Abstract
Background
COVID-19 can be asymptomatic in a substantial proportion of patients. The assessment and management of these patients constitute a key element to stop dissemination.
Aim
To describe the assessment and treatment of asymptomatic infection in patients with a confirmed diagnosis of COVID-19.
Methods
We searched five databases and search engines for preprints/preproofs, up to August 22, 2020. We included cohort, cross-sectional, and case series studies, reporting the assessment and management of asymptomatic individuals. We extracted data on total discharges with negative PCR, length of hospitalization, treatment, and number of patients who remained asymptomatic. A random-effects model with inverse variance method was used to calculate the pooled prevalence.
Results
41 studies (nine cross-sectional studies, five retrospective studies and 27 reports/case series; 647 asymptomatic individuals), were included, of which 47% were male (233/501). The age of patients was between 1month and 73 years. In patients who became symptomatic, length of hospitalization mean was 13.6 days (SD 6.4). Studies used lopinavir/ritonavir, hydroxychloroquine plus ritonavir/lopinavir, hydroxychloroquine with and without azithromycin, ribavirin plus interferon and interferon alfa. The proportion of individuals who remained asymptomatic was 91% (463/588 patients; 95%CI: 78.3%–98.7%); and asymptomatic individuals discharged with negative PCR was 86% (102/124 individuals; 95%CI: 58.4%–100%).
Conclusions
There is no standard treatment for asymptomatic COVID-19 individuals. There are no studies of adequate design to make this decision. It has been shown that most asymptomatic individuals who were followed have recovered, but this cannot be attributed to standard treatment.
Keywords: SARS-CoV-2, COVID-19, Asymptomatic, Systematic review
1. Introduction
The novel coronavirus disease (COVID-19 or SARS-CoV-2 infection) pandemic is currently of great global concern [1,2]. The spectrum of presentations of those infected with SARS-CoV-2 can range from asymptomatic to mild upper respiratory tract infections to severe acute respiratory syndrome and death in humans. The coronavirus group includes the coronavirus that causes Middle East Respiratory Syndrome and the virus that causes Severe Acute Respiratory Syndrome (SARS-CoV) [3,4]. The expansion of the pandemic was rapid and uncontrolled since transmission events have been occurring between susceptible and infectious individuals. The intensive global spread of the infection led the World Health Organization (WHO) to declare SARS-CoV-2 infection as a pandemic [5,6].
The clinical features vary, but the most common early symptoms, in those who are symptomatic, are fever (98%), cough (76%), and myalgia or fatigue (44%) [2]. Less common symptoms are sputum production (28%), headache (8%), hemoptysis (5%), and diarrhea (3–6%) [7,8]. Dyspnea does not appear in all patients, some patients may have lymphopenia, and others may have abnormal changes on chest computed tomography (CT) scan. However, some patients do not present symptoms previous to the diagnosis, being described as asymptomatic individuals [9,10].
Recent studies of SARS-CoV-2 include individuals who did not have symptoms but were screened for infection because they had family members, close contact, or had been in countries with confirmed cases [11,12]. In many cases, the test had to be done more than once for confirmation [13]. Despite being asymptomatic, medical isolation is recommended due to their high capability of transmission [1].
The evaluation of asymptomatic carriers is difficult and data are limited, but of utmost importance because of their potential to spread the virus in the general population [12]. Currently, there is no synthesis of information on the assessment and management of asymptomatic SARS-CoV-2 infection. Such an evidence synthesis could improve evidence-based decisions. Therefore, our aim was to conduct a systematic review to describe the assessment and treatment of asymptomatic infection in individuals with a confirmed diagnosis of COVID-19.
2. Methods
2.1. Protocol
A systematic review of the literature was performed following the PRISMA recommendations (Preferred Reporting Items for Systematic reviews and Meta-Analyses, 2009) [14]. The complete protocol was registered in PROSPERO (CRD42020176244). Asymptomatic individuals were defined as subjects without any symptoms at the time of diagnosis.
2.2. Data sources
The search was performed in the following databases: PubMed, Scopus, Web of Science, Ovid-Medline, Embase, and search engines for preprints/preproofs (“Other sources”, https://www.medrxiv.org). We included case-control studies, cohorts, cross-sectional, reports, and case series studies, as published articles or in their preprints/preproofs versions. There were no language restrictions. We searched records up to August 22, 2020. We included studies that assessed and treated asymptomatic infection in individuals with a confirmed diagnosis of SARS-CoV-2 by RT-PCR and described at least one of the outcomes of interest. Experimental studies, systematic reviews, narrative reviews, conference proceedings, editorials, and letters to the editor without original data were excluded.
2.3. Outcomes
The primary outcome was total discharges with negative RT-PCR at the end of the follow-up. Additional outcomes of interest were length of hospitalization, treatment in asymptomatic infection individuals, and number of individuals who remained asymptomatic.
2.4. Study selection
After the search, two authors (CDA and CSR) independently conducted the review by title and abstract according to the inclusion and exclusion criteria. Relevant studies were selected and searched by full text for the next phase of assessment. Discrepancies were consulted with another author (JBM), and a consensus was reached. The selection of articles in each phase of the review process was made in the Endnote X9 program.
2.5. Data extraction
Two authors (FOGS and CAAR) extracted the data using elaborate excel formats. Again, discrepancies were discussed and resolved with another author (JBM). The data extracted from each study were: study information (first author, year of publication, type of study and country), number of cases or participants, type of treatment, type of risk contact, length of asymptomatic period and outcomes follow-up. The individual definitions of each study were not considered.
2.6. Risk of bias assessment
Cohorts and case control studies were assessed with Newcastle–Ottawa scale [15]. For the cross-sectional studies, we used the modified Newcastle–Ottawa scale (NOS) tool [16]. For case reports and cases series, the studies were evaluated with a tool based methodological quality and synthesis of case series and case reports.
2.7. Statistical analysis
A random-effects model with inverse variance method and Freeman-Tukey double arcsine transformation was used to calculate the pooled prevalence rates as well as their 95% confidence intervals (CIs). DerSimonian-Laird estimator for Tau2 was used. Heterogeneity between the studies was evaluated by I2 statistics, and I2 > 50% or P < 0.05 indicated significant heterogeneity. The analysis was performed in R 3.5.2, using meta package.
2.8. Ethical considerations
This is a systematic review of published and open access information so no ethics committee approval was required.
