Abstract
Objective:
Behavioral inhibition (BI) is an early developing trait associated with cautiousness and development of clinical depression and anxiety. Little is known about the neural basis of BI and its predictive importance concerning risk for internalizing disorders. We looked at functional connectivity of the default-mode network (DMN) and salience network (SN), given their respective roles in self-relational and threat processing, in the risk for internalizing disorders, with an emphasis on determining the functional significance of these networks for BI.
Method:
We used functional magnetic resonance imaging to scan, during the resting state, children and adolescents 8 to 17 years of age who were either at high familial risk (HR; n = 16) or low familial risk (LR; n = 18) for developing clinical depression and/or anxiety. Whole-brain DMN and SN functional connectivity were estimated for each participant and compared across groups. We also compared the LR and HR groups on levels of BI and anxiety, and incorporated these data into follow-up neurobehavioral correlation analyses.
Results:
The HR group, relative to the LR group, showed significantly decreased DMN connectivity with the ventral striatum and bilateral sensorimotor cortices. Within the HR group, trait BI increased as DMN connectivity with the ventral striatum and sensorimotor cortex decreased. The HR and LR groups did not differ with respect to SN connectivity.
Conclusion:
Our findings show, in the risk for internalizing disorders, a negative functional relation between brain regions supporting self-relational processes and reward prediction. These findings represent a potential neural substrate for behavioral inhibition in the risk for clinical depression and anxiety.
Keywords: depression risk, default-mode, ventral striatum, behavioral inhibition, fMRI
Major depressive disorder (MDD) is among the leading causes of disability worldwide.1,2 Given the substantial contribution of MDD to the global burden of disease, a great deal of research has focused on the etiology and pathophysiology of depression, and has implicated neural, genetic, and environmental factors.3–6 To better understand trajectories toward MDD on a developmental time-scale, significant attention has been devoted to studying the children without depression of adults with clinical depression.7–12 In this context, prospective longitudinal work has shown that the children of parents with depression are 3 times more likely to develop a depressive or bipolar disorder than are children without a parent with depression.13 Moreover, having a familial history of depression opens the door to a broader spectrum of risk for psychopathology, with children of parents with depression at significantly elevated risk for developing substance use and anxiety disorders.13 Therefore, although these children are most at risk for developing a mood disorder among other Axis I disorders,13 they are best understood in terms of pluripotency for internalizing and substance use disorders more generally.
The recognized importance of identifying risk factors for depression and anxiety has motivated a new generation of research comparing samples at high to low familial risk on a variety of biological dimensions. Neural investigations of children who have never been depressed who have family members with depression have typically used established experimental paradigms from affective neuroscience to identify neural functional abnormalities in young persons at elevated risk for MDD. These studies have found that adolescents and young adults most at risk exhibit neural responses to emotionally valenced stimuli similar to those that characterize adults with mood and/or anxiety disorders.14,15 Specifically, researchers have observed, in the risk for MDD, increased response of limbic prefrontal cortical networks and decreased response of dorsal prefrontal cortical networks under conditions of negative affective challenge. For example, a recent study incorporating sad mood induction found greater activation of the amygdala in the risk for MDD11; another study of neural response to sad film clips found increased activation of the insula and right caudate nucleus9 in youth at high versus low risk for depression. Similarly, a recent study of neural response during fearful-face processing found greater amygdala and nucleus accumbens activation to fearful faces in children and teens at risk for MDD.7 Highlighting a failure of dorsal prefrontal cortical response under negative affective conditions in the risk for depression, another fear-processing study reported decreased response to fearful faces in the dorsolateral prefrontal cortex (DLPFC) in high- versus low-risk groups.10 Similarly, researchers examining the neural correlates of regulation of an induced sad mood reported decreased activation in DLPFC in high-risk participants.11
