Abstract
Background
The Omega‐3 Index is a test that measures the amount of the long‐chain omega‐3 polyunsaturated fatty acids (n‐3 PUFAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in red blood cell membranes, which is expressed as a percentage of all fatty acids. However, alpha‐linolenic acid (ALA) from flaxseed oil, which is a short‐chain n‐3 PUFA, is often promoted in pet feed as a n‐3 source, implicitly assuming it is an effective precursor of EPA and DHA.
Objective
This study was aimed to compare the effect of supplementation with a plant‐based short‐chain n‐3 PUFA source (flaxseed oil, FSO) with a marine long‐chain n‐3 PUFA source (astaxanthin krill oil, AKO) to increase the Omega‐3 Index in dogs.
Methods
Ten adult Alaskan Huskies of both genders were supplemented daily with 1,155 mg of EPA/DHA from AKO, whereas another 10 dogs received 1,068 mg ALA from flaxseed oil for 6 weeks. Fatty acid and Omega‐3 Index measurements of the two groups were taken after 0, 3 and 6 weeks for comparison.
Results
The EPA and DHA concentrations increased significantly only in the dogs fed with AKO resulting in a significant increase in mean Omega‐3 Index, from 1.68% at baseline to 2.7% after 6 weeks of supplementation (p < .0001). On the contrary, both EPA and DHA concentrations decreased significantly in the dogs fed with FSO, which led to a significant decrease in mean Omega‐3 Index from 1.6% at baseline to 0.96% at study end (p < .0001).
Conclusions
The results showed that supplementation of AKO from Antarctic krill led to a significant increase in the Omega‐3 Index in comparison to FSO in dogs. This suggests that preformed marine EPA and DHA sources are needed in dog feeds, as the dietary requirements proposed by feed industry organizations are not met with conversion from short‐chain n‐3 fatty acids.
Keywords: Alpha‐linolenic acid, astaxanthin krill oil, flaxseed oil, Omega‐3 Index, omega‐3 long‐chain polyunsaturated fatty acids
This study investigated supplementation of two different omega‐3 fatty acid sources i.e. plant (flaxseed oil, FSO) versus marine (astaxanthin krill oil, AKO) to compare the effects of short‐ versus long‐chain polyunsaturated fatty acids (PUFAs) on the Omega‐3 Index in dogs. The results suggest that preformed marine EPA and DHA sources are needed in dog feeds, as the dietary requirements are not met with conversion from equal dosage of the short‐chain omega‐3 PUFA precursor, ALA from flaxseed oil.

1. INTRODUCTION
Omega‐3 polyunsaturated fatty acids (n‐3 PUFAs) are a family consisting of both short‐ and long‐chain n‐3 fatty acids. The shortest chain fatty acid in this family is α‐linolenic acid (ALA, 18:3n‐3). ALA is a precursor for the long‐chain and more unsaturated n‐3 long‐chain PUFAs, such as eicosapentaenoic acid (20:5n‐3, EPA), docosapentaenoic acid (22:5n‐3, DPA) and docosahexaenoic acid (22:6n‐3, DHA) (Calder, 2013).
Long‐chain n‐3 PUFAs, especially EPA and DHA, are essential in companion animals for normal growth and prevention of several diseases, such as cardiovascular (Smith et al., 2007), renal (Brown et al., 1998), gastrointestinal (Trepanier, 2009), orthopaedic (Fritsch et al., 2010) and dermatological diseases (Mueller et al., 2004), as well as for better retinal (Bauer et al., 2006) and immune functions (Abba et al., 2005).
Based on a growing body of evidence on the importance of long‐chain n‐3 PUFAs, EPA and DHA have been recommended for dogs in the Nutrient Requirements of Dogs, published by the National Research Council (NRC) (Lenox, 2016). As per this recommendation, dogs in the growing phase require 0.13 g/kcal and adult dogs 0.11 g/kcal of EPA and DHA, respectively. Besides, the Canine Nutrition Expert Subcommittee of the Association of American Feed Control Officials (AAFCO) established a dietary minimum concentration of EPA plus DHA at 0.05% on dry matter basis (0.1 g/kcal) for growing and reproductive dogs (Lenox, 2016).
Nevertheless, the commercial feeds for dogs vary greatly in their EPA and DHA content, with some feeds having almost negligible or very low amounts of these fatty acids (Ahlstrøm et al., 2004). Besides, because labelling legislations do not clearly distinguish between the two groups of n‐3 PUFAs, that is n‐3 short‐ versus long‐chain (Turchini et al., 2012), some feed labels claim to be rich in n‐3 fatty acids, even though they contain only short‐chain PUFAs that are not directly linked to health benefits (Chen et al., 2020). For example many commercial feeds for dogs are supplemented with vegetable oils such as flaxseed oil (FSO), canola oil, walnut oil and soybean oil that can only provide dogs with the short‐chain n‐3 FAs, ALA (Lenox, 2016). It should be noted that ALA plays a role in cell metabolism via conversion to the longer and more unsaturated forms, EPA, DPA and DHA (Figure 1). One of the initial steps, the addition of a double bond to ALA by the ∆6‐desaturase enzyme, is the rate‐limiting step in the conversion pathway and contributes to the reported low conversion efficiency of ALA to the longer‐chain PUFAs in mammals (Calder, 2013). On the other hand, preformed long‐chain PUFAs can be found in fatty fish, such as menhaden, anchovies, herring and mackerel or algae and krill (Burri et al., 2016; Dahms et al., 2019).