3. Results
3.1. Selection of studies
The search yielded 1683 results. After duplicates were excluded, 1415 titles and abstracts were reviewed, 1359 of these were excluded, and 56 scientific papers were evaluated in detail. Finally, 41 studies were included for the qualitative synthesis [9,[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]], and 38 studies were included for the quantitative synthesis (Fig. 1 ).
Fig. 1.
Flowchart of study selection.
3.2. Characteristics of the studies included
All studies were published in 2020. Twenty-eight studies were performed out in China, four studies were performed in Italy, four studies were developed in USA, one study was performed in Iran, one study in Malaysia, one study was performed in France, one study in Republic of Korea and one study was performed in South Korea. Nine cross-sectional studies, five retrospective studies, and twenty-seven reports/case series were included. Overall, studies included 647 individuals who were asymptomatic on admission to healthcare centres; 47% (233/501) were male. Individuals were aged from 1 month to 73 years. Individuals included had a confirmed diagnosis of SARS-CoV-2 by RT-PCR (Table 1 ). All individuals in included studies informed that they had contact with confirmed COVID-19 cases, for example, contact with an infected person or Wuhan citizen, contact with an infected family member, or health staff.
Table 1.
Characteristics of included studies in systematic review.
| Author | Year | Type of study | Country | Total of asymptomatic individuals | Male (n, %) | Age (mean, SD) | Type of risk contact | Length of asymptomatic period | Clinical and imaging features | Treatment | Outcomes of patients at the end of study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu et al. | 2020 | Cross-sectional | China | 24 | 8 (33.3) | 32.5 (IQR 5–95) | All were close contacts of COVID-19 patients in Nanjing | 5 patients (20.8%) developed symptoms during follow-up. Median length of asymptomatic period: 1 day (2 patients with 0 day, 2 patients with 1 day, and 1 patient with 2 days) | Clinical: All the five cases developed fever without chills, with body temperatures ranges 36.5 °C–38.0 °C, but none presented high fever (>39 °C). One case also had cough, fatigue and nasal congestion. Another case presented cough, fatigue, dizziness and arthralgia. Images: Twelve (50.0%) cases showed ground-glass or patchy shadows in lungs in their chest CT images. Five (20.8%) cases showed stripe shadows in lungs, an atypical image finding. | 21 cases (87.5%) received antiviral therapy. One case also received antibiotics therapy, antifungal therapy plus immunoglobin therapy. Immunoglobin therapy was also given to 2 cases. All these cases were treated with interferon atomization. None of the cases developed severe pneumonia, requiring systemic corticosteroids treatment, mechanical ventilation, or admission to ICU. | 18 cases (75.0%) had the virus cleared (2 continuous negatives of nucleic acid tests), among whom 9 cases were discharged from the hospital while the rest 9 were kept in hospital for further observation. Six cases had nucleic acid tests reversed to positive after one negative result. Of particular concern, one case showed positive again even after the continuous negative of nucleic acid tests. |
| Tuo Ji et al. | 2020 | Cross-sectional | China | 41 | Not reported | Not reported | They had epidemiological clues for COVID-19 contact | Not mentioned | Not mentioned | Not mentioned | After quarantined for at least 14 days, all the persons had no signs of illness |
| Kimball et al. | 2020 | Cross-sectional | USA | 13 | Not reported | Not reported | They had history of exposure to epidemic areas or close contact with an infected individual. | 3 cases stay asymptomatic during the follow-up 1 week. The rest 10 cases developed symptoms and the mean interval from testing to symptom onset in the presymptomatic residents was 3 days | Clinical: fever (eight residents), malaise (six), and cough (five). | Not mentioned | Not mentioned |
| Wang Xiaobing et al. | 2020 | Cross-sectional | China | 30 | Not reported | Not reported | They had history of exposure to epidemic areas or close contact with an infected individual. | 14 cases stay asymptomatic during the follow-up 24 days | Clinical: 16 cases developed symptoms, Fever occurred in 6 of 30 ones (20%), with cough in 8 of 30 (26.7%), myalgia in 3 of 30 (10%), dyspnea in 2 of 30(6.7%), runny nose in 1 of 30(3.3%), nasal congestion in 1 of 30 (3.3%) and abdominal pain in 3.3%. Images: Not mentioned | Not mentioned | 2(2.0%) patients with aggravation of illness during follow up(n = 100) |
| Wang Y.et al. | 2020 | Cross-sectional | China | 55 | 22 (40%) | 49 (IQR 2–69) | Close contact with family member diagnosticated with SARS-CoV-2 infection | 70.9% developed symptoms during follow-up. 1–7 days | Clinical: Seven cases had mild cough and seven cases had low fever 3–5 days later (two cases presenting both cough and fever, which later complicated with hypoxia (SpO2 = 90%) and restlessness). Images: 16 (29.1%; 16/55) patients showed normal first chest CT. 37 cases showed pneumonia in first chest CT | Lopinavir/Ritonavir was given to all cases as initial therapy for 7 days. Two cases with hypoxia received intravenous immunoglobulin 10 g/day and methylprednisolone (1–2 mg/kg/day) therapy for 3 days. Heated humidified high-flow nasal cannula (HHHFNC) was used for 5 days. Eventually, the two patients recovered without complication | None of the cases were admitted to ICU. All the cases recovered and were discharged home |
| Meng et al. | 2020 | Cross-sectional | China | 58 | 26(44.8%) | 42.6 (16.6) | Epidemiological history (100%) | 3.71 ± 2.86 days | Clinical: eight patients developed fever; nine patients developed cough; eight patients developed fatigue; two patients developed shortness of breath; and one patient developed diarrhea. Images: frequent findings in CT were multiple lesions (62.1%), bilateral lesions (41.4%), peripheral distribution (75.9%) and ground glass opacities (51.7%) | Not mentioned | All patients were discharged after treatment. |
| Breslin et al. | 2020 | Cross-sectional | USA | 14 | 0 (0) | Not reported | Not mentioned | 6 patients developed symptoms within the first seven days after positive swab result. 4 stays asymptomatic. | Clinical: 8 patients developed fever intra or post-partum. 6 patients developed cough, myalgias, chest pain, anosmia, and/or dysgeusia. 2 patients were admitted to ICU due to obstetrical complications including respiratory distress. Images: Not mentioned | Patients with intra or postpartum fever received antibiotics for suspected intraamniotic infection or endometritis | 13 patients (including 1 ICU-admitted patient) were discharged. The other remaining patient stays in ICU with renal Insufficiency without mechanical ventilation nor dialysis |
| Kong et al. | 2020 | Cross-sectional | China | 100 | 55 (55.0%) | 37.7 ± 19.0 | History of recent travel or residence in the outbreak area or Contact history with COVID-19 patients. | 17 (27.4%) developed relevant symptoms days (median: 7 days, range: 1–13 days) after diagnosis |
