a better understanding of the immune mechanisms driving CVD progression. While several studies support the role of CMV in cardiovascular disease, these data are primarily from HIV-negative persons and results are conflicting. The increase in circulating CMV-specific memory T cells in HIV-positive persons provides an ideal ‘experiment of nature’ to identify relationships between CMV-specific CD4+ T cells and CVD endpoints, which can be further explored in the general population. Here we show that CMV-specific CD4+ T cells against the gB protein are C-G-C+ and associated with subclinical atherosclerosis in HIV-positive persons. Future studies will investigate the epitopes and immune function of plaque-resident cells to determine whether they function as cytotoxic CD4+ T cells, helper cells that influence CD8+ T cell function, or have another unique role.
Supplementary Material
Sources of Funding:
This work was funded by NIH grants K23 100700 (JK), K12 HL143956 (JK and CW), R01 DK112262 (JK and CW), HL131977 (JB), R56 DK108352 (JK), DK095811 (MJT), DK111949 (MJT), the Vanderbilt Clinical and Translational Science award from NCRR/NIH grant UL1 RR024975, the Vanderbilt Infection Pathogenesis and Epidemiology Research Training Program (VIPER) grant T32 AI007474, CTSA award no. KL2 TR002245 from the National Center for Advancing Translational Sciences, and the Tennessee Center for AIDS Research grant P30 AI110527. The funding authorities had no role in study design; data collection, analysis, or interpretation; decision to publish; or preparation of the manuscript.
Abbreviations and Acronyms
- ART
Antiretroviral therapy
- C~G~C
CX3CR1+ GPR56+ CD57+
- CD4
Cluster of differentiation 4
- CD57
Cluster of differentiation 57
- CD8
Cluster of differentiation 8
- CIMT
Carotid intima-media thickness
- CMV
Cytomegalovirus
- CT
Computed tomography
- CVD
Cardiovascular disease
- CX3CR1
C-X3-C Motif Chemokine Receptor 1
- DAPI
4′,6-diamidino-2-phenylindole
- DNA
Deoxyribonucleic acid
- DYS
DYSNTHSTRYV
- ECG
Electrocardiogram
- ELISA
Enzyme-linked immunosorbent assay
- FFPE
Formalin fixed paraffin embedded
- FMD
Brachial artery flow-mediated dilation
- gB
Glycoprotein B
- GPR56
G protein-coupled receptor 56
- HIV
Human immunodeficiency virus
- HRP
Horseradish Peroxidase
- HsCRP
High sensitivity C-reactive protein
- ICAM1
intercellular adhesion molecule 1
- IHC
Immunohistochemical
- IMT
Intima-media thickness
- KLRB1
Killer cell lectin like receptor B1
- KLRG1
Killer cell lectin like receptor G1
- MHC
Major histocompatibility complex
- RNA
Ribonuclease A
- TEM
T effector memory
- TEMRA
T effector memory CD45RA+ cells
Footnotes
Disclosure: Dr. Koethe has served as a consultant to Merck & Co., Gilead Sciences, ViiV Healthcare, and Theratechnologies, and received research support from Merck & Co. and Gilead Sciences. The authors have no other competing interests or financial disclosures.
REFERENCES
- 1.Simmonds J, Fenton M, Dewar C, Ellins E, Storry C, Cubitt D, Deanfield J, Klein N, Halcox J, Burch M. Endothelial dysfunction and cytomegalovirus replication in pediatric heart transplantation. Circulation. 2008;117:2657–2661 [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in hiv-infected and non-hiv-infected patients in a us health care system. J Acquir Immune Defic Syndr. 2012;60:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between hiv infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using québec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–253 [DOI] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. Hiv infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So-Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez-Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between hiv infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: Results from the veterans aging cohort study. JAMA Cardiol. 2017;2:536–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, Budoff M, Butt AA, Gibert C, Goetz MB, Gottdiener J, Gottlieb S, Justice AC, Leaf D, McGinnis K, Rimland D, Rodriguez-Barradas MC, Sico J, Skanderson M, Tindle H, Tracy RP, Warner A, Freiberg MS. Hiv infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris SR, Zhao M, Smith DM, Vargas MV, Little SJ, Gianella S. Longitudinal viral dynamics in semen during early hiv infection. Clin Infect Dis. 2017;64:428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abana CO, Pilkinton MA, Gaudieri S, Chopra A, McDonnell WJ, Wanjalla C, Barnett L, Gangula R, Hager C, Jung DK, Engelhardt BG, Jagasia MH, Klenerman P, Phillips EJ, Koelle DM, Kalams SA, Mallal SA. Cytomegalovirus (cmv) epitope-specific cd4(+) t cells are inflated in hiv(+) cmv(+) subjects. J Immunol. 2017;199:3187–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komanduri KV, Donahoe SM, Moretto WJ, Schmidt DK, Gillespie G, Ogg GS, Roederer M, Nixon DF, McCune JM. Direct measurement of cd4+ and cd8+ t-cell responses to cmv in hiv-1-infected subjects. Virology. 2001;279:459–470 [DOI] [PubMed] [Google Scholar]