FIGURE 1.
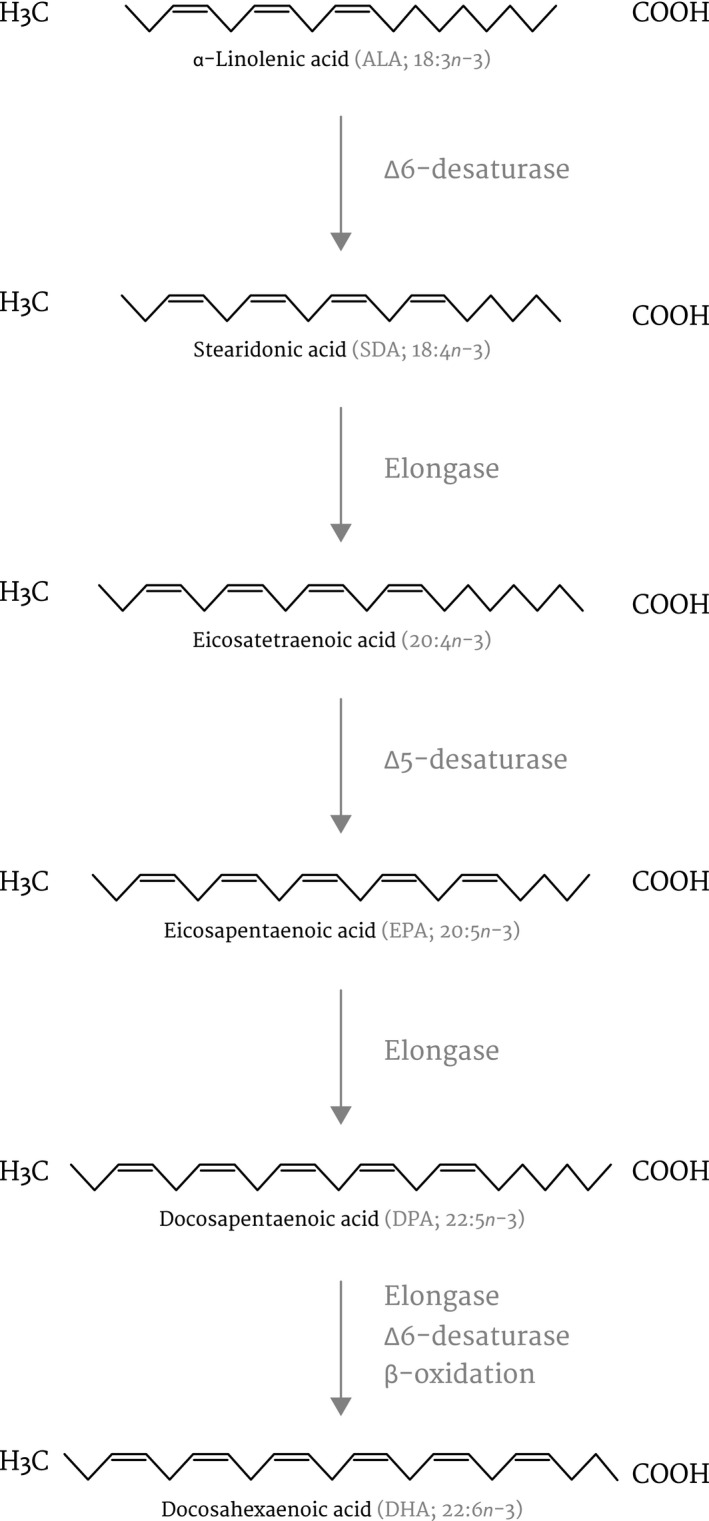
Conversion pathway for alpha‐linolenic acid (ALA) to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)
Krill are small crustaceans from the Euphausiacea family, which consists of 86 species (Spiridonov & Casanova, 2010). Krill meal is made from the largest one, Antarctic krill (Euphausia superba), which is harvested in the Southern Ocean, and is a sustainable source for marine long‐chain n‐3 PUFAs. One krill product suitable for pets is astaxanthin krill oil (AKO). It contains EPA and DHA, as well as the strong antioxidant astaxanthin.
This study investigated the supplementation of two different n‐3 sources, that is plant (FSO) versus marine (AKO) to compare the effect of short‐ versus long‐chain PUFAs, given in equal doses, on the Omega‐3 Index (i.e. the amount of EPA and DHA in red blood cell membranes) in dogs. For the first time, AKO was evaluated as a source of marine n‐3 long‐chain PUFAs in a study with dogs.
2. MATERIALS AND METHODS
2.1. Ethics, animals and diet
Twenty adult Alaskan husky sled dogs of both genders were included in the 6‐week study. The age range of the dogs was from 1 to 6 years with a mean age of 3 years. The weight ranged from 17.8 to 26.1 kg, with a mean weight of 22.2 kg (Table 1).
TABLE 1.