Cough, Pharyngalgia or Runny nose, Fever, Chills or fatigue, Muscle aches or headaches. Among the 60 asymptomatic cases who demonstrated positive CT findings, 37 cases showed typical multiple peripheral patchy ground glass opacities, some of who showed parenchymal consolidation, interlobular septal thickening, bronchial wall thickening and halo signs or reverse-halo signs. Eighteen cases showed single or several scattered ground glass opacities. 5 cases showed nodular ground glass opacities. |
antiviral therapy if the CT imaging showed positive findings for pneumonia. | There were no deaths in the asymptomatic group. All other patients were discharged from the hospital |
| Lu Y et al. | 2020 | Cross-sectional | China | 29 | 17 (58.6%) | 7 (interquartile range 6–11) | Not mentioned | Stay asymptomatic | Non symptoms. 9 (32%) has pneumonia in chest radiological study | All patients were administered antiviral therapy, of which interferon-α nebulization was the most frequently used. None of the patients required oxygen therapy. |
All patients discharge after 10 days |
| Ma et al. | 2020 | Retrospective cohort study | China | 11 | 6(54.5) | 23(range 1–60) | 3 were residents of Wuhan,1 Wuhan visitor, 7 close contact with confirmed case | Stay asymptomatic | 7 have patchy shadows or ground glass opacity on CT | All patients received antiviral treatment, including lopinavir/ritonavir tablets, arbidol, and inhalation of recombinant human interferon |
9(81.8) of them discharge at the end of the follow-up, and 2 remain hospitalized because they still positive |
| Wang Y et al. | 2020 | Retrospective cohort study | China | 63 | 34(54%) | 39.30 ± 16.45 | 17(27%) Exposure history in Hubei and 18(28.6%) family cluster | Stay asymptomatic | Non symptoms.29 had anormal chest CT findings | Antiviral treatment with α-Interferon inhalation and Lopinavir/Ritonavir oral with Thymosin injection | All patients were discharged |
| Xu et al. | 2020 | Retrospective cohort study | China | 15 | 10 (66.7) | 27.0 (17.0, 36.0) | Contact with suspected or confirmed patients | Stay asymptomatic | Non symptoms. Ground‐glass opacity (40%) and pneumonia(52%) | interferon α‐2b, arbidol, lopinavir/ritonavir | All patients were discharged |
| London et al. | 2020 | Retrospective cohort study | USA | 22 | 0(0%) | 30.5 (interquartile range 24.5–34.8) | Testing for COVID-19 became universal for all antepartum and labor and delivery admissions | Stay asymptomatic | Non symptoms. Images nor mentioned | No treatment | All discharge after 10 days |
| Qiu et al. | 2020 | Retrospective cohort study | China | 10 | Not reported | Not reported | They had history of exposure to epidemic areas or close contact with an infected individual. | Stay asymptomatic | Non symptoms. Images: No abnormal radiographic | Interferon alfa treatment | In one case: 10 days to become SARS-CoV-2 PCR-negative. All patients were cured. |
| Albano et al. | 2020 | Case series | Italy | 6 | 2 (33.3) | 62.2 (8.7) | Not mentioned | 2 patients developed symptoms | Clinical: Case 4: several days later of the diagnosis, fever and dyspnoea appeared Case 7: Fever and cough appeared one day after the scan. Images: No reported | Hydroxychloroquine plus Ritonavir/Lopinavir (3 cases) | Not mentioned |
| Dong et al. | 2020 | Case series | China | 1 | 1(100%) | 26 | Nurse, contact with an infected person | Stay asymptomatic | Non symptoms. Chest CT with no sign of pneumonia | Treated with antiviral drugs including Arbidol and Prezcobix (Darunavir and Cobicistat tablets) | Discharged 4 days later after testing negative in two consecutive RT-PCR assays |
| Lin et al. | 2020 | Case report | China | 1 | 1(100%) | 61 | Close contact with a novel coronavirus pneumonia patient more than 10 days prior admission | Since admission, the patient has remained with only mild shortness of breath after activity on the 11th day of admission | Clinical: Mild shortness of breath after activity on the 11th day of admission. Images: Day 1: CT showed multiple ground glass opacities in the right lung. Day 3, CT revealed an enlarged lesion with small areas of consolidation in the center. Day 6, CT showed a further increase of lesions. Day 9, CT showed the lesions progressed further and involved both lungs, with thickened interlobular septa around the lesion in the upper lobe of the right lung; in addition, there were small bilateral pleural effusions. Day 23, CT showed that pleural effusions had resolved, and bilateral pulmonary lesions improved | During hospitalization the main treatment has been oral antiviral drugs (Lopinavir and Ritonavir tablets), interferon and methylprednisolone | Day 23: Patient remains hospitalized because his nucleic acid test is still positive |
| Ling et al. | 2020 | Case report | China | 4 | Not reported | Not reported | The majority patients had a history of exposure in Wuhan or to infected patients | Stay assymptomatic | Non symptoms. Images: Chest CT images showed no significant abnormalities | Not mentioned | 2 of 4 patients subsequently presented two consecutives negative nucleic acid detection at least 24 h apart and finally recovered |
| Nicastri et al. | 2020 | Case report | Italy | 1 | 1(100%) | Not reported | Contact with Wuhan person | The patients developed signs during follow-up | Clinical: Day 2: Mild conjunctivitis. Day 10–11: Tonsillar exudate. Images: Chest CT were normal | Lopinavir/ritonavir | The isolation regimen was stopped, and the patient discharged at the end of 14-day quarantine after obtaining two SARS-CoV-2 negative samples 24 h apart near |
| Polverari et al. | 2020 | Case report | Italy | 1 | 1(100%) | 73 | Patient declared no suspected expositions to infected people | 3 days | Images: PET/CT revealed the presence of bilateral, diffuse and peripheral predominant ground-glass opacities in the lower lobes | Not mentioned | Patient with non-small cells lung cancer, 3 days after the diagnosis intensive care unit was necessary for rapid disease progression and severe respiratory distress syndrome. |
| Poli et al. | 2020 | Case report | Italy | 1 | 1(100%) | 1 month | Close contact with the grandfather who was later hospitalized for COVID-19 | Stay asymptomatic | Non symptoms. Images not reported | Not mentioned | Not mentioned |
| Bai et al. | 2020 | A case report of familial cluster | China | 1 | 0 | 20 | lives in Wuhan | Stay asymptomatic | Non symptoms. Images: Chest CT images showed no significant abnormalities | Not mentioned | Not mentioned |
| Chan et al. | 2020 | A case report of familial cluster | China | 1 | 1(100%) | 10 | close contacts of COVID-19 patient | Stay asymptomatic | Non symptoms. Images: ground-glass lung opacities | Not mentioned | Admitted to hospital under isolation, supportive care, and remained stable at 9th day |
| Le et al. | 2020 | A case report of familial cluster | China | 1 | 1(100%) | 55 | Contact with Wuhan person | Stay asymptomatic | Non symptoms. Images not mentioned | Not mentioned | Patient was discharged after 2 consecutive negative PCR results |