The extant research examining the functional neural substrates of familial risk for MDD shows a pattern of neural response in children at elevated risk for depression that is common with that observed in adults with depression15 and anxiety.14 In furthering our understanding of neural-level foundations of familial risk for developing internalizing disorders, however, it is important that we incorporate knowledge of the intermediate behavioral phenotypes in the trajectory from familial risk for depression to the spectrum of internalizing disorders for which the children of parents with depression are at risk. Specifically, we propose that although it is important to consider the neural correspondence between risk for depression and MDD and clinical anxiety, it is critical that we address the more proximate objective of identifying links between neural function and emotional traits that are expressed abnormally in children at high risk—traits that could be crucial in the transition from mental health to mental illness. Given that higher levels of anxiety—as broadly construed—in children have been shown in longitudinal investigations to predict higher rates of clinically significant depression16 and anxiety,17 it is reasonable to investigate the neural underpinnings of anxious behaviors as a phenotypic link between the risk for depression and expression of MDD and/or clinical anxiety. Therefore, we examined, in the present investigation, the neural correlates of behavioral inhibition (BI) in the context of familial risk for mood and anxiety disorders. BI is a temperamental characteristic linked to fear-associated behaviors such as shyness and cautiousness18; moreover, BI has been observed reliably in infancy19,20 and shown to be stable across childhood.21 Elevated BI has been observed in children of parents with MDD18 and is a risk factor for the development of clinical anxiety,22 which in turn is a risk factor for depression.23,24 Moreover, elevated BI directly predicts subsequent development of depression25; furthermore, heightened expression of this trait persists into and beyond the first episode of MDD.26
We examined BI in the familial risk for mood and anxiety disorders from the perspective of resting-state functional magnetic resonance imaging (fMRI) investigations of the brain’s intrinsic functional connectivity. Such investigations have consistently found correlated activity among distinct brain networks in a task-free context.27 To date, researchers have identified at least 7 networks that show distinct functions and patterns of interrelated activity.28 In the present investigation, we compared youth at high versus low familial risk for internalizing disorders with respect to connectivity of 2 of the most reliably observed intrinsic networks: the default-mode network (DMN) and the salience network (SN). The DMN, comprising the ventromedial prefrontal cortex, posterior cingulate cortex, and inferior parietal lobe, is a postulated neural substrate for encoding and elaborating on information from an egocentric reference frame.29,30 Consistent with this formulation, functional abnormalities in the DMN have been found to be correlated with levels of self-relational, ruminative thinking in MDD.31,32 The SN, which comprises the amygdala and fronto-insular and dorsal anterior cingulate cortices, is hypothesized to subserve processing of personally and biologically relevant stimuli.33 Furthermore, meta-analyses of functional neuroimaging data have shown this network to play a significant role in depression17 and anxiety.14
Given that conceptualizations of self-relational cognition (e.g., introversion, shyness) and biological relevance (e.g., threat sensitivity, caution) in BI map well onto the postulated functions of the DMN and SN, respectively, we hypothesized that, in addition to documenting greater BI in youth at familial risk for depression and/or anxiety, we would observe significant differences between high- and low-risk groups in DMN and SN connectivity in limbic and sensorimotor networks necessary for motivating and executing behaviors. We further postulated that, within the high-risk group, the degree of DMN and SN connectivity in regions that show differential connectivity between groups would be correlated significantly with levels of BI. More specifically, we postulated that a qualitative shift in the functional characteristics of these regions, as evidenced by markedly abnormal connectivity with the DMN and/or SN, in the high-risk group would have a unique association with BI in this group.
METHOD
Participants
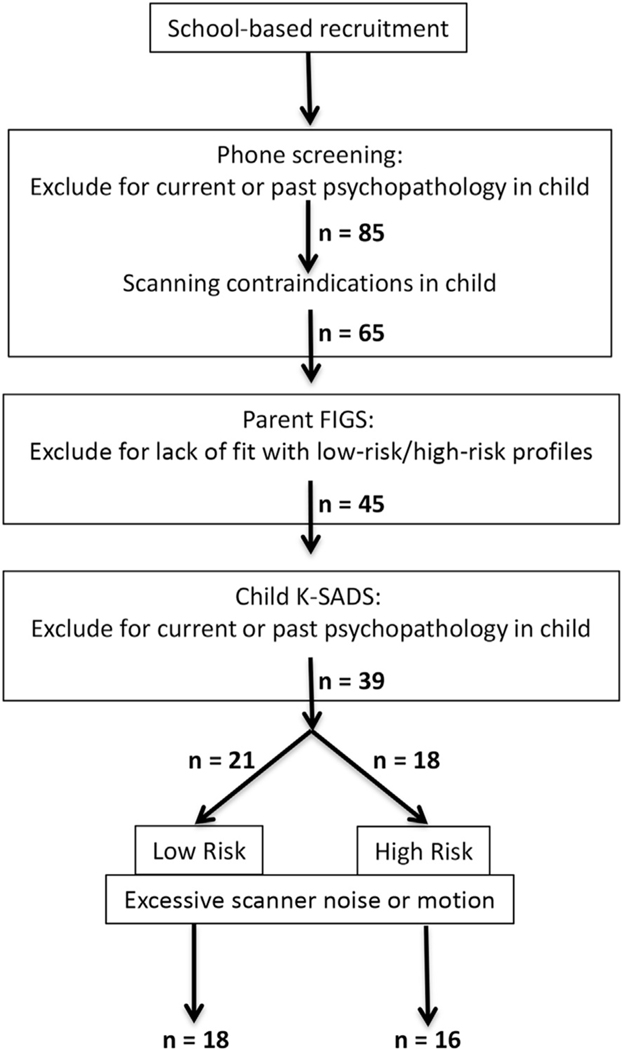
