Abstract
Evidence in humans suggests a correlation between nicotine smoking and severe respiratory symptoms with COVID-19 infection. In lung tissue, angiotensin-converting enzyme 2 (ACE2) appears to mechanistically underlie viral entry. Here, we investigated whether e-cigarette vapor inhalation alters ACE2 and nicotinic acetylcholine receptor (nAChR) expression in male and female mice. In male lung, nicotine vapor inhalation induced a significant increase in ACE2 mRNA and protein, but surprisingly, these differences were not found in females. Further, both vehicle and nicotine vapor inhalation downregulated α5 nAChR subunits in both sexes, while differences were not found in α7 nAChR subunit expression. Finally, blood ACE2 levels did not differ with exposure, indicating that blood sampling is not a sufficient indicator of lung ACE2 changes. Together, these data indicate a direct link between e-cigarette vaping and increased ACE2 expression in male lung tissue, which thereby reveals an underlying mechanism of increased vulnerability to coronavirus infection in individuals vaping nicotine.
Keywords: Nicotine, E-cigarette, ACE2, COVID-19, Individual differences, Lung
1. Introduction
Worldwide, over one billion people smoke nicotine cigarettes, resulting in greater than eight million deaths per year (Gowing et al., 2015; WHO, 2008, 2020c). These devastating statistics have been recently paralleled by the increasing mortality rate due to the COVID-19 pandemic, which has been induced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (WHO, 2020a, b). With these infections, symptoms range from asymptomatic or mild (e.g., fever and cough) to severe (e.g., pneumonia and death) (Guan et al., 2020). Given that cigarettes and other chemical constituents in vape solutions may induce inflammation and damage lung tissue (Lerner et al., 2015; Wang et al., 2019; Wu et al., 2014) and pulmonary tissue appears to function as a short-term depot for nicotine binding (Brewer et al., 2004), individuals consuming e-cigarette products may be at a higher risk for SARS-CoV-2 viral infection and related pathology. Indeed, recent reports indicate higher rates of infection in teens and young adults using e-cigarettes (Gaiha et al., 2020), and a link between e-cigarette use and increased risk of death with COVID-19 infection has been documented in adults (Kashyap et al., 2020).
E-cigarettes, which allow users to inhale vaporized liquid nicotine rather than the smoke from burning tobacco, have increased in popularity in the US and Europe, with an estimated 2.5 million users in the US alone (Editorial, 2014; Syamlal et al., 2016). The spread of the e-cigarette market has been rapid, despite many unanswered questions about their safety, efficacy in reducing dependence, and overall impact on public health. At present, a wide range of people across demographics consume e-cigarettes, including individuals who were not previously tobacco smokers (Adkison et al., 2013; Grana et al., 2014), and these numbers have dramatically risen in more recent years, especially among youth (Evans-Polce et al., 2020). Previous studies have shown that lung inflammation and suppression of the immune response are found following exposure to a variety of chemicals in e-cigarette vapor, including nicotine (Lerner et al., 2015; Wang et al., 2019; Wu et al., 2014). Interestingly, sex-specific effects have also been documented in both lung disease and nicotine’s actions. For instance, in pulmonary fibrosis rat models, higher mortality and fibrosis has been observed in females, and ovariectomy is protective, whereas estrogen exacerbates, the disease pathology (Gharaee-Kermani et al., 2005; Redente et al., 2011; Sathish et al., 2015). Further, following acute e-cigarette vapor exposure, increased pro‐inflammatory cytokine release occurs in male, but not female, mice (Wang et al., 2019). Sex-specific effects have also been documented with lung disease in humans for chronic obstructive pulmonary disease (COPD), interstitial pulmonary fibrosis and asthma (Sathish et al., 2015; Wang et al., 2019). Thus, individual differences in disease onset and progression may not only be due to the varying patterns of drug use across time but may also be attributed to innate biological differences within each sex.
Following vapor inhalation, chemicals quickly become in contact with lung tissue, leading to high levels of drug absorption given the complex vasculature network within the organ. It is thereby not surprising that cigarette smoking is linked to lung cancer and cardiovascular and pulmonary disease across many diverse populations worldwide (GBD Collaborators, 2017). Through its actions on the nicotinic acetylcholine receptors (nAChRs), nicotine exposure induces multiple effects on pulmonary mechanisms, including the renin-angiotensin system (RAS). Angiotensin-converting enzyme 2 (ACE2) is localized in the cellular membrane, with an enzymatically active domain on the external membrane surface, and systemwide, ACE2 is found in lungs, arteries, heart, kidney and intestines (Hamming et al., 2004). Cleavage of the extracellular active domain results in a soluble protein that can be measured in the blood (Patel et al., 2014). ACE2 has been well-described for its involvement in the conversion of Angiotensin II to Angiotensin-(1–7) (Cohen et al., 2020), and more recently, has been functionally described as mediating virus entry and cell fusion for coronaviruses (Zhou et al., 2020). While ACE2 expression in lung tissue appears to be upregulated in cigarette smokers, including those with COPD (Brake et al., 2020; Cai et al., 2020; Leung et al., 2020), it is unknown as to whether this correlation is due to smoke exposure or other pre-existing environmental or biological factors. Moreover, these effects could also be due to the numerous toxins, carcinogens, and other constituents found in tobacco cigarette smoke, chemicals which are not present in e-cigarette vapor solutions. Thus, while these findings suggest that smokers have an increased risk of COVID-19 infection and related complications based on increased ACE2 expression, it is unclear as to whether this represents a causal relationship between nicotine vapor with ACE2, as well as which factors in nicotine products mitigate these effects.
In these studies, we examined the effects of five days of e-cigarette vapor inhalation, either with or without nicotine, on lung tissue in male and female mice. We show that nicotine vapor inhalation induces a significant increase in both ACE2 mRNA expression and ACE2 cell density in the lungs of male mice, effects which were not present in female lung tissue. We next examined for differences in the expression of nicotinic acetylcholine receptor (nAChR) subunits, the direct site of action for nicotine in lungs. Our studies focused on the nAChR subunits that have been implicated in lung cancer and immune function, namely the α5 and α7 nAChR subunits. Surprisingly, for the α5 nAChR subunit, both vehicle and nicotine vapor inhalation resulted in a significant downregulation in both males and females. However, no differences were found between groups for the α7 nAChR subunit. Finally, blood plasma was examined, but differences were not found in ACE2 enzyme protein expression, thereby revealing that blood sampling for ACE2 does not correlate with changes in lung expression.