| Lu S. et al. | 2020 | A case report of familial cluster | China | 2 | 0 | Not reported | Relatives (elder sister and son) of a coronavirus confirmed patients | Both patients stay asymptomatic | Non symptoms. Images: Patient B: Chest CT showed multiple patchy and ground glass shadows in both lungs. Patient C: No signs in chest CT | Both patients were given ribavirin plus interferon antiviral and symptomatic treatment | Not mentioned |
| Pan et al. | 2020 | A case report of familial cluster | China | 2 | 1(50%) | Not reported | Contact with Wuhan person | Stay asymptomatic | Non symptoms. Images: Normal CT chest | Not mentioned | Not mentioned |
| Qian et al. | 2020 | A case report of familial cluster | China | 2 | 1(100%) | Not reported | Husband and one grandchildren of an index case | Both patients stay asymptomatic | Non symptoms. Images nor mentioned | Not mentioned | Not mentioned |
| Tong et al. | 2020 | A case report of familial cluster | China | 3 | 1(33.3%) | 28(IQR 12–42) | Wife of an index case. Son and wife of another index case | Patients stay asymptomatic | Non symptoms. Images nor mentioned | Not mentioned | Not mentioned |
| Ye et al. | 2020 | A case report of familial cluster | China | 3 | 3(100%) | 28(IQR 23–50) | Contact with Wuhan person | One case stay asymptomatic, the rest developed symptoms 1 and 2 days later. during follow-up 4 weeks | Clinical: 2 patients developed fever and cough. Images: Chest CT images showed Ground-glass changes (Case 3 and 5). No abnormalities of Case 2 | Not mentioned | In case 2: 10 days to become PCR-negative. The rest still hospitalized and PCR-positive |
| Zhang et al. | 2020 | A case report of familial cluster | China | 1 | 1(100%) | 10 | Contact with an infected person | Stay asymptomatic | Non symptoms. Images: chest radiograph demonstrated ground glass opacities | Not mentioned | 22 days later the patient was discharged. |
| Sutton et al. | 2020 | Case series | USA | 29 | 0 (0) | Not reported | Lives in New York | 26 patients stay asymptomatic and three patients developed fever before postpartum discharge (median length of stay, 2 days) | Clinical: Fever developed in 3 patients before postpartum discharge | Two febrile patients received antibiotics for presumed endomyometritis (although 1 patient did not have localizing symptoms), and one patient received supportive care | Not mentioned |
| Du et al. | 2020 | Case series | China | 8 | 5 (62.5%) | Not reported | Familial cluster | 5.43 ± 6.33 days. | Clinical: three patients developed mild symptoms, and five patients developed conventional symptoms. Images: frequent findings were lung injury (five patients) and bilateral lesions (three patients). | Treated according to the plan of the National Health Commission (trial version 5) | Not mentioned |
| Samsami et al. | 2020 | Case series | Iran | 8 | 5 (62%) | 49.7(13.1) | 5 patients had history of close contact with a suspected COVID-19 case | Two patients experienced mild symptoms during hospitalization. Six patients remained asymptomatic | Clinical: Two patients developed fever, cough, and myalgia. Images: Chest CT showed findings compatible with pneumonia in all patients | All patients received hydroxychloroquine with or without azithromycin | All patients were discharged from hospital. None of the patients required ICU |
| Zhou et al. | 2020 | Case series | China | 13 | Not reported | Not reported | Close contact of confirmed cases | 10 patients remained asymptomatic and three patients developed symptoms at the second day of hospitalization | Images: 12 patients showed multiple ground-glass opacities and four of these showed radiographic progression during hospitalization, but all showed improvement before discharge. One patient had no evidence of radiographic abnormalities consistent with COVID-19. Clinical: Three patients developed symptoms at the second day of hospitalization (sore throat, non-productive cough, chest distress, and diarrhea). |
Not mentioned | All patients tested SARS-CoV-2-RT-PCR-negative at a median time of 13 days (range 3–19 days). |
| See et al. | 2020 | Case series | Malaysia | 4 | 3 (75%) | 6.4 (4.3) | Contact with an infected person in china | Only one patient remained asymptomatic | Clinical: case 1 had fever and diarrhea, case 2 has fever and upper respiratory tract symptoms, and case 3 had mild cough and wheeze. Images: chest X-ray showed opacities in two patients. No information on the rest. | Paracetamol in two patients, penicillin V in one patient | All patients recovered |
| An et al. | 2020 | Case series | China | 25 | Not reported | Not reported | 22 were family members in care of confirmed patients with COVID-19. 3 patients were cleaning of medical waste and hospital-transportation staff | 16 stars asymptomatic. Nine patients developed symptoms. | Clinical: 9 patients developed mild cough and/or other symptoms. Images: 24 patients had abnormal CT findings in the lung. Approximately two-thirds had an involvement of a single lobe, and two-thirds had only a ground-glass density shadow. | The 9 symptomatic patients received chloroquine 500 mg twice daily for seven days and Arbidol 200 mg three times a day for no more than ten days. | All patients recovered. 16 recovered without any symptoms during the follow-up, and 9 recovered with resolved symptoms. |
| Danis et al. | 2020 | Case series | France | 1 | Not reported | Not reported | Contact with an infected person | Stay asymptomatic | Non symptoms. Images nor mentioned | No anti-viral treatment | The symptoms of all cases resolved rapidly, without anti-viral treatment |
| Chang M. et al. | 2020 | Case series | Republic of Korea | 10 | 6(60%) | 65 ± 12.8 years | Contact history with COVID-19 patients | Stay asymptomatic | Non symptoms. All patients (100%) had ground glass opacity (GGO) on chest CT predominantly distributed peripherally and posteriorly | hydroxychloroquine sulfate and lopinavir/ritonavir | All patients were discharged from hospital. None of the patients required ICU |
| Kim et al. | 2020 | Case series | Korea of South | 10 | 4(40%) | 31 years (interquartile range 17.8–55.8 years). | Contact with confirmed COVID-19 case |
7 patients stay asymptomatic and 3 patients developed symptoms 1 or 2 days later the diagnosis | Three patients who were asymptomatic on admission developed myalgia, fever, and a cough. | No anti-viral treatment | It was found that RT-PCR was indeterminate or negative 14 days after diagnosis in entirely asymptomatic individuals |
| Song et al. | 2020 | Case series | China | 8 | 5(62.5%) | 10.1 ± 4.3 | Family members confirmed with COVID-19 prior to children | Stay asymptomatic | Non symptoms, patchy, GGOs | Azithromycin, Oseltamivir, Arbidol, Traditional Chinese medicine | All patients were discharged |
| Yang et al. | 2020 | Case series | China | 23 | 11(33.3%) | 37 (26–45) | Not mentioned | Stay asymptomatic | Non symptoms. Images nor mentioned | No mentioned | No deaths reported |
SD= Standard deviation; IQR: interquartile range.