- 11.Lichtner M, Cicconi P, Vita S, Cozzi-Lepri A, Galli M, Lo Caputo S, Saracino A, De Luca A, Moioli M, Maggiolo F, Marchetti G, Vullo V, d’Arminio Monforte A, Study IF. Cytomegalovirus coinfection is associated with an increased risk of severe non-aids-defining events in a large cohort of hiv-infected patients. J Infect Dis. 2015;211:178–186 [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Peng G, Bai J, He B, Huang K, Hu X, Liu D. Cytomegalovirus infection and relative risk of cardiovascular disease (ischemic heart disease, stroke, and cardiovascular death): A meta-analysis of prospective studies up to 2016. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566 [PubMed] [Google Scholar]
- 14.Petrakopoulou P, Kübrich M, Pehlivanli S, Meiser B, Reichart B, von Scheidt W, Weis M. Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation. 2004;110:II207–212 [DOI] [PubMed] [Google Scholar]
- 15.Stone SF, Price P, Khan N, Moss PA, French MA. Hiv patients on antiretroviral therapy have high frequencies of cd8 t cells specific for immediate early protein-1 of cytomegalovirus. AIDS. 2005;19:555–562 [DOI] [PubMed] [Google Scholar]
- 16.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG. Cytomegalovirus-specific t cells persist at very high levels during long-term antiretroviral treatment of hiv disease. PLoS One. 2010;5:e8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harari A, Rizzardi GP, Ellefsen K, Ciuffreda D, Champagne P, Bart PA, Kaufmann D, Telenti A, Sahli R, Tambussi G, Kaiser L, Lazzarin A, Perrin L, Pantaleo G. Analysis of hiv-1- and cmv-specific memory cd4 t-cell responses during primary and chronic infection. Blood. 2002;100:1381–1387 [DOI] [PubMed] [Google Scholar]
- 18.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, Nasir K, Grinspoon SK. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in hiv-infected men. AIDS. 2010;24:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha A, Ma Y, Scherzer R, Hur S, Li D, Ganz P, Deeks SG, Hsue PY. Role of t-cell dysfunction, inflammation, and coagulation in microvascular disease in hiv. J Am Heart Assoc. 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parrinello CM, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt PW, Deeks SG, Hodis HN, Kaplan RC. Cytomegalovirus immunoglobulin g antibody is associated with subclinical carotid artery disease among hiv-infected women. J Infect Dis. 2012;205:1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pachnio A, Ciaurriz M, Begum J, Lal N, Zuo J, Beggs A, Moss P. Cytomegalovirus infection leads to development of high frequencies of cytotoxic virus-specific cd4+ t cells targeted to vascular endothelium. PLoS Pathog. 2016;12:e1005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362 [DOI] [PubMed] [Google Scholar]
- 23.Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649 [DOI] [PubMed] [Google Scholar]
- 24.Wanjalla CN, McDonnell WJ, Barnett L, Simmons JD, Furch BD, Lima MC, Woodward BO, Fan R, Fei Y, Baker PG, Ram R, Pilkinton MA, Mashayekhi M, Brown NJ, Mallal SA, Kalams SA, Koethe JR. Adipose tissue in persons with hiv is enriched for cd4. Front Immunol. 2019;10:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, Rosales SL, Fu Z, Picarda G, Burel J, Zapardiel-Gonzalo J, Tennekoon RN, De Silva AD, Premawansa S, Premawansa G, Wijewickrama A, Greenbaum JA, Vijayanand P, Weiskopf D, Sette A, Peters B. Unique phenotypes and clonal expansions of human cd4 effector memory t cells re-expressing cd45ra. Nature communications. 2017;8:1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong KL, Schlickeiser S, Vogt K, Boës D, Stanko K, Appelt C, Streitz M, Grütz G, Stobutzki N, Meisel C, Iwert C, Tomiuk S, Polansky JK, Pascher A, Babel N, Stervbo U, Sauer I, Gerlach U, Sawitzki B. Killer-like receptors and gpr56 progressive expression defines cytokine production of human cd4. Nat Commun. 2019;10:2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Hochst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, Flecken T, Giesen D, Engel D, Jung S, Busch DH, Protzer U, Thimme R, Mann M, Kurts C, Schultze JL, Kastenmuller W, Knolle PA. Functional classification of memory cd8(+) t cells by cx3cr1 expression. Nature communications. 2015;6:8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T. Dual functions of fractalkine/cx3c ligand 1 in trafficking of perforin+/granzyme b+ cytotoxic effector lymphocytes that are defined by cx3cr1 expression. J Immunol. 2002;168:6173–6180 [DOI] [PubMed] [Google Scholar]