Gender (female, F or male, M), weight and age of the 20 Alaskan husky sled dogs enrolled in the study
| Gender | Age (y) | Weight (kg) |
|---|---|---|
| Flaxseed oil | ||
| F | 4 | 20 |
| F | 4 | 20 |
| M | 2 | 21 |
| F | 2 | 17.9 |
| M | 3 | 21.1 |
| M | 6 | 23.5 |
| M | 6 | 26.1 |
| M | 3 | 23.5 |
| F | 1 | 18 |
| M | 1 | 20.7 |
| Mean | 3.2 | 21.18 |
| Astaxanthin krill oil | ||
| F | 3 | 21.2 |
| M | 6 | 24.4 |
| M | 4 | 25.5 |
| M | 4 | 24.8 |
| M | 2 | 27.5 |
| M | 6 | 22.9 |
| M | 6 | 23.3 |
| M | 1 | 22.3 |
| M | 1 | 22 |
| F | 1 | 17.8 |
| Mean | 3.4 | 23.17 |
The participating dogs were used to regular blood sample collection, because of their dog sled race experience and any discomfort was minimized by sample collection in a known environment at their kennel. Guidelines by the Institutional Animal Ethics Committee were followed, but comparing two different feeds fed to dogs, where no adverse effects of the feeds are expected, does not require an approval according to the Norwegian regulation of animal experimentation.
During the study period of 42 days, the dogs were trained in a light treadmill workout for one hour per day. The dogs were randomly divided into two groups with 10 dogs in each group, but it was ensured that an almost even age distribution was achieved. The AKO group consisted of two females and eight males and there were four females and six males in the FSO group. One of the groups received 1,155 mg of EPA/DHA from AKO (QRILLTM AstaOmega oil blend, Aker BioMarine Antarctic AS, Lysaker, Norway) as a source of long‐chain PUFAs, and the second group received 1,068 mg ALA from FSO (Biofarmab, Sweden) as a source of short‐chain PUFAs.
The AKO supplement used was a mix of an astaxanthin‐rich triglyceride krill oil and a phospholipid krill oil in an 80/20‐mix. Color tags, blue or red, on each dog collar and doghouse were used to identify the group each dog belonged to. All 20 dogs were fed the same commercial base feed for highly active dogs 10 days before and also throughout the whole trial period, which contained 419 kcal/100g, 540 mg ALA/100 g and no EPA or DHA (Table 2). Prior to the washout period, the dogs were given a high‐performance commercial feed containing 0.45% EPA and DHA from fish oil.
TABLE 2.
Experimental diet formulation of the commercial feed
| Ingredients | |
|---|---|
| Proteins | 28.2% |
| Fat | 24.1% |
| Carbohydrates | 33% |
| Fiber | 1.6% |
| Water | 7.5% |
| Calcium | 0.95% |
| Phosphorous | 0.7% |
| Sodium | 0.36% |
| Potassium | 0.75% |
| Magnesium | 0.08% |
| n−3 fatty acids | 0.53% |
| n−6 fatty acids | 4.43% |
| α‐linolenic acid | 540 mg/100g |
| Vitamin A | 6,346 IU/kg |
| Vitamin D | 513 IE/kg |
| Vitamin E | 605 IU/kg |
| Vitamin C | 101 mg/kg |
| Βeta‐carotene | 1.5 mg/kg |
| Energy | 419 kcal/100g |
The active and the control supplements given in addition to the base feed were provided to the kennel in airtight and feed grade approved 500 ml pump bottles. The bottles were acquired from GAPLAST GmbH (Saulgrub‐Altenau, Germany). The full fatty acid profiles of AKO and FSO were analysed and are presented in Table 3. From the supplements, the dogs in the AKO group received 1,155 mg of EPA/DHA daily, whereas the dogs in the FSO group received an almost equal amount of 1,068 mg ALA per day for the study period of 6 weeks. Dogs were fed once per day, in the late evening. The supplements were given together with dry food to ensure that enough digestive enzymes are released to digest the additional fat added. The feed was fed at the maintenance level of highly trained athletes in low training/recovery/vacation mode and corresponds to almost double the amount required by the general house dog.
TABLE 3.
Composition of astaxanthin krill oil and flaxseed oil in percent
| Fatty acids (%) | Astaxanthin krill oil | Flaxseed oil |
|---|---|---|
| ∑Saturated | 33.5 | 7.7 |
| ∑Monounsaturated | 28.7 | 19.5 |
| ∑ Polyunsaturated n−6 | 1.4 | 15.7 |
| ∑ Polyunsaturated n−3 | 16.1 | 56.4 |
| ∑Polyunsaturated | 18.8 | 72.1 |
| n−6/n−3 | 0.09 | 0.28 |
| EPA + DHA | 12.4 | <0.1 |
| ALA | 0.5 | 56.4 |
| SDA | 2.1 | <0.1 |
| Astaxanthin esters | 804 mg/kg | 0 |
| Peroxide a | <2 meq/kg | 3.55 meq/kg |
Abbreviations: ALA, α‐linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; SDA, stearidonic acid.
Given as mean of four repetitive analyses from various bottles.
2.2. Blood sampling and Omega‐3 Index
Dogs in both groups were physically examined by a veterinarian prior to blood sample collection. During this examination, 6–8 ml of venous blood was collected from the cephalic vein into a vacutainer containing EDTA for plasma collection. Red blood cells (RBC) and plasma were separated by centrifugation at 3,000 rpm for 15 min at room temperature and kept on dry ice until stored at −80°C. Omega‐3 Index was measured in isolated RBCs at Omega Quant, University of South Dakota School of Medicine, USA (Harris et al., 2004). Omega‐3 Index is given as EPA + DHA in red blood cells expressed as a percentage of total identified fatty acids.