2. Materials and methods
2.1. Study design
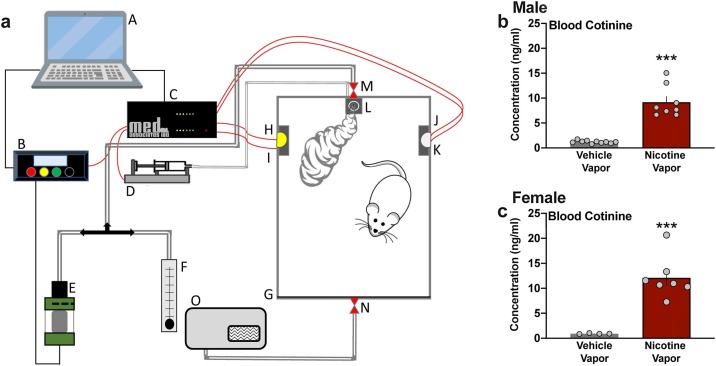
The goal of these studies was to examine the effects of e-cigarette vapor inhalation, either with or without nicotine, on protein expression in lung tissue of male and female mice. Mice were exposed to vaporized nicotine (7.5 mg/mL, free base) or vehicle during one hr daily sessions across five consecutive days in a sealed chamber (28.58 cm x 17.78 cm x 15.24 cm) with regulated airflow (1 L/min) (LJARI Vapor Chambers). For each session, animals were exposed to one puff every five min for a total of 12 puffs per hr session; each puff administration allowed for ∼40 s of vapor exposure, as previously described (Lallai et al., 2021). The vapor chamber configuration is illustrated in Fig. 1 a. A separate control group of mice were handled each day for five consecutive days. Animals were assigned randomly to each treatment group. Investigators were blinded for all experiments, until statistical analysis of the final data sets.
Fig. 1.
Vaporized nicotine inhalation significantly increases blood cotinine levels in male and female mice. (a) Schematic diagram illustrates the vapor exposure system. Diagram denotes the following components of the apparatus: A, computer; B, panel vapor and voltage control; C, controller; D, syringe pump for liquid food delivery; E, cartridge; F, air flow regulator; G, plexiglass sealed box; H, left lever; I, left cue light; J, right lever; K, right cue light; L, food dispenser; M, air in; N, air out; O, vacuum pump. (b-c) Exposure to nicotine vapor (7.5 mg/mL) across a 1-hr session induced a substantial increase in blood cotinine, the main metabolite of nicotine, in both male (b) and female (c) mice. Blood samples were collected 2 h after the first vapor puff administration. Individual data points are represented on each graph, and graph bars express as the mean ± SEM ***p < 0.001.
2.2. Animals
Adult male and female C57BL/6 J wildtype mice (Jackson Labs) were housed 2–3 per cage and maintained in an environmentally controlled vivarium on a 12h:12 h reversed light: dark cycle. Food and water were provided ad libitum. All procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The University of California, Irvine.
2.3. Drugs
(-)-Nicotine hydrogen tartrate salt (MP Biomedicals) was dissolved in vehicle (50:50 propylene glycol and vegetable glycerin) for a dose of 7.5 mg/mL (free base, pH 7.4). Ketamine (KetaVed, Patterson Veterinary) and xylazine (AnaSed, Patterson Veterinary) were diluted in sterile saline and simultaneously injected intraperitoneally at a dose of 100 and 10 mg/kg, respectively, for mouse perfusions.
2.4. Blood collection and lung extractions
Two hours after the last vapor exposure session, mice were placed under 4% isoflurane anesthesia, and blood was collected from the facial vein. Blood was centrifuged for 10 min at 500 x g, and plasma was collected and then centrifuged again at 2000 x g for 10 min to remove any trace of residual cells. Mice were sacrificed, and lung tissue was removed, flash frozen, and stored at -80°C. Each sample was analyzed for multiple RT-qPCR assays based on the amount of RNA obtained from each subject. For immunohistochemistry, a second cohort of mice was anesthetized with ketamine/xylazine and perfused through the ascending aorta with 0.9 % saline, followed by 4 % paraformaldehyde in the phosphate buffer solution (PBS; pH 7.4). Lungs were harvested, postfixed for 24 h in 4 % paraformaldehyde, and then cryoprotected in 30 % sucrose in PBS. After at least 72 h, lungs were cut into 20 μm coronal sections on a cryostat, frost mounted on slides, and stored at -80°C until processing for immunofluorescence.
2.5. RNA extraction and cDNA synthesis
RNA was extracted from homogenized tissue with Trizol reagent (Ambion Life Techniologies) via the manufacturer’s protocol. The quality of the RNA was determined by a NanoDrop 2000 spectrophotometer (ThermoScientific). For each sample, 100 ng of total RNA was reverse transcribed into cDNA with the iScript cDNA synthesis kit (Cat # 1708891; Bio-Rad Laboratories).
2.6. RT-qPCR mRNA quantification
Real-time quantitative polymerase chain reaction was performed on for angiotensin-converting enzyme 2 (ACE2), α5 and α7 nicotinic acetylcholine receptor (nAChR) subunits, and the housekeeping gene, β-actin (Actb). TaqMan Universal Master Mix II with real time PCR gene expression assays for Ace2 (Mm01159003_m1, Cat# 4331182 ThermoFisher), Chrna5 (Mm00616329_m1, ThermoFisher, Cat# 4331182), Chrna7 (Mm01312227_m1, ThermoFisher, Cat# 4351372), and Actb (Mm02619580_g1, ThermoFisher, Cat# 4331182) were utilized according to manufacture parameters (Applied Biosystems). Samples were tested in duplicate and quantified with a CFX96 RT-qPCR system (Biorad). Normalized gene expression (2ΔCt) was calculated with the equation 2^(β−actin Ct – target mRNA Ct). For nAChR subunit α5 genes, normalized values were multiplied by 10,000 to represent data as whole numbers, and for the nAChR subunit α7 genes, normalized values were multiplied by 1,000. After samples were processed, group assignment was revealed to permit comparisons of the data.
2.7. Blood analysis
The concentration of cotinine was determined with the Mouse/Rat Cotinine ELISA kit (Cat# 100902; OriGene Technologies). ACE2 protein concentration in blood plasma was determined by using the Mouse ACE2 ELISA Kit (Cat# ab213843; Abcam). Both assays were performed according to the manufacturer's instructions. For the quantification of ACE2 in blood, pg/ml values were multiplied by 0.001 to represent data as ng/ml.
2.8. Immunofluorescence staining
Fixed sections of lungs were washed in PBS (0.1 M, pH 7.4) containing 0.5 % Triton X-100 and then pre-treated using heat-mediated antigen retrieval with sodium citrate buffer (50 mM; pH 6) at 96 °C. Non-specific staining was blocked with 10 % normal donkey serum in PBS (0.1 M) for 60 min. Sections were next incubated overnight at 4 °C in primary antibody (goat anti-mACE2, 1:100; Cat # AF3437; R&D Systems) in blocking solution. Thereafter, sections were washed 0.1 M PBS containing 0.5 % Triton X-100 and then incubated with Alexa Fluor 594 donkey anti-goat (1:200; Cat # 705−585-147; Jackson Laboratory) secondary antibody at room temperature for 120 min. The slides were next rinsed in PBS (0.1 M) and coverslipped with Vectashield mounting medium with DAPI (Vector Laboratories). The analysis of the ACE2 positive cells was performed using a fluorescent microscope and MicroBrightField Stereo Investigator software (MBF Bioscience). For each subject, an entire lung section was traced using 10x magnification to obtain mm2 values, and cell counting was performed under 40x magnification. Results are presented as density of cells/mm2.
