Abstract
Stroke is a leading cause of disability and mortality, with limited treatment options. After stroke injury, microglia and CNS‐resident macrophages are rapidly activated and regulate neuropathological processes to steer the course of functional recovery. To accelerate this recovery, microglia can engulf dying cells and clear irreparably‐damaged tissues, thereby creating a microenvironment that is more suitable for the formation of new neural circuitry. In addition, monocyte‐derived macrophages cross the compromised blood‐brain barrier to infiltrate the injured brain. The specific functions of myeloid lineage cells in brain injury and repair are diverse and dependent on phenotypic polarization statuses. However, it remains to be determined to what degree the CNS‐invading macrophages occupy different functional niches from CNS‐resident microglia. In this review, we describe the physiological characteristics and functions of microglia in the developing and adult brain. We also review (a) the activation and phenotypic polarization of microglia and macrophages after stroke, (b) molecular mechanisms that control polarization status, and (c) the contribution of microglia to brain pathology versus repair. Finally, we summarize current breakthroughs in therapeutic strategies that calibrate microglia/macrophage responses after stroke.
Keywords: neuroinflammation, phagocytosis, polarization, repopulation, transplantation
Short abstract
The present review summarizes recent advances in microglial research in relation to stroke with emphases on microglial/macrophage phenotypic polarization and function in brain injury and repair. It also reviews the physiological characteristics and functions of microglia in the developing and adult brain, and describes current breakthroughs in therapeutic strategies that calibrate microglia/macrophage responses after stroke.
1. INTRODUCTION
Stroke is a leading cause of long‐term disability and mortality across the globe. There are two major types of stroke: ischemic stroke and intracerebral hemorrhage (ICH). Ischemic stroke accounts for ~70%–80% of all stroke cases, and ICH accounts for ~10%–20%. 1 Ischemic stroke and ICH trigger blood‐brain barrier (BBB) disruption, neuroinflammation cascades, and neuronal death, leading to severe neurological deficits. 2 , 3 Treatments against both the acute and chronic phases of stroke are limited, 4 , 5 , 6 necessitating research into therapeutic strategies to attenuate brain injury and facilitate functional recovery in stroke patients. The cellular and molecular mechanisms that determine stroke onset and injury progression are only beginning to be identified with the help of modern genetic research tools.
Microglia, the resident immune cells of the CNS, are widely distributed across the brain and spinal cord and form an integral part of the neurovascular unit. One of the major functions of microglia is to constantly survey the CNS microenvironment and maintain brain homeostasis. 7 , 8 Stroke injury induces rapid activation and migration of microglia to the lesion sites. 9 Microglia can undergo profound morphological changes (from ameboid to hyper‐ramified) and phenotypic polarization (from quiescent to active), culminating in the release of cytokines, chemokines, and other immune modulators. Microglial functions are largely dependent on their pro‐ or anti‐inflammatory phenotype at specific pathophysiological stages and in specific brain regions after stroke, underscoring the importance of both spatial and temporal dimensions in microglia dynamics. 7 In general, pro‐inflammatory microglia secrete detrimental cytokines and molecules that aggravate brain injury, whereas anti‐inflammatory microglia promote brain repair and facilitate neurological recovery. 10 As microglia harbor the inherent mechanisms to rapidly switch between detrimental vs. beneficial functions, modulating these characteristics may be suitable for the development of novel therapeutics for stroke. In this review, we summarize microglial biology and discuss their polarization and modulatory mechanisms, both in terms of brain injury during stroke and brain restoration during the post‐stroke recovery phase. Lastly, we describe recent breakthroughs in therapeutic strategies that target microglial responses after experimental stroke.
2. PHYSIOLOGICAL CHARACTERISTICS AND FUNCTIONS OF MICROGLIA IN THE BRAIN
2.1. Morphology of microglia in the developing and adult healthy brain
Microglia were first defined by Pío Del Río‐Hortega over a century ago. 11 Microglial cells first develop from primitive myeloid progenitors in the yolk sac and migrate to the CNS from embryonic day 8.5, until BBB closure on embryonic day 13. In the embryonic and early developmental stages, microglial development and differentiation precedes other CNS cells. 12 Microglia harbor a unique ameboid morphology during fetal brain development, and have distinct molecular and functional properties compared to adult microglia. 13
In the healthy adult brain, microglia numbers and spatial distribution are maintained by a process of self‐renewal. 14 The density of microglia is region‐specific, with higher numbers in the hippocampus and olfactory bulb, and lower numbers within fiber tracts. 15 There is also substantial variability in the size and morphology of microglia in different brain regions. 16 For example, microglia in gray matter have smaller somas than white matter, with process extensions. Microglia continually extend and retract these branches during CNS surveillance. 17 To facilitate migration from gray matter into white matter tracts, microglia transform from a ramified into an amoeboid shape, with rounded cell bodies and processes that orient along the direction of the fibers. 18 , 19 After exposure to challenges in the microenvironment, microglia transform into a more spherical shape and spring into action against toxic stimuli. 17 , 20 Aside from exposure to environmental challenges, aging also impacts microglial morphologies, with natural shortening of processes and an increase in somal volumes in aged brains. 21
2.2. Microglial function in the developing and adult healthy brain
Microglia maintain CNS homeostasis in both adult and developing brains. Microglia are aptly named the “engineers” of the CNS, as they participate in neurogenesis, programmed cell death, synapse elimination, and neural circuit formation during development. 22 , 23 During early development, neurotrophic factors secreted by microglia enhance neurogenesis and promote the survival and differentiation of specific neuronal lineages. 24 , 25 Additionally, immature neurons that undergo programmed cell death are cleared by microglia without triggering inflammatory processes. 26 , 27 Microglia also remodel the CNS by selectively pruning redundant neuronal processes and defective synapses that have the potential to hinder the maturation of neuronal circuitry. 28 , 29 Ultimately, the physiological roles of microglia help establish and strengthen functional and mature neuronal circuits during brain development.
In adult brains, microglia actively scan the brain microenvironment by extending and retracting their processes. Alterations in the brain microenvironment elicit rapid and profound changes in microglial morphologies and functions, inducing the expression of several pro‐survival molecules. Microglia also actively interact with neurons, influence neuronal regeneration, proliferation, migration, and secrete neurotrophic factors, such as insulin‐like growth factor‐1 (IGF‐1), to promote the health and survival of neighboring neurons. 30
Under pathological conditions, microglia are a critical component of the innate immune sentinel network and serve as the first line of defense in the CNS. Phagocytosis is an established microglial function designed for removal of cellular debris or dead cells, without necessarily initiating an inflammatory response. 31 , 32 Microglia quickly detect pathogens, including bacteria, fungi, and viruses that enter the brain parenchyma and respond by producing pro‐ and/or anti‐inflammatory cytokines, chemokines, and complement proteins. These reactions are designed to promote pathogen clearance, although at times may cause intense, collateral inflammatory reactions and exacerbate CNS pathology. On the other hand, the collateral inflammatory reactions and highly reactive cellular milieu may be essential to block and clear pathogenic infections and prevent organismal death.
3. MICROGLIAL POLARIZATION AND THEIR MODULATORY MECHANISMS AFTER STROKE
3.1. Microglial activation and phenotypic polarization after stroke
Microglia are among the first cells to respond to acute brain injury, with their activation lasting months after injury onset. In a transient ischemic stroke model, activated Iba1+ microglia emerge in the infarct core area within 24 hours and peak within 4–7 days after reperfusion. In the peri‐infarct region, microglia accumulate much earlier, within ~3.5 hours and peak at 7 days post‐reperfusion. 33 Activated microglia undergo rapid morphological changes, including thickening of processes and hypertrophy of somata. Activated microglia also upregulate cell surface markers and secrete pro‐ or anti‐inflammatory cytokines. Early studies classified activated microglia into “detrimental” M1 versus “protective” M2 subtypes according to their protein/cytokine expression profiles. M1 microglia secrete pro‐inflammatory cytokines, such as TNF‐α, IL‐1β, IL‐12, and IL‐23, and they can be detected by cell surface markers such as CD16, CD32, and CD86. In contrast, M2 microglia secrete anti‐inflammatory cytokines such as TGF‐β, IL‐4, IL‐10, IL‐13, and growth factors such as vascular endothelial growth factor (VEGF), brain‐derived neurotrophic factor (BDNF). In general, M2 microglia can be identified by CD206 and Arg1, among other markers. 34 , 35 The view that microglia are dichotomous and exclusively express either M1 or M2‐specific markers is the subject of recent controversy and regarded as oversimplified. For example, in the case of traumatic brain injury, M1/M2 microglial polarization may be concurrent 36 and with the emergence of single‐cell technologies and mass cytometry, novel microglial subtypes with distinct and complex molecular signatures have been identified. 37 , 38 Despite these observations and critiques, the terms “M1” and “M2” continue to be useful in distinguishing microglial phenotypes based on protein marker expression or pro‐ versus anti‐inflammatory functional effects.