2.3. Statistical analysis
A mixed‐design one‐way ANOVA was employed to test the effect of supplement type (AKO and FSO; between subject factor) on RBC membrane fatty acid composition. Paired sample t‐tests were used to test for within‐group changes across time points for individual fatty acids. Alpha was set at 0.05.
3. RESULTS
3.1. Omega‐3 Index
The n‐3 analysis from RBCs revealed that baseline levels of Omega‐3 Index were similar in the AKO and the FSO groups. The Omega‐3 Index levels in the AKO group significantly increased after 3 weeks, when compared with the baseline levels, as shown in Figure 2. These levels were significantly higher also after 6 weeks in the AKO group compared with the FSO group (p < .0001). On the contrary, the Omega‐3 Index in the FSO group significantly decreased after 3 and 6 weeks, when compared with the baseline levels, as shown in Figure 2.
FIGURE 2.
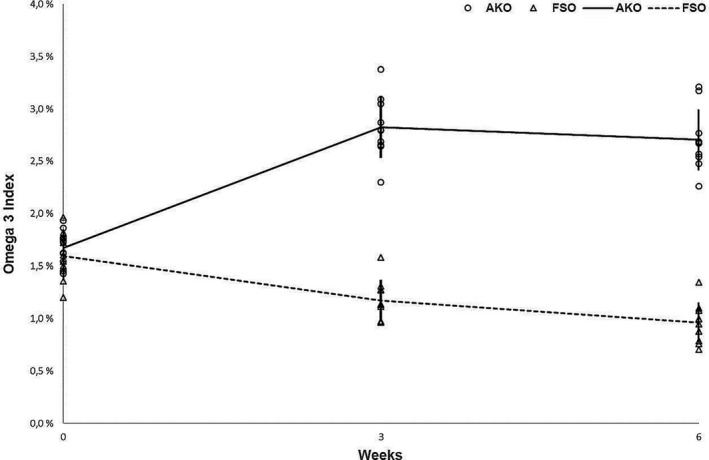
The mean omega‐3 index ± SD values (%) at the three time points, baseline (0 weeks), mid (3 weeks) and end (6 weeks) of the study period by supplement type
3.2. N‐6/n‐3 ratio
As shown in Figure 3, the n‐6/n‐3 ratio was similar in the AKO and the FSO groups at baseline with 15.91 and 16.51, respectively. The n‐6/n‐3 ratio in the AKO group significantly decreased after 3 weeks to 9.93, when compared with the baseline levels (Figure 3). These levels were significantly lower also after 6 weeks (10.16) in the AKO group (p < .0001).
FIGURE 3.
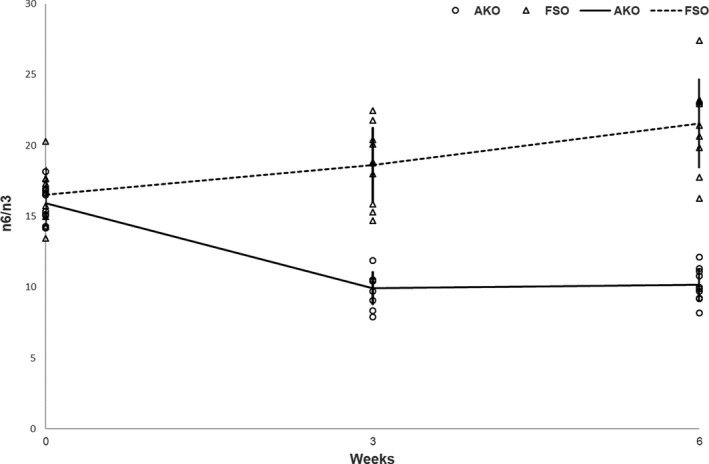
The mean n‐6/n‐3 ± SD values at the three time points, baseline (0 weeks), mid (3 weeks) and end (6 weeks) of the study period by supplement type
On the other hand, a significant increase in the n‐6/n‐3 ratio was observed in the FSO group from baseline to 3 and 6 weeks of feeding being 18.62 and 21.57, respectively (Figure 3).
3.3. Individual fatty acids
The RBC membrane fatty acid composition at 0, 3 and 6 weeks in the two groups is shown in Table 4. In general, a significant increase in n‐3 fatty acids and a significant decrease in n‐6 fatty acids were observed only in the AKO group after 3 and 6 weeks (p < .0001). The levels of n‐3 fatty acids decreased significantly in the FSO group both after 3 and 6 weeks (p < .0001). The levels of n‐6 fatty acids increased after 3 weeks in the FSO group and then decreased after 6 weeks.
TABLE 4.