2.9. Statistical analyses
Data were analyzed by a t-test or one-way analysis of variance (ANOVA), followed by Bonferroni post-hoc test with correction for multiple comparisons (GraphPad Prism), as appropriate. The criterion for significance was set at p < 0.05.
3. Results
3.1. E-cigarette vapor inhalation results in high cotinine blood levels
Mice were exposed to passive nicotine or vehicle vapor in sealed chambers (Fig. 1a). To assess and validate the level of nicotine exposure induced with e-cigarette vapor inhalation, blood samples were analyzed from male and female mice two hours after the first vapor puff exposure. Nicotine’s main metabolite, cotinine, was assayed in blood plasma for comparison between the vapor groups. Nicotine vapor exposure induced a significant increase in cotinine levels in both males (Fig. 1b) (unpaired t-test, t(16) = 8.079, p < 0.0001) and females (Fig. 1c) (unpaired t-test, t(9) = 5.228, p = 0.0005). Of note, this level of blood cotinine is in the range of that found in previous studies with rodents and humans (Chen et al., 2020b; Lefever et al., 2017; Matta et al., 2007; Velez de Mendizabal et al., 2015), which supports the translational relevance of the protocol. Therefore, these findings thereby validate the presence of systemic nicotine exposure via vapor inhalation for this model system.
3.2. Nicotine vapor inhalation increases ACE2 mRNA expression and cell density in male lung tissue
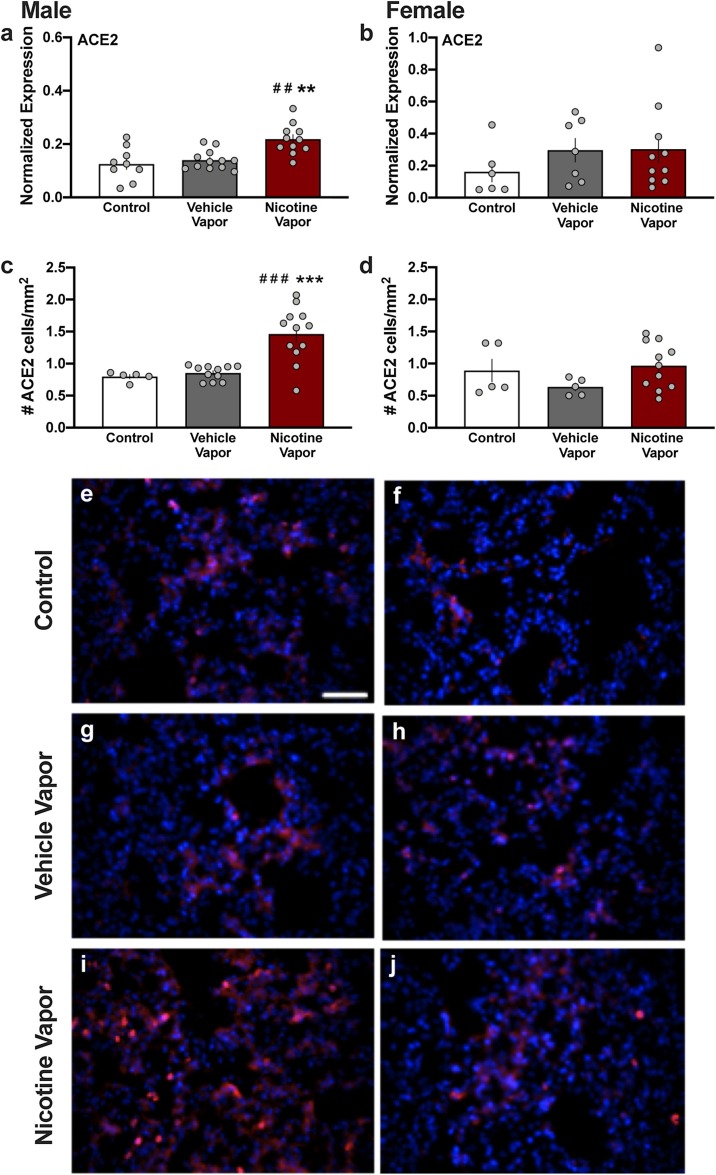
Following five consecutive treatment days, lung tissue was extracted one hour following the end of the vapor session. Lung tissue was examined for mRNA expression of ACE2 with RT-qPCR. In male mice, a significant increase in ACE2 mRNA was found (One-way ANOVA, F(2,29) = 9.880, p = 0.0005, R2 = 0.4053; post-hoc, Control vs. Nicotine p = 0.0012, Vehicle vs. Nicotine p = 0.0032) (Fig. 2 a). In contrast, females exposed to nicotine vapor did not exhibit a significant difference in ACE2 mRNA compared to the control groups (Fig. 2b) (One-way ANOVA, F(2,20) = 0.847, p = 0.4434, R2 = 0.0781). A second cohort of mice were treated as above for five consecutive days but were then perfused one hour after the end of the vapor session. Immunohistochemical analysis of ACE2 protein expression was performed in lung parenchyma, which is characterized by a large number of thin-walled alveoli (Suki et al., 2011). Nicotine vapor inhalation in males induced a statistically significant increase in the density of ACE2 positive cells (Fig. 2c) (One-way ANOVA, F(2,25) = 15.69, p < 0.0001, R2 = 0.5565; post-hoc, Control vs. Nicotine p = 0.0007, Vehicle vs. Nicotine p = 0.0001), which was consistent with the mRNA findings reported in Fig. 2a. In contrast, females exposed to nicotine vapor did not exhibit a difference in the density of ACE2-positive cells, as compared to control and vehicle vapor inhalation (Fig. 2d) (One-way ANOVA, F(2,18) = 1.733, p = 0.2050, R2 = 0.1615). The quantitative results are evidenced in representative images of the tissue across groups. Low levels of ACE2 immunoreactive cells were observed in males and females of the control (Fig. 2e, f) and vehicle vapor inhalation (Fig. 2g, h) groups. In contrast, a higher level of ACE2 immunoreactivity was evidenced with nicotine vapor inhalation in males (Fig. 2i), but not females (Fig. 2l). Together, these results reveal that nicotine vapor inhalation induces an increase in the density of ACE2-positive cells in the male lung.
Fig. 2.