Several groups have examined the spatiotemporal activation and polarization of microglia after stroke. 39 , 40 In ischemic stroke, activated microglia express M2 phenotypic markers in the acute stage. However, they gradually switch toward M1 phenotype at about 1 week and last for a few weeks after injury (Figure 1). 41 This phenotype shift may result from the recruitment of M1 microglia/macrophages to the injury site and the M2‐to‐M1 transition of local activated microglia/macrophages. In ICH, an M1 to M2 microglial/macrophage transition is observed in both collagenase‐induced ICH and blood‐induced ICH. 42 This transition usually happened within 1 week after injury and can last for at least 14 days after injury (Figure 1). 42 The reasons for the transition need further studies, but one of the major possibility might be the infiltration of M2‐like circulating blood monocytes or macrophages.
FIGURE 1.
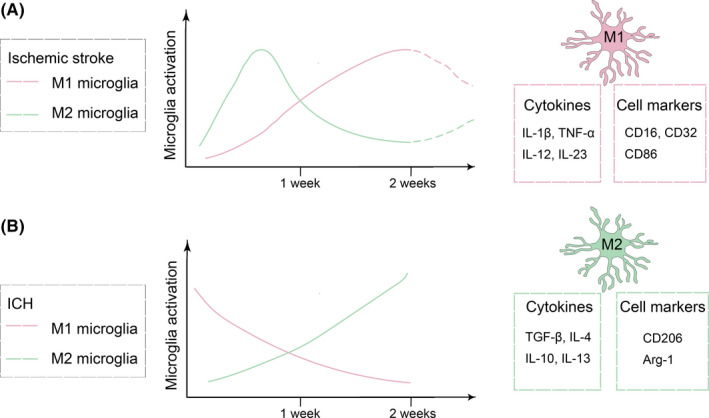
Temporal activation and polarization of microglia in ischemic stroke and intracerebral hemorrhage. (A) In ischemic stroke, microglia gradually switch from M2‐phenotype toward M1‐phenotype within 1 week after injury. (B) In the intracerebral hemorrhage (ICH) model, microglia with M1‐phenotype exhibit a decreasing trend, while M2‐like microglia exhibit an increasing trend in the first two weeks after injury. A few cell markers and cytokines expressed by M1 and M2 microglia are also shown
Imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) have enabled the examination of microglial activation in clinical stroke. Although patient brains showed activated microglia in acute, sub‐acute, and chronic phases after ischemic stroke, 43 the specific activation patterns in the infarct core and peri‐infarct regions vary across individuals. In some patients, microglia are initially chiefly activated in the ischemic core within 24 to 48 hours after stroke and only then gradually extend to the peri‐infarct area. 44 In another study, activated microglia increased in both the infarct core and peri‐infarct areas 7 days after stroke and the activation gradually decreased over time. 45 These divergent patterns of microglial activation may reflect differences in the initial severity of the injury, the status of the BBB in the ischemic core versus penumbra, and the absence or presence of sepsis in stroke patients. This clinical research area warrants further exploration. For example, clinician scientists might examine microglial activation in stroke patients early after injury and correlate these spatiotemporal patterns with subsequent neurological outcomes.
3.2. Molecules and mechanisms modulating microglial phenotype polarization after stroke
Below, we have summarized some of the more widely reported modulators of microglial phenotypic polarization post‐stroke injury (Figure 2).
FIGURE 2.
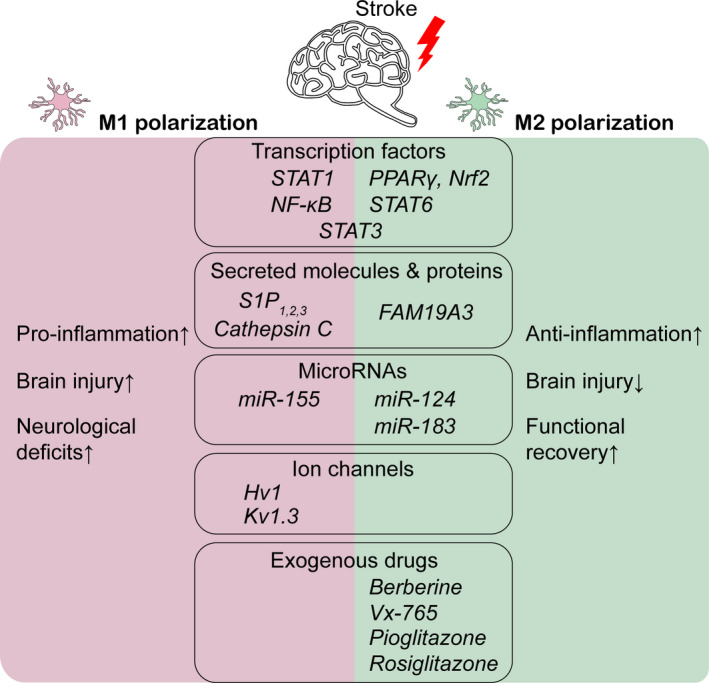
Modulators of microglia phenotype polarization after stroke. Molecules stimulating microglia toward M1 pro‐inflammatory polarization may exacerbate brain injury and increase neurological deficits. Anti‐inflammatory M2 microglia attenuate brain injury and promote functional recovery after stroke. This list is not meant to be exhaustive
3.2.1. Transcription factors
Peroxisome proliferator‐activated receptor gamma (PPARγ) belongs to a superfamily of nuclear receptors that contribute to antioxidant and anti‐inflammatory responses. 46 PPARγ agonists such as rosiglitazone 47 or PPARγ activating factors, such as 1, 25‐dihydroxyvitamin D3 (1, 25‐D3), recombinant human fibroblast growth factor 21 (rhFGF21), and oleic acid 48 , 49 , 50 attenuate brain inflammation and protect against ischemic injury in murine models of stroke. Specifically, microglia respond with a decrease in M1 phenotypic markers and a parallel increase in M2 markers after PPARγ activation. In ICH induced by intraparenchymal injections of blood, treatment with PPARγ agonists decreased M1 cytokine levels and promoted microglial phagocytosis of extravasated red blood cells, thereby mitigating the cytotoxic insult and encouraging hematoma resolution. 51 , 52
Members of the signal transducer and activator of transcription (STAT) family of transcription factors play key roles in cellular proliferation, immunity, and inflammation. 53 , 54 STAT1 controls M1 microglia/macrophage activation, 55 whereas STAT6 promotes M2‐phenotypic polarization 56 after cerebral ischemia. The effect of STAT3 is likely to be context‐dependent: In mice with collagenase‐induced ICH, phosphorylated (active) STAT3 was mainly present within activated microglia and macrophages, and inhibition of pSTAT3 reduced brain edema. 57 Conversely, microglia may be propelled toward the protective, anti‐inflammatory M2‐phenotype by modulating the STAT3 signaling pathway in ischemic injury models. 58 , 59
Other transcription factors implicated in microglial polarization after stroke injury include the NF‐κB and Nrf2‐related signaling pathways. Nuclear factor‐κB (NF‐κB) is a transcription factor that regulates the expression of M1‐signature genes. Activation of NF‐κB by toll‐like receptors (TLR2 and TLR4) increases pro‐inflammatory cytokine levels in ischemic stroke and ICH. 60 , 61 , 62 , 63 The nuclear factor erythroid 2‐related factor 2 (Nrf2) and its signaling pathway encourages microglia/macrophage polarization toward the M2 phenotype in ischemic stroke and ICH. 64 , 65 Hence, the net balance between NF‐κB versus Nrf2 pathway activation may partly determine the course of the injury and recovery phases after stroke.