Mean ± SE fatty acid composition of RBC membranes (%) across the study period by supplement type (Astaxanthin krill oil, AKO and flaxseed oil, FSO)
| Fatty acid (%) | Diet | 0 weeks | 3 weeks | 6 weeks | |
|---|---|---|---|---|---|
| ∑Trans | AKO | 0.47 ± 0.01 | 0.48 ± 0.01 | 0.36 ± 0.01 | |
| FSO | 0.46 ± 0.00 | 0.48 ± 0.01 | 0.37 ± 0.01 | ||
| ∑Saturated | AKO | 43.40 ± 0.15 | 43.52 ± 0.22 | 46.03 ± 0.05 | |
| FSO | 43.08 ± 0.25 | 43.62 ± 0.30 | 46.57 ± 0.22 | ||
| ∑Monounsaturated | AKO | 12.34 ± 0.14 | 11.86 ± 0.12 | 10.97 ± 0.12 | |
| FSO | 12.59 ± 0.17 | 12.05 ± 0.14 | 10.99 ± 0.12 | ||
| Polyunsaturated | 18:3 n−3 (ALA) |
AKO |
0.28 ± 0.02 | 0.24 ± 0.01a | 0.18 ± 0.01a |
| FSO | 0.30 ± 0.02 | 0.37 ± 0.01b | 0.27 ± 0.01b | ||
| 20:5 n−3 (EPA) | AKO* | 0.69 ± 0.03a | 1.67 ± 0.07a | 1.52 ± 0.06a | |
| FSO | 0.65 ± 0.03a | 0.53 ± 0.02b | 0.43 ± 0.02b | ||
| 22:5 n−3 (DPA) | AKO* | 0.65 ± 0.04a | 1.02 ± 0.08a | 0.98 ± 0.07a | |
| FSO | 0.64 ± 0.04a | 0.74 ± 0.07b | 0.67 ± 0.05b | ||
| 22:6 n−3 (DHA) | AKO* | 0.99 ± 0.04a | 1.16 ± 0.05a | 1.18 ± 0.05a | |
| FSO | 0.95 ± 0.06a | 0.65 ± 0.04b | 0.54 ± 0.04b | ||
| 18:2 n−6 (Linoleic) |
AKO |
17.02 ± 0.47 | 15.93 ± 0.46 | 14.08 ± 0.22 | |
| FSO | 16.98 ± 0.93 | 16.22 ± 0.34 | 14.29 ± 0.25 | ||
| 20:4 n−6 (Arachidonic) |
AKO |
20.99 ± 0.47 | 21.05 ± 0.41 | 21.42 ± 0.33 | |
| FSO | 21.21 ± 0.82 | 21.95 ± 0.41 | 22.33 ± 0.34 | ||
| ∑n−3 | AKO* | 2.60 ± 0.06a | 4.09 ± 0.16a | 3.86 ± 0.13a | |
| FSO | 2.54 ± 0.10a | 2.28 ± 0.11b | 1.90 ± 0.10b | ||
| ∑n−6 | AKO | 41.19 ± 0.24a | 40.05 ± 0.12a | 38.78 ± 0.18a | |
| FSO | 41.34 ± 0.19a | 41.57 ± 0.22b | 40.19 ± 0.23b |
Abbreviations: ALA, α‐linolenic acid, AKO, astaxanthin krill oil, DHA: docosahexaenoic acid, DPA: docosapentaenoic acid, EPA: eicosapentaenoic acid, FSO: flaxseed oil.
Denotes a significant difference in within‐group (row‐wise) values from week 0 to 6 at p < .05. Besides, different superscript symbols (a, b) denote a significant difference between groups (column‐wise) values at p < .05.
A significant increase in both EPA and DHA was observed in the AKO group after 3 weeks to 1.67 and 1.16%, respectively, and after 6 weeks to 1.52 and 1.18%, respectively, in comparison to baseline values of 0.69 and 0.99%. The FSO group had a significant reduction in both EPA (0.65% at baseline, 0.53% at 3 weeks and 0.43% at 6 weeks), and DHA levels (0.95% at baseline, 0.65% at 3 and 0.54% at 6 weeks) (Table 4).
Furthermore, a significant increase in DPA concentrations was observed in the AKO group (0.65% baseline, 1.02% at 3 and 0.98% at 6 weeks) (Table 4). Even though there was an increase in DPA levels in the FSO group after 3 weeks, these levels dropped to baseline levels at the end of the study (0.64% baseline, 0.74 at 3 weeks and 0.67% at 6 weeks).
3.4. Sum of fatty acids
It is evident from Table 4, that both the AKO and FSO group showed a significant reduction in trans fatty acids at the study end to 0.36 and 0.37%, respectively, in comparison to baseline levels of 0.47 and 0.46%, respectively. Similarly, also the monounsaturated fatty acids decreased across the study period (AKO: 12.34 to 10.97% and FSO: 12.59 to 10.99%). Moreover, there was a significant increase in RBC membrane saturated fatty acids in both groups at 6 weeks (AKO: 43.4 to 46.03% and FSO: 43.08% to 46.57%).
4. DISCUSSION
This study was conducted to quantify differences of short‐ versus long‐chain PUFA supplementation with FSO and AKO on levels of Omega‐3 Index and n‐6/n‐3 ratios in RBCs in dogs. The Omega‐3 Index was chosen as a marker for whole‐body omega‐3 status, as the erythrocyte membrane represents a prototype cell membrane of which the fatty acid composition correlates to other tissue membranes (Fenton et al., 2016). The index has the advantage of being an easily available, non‐invasive marker for tissue PUFA levels. Moreover, RBCs have the advantage to represent long‐term omega‐3 intake with less variability than plasma measurements (Harris, 2013).
Based on the results obtained, it was evident that only dogs receiving feeds with long‐chain PUFAs had a significant increase in the levels of EPA and DHA in their RBCs, in comparison to dogs that received feed containing short‐chain PUFAs. The increase in EPA and DHA led to a significant increase in the Omega‐3 Index (Figure 2), and a significant reduction in the n‐6/n‐3 ratio (Figure 3) in the RBCs of the dogs in the AKO group, in comparison to the dogs that received FSO in their feeds.