Nicotine vapor inhalation increases ACE2 mRNA and protein expression in male, but not female, lung. (a-b) After five consecutive days of vapor exposure, lung tissue was analyzed for the expression of ACE2 mRNA. (a) Increased ACE2 mRNA expression was found in lung tissue of male mice exposed to nicotine vapor, as compared to control (no vapor exposure) and vehicle vapor exposure. (b) In females, no significant differences were found in ACE2 mRNA expression among exposure conditions. (c-d) Lung tissue was also examined for ACE2 protein expression. (c) Quantification of the number of ACE2-positive cells in lung tissue revealed that nicotine vapor significantly increased ACE2 protein expression in male mice, as compared to the control and vehicle vapor groups. (d) The number of ACE2-positive cells did not differ among groups in female lung tissue. Individual data points are represented on each graph, and graph bars express as the mean ± SEM. ##p < 0.01 vs vehicle vapor, ###p < 0.001 vs vehicle, **p < 0.01 vs control, ***p < 0.001 vs control. (e-j) Representative images of ACE2 immunoreactivity (red) in lung tissue following exposure. In males, high levels of ACE2 immunoreactivity can be evidenced in lungs exposed to nicotine vapor (i), as compared to control (e) and vehicle vapor (g). For females, representative images demonstrate similar levels of lung ACE2 protein expression for the control (f), vehicle vapor (h) and nicotine vapor (j) groups. DAPI: Blue. Scale bar =50 μm.
3.3. α5 nicotinic receptor subunit expression decreases following e-cigarette vapor inhalation
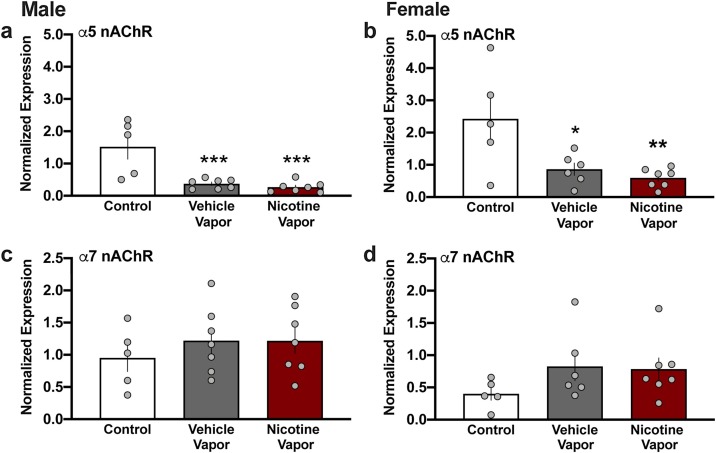
In the lungs, nicotine directly acts on the nicotinic acetylcholine receptors (nAChRs), which are composed of varying α and β subunit combinations. Lung nAChRs containing the α5 subunits have been implicated in lung cancer (Kuryatov et al., 2011; Ma et al., 2014; Sun and Ma, 2015), whereas those containing the α7 subunit are involved in immune and inflammatory responses (Fujii et al., 2007; Thomsen and Mikkelsen, 2012; Zhang et al., 2015). Interestingly, both vehicle and nicotine vapor inhalation resulted in a downregulation in α5 nAChR mRNA; this vapor-mediated effect was found in both males (Fig. 3 a) (One-way ANOVA, F(2,16) = 13.07, p = 0.0004, R2 = 0.6203; post-hoc, Control vs. Vehicle p = 0.0015, Control vs. Nicotine p = 0.0007) and females (Fig. 3b) (One-way ANOVA, F(2,15) = 6.795, p = 0.0079, R2 = 0.4753; post-hoc, Control vs. Vehicle p = 0.0329, Control vs. Nicotine p = 0.0094). In contrast, no significant differences were found in α7 nAChR subunit expression in the lungs of either males (Fig. 3c) (One-way ANOVA, F(2,16) = 0.5021, p = 0.6145, R2 = 0.0591) or females (Fig. 3d) (One-way ANOVA, F(2,15) = 1.522, p = 0.2502, R2 = 0.1687).
Fig. 3.
Nicotine vapor inhalation alters nicotinic receptor subunit mRNA expression in lungs. Following treatment, expression of the nicotinic acetylcholine receptor (nAChR) subunits were examined in lung tissue. (a) Males exposed to either vehicle vapor or nicotine vapor exhibited a significant decrease in expression of α5 nAChR mRNA compared to the control condition. (b) In females, vehicle vapor or nicotine vapor exposure decreased expression of α5 nAChR mRNA in lung tissue compared to the control condition. (c) In males, no differences were found in the expression of α7 mRNA among groups. (d) In females, α7 mRNA exhibited no differences in expression following vapor exposure. Individual data points are represented on each graph, and graph bars express as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.0001 vs control group.
3.4. Undetectable changes in circulating ACE2 expression in blood following e-cigarette vapor inhalation
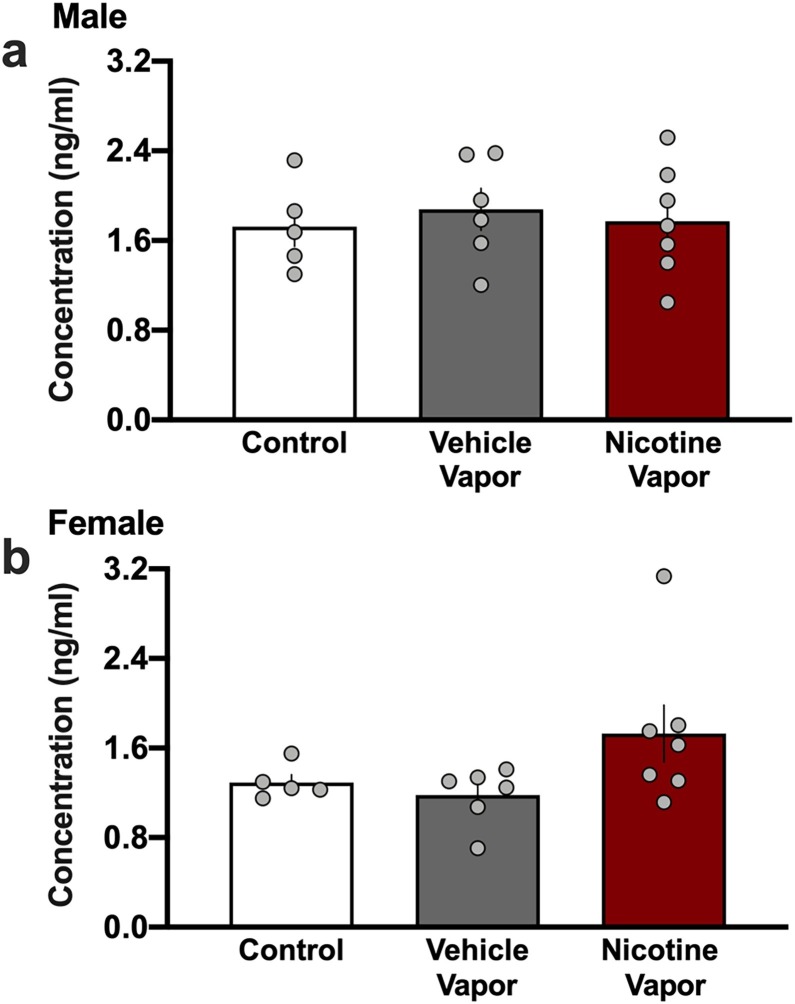
Given that the enzymatically active domain of ACE2 can become cleaved, resulting in soluble ACE2 protein circulating throughout the blood (Patel et al., 2014), we next examine blood plasma collected from subjects at the time of sacrifice. We hypothesized that if increased ACE2 expression was found in lung tissue, a detectable change in the blood could serve as a biomarker of drug exposure and the related changes in lung ACE2 expression. ACE2 was detected in the blood of all subjects, but comparisons among the groups did not find any treatment-related differences in males (Fig. 4 a) (One-way ANOVA, F(2,15) = 0.1710, p = 0.8445, R2 = 0.0223) or females (Fig. 4b) (One-way ANOVA, F(2,15) = 2.646, p = 0.1037, R2 = 0.2608). Thus, these findings indicate that blood sample analysis for ACE2 expression is not sufficient to reflect the localized changes occurring in the lungs following e-cigarette nicotine inhalation.