3.2.2. MicroRNAs
MicroRNAs (miRNAs) are small, non‐coding RNAs that bind to mRNAs and play important roles in the regulation of gene expression at the post‐transcriptional level. A number of studies have elucidated the role of specific miRNAs in microglia polarization. 66 , 67 , 68 For example, miR‐124 modulates and shifts microglia from M1 to M2 phenotype in the sub‐acute phase after stroke 66 miR‐155, which is a well‐established microglia phenotype modulator, could promote M1 polarization after stroke. 69 miR‐183 reduces pro‐inflammatory IL‐1β, IL‐6, and TNF‐α in microglia via NF‐κB signaling in the middle cerebral artery occlusion (MCAO) model. 67
3.2.3. Secreted molecules and proteins
Sphingosine 1‐phosphate (S1P) is a lipid mediator secreted into the extracellular environment to regulate cell migration, differentiation, and survival 70 and has emerged as a microglia modulatory target in stroke. 71 , 72 , 73 In a brain ischemia model, signaling through the S1P1 receptor triggers microglial activation and impacts microglial polarization. 71 An antagonist of S1P1 activity, AUY954, attenuated the activation of M1‐relevant molecules ERK1/2 and p38, but increased activation of the M2‐relevant, Akt molecule. 71 Similarly, the S1P2‐specific receptor antagonist JTE013 suppressed M1‐relevant NF‐κB, ERK1/2, and JNK activation in activated microglia. 72 The S1P3 inhibitor CAY10444 mitigates ischemia‐induced neurological deficits, reduces microglia activation, and impacts pro‐inflammatory M1 polarization as well as phosphorylation of ERK1/2, p38 MAPK, and Akt. 73 Other secreted molecules also regulate microglia phenotype polarization. FAM19A3, a secreted protein in the brain, promotes polarization toward the M2 phenotype and ameliorates cerebral ischemia. 74
3.2.4. Ion channels
The ion channels Hv1 and Kv1.3 also regulate microglia polarization and inflammation. 75 , 76 Hv1−/− mice exhibit smaller infarcts and superior neurological performance, which are associated with a microglial polarity switch from M1 to M2. 75 Genetic knockout of Kv1.3 in microglia reduces the expression of LPS‐induced pro‐inflammatory mediators, including IL‐1β, TNF‐α, IL‐6, and iNOS, and reduces the LPS‐induced impairment in hippocampal long‐term potentiation. 76
3.2.5. Exogenous drugs
Several exogenous compounds and drugs are able to modulate microglial phenotype in experimental studies. 77 , 78 , 79 , 80 For example, the naturally occurring chemical compound berberine may protect against ischemic stroke by modulating microglial polarization. 77 , 78 Vx‐765, a small‐molecule caspase‐1 inhibitor, mediates M2 microglia/macrophage polarization by suppressing NF‐κB activation. 79 As mentioned previously, widely used PPARγ agonists such as pioglitazone and rosiglitazone can propel microglia toward anti‐inflammatory phenotypes. 81 The repurposing of these drugs may pave a new avenue for stroke treatment.
3.3. Contribution of infiltrated peripheral myeloid cells to stroke pathology and repair
Microglia are not the sole myeloid cell in the CNS even under resting, physiological conditions. Although microglia dominate in brain parenchyma, border associated macrophages (BAM) also reside in specialized compartments of the CNS and are named after the locations they inhabit, including leptomeningeal macrophages, choroid‐plexus macrophages, and perivascular macrophages. 82 CNS myeloid cells are distinct from peripheral myeloid cells in several aspects. Unlike peripheral monocytes that are constantly renewed from bone marrow stem cells, CNS myeloid cells undergo self‐renewal. 83 In addition, the turnover rate for CNS myeloid cells is much slower than peripheral myeloid cells, rendering them more vulnerable to loss of population numbers from toxic insults. It has also been reported that CD163+ BAM are reprogrammed after acute stroke, resulting in leukocyte chemotaxis and neurological impairments. 84 , 85 Perhaps for these reasons, BAM depletion reduces acute ischemic brain injury.
Under pathological conditions such as stroke, monocyte‐derived macrophages may cross the compromised BBB and invade the injured brain, thereby diversifying the population of myeloid cells and increasing their functional repertoires. Immature monocytes infiltrate into the infarct border within 24 hours of stroke and differentiate into mature phagocytes within the lesion boundaries. 86 Peripheral macrophages are most abundant in the ischemic brain within 3–7 days after transient focal cerebral ischemia, and the numbers decrease thereafter. 87 , 88 Mice with selective deletion of CCR2, a molecule essential for monocyte infiltration, show a delay, rather than abolishment of post‐stroke inflammation, which is accompanied by reduced angiogenesis and worse neurological performance. 89 However, further analyses in the same report indicated heterogeneities within the infiltrating CCR2+ monocytes, which may contribute to acute neuroinflammation and long‐term functional recovery. A recent study employing Cxcr4‐CreER‐mediated lineage‐tracing mice successfully distinguished hematopoietic stem cell‐derived monocytes from microglia and other tissue‐resident macrophages, thereby identifying critical functions of blood‐borne monocytes in initiation of a robust defense response after acute cerebral ischemia. 90
Microglia, brain‐resident macrophages, and peripheral monocyte‐derived macrophages occupy distinct functional niches, playing important and diverse roles in the ischemic brain. Distinguishing between these cells has been a technical challenge, as these cells all belong to a large family of mononuclear phagocytes and therefore share commonalities in gene expression. However, with recent advances in single‐cell technologies, several microglia‐specific markers (eg, Tmem119, P2ry12, and Sall1) and macrophage‐specific markers (eg, Mertk) have been identified and are now used to specify myeloid lineage cells. 91 Furthermore, a number of new technical approaches can distinguish the roles of microglia and macrophages in stroke. Bone marrow chimeric mice are frequently used to examine the spatial distributions of microglia versus peripheral macrophages in the ischemic brain and to explore the contribution of infiltrating immune cells. 56 , 92 , 93 Recent studies also have leveraged the cre‐loxp system and the development of novel cell type‐specific cre mice to further enhance our ability to distinguish the functions of distinct myeloid cells. For example, Tmem119‐CreER mice and P2ry12‐CreER mice have been generated and are available for brain‐resident microglia‐specific exploration. 94 , 95 Cxcr4‐CreER mice are also valuable tools for research on hematopoietic stem cell‐derived monocytes. 90 In the wake of rapid technological advances, these novel approaches are expected to shed light on mechanistic underpinnings of the different myeloid compartments in stroke injury and post‐stroke brain repair.
4. DIVERSE FUNCTIONS OF ACTIVATED MICROGLIA/MACROPHAGES IN STROKE
4.1. Impact on BBB integrity
Microglia are vital components of the neurovascular unit and regulate BBB integrity after stroke. 96 Microglia are activated acutely after stroke and upregulate the expression of transcription factors, including hypoxia‐inducible factor‐1 (HIF‐1) and NF‐κB, as well as reactive oxygen species (ROS) and nitric oxide (NO). The latter molecules contribute to endothelial damage and BBB hyperpermeability. 41 , 97 In addition, perivascular microglial/macrophage activation elevates the release of pro‐inflammatory cytokines, such as IL‐6, IL‐1β, and TNFα, all of which are known to be detrimental to BBB integrity during the early phase of ischemia. 98 , 99 , 100 , 101 Endothelial cells are also activated by these pro‐inflammatory cytokines, leading to an increase in the expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM‐1), P‐selectin, and vascular cell adhesion protein (VCAM), which in turn enhance the infiltration of circulating leukocytes into brain parenchyma, leading to further brain inflammation in late phases after stroke. 102 In addition, in vivo two‐photon live imaging suggests that activated microglia/macrophages can migrate to blood vessels and engulf endothelial cells by phagocytosis soon after reperfusion, which causes an eventual breakdown of the BBB. 103 In contrast, anti‐inflammatory microglia/macrophages may facilitate long‐term neurovascular remodeling and improve neurological functions at late phases after stroke. 104 A recent study found that depletion of microglia damages BBB integrity after ischemic stroke, perhaps due to the neutrophils recruited into the infarct area, which are not engulfed because of the depletion of microglia. 105 In sum, these studies reveal diverse roles for microglia in stroke, which may be influenced by injury stage, pro‐ versus anti‐inflammatory status, cell‐cell interactions with non‐microglial cells, and the nature of the surrounding cellular milieu.