The results from this study demonstrated that long‐chain n‐3 PUFAs are a better source to increase the Omega‐3 Index in dogs in comparison to the short‐chain n‐3 PUFAs. The results also suggest that supplementation with AKO is an efficient mean to increase long‐chain n‐3 levels in the diet of dogs important for whole‐body homeostasis and to reduce the risk of various diseases (Bauer, 2011; Filburn & Griffin, 2005; Kaur et al., 2020). As krill is a sustainable n‐3 source, its dietary inclusion into pet feed might help to alleviate the pressure on wild fish stocks (Naylor et al., 2000).
The obtained results are in alignment with other reports, such as a study carried out by Mueller et al., 2005, where the authors measured the plasma concentrations of essential fatty acids in dogs (Mueller et al., 2005). One group of dogs were supplemented with FSO and the second group with preformed EPA and DHA. The authors only found a significant increase in EPA and DHA levels associated with a decrease in ARA in the dogs fed with preformed EPA and DHA. However, only ALA levels increased in the dogs with FSO supplementation (Naylor et al., 2000). Similarly, in a study by Bauer et al., 2004, 12 lactating bitches were fed with ALA that approximated 18–37 g of ALA/day (depending on the stage of gestation and during lactation), from the time of insemination throughout gestation, parturition and lactation (Bauer et al., 2004). For comparison, our study provided 1,068 mg of ALA per day to the dogs in the FSO group. The authors observed only a significant increase in ALA levels, with no enrichment in long‐chain PUFAs (EPA, DPA and DHA) in the milk, as well as during the lactation and gestation period in bitches (Bauer et al., 2004). Based on these results, the authors suggested that ALA is not an effective precursor for n‐3 long‐chain PUFAs.
Over the past 20 years, there has been an increasing interest in the roles of n‐3 long‐chain PUFAs, whereas the importance of short‐chain PUFAs, such as ALA in mammals is still not fully elucidated (Sinclair et al., 2002). A particular consideration is, whether this fatty acid has an essential role in physiology in its own right or whether its major function is to serve as a precursor of the long‐chain and more unsaturated n‐3 PUFAs, such as EPA, DPA and DHA. So far, in the light of the existing literature, it seems that the role of ALA is mainly evident as a precursor for n‐3 long‐chain PUFAs. However, the scientific evidence is rather limited in dogs, with many studies not finding an increase in EPA or DHA after ALA supplementation through FSO (Bauer et al., 2004; Mueller et al., 2005). Also in other species such as horses (Hess et al., 2012), guinea pigs (Fu & Sinclair, 2000), cats (Rivers et al., 1975) and humans (Gerster, 1998) has a significant increase in the circulating levels of EPA and DHA been reported, when preformed EPA and DHA were supplemented in contrast to ALA supplementation that gave only negligible or no increase in circulating EPA and DHA levels.
The rate‐limiting enzyme is delta‐6‐desaturase, which converts ALA to stearidonic acid (SDA), before elongation to EPA by an elongase (Gregory et al., 2011). The AKO used contained 2.1% SDA (Table 3), but it remains unclear how much was converted to EPA and has contributed to the Omega‐3 Index in the AKO‐supplemented dogs.
In this study, we found a significant decrease in EPA and DHA RBC levels in the FSO group after 3 and 6 weeks. This indicates that the washout period of 10 days before the study start was not long enough to fully reduce the n‐3 levels after feeding a diet that included EPA and DHA. It has been suggested previously that membrane concentrations of EPA and DHA follow first‐order elimination kinetics with different kinetics of EPA and DHA (Hals et al., 2017). It might therefore be advisable that 8 weeks of washout are considered for future studies.
The increase in EPA/DHA levels can only be achieved with a relative amount of decrease in the other fatty acids in the membranes of RBCs. In this study, the increase in EPA/DHA levels was paralleled with a compensatory decrease in the total n‐6 fatty acid levels in the AKO group, which led to a reduced n‐6 to n‐3 PUFA ratio. N‐3 fatty acids are known for their anti‐inflammatory effects as opposed to the pro‐inflammatory n‐6 fatty acids. Hence, an increase in EPA/DHA levels in combination with a decrease in the n‐6/n‐3 PUFA ratio, in the AKO group, would be beneficial in providing the dogs with a more anti‐inflammatory immune state. These changes are of functional relevance to the health of dogs and it is therefore of interest to increase their Omega‐3 Index, associated with a parallel decline in n‐6/n‐3 PUFA ratio.
5. CONCLUSION
This study demonstrated that a QRILLTM AstaOmega krill oil blend is a better source than FSO for increasing concentrations of essential long‐chain PUFAs in the RBCs of dogs. Hence, the authors emphasize that attention should be paid to the type of fat source that is used in standalone supplements or when diets for dogs are formulated, in order to assure a sufficient amount of long‐chain n‐3 PUFAs in the diet.
CONFLICT OF INTEREST
All the authors are employees of Aker Biomarine Antarctic AS, Norway that has provided the astaxanthin krill oil and has sponsored the study. The authors declare that they have no other competing interests.