Fig. 4.
ACE2 expression in the blood was not significantly altered by vehicle or nicotine vapor exposure. (a) Blood plasma collected from male mice across groups did not differ in the expression of ACE2 protein. (b) Female blood plasma did not significantly differ in the level of ACE2 protein among groups. Individual data points are represented on each graph, and graph bars express as the mean ± SEM.
4. Discussion
In the current studies, we found a significant increase in ACE2 mRNA expression and cell density in male lung tissue following nicotine vapor inhalation. Surprisingly, this difference was sex-specific, as nicotine vapor inhalation was ineffective in altering ACE2 mRNA or protein expression in female mice. We also found that both vehicle and nicotine vapor inhalation resulted in a downregulation of the α5 nAChR subunit in both sexes, whereas mRNA expression of the α7 nAChR subunit were unaltered, in lung tissue. Finally, examination of the blood did not reveal any differences in circulating ACE2 levels following vapor inhalation, indicating blood levels of circulating ACE2 are not necessarily representative of changes in lung expression. Together, these findings provide direct evidence supporting the hypothesis that nicotine e-cigarette use increases ACE2 expression in male lung tissue, which could thereby propagate altered susceptibility to viral infection.
A variety of nAChR subunits have been documented within the lungs in humans (Lam et al., 2007, 2016), non-human primates (Shorey-Kendrick et al., 2015) and rodents (Grau et al., 2007). In the current studies, we examined expression of α5 and α7 nAChR subunits. In lung tissue, the α5 nAChR subunit is thought to combine with α4 and β2 subunits to form a functional heteromeric receptor subtype, whereas α7 nAChR subunits combine together by themselves to form a functional homomeric receptor. Several lines of evidence have implicated the α5 and α7 nAChRs in immune function. For instance, α7 nAChRs regulate B lymphocyte development and survival (Skok et al., 2006), α7 nAChR-containing CD11b splenic cells enter lungs during infection (Huang et al., 2019), lung group 2 innate lymphoid cells express α7 nAChRs (Huang et al., 2019), and mice with genetic knockout of the α7 nAChR exhibit exacerbated immune reactivity with protein antigen administration (Skok et al., 2005). Toxin-induced peripheral inflammation is reduced in mice lacking the α5 nAChR subunit (Bagdas et al., 2015), and T-cells and mast cells can express α5 nAChR subunits (Chernyavsky et al., 2009; Kindt et al., 2008). While we did not find any differences in α7 nAChR mRNA expression, the evidenced downregulation in α5 nAChR subunit mRNA could potentially be related to changes in immune cell signaling following chronic inhalation of the vehicle solution containing propylene glycol and vegetable glycerin. Moreover, activation of nAChR signaling has been implicated in lung cancer development and progression. For instance, agonist-mediated activation of nAChRs increases the proliferation of bronchial cancer cells in vitro, an effect that can be reversed by the general nAChR antagonist mecamylamine (Arredondo et al., 2006). It has further been suggested that lung cancer is specifically mediated by nAChRs containing the α5 subunit. In humans, allelic variation in the α5 subunit reduces α4β2α5 nAChR function and is associated with an increased vulnerability for lung cancer (Kuryatov et al., 2011; Li et al., 2011). Nicotine activation of α5-containing nAChRs in non-small cell lung cancer leads to downstream activation of several pathways that promote cancerous proliferation and metastasis, such as JAK2/STAT3, HIF-1α/VEGF and Jab1/Csn5, as demonstrated in vitro (Chen et al., 2020a; Ma et al., 2014; Sun and Ma, 2015; Sun et al., 2017; Zhang et al., 2017). Further, shRNA-mediated knockdown of the α5 nAChR subunit appears to inhibit lung tumor growth in vivo (Sun et al., 2017). In the current studies, we found that both the vehicle vapor, as well as nicotine vapor, induced a downregulation in α5 nAChR mRNA in both male and female lung tissue. This unexpected result suggests that chronic nicotine vape exposure reduces the expression of α5 nAChR signaling in normal lung tissue, which could serve to limit the ability of subsequent nicotine exposure to exert detrimental effects on the cells. Indeed, nicotine-induced activation of STAT3 can result in a STAT3-mediated downregulation in α5 nAChR expression in lung (Zhang et al., 2017), which would be consistent with the downregulation observed in the current studies. However, additional studies are necessary to determine the specific mechanisms leading to α5 nAChR downregulation, including identification of which nAChR-expressing cells (e.g., local epithelial and infiltrating immune cells) are specifically affected by such vapor exposure.
E-cigarette nicotine vapor inhalation resulted in an increase in ACE2 in the lungs of male, but not female, mice. This surprising sex-specific effect infers an increased susceptibility to the harmful effects of nicotine vaping in males. The precise mechanisms underlying these effects are unclear at present. One possibility is that estrogen can serve as an allosteric modulator of nAChRs (Paradiso et al., 2001), which may underlie differential effects of nicotine in lung tissue. While the current studies didn’t control for estrus cycle, it should be noted that the five day exposure time frame in these studies would have allowed for nicotine exposure to occur throughout all of the stages of the mouse estrus cycle across subjects. Another possibility is that males and females differ in nicotine metabolism (Kyerematen et al., 1988). Nicotine is metabolized into cotinine through the action of the cytochrome P4502A6 (CYP2A6) in humans, which is highly homologous to the mouse isoform CYP2A5 (Chen et al., 2020b; Raunio and Rahnasto-Rilla, 2012). Interestingly, females exhibit a shorter half-life and a quicker elimination of nicotine to yield a significantly higher elimination rate value (Benowitz, 1999; Prather et al., 1993). Thus, while the vapor exposure patterns were the same in these studies, an increased metabolism in females could have limited the net duration of nicotine’s actions. Finally, it is possible that the male and female subjects could have differed in their inhalation rates during the vapor exposure period. However, these possibilities need to be addressed in further studies.