4.2. Impact on neurogenesis
As mentioned above, microglia density is high in the hippocampus and olfactory bulb, two regions that undergo neurogenesis even into adulthood. Release of pro‐ or anti‐inflammatory cytokines by microglia influences the proliferation and differentiation of neural stem/progenitor cells (NPCs) and neurogenesis after stroke. 106 In an ex vivo study, live sections from ischemic brains incubated with conditioned media from M2‐microglia showed an increase in the proliferation and differentiation of NPCs within the subventricular zone (SVZ) neurogenic niche. 107 Strategies to switch microglia/macrophages from the M1 to M2 phenotype promote proliferation and differentiation of NPCs and increase neuronal densities in experimental stroke models. 108 , 109 , 110 An appropriate inflammatory milieu is likely to provide a suitable microenvironment for neurogenesis but detailed mechanisms underlying the inflammatory responses that regulate neurogenesis remain unknown. Furthermore, microglia might also influence neurogenesis by regulating the migration of NPCs. Recently, a distinct population of microglia with special genetic profiles were shown to assist in the migration of NPCs from the SVZ and integration of NPCs into other brain regions, but these mechanisms remain to be verified in stroke models. 111
4.3. Impact on angiogenesis
The impact of microglia/macrophage activation on post‐stroke neurovascular remodeling and angiogenesis predominantly depends upon polarization phenotype. 106 Titrating microglia/macrophage activation to an anti‐inflammatory phenotype may facilitate angiogenesis in experimental stroke. 112 , 113 , 114 , 115 A recent study transplanted IL‐4 (M2)‐polarized BV2 microglia into mouse ischemic brains and identified higher angiogenin expression. 116 VEGF is a major participant in the regulation of normal and pathological angiogenesis. Microglia/macrophages mediate vascular sprouting via the VEGF signaling pathway by directly expressing VEGF isoforms at the later phases of stroke. 117 Moreover, microglia can also indirectly promote the release of VEGF‐A and platelet‐derived growth factor‐BB (PDGF‐BB) from endothelial cells, thereby enhancing angiogenesis in microglia and endothelial co‐culture cell systems. 118
4.4. Impact on white matter integrity
At acute phases of stroke, overexpression of pro‐inflammatory factors such as iNOS and TNF‐α by activated microglia/macrophages may play a pivotal role in damaging oligodendroglial progenitor cells (OPCs) and oligodendrocytes (OLs), resulting in white matter impairment. 119 , 120 , 121 However, shifting microglia/macrophages toward M2‐phenotypes may promote white matter integrity and oligodendrogenesis in the sub‐acute or chronic phases after stroke. 58 , 122 , 123 , 124 , 125 For example, deletion of Na+/H+ exchanger, Nhe1, in microglia/macrophages successfully promotes the expression of anti‐inflammatory cytokines, including Ym1, TGF‐β, and IL‐10, which subsequently stimulates white matter remyelination at 14 days after stroke. 126 In addition, the phagocytic capabilities of microglia/macrophages enable engulfment of myelin debris and provide neuroprotection in stroke. 127 In an experimental ICH model, white matter fibers within the hematoma may be used by microglia/macrophages as a scaffold to infiltrate into the hematoma and assist in its clearance. 128
4.5. Impact on neuroplasticity
Neuroplasticity after stroke includes not only restoration of neural networks and circuitry, but also rewiring of functional connections. In recent years, there is a growing consensus that microglia/macrophages play a central role in regulating neuroplasticity, although the exact underlying mechanisms remain poorly understood. Experimental evidence suggests that microglia/macrophages can rapidly modify neuronal activity and modulate synaptic function, thereby supporting recovery from stroke injuries. 129 After transient experimental cerebral ischemia, the duration of contact between microglia/macrophage processes and synapses is substantially increased and is frequently followed by the removal of the presynaptic boutons. 130 Further, some synapses in ischemic areas disappear after the establishment of these prolonged microglia‐neuron interactions, indicating that these glial cells support an increase in synaptic turnover. 130 Functionally, a heightened neuroinflammatory response in microglia/macrophages can rapidly modify neuronal activity and modulate synaptic function. Specifically, it was found that activation of microglia/macrophages by an inflammatory stimulus may drive long‐term synaptic depression (LTD) in neurons by NADPH oxidase, one of the main mediators of neurotoxicity in stroke. 131 Studies examining the differential impact of microglia/macrophage phenotypic polarization on synaptic plasticity after stroke are needed.
5. THERAPEUTIC OPPORTUNITIES AND CHALLENGES IN TARGETING MICROGLIA/MACROPHAGES IN STROKE
5.1. Cell‐based therapy: Microglial transplantation and alloreplacement
Microglia transplantation or alloreplacement (by non‐self cells) has been proposed to attenuate brain injury and improve neurological outcomes after stroke. In a rat MCAO model, exogenous microglia injected into the cerebral ventricles migrate to the lesion site, reducing the associated neurodegeneration and behavioral deficits. 132 , 133 In chronic cerebral ischemia, transplantation of HMO6 cells (a human microglia cell line) inhibited ischemia‐induced white matter damage by reducing MMP‐2 in microglia. 134 Although these studies transplanted untreated microglia, others advocate use of polarized (typically M2) microglia in preclinical models. Transplantation of IL‐4‐pretreated BV2 microglia into recipient mice 45 minutes after MCAO ameliorated ischemia‐induced brain damage and promoted angiogenesis. 116 Thus, microglial transplantation has emerged as a novel therapeutic strategy to ameliorate stroke injury and improve neurological function. However, some studies have shown contradictory results. In a permanent cerebral ischemia model, rats were injected with microglia through the tail vein at 24 hours after stroke injury. These cells, however, failed to protect the brain and improve neurological function. 135 These discrepancies might be because of differences in animal models, transplantation strategies, intervention delivery routes and regimens, and other features of the experimental paradigms. In addition to direct transplantation of microglia, some studies have explored cell‐based therapies by transferring monocytes/macrophages, 136 bone marrow stromal cells, 137 umbilical cord cells, 138 , 139 and mesenchymal stem cells 140 into recipient animals after stroke; the transplanted cells modulated inflammatory responses and exerted neuroprotection.
One significant limitation of the traditional transplantation method is the low replacement efficiency, which may be another reason for inconsistent therapeutic outcomes of microglia transplantation or alloreplacement. In 2020, Xu et al. reported three highly efficient strategies for microglia replacement globally in the CNS or in specific brain regions. 141 Compared to traditional methods, they created niches in the mouse brain that were microglia‐free, prior to non‐self cell transplantation. Specifically, PLX5622‐formulated chow and whole‐body irradiation were employed to deplete CNS‐resident microglia before transplantation. These microglia‐free niches aided survival and engraftment of transplanted cells. The transplantation strategies also open up a window for treatment of microglia‐associated CNS disorders, aside from stroke. 141 Although microglia alloreplacement showed therapeutic promise, more studies are needed to understand the exact role, mechanism, and integration of grafted microglia and their impact on outcomes after stroke.
5.2. Harnessing the protective molecules synthesized and released by microglia/macrophages
Protective microglia/macrophages produce beneficial molecules to attenuate brain damage and promote recovery after stroke. For example, TGF‐α derived by M2‐microglia enhances proliferation and differentiation of NPCs in the SVZ of mice after cerebral ischemia. 107 Exosomes derived from M2‐microglia/macrophages carry molecules such as miR‐124 that can protect mice from post‐stroke cognitive impairment and promote functional recovery. 142 , 143 , 144 , 145 The matricellular glycoprotein osteopontin (OPN) produced by infiltrating macrophages re‐establishes the integrity of the BBB after ischemic stroke. 146 Similarly, inhibitors that block the detrimental factors produced by microglia/macrophages can also achieve neuroprotection after stroke. For example, adiposomes derived from microglia have pro‐inflammatory and/or pro‐death effects in ischemic brains, and inhibiting adiposome formation with chemicals such as NS‐398 reduces neuroinflammation, brain infarct volume, and motor deficits. 147 M1‐phenotype microglia secrete TNF‐α, which may disintegrate the BBB and induce endothelial necroptosis after ischemic stroke. However, infliximab, a drug that inhibits TNFα, reduces these pathologies and improves neurological scores. 100 Despite these studies demonstrating the therapeutic potential of modulating microglia‐derived soluble factors, many challenges remain to translate these findings into clinical application.