- 29.Gordon CL, Lee LN, Swadling L, Hutchings C, Zinser M, Highton AJ, Capone S, Folgori A, Barnes E, Klenerman P. Induction and maintenance of cx3cr1-intermediate peripheral memory cd8(+) t cells by persistent viruses and vaccines. Cell Rep. 2018;23:768–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg A, Gianella S, Nakazawa M, Trout R, Spector SA. Association of cytomegalovirus dna and immunologic markers of cardiovascular disease. Open Forum Infect Dis. 2019;6:ofz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koethe JR, Jenkins CA, Petucci C, Culver J, Shepherd BE, Sterling TR. Superior glucose tolerance and metabolomic profiles, independent of adiposity, in hiv-infected women compared with men on antiretroviral therapy. Medicine (Baltimore). 2016;95:e3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with hiv on long-term antiretroviral therapy. AIDS. 2016;30:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of t cell and macrophage activation with arterial vascular health in hiv. AIDS Res Hum Retroviruses. 2017;33:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koethe JR, Jenkins CA, Furch BD, Lake JE, Barnett L, Hager CC, Smith R, Hulgan T, Shepherd BE, Kalams SA. Circulating markers of immunologic activity reflect adiposity in persons with hiv on antiretroviral therapy. J Acquir Immune Defic Syndr. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owens CD, Wake N, Conte MS, Gerhard-Herman M, Beckman JA. In vivo human lower extremity saphenous vein bypass grafts manifest flow mediated vasodilation. J Vasc Surg. 2009;50:1063–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen PL, Jarolim P, Basaria S, Zuflacht JP, Milian J, Kadivar S, Graham PL, Hyatt A, Kantoff PW, Beckman JA. Androgen deprivation therapy reversibly increases endothelium-dependent vasodilation in men with prostate cancer. J Am Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nohria A, Kinlay S, Buck JS, Redline W, Copeland-Halperin R, Kim S, Beckman JA. The effect of salsalate therapy on endothelial function in a broad range of subjects. J Am Heart Assoc. 2014;3:e000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckman JA, Goldfine AB, Dunaif A, Gerhard-Herman M, Creager MA. Endothelial function varies according to insulin resistance disease type. Diabetes Care. 2007;30:1226–1232 [DOI] [PubMed] [Google Scholar]
- 40.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275 [DOI] [PubMed] [Google Scholar]
- 41.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, Wang Z, Remark R, Li JR, Pina C, Faries C, Awad AJ, Moss N, Bjorkegren JLM, Kim-Schulze S, Gnjatic S, Ma’ayan A, Mocco J, Faries P, Merad M, Giannarelli C. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otsuka F, Kramer MC, Woudstra P, Yahagi K, Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR, Joner M, Virmani R. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis. 2015;241:772–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacre K, Hunt PW, Hsue PY, Maidji E, Martin JN, Deeks SG, Autran B, McCune JM. A role for cytomegalovirus-specific cd4+cx3cr1+ t cells and cytomegalovirus-induced t-cell immunopathology in hiv-associated atherosclerosis. AIDS. 2012;26:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savva GM, Pachnio A, Kaul B, Morgan K, Huppert FA, Brayne C, Moss PA, Study MRCCFaA. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12:381–387 [DOI] [PubMed] [Google Scholar]
- 45.Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: The atherosclerosis risk in communities (aric) study. Arch Intern Med. 2000;160:2027–2032 [DOI] [PubMed] [Google Scholar]
- 46.Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Comstock GW, Szklo M. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996;94:922–927 [DOI] [PubMed] [Google Scholar]
- 47.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, Deeks SG. Increased carotid intima-media thickness in hiv patients is associated with increased cytomegalovirus-specific t-cell responses. AIDS. 2006;20:2275–2283 [DOI] [PubMed] [Google Scholar]
- 48.Koethe JR, McDonnell W, Kennedy A, Abana CO, Pilkinton M, Setliff I, Georgiev I, Barnett L, Hager CC, Smith R, Kalams SA, Hasty A, Mallal S. Adipose tissue is enriched for activated and late-differentiated cd8+ t cells and shows distinct cd8+ receptor usage, compared with blood in hiv-infected persons. J Acquir Immune Defic Syndr. 2018;77:e14–e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shnayder M, Nachshon A, Krishna B, Poole E, Boshkov A, Binyamin A, Maza I, Sinclair J, Schwartz M, Stern-Ginossar N. Defining the transcriptional landscape during cytomegalovirus latency with single-cell rna sequencing. MBio. 2018;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein JH, Yeh E, Weber JM, Korcarz C, Ridker PM, Tawakol A, Hsue PY, Currier JS, Ribaudo H, Mitchell CKC. Brachial artery echogenicity and grayscale texture changes in hiv-infected individuals receiving low-dose methotrexate. Arterioscler Thromb Vasc Biol. 2018;38:2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic hiv infection. Curr Opin HIV AIDS. 2016;11:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