A further interesting consideration is that a multitude of different chemical constituents are often found in e-cigarette products on the market. The most prevalent among these are flavorants, which make the products more palatable for the user and thereby promote use, especially among youth and other vulnerable populations (Arrazola et al., 2014; D’Silva et al., 2012; Lallai and Fowler, 2017; Litt et al., 2016). Given the many different chemicals introduced into e-cigarette products to produce the various flavor profiles, it has been challenging to obtain a clear understanding of the potential synergistic effects of all these chemicals with nicotine on lung health. However, it has been found that flavorants can interact with the metabolism of nicotine through CYP2A6 (Stratton et al., 2018), which can alter nicotine’s duration of action. Further, a large proportion of youth preferentially consume flavored nicotine products, with menthol as one of the most popular for tobacco cigarettes (Jackson et al., 2020; Villanti et al., 2017). In smokers with chronic obstructive pulmonary disease, more severe lung inflammation is associated with menthol cigarette smoking compared to non-mentholated products (Park et al., 2015), and in mice, sub-chronic exposure to the extracts of menthol cigarette smoke induces lung inflammation (Lin et al., 2018). Although the putative action of menthol and other flavorants on ACE2 has not yet been elucidated, one may predict that menthol would act synergistically with nicotine to exert detrimental effects, although this needs to be investigated in further studies.
These studies provide evidence to support a biological link between nicotine vaping and increased susceptibility to coronavirus infection via ACE2 upregulation, but potential limitations should be considered. First, the receptor binding motif of SARS-CoV-2 has high affinity for the human ACE2 isoform, but not the mouse or rat isoform, and as such, further validation studies are required in mice expressing the humanized ACE2 protein with co-exposure to the SARS-CoV-2 virus and e-cigarette vapor. Second, the current studies examined lung tissue after five days of daily one-hour exposure periods. Given that humans exhibit varying patterns of use throughout each day and across years/decades, varying levels of exposure may produce differential effects, with higher levels of exposure expected to increase pathological effects across time. Thus, it is possible that ACE2 could become altered in females with a higher exposure level, although this needs to be directly investigated. It should also be noted that the results reported herein may have important implications for second-hand exposure. Since the mice were exposed to vapor in chambers, rather than direct oral/nasal administration, the effects evidenced are applicable to the second-hand exposure produced when individuals, including children, are in close proximity to those actively vaping e-cigarettes. Finally, conflicting evidence of nicotine with COVID-19 has been reported. For instance, some reports indicate a lower incidence of smokers in those hospitalized with COVID-19 (Farsalinos et al., 2020); however, this assumes that everyone in the population had equal likelihood of being exposed to the virus, which may not be a correct assumption. Indeed, opposing results have been reported with higher rates of infection in teens and young adults using e-cigarettes (Gaiha et al., 2020), and a link between e-cigarette use and increased risk of death with COVID-19 infection has been documented in adults (Kashyap et al., 2020). Thus, one needs to take into account other factors, such as the route of exposure and time of exposure relative to the time of infection. For instance, it may be that nicotine predisposes an individual to risk of lung infection, given the current findings with ACE2 expression in the lungs and noted epidemiological data from the US, but following infection, nicotine may have some beneficial anti-inflammatory effects for treatment within other organ systems.
5. Conclusions
Here, we found an upregulation in expression of ACE2 mRNA and cell density with e-cigarette vapor inhalation in male, but not female, lungs. These nicotine-mediated effects were not reflected in analysis of ACE2 expression in blood plasma, thereby limiting the potential of circulating ACE2 to be used as a biomarker of representative lung levels. Further studies should be directed at determining whether e-cigarette nicotine inhalation subsequently leads to sex-specific altered pathology and lung function following active viral SARS-CoV-2 infection, and whether these effects on ACE2 can persist long-term following cessation of e-cigarette use. Finally, given the dramatic increase in the use of e-cigarettes by adolescents in the United States and higher incidence of COVID-19 infection found in e-cigarette users (Gaiha et al., 2020; Livingston et al., 2019), these findings reveal a putative biological underpinning that likely mitigates this increased susceptibility, thereby highlighting the need to inform policy to further restrict e-cigarette use among youth.
CRediT authorship contribution statement
Valeria Lallai: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Letizia Manca: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization. Christie D. Fowler: Conceptualization, Methodology, Validation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the NIH National Institute on Drug Abuse (DP1 DA039658 and R01 DA051831 to CDF) and Tobacco-Related Disease Research Program (TRDRP) (T30FT0967 to VL).