5.3. Gene correction or manipulation of intracellular phenotypic switches in microglia/macrophages
Recent studies have identified several intracellular molecular switches that control phenotypic changes in microglia/macrophages. Precise gene correction or manipulation of these switches may lead to novel therapies that boost repair functions of microglia/macrophages after stroke. miRNAs are important intracellular molecular switches that regulate microglial phenotypic change. 148 As described above, miRNA‐124 is one such potential miRNA for stroke therapy. Injection of miRNA‐124 to mice with stroke injury shifts the polarization of microglia from pro‐inflammatory to the anti‐inflammatory phenotype and upregulates the expression of Arg‐1, which is associated with neuronal protection and post‐stroke recovery. 149 , 150 On the other hand, the well‐characterized miRNA‐155 promotes microglia M1 polarization, and siRNA‐mediated knockdown of miRNA‐155 in BV2 microglial cells mitigates pro‐inflammatory damage induced by LPS. 151 In addition to miRNAs, strategies targeted at modulating the expression of other molecules, such as STATs and PPARγ, also have the potential to promote stroke recovery. 56 , 152
Precise gene correction in microglia offers important insights into neurological diseases. Triggering receptor expressed on myeloid cells 2 (TREM2) is upregulated in microglia of mice subjected to MCAO. 153 Knockdown of microglial TREM2 intensifies the pro‐inflammatory response and exacerbates brain injury, due perhaps to the phagocytic function of TREM2, which helps clear apoptotic cell debris. 154 For precise gene correction, adeno‐associated viruses (AAVs) and retroviruses are most commonly used as vehicles for gene delivery. Intrahippocampal injection of AAV particles containing RNAi for silencing cyclin‐dependent kinase 5 (CDK5) prevents microglial hyperreactivities, hippocampal degeneration, and cognitive dysfunction after cerebral ischemia. 155 However, as AAV does not specifically target microglia per se, translatable strategies employing recombinant AAVs with microglia cell‐specific, promoter‐driven expression are urgently needed.
5.4. Repopulation/rejuvenation of microglia/macrophages
Microglia/macrophage repopulation or rejuvenation by genetic or pharmacological tools has potential in modulating neurological functions in different brain disorders. 156 , 157 In 24‐month‐old mice, depletion and repopulation of microglia with the colony‐stimulating factor 1 (CSF1) receptor inhibitor PLX5622 improves spatial memories and reverses age‐related changes in gene expression in neurons. 158 Repopulation of microglia in organotypic hippocampal slice culture after removal of PLX3397, another widely used CSF1 receptor inhibitor, provides an anti‐inflammatory, trophic environment and returns pro‐inflammatory cytokines to normal levels. 159 The source of the repopulated microglia remains an obvious and important question to answer. Some studies support the view that peripheral myeloid cells may be engrafted to occupy the CNS microglial niches after microglia depletion. 160 , 161 Others believe that microglial repopulation relies on self‐renewal of CNS‐resident microglia. 162 , 163 In either case, there is general agreement that freshly repopulated microglia tend to exhibit a neuroprotective and pro‐regenerative phenotype, which facilitates brain repair and alleviates neurological deficits after aging or brain injury.
In a model of ~80% hippocampal neuronal loss, microglial elimination followed by repopulation promoted recovery of cognitive function in mice by regulating synaptic plasticity. 164 In traumatic brain injury (TBI), although microglial depletion does not alter cognitive performance, repopulating microglia attenuate cognitive deficits and promote neurogenesis via IL‐6 trans‐signaling. 165 CSF1R inhibition effectively and sustainably depletes microglia in two experimental models of ICH (induced by injection of collagenase or autologous blood) and attenuates ICH‐induced neurological deficits and edema by promoting BBB integrity. 166 In transient focal cerebral ischemia, depletion of microglia by administration of PLX3397 for 21 days exacerbates neurological deficits and brain infarction at 24 hours after MCAO. 167 , 168 However, microglial repopulation occurs within 2 weeks after withdrawing PLX3997 from the diet, and repopulation reverses the brain infarction induced by microglial depletion. 168 Although microglia repopulation after depletion shows therapeutic potential against stroke, there are still limitations of the approach. For example, pharmacological and systemic depletion of microglia may have adverse effects on peripheral immune cells that also express the targeted receptor. Although CSF1R inhibitors have been examined in clinical settings (eg, NCT01329991, NCT01282684) for their safety and tolerability, further studies are needed to better understand the underlying mechanisms of microglia depletion and repopulation and to improve the specificity of this approach for clinical application.
5.5. Challenges in the therapeutic targeting of microglia in stroke injury
Although extensive preclinical studies support the therapeutic potential of targeting microglia for stroke, several hurdles remain before this approach can be translated into effective clinical use. First, microglia switch their phenotypes dynamically in a spatiotemporal pattern after brain injuries, including stroke. Therefore, therapeutic targeting of microglia phenotype must be precise in terms of ischemic phases, brain regions, and timing. Second, the molecules and signaling pathways that underlie microglial phenotypic regulation may have other functions. For example, miRNAs not only participate in microglia‐induced responses, but also multiple other neuropathological responses within other cell types. Manipulation of these factors may generate unwanted bystander effects. Although genetic tools have improved our mechanistic understanding of the contribution of microglia to stroke outcomes, these may not be feasible in patients or sufficiently safe. Third, the critical impact of aging and sex differences on microglial function are largely neglected in preclinical studies but are likely to influence clinical outcomes. Fourth, preclinical stroke models do not fully recapitulate the heterogeneity of the human disease—a major reason for failure of clinical translation of neuroprotectants. Similarly, heterogeneities exist at the cellular level, as microglial polarization phenotypes are not mutually exclusive. Finally, indiscriminate targeting of all microglia may elicit changes in nearby cells (eg, astroglia) to compensate for loss of innate immune function. Thus, fine‐tuning the immune response may be superior as a therapeutic approach, rather than systemic and nonspecific loss of a cell type that serves as a first‐responder to injury and disease.
6. SUMMARY
This review provides an overview of microglia in brain injury and repair after stroke. As the major innate immune cells of the CNS, microglia are the professional phagocytes of the brain and spinal cord and also participate in numerous developmental events, including neurogenesis and neural circuit formation. In the adult brain, microglia continue to serve as immune sentinels to monitor the microenvironment and maintain homeostasis. After stroke injury, microglia undergo rapid morphological changes, polarizing into pro‐ or anti‐inflammatory phenotypes, and steering the course of further degeneration or eventual recovery. Microglia/macrophage polarization can be regulated by several modulators, including transcription factors, microRNAs, and drug delivery. Targeting microglial phenotype through these modulators may help combat stroke injury and encourage tissue repair and functional recovery.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENT
This project was supported by the University of Pittsburgh School of Medicine. X. J. is supported by the American Heart Association Postdoctoral Fellowship 18POST33960201. X. H. is supported by VA Merit Review Grants. We thank Patricia Strickler for administrative support.
Lyu, Xie and Bhatia are contributed equally to this work.
Contributor Information
Xiaoming Hu, Email: xij24@pitt.edu, Email: hux2@upmc.edu.