Eighteen children and adolescents (aged 8–17 years; 9 male and 9 female) at low risk (LR), and 16 children and adolescents (aged 8–17 years; 8 male and 8 female) at high risk (HR) for developing a mood and/or anxiety disorder, participated in this study, along with their mothers. Figure 1 provides a flow chart depicting the recruitment and screening processes. Children and adolescents, regardless of familial psychiatric history, were recruited from area schools and then phone screened for the presence of personal psychiatric history and scanning contraindication. Qualifying individuals were then assessed diagnostically by a trained psychiatric interviewer (J.S.) using the Kiddie Schedule of Affective Disorders and Schizophrenia (K-SADS).34 Mothers were assessed for mental disorders via the Structured Clinical Interview for DSM-IV-TR (SCID).35 Corroboration of both child and mother diagnoses was obtained using an unstructured diagnostic interview with a psychiatrist; in all cases, the psychiatric interviewer and psychiatrist agreed on the diagnosis determined by the interviewer. Mental health histories from first-degree relatives (mother, father, siblings) and second-degree relatives (genetically related aunts, uncles, and grandparents) were obtained from mothers using the Family Interview for Genetic Studies (FIGS).36 The FIGS is a clinician-administered instrument for systematically collecting psychiatric data about relatives in family studies of psychiatric disorders. For both HR and LR participants, mothers provided full first-degree family histories; once HR status was determined, second-degree histories were not acquired. Only for LR participants were second-degree histories investigated; this was done to ensure a “clean” family history for children and adolescents in the LR group. Thus, LR participants both did not meet criteria for any psychiatric disorder and had no first- or second-degree relative with a major psychiatric disorder. The HR participants also did not meet criteria for any current or past psychiatric disorder but had at least 1 first-degree relative with a diagnosis of MDD or bipolar disorder (BD). As mentioned here, we included in the group at elevated risk for MDD children and adolescents with a first-degree relative with either MDD or BD. We made this methodological decision based on the significant genetic relation between MDD and BD as revealed by data showing that the monozygotic twin of a proband with BD is twice as likely to have MDD as the dizygotic twin of a proband with BD. Importantly, in the context of the present study of familial depression, the dizygotic twin of a proband with BD is approximately 3 times as likely to have MDD as BD; this indicates that, with the exception of monozygotic twins, familial BD is more associated with MDD than is BD.37
FIGURE 1.
Flow chart depicting sequence of recruitment, screening, and assessment of participants. Note: FIGS = Family Interview for Genetic Studies; K-SADS = Kiddie Schedule of Affective Disorders and Schizophrenia.
Children and adolescents completed self-report versions of the Children’s Depression Inventory (CDI),38 the Multidimensional Anxiety Scale for Children (MASC),39,40 and the Behavioral Inhibition Questionnaire (BIQ).41 Importantly, in light of the relatively broad age range of participants included in this study, the validity and reliability of the CDI, MASC, and BIQ have been established in children and adolescents from elementary through high school age.38,40,41 To identify any demographic and cognitive differences between LR and HR groups, we administered and compared groups with respect to the Pubertal Development Scale,42 the Wechsler Abbreviated Scale of Intelligence,43 the Hollingshead Four Factor Index of Social Status,44 and the Edinburgh Handedness Inventory.45
Resting-State fMRI
To identify the DMN and SN in each participant, we conducted seed-based functional connectivity analysis46 using DMN and SN seed regions, that is, 3 seed regions averaged together to calculate a single statistical map for each network, optimized for pediatric samples,47 on whole-head resting-state fMRI data. We de-noised the fMRI data using despiking and low-pass filtering algorithms available in Analysis of Functional NeuroImages (AFNI)48 distribution. We further corrected the fMRI data for influences of measurement artifacts,49 cardiac pulsatility and respiratory motion,50 and changes in respiratory rate51 and heart rate.52 In addition, we applied to the data (as noise regressors) translational and rotational motion regressors and their first derivatives, as well as regressors for unmodeled residual noise53 (see Figure S1, available online). Finally, we applied a censoring/scrubbing procedure for removing effects on fMRI signal of participant motion greater than 0.2 mm during a given acquisition. Supplement 1, available online, provides a full description of each of the procedures briefly presented here.