3.3. Assessment of risk of bias
Regarding the selection domain, all studies had complete fulfillment criteria in each question. Regarding the comparability domain, only two studies [48,57] performed descriptive analyses by controlling for relevant factors (i.e., age groups and familial clusters). Regarding the outcome domain, most of the studies had complete stars in each question, except one study [50], which did not perform a statistical test. The number of stars ranged from seven to eight (Appendix Table 1).
3.4. Primary outcomes of asymptomatic individuals with COVID-19
The mean length of hospitalization was 13.6 (SD 6.4) days [24,32,37,48,55,56,58,59] Regarding the length of asymptomatic period, twenty-one studies reported that all assessed individuals remained asymptomatic [9,19,[21], [22], [23], [24],30,[32], [33], [34], [35],38,39,41,42,45,47,49,52,53,55], nineteen studies reported that some individuals developed symptoms during hospitalization or follow-up, and one study did not report any follow-up. One study describes that the only asymptomatic individual throughout the study, was asymptomatic except for slight shortness of breath during activity [31]; one study did not report the length of time its individual remains asymptomatic [48]. In general, it was not possible to quantify the average time individuals remained asymptomatic from diagnosis and during hospitalization.
Twenty-one studies reported drug management. Eleven studies used lopinavir/ritonavir and another antivirals [9,17,22,24,29,31,34,35,37,45,49]; three studies used hydroxychloroquine plus ritonavir/lopinavir [17,22,43]; another study used ribavirin plus interferon [9], or interferon alfa only [42]. One study reported the use of antibiotics and antifungal therapy [26].
At the end of follow-up, no study reported death as an outcome of an asymptomatic infection. In one cross sectional-study, 29% had a positive test (nucleic acid) after previous negative results [26]. The rest of the cases were discharged or remained hospitalized for further observation after having the virus cleared; however, one patient remained hospitalized due to a positive test even on the 23rd day [31].
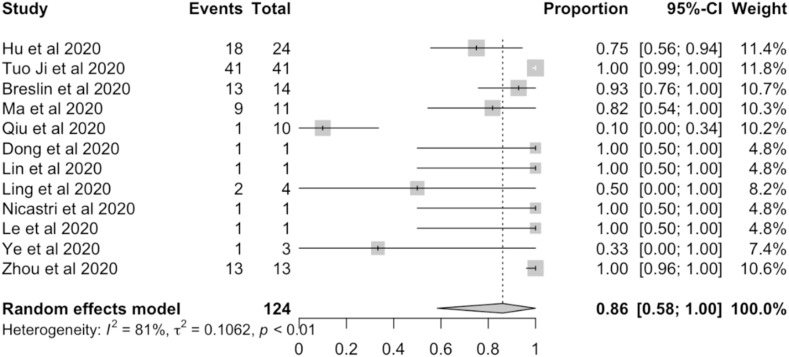
Random-effects meta-analyses were carried out using the number of individuals who remained asymptomatic until the end of study, patients discharged with negative PCR, and a total of asymptomatic individuals. The proportion of individuals who remained asymptomatic was 91% (463/588 individuals; 95%CI: 78.3%–98.7%). The meta-analysis indicated that between-study variability was high (Tau2 0.09; heterogeneity I2 = 85.7% [81.2%–89%] p-value of <0.0001) (Fig. 2 ). The prevalence of asymptomatic individuals discharged with negative PCR were 86% (102/124 individuals; 95%CI: 58.4%–100%). The meta-analysis indicated that between-study variability was high (Tau2 0.1; heterogeneity I2 = 80.5% [66.9%–88.5%] p-value of <0.0001) (Fig. 3 ).
Fig. 2.
Prevalence meta-analysis of patients who remained asymptomatic (events) and asymptomatic individuals (total).
Fig. 3.
Prevalence meta-analysis of discharged patients (events) and asymptomatic individuals (total).
4. Discussion
4.1. Main findings
Our study clinically described hospitalized individuals who had contact with persons who had COVID-19 confirmed or suspected infection, such as health care workers or Wuhan citizens. The included studies report that most individuals remained asymptomatic until discharge. Regarding the imaging characteristics, most patients showed ground-glass or patchy shadows in lungs, and consolidation patterns in first chest CT. In this systematic review, the most prescribed treatment was an antiviral drug combination, most notably lopinavir/ritonavir although, other studies used immunoglobulin therapy. 86% of asymptomatic individuals recovered (or tested negative after first positive test) and were discharged home.