Xiaoyan Jiang, Email: xij24@pitt.edu, Email: hux2@upmc.edu.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. Lancet Neurol. 2009;8(4):355‐369. [DOI] [PubMed] [Google Scholar]
- 2. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2018;134(Pt B):240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hankey GJ. Stroke. Lancet. 2017;389(10069):641‐654. [DOI] [PubMed] [Google Scholar]
- 5. Hankey GJ. Secondary stroke prevention. Lancet Neurol. 2014;13(2):178‐194. [DOI] [PubMed] [Google Scholar]
- 6. Ren H, Han R, Chen X, et al. Potential therapeutic targets for intracerebral hemorrhage‐associated inflammation: an update. J Cereb Blood Flow Metab. 2020;40(9):1752‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018‐1027. [DOI] [PubMed] [Google Scholar]
- 8. Xie D, He M, Hu X. Microglia/macrophage diversities in central nervous system physiology and pathology. CNS Neurosci Ther. 2019;25(12):1287‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurogibol. 2017;157:247‐272. [DOI] [PubMed] [Google Scholar]
- 10. Qin C, Zhou LQ, Ma XT, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35(5):921‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R‐H. P. Estudios sobre la neuroglía. La microglía y su transformación en células en bastoncito y cuerpos gránulo‐adiposos. Trab Lab Invest Biol Univ Madrid. 1920;18:37‐82. [Google Scholar]
- 12. Frost JL, Schafer DP. Microglia: architects of the developing nervous system. Trends Cell Biol. 2016;26(8):587‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cengiz P, Zafer D, Chandrashekhar JH, et al. Developmental differences in microglia morphology and gene expression during normal brain development and in response to hypoxia‐ischemia. Neurochem Int. 2019;127:137‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikodemova M, Kimyon RS, De I, Small AL, Collier LS, Watters JJ. Microglial numbers attain adult levels after undergoing a rapid decrease in cell number in the third postnatal week. J Neuroimmunol. 2015;278:280‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kongsui R, Beynon SB, Johnson SJ, Walker FR. Quantitative assessment of microglial morphology and density reveals remarkable consistency in the distribution and morphology of cells within the healthy prefrontal cortex of the rat. J Neuroinflammation. 2014;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes V. Microglia: The constant gardeners. Nature. 2012;485(7400):570‐572. [DOI] [PubMed] [Google Scholar]
- 18. Arcuri C, Mecca C, Bianchi R, Giambanco I, Donato R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Front Mol Neurosci. 2017;10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torres‐Platas SG, Comeau S, Rachalski A, et al. Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation. 2014;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314‐1318. [DOI] [PubMed] [Google Scholar]
- 21. Perkins AE, Piazza MK, Deak T. Stereological analysis of microglia in aged male and female fischer 344 rats in socially relevant brain regions. Neuroscience. 2018;377:40‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harry GJ. Microglia during development and aging. Pharmacol Ther. 2013;139(3):313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schafer DP, Stevens B. Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol. 2015;7(10):a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ueno M, Fujita Y, Tanaka T, et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543‐551. [DOI] [PubMed] [Google Scholar]
- 25. Trang T, Beggs S, Salter MW. Brain‐derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol. 2011;7(1):99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells‐2. J Exp Med. 2005;201(4):647‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hristova M, Cuthill D, Zbarsky V, et al. Activation and deactivation of periventricular white matter phagocytes during postnatal mouse development. Glia. 2010;58(1):11‐28. [DOI] [PubMed] [Google Scholar]
- 28. Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456‐1458. [DOI] [PubMed] [Google Scholar]
- 29. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron. 2012;74(4):691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bilbo S, Stevens B. Microglia: the brain's first responders. Cerebrum. 2017;2017:14. [PMC free article] [PubMed] [Google Scholar]
- 31. Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31(45):16064‐16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen RX, Shen H, Huang SX, et al. P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci Ther. 2020;26(4):416‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium‐binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32(5):1208‐1215. [DOI] [PubMed] [Google Scholar]
- 34. Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lambertsen KL, Finsen B, Clausen BH. Post‐stroke inflammation‐target or tool for therapy? Acta Neuropathol. 2019;137(5):693‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morganti JM, Riparip LK, Rosi S. Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PLoS One. 2016;11(1):e0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stratoulias V, Venero JL, Tremblay ME, Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019;38(17):e101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jordao MJC, Sankowski R, Brendecke SM, et al. Single‐cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363(6425):eaat7554. [DOI] [PubMed] [Google Scholar]
- 39. Kluge MG, Abdolhoseini M, Zalewska K, et al. Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post‐stroke. J Cereb Blood Flow Metab. 2019;39(12):2456‐2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rodhe J, Burguillos MA, de Pablos RM, et al. Spatio‐temporal activation of caspase‐8 in myeloid cells upon ischemic stroke. Acta Neuropathol Commun. 2016;4(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063‐3070. [DOI] [PubMed] [Google Scholar]
- 42. Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13(7):420‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krupinski J, Kaluza J, Kumar P, Kumar S. Immunocytochemical studies of cellular reaction in human ischemic brain stroke. MAB anti‐CD68 stains macrophages, astrocytes and microglial cells in infarcted area. Folia Neuropathol. 1996;34(1):17‐24. [PubMed] [Google Scholar]
- 45. Gulyas B, Toth M, Schain M, et al. Evolution of microglial activation in ischaemic core and peri‐infarct regions after stroke: a PET study with the TSPO molecular imaging biomarker [((11))C]vinpocetine. J Neurol Sci. 2012;320(1–2):110‐117. [DOI] [PubMed] [Google Scholar]
- 46. Neve BP, Fruchart JC, Staels B. Role of the peroxisome proliferator‐activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol. 2000;60(8):1245‐1250. [DOI] [PubMed] [Google Scholar]
- 47. Li Y, Zhu ZY, Lu BW, et al. Rosiglitazone ameliorates tissue plasminogen activator‐induced brain hemorrhage after stroke. CNS Neurosci Ther. 2019;25(12):1343‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo T, Wang Y, Guo Y, et al. 1, 25–D3 protects from cerebral ischemia by maintaining bbb permeability via PPAR‐gamma activation. Front Cell Neurosci. 2018;12:480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Wang D, Liu F, Zhu L, et al. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J Neuroinflammation. 2020;17(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song J, Kim YS, Lee DH, et al. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci Rep. 2019;9(1):10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator‐activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61(4):352‐362. [DOI] [PubMed] [Google Scholar]
- 52. Zhao X, Grotta J, Gonzales N, Aronowski J. Hematoma resolution as a therapeutic target: the role of microglia/macrophages. Stroke. 2009;40(3 Suppl):S92‐94. [DOI] [PubMed] [Google Scholar]
- 53. Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of Jak‐STAT signaling in the immune system. Nat Immunol. 2017;18(4):374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK‐STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77(5):521‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boddaert J, Bielen K, sJongers B et al. CD8 signaling in microglia/macrophage M1 polarization in a rat model of cerebral ischemia. PLoS One. 2018;13(1):e0186937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cai W, Dai X, Chen J, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight. 2019;4(20);e131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim CK, Ryu WS, Choi IY, et al. Detrimental effects of leptin on intracerebral hemorrhage via the STAT3 signal pathway. J Cereb Blood Flow Metab. 2013;33(6):944‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qin C, Fan WH, Liu Q, et al. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48(12):3336‐3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu ZJ, Ran YY, Qie SY, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti‐inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019;25(12):1353‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu R, Liao XY, Pan MX, et al. glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of m1 microglial polarization via the NF‐kappaB p65/Hif‐1alpha signaling pathway. J Immunol. 2019;202(6):1704‐1714. [DOI] [PubMed] [Google Scholar]
- 61. Lin S, Yin Q, Zhong Q, et al. Heme activates TLR4‐mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang YC, Zhou Y, Fang H, et al. Toll‐like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann Neurol. 2014;75(6):876‐889. [DOI] [PubMed] [Google Scholar]
- 63. Jin X, Liu MY, Zhang DF, et al. Baicalin mitigates cognitive impairment and protects neurons from microglia‐mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF‐kappaB signaling pathway. CNS Neurosci Ther. 2019;25(5):575‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization‐new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Naito Y, Takagi T, Higashimura Y. Heme oxygenase‐1 and anti‐inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83‐88. [DOI] [PubMed] [Google Scholar]