Comparing Intrinsic Networks Between Groups
We compared the LR and HR groups with respect to DMN and SN connectivity by conducting voxel-wise t tests that included age and gender as noise covariates, as opposed to effects of interest, given that we were underpowered to use a factorial design. Using AFNIS’s 3dClustSim (see Table S1, available online, for additional information) applied to a gray matter mask constructed from our pediatric template brain, we calculated voxelwise and cluster thresholds (p = .025; k = 21 voxels) to keep familywise error at αcorrected = 0.05.
Neural–Behavioral Correlation Analyses
Within each group separately, we computed correlations of BIQ and MASC scores with fit coefficients from regions that showed between-group differences in intrinsic network connectivity. While examining correlations between the neural data and BIQ scores extended from the study’s hypotheses, which were therefore not subject to multiple comparisons correction, we also computed correlations with MASC scores to investigate the specificity of any significant neurobehavioral correlations involving BIQ scores. Moreover, before conducting these separate correlation analyses, we computed the correlations between the BIQ and MASC to ensure that these measures were not significantly related to one another (see Table S2, available online). We conducted these analyses separately within each group to avoid identifying a correlation in the data that aliased already-identified between-group differences. To determine the significance of computed correlations, we used a criterion of α = 0.05, 2-tailed, uncorrected for multiple comparisons.
RESULTS
Demographic and Clinical Characteristics
Demographic and questionnaire data for the LR and HR groups are presented in Table 1. The 2 groups did not differ with respect to age, gender composition, handedness, intelligence, socioeconomic status, or pubertal status (all p > .10). None of the participants met criteria for an Axis I disorder, and the groups did not significantly differ with respect to levels of depressive symptomatology as indexed with the CDI (p > .10). The LR and HR groups differed significantly on the measures of behavioral inhibition (BIQ) and anxiety (MASC), with the HR group reporting significantly higher levels of behavioral inhibition and anxiety than did the LR group (both p < .05). In neither group did BIQ, CDI, and MASC scores significantly correlate with one another (all p > .10; see Table S2, available online). For descriptive purposes, we present in Table S3 (available online) first-degree family psychiatric profiles for the HR group.
TABLE 1.
Demographics and Clinical and Behavioral Data
| High-Risk Mean (SE)/Frequency | Low-Risk Mean (SE)/Frequency | p | |
|---|---|---|---|
| Gender | 8 female, 8 male | 9 female, 9 male | >.10 |
| Age, y | 13.2 (0.7) | 12.6 (0.7) | >.10 |
| PDS Pubertal Status | 13.4 (1.2) | 14.6 (1.2) | >.10 |
| WASI | 115.1 (3.5) | 112.4 (1.9) | >.10 |
| Hollingshead SES Index | 53.5 (2.7) | 54.4 (2.8) | >.10 |
| EHI Laterality Quotient | 85.5 (3.9) | 72.6 (10.9) | >.10 |
| CDI Total t score | 41.9 (1.6) | 40.2 (1.4) | >.10 |
| MASC Total t score | 44.8 (1.9) | 38.8 (1.6) | <.05 |
| BIQ raw score | 90.4 (4.6) | 64.8 (4.9) | <.05 |
| TRs excluded from analysis | 2.4 (0.9) | 2.6 (1.0) | >.10 |
Note: Boldface text indicates statistically significant probability values. BIQ = Behavioral Inhibition Questionnaire; CDI = Child Depression Inventory; EHI = Edinburgh Handedness Inventory; MASC = Multidimensional Anxiety Scale for Children; PDS = Pubertal Development Scale; SE = standard error of the mean; SES = socioeconomic status; TR = time to repeat; WASI = Wechsler Abbreviated Scale of Intelligence.
Imaging Data Quality Measures
We found generally low participant motion during scanning and high temporal signal-to-noise ratio (TSNR). Estimates of framewise displacement were 0.057 mm (standard error of the mean [SE] = 0.01 mm) in the HR group and 0.066 mm (SE = 0.01 mm) in the LR group. TSNR averaged 143.21 (SE = 35.91) in the HR group and 141.01 (SE = 33.24) in the LR group. The groups did not differ with respect to average framewise displacement (t32 = 0.936, p > .10), percentage of censored TRs (t32 = 0.349, p > .10), or average TSNR (t32 = 0.694, p > .10). For visual quality inspection, readers are directed to Figure S2, available online, in which we present a DMN connectivity map averaged across all participants.
Between-Group Differences in Intrinsic Network Connectivity
SN Connectivity.
We observed no significant group difference in brainwide SN connectivity.
DMN Connectivity.