AUTHOR CONTRIBUTION
Tonje Elisabeth Dominguez: Conceptualization; Formal analysis; Investigation. Kiranpreet Kaur: Writing‐original draft. Lena Burri: Conceptualization; Supervision; Writing‐review & editing.
Dominguez TE, Kaur K, Burri L. Enhanced omega‐3 index after long‐ Versus short‐chain omega‐3 fatty acid supplementation in dogs. Vet Med Sci.2021;7:370–377. 10.1002/vms3.369
REFERENCES
- Abba, C. , Mussa, P. P. , Vercelli, A. , & Raviri, G. (2005). Essential fatty acids supplementation in different‐stage atopic dogs fed on a controlled diet. Journal of Animal Physiololgy & Animal Nutrition, 89(3–6), 203–207. 10.1111/j.1439-0396.2005.00541.x [DOI] [PubMed] [Google Scholar]
- Ahlstrøm, Ø. , Krogdahl, Å. , Vhile, S. G. , & Skrede, A. (2004). Fatty Acid Composition in Commercial Dog Foods. American Society for Nutritional Sciences. Journal of Nutrition, 134, 2145S–S2147. 10.1093/jn/134.8.2145S [DOI] [PubMed] [Google Scholar]
- Bauer, J. E. (2011). Therapeutic use of fish oils in companion animals. Journal of the American Veterinary Medical Association, 239, 1441–1451. 10.2460/javma.239.11.1441 [DOI] [PubMed] [Google Scholar]
- Bauer, J. E. , Heinemann, K. M. , Bigley, K. E. , Lees, G. E. , & Waldron, M. K. (2004). Maternal diet alpha‐linolenic acid during gestation and lactation does not increase docosahexaenoic acid in canine milk. Journal of Nutrition, 134, 2035S–S2038. [DOI] [PubMed] [Google Scholar]
- Bauer, J. E. , Heinemann, K. M. , Lees, G. E. , & Waldron, M. K. (2006). Retinal functions of young dogs are improved and maternal plasma phospholipids are altered with diets containing long‐chain n‐3 polyunsaturated fatty acids during gestation, lactation, and after weaning. Journal of Nutrition, 136, 1991S–S1994. 10.1093/jn/136.7.1991S [DOI] [PubMed] [Google Scholar]
- Brown, S. A. , Brown, C. A. , Crowell, W. A. , Barsanti, J. A. , Allen, T. , Cowell, C. , Finco, D. R. (1998). Beneficial effects of chronic administration of dietary omega‐3 polyunsaturated fatty acids in dogs with renal insufficiency. Journal of Laboratory and Clinical Medicine, 131, 447–455. [DOI] [PubMed] [Google Scholar]
- Burri, L. , Hoem, N. , Monakhova, Y. B. , & Diehl, B. W. (2016). Fingerprinting krill Oil by 31 P, 1 H and 13 C NMR spectroscopies. Journal of the American Oil Chemists Society, 93, 1037–1049. https://aocs.onlinelibrary.wiley.com/doi/abs/10.1007/s11746‐016‐2836‐3 [Google Scholar]
- Calder, P. (2013). Omega‐3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? British Journal of Clinical Pharmacology, 75, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Deng, G. , Zhou, Q. , Chu, X. , Su, M. , Wei, Y. , Li, L. , Zhang, Z. (2020). Effects of eicosapentaenoic acid and docosahexaenoic acid versus α‐linolenic acid supplementation on cardiometabolic risk factors: A meta‐analysis of randomized controlled trials. Food Funct, 26, 1919–1932. [DOI] [PubMed] [Google Scholar]
- Dahms, I. , Bailey‐Hall, E. , Sylvester, E. , Parenteau, A. , Yu, S. , Karagiannis, A. , Roos, F. , & Wilson, J. (2019). Safety of a novel feed ingredient, Algal Oil containing EPA and DHA, in a gestation‐lactation‐growth feeding study in Beagle dogs. PLoS One, 14, e0217794. 10.1371/journal.pone.0217794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton, J. I. , Gurzell, E. A. , Davidson, E. A. , & Harris, W. S. (2016). Red blood cell PUFAs reflect the phospholipid PUFA composition of major organs. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 112, 12–23. 10.1016/j.plefa.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Filburn, C. R. , & Griffin, D. (2005). Canine plasma and erythrocyte response to a docosahexaenoic acid‐enriched supplement: Characterization and potential benefits. Veterinary Therapeutics, 6, 29–42. [PubMed] [Google Scholar]
- Fritsch, D. , Allen, T. A. , Dodd, C. E. , Jewell, D. E. , Sixby, K. A. , Leventhal, P. S. , & Hahn, K. A. (2010). Dose‐titration effects of fish oil in osteoarthritic dogs. Journal of Veterinary Internal Medicine, 24, 1020–1026. 10.1111/j.1939-1676.2010.0572.x [DOI] [PubMed] [Google Scholar]
- Fu, Z. , & Sinclair, A. J. (2000). Increased‐linolenic acid intake in‐creases tissue‐linolenic acid content and apparent oxidation with little effect on tissue docosahexaenoic acid in the guinea pig. Lipids, 35, 395–400. https://link.springer.com/article/10.1007/s11745‐000‐537‐7 [DOI] [PubMed] [Google Scholar]