- 66. Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA‐124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP‐alpha‐PU.1 pathway. Nat Med. 2011;17(1):64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xiang B, Zhong P, Fang L, Wu X, Song Y, Yuan H. miR‐183 inhibits microglia activation and expression of inflammatory factors in rats with cerebral ischemia reperfusion via NF‐kappaB signaling pathway. Exp Ther Med. 2019;18(4):2540‐2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bernstein DL, Zuluaga‐Ramirez V, Gajghate S, et al. miR‐98 reduces endothelial dysfunction by protecting blood‐brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab. 2020;40(10):1953‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pena‐Philippides JC, Caballero‐Garrido E, Lordkipanidze T, Roitbak T. In vivo inhibition of miR‐155 significantly alters post‐stroke inflammatory response. J Neuroinflammation. 2016;13(1):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mendelson K, Evans T, Hla T. Sphingosine 1‐phosphate signalling. Development. 2014;141(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gaire BP, Bae YJ, Choi JW. S1P1 regulates M1/M2 polarization toward brain injury after transient focal cerebral ischemia. Biomol Ther (Seoul). 2019;27:522‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sapkota A, Gaire BP, Kang MG, Choi JW. S1P2 contributes to microglial activation and M1 polarization following cerebral ischemia through ERK1/2 and JNK. Sci Rep. 2019;9(1):12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gaire BP, Song MR, Choi JW. Sphingosine 1‐phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J Neuroinflammation. 2018;15(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shao Y, Deng T, Zhang T, Li P, Wang Y. FAM19A3, a novel secreted protein, modulates the microglia/macrophage polarization dynamics and ameliorates cerebral ischemia. FEBS Lett. 2015;589(4):467‐475. [DOI] [PubMed] [Google Scholar]
- 75. Tian DS, Li CY, Qin C, Murugan M, Wu LJ, Liu JL. Deficiency in the voltage‐gated proton channel Hv1 increases M2 polarization of microglia and attenuates brain damage from photothrombotic ischemic stroke. J Neurochem. 2016;139(1):96‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Di Lucente J, Nguyen HM, Wulff H, Jin LW, Maezawa I. The voltage‐gated potassium channel Kv1.3 is required for microglial pro‐inflammatory activation in vivo. Glia. 2018;66(9):1881‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu J, Cao D, Guo C, et al. berberine facilitates angiogenesis against ischemic stroke through modulating microglial polarization via AMPK signaling. Cell Mol Neurobiol. 2019;39(6):751‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu DQ, Chen SP, Sun J, et al. Berberine protects against ischemia‐reperfusion injury: a review of evidence from animal models and clinical studies. Pharmacol Res. 2019;148:104385. [DOI] [PubMed] [Google Scholar]
- 79. Li Q, Dai Z, Cao Y, Wang L. Caspase‐1 inhibition mediates neuroprotection in experimental stroke by polarizing M2 microglia/macrophage and suppressing NF‐kappaB activation. Biochem Biophys Res Commun. 2019;513(2):479‐485. [DOI] [PubMed] [Google Scholar]
- 80. Hong Shi DC, Gao Y. Potential role of omega‐3 polyunsaturated fatty acids in ischemic stroke: review on clinical and preclinical studies. Condition Med. 2019;2(4):152‐163. [Google Scholar]
- 81. Gamdzyk M, Lenahan C, Tang J, Zhang JH. Role of peroxisome proliferator‐activated receptors in stroke prevention and therapy‐The best is yet to come? J Neurosci Res. 2020;98(11):2275‐2289. [DOI] [PubMed] [Google Scholar]
- 82. Kierdorf K, Masuda T, Jordao MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20(9):547‐562. [DOI] [PubMed] [Google Scholar]
- 83. Prinz M, Erny D, Hagemeyer N. Erratum: Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol. 2017;18(8):951. [DOI] [PubMed] [Google Scholar]
- 84. Pedragosa J, Salas‐Perdomo A, Gallizioli M, et al. CNS‐border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol Commun. 2018;6(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rajan WD, Wojtas B, Gielniewski B, et al. Defining molecular identity and fates of CNS‐border associated macrophages after ischemic stroke in rodents and humans. Neurobiol Dis. 2020;137:104722. [DOI] [PubMed] [Google Scholar]
- 86. Gliem M, Mausberg AK, Lee JI, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71(6):743‐752. [DOI] [PubMed] [Google Scholar]
- 87. Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 88. Breckwoldt MO, Chen JW, Stangenberg L, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci USA. 2008;105(47):18584‐18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pedragosa J, Miro‐Mur F, Otxoa‐de‐Amezaga A, et al. CCR2 deficiency in monocytes impairs angiogenesis and functional recovery after ischemic stroke in mice. J Cereb Blood Flow Metab. 2020;40(1_suppl):S98‐S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Werner Y, Mass E, Ashok Kumar P, et al. Cxcr4 distinguishes HSC‐derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nat Neurosci. 2020;23(3):351‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sousa C, Biber K, Michelucci A. Cellular and molecular characterization of microglia: a unique immune cell population. Front Immunol. 2017;8:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tanaka R, Komine‐Kobayashi M, Mochizuki H, et al. Migration of enhanced green fluorescent protein expressing bone marrow‐derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117(3):531‐539. [DOI] [PubMed] [Google Scholar]
- 93. Yang Y, Liu H, Zhang H, et al. ST2/IL‐33‐dependent microglial response limits acute ischemic brain injury. J Neurosci. 2017;37(18):4692‐4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kaiser T, Feng G. Tmem119‐EGFP and Tmem119‐CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro. 2019;6(4):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McKinsey GL, Lizama CO, Keown‐Lang AE, et al. A new genetic strategy for targeting microglia in development and disease. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. da Fonseca AC, Matias D, Garcia C, et al. The impact of microglial activation on blood‐brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kahles T, Luedike P, Endres M, et al. NADPH oxidase plays a central role in blood‐brain barrier damage in experimental stroke. Stroke. 2007;38(11):3000‐3006. [DOI] [PubMed] [Google Scholar]
- 98. Richter F, Eitner A, Leuchtweis J, Bauer R, Lehmenkuhler A, Schaible HG. Effects of interleukin‐1ss on cortical spreading depolarization and cerebral vasculature. J Cereb Blood Flow Metab. 2017;37(5):1791‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677‐1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen AQ, Fang Z, Chen XL, et al. Microglia‐derived TNF‐alpha mediates endothelial necroptosis aggravating blood brain‐barrier disruption after ischemic stroke. Cell Death Dis. 2019;10(7):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhao J, Mu H, Liu L, et al. Transient selective brain cooling confers neurovascular and functional protection from acute to chronic stages of ischemia/reperfusion brain injury. J Cereb Blood Flow Metab. 2019;39(7):1215‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jiang X, Andjelkovic AV, Zhu L, et al. Blood‐brain barrier dysfunction and recovery after ischemic stroke. Prog Neurogibol. 2018;164:144‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jolivel V, Bicker F, Biname F, et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015;129(2):279‐295. [DOI] [PubMed] [Google Scholar]
- 104. Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood‐brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Otxoa‐de‐Amezaga A, Miro‐Mur F, Pedragosa J, et al. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol. 2019;137(2):321‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang X, Xuan W, Zhu ZY, et al. The evolving role of neuro‐immune interaction in brain repair after cerebral ischemic stroke. CNS Neurosci Ther. 2018;24(12):1100‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Choi JY, Kim JY, Kim JY, Park J, Lee WT, Lee JE. M2 phenotype microglia‐derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp Neurobiol. 2017;26(1):33‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yang L, Tucker D, Dong Y, et al. Photobiomodulation therapy promotes neurogenesis by improving post‐stroke local microenvironment and stimulating neuroprogenitor cells. Exp Neurol. 2018;299(Pt A):86‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xiang J, Zhang X, Fu J, Wang H, Zhao Y. USP18 overexpression protects against focal cerebral ischemia injury in mice by suppressing microglial activation. Neuroscience. 2019;419:121‐128. [DOI] [PubMed] [Google Scholar]
- 110. Geng W, Tang H, Luo S, et al. Exosomes from miRNA‐126‐modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 2019;11(2):780‐792. [PMC free article] [PubMed] [Google Scholar]
- 111. Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de Menezes JR, Nedergaard M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci. 2015;35(34):11848‐11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Song D, Zhang X, Chen J, et al. Wnt canonical pathway activator TWS119 drives microglial anti‐inflammatory activation and facilitates neurological recovery following experimental stroke. J Neuroinflammation. 2019;16(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jin Q, Cheng J, Liu Y, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131‐142. [DOI] [PubMed] [Google Scholar]
- 114. Logan AC, Katzman MA, Balanza‐Martinez V. Natural environments, ancestral diets, and microbial ecology: is there a modern "paleo‐deficit disorder"? Part II. J Physiol Anthropol. 2015;34:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shang K, He J, Zou J, et al. Fingolimod promotes angiogenesis and attenuates ischemic brain damage via modulating microglial polarization. Brain Res. 2020;1726:146509. [DOI] [PubMed] [Google Scholar]
- 116. Tian Y, Zhu P, Liu S, et al. IL‐4‐polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv Clin Exp Med. 2019;28(4):421‐430. [DOI] [PubMed] [Google Scholar]