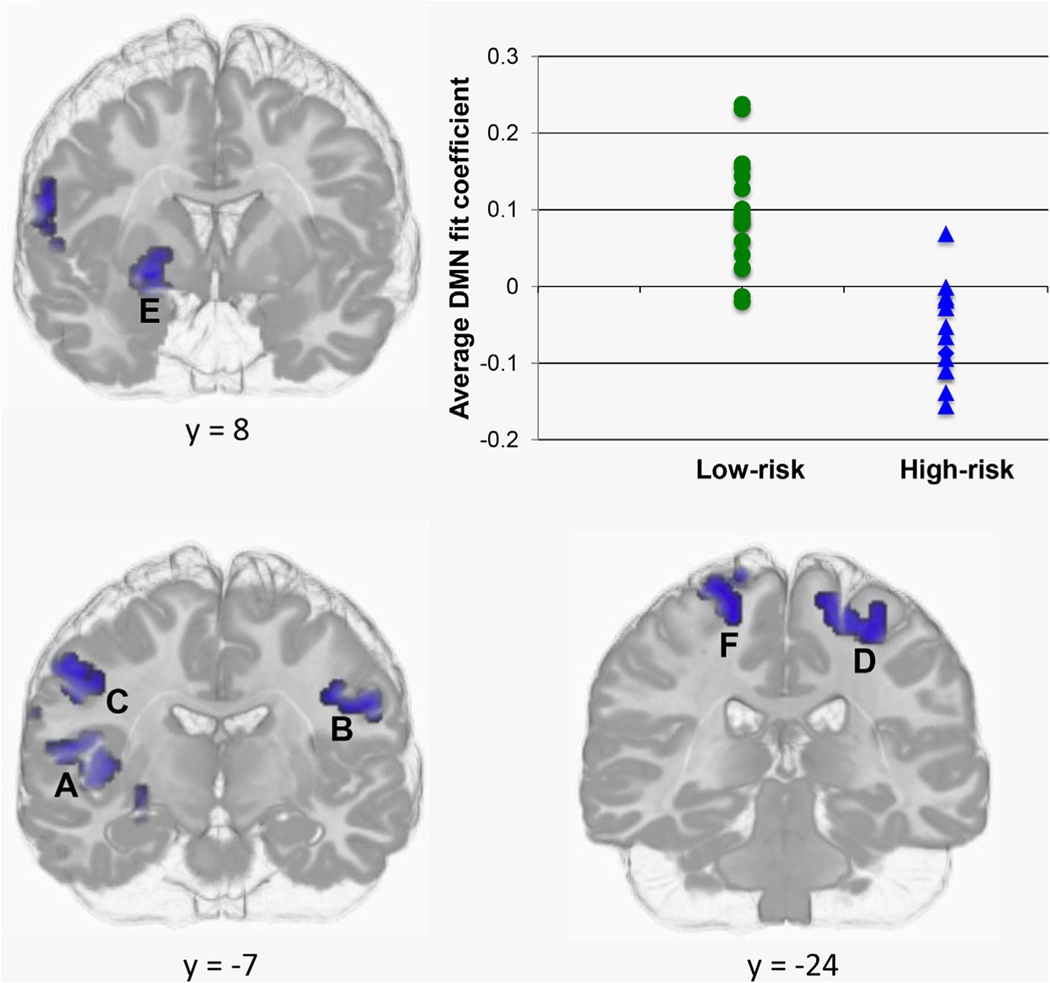
Our comparisons of DMN connectivity maps revealed significantly reduced DMN connectivity in the HR relative to the LR group in the ventral striatum and motor and sensory regions. Specifically, the HR group showed negative connectivity with the DMN in a part of the right ventral striatum considered the limbic component of the striatum54 as well as bilateral pre- and postcentral gyri and right posterior insular cortex; conversely, the LR group showed positive connectivity between the DMN and these structures (Figure 2). In Table 2, we present the centers of mass, cluster sizes, and between-groups effect sizes (Cohen’s d55) associated with each of these regions.
FIGURE 2.
Decreased connectivity with the default-mode network (DMN) in right ventral striatum, and bilateral pre- and postcentral gyri in high-risk versus low-risk participant groups. Note: Given the similar between-group differences in DMN connectivity in regions identified in the analysis, we show, in the scatter plot, averaged fit coefficients across these regions for each participant in each group. (See Table 2 for coordinates and sizes of clusters A to F in this figure.)
TABLE 2.