Edited by Dr. M.D. Coleman
References
- Adkison S.E., O’Connor R.J., Bansal-Travers M., Hyland A., Borland R., Yong H.H., Cummings K.M., McNeill A., Thrasher J.F., Hammond D., Fong G.T. Electronic nicotine delivery systems: international tobacco control four-country survey. Am. J. Prev. Med. 2013;44:207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola R.A., Neff L.J., Kennedy S.M., Holder-Hayes E., Jones C.D., Centers for Disease, C., Prevention Tobacco use among middle and high school students--United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014;63:1021–1026. [PMC free article] [PubMed] [Google Scholar]
- Arredondo J., Chernyavsky A.I., Grando S.A. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J. Cancer Res. Clin. Oncol. 2006;132:653–663. doi: 10.1007/s00432-006-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D., AlSharari S.D., Freitas K., Tracy M., Damaj M.I. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem. Pharmacol. 2015;97:590–600. doi: 10.1016/j.bcp.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N.L. Nicotine addiction. Prim. Care. 1999;26:611–631. doi: 10.1016/s0095-4543(05)70120-2. [DOI] [PubMed] [Google Scholar]
- Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J. Clin. Med. 2020:9. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B.G., Roberts A.M., Rowell P.P. Short-term distribution of nicotine in the rat lung. Drug Alcohol Depend. 2004;75:193–198. doi: 10.1016/j.drugalcdep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Cai G., Bosse Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020;201:1557–1559. doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Jia Y., Zhang Y., Zhou D., Sun H., Ma X. alpha5-nAChR contributes to epithelial-mesenchymal transition and metastasis by regulating Jab1/Csn5 signalling in lung cancer. J. Cell. Mol. Med. 2020;24:2497–2506. doi: 10.1111/jcmm.14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Fowler J.P., Wang J., Watson C.J.W., Sherafat Y., Staben A., Lazarus P., Denton T.T., Fowler C.D. The novel CYP2A6 inhibitor, DLCI-1, decreases nicotine self-administration in mice. J. Pharmacol. Exp. Ther. 2020;372:21–29. doi: 10.1124/jpet.119.260653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky A.I., Arredondo J., Galitovskiy V., Qian J., Grando S.A. Structure and function of the nicotinic arm of acetylcholine regulatory axis in human leukemic T cells. Int. J. Immunopathol. Pharmacol. 2009;22:461–472. doi: 10.1177/039463200902200223. [DOI] [PubMed] [Google Scholar]
- Cohen J.B., Hanff T.C., Bress A.P., South A.M. Relationship between ACE2 and other components of the renin-angiotensin system. Curr. Hypertens. Rep. 2020;22:44. doi: 10.1007/s11906-020-01048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva J., Boyle R.G., Lien R., Rode P., Okuyemi K.S. Cessation outcomes among treatment-seeking menthol and nonmenthol smokers. Am. J. Prev. Med. 2012;43:S242–248. doi: 10.1016/j.amepre.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Editorial Clearing the smoke. Nat. Neurosci. 2014;17:1013. doi: 10.1038/nn.3777. [DOI] [PubMed] [Google Scholar]
- Evans-Polce R.J., Veliz P., Boyd C.J., McCabe S.E. Initiation Patterns and Trends of E-Cigarette and Cigarette Use Among U.S. Adolescents. J. Adolesc. Health. 2020;66:27–33. doi: 10.1016/j.jadohealth.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Angelopoulou A., Alexandris N., Poulas K. COVID-19 and the nicotinic cholinergic system. Eur. Respir. J. 2020:56. doi: 10.1183/13993003.01589-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y.X., Fujigaya H., Moriwaki Y., Misawa H., Kasahara T., Grando S.A., Kawashima K. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. J. Neuroimmunol. 2007;189:69–74. doi: 10.1016/j.jneuroim.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Gaiha S.M., Cheng J., Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J. Adolesc. Health. 2020;67:519–523. doi: 10.1016/j.jadohealth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaee-Kermani M., Hatano K., Nozaki Y., Phan S.H. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 2005;166:1593–1606. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing L.R., Ali R.L., Allsop S., Marsden J., Turf E.E., West R., Witton J. Global statistics on addictive behaviours: 2014 status report. Addiction. 2015;110:904–919. doi: 10.1111/add.12899. [DOI] [PubMed] [Google Scholar]
- Grana R., Benowitz N., Glantz S.A. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau V., Wilker S., Hartmann P., Lips K.S., Grando S.A., Padberg W., Fehrenbach H., Kummer W. Administration of keratinocyte growth factor (KGF) modulates the pulmonary expression of nicotinic acetylcholine receptor subunits alpha7, alpha9 and alpha10. Life Sci. 2007;80:2290–2293. doi: 10.1016/j.lfs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for, C Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhao C., Su X. Neuroimmune regulation of lung infection and inflammation. QJM. 2019;112:483–487. doi: 10.1093/qjmed/hcy154. [DOI] [PubMed] [Google Scholar]
- Jackson M., Singh K.P., Lamb T., McIntosh S., Rahman I. Flavor preference and systemic immunoglobulin responses in E-cigarette users and waterpipe and tobacco smokers: a pilot study. Int. J. Environ. Res. Public Health. 2020:17. doi: 10.3390/ijerph17020640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap V.K., Dhasmana A., Massey A., Kotnala S., Zafar N., Jaggi M., Yallapu M.M., Chauhan S.C. Smoking and COVID-19: adding fuel to the flame. Int. J. Mol. Sci. 2020;21:6581. doi: 10.3390/ijms21186581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt F., Wiegand S., Niemeier V., Kupfer J., Loser C., Nilles M., Kurzen H., Kummer W., Gieler U., Haberberger R.V. Reduced expression of nicotinic alpha subunits 3, 7, 9 and 10 in lesional and nonlesional atopic dermatitis skin but enhanced expression of alpha subunits 3 and 5 in mast cells. Br. J. Dermatol. 2008;159:847–857. doi: 10.1111/j.1365-2133.2008.08774.x. [DOI] [PubMed] [Google Scholar]
- Kuryatov A., Berrettini W., Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol. Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyerematen G.A., Owens G.F., Chattopadhyay B., deBethizy J.D., Vesell E.S. Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab. Dispos. 1988;16:823–828. [PubMed] [Google Scholar]
- Lallai V., Fowler C.D. More than just chillin’: interactive effects of menthol and nicotine in drug reward. Neuropsychopharmacology. 2017;42:2283–2284. doi: 10.1038/npp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallai V., Chen Y.C., Roybal M.M., Kotha E.R., Fowler J.P., Staben A., Cortez A., Fowler C.D. Nicotine e-cigarette vapor inhalation and self-administration in a rodent model: sex- and nicotine delivery-specific effects on metabolism and behavior. Addict. Biol. 2021:e13024. doi: 10.1111/adb.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D.C., Girard L., Ramirez R., Chau W.S., Suen W.S., Sheridan S., Tin V.P., Chung L.P., Wong M.P., Shay J.W., Gazdar A.F., Lam W.K., Minna J.D. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–4647. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- Lam D.C., Luo S.Y., Fu K.H., Lui M.M., Chan K.H., Wistuba I.I., Gao B., Tsao S.W., Ip M.S., Minna J.D. Nicotinic acetylcholine receptor expression in human airway correlates with lung function. Am. J. Physiol. Lung Cell Mol. Physiol. 2016;310:L232–239. doi: 10.1152/ajplung.00101.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever T.W., Lee Y.O., Kovach A.L., Silinski M.A., Marusich J.A., Thomas B.F., Wiley J.L. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend. 2017;172:80–87. doi: 10.1016/j.drugalcdep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S., Robinson R., Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10:e0116732. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., Dorscheid D.R., Sin D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020:55. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., McCollum M., Bracamontes J., Steinbach J.H., Akk G. Functional characterization of the alpha5(Asn398) variant associated with risk for nicotine dependence in the alpha3beta4alpha5 nicotinic receptor. Mol. Pharmacol. 2011;80:818–827. doi: 10.1124/mol.111.073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A.H., Liu M.H., Ko H.K., Perng D.W., Lee T.S., Kou Y.R. Menthol cigarette smoke induces more severe lung inflammation than non-menthol cigarette smoke does in mice with subchronic exposure - role of TRPM8. Front. Physiol. 2018;9:1817. doi: 10.3389/fphys.2018.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M.D., Duffy V., Oncken C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob. Control. 2016;25:ii67–ii72. doi: 10.1136/tobaccocontrol-2016-053223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston C.J., Freeman R.J., Costales V.C., Westhoff J.L., Caplan L.S., Sherin K.M., Niebuhr D.W. Electronic nicotine delivery systems or E-cigarettes: american college of preventive medicine’s practice statement. Am. J. Prev. Med. 2019;56:167–178. doi: 10.1016/j.amepre.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Ma X., Jia Y., Zu S., Li R., Jia Y., Zhao Y., Xiao D., Dang N., Wang Y. alpha5 Nicotinic acetylcholine receptor mediates nicotine-induced HIF-1alpha and VEGF expression in non-small cell lung cancer. Toxicol. Appl. Pharmacol. 2014;278:172–179. doi: 10.1016/j.taap.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Matta S.G., Balfour D.J., Benowitz N.L., Boyd R.T., Buccafusco J.J., Caggiula A.R., Craig C.R., Collins A.C., Damaj M.I., Donny E.C., Gardiner P.S., Grady S.R., Heberlein U., Leonard S.S., Levin E.D., Lukas R.J., Markou A., Marks M.J., McCallum S.E., Parameswaran N., Perkins K.A., Picciotto M.R., Quik M., Rose J.E., Rothenfluh A., Schafer W.R., Stolerman I.P., Tyndale R.F., Wehner J.M., Zirger J.M. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Paradiso K., Zhang J., Steinbach J.H. The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J. Neurosci. 2001;21:6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Foreman M.G., Demeo D.L., Bhatt S.P., Hansel N.N., Wise R.A., Soler X., Bowler R.P. Menthol cigarette smoking in the COPDGene cohort: relationship with COPD, comorbidities and CT metrics. Respirology. 2015;20:108–114. doi: 10.1111/resp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Clarke N., Wang Z., Fan D., Parajuli N., Basu R., Putko B., Kassiri Z., Turner A.J., Oudit G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014;66:167–176. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Prather R.D., Tu T.G., Rolf C.N., Gorsline J. Nicotine pharmacokinetics of Nicoderm (nicotine transdermal system) in women and obese men compared with normal-sized men. J. Clin. Pharmacol. 1993;33:644–649. doi: 10.1002/j.1552-4604.1993.tb04718.x. [DOI] [PubMed] [Google Scholar]
- Raunio H., Rahnasto-Rilla M. CYP2A6: genetics, structure, regulation, and function. Drug Metabol. Drug Interact. 2012;27:73–88. doi: 10.1515/dmdi-2012-0001. [DOI] [PubMed] [Google Scholar]
- Redente E.F., Jacobsen K.M., Solomon J.J., Lara A.R., Faubel S., Keith R.C., Henson P.M., Downey G.P., Riches D.W. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;301:L510–518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V., Martin Y.N., Prakash Y.S. Sex steroid signaling: implications for lung diseases. Pharmacol. Ther. 2015;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey-Kendrick L.E., Ford M.M., Allen D.C., Kuryatov A., Lindstrom J., Wilhelm L., Grant K.A., Spindel E.R. Nicotinic receptors in non-human primates: analysis of genetic and functional conservation with humans. Neuropharmacology. 2015;96:263–273. doi: 10.1016/j.neuropharm.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skok M., Grailhe R., Changeux J.P. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur. J. Pharmacol. 2005;517:246–251. doi: 10.1016/j.ejphar.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Skok M., Grailhe R., Agenes F., Changeux J.P. The role of nicotinic acetylcholine receptors in lymphocyte development. J. Neuroimmunol. 2006;171:86–98. doi: 10.1016/j.jneuroim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Stratton K., Kwan L.Y., Eaton D.L. The National Academies Press; Washington, DC: 2018. Public Health Consequences of E-Cigarettes. [PubMed] [Google Scholar]
- Suki B., Stamenovic D., Hubmayr R. Lung parenchymal mechanics. Compr. Physiol. 2011;1:1317–1351. doi: 10.1002/cphy.c100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Ma X. alpha5-nAChR modulates nicotine-induced cell migration and invasion in A549 lung cancer cells. Exp. Toxicol. Pathol. 2015;67:477–482. doi: 10.1016/j.etp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Sun H.J., Jia Y.F., Ma X.L. Alpha5 nicotinic acetylcholine receptor contributes to nicotine-induced lung cancer development and progression. Front. Pharmacol. 2017;8:573. doi: 10.3389/fphar.2017.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamlal G., J.A, King B.A., Mazurek J.M. Electronic Cigarette Use Among Working Adults — United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65:557–561. doi: 10.15585/mmwr.mm6522a1. [DOI] [PubMed] [Google Scholar]
- Thomsen M.S., Mikkelsen J.D. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J. Neuroimmunol. 2012;251:65–72. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Velez de Mendizabal N., Jones D.R., Jahn A., Bies R.R., Brown J.W. Nicotine and cotinine exposure from electronic cigarettes: a population approach. Clin. Pharmacokinet. 2015;54:615–626. doi: 10.1007/s40262-014-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti A.C., Johnson A.L., Ambrose B.K., Cummings K.M., Stanton C.A., Rose S.W., Feirman S.P., Tworek C., Glasser A.M., Pearson J.L., Cohn A.M., Conway K.P., Niaura R.S., Bansal-Travers M., Hyland A. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH study (2013–2014) Am. J. Prev. Med. 2017;53:139–151. doi: 10.1016/j.amepre.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Khan N.A., Muthumalage T., Lawyer G.R., McDonough S.R., Chuang T.D., Gong M., Sundar I.K., Rehan V.K., Rahman I. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadv. 2019;1:609–623. doi: 10.1096/fba.2019-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2008. WHO Report on the Global Tobacco Epidemic: MPOWER Geneva. [Google Scholar]
- WHO . 2020. Coronavirus Disease (COVID-19) Outbreak.https://www.who.int/health-topics/coronavirus#tab=tab_1 [Google Scholar]
- WHO . 2020. Coronavirus Disease (COVID-19) Pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- WHO . 2020. Tobacco.https://www.who.int/news-room/fact-sheets/detail/tobacco [Google Scholar]
- Wu Q., Jiang D., Minor M., Chu H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9:e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Yu J.Y., Liu L.Q., Peng L., Chi F., Wu C.H., Jong A., Wang S.F., Cao H., Huang S.H. Alpha7 nicotinic acetylcholine receptor is required for blood-brain barrier injury-related CNS disorders caused by Cryptococcus neoformans and HIV-1 associated comorbidity factors. BMC Infect. Dis. 2015;15:352. doi: 10.1186/s12879-015-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jia Y., Li P., Li H., Xiao D., Wang Y., Ma X. Reciprocal activation of alpha5-nAChR and STAT3 in nicotine-induced human lung cancer cell proliferation. J. Genet. Genomics. 2017;44:355–362. doi: 10.1016/j.jgg.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]