- Gerster, H. (1998). Can adults adequately convert‐linolenic acid toeicosapentaenoic acid and docosahexaenoic acid? International Journal for Vitamin and Nutrition Research, 68, 159–173. [PubMed] [Google Scholar]
- Gregory, M. K. , Gibson, R. A. , Cook‐Johnson, R. J. , Cleland, L. G. , & James, M. J. (2011). Elongase reactions as control points in long‐chain polyunsaturated fatty acid synthesis. PLoS One, 6, e29662. 10.1371/journal.pone.0029662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hals, P. , Wang, X. , Piscitelli, F. , Marzo, V. D. , & Xiao, Y. F. (2017). The time course of erythrocyte membrane fatty acid concentrations during and after treatment of non‐human primates with increasing doses of an omega‐3 rich phospholipid preparation derived from krill‐oil. Lipids Health, 16, 16. 10.1186/s12944-017-0414-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, W. S. (2013). Assessing fatty acid biostatus: Red blood cells or plasma? Lipid Technology, 25, 179–181. 10.1002/lite.201300290 [DOI] [Google Scholar]
- Harris, W. S. , Sands, S. A. , Windsor, S. L. , Ali, H. A. , Stevens, T. L. , Magalski, A. , Porter, C. B. , & Borkon, A. M. (2004). Omega‐3 fatty acids in cardiac biopsies from heart transplantation patients: Correlation with erythrocytes and response to supplementation. Circulation, 110, 1645–1649. 10.1161/01.CIR.0000142292.10048.B2 [DOI] [PubMed] [Google Scholar]
- Hess, T. M. , Rexford, J. K. , Hansen, D. K. , Harris, M. , Schauermann, N. , Ross, T. , Engle, T. E. , Allen, K. G. , Mulligan, C. M. (2012). Effects of two different dietary sources of long chainomega‐3, highly unsaturated fatty acids on incorporation into the plasma, red blood cell, and skeletal muscle in horses. Journal of Animal Science, 90, 3023–3031. [DOI] [PubMed] [Google Scholar]
- Kaur, H. , Singla, A. , Singh, S. , Shilwant, S. , & Kaur, R. (2020). Role of Omega‐3 Fatty Acids in Canine Health: A Review. International Journal of Current Microbiology and Applied Sciences, 9, 2283–2293. 10.20546/ijcmas.2020.903.259 [DOI] [Google Scholar]
- Lenox, C. E. (2016). ROLE OF DIETARY FATTY ACIDS IN DOGS & CATS : https://todaysveterinarypractice.com/wp‐content/uploads/sites/4/2016/08/TVP_2016‐0910_NN‐FattyAcids.pdf
- Mueller, R. S. , Fettman, M. J. , Richardson, K. , Hansen, R. A. , Miller, A. , Magowitz, J. , & Ogilvie, G. K. (2005). Plasma and skin concentrations of polyunsaturated fatty acids before and after supplementation with n‐3 fatty acids in dogs with atopic dermatitis. American Journal of Veterinary Research, 66, 868–873. 10.2460/ajvr.2005.66.868 [DOI] [PubMed] [Google Scholar]
- Mueller, R. S. , Fieseler, K. V. , Fettman, M. J. , Zabel, S. , Rosychuk, R. A. W. , Ogilvie, G. K. , & Greenwalt, T. l. (2004). Effect of omega‐3 fatty acids on canine atopic dermatitis. Journal of Small Animal Practice, 45, 293–297. 10.1111/j.1748-5827.2004.tb00238.x [DOI] [PubMed] [Google Scholar]
- Naylor, R. L. , Goldburg, R. G. , Primavera, J. H. , Kautsky, N. , & Troell, M. (2000). Effect of aquaculture on world fish supplies. Nature, 405, 1017–1024. 10.1038/35016500 [DOI] [PubMed] [Google Scholar]
- Rivers, J. P. , Sinclair, A. J. , & Crawford, M. A. (1975). Inability of the cat to desaturate essential fatty acids. Nature, 258, 171–173. 10.1038/258171a0 [DOI] [PubMed] [Google Scholar]
- Sinclair, A. J. , Attar‐Bashi, N. M. , & Li, D. (2002). What is the role of α‐linolenic acid for mammals? Lipids, 37, 1113–1123. [DOI] [PubMed] [Google Scholar]
- Smith, C. E. , Freeman, L. M. , Rush, J. E. , Cunningham, S. M. , & Biourge, V. (2007). Omega‐3 fatty acids in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. Journal of Veterinary Internal Medicine, 21, 265–273. 10.1111/j.1939-1676.2007.tb02959.x [DOI] [PubMed] [Google Scholar]
- Spiridonov and Casanova (2010). Order Euphausiacea Dana, 1852. Koninklijke Brill NV, Leiden: The Crustacea. https://www.researchgate.net/publication/259867076_Order_Euphausiacea_Dana_1852/citations [Google Scholar]
- Trepanier, L. (2009). Idiopathic inflammatory bowel disease in cats. Rational treatment selection. Journal of Feline Medicine and Surgery, 11, 32–38. 10.1016/j.jfms.2008.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchini, G. M. , Nichols, P. D. , Barrow, C. , & Sinclair, A. J. (2012). Jumping on the omega‐3 bandwagon: Distinguishing the role of long‐chain and short‐chain omega‐3 fatty acids. Critical Reviews in Food Science and Nutrition, 52, 795–803. 10.1080/10408398.2010.509553 [DOI] [PubMed] [Google Scholar]