Cluster Sizes and Centers of Mass (Montreal Neurological Institute) in Regions Showing Decreased Default Mode Network Connectivity in High-Risk Relative to Low-Risk Groups
| Region | Cluster size (voxels) | X | Y | Z | Cohen’s d |
|---|---|---|---|---|---|
| A. Right posterior insula | 53 | 43 | −11 | 6 | 1.95 |
| B. Left sensorimotor cortex | 32 | −44 | −13 | 33 | 1.59 |
| C. Right sensorimotor cortex | 30 | 52 | −6 | 39 | 1.46 |
| D. Left sensorimotor cortex | 28 | −23 | −25 | 60 | 1.92 |
| E. Right ventral striatum | 25 | 24 | 0 | −9 | 2.29 |
| F. Right sensorimotor cortex | 21 | 19 | −25 | 71 | 1.35 |
Neural Behavioral Correlations
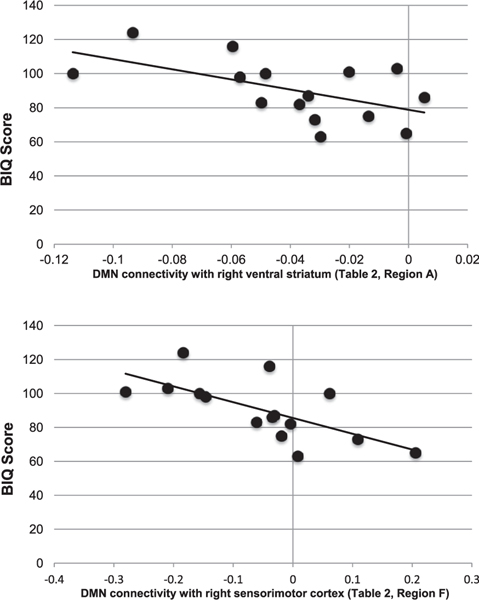
Within the HR group alone, we found that BIQ scores were significantly correlated with connectivity between the DMN and right ventral striatum and right pre- and postcentral gyri (HR group: r14 = −0.55 and −0.67, respectively, both p < .05 (Figure 3); LR group: r16 = −0.29 and 0.41, respectively, both p > .10). The correlation between BIQ scores and DMN connectivity was significantly different between groups for the pre- and postcentral gyri (p < .05) but not the ventral striatum (p > .10). MASC scores were not significantly correlated with DMN connectivity in any brain region within either group (all p > .10).
FIGURE 3.
Scatter plots depicting significant correlations in the high-risk (HR) participant group between Behavioral Inhibition Questionnaire (BIQ) scores and default-mode network (DMN) connectivity in regions showing differential connectivity between groups. Note: In only the HR group, decreasing DMN connectivity with the ventral striatum (top) and sensorimotor cortex (bottom) significantly predicts increasing BIQ scores.
DISCUSSION
In the present study, we used fMRI-based intrinsic functional connectivity analysis to examine the neural substrates of BI in children and adolescents at familial risk for internalizing disorders such as depression and anxiety. As expected, although no child or adolescent in our study met criteria for any Axis I psychopathology, participants in the HR group scored significantly higher than did those in the LR group with respect to BI. Furthermore, in the HR group alone, we observed significant anticorrelations of DMN activity with activity in the right ventral striatum and bilateral sensorimotor cortices. Moreover, we found only in the HR group that stronger anticorrelations of DMN activity with activity in the right ventral striatum and right sensorimotor cortex were associated with higher levels of trait BI. As we detail below, these findings present an intuitive neural substrate for BI in the risk for internalizing disorders such as depression and anxiety.
Proposed Neural Model of BI in Risk for Depression and Anxiety
Our results implicate the DMN and ventral striatum in behavioral inhibition associated with familial risk for internalizing disorders. In understanding how the abnormal relation between these regions could constitute a neural substrate for BI in children or adolescents at risk for a clinically significant depressive and/or anxiety disorder, it is important to consider the functioning of and connectivity between these regions. First, the DMN has been hypothesized to undergird internally directed, stimulus-independent thinking.56 Consistent with this formulation, this network has been found to be active during periods of self-reported mind wandering in experience-sampling fMRI paradigms57 Recent meta-analytic syntheses indicate that the composition of the stimulus-independent thought supported by the DMN is broad, with this system supporting autobiographical memory retrieval, theory of mind operations, and prospection.30 These characteristics of the DMN converge to suggest that this neural system supports the construction of internally represented models of current and past events as well as future plans and outcomes.30
In better understanding the present data, it is important to consider the role of the ventral striatum in guiding behavior. According to actor–critic models of striatal functioning,58 the ventral striatum serves as the neural substrate for generating predictions of reward and punishment by receiving information about the internal and external environments and then computing predictions about rewards or punishments associated with these stimuli. Furthermore, it is important to note that the ventral striatum, in its role within the limbic subdivision of the cortico-striatal–pallido–thalamic loop, receives input about the state of the internal environment from ventral prefrontal and anterior cingulate regions considered to be hubs of the DMN.59 Finally, it is essential to consider that although higher ventral striatum activity has been linked most frequently to stimulus-seeking behaviors,60 recent work from Levita et al. has shown that deactivation of the ventral striatum plays a significant role in passive avoidance behaviors associated with behavioral inhibition and anxiety.61 Specifically, Levita et al. observed greater deactivation of the ventral striatum when participants withheld a button press during scanning to avoid seeing a noxious versus a nonnoxious stimulus; in addition, greater ventral striatum deactivation during passive avoidance was found to be associated with higher levels of state anxiety.61