4.2. What is known in the literature about our research?
COVID-19 has respiratory and systemic implications. The clinical and epidemiological characteristics are comparable with SARS [60]. Diagnosis is not only based on the symptoms but also on the history of exposure to the virus. Thus, effective tests are required to recognize patients regardless of the presence of symptoms.
RT-PCR and other laboratory tests can detect asymptomatic cases, and confirm asymptomatic infection [61], but they have the limitation that their sensitivity with one test is not optimal and two tests are ideally needed to optimize detection capacity [62]. Then, only knowing this dissemination capacity will allow us to understand the rapid spread and propose measures to stop and control the pandemic. In this way, the transmission of COVID-19 and the spectrum of disease can be understood, and the spread of the pandemic controlled by using these diagnostic tools [63].
The mechanisms by which asymptomatic carriers transmit the SARS-CoV-2 and the extent of such transmission are still unclear. There are several reports in the medical literature such as one from Bai et al. [19], who report a positive case who remained asymptomatic for more than 20 days. An individual becomes an asymptomatic carrier when their antiviral defense is strong. In this sense, the immune response limits the infection but cannot completely block the replication of SARS-CoV-2 [64]. So, the spread of the infection from asymptomatic persons may occur. However, the low viral load indicates a relatively low risk of transmission to other individuals. However, if the immune response against SARS-CoV-2 is dissociated from viral replication, the viral load is higher, so the risk of community transmission is significantly higher too [64].
4.3. Identification of asymptomatic people to control the spread of the disease
Asymptomatic individuals generate uncertainty for identification, diagnosis, and treatment, which compromises infection control and the spread of the disease [65]. Also, individuals with mild, nonspecific, and asymptomatic symptoms are difficult to identify and quarantine [66]. Based on the evidence obtained, we observed that the viral load is usually low. If this is also the case for SARS-CoV-2, the risk should remain low. Studies on the natural history of SARS-CoV-2 infection in humans are urgently needed [67].
The monitoring of viral loads, clinical presentations, and antibody titers over time in such asymptomatic persons is necessary to provide important information. Information is needed on how many of the asymptomatic individuals will develop symptoms in a later phase, whether virus shedding from the subjects is indeed less robust, and how often they may transmit SARS-CoV-2 to others [3].
Symptoms of COVID-19 are non-specific and the disease presentation can range from asymptomatic to severe pneumonia and death [12]. Some studies suggest that pre-symptomatic or asymptomatic carriers may cause COVID-19 transmission. It cannot be established whether the greatest proportion of contagion resides in asymptomatic individuals or in those who have already developed symptoms before the diagnosis is established [61,68,69]. So, a standardized definition of asymptomatic cases is important for assessing the true severity of disease and for optimizing public health control [70].
Based on the included reports, the following definitions can be proposed: 1) The pre-symptomatic case includes an infected individual (confirmed with RT-PCR test) in their incubation period that currently is without symptoms, but, he/she develops symptoms in the future. This is a retrospective definition [28]. 2) The paucisymptomatic case includes a confirmed RT-PCR test infected patient with mild upper respiratory infection symptoms, such as cough, mild conjunctivitis, or mild tonsillar exudate [37]. Finally, 3) an asymptomatic case is a confirmed RT-PCR test infected individual without any respiratory or other symptom during all the period of infection until the discharge of the patient with two sequential RT-PCR negative tests. This is also a retrospective definition.
During the current pandemic situation, where daily surveillance is necessary, we can consider a “potential asymptomatic individual” to be one with confirmed RT-PCR test without any respiratory or other symptom in the last two weeks till the diagnosis date. If the patient develops any sign or symptom related to the infection, it automatically is cataloged as a pre-symptomatic or paucisymptomatic patient. Additionally, we must consider dermatological [71], neurological [72], and gastrointestinal [73] signs and symptoms as atypical presentations of COVID-19 presentation.
Currently, most national and international infectious diseases societies recommend SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to the virus. Known exposure was defined as direct contact with a laboratory confirmed case of COVID-19. Additionally, it is important to remember that an asymptomatic individual, depending on timing of exposure, may be a presymptomatic individual that may develop symptoms later, and then should be followed up [23,26,[74], [75], [76], [77]].
4.4. How should asymptomatic individuals with COVID-19 be treated?
Asymptomatic individuals may remain in that state and may not develop moderate-severe symptoms (viral pneumonia and hypoxia) so hospitalization is not essential, and home isolation with monitoring is the first measure of care [78]. This home isolation should be monitored remotely and to date, there are few protocols in place on this topic. In depth surveillance is needed for individuals who have risk factors for severe disease despite their asymptomatic condition, because of the risk of progression to symptomatic disease with severe outcome in the second week after the onset of any symptoms [79]. Overall, management of patients who warrant hospitalization consists of ensuring appropriate infection control and supportive care (including oxygenation and potentially ventilatory support for acute respiratory distress syndrome) [80].
In our study we did not find strong evidence for the use of treatment schemes (with or without drugs), so it is not possible to provide therapeutic guidelines for the treatment of asymptomatic infections [81].
4.5. What does our study add to the literature?
In relation to the identification of the SARS-CoV-2, the included studies used molecular techniques, which are the first line to confirm suspected cases. The RT-PCR has proven to be a sensitive and specific method for the detection of the agent in respiratory samples even for asymptomatic persons. Following these techniques, the studies reported a prevalence of asymptomatic infection ranging from 4% to 80% [82], defining an asymptomatic case as a laboratory-confirmed case that does not develop symptoms. These individuals can continue spreading from person to person in the community and at health facilities. Additionally, our study adds a proposal for the standardization concept for pre-symptomatic, paucisymptomatic, and asymptomatic individuals.
On the other hand, we found no guidelines or evidence-based consensus that strongly recommend treatment for asymptomatic individuals. Several investigators have proposed specific treatment regimens ranging from drugs to treat symptoms to the use of anti-viral combinations such as lopinavir/ritonavir, although the effectiveness of such regimens is still in question. It is not known whether lowering viral load in asymptomatic individuals will have any impact on reducing transmission. Despite that, most of the asymptomatic individuals have good overall clinical outcomes without complications.