- 117. Zhao X, Eyo UB, Murugan M, Wu LJ. Microglial interactions with the neurovascular system in physiology and pathology. Dev Neurobiol. 2018;78(6):604‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ding X, Gu R, Zhang M, et al. Microglia enhanced the angiogenesis, migration and proliferation of co‐cultured RMECs. BMC Ophthalmol. 2018;18(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Deng Y, Lu J, Sivakumar V, Ling EA, Kaur C. Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain Pathol. 2008;18(3):387‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Uchida H, Fujita Y, Matsueda M, et al. Damage to neurons and oligodendrocytes in the hippocampal CA1 sector after transient focal ischemia in rats. Cell Mol Neurobiol. 2010;30(7):1125‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee J, Hamanaka G, Lo EH, Arai K. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther. 2019;25(12):1290‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age‐related long‐term deficits in neurological function after stroke. Exp Neurol. 2015;272:109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Han L, Cai W, Mao L, et al. Rosiglitazone promotes white matter integrity and long‐term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628‐2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu LQ, Liu XR, Zhao JY, et al. Brain‐selective mild hypothermia promotes long‐term white matter integrity after ischemic stroke in mice. CNS Neurosci Ther. 2018;24(12):1275‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dai X, Chen J, Xu F, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab. 2020;40(3):639‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Song S, Wang S, Pigott VM, et al. Selective role of Na(+) /H(+) exchanger in Cx3cr1(+) microglial activation, white matter demyelination, and post‐stroke function recovery. Glia. 2018;66(11):2279‐2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu Y, Wu C, Hou Z, et al. Pseudoginsenoside‐F11 accelerates microglial phagocytosis of myelin debris and attenuates cerebral ischemic injury through complement receptor 3. Neuroscience. 2020;426:33‐49. [DOI] [PubMed] [Google Scholar]
- 128. Chen J, Koduri S, Dai S, et al. Intra‐hematomal white matter tracts act as a scaffold for macrophage infiltration after intracerebral hemorrhage. Transl Stroke Res. 2020;22:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sandvig I, Augestad IL, Haberg AK, Sandvig A. Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur J Neurosci. 2018;47(12):1414‐1428. [DOI] [PubMed] [Google Scholar]
- 130. Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974‐3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang J, Malik A, Choi HB, Ko RW, Dissing‐Olesen L, MacVicar BA. Microglial CR3 activation triggers long‐term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82(1):195‐207. [DOI] [PubMed] [Google Scholar]
- 132. Kitamura Y, Takata K, Inden M, et al. Intracerebroventricular injection of microglia protects against focal brain ischemia. J Pharmacol Sci. 2004;94(2):203‐206. [DOI] [PubMed] [Google Scholar]
- 133. Kitamura Y, Yanagisawa D, Inden M, et al. Recovery of focal brain ischemia‐induced behavioral dysfunction by intracerebroventricular injection of microglia. J Pharmacol Sci. 2005;97(2):289‐293. [DOI] [PubMed] [Google Scholar]
- 134. Narantuya D, Nagai A, Sheikh AM, et al. Microglia transplantation attenuates white matter injury in rat chronic ischemia model via matrix metalloproteinase‐2 inhibition. Brain Res. 2010;1316:145‐152. [DOI] [PubMed] [Google Scholar]
- 135. Jiang C, Wang J, Yu L, et al. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav Brain Res. 2013;250:222‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Garcia‐Bonilla L, Brea D, Benakis C, et al. Endogenous protection from ischemic brain injury by preconditioned monocytes. J Neurosci. 2018;38(30):6722‐6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ohtaki H, Ylostalo JH, Foraker JE, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105(38):14638‐14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Womble TA, Green S, Shahaduzzaman M, et al. Monocytes are essential for the neuroprotective effect of human cord blood cells following middle cerebral artery occlusion in rat. Mol Cell Neurosci. 2014;59:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nadia Sadanandan MS, Park YJ, Cho J, Borlongan CV, Jea‐Young L. The potential of umbilical cord to afford natural conditioning in three different mesenchymal stem cell populations for stroke therapy. Condition Med. 2020;2(4):199‐203. [Google Scholar]
- 140. Wang LQ, Lin ZZ, Zhang HX, et al. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci Ther. 2014;20(4):317‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu Z, Rao Y, Huang Y, et al. Efficient strategies for microglia replacement in the central nervous system. Cell Rep. 2020;32(6):108041. [DOI] [PubMed] [Google Scholar]
- 142. Song Y, Li Z, He T, et al. M2 microglia‐derived exosomes protect the mouse brain from ischemia‐reperfusion injury via exosomal miR‐124. Theranostics. 2019;9(10):2910‐2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yang HC, Zhang M, Wu R, et al. C‐C chemokine receptor type 2‐overexpressing exosomes alleviated experimental post‐stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J Stem Cells. 2020;12(2):152‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zheng Y, He R, Wang P, Shi Y, Zhao L, Liang J. Exosomes from LPS‐stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7(5):2037‐2049. [DOI] [PubMed] [Google Scholar]
- 145. Li F, Zhao L, Shi Y, Liang J. Edaravone‐loaded macrophage‐derived exosomes enhance neuroprotection in the rat permanent middle cerebral artery occlusion model of stroke. Mol Pharm. 2020;17(9):3192‐3201. [DOI] [PubMed] [Google Scholar]
- 146. Gliem M, Krammes K, Liaw L, van Rooijen N, Hartung HP, Jander S. Macrophage‐derived osteopontin induces reactive astrocyte polarization and promotes re‐establishment of the blood brain barrier after ischemic stroke. Glia. 2015;63(12):2198‐2207. [DOI] [PubMed] [Google Scholar]
- 147. Lin CH, Liao LY, Yang TY, et al. Microglia‐derived adiposomes are potential targets for the treatment of ischemic stroke. Cell Mol Neurobiol. 2019;39(5):591‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Guo Y, Hong W, Wang X, et al. MicroRNAs in microglia: how do micrornas affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front Mol Neurosci. 2019;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hamzei Taj S, Kho W, Aswendt M, et al. Dynamic modulation of microglia/macrophage polarization by miR‐124 after focal cerebral ischemia. J Neuroimmune Pharmacol. 2016;11(4):733‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M. MiRNA‐124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials. 2016;91:151‐165. [DOI] [PubMed] [Google Scholar]
- 151. Yin H, Song S, Pan X. Knockdown of miR‐155 protects microglia against LPS‐induced inflammatory injury via targeting RACK1: a novel research for intracranial infection. J Inflamm (Lond). 2017;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR‐gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci. 2007;28(5):244‐249. [DOI] [PubMed] [Google Scholar]
- 153. Kurisu K, Zheng Z, Kim JY, et al. Triggering receptor expressed on myeloid cells‐2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J Cereb Blood Flow Metab. 2019;39(10):1906‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wu R, Li X, Xu P, et al. TREM2 protects against cerebral ischemia/reperfusion injury. Mol Brain. 2017;10(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gutierrez‐Vargas JA, Munera A, Cardona‐Gomez GP. CDK5 knockdown prevents hippocampal degeneration and cognitive dysfunction produced by cerebral ischemia. J Cereb Blood Flow Metab. 2015;35(12):1937‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Waisman A, Ginhoux F, Greter M, Bruttger J. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol. 2015;36(10):625‐636. [DOI] [PubMed] [Google Scholar]
- 157. Li LZ, Huang YY, Yang ZH, Zhang SJ, Han ZP, Luo YM. Potential microglia‐based interventions for stroke. CNS Neurosci Ther. 2020;26(3):288‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Elmore MRP, Hohsfield LA, Kramar EA, et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 2018;17(6):e12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Coleman LG Jr, Zou J, Crews FT. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J Neuroinflammation. 2020;17(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Varvel NH, Grathwohl SA, Baumann F, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109(44):18150‐18155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lund H, Pieber M, Parsa R, et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia‐like cells. Nat Commun. 2018;9(1):4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bruttger J, Karram K, Wortge S, et al. Genetic cell ablation reveals clusters of local self‐renewing microglia in the mammalian central nervous system. Immunity. 2015;43(1):92‐106. [DOI] [PubMed] [Google Scholar]
- 163. Huang Y, Xu Z, Xiong S, et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci. 2018;21(4):530‐540. [DOI] [PubMed] [Google Scholar]
- 164. Rice RA, Pham J, Lee RJ, Najafi AR, West BL, Green KN. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia. 2017;65(6):931‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Willis EF, MacDonald KPA, Nguyen QH, et al. Repopulating microglia promote brain repair in an IL‐6‐dependent manner. Cell. 2020;180(5):833‐846 e816. [DOI] [PubMed] [Google Scholar]
- 166. Li M, Li Z, Ren H, et al. Colony stimulating factor 1 receptor inhibition eliminates microglia and attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2017;37(7):2383‐2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jin WN, Shi SX, Li Z, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab. 2017;37(6):2224‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Szalay G, Martinecz B, Lenart N, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