Based on our findings that the DMN shows an anticorrelated relation with the ventral striatum that predicts elevated BI in at-risk children and adolescents, and on accounts of the functionality of the DMN and ventral striatum presented above, we speculate that DMN-mediated information pertinent to the internal environment is passed via cortico-striatal tracts to the ventral striatum where, in high-risk children or adolescents, deactivation of the ventral striatum occurs, similar to that associated with passive avoidance,61 which facilitates generation of negative outcome prediction. We propose that such a process linking information from the internal milieu with negative outcome prediction represents a biological substrate for BI in the risk for depression and anxiety. Although methodological limitations62 prevent us from inferring an inhibitory relation between the DMN and ventral striatum in children or adolescents at risk for MDD, we note that the distinction between patterns of positive and negative functional connectivity measured with fMRI is not arbitrary and, indeed, has been shown to mark qualitatively different psychological processes.63
Several limitations of the present study should be considered. First, we assessed children and adolescents over a relatively broad age range (8–17 years). Although the average age and range of ages across our high- and low-risk samples were well matched, variance in emotional functioning and neural connectivity over this age range may have reduced the sensitivity of our analyses. Moreover, this study incorporates a relatively small sample size; thus, we hasten to point out the preliminary nature of the findings presented here. Although the effect sizes observed were large (all Cohen’s d > 1), it will be important, in larger future efforts, to test the reliability of our findings. Moreover, we used self-report measures of behavioral inhibition and anxiety in this study; although the self-report forms that we used have been validated in children and adolescents, collecting convergent reports from teachers and parents would have further strengthened our results. Finally, our mood-disorder proband sample had unusually low levels of comorbid anxiety, indicating that this sample might not have been sufficiently representative to warrant generalization from our high-risk sample to the broader population of individuals at risk for internalizing disorders.
The results presented here support an intuitive and neuroanatomically viable neural rendering of BI in the risk for depression and anxiety. Our findings show an inhibitory relation between the DMN and ventral striatum in the risk for internalizing disorders and present an intriguing contrast with existing data showing increased striatal reactivity to cues signaling impending gain or loss in behaviorally inhibited relative to noninhibited adolescents,64 a finding that has been replicated.65 Although there has been some speculation that this might relate to the role of the ventral striatum in generating both negative and positive outcome predictions,66 the present findings indicate that the distinction between internally and externally generated predictive cues could be valuable in formulating future research.
Supplementary Material
Acknowledgments
The authors thank the Warren Foundation for funding the research presented in this article.
Footnotes
Disclosure: Drs. Molfese, Marx, Thomason, Gotlib, Drevets, Hamilton, Ms. Frost Bellgowan, Mr. Glen, and Ms. Santiago report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder—results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–3105. [DOI] [PubMed] [Google Scholar]
- 3.Drevets WC, Raichle ME. Neuroanatomical circuits in depression—implications for treatment mechanisms. Psychopharmacol Bull. 1992;28: 261–274. [PubMed] [Google Scholar]
- 4.Foland-Ross LC, Hardin MG, Gotlib IH. Neurobiological markers of familial risk for depression. In: Cowen PJ, Sharp T, Lau JY, eds. Behavioral Neurobiology of Depression and Its Treatment, Vol 14. Berlin: Springer-Verlag; 2013:181–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. [DOI] [PubMed] [Google Scholar]
- 6.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monk CS, Klein RG, Telzer EH, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. [DOI] [PubMed] [Google Scholar]
- 8.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levesque ML, Beauregard M, Ottenhof KW, et al. Altered patterns of brain activity during transient sadness in children at familial risk for major depression. J Affect Disord. 2011;135:410–413. [DOI] [PubMed] [Google Scholar]
- 10.Mannie ZN, Taylor MJ, Harmer CJ, Cowen PJ, Norbury R. Frontolimbic responses to emotional faces in young people at familial risk of depression. J Affect Disord. 2011;130:127–132. [DOI] [PubMed] [Google Scholar]
- 11.Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. J Abnorm Psychol. 2012;121:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olino TM, McMakin DL, Morgan JK, et al. Reduced reward anticipation in youth at high-risk for unipolar depression: a preliminary study. Dev Cogn Neurosci. 2014;8:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieb R, Isensee B, Hofler M, Pfister H, Wittchen HU. Parental major depression and the risk of depression and other mental disorders in offspring—a prospective-longitudinal community study. Arch Gen Psychiatry. 2002;59:365–374. [DOI] [PubMed] [Google Scholar]
