Abstract
Some hospitals have faced a surge of patients with COVID-19, while others have not. We assessed whether COVID-19 burden (number of patients with COVID-19 admitted during April 2020 divided by hospital certified bed count) was associated with mortality in a large sample of US hospitals. Our study population included 14,226 patients with COVID-19 (median age 66 years, 45.2% women) at 117 hospitals, of whom 20.9% had died at 5 weeks of follow-up. At the hospital level, the observed mortality ranged from 0% to 44.4%. After adjustment for age, sex, and comorbidities, the adjusted odds ratio for in-hospital death in the highest quintile of burden was 1.46 (95% CI, 1.07-2.00) compared to all other quintiles. Still, there was large variability in outcomes, even among hospitals with a similar level of COVID-19 burden and after adjusting for age, sex, and comorbidities.
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Internal Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
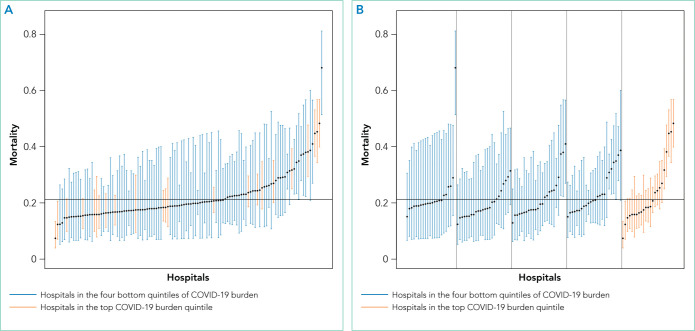
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). The 22 hospitals reporting zero deaths admitted a median of six patients with COVID-19 (maximum, 17). After adjustment for age, sex, and comorbidities, the adjusted odds ratio for in-hospital death in the most burdened hospitals was 1.46 (95% CI, 1.07-2.00) compared to hospitals in the bottom four quintiles of burden. The adjusted in-hospital mortality rate for each study hospital is shown in the Figure.
TABLE.
Characteristics and Outcomes of 14,226 Patients Admitted to US Hospitals With COVID-19
| Characteristic | No. of patients | Observed mortality, No. (%)a |
|---|---|---|
| All patients | 14,226 | 2,972 (20.9) |
|
| ||
| Age, y | ||
| <20 | 132 | 2 (1.5) |
| 20-44 | 1,742 | 60 (3.4) |
| 45-54 | 1,697 | 145 (8.5) |
| 55-64 | 2,947 | 428 (14.5) |
| 65-74 | 3,170 | 765 (24.1) |
| 75-84 | 2,592 | 838 (32.3) |
| 85+ | 1,946 | 734 (37.7) |
|
| ||
| Sex | ||
| Female | 6,425 | 1,219 (19.0) |
| Male | 7,801 | 1,753 (22.5) |
|
| ||
| Regionb | ||
| Midwest | 2,009 | 369 (18.4) |
| Northeast | 10,421 | 2,334 (22.4) |
| South | 1,176 | 169 (14.4) |
| West | 620 | 100 (16.1) |
|
| ||
| Comorbid conditionsc | ||
| Congestive heart failure | 2,110 | 734 (34.8) |
| Chronic lung disease | 2,700 | 628 (23.3) |
| Chronic kidney disease | 3,231 | 1,038 (32.1) |
| Hypertension | 8,298 | 2,097 (25.3) |
| Diabetes | 5,210 | 1,334 (25.6) |
| Obesity | 2,175 | 428 (19.7) |
|
| ||
| Hospital COVID-19 burden, quintile (admits/bed) | ||
| Lowest (0.004-0.05) | 210 | 32 (15.2) |
| Second (0.05-0.10) | 525 | 71 (13.5) |
| Third (0.10-0.20) | 1,002 | 164 (16.4) |
| Fourth (0.20-0.39) | 2,906 | 525 (18.1) |
| Highest (0.39-2.03) | 9,583 | 2,180 (22.7) |
|
| ||
| Hospital size (beds)d | ||
| <200 | 2,766 | 456 (16.5) |
| 200-699 | 4,692 | 973 (20.7) |
| >700 | 6,768 | 1,543 (22.8) |
In-hospital mortality through 5 weeks of follow-up.
Based on Census Bureau Regions and Divisions. United States Census Bureau. Accessed March 2, 2021. https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
Diagnoses abstracted from International Statistical Classification of Diseases, Tenth Revision data per the methodology described in the Methods section of this article.
Certified bed count from December 2019 Centers for Medicare & Medicaid Services data.8
FIG.
In-Hospital Mortality Rates for Patients With COVID-19 at 117 US Hospitals. (A) The caterpillar plot presents the in-hospital mortality rate for patients with COVID-19 at each of the study hospitals adjusted for age, sex, and comorbidities. The y-axis represents in-hospital mortality. Hospitals are arrayed along the x-axis, ordered by adjusted mortality (point estimate with error bars representing 95% CI). The solid line depicts the adjusted mortality for the entire sample. (B) The caterpillar plot groups the same 117 hospitals by quintile of COVID-19 burden and orders hospitals by adjusted mortality within each group.
Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on inter-hospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
Acknowledgments
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
Footnotes
Find additional supporting information in the online version of this article.
Disclosures: Dr Hu is the chief executive officer of CarePort Health. Mr. Martin is the director of Post-Acute Care Analytics at CarePort Health. No other disclosures were reported.
Funding: Drs Boscardin, Covinsky, and Smith are supported by the UCSF Pepper Center grant P30AG044281. The funder had no role in the design, conduct, or interpretation of the study, or the decision to publish. Dr Covinsky was supported by grants from the National Institute on Aging during the conduct of the study.
Access to Data: Mr Martin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would be happy to share statistical code used to generate results.
References
- 1.Centers for Disease Control and Prevention; [Accessed December 29, 2020]. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated November 2, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html. [PubMed] [Google Scholar]
- 2.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1–12. doi: 10.1001/jamainternmed.2020.4568. doi: 10.1001/jamainternmed.2020.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1–3. doi: 10.1007/s11606-020-06084-7. [DOI] [PMC free article] [PubMed]
- 5.Centers for Disease Control and Prevention; [Accessed June 2, 2020]. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf. [Google Scholar]
- 6.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 7.United States Census Bureau; [Accessed March 2, 2021]. About the American Community Survey. Updated January 4, 2021. https://www.census.gov/programs-surveys/acs/about.html. [Google Scholar]
- 8.Centers for Medicare Medicaid Services; [Accessed March 2, 2021]. Provider of service files. Revised January 15, 2020. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file. [Google Scholar]
- 9.Ash AS, Fienberg SE, Louis TA, et al. Committee of Presidents of Statistical Societies white paper; Jan, 2012. [Accessed March 1, 2021]. Statistical issues in assessing hospital performance. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf. [Google Scholar]
- 10.Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;6(4):e17401. doi: 10.1371/journal.pone.0017401. doi: 10.1371/journal.pone.0017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33–41. doi: 10.7326/M20-3905. doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]