4.6. Limitations
Our study has some limitations. First, we included observational studies that do not have a comparison group. Second, the published reports and case series do not provide enough level of evidence for decision making, so this systematic review is oriented to the description and explanation of the findings, and not to validate or refute based on the evidence. Third, although the evaluation and clinical evolution in most of the studies included in this review have a common factor, we cannot conclude that the treatments adopted in each case are effective in considering them as standards in other disease contexts. The scientific production the numbers of papers on COVID-19 are increasing day by day [83] with new evidence on the identification, management of role in community transmission of asymptomatic individuals so it is possible that our findings will be quickly outdated. Finally, another limitation is that most of the included studies are from China [84], some from the USA and Korea, very few from central Europe, one study from Iran and one from Malaysia, but none from Oceania, and from Latin America, as these regions came later to research on COVID-19, compared to China and USA.
5. Conclusions
Early recognition of individuals at risk of COVID-19 infection is the most effective prevention measure to avoid the spread of the disease. Individuals at risk of infection are those who are in direct contact with positive patients whether or not they have developed symptoms. The diagnosis of all asymptomatic individuals follows the same protocol as patients with symptoms. There is no standard treatment for asymptomatic COVID-19 individuals. There are no studies of adequate design to make this decision. Most infected asymptomatic cases, seven out of ten, remain asymptomatic. All individuals at risk require immediate diagnostic evaluation to avoid spreading the disease. Most of the asymptomatic individuals have overall good clinical outcomes without complications. Protocols and guidelines are needed to remotely monitor and guide care for asymptomatic cases.
Declaration of competing interest
None for all authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2021.102058.
Funding
There was no specific funding for this project.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020:33. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khot W.Y., Nadkar M.Y. The 2019 novel coronavirus outbreak - a global threat. J Assoc Phys India. 2020;68:67–71. [PubMed] [Google Scholar]
- 6.Rabaan A.A., Al-Ahmed S.H., Haque S., Sah R., Tiwari R., Malik Y.S., et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Inf Med. 2020;28:174–184. [PubMed] [Google Scholar]
- 7.Su L., Ma X., Yu H., Zhang Z., Bian P., Han Y., et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microb Infect. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villamizar-PeNa R., Gutierrez-Ocampo E., Rodriguez-Morales A.J. Pooled prevalence of diarrhea among COVID-19 patients. Clin Gastroenterol Hepatol. 2020;18:2385–2387. doi: 10.1016/j.cgh.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo G.G., Gao S.J. Global health concerns stirred by emerging viral infections. J Med Virol. 2020;92:399–400. doi: 10.1002/jmv.25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J.Y., Yun J.G., Noh J.Y., Cheong H.J., Kim W.J. Covid-19 in South Korea - challenges of subclinical manifestations. N Engl J Med. 2020;382:1858–1859. doi: 10.1056/NEJMc2001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Tawfiq J.A. Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19) Trav Med Infect Dis. 2020;35:101608. doi: 10.1016/j.tmaid.2020.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez-Moreno C.A., Rodriguez-Morales A.J. Testing Dilemmas: post negative, positive SARS-CoV-2 RT-PCR - is it a reinfection? Trav Med Infect Dis. 2020;35:101743. doi: 10.1016/j.tmaid.2020.101743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S., et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albano D., Bertagna F., Bertoli M., Bosio G., Lucchini S., Motta F., et al. Incidental findings suggestive of COVID-19 in asymptomatic patients undergoing nuclear medicine procedures in a high-prevalence region. J Nucl Med. 2020;61:632–636. doi: 10.2967/jnumed.120.246256. [DOI] [PubMed] [Google Scholar]
- 18.An P., Song P., Wang Y., Liu B. Asymptomatic patients with novel coronavirus disease (COVID-19) Balkan Med J. 2020;37:229–230. doi: 10.4274/balkanmedj.galenos.2020.2020.4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K., et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang M.C., Hur J., Park D. Chest computed tomography findings in asymptomatic patients with COVID-19. medRxiv. 2020 doi: 10.1159/000509334. 2020.05.09.20096370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danis K., Epaulard O., Benet T., Gaymard A., Campoy S., Botelho-Nevers E., et al. Cluster of coronavirus disease 2019 (COVID-19) in the French alps, february 2020. Clin Infect Dis. 2020;71:825–832. doi: 10.1093/cid/ciaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X., Cao Y.Y., Lu X.X., Zhang J.J., Du H., Yan Y.Q., et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75:1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du W., Yu J., Wang H., Zhang X., Zhang S., Li Q., et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection. 2020;48:445–452. doi: 10.1007/s15010-020-01427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.E., Jeong H.S., Yu Y., Shin S.U., Kim S., Oh T.H., et al. Viral kinetics of SARS-CoV-2 in asymptomatic carriers and presymptomatic patients. Int J Infect Dis. 2020;95:441–443. doi: 10.1016/j.ijid.2020.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K., et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - king county, Washington, march 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong W., Wang Y., Hu J., Chughtai A., Pu H. Clinical Research Collaborative Group of Sichuan Provincial People's H. Comparison of clinical and epidemiological characteristics of asymptomatic and symptomatic SARS-CoV-2 infection: a multi-center study in Sichuan Province, China. Trav Med Infect Dis. 2020:101754. doi: 10.1016/j.tmaid.2020.101754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le T.Q.M., Takemura T., Moi M.L., Nabeshima T., Nguyen L.K.H., Hoang V.M.P., et al. Severe acute respiratory syndrome coronavirus 2 shedding by travelers, vietnam, 2020. Emerg Infect Dis. 2020;26:1624–1626. doi: 10.3201/eid2607.200591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin C., Ding Y., Xie B., Sun Z., Li X., Chen Z., et al. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin Imag. 2020;63:7–9. doi: 10.1016/j.clinimag.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling Z., Xu X., Gan Q., Zhang L., Luo L., Tang X., et al. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur J Radiol. 2020;126:108956. doi: 10.1016/j.ejrad.2020.108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.London V., McLaren R., Jr., Atallah F., Cepeda C., McCalla S., Fisher N., et al. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. 2020;37:991–994. doi: 10.1055/s-0040-1712164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y., Li Y., Deng W., Liu M., He Y., Huang L., et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in wuhan. Pediatr Infect Dis J. 2020;39:e95–e99. doi: 10.1097/INF.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y., Xu Q.N., Wang F.L., Ma X.M., Wang X.Y., Zhang X.G., et al. Characteristics of asymptomatic patients with SARS-CoV-2 infection in Jinan, China. Microb Infect. 