- 14.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am J Psychiatry. 2012;169:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole DA, Peeke LG, Martin JM, Truglio R, Seroczynski AD. A longitudinal look at the relation between depression and anxiety in children and adolescents. J Consult Clin Psychol. 1998;66:451–460. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: prospective prediction of panic attacks and Axis I pathology. J Psychiatr Res. 2006;40: 691–699. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum JF, Biederman J, Hirshfeld-Becker DR, et al. A controlled study of behavioral inhibition in children of parents with panic disorder and depression. Am J Psychiatry. 2000;157:2002–2010. [DOI] [PubMed] [Google Scholar]
- 19.Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. [DOI] [PubMed] [Google Scholar]
- 20.Hirshfeld-Becker DR, Biederman J, Henin A, et al. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28: 225–233. [DOI] [PubMed] [Google Scholar]
- 21.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral-inhibition in children. Child Dev. 1987;581459–581473. [PubMed] [Google Scholar]
- 22.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64:903–912. [DOI] [PubMed] [Google Scholar]
- 24.Pine DS, Cohen P, Gurley D, Brook J, Ma YJ. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. [DOI] [PubMed] [Google Scholar]
- 25.Gladstone GL, Parker GB. Is behavioral inhibition a risk factor for depression? J Affect Disord. 2006;95:85–94. [DOI] [PubMed] [Google Scholar]
- 26.Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. [DOI] [PubMed] [Google Scholar]
- 27.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. [DOI] [PubMed] [Google Scholar]
- 28.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. [DOI] [PubMed] [Google Scholar]
- 31.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu XL, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state Default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. [DOI] [PubMed] [Google Scholar]
- 33.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208–1208. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R Personality-Disorders (SCID-I). J Personal Disord. 1995;9:83–91. [Google Scholar]
- 36.NIMH. NIMH Genetics Initiative: FAMily Interview for Genetic Studies (FIGS). Rockville, MD: National Institute of Mental Health; 1992. [Google Scholar]
- 37.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs M. The Childrens Depression Inventory (CDI). Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 39.March JS, Parker JDA, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. [DOI] [PubMed] [Google Scholar]
- 40.March JS, Sullivan K, Parker J. Test-retest reliability of the multidimensional anxiety scale for children. J Anxiety Disord. 1999;13:349–358. [DOI] [PubMed] [Google Scholar]
- 41.Broeren S, Muris P. A psychometric evaluation of the behavioral inhibition questionnaire in a non-clinical sample of dutch children and adolescents. Child Psychiatry Hum Dev. 2010;41:214–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bond L, Clements J, Bertalli N, et al. A comparison of self-reported puberty using the Pubertal Development Scale and the Sexual Maturation Scale in a school-based epidemiologic survey. J Adolesc. 2006;29:709–720. [DOI] [PubMed] [Google Scholar]
- 43.Axelrod BN. Validity of the Wechsler Abbreviated Scale of Intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002;9:17–23. [DOI] [PubMed] [Google Scholar]
- 44.Bornstein MH, Hahn CS, Suwalsky JTD, Haynes OM. Socioeconomic status, parenting, and child development: The Hollingshead Four-Factor Index of Social Status and the Socioeconomic Index of Occupations. In: Bornstein MH, Bradley RH, eds. Socioeconomic Status, Parenting, and Child Development. Mahwah, NJ: Lawrence Erlbaum; 2003:29–82. [Google Scholar]
- 45.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 46.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomason ME, Dennis EL, Joshi AA, et al. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011;55:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 49.Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. [DOI] [PubMed] [Google Scholar]
- 51.Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marx M, Pauly KB, Chang C. A novel approach for global noise reduction in resting-state fMRI: APPLECOR. Neuroimage. 2013;64:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Compar Neurol. 2004; 476:330–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum; 1988. [Google Scholar]
- 56.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106: 8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. [DOI] [PubMed] [Google Scholar]
- 59.Alexander GE, Delong MR, Strick PL. Parallel Organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 60.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levita L, Hoskin R, Champi S. Avoidance of harm and anxiety: a role for the nucleus accumbens. Neuroimage. 2012;62:189–198. [DOI] [PubMed] [Google Scholar]
- 62.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. [DOI] [PubMed] [Google Scholar]
- 63.Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26:6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guyer AE, Choate VR, Detloff A, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helfinstein SM, Fox NA, Pine DS. Approach-withdrawal and the role of the striatum in the temperament of behavioral inhibition. Dev Psychol. 2012;48:815–826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.