2020;22:212–217. doi: 10.1016/j.micinf.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng H., Xiong R., He R., Lin W., Hao B., Zhang L., et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020;81:e33–e39. doi: 10.1016/j.jinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicastri E., D'Abramo A., Faggioni G., De Santis R., Mariano A., Lepore L., et al. Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.11.2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poli P., Timpano S., Goffredo M., Padoan R., Badolato R. Asymptomatic case of Covid-19 in an infant with cystic fibrosis. J Cyst Fibros. 2020;19:e18. doi: 10.1016/j.jcf.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polverari G., Arena V., Ceci F., Pelosi E., Ianniello A., Poli E., et al. (18)F-Fluorodeoxyglucose uptake in patient with asymptomatic severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019) referred to positron emission tomography/computed tomography for NSCLC restaging. J Thorac Oncol. 2020;15:1078–1080. doi: 10.1016/j.jtho.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian G., Yang N., Ma A.H.Y., Wang L., Li G., Chen X., et al. COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin Infect Dis. 2020;71:861–862. doi: 10.1093/cid/ciaa316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samsami M., Zebarjadi Bagherpour J., Nematihonar B., Tahmasbi H. COVID-19 pneumonia in asymptomatic trauma patients; report of 8 cases. Arch Acad Emerg Med. 2020;8:e46. [PMC free article] [PubMed] [Google Scholar]
- 44.See K.C., Liew S.M., Ng D.C.E., Chew E.L., Khoo E.M., Sam C.H., et al. COVID-19: four paediatric cases in Malaysia. Int J Infect Dis. 2020;94:125–127. doi: 10.1016/j.ijid.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song W., Li J., Zou N., Guan W., Pan J., Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19) J Clin Virol. 2020;127:104377. doi: 10.1016/j.jcv.2020.104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong Z.D., Tang A., Li K.F., Li P., Wang H.L., Yi J.P., et al. Potential presymptomatic transmission of SARS-CoV-2, zhejiang province, China, 2020. Emerg Infect Dis. 2020;26:1052–1054. doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuo Ji H.-L.C., Xu Jing, Wu Ling-Ning, Li Jie-Jia, Chen Kai, Qin Gang. Lockdown contained the spread of 2019 novel coronavirus disease in Huangshi city, China: early epidemiological findings | Clinical Infectious Diseases | Oxford Academic. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R., et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26:1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Liu Y., Liu L., Wang X., Luo N., Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in shenzhen, China. J Infect Dis. 2020;221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Tong J., Qin Y., Xie T., Li J., Li J., et al. Characterization of an asymptomatic cohort of SARS-COV-2 infected individuals outside of Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu T., Huang R., Zhu L., Wang J., Cheng J., Zhang B., et al. Epidemiological and clinical features of asymptomatic patients with SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25944. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye F., Xu S., Rong Z., Xu R., Liu X., Deng P., et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 2020;94:133–138. doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Tian S., Lou J., Chen Y. Familial cluster of COVID-19 infection from an asymptomatic. Crit Care. 2020;24:119. doi: 10.1186/s13054-020-2817-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X., Li Y., Li T., Zhang W. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26:957–959. doi: 10.1016/j.cmi.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye F., Xu S., Rong Z., Xu R., Liu X., Deng P., et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ki M., Task Force for -nCo V. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.M., Hayashi K., et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okamaoto K., Shirato K., Nao N., Saito S., Kageyama T., Hasegawa H., et al. An assessment of real-time RT-PCR kits for SARS-CoV-2 detection. Jpn J Infect Dis. 2020 doi: 10.7883/yoken.JJID.2020.108. [DOI] [PubMed] [Google Scholar]
- 63.Hu Z.B., Song C. [Screening and management of asymptomatic infection of 2019-novel coronavirus] Zhonghua Yufang Yixue Zazhi. 2020;54:484–485. doi: 10.3760/cma.j.cn112150-20200229-00220. [DOI] [PubMed] [Google Scholar]
- 64.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microb Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gates B. Responding to covid-19 - a once-in-a-century pandemic? N Engl J Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 66.Han Y., Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J Med Virol. 2020;92:639–644. doi: 10.1002/jmv.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epidemiology Working Group for Ncip Epidemic Response CCfDC, Prevention [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020 doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu X., Yang R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir Viruses. 2020;14:474–475. doi: 10.1111/irv.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leung N.H., Xu C., Ip D.K., Cowling B.J. Review article: the fraction of influenza virus infections that are asymptomatic: a systematic review and meta-analysis. Epidemiology. 2015;26:862–872. doi: 10.1097/EDE.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunt M., Koziatek C. A case of COVID-19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases Emerg Med. 2020;4:219–221. doi: 10.5811/cpcem.2020.3.47349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanson K.E., Caliendo A.M., Arias C.A., Hayden M.K., Englund J.A., Lee M.J., et al. The infectious diseases society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.IDSA. IDSA Guidelines on the diagnosis of COVID-19: molecular diagnostic testing. 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/
- 77.Saaavedra-Trujillo C.H., et al. Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID-19 en establecimientos de atención de la salud - recomendaciones basadas en consenso de expertos e informadas en la evidencia. Infectio. 2020;24:1–102. [Google Scholar]
- 78.Adhikari S.P., Meng S., Wu Y.J., Mao Y.P., Ye R.X., Wang Q.Z., et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., et al. Epidemiology of covid-19 in a long-term care facility in king county, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del Rio C., Malani P.N. COVID-19-New insights on a rapidly changing epidemic. J Am Med Assoc. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 82.Carl Heneghan J.B., Jefferson Tom. 2020. COVID-19: what proportion are asymptomatic? The Centre for Evidence-Based Medicine develops, promotes and disseminates better evidence for healthcare. [Google Scholar]
- 83.Torres-Salinas D. Ritmo de crecimiento diario de la producción científica sobre Covid-19. Análisis en bases de datos y repositorios en acceso abierto. El Prof Inf. 2020;29 [Google Scholar]
- 84.Bonilla-Aldana D.K., Quintero-Rada K., Montoya-Posada J.P., Ramirez-Ocampo S., Paniz-Mondolfi A., Rabaan A.A., et al. SARS-CoV, MERS-CoV and now the 2019-novel CoV: have we investigated enough about coronaviruses? - a bibliometric analysis. Trav Med Infect Dis. 2020;33:101566. doi: 10.1016/j.tmaid.2020.101566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.